Introduction
Bronchial thermoplasty (BT) is a novel therapeutic
option for severe asthma. BT may significantly improve the lung
function, reduce the times of acute attack and the dosage of
glucocorticoids in patients with severe asthma with a predicted
forced expiratory volume in 1 sec (FEV1) percentage of
<60%, which is safe and effective (1). Furthermore, a study demonstrated that
BT may be confidently offered to patients with asthma with an
FEV1 that is 30-50%, prediction of no risk of more
frequent or more severe adverse events and with an expected
response of the same degree as that of patients with better lung
function (2). BT primarily targets
the bronchial smooth muscle (>3 mm diameter), delivering thermal
energy (heated to 65˚C) to reduce the airway smooth muscle mass
(3,4). It reduces fibroblast remodelling
through modifying the function of epithelial cells, particularly by
reducing heat shock protein 60 secretion and subsequent signaling
pathways that regulate protein arginine methyltransferase 1
expression (5). A total of 2
studies on the short-term outcomes of BT have reported that,
despite its long-term effectiveness, BT may cause early radiologic
modifications, including consolidation, ground-glass opacities and
atelectasis (6,7). However, neither of these studies
presented data on bronchoscopic findings. In August 2014, a patient
with severe asthma and its associated complications presented at
our center. The patient had earlier undergone upper lobe
atelectasis and pneumothorax followed by a third BT procedure
(8). Henceforth, in our center,
chest radiography and close follow-up were performed routinely in
all patients after each BT session. The present study reports on a
case of pneumothorax after BT procedure and retrospectively
analyzed the short-term radiologic and bronchoscopic modifications
in 12 patients with severe asthma after a total of 33 BT
sessions.
Materials and methods
Patients
The present study retrospectively analyzed the data
of 12 patients with severe asthma who were voluntarily treated with
BT at Guangzhou Institute of Respiratory Health, State Key
Laboratory of Respiratory Disease, The First Affiliated Hospital of
Guangzhou Medical University in China between July 2014 and October
2017. The inclusion criteria were as follows: i) Subjects with
asthma aged 18-65 years; ii) severe asthma (9): Drug therapy was required for Level-4
and -5 in the past year (large dose of inhaled
corticosteroids/long-acting β2-agonists or leukotriene
modifier/theophyline), or the systemic corticosteroid treatment
lasted ≥50% of the time to prevent ‘uncontrollable’ asthma; or the
‘uncontrollable’ asthma still occurred in spite of the above
treatment; iii) non-smoker for ≥1 year (if former smoker, <10
pack-years total smoking history). The exclusion criteria were as
follows: i) Participation in another trial within 6 weeks of the
baseline period involving respiratory intervention; ii)
post-bronchodilator FEV1 percentage of <65%; iii) 3
or more hospitalizations for exacerbations of asthma in the
previous year; OR a history of life-threatening asthma, defined by
past intubations for asthma, or intensive care unit (ICU) admission
for asthma within the prior 24 months; iv) a history of recurrent
lower respiratory tract infections requiring antibiotics (more than
3 in the past 12 months); v) a history of recurrent oral steroid
use for asthma (4 or more pulses of oral steroids in the past 12
months); vi) known sensitivity to medications required to perform
bronchoscopy (such as lidocaine, atropine and benzodiazepines);
vii) known systemic hypersensitivity or contraindication to
methacholine chloride or other parasympathomimetic agents; viii)
acute respiratory infection; ix) other respiratory diseases
including emphysema, cystic fibrosis, vocal cord dysfunction,
mechanical upper airway obstruction, Churg-Strauss syndrome or
allergic aspergillosis; x) segmental atelectasis, lobar
consolidation, significant or unstable pulmonary infiltrate, or
pneumothorax confirmed by chest radiography; xi) cardiovascular
disease including myocardial infarction, angina, cardiac
dysfunction, cardiac dysrhythmia, conduction defect, cardiomyopathy
or stroke; xii) known aortic aneurysm; xiii) significant comorbid
illness including cancer, renal failure, liver disease or cerebral
vascular disease; xiv) uncontrolled hypertension; xv) implanted
electrical stimulation device; xvi) known coagulopathy; xvii) any
other medical condition that may interfere with study participation
in the opinion of the investigator. The study obtained approval
from the Human Research Ethics Committee of Guangzhou Institute of
Respiratory Health, State Key Laboratory of Respiratory Disease,
The First Affiliated Hospital of Guangzhou Medical University
(China). The patients used high doses of inhalation
corticosteroids/long-acting beta-agonists and 25% were also treated
with oral or systemic corticosteroids. These 12 patients underwent
a total of 33 BT sessions. Oral prednisolone tablets (40 mg QD)
were given three days prior to BT and three days after BT. Chest
imaging evaluations included chest radiography and/or computed
tomography (CT) and were routinely performed prior to BT. The
demographic and clinical data of these 12 patients are presented in
Table I.
 | Table ICharacteristics of patients with
severe asthma treated by BT (n=12). |
Table I
Characteristics of patients with
severe asthma treated by BT (n=12).
| Parameter | Value |
|---|
| Sex
(male/female) | 4/8 |
| Age (years) | 48.1±2.9 |
| Clinical status
before BT |
|
Asthma
duration (years) | 9.8±2.4 |
|
Number of
hospital admissions in the previous year per patient | 2.1±0.4 |
|
Number of
patients taking long-term oral corticosteroid therapy | 3 (25.0) |
|
Eosinophils
in blood (%) | 0.9 (0.5-6.2) |
|
Total IgE
(kU/l) | 240.1±63.3 |
|
Eosinophils
in induced sputum (%) | 13.5
(6.5-58.5) |
|
FEV1
pre-β2-agonists predicted (%) | 56.2±10.5 |
|
FVC
predicted (%) | 93.8±20.4 |
|
MMEF
predicted (%) | 37.1±22.6 |
| Complications |
|
Mild
bronchiectasis | 2 (16.7) |
|
Nasosinusitis | 8 (66.7) |
|
Hypertension | 3 (25.0) |
|
Diabetes | 1 (8.3) |
|
OSAHS | 1 (8.3) |
BT
BT was performed on each patient for three sessions
at intervals of at least three weeks (10,11),
which was supposed to ensure adequate healing of previously treated
segments prior to proceeding with any further treatment. The right
lower lobe was treated during the first session, the left lower
lobe during the second session and the right and left upper lobes
during the third session (3). The
right middle lobe was not treated due to theoretical concerns of
inducing right middle lobe syndrome stemming from the long and
narrow lobar airway typically leading to the right middle lobe. To
date, no experience in treating the right middle lobe with
bronchial thermoplasty has been published (11).
