Introduction
Diabetic nephropathy (DN) is one of the most common
and serious chronic complications of diabetes. Without appropriate
treatment, DN can progress to end-stage renal disease (1,2);
however, the pathogenesis of DN is complex and unclear. Previous
studies have revealed that DN is associated with genetic
susceptibility, abnormal renal hemodynamics, oxidative stress and
inflammatory response (3-5).
The p38MAPK signaling pathway is an important branch of the MAPK
pathway, which is activated in the kidney, and can promote
glomerulosclerosis and tubulointerstitial fibrosis by participating
in inflammatory reactions and oxidative stress (6). Nucleotide-binding oligomerization
domain-like receptor protein 3 (NLRP3) inflammasome is a molecular
platform that activates caspase-1. The NLRP3 inflammasome can
regulate the maturation and secretion of pro-inflammatory
cytokines, and can thus regulate the chronic inflammatory response
(7,8). Sustained hyperglycemia in type 2
diabetes mellitus (T2DM) can activate the p38MAPK signaling pathway
and the NLRP3 inflammasome, which can further affect the occurrence
and development of DN by regulating the expression of downstream
related factors (9-11)
Therefore, studying changes in the p38MAPK signaling pathway and
NLRP3 inflammasome in the kidneys of patients with diabetes may be
of great significance for further study of the pathogenesis of
DN.
At present, the clinical treatment of T2DM includes
improving diet, appropriate exercise, drug control of blood glucose
and surgery (12-14).
Most of these drugs can control blood glucose well, but long-term
use is prone to drug resistance and adverse reactions (3,14). For
example, oral hypoglycemic drugs such as sulfonylureas, biguanides
and insulin sensitizers can cause drowsiness and gastrointestinal
reactions (13). After subcutaneous
injection, allergic reactions, sarcoidosis and blood stasis may
occur. In addition, it may cause lipoatrophy under the skin, which
will lead to unstable insulin absorption, and it is very difficult
to resolve spontaneously once it occurs (14). Therefore, it is necessary to
identify a drug with reliable therapeutic effect, fewer adverse
reactions and good kidney protection.
Sericin is a water extract obtained from the cocoon
of silkworms (Bombyx mori) and is a natural water-soluble
protein. It has been demonstrated that sericin can effectively
reduce blood glucose in rats with T2DM and protect against
diabetes-induced kidney damage (15,16).
However, the underlying mechanism is currently unclear. The present
study aimed to investigate the protective effects and underlying
mechanism of sericin on kidney injury in rats with T2DM. Changes in
key proteins associated with the p38MAPK signaling pathway and
NLRP3 inflammasome were analyzed. The present findings may provide
novel insights for the prevention and treatment of T2DM and its
complications.
Materials and methods
Animals
Male Sprague-Dawley rats (n=42; weight, 170-190 g;
age, 5-6 weeks) were purchased from Beijing Vital River Laboratory
Animal Technology Co., Ltd. and were maintained under standard
conditions (Temperature, 20-24˚C; humidity, 50-55%; free access to
food and water; 12/12 h light/dark cycle). The health and behaviors
of the rats were monitored every day. The main humane endpoints of
reduced heart rate and respiration rate were used to determine when
animals should be euthanized. All animal experimental procedures
were approved by the Ethics Committee of Chengde Medical University
(Chengde, China).
Establishment of a rat model of
T2DM
A rat model of T2DM was established in 30 rats
according to previous studies (17-19).
Briefly, rats were fed a high-fat and high-sugar diet (59% of basic
feed, 20% of white sugar, 3% of egg yolk, 18% of lard) for 4 weeks
(20,21), and were then intraperitoneally
injected with 35 mg·kg-1·d-1 streptozotocin
(Sigma-Aldrich; Merck KGaA) for 2 days. If fasting blood glucose
was ≥11.1 mmol·l-1 for three consecutive measurements at
72 h after the last injection, the T2DM model was considered
successfully established. During model establishment, six rats died
(22-24).
Animal grouping and treatment
After successful T2DM modeling, the rats continued
to be fed with a high-fat and high-sugar diet for 4 weeks (17). Subsequently, the T2DM model rats
were randomly divided into the T2DM model group and the sericin
group (n=12 rats/group). Rats in the sericin and T2DM model groups
were administered 2.4 g·kg-1·d-1 sericin by
gavage and an equal volume of normal saline, respectively, for 35
days. In parallel, rats in the normal group (fed with a normal diet
ad libitum; n=12) were administered an equal volume of
normal saline for 35 days. After treatment for 35 days, the rats in
each group were fasted for 12 h. Blood samples were collected via
the tail vein for blood glucose testing once a week; 5-10 µl blood
was collected each time. Kidney tissues were collected following
anesthesia (intraperitoneal injection of 300 mg/kg 10% chloral
hydrate) and sacrifice by decapitation. During anesthesia, the rats
did not exhibit any signs of peritonitis, pain or discomfort.
Detection of blood glucose levels
Blood samples from each group of rats were
centrifuged at 800 x g for 20 min at 4˚C. The serum was then
collected and glucose levels were detected using the Beckman
Coulter AU5800 Automatic Biochemical Analyzer (Beckman Coulter,
Inc.).
H&E staining
The kidney morphology of each group was observed by
H&E staining. Briefly, kidney samples were fixed in Bouins
fixative liquid [a mixture of picric acid saturated liquid (1.22%),
formaldehyde and glacial acetic acid in a ratio of 15:5:1] at room
temperature for 24 h, embedded in paraffin and cut into 5-µm
sections. The sections were stained with hematoxylin (room
temperature for 7 min), eosin (room temperature for 1 min),
dehydrated in gradient ethanol and incubated with xylene (room
temperature for 30 min). The samples were then observed under a
light microscope (OLYMPUS BH-2; Olympus Corporation).
Immunohistochemistry
Kidney tissue sections were incubated with 3%
H2O2-methanol for 30 min at 37˚C to
inactivate endogenous peroxidase activity. Antigen retrieval was
then performed in a microwave for 8 min with 0.01 M citrate buffer
(pH, 6.0). Subsequently, tissue sections were incubated with the
following primary antibodies at 4˚C overnight: MKK6 (1:100; cat.
no. A2575; Wuhan Aibotek Biotechnology Co., Ltd.), phosphorylated
(p)-p38MAPK (1:50; cat. no. sc-166182; Santa Cruz Biotechnology,
Inc.), NF-κB (1:50; cat. no. ab16502; Abcam), NLRP3 (1:100; cat.
no. DF7438; Affinity Biosciences), caspase-1 (1:200; cat. no.
