Introduction
X-rays are a type of ionizing radiation widely used
in medicine, such as during imaging examinations and radiotherapy
of malignant tumors (1). Previous
studies have shown that high dose X-rays can lead to bone loss,
which increases the risk of fractures (2,3) that
do not heal easily (4,5).
Luckey previously proposed the theory of excitation
effect of low dose X-ray irradiation (LDI), which has become a
focus of interest over the years (6). Previous studies investigating bone
tissue have revealed that LDI promotes osteoid matrix proliferation
and mineralization (7,8). Furthermore, low dose X-rays are widely
used in different branches of medicine, particularly in orthopedics
(1). Patients with orthopedic
diseases, including fracture and lumbar degenerative diseases often
receive multiple doses of X-rays during therapy (9,10). Due
to this, low dose X-rays have vital significance.
Previous research has shown that LDI could promote
osteoblast proliferation and differentiation, whilst high dose
X-rays can lead to bone loss (8,9,11,12).
In a previous study, LDI promoted osteoblast differentiation and
accelerated fracture healing in mice (12,13).
Additionally, Park et al (7)
demonstrated that doses of 1 and 2 Gy X-ray radiation significantly
increased differentiation and mineralization of osteoblasts.
However, the molecular mechanism by which LDI exerts its effects
has not been elucidated. LDI may be associated with altered gene
expression that is related to signal transduction, cell cycle
regulation and cytoskeleton reorganization (14).
Microarrays demonstrated that LDI induced
significant upregulation of LIM domain kinase 2 (LIMK2), and that
this was associated with cytoskeleton reorganization (15-17).
Furthermore, Kurpinski et al (18) reported that cytoskeleton and
receptor signaling of human mesenchymal stem cells were uniquely
activated in response to 0.1 Gy LDI.
The current study hypothesized that LDI may cause
cytoskeleton rearrangement and affect cell morphology. Changes in
cytoskeleton reorganization may be the structural basis for the
exchange of intracellular and extracellular information following
LDI stimulation. The changes in adhesion signals may also affect
biological processes, such as osteoblast proliferation and
differentiation (19,20).
The cytoskeleton is mainly composed of actin
microfilaments, which act as a mechanical support framework that
maintains cell morphology, transduces various intracellular signals
(21) and serves a crucial role in
cell adhesion, motility, division and differentiation (22). Additionally, Ricci et al
(23) reported that intact
cytoskeletal actin filaments contribute to cell survival. The
cytoskeleton is sensitive to extracellular stimuli, which can cause
rapid reorganization of the actin cytoskeleton (23). Various stimuli, including fluid
shear stress, magnetic field and ultrasound, regulate the actin
cytoskeleton (24-27).
The appropriate intensity of these stimuli may promote the
reorganization of the cytoskeleton, thereby altering cell functions
(24).
Nonetheless, few studies have addressed the effect
of LDI on the osteoblast cytoskeleton. Onoda et al (28) demonstrated that LDI induced the
reorganization of fiber (F) actin microfilaments of pulmonary
microvascular endothelial cells. However, 24 h post-irradiation,
the depolymerized microfilaments reverted to their pre-irradiation
states. These results indicated that various complex pathways may
regulate the process of actin reorganization.
Small GTPases of the Rho family have been researched
extensively. RhoA and its effectors serve a crucial role in
regulating cytoskeleton arrangement and various essential cellular
processes, including proliferation and differentiation (29,30).
RhoA is the prototypical member of the Rho family and responds to
plasma membrane receptors for various stimuli, including cytokines
and environmental stress (31).
Furthermore, RhoA controls stress-mediated fiber formation by
regulating certain downstream key proteins (31,32).
The most extensively studied RhoA effector protein is
Rho-associated kinase (ROCK) 1, which mediates actin contractility
by phosphorylating myosin light chains (31,32).
Moreover, ROCK proteins may phosphorylate and activate LIMKs, which
phosphorylate cofilin (31,32). Cofilin is involved in the
reorganization of the actin cytoskeleton (33).
The present study investigated the effects of X-ray
irradiation on the morphology and cytoskeleton of MC3T3-E1 cells.
Additionally, the roles of the RhoA/ROCK pathway in this process
were investigated to determine the biological effects of LDI.
Materials and methods
Cell culture
Pre-osteoblastic MC3T3-E1 cells were obtained from
the Institute of Biochemistry and Cell Biology. A total of
1x105 cells were cultured in α-MEM supplemented with 10%
FBS, 5 mM β-glycerophosphate, 50 µg/ml ascorbic acid and 100 nM
dexamethasone (all Sigma-Aldrich; Merck KGaA) at 37˚C with 5%
CO2. Fresh medium was replaced every three days and
cells were sub-cultured at 80% confluency.
Irradiation of osteoblastic cells
A total of 2x104 MC3T3-E1 cells were
exposed to 0 (control), 0.5 or 5 Gy X-ray irradiation (at a rate of
200 cGy/min) emitted by a medical linear accelerator (Siemens
Primus) at room temperature using a 6 MV radiation source. The time
at irradiation was defined day 0.
Transmission electron microscopy
(TEM)
MC3T3-E1 cells were cultured for 3 days, fixed in
2.5% glutaraldehyde for 2 h at 4˚C and rinsed three times (15 min
each time) with 0.1 M sodium phosphate buffered saline (PBS).
Post-fixation was then performed with 1% osmium tetroxide (Beyotime
Institute of Biotechnology) for 3 h at room temperature.
Subsequently, the cells were dehydrated through an ascending
gradient of ethanol and immersed in acetone. Samples were then
embedded and double stained in 2% aqueous uranyl acetate and
Satoh's lead citrate at 4˚C for 2 h, and observed with a
transmission electron microscope (magnification, x5,000;
JEM-1200EX; JEOL, Ltd.) operated at 85 kV.
Labeling of F-actin cytoskeleton for
fluorescence microscopy
MC3T3-E1 cells were seeded (1.0x104
cells/ml) on poly-L-lysine-coated glass coverslips in 24-well
plates and cultured in an osteogenic differentiation medium
(containing 50 µg/ml ascorbic acid and 10 mM β-glycerophosphate;
Beyotime Institute of Biotechnology). Following X-ray irradiation,
cells were gently washed with PBS at 37˚C at time points 2 h, 1, 3
or 5 days post-irradiation. Cells were then fixed with 4%
paraformaldehyde (pH 7.4) for 20 min at room temperature and
permeabilized with 0.1% Triton X-100 for 5 min at room temperature.
Cells were then washed three times with PBS and blocked with 1%
bovine serum albumin (Beyotime Institute of Biotechnology) for 30
min at room temperature and incubated with FITC-conjugated
phalloidin (Sigma-Aldrich; Merck KGaA; 1:100) in the dark for 1 h
at room temperature. Cell nuclei were stained with 100 nM DAPI for
10 min at room temperature, followed by analysis using a
fluorescence microscope (magnification, x200).
Actin is the main component of the cytoskeleton
(21). FITC-conjugated phalloidin
is a specific dye for F-actin and glows green after binding to
polymerized F-actin (23). F-actin
expression can be quantitatively analyzed according to the
intensity of green fluorescence in cells. Therefore, the mean
fluorescence intensity of each group of cells was calculated to
compare the content of actin in each group.
ImageJ software (version 1.8.0; National Institutes
of Health) was used to randomly analyze the images of six cells
with clear boundaries within the slide and the average fluorescence
intensity of each cell (average fluorescence intensity=fluorescence
intensity/cell area) was measured as the analysis index. Data are
presented as the mean ± standard deviation, and represented the
mean fluorescence intensity. ‘Area’ represents the total area of
cells counted, ‘Min’ represents the lowest fluorescence intensity
and ‘IntDen’ represents the total fluorescence intensity.
Total RNA extraction and gene
expression analysis by reverse transcription-quantitative PCR
(RT-qPCR)
MC3T3-E1 cells were cultured in osteogenic
differentiation medium following X-ray irradiation. The cells were
harvested on days 3 and 7 after irradiation. Total RNA was
extracted using TRIzol® (Invitrogen, Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. cDNA
was obtained from total RNA (1 µg) using RevertAid First Strand
cDNA Synthesis kit (cat. no. K1622; Fermentas; Thermo Fisher
Scientific, Inc.). The reaction system was incubated at 42˚C for 1
h, and treated at 70˚C for 10 min. The first cDNA strand was
obtained and stored at -20˚C. qPCR was performed in a total volume
of 20 µl, which consisted of 1 µl cDNA (500 ng), 1 µl gene-specific
10 µM PCR primer-pair stock and 10 µl SsoFast™ EvaGreen®
Mix (Bio-Rad Laboratories, Inc.) using the Bio-Rad CFX96 system
according to the manufacturer's protocol. The expression of RhoA,
ROCK1, LIMK2 and β-actin was detected by RT-PCR. The specific
primers used are listed in Table I.