Evaluations after BT
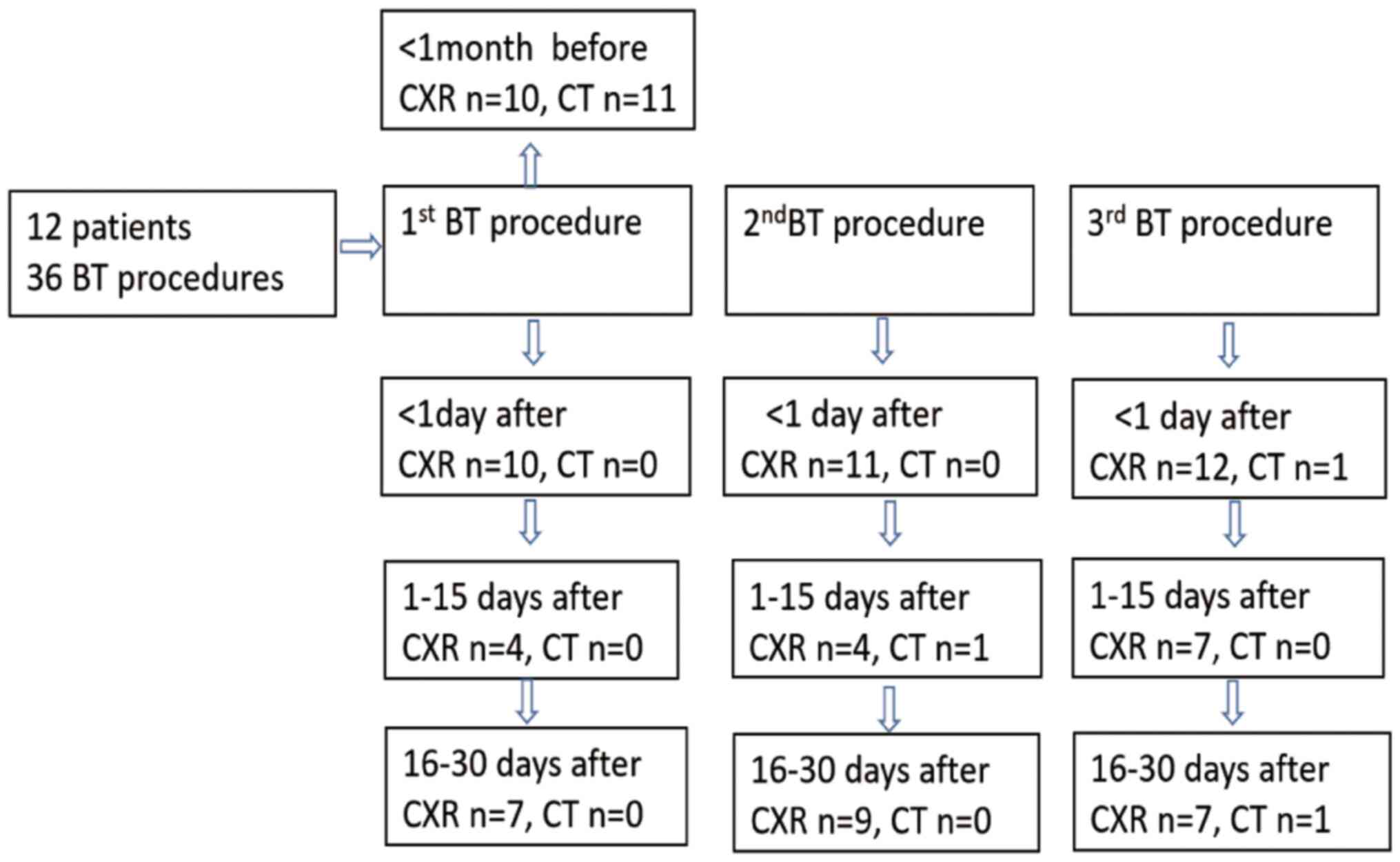
A flow chart of radiologic examinations after BT is
presented in Fig. 1. Chest
radiographs were obtained within 18-24 h after 33 BT sessions
(chest radiography began after the third BT session in case 1 and
the second BT session in case 2), as various studies indicated that
radiological abnormalities were observed immediately after BT
treatment (6,7,12) and
another study reported that a moderate temperature increase of BT
causes autolysis and microvascular hemorrhage resulting in
inflammation and edema after BT resembling ground-glass
abnormalities (7). All possible
radiologic modifications - peribronchial consolidations,
atelectasis, pleural effusion, effusion in oblique fissures,
pleural thickening and pneumothorax - were assessed by experienced
radiologists. Symptoms such as fever, cough, chest pain, wheezing
and dyspnea were also recorded from a systemic review of the
clinical charts. After BT, bronchoscopic interventions were applied
as necessary based on symptoms and radiologic findings. Follow-up
visits were scheduled to take place 2-15 days after each BT
session, as the percentage of atelectasis among 32 abnormal
radiographs which were performed within 18-24 h after BT was
>50%. Follow-up visits were scheduled to take place 16-30 days
after each BT session, which was for the purpose of checking
whether the patient was suitable for treatment with the next BT
session. Radiologic findings during the two follow-up visits were
also recorded.
Statistical analysis
Variables with a normal distribution are expressed
as the mean ± standard deviation and comparisons between these
groups were evaluated using the independent-samples t-test.
Variables with a skewed distribution were expressed as the median
[interquartile range (25th-75th percentile)] and comparisons
between the groups were performed using the Mann-Whitney U-test.
For categorical variables, the values are expressed as n (%) in
each category and compared using the χ2 test as
appropriate. P<0.05 was considered to indicate statistical
significance. All statistical analyses were performed using SPSS
version 16.0 software (SPSS, Inc.).
Results
Radiologic and clinical findings
during the immediate post-operative period (day 1).
A total of 8 female and 4 male patients were
included in the current study (mean age, 48 years). All patients
underwent chest radiography (n=10) or CT (n=11) prior to the first
BT session with 11 patients having no atelectasis, bronchiectasis,
peribronchial consolidations, pleural effusion, effusion in oblique
fissures, pleural thickening or pneumothorax.
Symptoms that occurred within 24 h after BT are
summarized in Table II. A total of
9 patients had symptoms including cough, expectoration, tachypnea,
wheezing, chest tightness, chest pain and dyspnea. None of the
patients developed a fever. Within 18-24 h after BT, 12 patients
underwent a total of 33 chest radiographs and 1 CT scan. Among
them, 32 radiographs exhibited modifications (Table III): Atelectasis (n=17, 53.1%;
Fig. 2C and D and Figs.
4A and 5A), peribronchial
consolidations (n=27, 84.4%; Figs.
2C-E, 3G and 4A), pleural effusion (n=6, 18.8%; Fig. 3B and C), effusion in oblique fissures (n=1,
3.1%), pleural thickening (n=2, 6.3%) and pneumothorax (n=1, 3.1%).
Only one CT scan was performed 24 h after BT, which revealed
atelectasis in the left lower lobe (Fig. 3B and C). After the BT of the lower lobes,
radiographs indicated early radiologic modifications in untreated
lobes in three radiographs (3/20, 15.0%; Fig. 2C). After BT of the upper lobes,
radiographs exhibited radiologic modifications in the untreated
lobes in six radiographs (6/12, 50%; Figs. 2E and 3G), which were manifested mainly as
peribronchial consolidations in the left lower lobe (n=5).