A0964; Wuhan Aibotek Biotechnology Co., Ltd.), IL-1β (1:200; cat.
no. AF5103; Affinity Biosciences) and IL-6 (1:200; cat. no. DF6087;
Affinity Biosciences). After washing with PBS, secondary antibodies
(1:300; Biotin labeled goat anti-rabbit IgG polymer; cat. no.
SP-9001; Beijing Zhong Shan-Golden Bridge Biological Technology
Co., Ltd.) were added and incubated at 37˚C for 40 min. After DAB
staining at room temperature for 2 min, hematoxylin counterstaining
was performed at room temperature for 3 min. Brownish yellow and/or
tan particles in the glomeruli and/or renal tubules were considered
positive staining. Six rats were randomly selected from each group,
and three non-continuous kidney slices were randomly selected from
each rat. Three non-overlapping fields containing glomeruli and
tubules were randomly selected for observation in each slice. The
integrated optical density value of the positive staining in each
visual field was calculated using Image-ProPlus 6.0 software (Media
Cybernetics, Inc.), and the average optical density value was taken
as the relative expression level of the target protein.
Western blotting
Total protein was extracted from kidney tissues
using the RIPA (cat. no. R0020, Beijing Soleibao Technology Co.,
Ltd.) and quantified using the BCA protein concentration
determination kit (cat. no. PC0020, Beijing Soleibao Technology
Co., Ltd.). Total protein (60 µg) was separated by SDS-PAGE on
10-12% gels and was then transferred onto PVDF membranes (cat. no.
IEVH10100; EMD Millipore). After blocking with 5% non-fat milk at
room temperature for 1 h, membranes were incubated with the
following primary antibodies at room temperature for 2 h: MKK6
(1:500; cat. no. A2575; Wuhan Aibotek Biotechnology Co., Ltd.),
p38MAPK (1:500; cat. no. AF6456; Affinity Biosciences), p-p38MAPK
(1:1,000; cat. no. sc-166182; Santa Cruz Biotechnology, Inc.),
NF-κB (1:2,000; cat. no. ab16502; Abcam), NLRP3 (1:1,000; cat. no.
DF7438; Affinity Biosciences), caspase -1 (1:500; cat. no. A0964;
Wuhan Aibotek Biotechnology Co., Ltd.) and β-actin (1:500; cat. no.
AF7018; Affinity Biosciences). The secondary antibody (1:10,000;
cat. no. 074-1806; KPL, Inc.) was then added and the membranes were
incubated at room temperature for 1.5 h. Subsequently, the
membranes were washed and subjected to color development using the
Super ECL Plus ultra-sensitive luminescent solution (cat. no.
P1050; Beijing Pulilai Gene Technology Company). The gray
intensities of each band were measured using Quantity One-v 4.6.2
software (Bio-Rad Laboratories, Inc.). The ratio of the gray value
of the target protein to β-actin was taken as the relative
expression level of each target protein.
ELISA
The detection of IL-1β and IL-6 in serum was
performed using the Rat IL-1β/IL-6 ELISA kit (RayBiotech, Inc.;
IL-1β, cat. no. Q63264; IL-6 cat. no. P20607), according to the
manufacturer's protocol. The absorbance was measured at 450 nm
using a microplate reader (ELX808; BioTek Corporation). The
corresponding concentration was then calculated.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from 100 mg kidney tissue
using the TaKaRa MiniBEST Universal RNA Extraction kit (cat. no.
9767; Takara Biotechnology Co., Ltd.). cDNA was then generated by
using the PrimeScript™ RT Master Mix (Takara Biotechnology Co.,
Ltd.; cat. no. RR036A) at 37˚C for 15 min, and 85˚C for 5 sec. The
mRNA expression levels of MKK6, p38MAPK, NF-κB, IL-1β, IL-6, NLRP3,
caspase-1 and GAPDH genes were detected with RT-qPCR. The primer
sequences are listed in Table I.
qPCR was performed using 12.5 µl SYBR Premix Ex TaqII (Takara
Biotechnology Co., Ltd.; cat. no. RR820A), 1 µl PCR forward primer,
1 µl PCR reverse primer, 2 µl cDNA and 8.5 µl ddH2O. The
thermocycling conditions were as follows: 95˚C for 30 sec, followed
by 40 cycles at 95˚C for 5 sec and 60˚C for 30 sec, and a final
step at 60˚C for 5 min. The relative expression level of each
target gene was analyzed using the
2-∆∆Cq method (25).
 | Table IPrimers used in this study. |
Table I
Primers used in this study.
| Gene | Sequence
(5'-3') | Primer length
(bp) |
|---|
| MKK6 | F:
AACGGCCCACGTATCCAGAG | 147 |
| | R:
CCACCAATCCACAGTAGGGTCA | |
| p38MAPK | F:
TTACCGATGACCACGTTCAGTTTC | 107 |
| | R:
AGCGAGGTTGCTGGGCTTTA | |
| NF-κB | F:
GATGGGACGACACCTCTACACATA | 130 |
| | R:
CCCAAGAGTCGTCCAGGTCA | |
| IL-1β | F:
CCCTGAACTCAACTGTGAAATAGCA | 111 |
| | R:
CCCAAGTCAAGGGCTTGGAA | |
| IL-6 | F:
ATTGTATGAACAGCGATGATGCAC | 150 |
| | R:
CCAGGTAGAAACGGAACTCCAGA | |
| NLRP3 | F:
CTGAAGCATCTGCTCTGCAACC | 87 |
| | R:
AACCAATGCGAGATCCTGACAAC | |
| Caspase-1 | F:
ACTCGTACACGTCTTGCCCTCA | 190 |
| | R:
CTGGGCAGGCAGCAAATTC | |
| GAPDH | F:
GGCACAGTCAAGGCTGAGAATG | 143 |
| | R:
ATGGTGGTGAAGACGCCAGTA | |
Statistical analysis
Data were analyzed using IBM SPSS Statistics 21.0
statistical software (IBM Corp.). Data are presented as the mean ±
SD of three independent experiments. Multi-group comparisons were
performed by one-way ANOVA and pairwise comparisons were conducted
using LSD-t post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Sericin reduces blood glucose levels
in rats
To investigate the effects of sericin on blood
glucose levels, blood glucose was measured using the glucose
oxidase method. The blood glucose of rats in the normal, T2DM model
and sericin groups was 10.83±2.03, 29.45±4.82 and 13.20±4.09
mmol·l-1, respectively (Table II). The blood glucose levels in the
T2DM model group were significantly higher than those in the normal
group (P<0.05), whereas those in the sericin group were
significantly lower than those in the T2DM model group
(P<0.05).
 | Table IIBlood glucose levels of each
group. |
Table II
Blood glucose levels of each
group.