The thermocycling conditions consisted of initial denaturation at
95˚C for 30 sec, followed by 40 cycles of 5 sec at 95˚C and 5 sec
at 60˚C, which were subsequently followed by the melting curve
test. The relative mRNA expression normalized to β-actin was
expressed as a fold change, which was calculated using the
comparative Ct (2-ΔΔCq) method (34) using the control group as a reference
with 2-ΔΔCq=1.
 | Table IPrimer sequences used for reverse
transcription-quantitative PCR. |
Table I
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Forward primer
sequence (5'-3') | Reverse primer
sequence (3'-5') | Amplification
length (bp) |
|---|
| β-actin |
GAGACCTTCAACACCCCAGC |
CCACAGGATTCCATACCCAA | 446 |
| RhoA |
CGCTTTTGGGTACATGGAGT |
GTGGGCTCAGTCAAAAGCTC | 79 |
| LIMK2 |
GTGGGCTCAGTCAAAAGCTC |
CCACAAGGGTGCAAAGAAAT | 284 |
| ROCK1 |
AGGCGGTGATGGCTATTATG |
CCCAACCAAAGAATCTGCAT | 190 |
Protein extraction and
immunoblotting
A total of 1x106 MC3T3-E1 cells were
lysed on ice with RIPA lysis buffer (Beyotime Institute of
Biotechnology) containing protease and phosphatase inhibitors
(Beyotime Institute of Biotechnology) after being cultured for 1, 3
or 5 days. The supernatant was collected by centrifugation at
12,000 x g for 15 min at 4˚C, and the protein concentration was
quantified using a bicinchoninic acid assay (Beyotime Institute of
Biotechnology). Protein samples (30 µg/lane) were resolved using
10% SDS-PAGE and transferred onto PVDF membranes (EMD Millipore).
Membranes were blocked with 5% non-fat dried milk in Tris-buffered
saline with 0.1% Tween 20 (TBST) for 2 h at room temperature.
Membranes were incubated overnight at 4˚C with the following
antibodies: Rabbit anti-cofilin (cat. no. 3312; dilution 1:1,000;
Cell Signaling Technology, Inc.), rabbit anti-ROCK1 (cat. no. 4035;
dilution 1:2,000; Cell Signaling Technology, Inc.), rabbit
anti-phospho-LIMK2 (cat. no. 3845; dilution 1:2,000; Cell Signaling
Technology, Inc.), rabbit anti-phospho-cofilin (cat. no. 3311;
dilution 1:2,000; Cell Signaling Technology, Inc.) and β-actin
(cat. no. 4967; dilution 1:3,000; Cell Signaling Technology, Inc.).
Following three washes with TBST, membranes were incubated with
horseradish peroxidase-conjugated anti-rabbit IgG secondary
antibody (cat. no. ab97200; dilution 1:2,000; Abcam) for 1 h at
room temperature. Immunoreactive bands were visualized using
enhanced chemiluminescence detection reagents (ECL; EMD Millipore)
and images were captured using a chemiluminescence imaging system
(Kodak). Densitometric analysis was performed using the ImageJ
software (version 1.8.0; National Institutes of Health).
RhoA activation assay
A total of 1x106 MC3T3-E1 cells were
harvested on days 1, 3 and 5 following X-ray irradiation and lysed
with RIPA buffer (Beyotime Institute of Biotechnology) buffer. the
protein concentration was determined using a bicinchoninic acid
protein assay kit (Beyotime Institute of Biotechnology). A total of
30 µl the supernatant was used to determine the expression of total
RhoA. The remaining supernatant was used to isolate GTP-bound RhoA
using an Active GTPase Pull-down kit (cat. no. 16116; Thermo Fisher
Scientific, Inc.), which used the glutathione
S-transferase-Rhotekin Rho binding domain (EMD Millipore),
according to the manufacturer's protocol. The eluted proteins were
then separated using 15% SDS-PAGE, transferred to PVDF membranes
(EMD Millipore). After blocking with 5% skim milk in TBST for 2 h
at room temperature, the membranes were incubated overnight with
specific anti-RhoA antibodies (1:100; cat. no. sc-418; Santa Cruz
Biotechnology, Inc.). Total RhoA protein was detected via western
blotting. Immunoreactive bands were visualized using ECL (EMD
Millipore) and band intensity was quantified using ImageJ v1.8.0
software.
Y-27632 inhibition of ROCK1
MC3T3-E1 cells were pretreated with 10 µmol/l
Y-27632 (EMD Millipore) at 37˚C for 30 min prior to X-ray
irradiation. After being cultured for 1, 3 or 5 days, cells in each
group were labeled with FITC-phalloidin to observe the changes in
the cytoskeleton using the protocols aforementioned. The
fluorescence intensity of the cells was quantitatively analyzed
using ImageJ v1.8.0 software.
Alkaline phosphatase staining
MC3T3-E1 cells were cultured in 24-well plates at a
density of 1x104 cells/well. Cells were then treated
with the 0, 0.5 and 5 Gy X-ray doses. The medium was discarded on
day 7. Alkaline phosphatase staining was carried out using the
Alkaline Phosphatase Assay Kit (cat. no. P0321, Beyotime Institute
of Biotechnology) at room temperature for 30 min according to the
manufacturer's protocol and observed using an inverted light
microscope (magnification, x10).
Alizarin red staining
MC3T3-E1 cells were cultured in 24-well plates at a
density of 1x104 cells/well and treated with 0, 0.5 and
5 Gy X-ray. The medium was changed once every 3 days and discarded
on day 12. After washing with PBS, cells were fixed with 4%
paraformaldehyde at room temperature for 20 min and were stained
with 1% alizarin red dye (pH 4.2) at room temperature for 30 min.
Mineralized nodules were visualized using a light microscope
(magnification, x20).
Statistical analysis
Data are presented as mean ± SD and all experiments
were performed in triplicate. Differences between the groups were
analyzed by one-way ANOVA followed by Student-Newman-Keuls test
using SPSS software (version 18.0; SPSS, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of irradiation on MC3T3-E1
cell morphology
MC3T3-E1 cells cultured in α-MEM supplemented with
10% FBS adhered and formed cell colonies with irregular morphology,
mainly exhibiting fusiform shape, and had large nuclei and
observable nucleoli. After 3 days, the cells aggregated in a
semi-confluent state, cell number increased and cell bodies were
markedly enlarged. After 5 days, the cells formed confluent
monolayers.
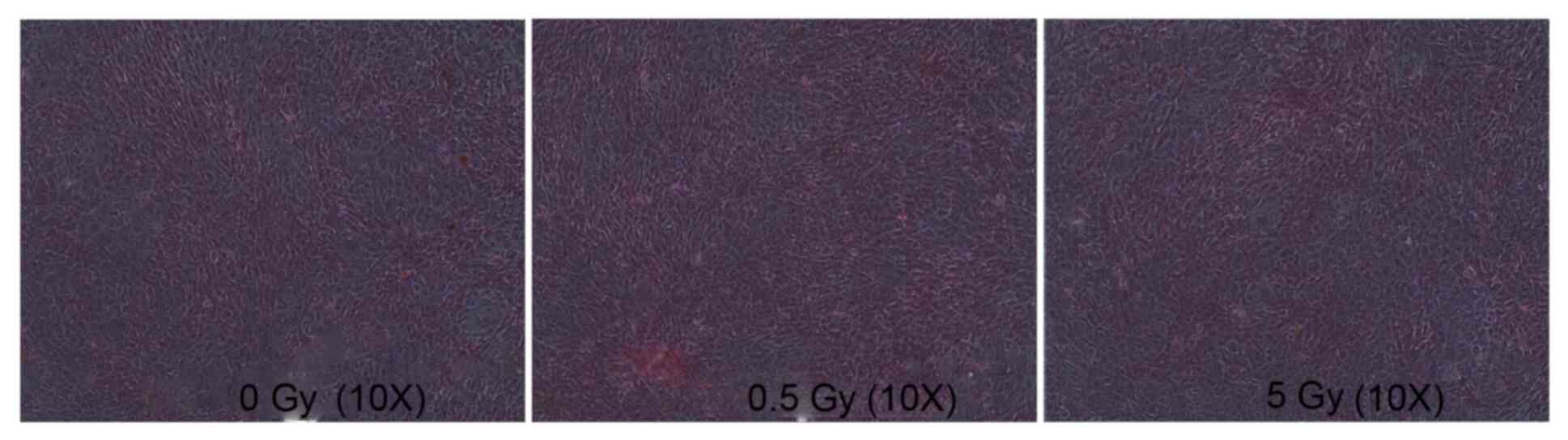
The unirradiated cells proliferated and exhibited
small cell volumes, plump cell bodies and abundant cytoplasm, with
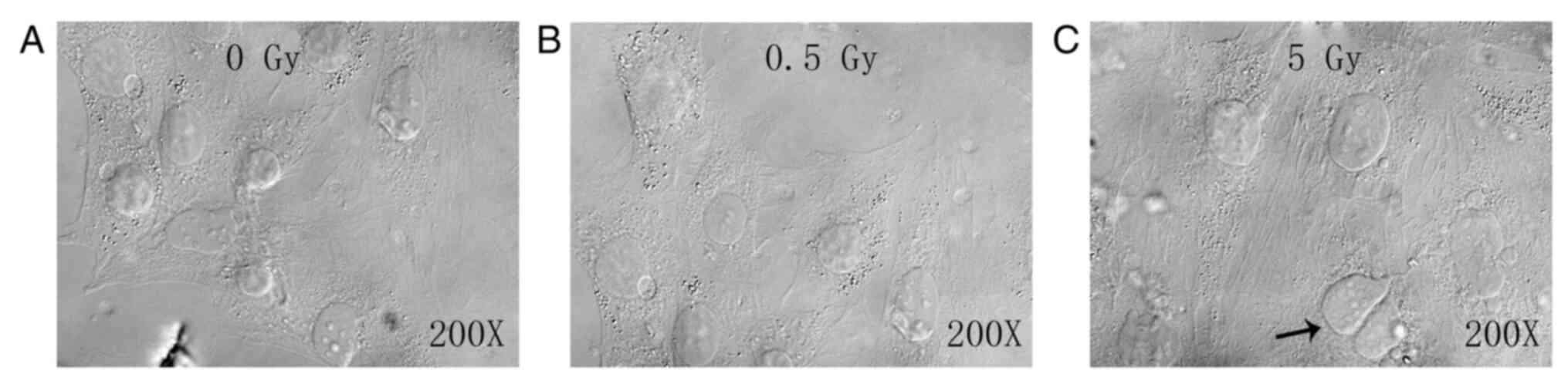
cells in the dividing phase exhibiting irregular shapes (Fig. 1A). No notable difference was
detected between the 0.5 Gy group and the control group (Fig. 1B). The 5 Gy group exhibited
decreased cell numbers, enlarged cell bodies, homogenous cytoplasm
and reduced refraction. Additionally, numerous black particles and
vacuoles were observed in the cytoplasm, along with multinucleated
giant cells (as indicated by arrow; Fig. 1C).