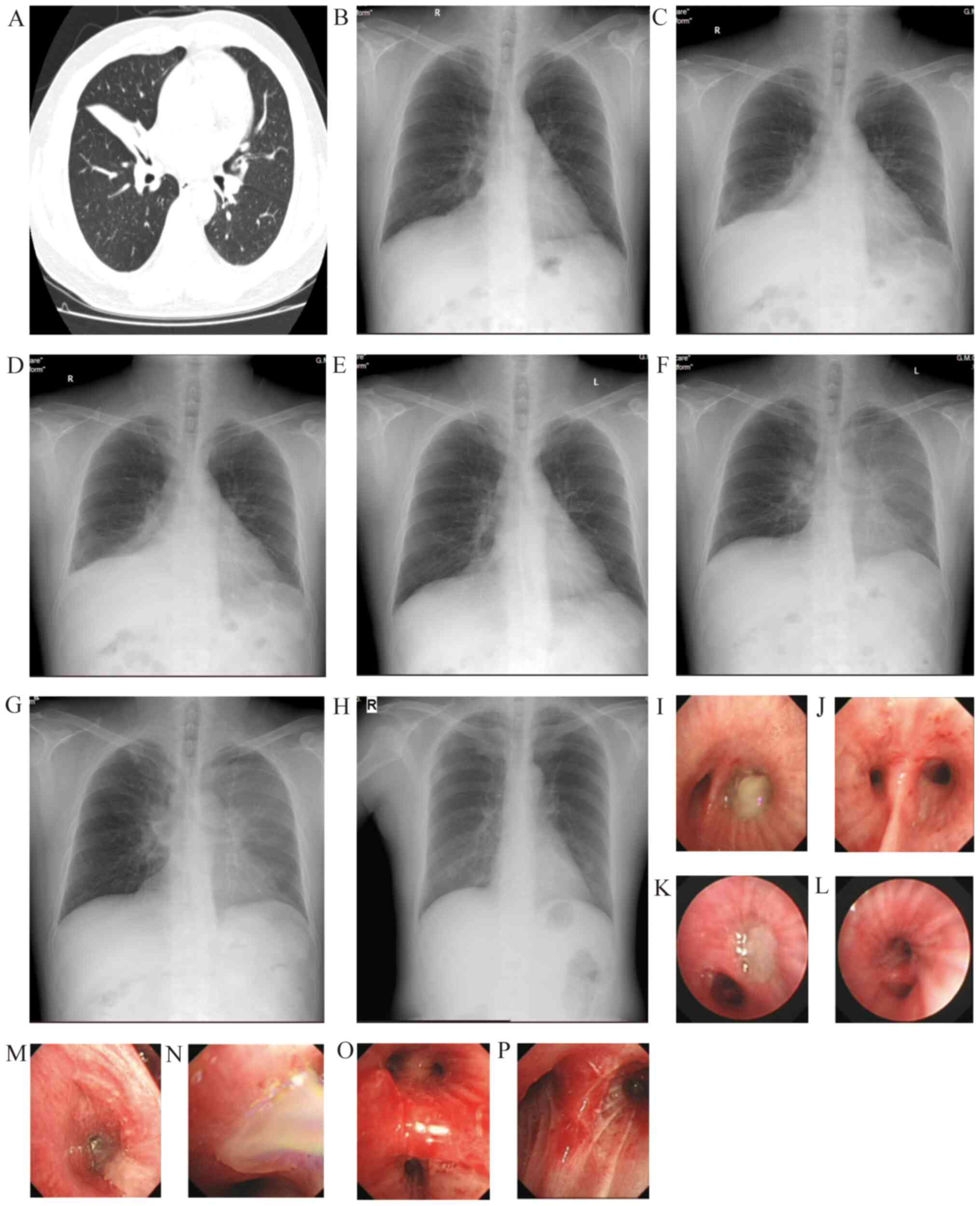 | Figure 2Representative images of a 45-year-old
male patient. (A) At 46 days prior to the first BT session,
atelectasis was observed in the right middle lung. (B) At four days
prior to the first BT session, slight infiltrates were present in
the right middle lung. (C) Within 24 h after the first BT session,
atelectasis was observed in the right middle lobe, along with
peribronchial consolidations in the right lower lung. (D) Within 24
h after the second BT session, atelectasis and peribronchial
consolidations were present in the left lower lobe. (E) Within 24 h
after the third BT session, peribronchial consolidations were
observed in the left lower lung. (F) Within five days after the
third BT session, peribronchial consolidations in the left lung
(upper and lower lobes) had increased. (G) Within 16 days after the
third BT session, peribronchial consolidations in the left lung
were the same as before. (H) Within five months after the third BT
session, the results were normal. At 4 days after the first BT
session, (I) sticky phlegm occluded the right lower lobe bronchus
and (J) the right lower lobe bronchus became patent after
treatment. At 2 days after the second BT session, (K) the left
lower lobe bronchus was occluded by yellowish phlegm and (L) the
basal and dorsal segments of the lower lobe became patent. At 1 day
after the third BT session, numerous white sticky phlegm plugs in
(M) the right upper lobe and (N) left upper lobe. Congestion and
swelling of bronchial mucosa in (O) left and (P) right upper lobes
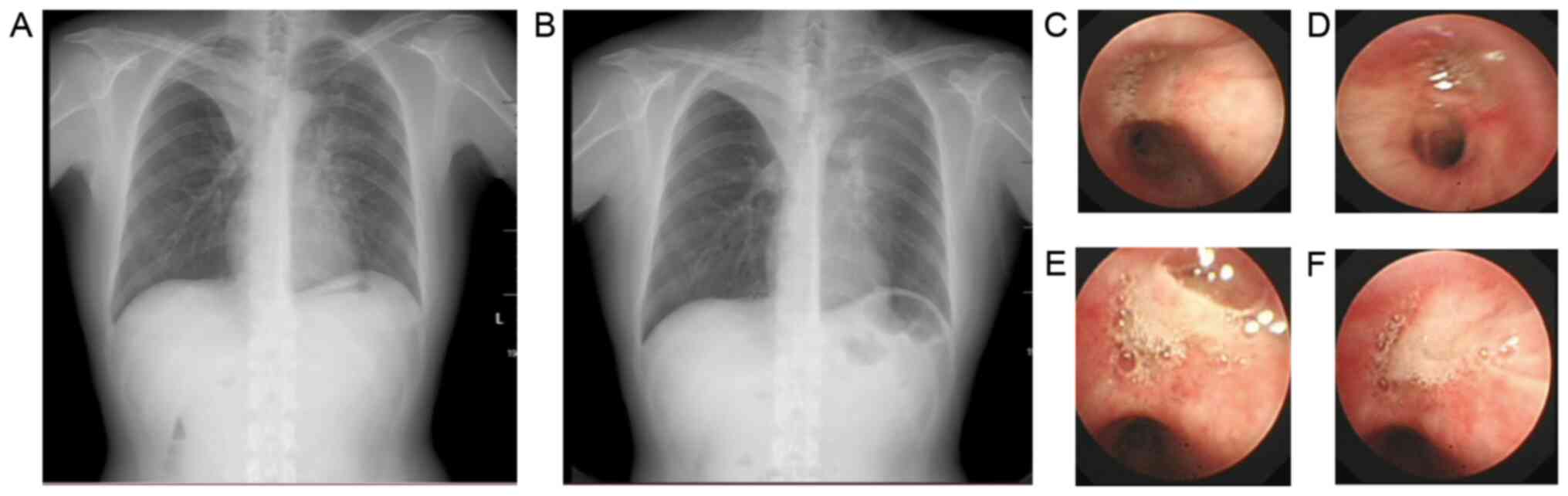
were present after treatment. Chest CTs are demonstrated in (A).