| | Normal group | T2DM model
group | Sericin groups |
|---|
| Blood glucose
(mmol·l-1) | 10.83±2.03 |
29.45±4.82a |
13.20±4.09b |
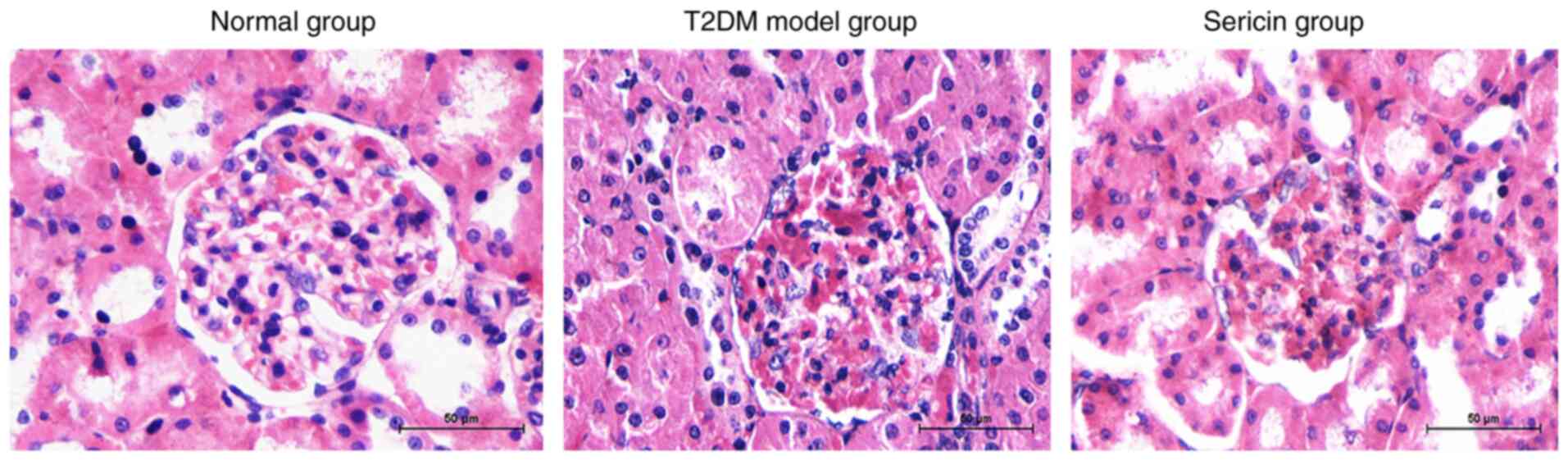
Sericin relieves the T2DM-induced
pathological changes to the rat kidney
To further address the effects of sericin, H&E
staining was conducted to detect the morphological structure of rat
kidneys in each group. Compared with in rats in the normal group,
renal glomerular hypertrophy and mesangial hyperplasia, thickened
basement membrane and extracellular matrix accumulation were
detected in the T2DM model group (Fig.
1). The renal pathological manifestations in the sericin group
were obviously reduced. The renal mesangial hyperplasia, basement
membrane thickening and renal interstitial fibrosis were milder in
the sericin group than those in the T2DM model group.
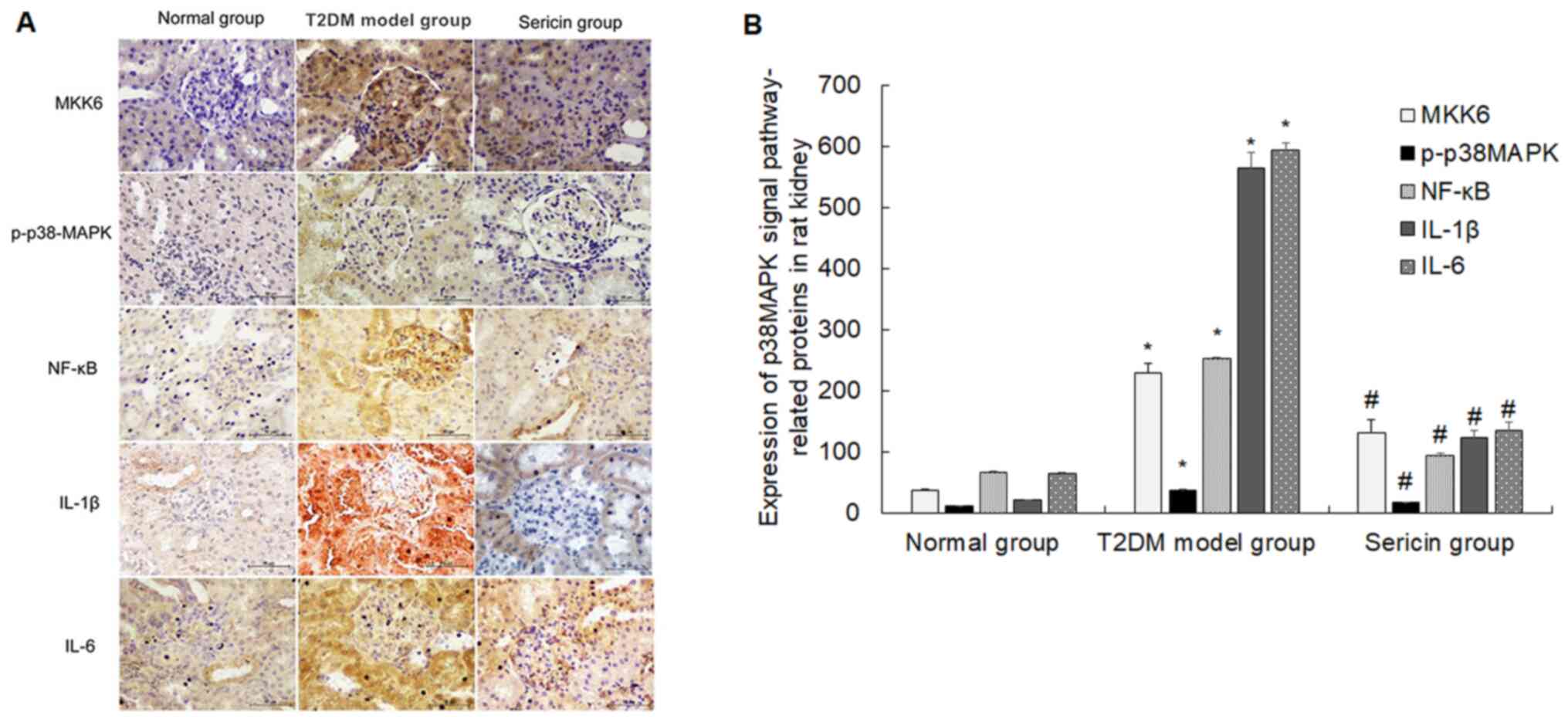
Sericin suppresses the expression of
p38MAPK signaling pathway-related proteins and genes in the rat
kidney
The present study measured the effects of sericin on
the expression levels of p38MAPK signaling pathway-related
proteins. The positive staining of MKK6, p-p38MAPK, NF-κB, IL-1β
and IL-6 protein was shown as brownish yellow and/or brown
granules, which were mainly distributed in renal tubular epithelial
cells and mesangial cells (Fig.