Observation of microstructural changes
by TEM
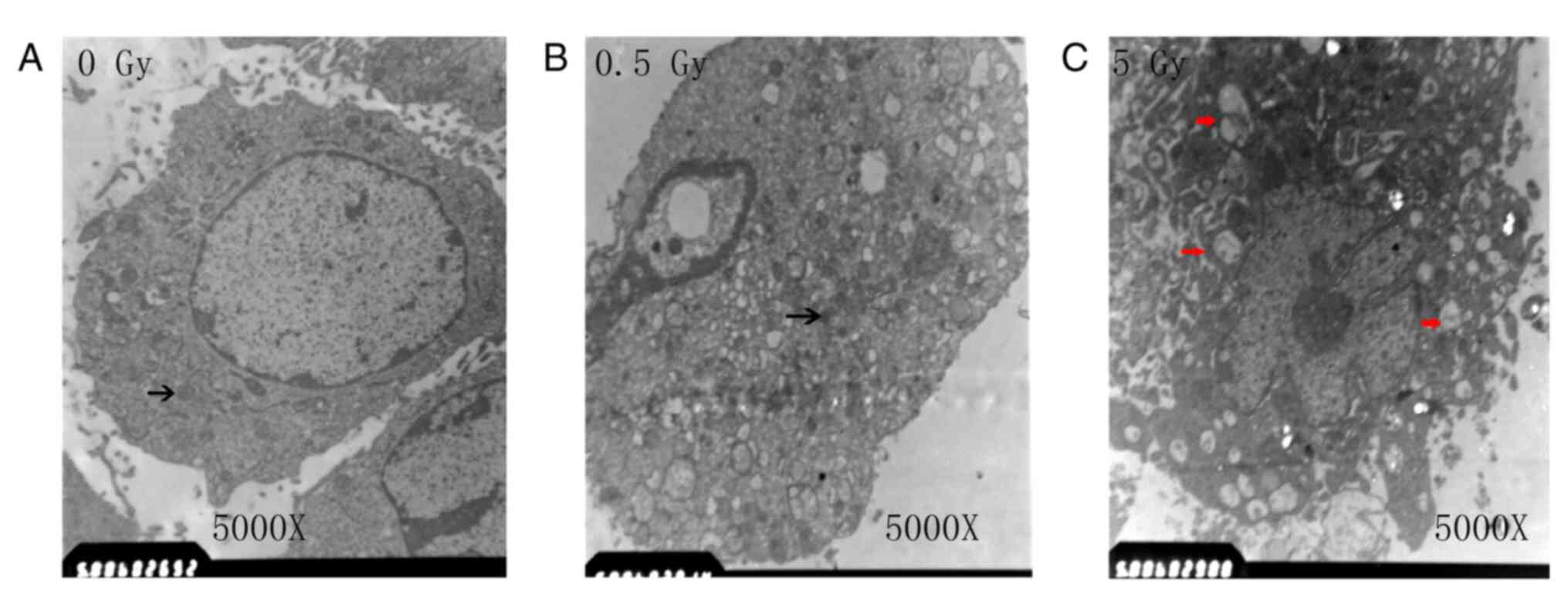
Cells in the control and the 0.5 Gy groups exhibited
abundant intracellular organelles and had observable nuclei.
Additionally, observation of intracellular Golgi bodies and
endoplasmic reticulum was common, while that of lysosomes was rare
(Fig. 2A and B). In the 5 Gy group, the nuclear
chromatin was condensed, the number of organelles decreased and the
number of enlarged vacuoles was increased (as indicated by the red
arrows; Fig. 2C).
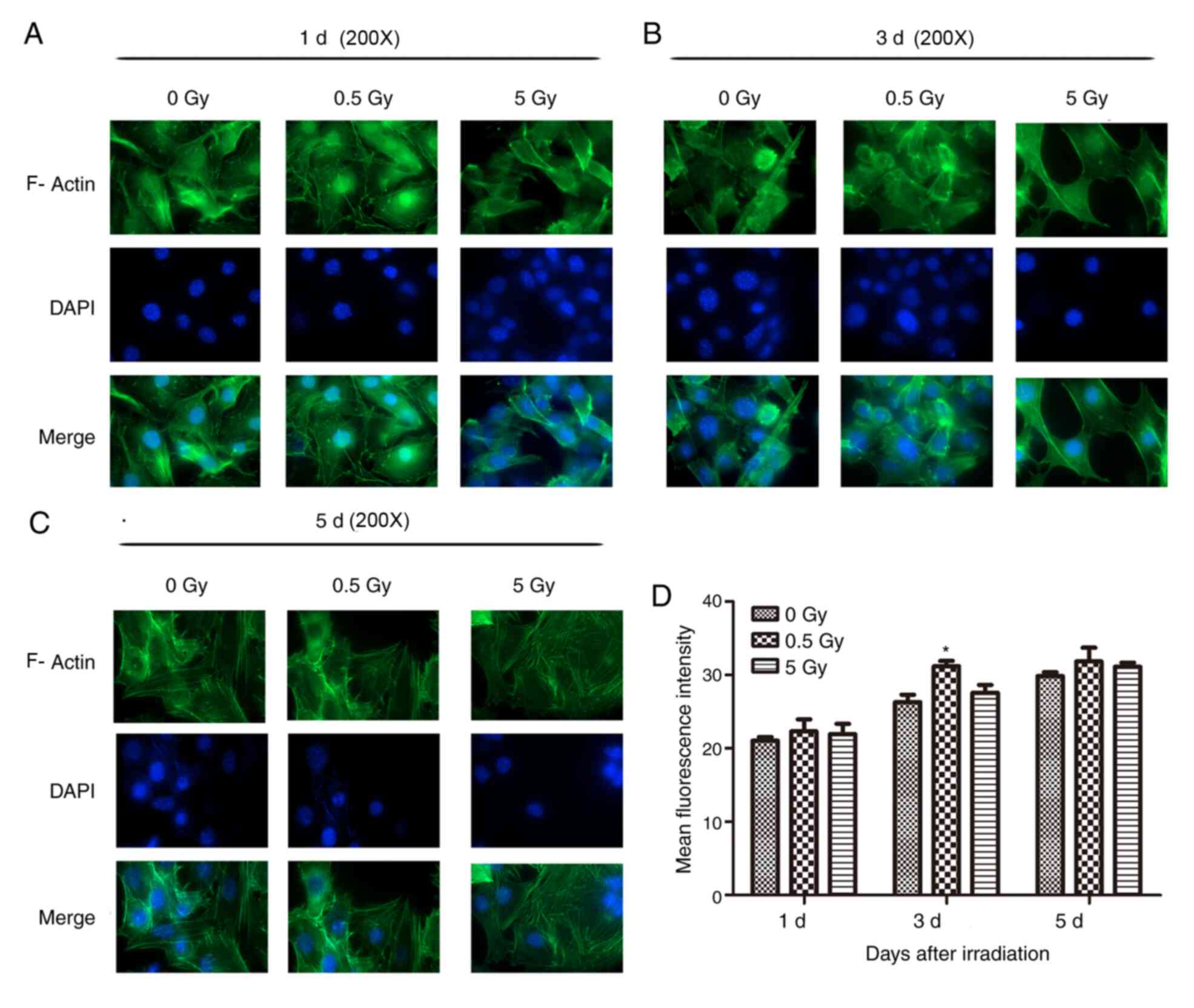
F-actin staining
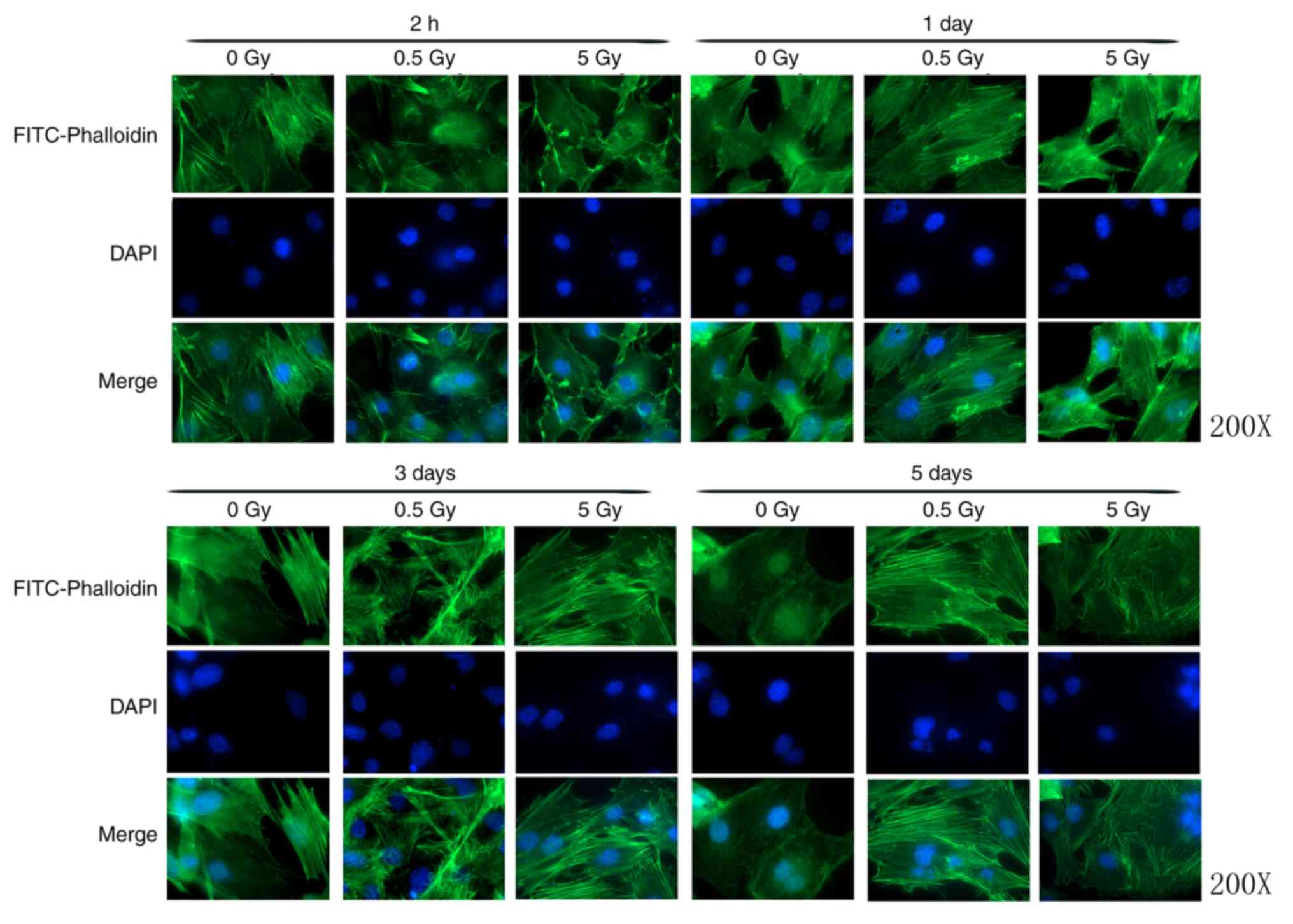
At 2 h post-irradiation, cells in the 0.5 and 5 Gy
groups exhibited decreased size and F-actin fluorescence intensity
compared with controls (Fig. 3).
Furthermore, the formation of stress fibers decreased and their
arrangement was sparse, disorganized, discontinuous or broken.
Additionally, the amount of crystallized actin increased in the
treated cells, particularly in the 5 Gy group. After 1 day, F-actin
began to thicken and rearranged in the 0.5 Gy group and
fluorescence intensity was significantly increased compared with
controls (Fig. 4). At 3 days
post-irradiation, fluorescence intensity in the 0.5 Gy group was
significantly increased compared with that in the 0 and 5 Gy groups
(Fig. 4). By day 5, F-actin
fluorescence intensity in the 0.5 Gy group returned to normal
(Fig. 4).
ImageJ software was used to randomly analyze the
images of six cells with clear boundaries within the slide and the
average fluorescence intensity of each cell (average fluorescence
intensity=fluorescence intensity/cell area) was measured as the
analysis index (Table II).
 | Table IIFluorescence intensity of fiber actin
in MC3T3-E1 cells following X-ray irradiation. |
Table II