X-rays are presented in (B, C-G). Bronchoscopy images are
demonstrated in (I-P). BT, bronchial thermoplasty. |
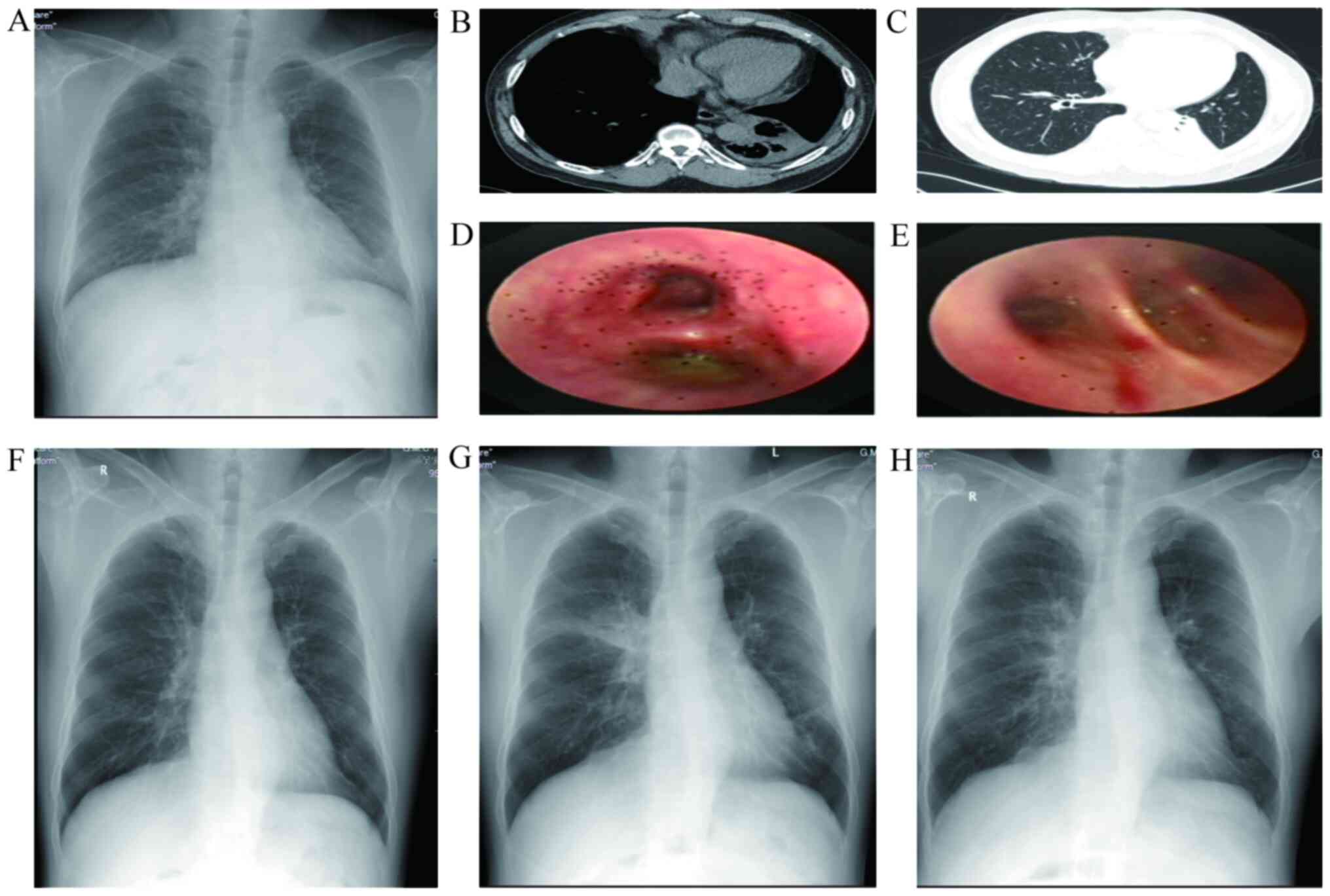 | Figure 3Representative images of a 50-year-old
male. (A) At three days prior to the first BT session, the
appearance was normal. Within 24 h after the second BT session,
pleural effusion (B) and left lower lung atelectasis (C) of the
left lung were observed. (D) At three days after the second BT
session, yellow viscous secretions were present in the left lower
lobe. (E) At 10 days after the second BT session, yellow viscous
secretions in the left lower lobe were observed. (F) At 15 days
after the second BT session, atelectasis had basically disappeared.