2A). The protein expression levels of MKK6, p-p38MAPK, NF-κB,
IL-1β and IL-6 were highest in the T2DM model group and lowest in
the normal group (Fig. 2B). The
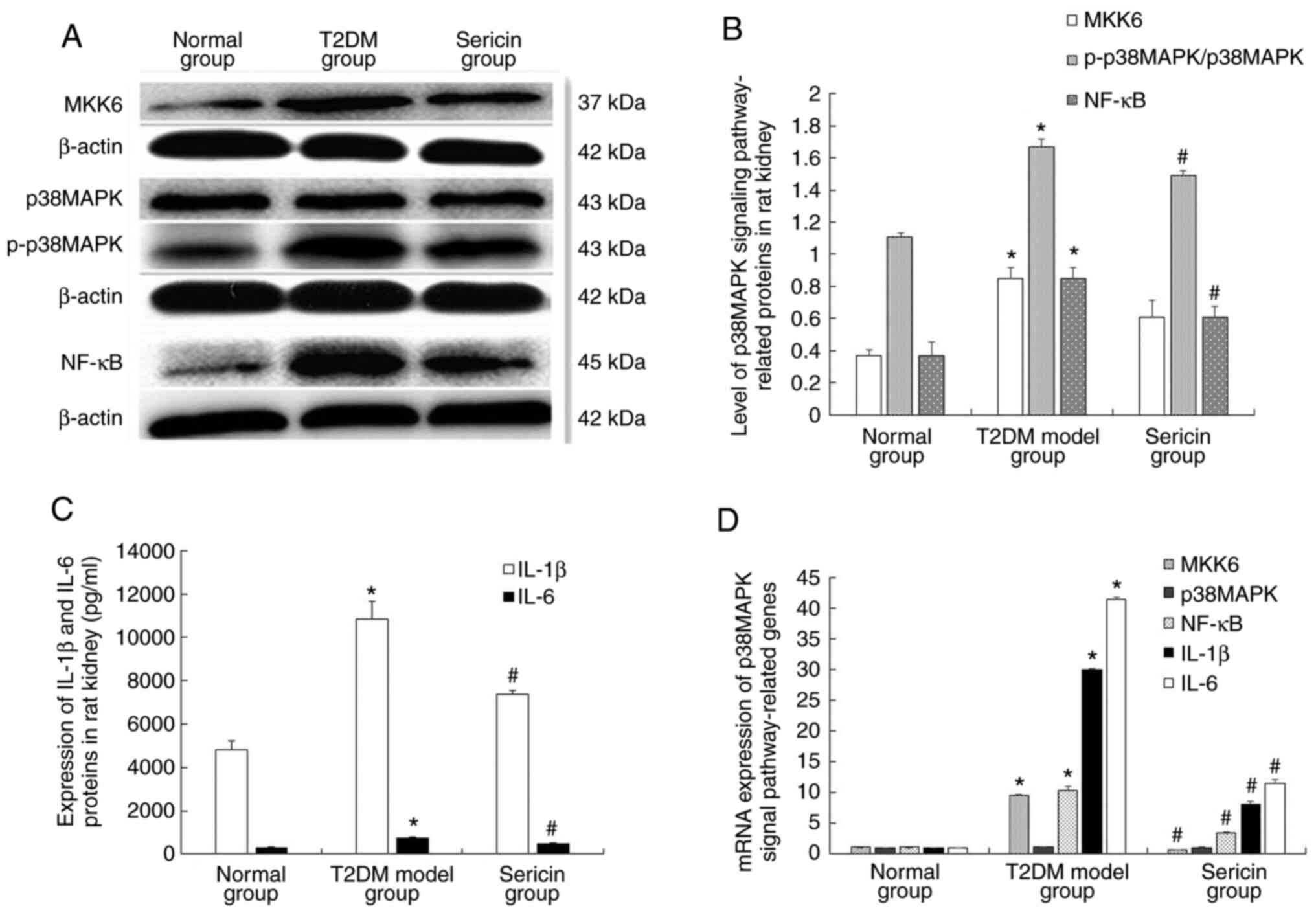
results of western blotting are shown in Fig. 3A. Clear MKK6, p38MAPK, p-p38MAPK,
NF-κB and β-actin bands were observed at 37, 43, 43, 45 and 42 kDa,
respectively. The protein expression levels of MKK6, p-p38MAPK and
NF-κB were highest in the T2DM model group and lowest in the normal
group (Fig. 3B). Levels of IL-1β
and IL-6 were further analyzed using ELISA (Fig. 3C). The levels of IL-1β and IL-6 in
the kidney tissue of the T2DM model group were significantly higher
than that of the normal group (P<0.05); while those in the
sericin group were significantly lower than that of the T2DM model
group (P<0.05). The mRNA expression levels of MKK6, p38MAPK,
NF-κB, IL-1β and IL-6 were measured using RT-qPCR (Fig. 3D). Statistically, the protein and
mRNA expression levels of MKK6, NF-κB, IL-1β and IL-6 in the
kidneys of the T2DM model group were significantly higher than
those in the normal group (P<0.05). Notably, the protein and
mRNA expression levels of MKK-6, NF-κB, IL-1β and IL-6 were
significantly lower in the sericin group compared with those in the
T2DM model group (P<0.05). The protein expression levels of
p-p38MAPK were significantly higher in the kidney of rats in the
T2DM model group than those in the normal group (P<0.05),
whereas the protein expression levels of p-p38MAPK were
significantly lower in the kidney of rats the sericin group
compared with those in the T2DM model group (P<0.05). However,
there was no significant difference in the mRNA and protein
expression levels of p38MAPK in each group of rats (P>0.05).
Taken together, these results indicated that sericin reduced the
expression of p38MAPK signaling pathway-related genes and proteins
in rat kidney samples.