Fluorescence intensity of fiber actin
in MC3T3-E1 cells following X-ray irradiation.
| A, 2 h
post-irradiation |
|---|
| Radiation dose
(Gy) | Area | Mean ± SD | Min | IntDen |
P-valuea |
|---|
| 0 | 256,956 | 29.107±13.296 | 15 | 7,479,102 | / |
| 0.5 | 250,396 | 25.329±12.209 | 12 | 6,342,378 | 0.045 |
| 5 | 248,358 | 27.021±13.049 | 12 | 6,710,960 | 0.036 |
| B, 1 day
post-irradiation |
| Radiation dose
(Gy) | Area | Mean ± SD | Min | IntDen | P-value |
| 0 | 213,267 | 30.865±14.266 | 22 | 8,075,451 | / |
| 0.5 | 270,394 | 37.176±16.750 | 16 | 10,052,197 | 0.038 |
| 5 | 200,453 | 36.728±15.782 | 12 | 7,362,259 | 0.525 |
| C, 3 days
post-irradiation |
| Radiation dose
(Gy) | Area | Mean ± SD | Min | IntDen | P-value |
| 0 | 264,269 | 35.645±17.213 | 10 | 9,419,783 | / |
| 0.5 | 253,611 | 38.687±18.072 | 15 | 9,811,449 | 0.047 |
| 5 | 285,670 | 36.039±12.128 | 12 | 10,295261 | 0.325 |
| D, 5 days
post-irradiation |
| Radiation dose
(Gy) | Area | Mean ± SD | Min | IntDen | P-value |
| 0 | 198,053 | 27.309±15.039 | 13 | 5,408,629 | / |
| 0.5 | 275,760 | 28.527±14.107 | 18 | 7,866,606 | 0.416 |
| 5 | 251,803 | 27.258±13.322 | 9 | 6,863,646 | 0.512 |
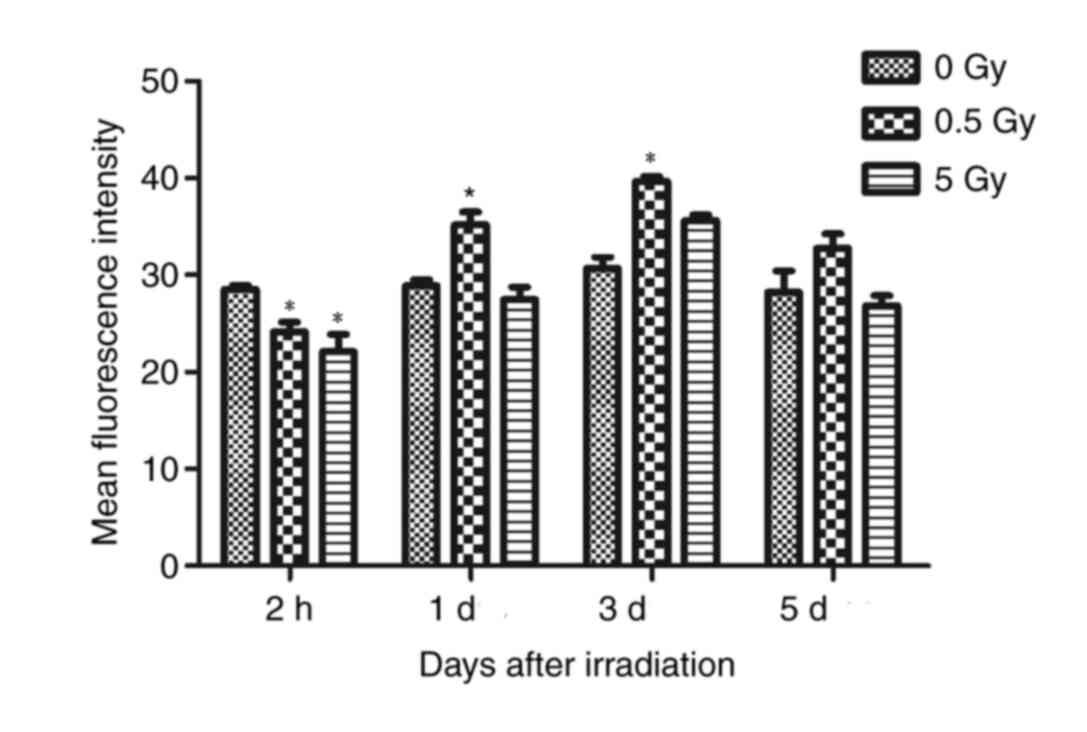
The results demonstrated that fluorescence intensity
of the 0.5 and 5 Gy groups were significantly decreased compared
with controls following 2 h of X-ray irradiation (P<0.05;
Fig. 4). At 24 h post-irradiation,
intensity in the 0.5 Gy group was significantly increased compared
with controls and the 5 Gy group (P<0.05; Fig. 4). The fluorescence intensity in the
5 Gy group was not significantly increased compared with controls
(P>0.05; Fig. 4). Following 3
days irradiation, the intensity in the 0.5 Gy group reached a peak
value that was significantly increased compared with the other two
groups (P<0.05; Fig. 4).