(G) Within 24 h after the third BT session, peribronchial
consolidations in the right upper lobe and left lower lobe were
present. (H) At three days after the third BT session, radiological
resolution in the right upper lobe and left lower lobe was
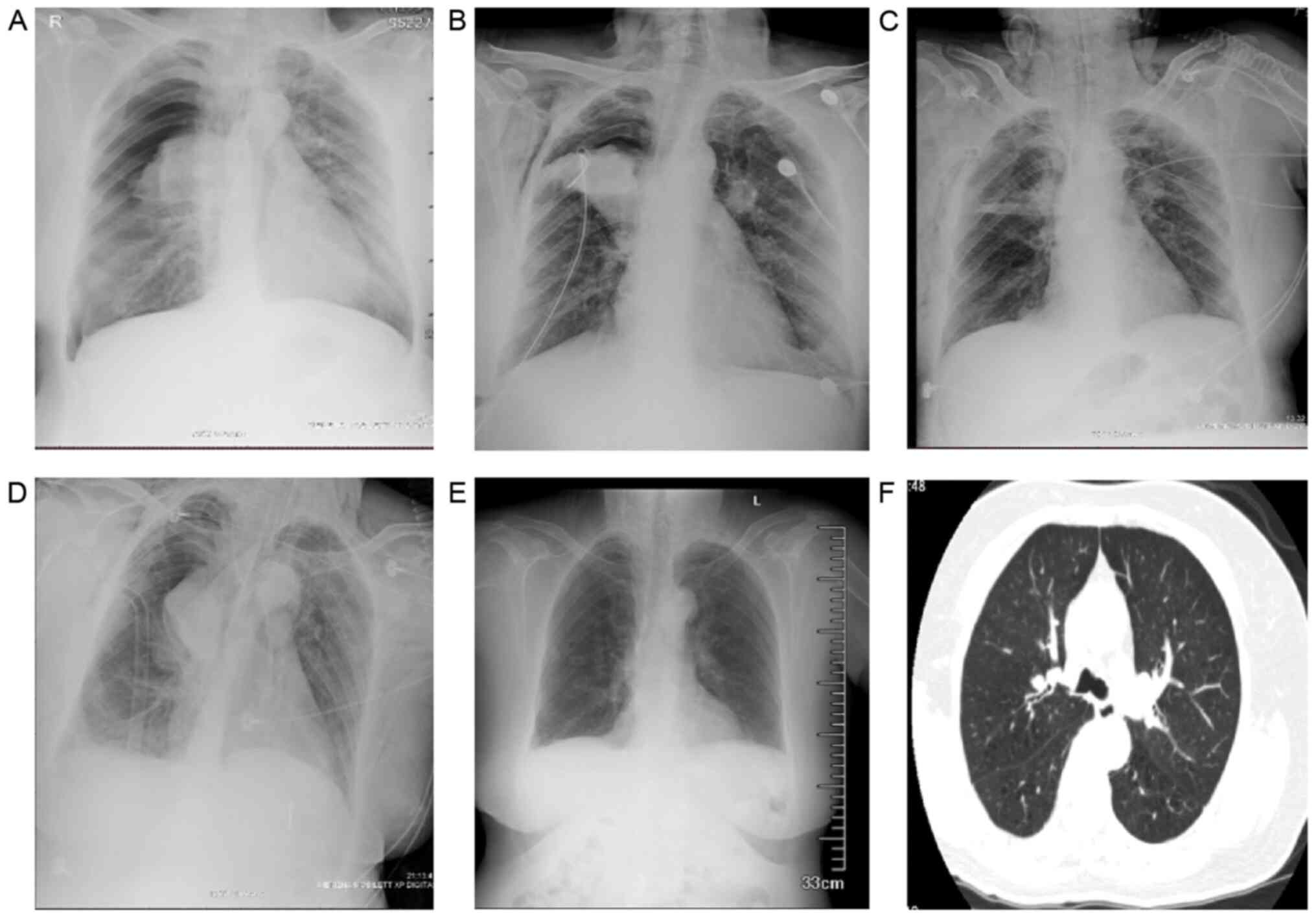
observed. X-rays are presented in (A, F, G and H). Chest CTs are
demonstrated in (B and C). Bronchoscopy images are presented in (D
and E). BT, bronchial thermoplasty. |
 | Table IIClinical data for the bronchoscopic
treatment and no-treatment groups after bronchial thermoplasty. |
Table II
Clinical data for the bronchoscopic
treatment and no-treatment groups after bronchial thermoplasty.
| A, Prior to the
first BT session |
|---|
| Parameter | Bronchoscopic
treatment group (n=7) | Non-bronchoscopic
treatment group (n=5) | P-value |
|---|
| Sex
(male/female) | 3/4 | 1/4 | 0.428 |
| Age (years) | 49.4±12.9 | 42.8±11.1 | 0.376 |
| BMI
(kg/m2) | 24.7±3.1 | 22.2±2.1 | 0.156 |
| Disease course
(years) | 11.6±9.2 | 7.4±7.3 | 0.421 |
| Smokers | 2 | 1 | 0.746 |
| Times of
hospitalization in the past year | 1.4±1.0 | 3.0±1.6 | 0.058 |
| Number of patients
with underlying disease | 5 | 4 | 0.746 |
| Nasosinusitis | 4 | 4 | |
| Mild
bronchiectasis | 2 | 0 | |
|
Hypertension | 3 | 0 | |
|
Diabetes | 1 | 0 | |
|
OSAHS | 1 | 0 | |
| Blood WBC count
(109/l) | 8.1±2.0 | 10.5±3.7 | 0.243 |
| Percentage of
neutrophils in blood (%) | 58.8
(53.8-60.9) | 66.9
(55.4-70.2) | 0.570 |
| Percentage of
eosinophils in blood (%) | 1.35 (0.2-5.6) | 0.9 (0.5-8.1) | 1.000 |
| Eosinophils in
induced sputum (%) | 13.0
(11.4-51.8) | 36.5
(1.8-58.3) | 0.806 |
| Neutrophils in
induced sputum (%) | 82.0
(38.9-85.4) | 58.0
(33.8-86.8) | 0.808 |
| TIgE (KU/l) | 207.0±178.8 | 286.4±282.1 | 0.561 |
| FVC predicted
(%) | 82.7±18.7 | 109.5±9.9 | 0.010 |
| FEV1
pre-β2-agonists predicted (%) | 65.1±22.0 | 87.8±9.0 | 0.038 |
| FEV1/FVC (%) | 64.6±11.2 | 69.4±11.5 | 0.489 |
| MMEF predicted
(%) | 32.5±21.4 | 43.6±25.1 | 0.429 |
| B, 24 h after three
BT sessions |
| Parameter | Bronchoscopic
treatment group (n=7) | Non-bronchoscopic
treatment group (n=5) | P-value |
|
Symptoms/complaints | | | |
|
Cough | 11 | 3 | 0.049 |
|
Expectoration | 11 | 3 | 0.049 |
|
Tachypnea | 4 | 0 | 0.077 |
|
Wheezing | 6 | 0 | 0.025 |
|
Chest
tightness | 4 | 0 | 0.077 |
|
Chest
pain | 1 | 0 | 0.398 |
|
Dyspnea | 1 | 0 | 0.398 |
| Radiologic
findings | 17 | 15 | |
| Lobar
atelectasis | 11 (64.7) | 0 (0) | <0.001 |
| Segmental
atelectasis | 0 (0) | 6 (40.0) | 0.004 |
| Peribronchial
consolidations | 13 (76.5) | 14 (93.3) | 0.197 |
| Average hospital
stays for 3 BT sessions | 4.4±1.8 | 2.2±0.7 | 0.017 |
 | Table IIIEarly radiological findings on the
day after bronchial thermoplasty. |
Table III
Early radiological findings on the
day after bronchial thermoplasty.
| Item | N (%) |
|---|
| Peribronchial
consolidations | 27 (84.4) |
| Atelectasis | 17 (53.1) |
| Pleural
effusion | 6 (18.8) |
| Oblique
effusion | 1 (3.1) |
| Pleural
thickening | 2 (6.3) |
| Pneumothorax | 1 (3.1) |
Radiological and bronchoscopic
findings after BT
Follow-up visits 2-15 days after
BT
A total of seven patients developed tachypnea, chest
tightness, chest pain, wheezing or dyspnea after a total of nine BT
sessions, and all of them had atelectasis, located in the treated
lobes in six cases (Figs.