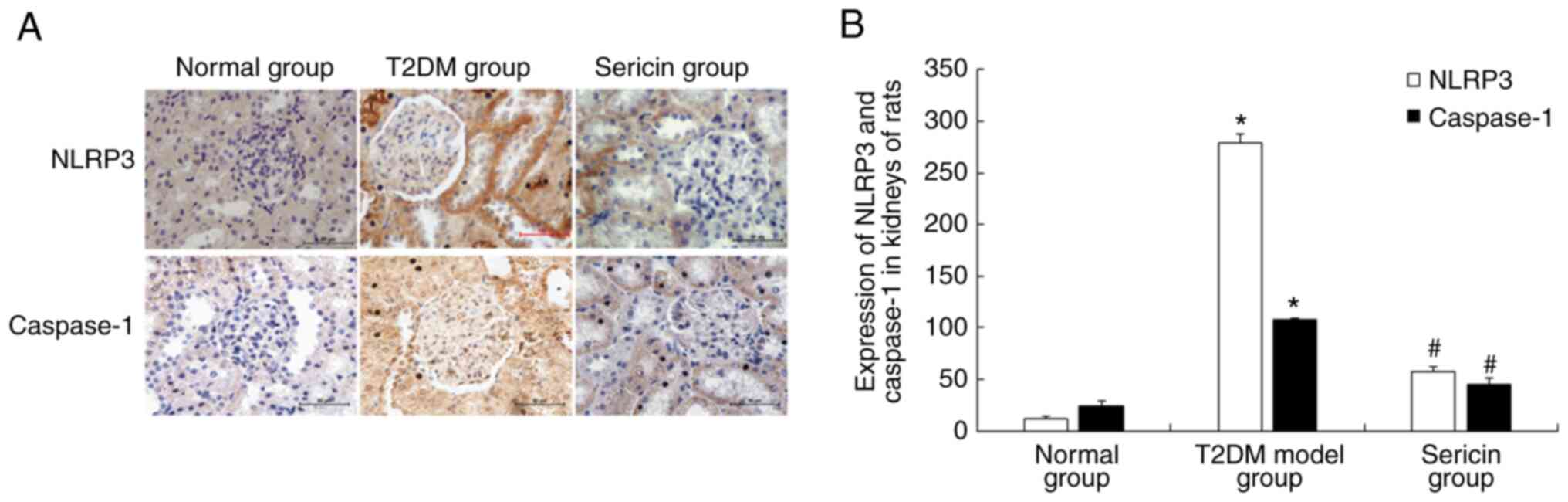
Sericin reduces the expression of
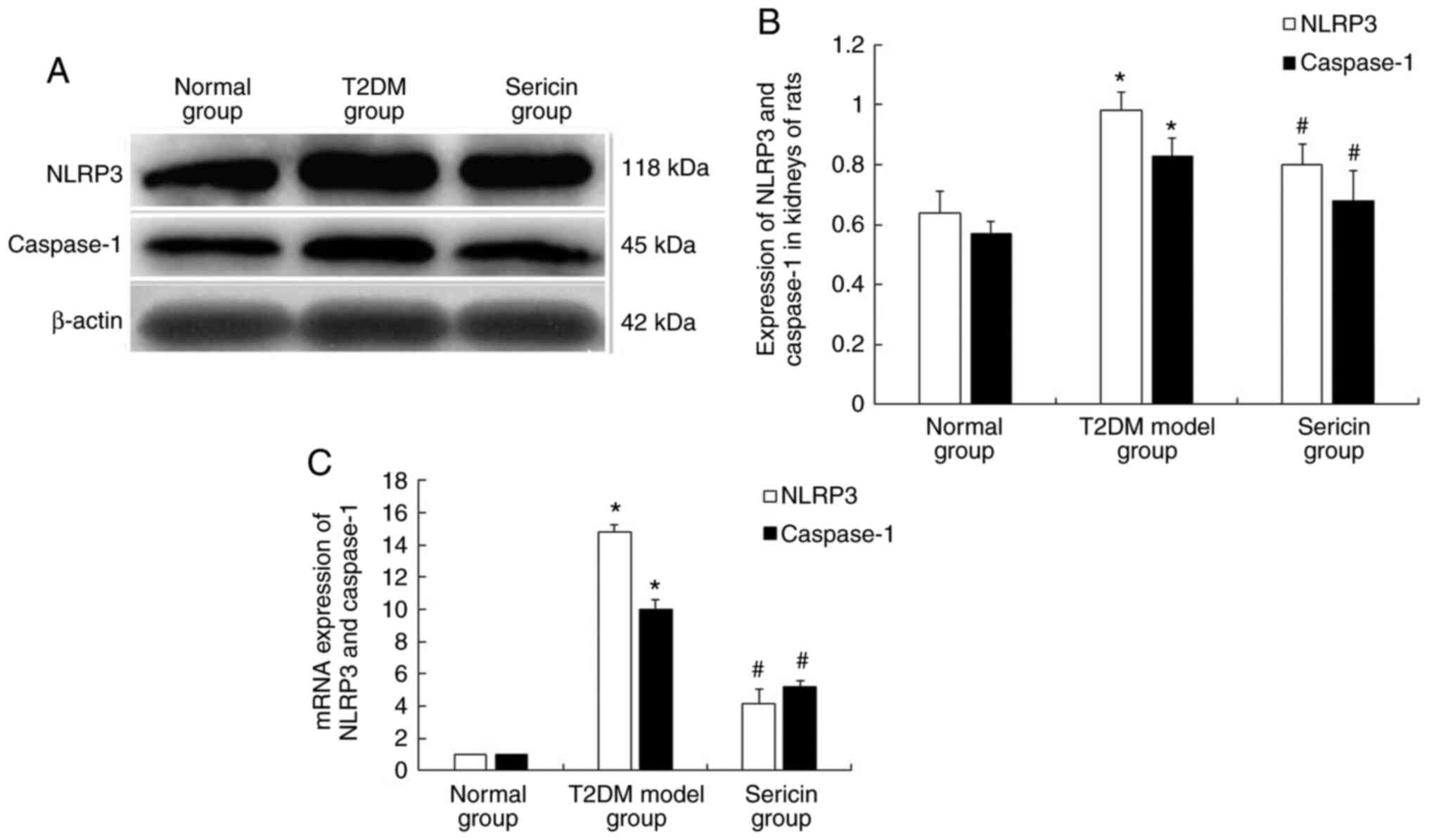
NLRP3 and caspase-1 in the kidneys of rats
The present study further tested the effects of
sericin on the expression levels of NLRP3 and caspase-1. Using
immunohistochemistry, NLRP3 and caspase-1 proteins were shown as
brownish yellow and/or tan granules. NLRP3 protein was mainly
expressed in the cytoplasm of renal tubular epithelial cells, and
caspase-1 protein was mainly expressed in renal tubular epithelial
cells and mesangial cells (Fig.
4A). The protein expression levels of NLRP3 and caspase-1 were
highest in the T2DM model group and lowest in the normal group
(Fig. 4B). The results of western
blotting are shown in Fig. 5A.