Additionally, the intensity in the 5 Gy group increased, but not
significantly compared with controls. At 5 days post-irradiation,
the fluorescence intensity of the 0.5 and 5 Gy groups decreased,
but not significantly when compared with controls (Fig. 4).
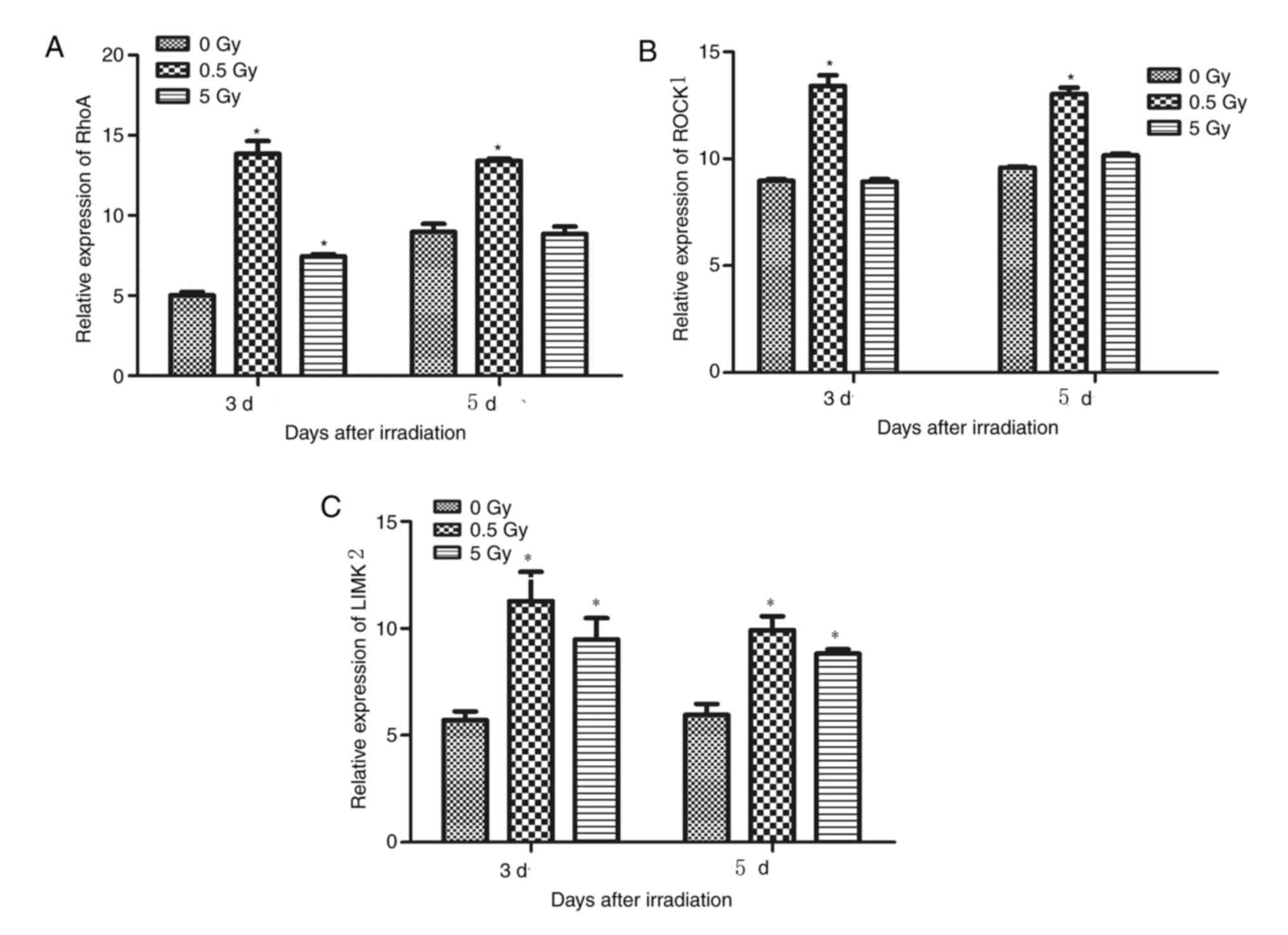
Effects of irradiation on RhoA, ROCK1
and LIMK2 expression
Previous studies have revealed that LIMK1 and LIMK2
are activated by Rho GTPases (26,29,35).
These molecules induce cytoskeleton reorganization by
phosphorylating and inactivating the actin depolymerization of
cofilin (35). The expression of
genes associated with the formation of actin filaments, including
RhoA, ROCK1 and LIMK2, were examined using RT-qPCR on days 3 and 5
days following irradiation. The results demonstrated that RhoA,
ROCK1 and LIMK2 expression was significantly upregulated following
irradiation with 0.5 Gy compared with those irradiated with 0 Gy
(P<0.05; Fig. 5A-C).
At 3 and 5 days post-irradiation, the expression of
RhoA in the 0.5 Gy group was significantly higher than that in the
control and 5 Gy groups (P<0.05; Fig. 5A). Additionally, RhoA was
significantly higher in the 5 Gy group compared controls 3 days
post-irradiation; however, no significant difference was detected
on day 5 (P>0.05; Fig. 5A).
LIMK2 expression in the 0.5 and 5 Gy groups was significantly
higher compared with controls at 3 and 5 days post-irradiation
(P<0.05; Fig. 5C), but no
significant difference was detected between the two groups
(Fig. 5C). ROCK1 expression in the
0.5 Gy group was significantly higher compared with the control and
5 Gy groups at days 3 and 5 (P<0.05), but no significant
difference was detected between the 5 and 0 Gy groups (Fig. 5B).