3-5) and in untreated lobe in one case (Fig. 2C). A total of 23 bronchoscopic
examinations were required in these patients (bronchoscopic
intervention group), which revealed that phlegm plugs occluded the
bronchus in the treated lobe, whereas there was a small amount of
white phlegm in the bronchus of the untreated lobe. Bronchoscopic
aspiration, fragmentation and washing were applied to remove the
secretions and plugs. At the same time, oral/systemic
glucocorticoids and expectorants were given to counteract airway
inflammation and cough up mucus. There were no positive results for
microorganisms. In one patient, bronchoscopic aspiration was
required after every BT session (Fig.
2K-P). In two patients, bronchoscopy was required after the
second BT session to remove phlegm plugs, requiring five
bronchoscopic sessions (Fig. 3D and
E). In four patients, bronchoscopy
was required after the third BT session to remove phlegm plugs.
Hence, a total of 10 chest radiographs were obtained from these
seven patients 2-15 days after BT sessions. Atelectasis was
resolved in 5 patients (5/7, 71.4%; Figs. 3F and 4E), peribronchial consolidations decreased
or were resolved in 4 patients (4/7, 57.1%; Figs. 3H and 4E) and pneumothorax was resolved in 1
patient (1/1, 100%; Fig. 5E).
The remaining five patients (including four who
developed segmental atelectasis within 18-24 h after BT and one
without atelectasis after BT) did not exhibit any obvious pulmonary
symptoms after BT and therefore did not undergo bronchoscopy
(non-bronchoscopic intervention group). All of these patients were
given ambroxol, myrtol standardized enteric-coated soft capsules
and inhaled medications. A total of five chest radiographs were
assessed at this follow-up, which indicated that the segmental
atelectasis resolved in three radiographs and peribronchial
consolidations decreased or disappeared in four radiographs (4/5,
80%).
The clinical data of the bronchoscopic and
non-bronchoscopic intervention groups are presented in Table II. Baseline pulmonary function,
average hospital stays for three BT sessions and post-operative
cough/expectoration, wheezing and radiologic modifications
(bronchial lobar or segmental atelectasis) were significantly
poorer in the bronchoscopic intervention group.
Follow-up visits 16-30 days after
BT
No bronchoscopy was required. A total of 23 chest
radiographic assessments revealed that 95.7% (22/23) of the
radiographic modifications observed at the previous examinations
were markedly decreased or disappeared. One exception was
peribronchial consolidations in the left lung (Fig. 2G).
Bronchoscopy and radiologic findings
after BT in three representative cases
A 66-year-old female retired worker presented with a
BMI of 26.2 kg/m2, an Asthma Control Test score of 18,
Asthma Control Questionnaire score of 1 and Mini-Asthma Quality of
Life Questionnaire score of 5. The patient was a non-smoker and had
hypertension for 11 years and abnormal glucose tolerance.
Furthermore, the patient had a 26-year history of asthma. At two
months prior to BT, the patient's lung function exhibited severe,
mixed ventilation dysfunction [FEV1% predicted, 34.0%;
FEV1/forced vital capacity (FVC), 49.8%] and chest CT
was normal. The patient did not have any symptoms after the first
or second BT session, and accordingly, no radiologic examination
was performed. At 12 h after the third BT session, the patient had
symptoms of wheezing, dyspnea and oxygen desaturation (89%), which
were treated with intravenous methylprednisolone. At 15 h after the
procedure, the symptoms of severe wheezing, chest tightness and
dyspnea continued to worsen with desaturation [saturated oxygen
pressure (SpO2), 88% under medium-flow oxygen
inhalation]. Bedside chest radiography revealed atelectasis in the
right upper lobe and pneumothorax surrounding the right upper lobe
(50-60% collapsed) (Fig. 5A). After
chest drain insertion, the above symptoms remained and type II
respiratory failure [pH 7.281; partial carbon dioxide pressure
(PaCO2), 51.9 mmHg; PaO2, 75.1 mmHg]
occurred. The patient was transferred to the ICU for noninvasive
ventilation. Bronchoscopy revealed swelling of the bronchial mucosa
and plugs blocking the bronchi of the left and right upper lobes,
which were removed by biopsy forceps and washing. At four days
after the third BT procedure, the chest radiograph indicated
resolved pneumothorax and relieved upper right lobe atelectasis
(Fig. 5C). The next day, dyspnea
had worsened (SpO2, 82%). Radiography indicated a
worsened right-sided pneumothorax, as well as more obvious
consolidation and atelectasis in the right upper lobe (Fig. 5D). The results of the blood gas
analysis were as follows: pH 7.524; PaO2, 61 mmHg;
PaCO2, 29.5 mmHg; and fraction of inspired oxygen, 90%).
After endotracheal intubation and mechanical ventilation,
bronchoscopy was performed daily to remove plugs, along with
administration of broad-spectrum antibiotics. After four days, the
intubation and thoracic drainage tube were successfully withdrawn
and sequential noninvasive mechanical ventilation was maintained.
On day 10, after BT, radiography indicated no atelectasis (Fig. 5E). On day 27, the patient's
radiographs were basically normal (Fig.
5F).
A 49-year-old male patient with a BMI of 26.8
kg/m2 had a 10-year history of asthma. The patient had a
20 pack-year smoking history but had not smoked for two years.
Furthermore, the patient obstructive sleep apnea, hypertension and
type 2 diabetes. At one week prior to BT, lung function tests
revealed severe obstructive ventilation dysfunction
(FEV1 predicted percentage, 49.7%; FEV1/FVC,
55.8%). Radiography revealed left lower lobe atelectasis and CT
indicated left lower lung atelectasis and pleural effusion of left
lung after the second BT session (Fig.
3B and C). The complete blood
count (CBC) was normal. The patient had symptoms of expectoration
and wheezing, and was treated with intravenous methylprednisolone
and non-invasive ventilation. The patient required three
bronchoscopic interventions, which revealed plugs at the left lower
lobe (Fig. 3D and E). On day 15 after BT, radiography
indicated that atelectasis had almost disappeared (Fig. 3F). On day 47, the patient underwent
a third BT session. Radiography performed within 24 h after this
session revealed peribronchial consolidations in the right upper
lobe and left lower lobe (Fig. 3G).
At the same time, the patient had symptoms of wheezing, which were
markedly alleviated after frequent nebulized intravenous
methylprednisolone treatment. After three days, radiography
indicated improvement (Fig.
3H).
A 46-year-old male presented with a 5-year history
of asthma and allergic rhinitis. The patient had a 1 pack-year
smoking history but had not smoked for seven years. In 2012, the
patient underwent tracheal intubation at our hospital due to a
severe asthma attack. The patient's lung function recorded in the
previous last year indicated severe, mixed ventilation dysfunction
(FEV1 predicted percentage, 41.9%; FEV1/FVC,
49.7%). Prior to the first BT session, lung function tests revealed
moderate obstructive ventilatory dysfunction (FEV1
predicted percentage, 61.9%; FEV1/FVC, 65.8%).