Clear NLRP3, caspase-1 and β-actin bands were seen at 118, 45 and
42 kDa, respectively. Consistently, the protein expression levels
of NLRP3 and caspase-1 were highest in the T2DM model group and
lowest in the normal group (Fig.
5B). RT-qPCR was conducted to detect their expression at the
mRNA level (Fig. 5C). The results
showed that the protein and mRNA expression levels of NLRP3 and
caspase-1 were significantly higher in the kidney of rats in the
T2DM model group than those in the normal group (P<0.05).
Conversely, their expression levels were significantly lower in the
kidney of rats in the sericin group compared with those in the T2DM
model group (P<0.05). These results indicated that sericin may
reduce the expression of NLRP3 and caspase-1 in the kidneys of
rats.
Discussion
T2DM is a chronic metabolic disease characterized by
insulin resistance and multiple organ damage, which often occurs in
the kidneys and can cause DN. DN is one of the serious
microvascular complications of T2DM (26,27).
Typical pathological changes of DN include hypertrophy of renal
cells, thickening of the glomerular basement membrane and
accumulation of the extracellular matrix, which can further cause
glomerulosclerosis and tubulointerstitial fibrosis, and eventually
lead to renal insufficiency or renal failure (28,29).
The pathogenesis of DN has not yet been fully elucidated, but
scholars have recognized that it is caused by the participation of
multiple risk factors in a certain genetic background, and the
inflammatory response is known to have an important role (30,31).
The p38MAPK signaling pathway can regulate the
inflammatory response of the kidney and is one of target pathways
of anti-inflammatory drugs (32).
The p38MAPK signaling pathway is characterized by a three-level
kinase cascade; under the action of hyperglycemia, MAPK kinase is
phosphorylated first, and then the threonine-tyrosine site in the
p38MAPK domain is phosphorylated to activate p38MAPK by activating
MKK6, which becomes p-p38MAPK (6,33). The
biological activity of p-p38MAPK further induces NF-κB, allowing it
to enter the nucleus from the cytoplasm, where it binds to the
target gene promoter or enhancer to activate IL-1β and IL-6, thus
inducing inflammation (34,35). IL-1β and IL-6 are important
regulators of inflammation. IL-1β can stimulate the proliferation
of mesangial cells and release reactive oxygen to participate in
kidney inflammation (36,37). IL-6 is involved in the inflammatory
response of the kidney through promoting mesangial cell
proliferation, extracellular matrix synthesis and local
infiltration of macrophages in renal tissues, and can accelerate
glomerular sclerosis, lead to tubular atrophy and interstitial
fibrosis, and thereby advance the progression of DN (37,38).
The present study revealed that fasting blood glucose, and the
expression levels of renal MKK6, p-p38MAPK, NF-κB, IL-1β and IL-6
were significantly higher in the T2DM model group compared with
those in the normal group. These findings indicated that under
hyperglycemia, the p38MAPK signaling pathway was activated in the
rat kidney, which may induce the activation of downstream
inflammatory cells and promote the expression of inflammatory
mediators. This may therefore lead to an increase in the production
of inflammatory factors that affect the kidney tissue, and cause
changes in kidney morphology and function. In addition,
hyperglycemia may stimulate the renal p38MAPK signaling pathway to
further induce oxidative stress and accelerate the process of renal
fibrosis, whereas oxidative stress can also activate the p38MAPK
signaling pathway. This vicious cycle may further promote the
occurrence and development of DN (34,39).
Notably, p38MAPK is biologically active only in the form of
p-p38MAPK; therefore, no significant difference was detected in
p38MAPK expression between the groups of rats in the present
study.
The NLRP3 inflammasome is an important part of the
immune inflammatory response and has been reported to serve an
important role in the occurrence and development of DN (40). The NLRP3 inflammasome consists of
NLRP3, caspase-1 and apoptosis-related speckle-like protein. Under
the stimulation of hyperglycemia, the domain of NLRP3 is changed
and the caspase-1 precursor is cleaved to generate active caspase-1
with hydrolase activity. Activated caspase-1 can promote the
processing of inactive IL-1β precursors, and also induce the
maturation and secretion of IL-1β to participate in the
inflammation of the kidney (41-43).
Notably, elevated IL-1β levels can also activate NF-κB in renal
tubular epithelial cells, further amplifying the inflammatory
response and accelerating the process of renal fibrosis (44). In the present study, the expression
levels of NLRP3 and caspase-1 were significantly higher in the
kidney of rats in the T2DM model group compared with those in the
normal group, thus suggesting that under hyperglycemic conditions,
the NLRP3 inflammasome may be activated, which could further
aggravate the inflammatory response of the kidney, causing
significant pathological changes in the kidneys of the T2DM model
group rats.
DN is a fatal chronic complication of diabetes. Once
it develops into end-stage renal disease, severe and complex
metabolic disorders often occur, resulting in poor prognosis
(45). Therefore, only with early
targeted prevention and treatment of DN can further development of
kidney damage be effectively controlled and delayed. At present,
the treatment of clinical DN is mainly based on hypoglycemic,
antihypertensive, lipid-lowering and anti-inflammatory therapies,
and other measures (46). Although
various hypoglycemic drugs can control blood glucose, long-term
administration is prone to induce drug resistance, and liver and
kidney damage. Therefore, a large number of studies have focused on
natural drugs that have a reliable hypoglycemic effect and exhibit
kidney protection (47-49).
Sericin is a natural water-soluble protein found in silk cocoons,
which is composed of 18 amino acids. In the molecular structure,
hydroxyl, carboxyl and amino groups of amino acids with strong
polar side groups account for the vast majority (50). Sericin is biodegradable and has no
toxic side effects on the human body. In China, silkworm cocoons
soaked in boiling water have been reported to reduce blood glucose
(51). Our previous studies also
demonstrated that sericin was able to lower blood glucose and
protect organs, such as the liver, kidney and islet cells (16,52,53);
however, the specific underlying mechanism is still unclear. In the
present study, the rats were treated with sericin by gavage. The
levels of fasting blood glucose, and the expression levels of MKK6,
p-p38MAPK, NF-κB, IL-1β, IL-6, NLRP3 and caspase-1 in the kidney
were lower in the sericin group than those in the T2DM model group.
In addition, the pathological changes in the kidneys of the sericin
group were markedly reduced. These findings indicated that the
expression of related factors in the p38MAPK signaling pathway and
NLRP3 inflammasome in the kidney of rats with T2DM changed
following gavage treatment with sericin. Therefore, sericin may
reduce blood glucose and renal injury in T2DM rats by regulating
these factors.
The present study had some limitations. Firstly,
there was a lack of experiments on the interaction between sericin
and the p38MAPK signaling pathway in models without hyperglycemia.