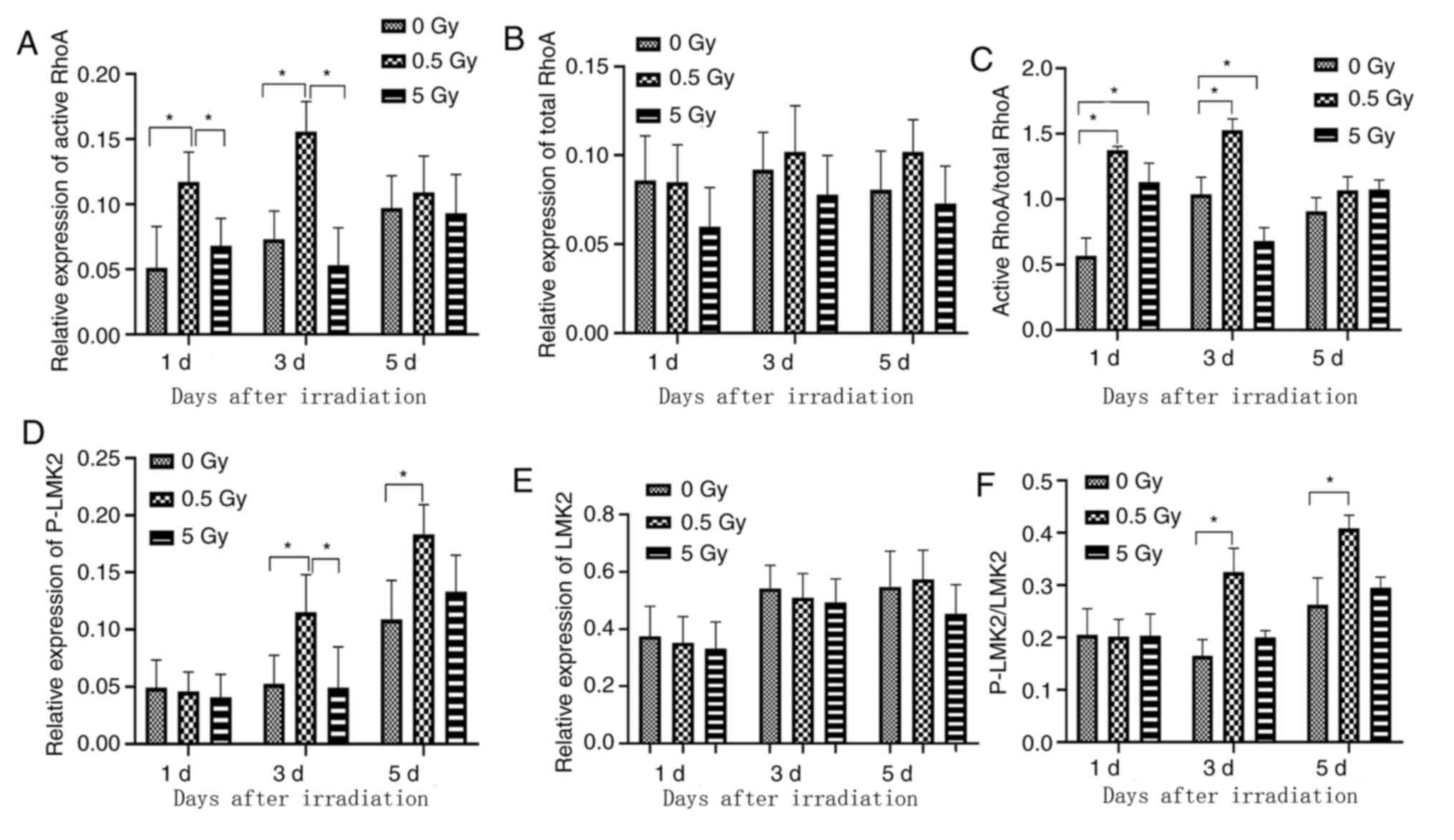
Synthesis of cytoskeletal
proteins
RhoA, ROCK1, LIMK2, phosphorylated LIMK2 (p-LIMK2),
cofilin and phosphorylated cofilin (P-cofilin) protein expression
was assessed by western blotting (Figs.
6-9) and RhoA, ROCK1, LIMK2, phosphorylated LIMK2 (p-LIMK2)
demonstrated similar expression levels when compared with mRNA
expression. The expression levels of cofilin and phosphorylated
cofilin (p-cofilin) were consistent with other cytoskeletal related
proteins.
RhoA activation via GTP-loading in X-ray-irradiated
osteoblasts was analyzed to determine the significance of RhoA in
radiation response. A low level of GTP-RhoA was detected in
non-irradiated (0 Gy) cells (Fig.
7A). This level increased significantly at 1 day
post-irradiation in the 0.5 Gy group compared with controls and the
5 Gy group (Fig. 7A), indicating
that RhoA was rapidly activated in osteoblasts. This increase was
most pronounced on day 3 in the 0.5 Gy group compared with controls
and the 5 Gy group (Fig. 7A). No
significant difference was observed in the total RhoA level between
groups (Fig. 7B). The variation
trend of active-RhoA/RhoA ratio in each group was similar to that
of active-RhoA (Fig. 7C).
Furthermore, P-LIMK2 expression significantly increased on days 3
and 5 following 0.5 Gy irradiation compared with controls and 5 Gy
groups (Fig. 7D). No significant
difference was observed in the total LIMK2 level between groups
(Fig. 7E). The variation trend of
p-LIMK2/LIMK2 ratio in each group was similar to that of p-LIMK2
(Fig. 7F). These results indicated
that RhoA was a critical downstream regulator of X-ray
irradiation-induced actin reorganization.
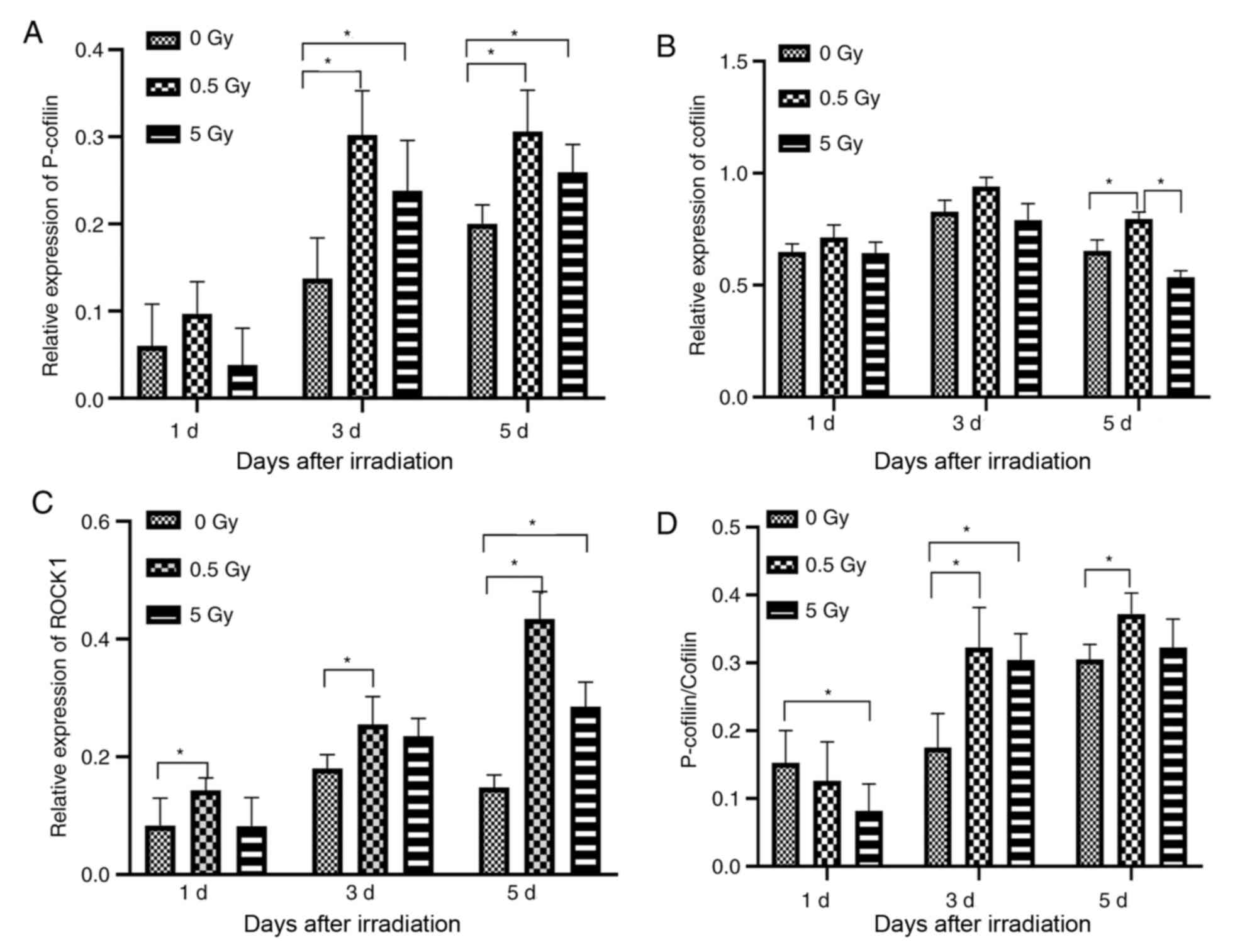
At 1 day post-irradiation, ROCK1 expression in the
0.5 Gy group was significantly increased compared with the control
group. On day 3, ROCK1 expression was also significantly increased
compared with controls (P<0.05, Fig.
9C) and reached a peak value at day 5 (P<0.05, Fig. 9C). Furthermore, P-cofilin level was
higher 1 day post-irradiation in the 0.5 Gy group compared to the
control and 5 Gy groups, but this difference was not statistically
significant (Fig. 9A). However,
there was a statistically significant increase in expression on
days 3 and 5 post-irradiation in the 0.5 Gy group compared with the
control groups (P<0.05; Fig.
9A). Additionally, P-cofilin expression was significantly
higher in the 5 Gy group compared with controls on days 3 and 5
(Fig. 9A). Furthermore, cofilin
expression was not significantly different between groups on days 1
and 3 post-irradiation; however, on day 5 expression was
significantly increased in the 0.5 Gy group compared with control
and 5 Gy groups (P<0.05; Fig.