Radiography performed within 24 h after the first BT session
revealed atelectasis in the right middle lobe and peribronchial
consolidations in the right lower lobe (Fig. 2C). The CBC revealed a white blood
cell count (WBC) of 23.55x109/l with 82.9% neutrophils. The patient
complained of a productive cough with yellow sticky phlegm. The
patient was given intravenous moxifloxacin, methylprednisolone and
frequent nebulized treatments. Bronchoscopy indicated that yellow,
sticky phlegm occluded the right lower lobe bronchus (Fig. 2I). After bronchoscopic washing and
ambroxol lavage, the right lower lobe bronchus became clear
(Fig. 2J) and the symptoms markedly
diminished. The patient underwent a second BT session 43 days
later. Within 24 h, radiography revealed atelectasis and
peribronchial consolidations in the left lower lobe (Fig. 2D). The CBC revealed a WBC of
18.6x109/l with 81.2% neutrophils and 0.2% eosinophils.
Bronchoscopy indicated that the left lower lobe bronchus was
occluded by yellowish phlegm (Fig.
2K). After 76 days, the patient underwent a third BT session
and on the following day, radiography indicated peribronchial
consolidations in the left lower lobe (Fig. 2E). Bronchoscopy revealed that
bronchi in the right and left upper lobes were occluded by numerous
white, sticky phlegm plugs (Fig.
2M-P). After five days, radiography suggested that
peribronchial consolidations in the left lung (upper and lower
lobes) had increased (Fig. 2F) and
the patient was still wheezing. After one week of administering
intravenous moxifloxacin, methylprednisolone and frequent nebulized
treatments, the wheezing diminished. At 5 months thereafter, the
patient's radiography results were normal (Fig. 2H).
Discussion
In a previous study by our group (8), a patient who suffered from
pneumothorax directly after BT was briefly described. To the best
of our knowledge, this was the first case that required intubation
and mechanical ventilation in an ICU after BT. In the present
study, this case was described in detail by displaying the chest
radiologic modifications. The collapse of the upper lobe due to
bronchus occlusion and excess traction on pleura was the major
cause of the localized pneumothorax. Further possible causes may be
old age, poor basic pulmonary function and underlying diseases.
Recently, Funatsu et al (13) reported on a case of pulmonary cyst
and pneumothorax after BT. Similar to our case, their patient
experienced hypoxemia and complete bilateral upper lobe atelectasis
after the third BT session. However, their case featured the sudden
emergence of a pulmonary cyst in the right middle lobe associated
with pneumothorax on post-operative day 6. They explained that
pneumothorax was caused by the rupture of the pulmonary cyst that
had been formed during the pulmonary function test after BT.
To the best of our knowledge, the present study was
the first to evaluate the short-term lung conditions following BT
based on both chest radiography and bronchoscopy. Abnormal
radiologic findings at 18-24 h after each BT session included
atelectasis, peribronchial consolidation, opacities, pleural
effusion, effusion in oblique fissures, pleural thickening and
pneumothorax. Bronchoscopic abnormalities at one day after each BT
session included sticky phlegm and mucus plugging in the
corresponding bronchi. In most cases, these radiologic
modifications were markedly decreased or had disappeared within one
month after the use of oral/systemic glucocorticoids, expectorants
and/or bronchoscopic interventions.
Recently, Debray et al (6) analyzed early CT modifications induced
by BT in 13 patients who had undergone a total of 27 BT sessions.
The chest CT performed on the day after BT revealed pulmonary
peribronchial consolidations and ground-glass opacities in all
treated lobes, as well as atelectasis (lobar and segmental). The
percentage of abnormal CT modifications was 68%. d'Hooghe et
al (7) analyzed 34 chest
radiographs and 16 chest CT scans of 12 severe asthmatic patients
directly after BT. Approximately 91% of the radiographs exhibited
modifications, including peribronchial consolidations (97%) and
atelectasis (29%). No bronchoscopic intervention was required. The
follow-up CT scan after six months indicated resolution of the
changes. d'Hooghe et al (7)
performed the chest X-ray within <5 h after BT and Debray et
al (6) performed the CT scan on
the day after BT. In the present study, the chest X-ray was
performed at 18-24 h after each BT session. In line with the
results of d'Hooghe et al (7) and Debray et al (6), similar chest radiologic modifications
were observed the present study. These two previous studies and
also the present study indicated that peribronchial consolidations
are the most frequent modifications shortly after BT. Atelectasis
at 18-24 h after BT occurred 53% of cases in the present patient
cohort, while Debray et al (6) and d'Hooghe et al (7) reported an incidence of 68 and 38%,
respectively. Debray et al (6) reported that CT changes were not
associated with respiratory symptoms. In comparison, the patients
with severe chest radiological modifications of the present study
had more symptoms.
Debray et al (6) reported that opacities occurred in 12
untreated lobes in one-third of patients on the day after BT. The
BT-untreated lobes involved the middle lobe (n=5) and the right
lower lobe (n=4). An explanation for this may be the diffusion of
heat shock of BT along the bronchial wall and extension of heat
shock through fissures (11).
d'Hooghe et al (7) reported
that 31% of the chest CTs had ground-glass opacities in the
neighboring non-BT-treated lobe. However, no abnormalities were
detected in the non-BT-treated right middle lobe. The flow of
mucosal blood and/or mucus runs down from the BT-treated upper
lobes to untreated lower lobes, which may be a reasonable
explanation for the radiological abnormalities being located only
in the non-BT-treated lower lobes in all of the patients of that
study. In the present study, 15% of chest radiographs exhibited
early modifications in the non-treated right upper lobes after BT
in the left lower lobes. However, after BT of the right and left
upper lobes, 50% of chest radiographs exhibited abnormalities
(mainly peribronchial consolidations) in untreated lower lobes. In
addition, bronchoscopic modifications in untreated lobes generally
included small amounts of white secretions in the bronchus and
swelling of the mucous membranes.
In the present study, 7 of the 12 patients required
bronchoscopic intervention after BT. The bronchoscopic abnormality
(mucus plugging) corresponded to imaging abnormalities of the same
lung lobe. The bronchoscopic intervention group exhibited severe
eosinophilic inflammation (median sputum eosinophil ratio, 13%)
which may contribute to the development of mucus plugging after BT.