Secondly, the phosphorylation levels of related factors in the
p38MAPK signaling pathway were not analyzed due to the limited
amount of kidney tissues and the unavailability of the antibody.
Further studies are thus warranted.
In conclusion, the present study demonstrated that
sericin reduced blood glucose, and inhibited activation of the
p38MAPK signaling pathway and NLRP3 inflammasome in the kidney of
rats with T2DM. These effects may reduce inflammation, weaken
T2DM-induced kidney damage, and delay the occurrence and
development of DN.
Acknowledgements
Not applicable.
Funding
The current study was supported by the National
Natural Science Foundation of China (grant no. 81441133) and the
Natural Science Foundation of Hebei Province (grant no.
H2013406096).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DL wrote the manuscript, performed the experiments
and analyzed the data. CC and DW collected the data. ZC and CS
conceived the idea, designed the study, provided technical support
and revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experimental procedures were approved by
the Ethics Committee of Chengde Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
He Y, Zhang L, Fang Z, Jiang H, Liu X and
Sun J: Regulation of Qizhen Jiangtang Granules on TGF-β1/Smad
signaling pathway in rats with diabetic nephropathy. J Anhui Univ
Tradit Chin Med. 39:50–55. 2020.
|
|
2
|
Zhang J: The effect of calcium dobesilate
capsule combined with ramipril in the treatment of early diabetic
nephropathy. Henan Medical Res. 28:2020–2021. 2019.
|
|
3
|
Chen X and Fang M: Oxidative stress
mediated mitochondrial damage plays roles in pathogenesis of
diabetic nephropathy rat. Eur Rev Med Pharmacol Sci. 22:5248–5254.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Manna K, Mishra S, Saha M, Mahapatra S,
Saha C, Yenge G, Nilesh Gaikwad, Pal R, Oulkar D, Banerjee K and
Das Saha K: Amelioration of diabetic nephropathy using pomegranate
peel extract-stabilized gold nanoparticles: Assessment of NF-κB and
Nrf2 signaling system. Int J Nanomedicine. 7:1753–1777.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sharma D, Bhattacharya P, Kalia K and
Tiwari V: Diabetic nephropathy: New insights into established
therapeutic paradigms and novel molecular targets. Diabetes Res
Clin Pract. 128:91–108. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cheng YX, Yang LL and Lin Y: Research
progress on the relationship between p38MAPK signaling pathway and
glomerular disease. Shandong Medical J. 58:98–101. 2018.
|
|
7
|
Shaw PJ, McDermott MF and Kanneganti TD:
Inflammasomes and autoimmunity. Trends Mol Med. 17:57–64.
2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Xuan HY, Chen YY and Wang CL: Inflammatory
bodies and kidney diseases. J Nephrol Dial Ren Transpl. 27:369–373.
2018.
|
|
9
|
Guo Y, Song Z, Zhou M, Yang Y, Zhao Y, Liu
B and Zhang X: Infiltrating macrophages in diabetic nephropathy
promote podocytes apoptosis via TNF-α-ROS-p38MAPK pathway.
Oncotarget. 8:53276–53287. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang X, Shi L, Han Z and Liu B:
Follistatin-like 3 suppresses cell proliferation and fibronectin
expression via p38MAPK pathway in rat mesangial cells cultured
under high glucose. Int J Clin Exp Med. 8:15214–15221.
2015.PubMed/NCBI
|
|
11
|
Luo B, Li B, Wang W, Liu X, Liu X, Xia Y,
Zhang C, Zhang Y, Zhang M and An F: Rosuvastatin alleviates
diabetic cardiomyopathy by inhibiting NLRP3 inflammasome and MAPK
pathways in a type 2 diabetes rat model. Cardiovasc Drugs Ther.
28:33–43. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xueyun X and Hanli C: The clinical effect
of Yiqi Huoxue Tongmai Decoction in adjuvant treatment of type 2
diabetic peripheral neuropathy. Inner Mongolia Tradit Chin Med.
39:9–11. 2020.
|
|
13
|
Lu M: Evaluation of the efficacy and drug
side effects of Baitangping and metformin in the treatment of type
2 diabetes. World Latest Medical Information Abstracts. 18:100–101.
2018.
|
|
14
|
Wang Q and Chang J: Subcutaneous injection
of insulin causes local skin adverse reactions. Chin J Dermatol.
48:65–67. 2015.
|
|
15
|
Fu XM, Zhong MR and Fu WL: Effect of
sericin on blood glucose and blood lipid in type 2 diabetic rats.
China J Gerontol. 31:103–105. 2011.
|
|
16
|
Song YX, Wang DD and Li DZ: Effect of
sericin on the expression of extracellular signal-regulated kinase
in the kidney of type 2 diabetic rats. China J Gerontol.
36:4050–4053. 2019.
|
|
17
|
Gao Y, Zhang M, Wu T, Xu M, Cai H and
Zhang Z: Effects of D-Pinitol on insulin resistance through
PI3K/Akt signaling pathway in type 2 diabetes mellitus rats. J
Agric Food Chem. 63:6019–6026. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yin M, Xu S, Wang Y, Sun X, Liang C, Li J
and Mu Y: Changes in the Wnt/β-catenin signaling pathway of the
aorta of type 2 diabetic rats and the regulatory effect of SIRT1.
Chin Pharmacol Bull. 32:337–342. 2016.
|
|
19
|
Zhang L, Wang L, Jia AO and Jia L: Effect
of water extract of okra on glucose and lipid metabolism in type 2
diabetic rats. Sci Technol Food Ind. 37:355–358, 363. 2016.
|
|
20
|
Chen S, Liang F and Wang H: High-fat,
low-sugar and high-fat, high-sugar diet to establish a rat model of
impaired glucose tolerance. Chin J Gerontol. 38:1930–1932. 2018.(In
Chinese).
|
|
21
|
Hao W, Li J and Jiang H: Advantages of
purified ingredient high-fat/high-sugar feed and its domestic
application status. J Hyg Res. 46:143–147. 2017.
|
|
22
|
You L, Lu K and Li Y: Intervention of
triptolide on the expression of glucose transporter type 1 and type
4 in the myocardium of diabetic rats. J Prac Med Tech. 22:573–575.
2015.
|
|
23
|
Dong Y: Effects of changes in serum T,
testicular ABP and INHB expression levels in hyperlipidemia and
diabetic rats on spermatogenic function, respectively. Guangxi
Medical University, Guangxi, 2018.
|
|
24
|
Zeng Z: The effect of telmisartan on the
PERK/ATF4/CHOP pathway of endoplasmic reticulum stress in rats with
diabetic myocardial damage. North China University of Technology,
Hebei, 2018.