9B). The expression of P-cofilin/cofilin was significantly
higher in the controls compared with the 0.5 and 5 Gy groups on day
1 (P<0.05; Fig. 9D). However,
P-cofilin/cofilin expression was significantly increased in the 0.5
and 5 Gy groups compared with controls on day 3, and between the
0.5 group and controls on day 5 (P<0.05; Fig. 9D). No significant difference was
detected between the 0.5 and 5 Gy groups on all timepoints tested
(Fig. 9D).
F-actin staining in cells pretreated
with ROCK1 inhibitor Y-27632 (10 µmol/l) for 30 min prior to X-ray
irradiation
The results demonstrated green fluorescence in all
cells 1 day following X-ray irradiation and Y-27632 pretreatment
(Table III, Fig. 10A-C). However, fluorescence
intensity was significantly weaker in the 0 Gy group compared with
other groups after 1 day. Deformed tension fibers and unorganized
thin filaments were observed (Fig.
10A). The fluorescence intensity of all groups was increased on
day 3 compared with the intensity on day 1 (Fig. 5B). This intensity was further
increased on day 5 and the tension fibers were organized and had
returned to baseline (Fig.
10C).
 | Table IIIFluorescence intensity of
Y-27632-pretreated MC3T3-E1 cells in each group. |
Table III
Fluorescence intensity of
Y-27632-pretreated MC3T3-E1 cells in each group.
| A, 1 day
post-irradiation |
|---|
| Radiation dose
(Gy) | Area | Mean ± SD | Min | IntDen |
|---|
| 0 | 277,623 | 22.035±11.503 | 13 | 6,117,423 |
| 0.5 | 272,308 | 23.513±11.986 | 15 | 6,402,778 |
| 5 | 190,825 | 22.761±17.157 | 13 | 4,343,367 |
| B, 3 days
post-irradiation |
| Radiation dose
(Gy) | Area | Mean ± SD | Min | IntDen |
| 0 | 252,417 | 26.306±15.477 | 7 | 6,640,082 |
| 0.5 | 232,329 | 31.277±11.777 | 15 | 7,266,554 |
| 5 | 260,815 | 26.502±13.742 | 13 | 6,912,119 |
| C, 5 days
post-irradiation |
| Radiation dose
(Gy) | Area | Mean ± SD | Min | IntDen |
| 0 | 238,070 | 30.361±16.946 | 10 | 7,227,973 |
| 0.5 | 284,840 | 32.241±14.872 | 13 | 9,183,526 |
| 5 | 284,334 | 30.307±16.136 | 3 | 8,617,311 |
At 1 day post-irradiation and -Y-27632 treatment, no
significant difference was detected in the fluorescence intensity
between the 0.5 and 5 Gy groups and controls (Fig. 10D). However, 3 days
post-irradiation and -treatment, the fluorescence intensity and the
number of tension fibers of all groups increased. Additionally, the
fluorescence intensity of cells in the 0.5 Gy group was
significantly increased compared with the control and 5 Gy groups
(P<0.05; Fig. 10D). By day 5,
the tension fibers were arranged into stress fibers, F-actin
gradually returned to baseline and no significant difference was
detected in fluorescence intensity among the groups (P>0.05;
Fig. 10D).
Identification of osteoblasts
Following alkaline phosphatase staining, the
cytoplasm of osteoblasts with purple granules, indicating staining
was positive for alkaline phosphatase expression on the cell
membrane surface of the osteoblasts (Fig. 11). Alkaline phosphatase serves as a
marker enzyme for the differentiation and maturation of osteoblast,
indicating the various stages of osteoblast differentiation
(36).
Following 21 days in culture, the osteoblasts
converged and showed multiple layers of overlapping growth. Their
cell arrangement centered on mineralized nodules. Following
alizarin red staining, multiple orange mineralized nodules of
various sizes were observed (Fig.
12). Mineralization is a critical biological characteristic of
osteoblasts (36). The results
showed a satisfactory mineralization function of the cultured
osteoblasts.
Discussion
The cytoskeleton is dynamic network of
interconnected polymers that regulates the mechanical properties of
cells and maintains cell morphology, cell division and
intracellular transportation (37,38).
Additionally, the cytoskeleton is closely associated with cell
migration, phagocytosis, pinocytosis and secretion (39,40)
and serves a major role in transducing a variety of intracellular
signals (41). Therefore, changes
in the cytoskeleton inevitably affect cell function.
The cytoskeleton mainly consists of microtubules,
microfilaments and intermediate filaments (21). Microfilaments are mainly composed of
actin in the form of free globular (G-) or F-actin (21). Mechanical stimulation causes
intracellular free G-actin aggregation, resulting in the formation
of F-actin (26). Microfilaments
serve a role in maintaining cell morphology, tight intercellular
connections and extracellular matrix adhesion and are sensitive to
ionizing radiation (42). The
dynamics of actin polymerization and depolymerization regulate cell
movement, adhesion and the cell division cycle (43). Ionizing radiation can damage the
membrane cytoskeleton, which affects cellular function (43).
In the current study, X-ray irradiation caused
intracellular morphological and microstructural changes in
osteoblasts. Cells irradiated by 0 and 0.5 Gy X-rays exhibited
abundant intracellular organelles, such as Golgi bodies and
endoplasmic reticulum, which are related to the enhancement of cell
proliferation and differentiation (13). However, in cells irradiated with 5
Gy X-rays, the nuclear chromatin condensed, the number of lysosomes
increased and the number of intracellular organelles decreased.
Additionally, various necrosis-related changes, including enlarged
vacuoles and homogeneous nuclear lysis, were observed. These
results were hypothesized to be related to direct cellular damage
caused by high doses of radiation.
A previous in vitro study demonstrated that
ionizing radiation can damage cellular actin networks and
endothelial cell barrier function (28). Human respiratory epithelial cell
line alu3 and 16HBEl40 were treated with 2-10 Gy irradiation and
the results demonstrated that F-actin was significantly reduced and
intracellular crystallized actin expression was increased. These
results indicated that radiation caused an increase in F-actin
depolymerization (44).
Furthermore, these cytoskeleton changes affected the connections
between cells, resulting in increased permeability and the
formation of cell gaps (44).
In the current study, unirradiated cells exhibited
normal cell morphology and F-actin was organized. At 2 h
post-irradiation, unirradiated cells shrank and became plump.
Subsequently, F-actin depolymerized and its fluorescence intensity
decreased, which indicated a disorganized and discontinuous state,
particularly in the 5 Gy group. The cause of these changes was
attributed to ionizing radiation, which lead to cytoskeleton
damage, fracture and collapse. However, the 0.5 Gy group exhibited
different changes. At 2 h post-irradiation, the arrangement of
microfilaments was disordered and discontinuous, similar to that of
the 5 Gy group. However, intracellular actin became thick and
rearranged 24 h post-irradiation. As a result, the fluorescence
intensity increased, indicating that F-actin expression was
significantly increased following LDI. Cytoskeleton rearrangement
occurred and was maintained for 5 days. Thus, the disordered
cytoskeleton arrangement was hypothesized to be due
radiation-induced damage, where an as yet unknown repair system had
been activated causing F-actin rearrangement. These changes were
similar to mechanical force-induced cytoskeleton reorganization
(26). Additionally, Ricci et
al (23) demonstrated that 0.5
Gy X-ray irradiation caused cytoskeleton reorganization and an
increase in the number of stress fibers in the cytoplasm. However,
the mechanisms involved in stimulating cellular signaling pathways
during DNA damage repair remain to be elucidated. In the current
study, F-actin expression post-irradiation decreased gradually in a
time-dependent manner; however, at day 7 F-actin fluorescence
intensity was similar to baseline, indicating that the cytoskeleton
changes induced by 0.5 Gy X-ray were reversible. Furthermore, the
F-actin cytoskeleton in MC3T3-E1 cells demonstrated decreased
fluorescence intensity and disordered F-actin when irradiated by 5
Gy.
The results of the current study demonstrated that
the expression of RhoA, LIMK2, ROCK1 and p-cofilin increased during
actin rearrangement following 0.5 Gy irradiation. These results
provided evidence that RhoA/ROCK/LIMK2/cofilin constituted a
pathway involved in the regulation of actin dynamics initiated by
X-ray irradiation. These results were in accordance with a previous
study by Rousseau et al (45). Furthermore, LIMK2 was linked to Rho
GTPases and, via direct substrate cofilin, to actin microfilaments.
A previous study demonstrated that Rho GTPases and their effector,
ROCK1, were involved in MC3T3-E1 cells in response to flow shear
stress, ultrasound and magnetic field (26). The results of the current study
demonstrated the potential sequence of signaling as RhoGTPases
regulating ROCK1, LIMK2 and cofilin to induce actin polymerization.
However, the polymerization decreased on the 5th day
post-irradiation as compared to the control group, which might be
related to negative feedback regulation in the cells.