However, the non-bronchoscopic group exhibited severe eosinophilic
inflammation (median sputum eosinophil ratio, 36.5%) and had milder
symptoms and imaging abnormalities after BT. Debray et al
(6) and d'Hooghe et al
(7) did not perform any induced
sputum analysis. Therefore, it is not known whether there was an
obvious eosinophilic airway inflammation in their patients
presenting with atelectasis after BT. It remains elusive whether
there is an association between eosinophilic airway inflammation
and mucus plugging of the airway after BT. Compared with the
non-bronchoscopic intervention group, the patients who required
bronchoscopic intervention had poorer lung function
(pre-β2-agonist mean FEV1 predicted
percentage, 65.1%) and were more likely to develop lung lobar
atelectasis (not segmental atelectasis), with their lobar bronchi
occluded by phlegm plugs. However, Debray et al (6) reported that the FEV1
predicted percentage in 13 patients was 60.5% and these patients
had a longer duration of asthma and more hospitalizations during
the past year. No bronchoscopic intervention was required for these
patients, which may be explained by their relatively younger age
(average, 40.8 years). Similarly, d'Hooghe et al (7) reported that no bronchoscopic
intervention was required for their patients. Facciolongo et
al (14) reported on a case of
recurrent lobar collapse occurring a few hours after two BT
sessions. The patient had acute respiratory failure and required
bronchoscopic removal of plugs. In the present study, seven
patients needed bronchoscopic intervention, but the intervention
was unable to relieve the radiological abnormality for one patient
after the third BT session. Further well-designed controlled
studies are warranted to determine whether bronchoscopic
intervention is required in patients developing lobar atelectasis
following BT.
In summary, to the best of our knowledge, the
present study reported the first case of pneumothorax following BT
in need of intubation. Furthermore, there was a high incidence of
early radiologic abnormalities (i.e., mainly atelectasis and
peribronchial consolidations) after BT. Bronchoscopy revealed
occlusion of the bronchus by phlegm plugs at the corresponding
sites. However, whether bronchoscopic intervention is needed for
atelectasis following BT requires further investigation. Besides
this, BT should be audited and recorded in detail and ideally
contribute to a framework of clinical trials in order to improve
risk-benefit evaluations and the selection of patients likely to
benefit from treatment.
Acknowledgements
The authors thank Dr Jiang Mei (Guangzhou Institute
of Respiratory Health, State Key Laboratory of Respiratory Disease,
The First Affiliated Hospital of Guangzhou Medical University) for
her assistance with the statistical analysis.
Funding
This research was funded by the Natural Science
Foundation of Guangdong Province (grant no. 2019A1515010622),
Professor K.F. Chung's Visiting Professor Project of Guangzhou
Institute of Respiratory Health (grant no. 500102010501069001) and
the National Natural Science Foundation of China (grant no.
81770017).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JX and SL conceived and designed the current study.
QZ, SL, MQ, SW and XZ provided administrative support. MQ, SW, XZ
and ZL searched the literature. JX, PH, ZW collected and collated
data. MQ, SW, ZL, CZ, YC, XL, QZ and KC analyzed and interpreted
the data. All authors wrote the manuscript and all authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study complied with the Declaration of Helsinki
and was approved by the Institutional Review Board Ethics Committee
of the First Affiliated Hospital of Guangzhou Medical University.
Written informed consent was obtained from each patient.
Patient consent for publication
The patients consented to the publication of their
data and images in the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Long F, Zhong D, Huang WT, Long L, Hu FB,
Fu P and Hu SY: Analysis of the safety and efficacy of bronchial
thermoplasty for severe asthma with the first second forced
expiratory volume (FEV(1)) as a percentage of the predicted value
(FEV(1)%pred)<60. Zhonghua Yi Xue Za Zhi. 100:2023–2027.
2020.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
2
|
Langton D, Ing A, Fielding D, Hersch N,
Sha J, Plummer V and Thien F: Safety and Effectiveness of Bronchial
Thermoplasty When FEV1 Is Less Than 50. Chest. 157:509–515.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pavord ID, Cox G, Thomson NC, Rubin AS,
Corris PA, Niven RM, Chung KF and Laviolette M: RISA Trial Study
Group: Safety and efficacy of bronchial thermoplasty in
symptomatic, severe asthma. Am J Respir Crit Care Med.
176:1185–1191. 2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dombret MC, Alagha K, Boulet LP, Brillet
PY, Joos G, Laviolette M, Louis R, Rochat T, Soccal P, Aubier M, et
al: Bronchial thermoplasty: A new therapeutic option for the
treatment of severe, uncontrolled asthma in adults. Eur Respir Rev.
23:510–518. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sun Q, Fang L, Roth M, Tang X,
Papakonstantinou E, Zhai W, Louis R, Heinen V, Schleich FN, Lu S,
et al: Bronchial thermoplasty decreases airway remodelling by
blocking epithelium-derived heat shock protein-60 secretion and
protein arginine methyltransferase-1 in fibroblasts. Eur Respir J.
54(1900300)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Debray MP, Dombret MC, Pretolani M, Thabut
G, Alavoine L, Brillet PY, Taillé C, Khalil A, Chanez P and Aubier
M: Early computed tomography modifications following bronchial
thermoplasty in patients with severe asthma. Eur Respir J.
49(1601565)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
d'Hooghe JNS, van den Berk IAH, Annema JT
and Bonta PI: Acute Radiological Abnormalities after Bronchial
Thermoplasty: A Prospective Cohort Trial. Respiration. 94:258–262.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang Q, Zhang X, Xie J, Qiu R, Chen Y,
Huang Z, He Y, Xian M, Li J and Li S: Bronchial thermoplasty in the
treatment of severe asthma. Zhonghua Jie He He Hu Xi Za Zhi.
39:183–188. 2016.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
9
|
Chung KF, Wenzel SE, Brozek JL, Bush A,
Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, et
al: International ERS/ATS guidelines on definition, evaluation and
treatment of severe asthma. Eur Respir J. 43:343–373.
2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cox G, Thomson NC, Rubin AS, Niven RM,
Corris PA, Siersted HC, Olivenstein R, Pavord ID, McCormack D,
Chaudhuri R, et al: AIR Trial Study Group: Asthma control during
the year after bronchial thermoplasty. N Engl J Med. 356:1327–1337.
2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mayse M, Laviolette M, Rubin A, Lampron N,
Simoff M, Duhamel D, Musani AI, Yung RC and Mehta AC: Clinical
pearls for bronchial thermoplasty. J Bronchology Interv Pulmonol.
14:115–123. 2007.
|
|
12
|
d'Hooghe JNS, Bonta PI, van den Berk IAH
and Annema JT: Radiological abnormalities following bronchial
thermoplasty: Is the pathophysiology understood? Eur Respir J.
50(1701537)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Funatsu A, Kobayashi K, Iikura M, Ishii S,
Izumi S and Sugiyama H: A case of pulmonary cyst and pneumothorax
after bronchial thermoplasty. Respirol Case Rep.
6(e00286)2017.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Facciolongo N, Menzella F, Lusuardi M,
Piro R, Galeone C, Castagnetti C, Cavazza A, Carbonelli C, Zucchi L
and Salsi PP: Recurrent lung atelectasis from fibrin plugs as a
very early complication of bronchial thermoplasty: A case report.
Multidiscip Respir Med. 10(9)2015.PubMed/NCBI View Article : Google Scholar
|