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yang D, Chen H, Liu Z, Chen H and Li Y:
Study on the correlation between cystatin C, retinol binding
protein, free fatty acid and the occurrence and development of type
2 diabetic nephropathy. Mark Immunoassay Clin. 27:1022–1025, 1032.
2020.
|
|
27
|
Guang S, Zhai R and Wang L: The value of
serum homocysteine, cystatin C, and superoxide dismutase in the
early diagnosis of type 2 diabetic nephropathy. Chin J Lab Diagn.
24:1114–1117. 2020.
|
|
28
|
Xie R, Zhang HP and Liu XH: PINCH-1
protein and diabetic nephropathy. Chin J Clin. 10:2933–2936.
2016.
|
|
29
|
Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J,
Shan Z, Liu J, Tian H, Ji Q, et al: Prevalence of diabetes among
men and women in China. N Engl J Med. 362:1090–1101.
2010.PubMed/NCBI
|
|
30
|
Guo L, Meng H and Gao A: Changes and
significance of inflammatory factors and oxidative stress
indicators in patients with diabetic nephropathy in different
stages. J Inner Mongolia Med Univ. 41:370–373. 2019.
|
|
31
|
Wang Y, Yang L and Cheng T: Inflammatory
pathogenesis of diabetic nephropathy and prevention and treatment
of traditional Chinese medicine. Chin J Exp Formulas. 24:200–207.
2018.
|
|
32
|
O'Neil JD, Ammit AJ and Clark AR: MAPK p38
regulates inflammatory gene expression via tristetraprol in: Doing
good by stealth. Int J Biochem Cell Biol. 94:6–9. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liu Z, Lv Y, Zhang Y, Liu F, Zhu L, Pan S,
Qiu C, Guo Y, Yang T and Wang J: Matrine-type alkaloids inhibit
advanced glycation end products induced reactive oxygen
species-mediated apoptosis of aortic endothelial cells in vivo and
in vitro by targeting MKK3 and p38MAPK signaling. J Am Heart Assoc.
6(e007441)2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chen DQ, Cao G, Chen H, Liu D, Su W, Yu
XY, Vaziri ND, Liu XH, Bai X, Zhang L and Zhao YY: Gene and protein
expressions and metabolomics exhibit activated redox signaling and
wnt/β-catenin pathway are associated with metabolite dysfunction in
patients with chronic kidney disease. Redox Biol. 12:505–521.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang Y, Lu SL and Mo ML: Effects of
expression of NF-κB, NLRP3 inflammatory bodies and apoptotic
factors in rats with diabetic nephropathy. Chin Tradit Med.
16:35–36. 2018.
|
|
36
|
Nitta T, Kanoh H, Inamori KI, Suzuki A,
Takahashi T and Inokuchi JI: Globo-series glycosphingolipids
enhance Toll-like receptor 4-mediated inflammation and play a
pathophysiological role in diabetic nephropathy. Glycobiology.
29:260–268. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Pan XY, Gong XH and Shen FX: Effects of
high glucose environment on the expression of IL-18, IL-1β, IL-6,
TNF-α in glomerular mesangial cells of HBZY-1 rats. China Integrat
Tradit Chin Western Med J Nephrol. 13:519–521. 2012.
|
|
38
|
Miyamoto S, Shikata K, Miyasaka K, Okada
S, Sasaki M, Kodera R, Hirota D, Kajitani N, Takatsuka T, Kataoka
HU, et al: Cholecystokinin plays a novel protective role in
diabetic kidney through anti-inflammatory actions on macrophage:
Anti-inflammatory effect of cholecystokinin. Diabetes. 61:897–907.
2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
He J, Chen X, Li B, Zhou W, Xiao J, He K,
Zhang J and Xiang G: Chaetocin induces cell cycle arrest and
apoptosis by regulating the ROS-mediated ASK-1/JNK signaling
pathways. Oncol Rep. 38:2489–2497. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yan Z, Liu S and Yan H: Research progress
on the role of NLRP3 inflammasome in diabetic nephropathy. Shandong
Med. 57:107–109. 2017.
|
|
41
|
Wang Z, Meng S, Cao L, Chen Y, Zuo Z and
Peng S: Critical role of NLRP3-caspase-1 pathway in age-dependent
isoflurane-induced microglial inflammatory response and cognitive
impairment. J Neuroinflammation. 15:109. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Patel MN, Carroll RG, Galván-Peña S, Mills
EL, Olden R, Triantafilou M, Wolf AI, Bryant CE, Triantafilou K and
Masters SL: Inflammasome priming in sterile inflammatory disease.
Trends Mol Med. 23:165–180. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Liu X, Zhang Z, Ruan J, Pan Y, Magupalli
VG, Wu H and Lieberman J: Inflammasome-activated gasdermin D causes
pyroptosis by forming membrane pores. Nature. 535:153–158.
2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Jiang CX, Wu L and Wu J: Curcumin analogs
inhibit ERK/JNK and NF-κB signaling pathways and exert
anti-inflammatory activities. Chin Tradit Herb Drugs. 47:2871–2876.
2016.
|
|
45
|
Zeng S and Zhou S: Research progress on
diabetic nephropathy and oxidative stress related substances. Chin
Med Eng. 28:37–40. 2020.
|
|
46
|
Wang Z, Yang L and Meng X: Discussion on
the research ideas of astragalus polysaccharides intervention in
the pathogenesis of diabetic nephropathy. Chin Med Eng. 33:1–5.
2020.
|
|
47
|
Li Y and Wang L: Research progress on
inhibitors of sodium-glucose cotransporter 2. Med J Chinese
People's Liberation Army: 9, 2019.
|
|
48
|
Zhou Y: Clinical efficacy of Bailing
capsule combined with metformin in patients with diabetic
nephropathy. Chin Tradit Patent Med: 2, 2019.
|
|
49
|
Zou Y: Advances in traditional Chinese and
western medicine for diabetic nephropathy. Int J Urol. 35:154–156.
2015.
|
|
50
|
Li G, Zhang H and Li J: Research status of
silkworm protein resources and its application in food industry.
Food Ind Sci Technol. 33:396–401. 2012.
|
|
51
|
Li SZ: Compendium of Materia Medica.
People's Medical Publishing House, Beijing, pp1561-1567, 1982.
|
|
52
|
Liu D, Wang X and Song C: Expression
changes of liver tumor necrosis factor-α in type 2 diabetic rats
and the protective effect of sericin. Chin J Gerontol.
35:6011–6013. 2015.
|
|
53
|
Li J, Liu D and Song C: Effect of sericin
on insulin protein expression in islet cells of type 2 diabetic
rats. Chin J Gerontol. 34:1549–1550. 2014.
|