The current study demonstrated that the cofilin
protein expression in the 0.5 and 5 Gy groups was higher than that
in the non-irradiation group. X-ray irradiation was hypothesized to
activate the Rho/ROCK pathway and increase the synthesis of
P-cofilin, which was derived from the intracellular synthesis of
cofilin. On days 3 and 5 following X-ray irradiation, the ratio of
P-cofilin/cofilin increased significantly in the 0.5 Gy group,
indicating that the synthetic cofilin was converted to P-cofilin.
Additionally, the fluorescence intensity of F-actin decreased in
cells pretreated with Y-27632. This further suggested that
LDI-induced cytoskeleton remodeling may occur through the
Rho/ROCK1/LIMK2/cofilin pathway.
Gabry et al (46) demonstrated that radiation causes
rapid rearrangement of actin in capillary endothelial cells,
leading to the activation the RhoA/ROCK signaling pathway. Rousseau
et al (45) revealed that
following 15 Gy X-ray irradiation, human microvascular endothelial
cells exhibited cytoskeleton rearrangement and RhoA expression
increased significantly. Previous studies have reported that 0.5 Gy
LDI shrinks murine and bovine pulmonary capillary endothelial cells
and increases intercellular space. These results were primarily
attributed to the increase in actin depolymerization (28,47).
Savla et al (44)
demonstrated that bovine pulmonary artery endothelial cell
permeability increased following 10 Gy irradiation, which disrupted
the integrity of F-actin and that the number of incomplete actin
crystals increased with the random distribution of F-actin in
cells. Furthermore, Kantak et al (48) revealed that ionizing radiation
induced the depolymerization of F-actin in vascular endothelial
cells, increasing in a dose-dependent manner. These results may be
related to cell type, cell sensitivity to radiation, culture
environment and radiation dose.
The RhoA/ROCK signaling pathway is involved in the
formation of the cytoskeleton, which promotes osteogenic
differentiation by altering contact areas between the cells
(49). Moreover, RhoGTPases are key
mediators of the Wnt signaling pathway, which can influence
cellular behavior through morphological and transcriptional changes
(9). Therefore, the RhoA/ROCK
signaling pathway may also interact with the Wnt signaling pathway,
participating in LDI to promote osteoblast differentiation.
The initiating factor in ionizing radiation-induced
RhoA activation has not been fully elucidated. Cells may produce
large amounts of ROS following irradiation (50-52).
RhoA has recently been discovered to be a target protein of
reactive oxygen species (ROS) (53,54)
ROS can activate multiple signaling pathways without ligand and
receptor binding, thereby activating RhoA. Furthermore, ROS can
activate stress-activated protein kinase (SAPK) family molecules,
including SAPK2/p38, which cause endothelial cell cytoskeleton
remodeling (55). Another putative
pathway is the formation of sphingolipid ceramides on the cell
membrane, which have been found to activate RhoA in vitro
(56). Sphingolipid ceramindes act
as critical messengers for transducing cellular stress (57). Additionally, Torroba et al
(58) revealed that the activation
of RhoA in epithelial cells requires a parallel signaling pathway
involving the activation of phosphatidylinositol 3-kinase.
In conclusion, the results of the current study
demonstrated that LDI caused reversible cytoskeleton
reorganization. At a radiation dose of 5 Gy, MC3T3-E1 cells
exhibited damaged cytoskeletons, and decreased and disorganized
actin content. The RhoA/ROCK1/LIMK2/cofilin pathway may be a
possible mechanism involved in these changes. Since cytoskeleton is
closely associated with cell function, further research into the
effects of LDI-induced actin reorganization on cell proliferation
and differentiation is imperative.
Acknowledgements
Not applicable.
Funding
The current study was funded by the National Natural
Science Foundation of China (grant no. 81171730). The funders did
not have a role in study design, data collection, data analysis,
decision to publish or the writing of the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QH and ZZ conceived and coordinated the study,
designed, performed and analyzed the experiments and wrote the
paper. LW, WS, QX, XZ and LZ performed data collection, data
analysis, and revised the paper. FY and QD designed the study,
carried out the data analysis and revised the paper. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fazel R, Krumholz HM, Wang Y, Ross JS,
Chen J, Ting HH, Shah ND, Nasir K, Einstein AJ and Nallamothu BK:
Exposure to low-dose ionizing radiation from medical imaging
procedures. N Engl J Med. 361:849–857. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Oh D and Huh SJ: Insufficiency fracture
after radiation therapy. Radiat Oncol J. 32:213–220.
2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Michel G, Blery P, Pilet P, Guicheux J,
Weiss P, Malard O and Espitalier F: Micro-CT analysis of
radiation-induced osteopenia and bone hypovascularization in Rat.
Calcif Tissue Int. 97:62–68. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wei RL, Jung BC, Manzano W, Sehgal V,
Klempner SJ, Lee SP, Ramsinghani NS and Lall C: Bone mineral
density loss in thoracic and lumbar vertebrae following radiation
for abdominal cancers. Radiother Oncol. 118:430–436.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Oest ME, Mann KA, Zimmerman ND and Damron
TA: Parathyroid hormone (1-34) transiently protects against
radiation-induced bone fragility. Calcif Tissue Int. 98:619–630.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Luckey T: Physiological benefits from low
levels of ionizing radiation. Health Phys. 43:771–789.
1982.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Park SS, Kim KA, Lee SY, Lim SS, Jeon YM
and Lee JC: X-ray radiation at low doses stimulates differentiation
and mineralization of mouse calvarial osteoblasts. BMB Rep.
45:571–576. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhou XZ, Zhang G, Dong QR, Chan CW, Liu CF
and Qin L: Low-dose X-irradiation promotes mineralization of
fracture callus in a rat model. Arch Orthop Trauma Surg.
129:125–132. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Schlessinger K, Hall A and Tolwinski N:
Wnt signaling pathways meet Rho GTPases. Genes Dev. 23:265–277.
2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Theocharopoulos N, Perisinakis K,
Damilakis J, Papadokostakis G, Hadjipavlou A and Gourtsoyiannis N:
Occupational exposure from common fluoroscopic projections used in
orthopaedic surgery. J Bone Joint Surg Am. 85:1698–1703.
2003.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pramojanee SN, Pratchayasakul W,
Chattipakorn N and Chattipakorn SC: Low-dose dental irradiation
decreases oxidative stress in osteoblastic MC3T3-E1 cells without
any changes in cell viability, cellular proliferation and cellular
apoptosis. Arch Oral Biol. 57:252–256. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xu W, Xu L, Chen M, Mao YT, Xie ZG, Wu SL
and Dong QR: The effects of low dose X-irradiation on osteoblastic
MC3T3-E1 cells in vitro. BMC Musculoskelet Disord.
13(94)2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chen M, Huang Q, Xu W, She C, Xie ZG, Mao
YT, Dong QR and Ling M: Low-dose X-ray irradiation promotes
osteoblast proliferation, differentiation and fracture healing.
PLoS One. 9(e104016)2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Murata K, Noda SE, Oike T, Takahashi A,
Yoshida Y, Suzuki Y, Ohno T, Funayama T, Kobayashi Y, Takahashi T
and Nakano T: Increase in cell motility by carbon ion irradiation
via the Rho signaling pathway and its inhibition by the ROCK
inhibitor Y-27632 in lung adenocarcinoma A549 cells. J Radiat Res.
55:658–664. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen M, Dong QR, Huang Q, Xu W and She C:
Effects of 0.5 Gy X-ray radiation on the profile of gene expression
in MC3T3-E1 osteoblasts. Zhonghua Yi Xue Za Zhi. 96:2659–2664.
2016.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
16
|
Kawano T, Zhu M, Troiano N, Horowitz M,
Bian J, Gundberg C, Kolodziejczak K and Insogna K: LIM kinase 1
deficient mice have reduced bone mass. Bone. 52:70–82.
2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Szezerbaty SKF, de Oliveira RF,
Pires-Oliveira DAA, Soares CP, Sartori D and Poli-Frederico RC: The
effect of low-level laser therapy (660 nm) on the gene expression
involved in tissue repair. Lasers Med Sci. 33:315–321.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kurpinski K, Jang DJ, Bhattacharya S,
Rydberg B, Chu J, So J, Wyrobek A, Li S and Wang D: Differential
effects of x-rays and high-energy 56Fe ions on human mesenchymal
stem cells. Int J Radiat Oncol Biol Phys. 73:869–877.
2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li X, Liu C, Li P, Li S, Zhao Z, Chen Y,
Huo B and Zhang D: Connexin 43 is a potential regulator in fluid
shear stress-induced signal transduction in osteocytes. J Orthop
Res. 31:1959–1965. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Moorer MC and Stains JP: Connexin43 and
the intercellular signaling network regulating skeletal remodeling.
Curr Osteoporos Rep. 15:24–31. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gotlieb AI: The endothelial cytoskeleton:
Organization in normal and regenerating endothelium. Toxicol
Pathol. 18:603–617. 1990.PubMed/NCBI
|
|
22
|
McBeath R, Pirone DM, Nelson CM,
Bhadriraju K and Chen CS: Cell shape, cytoskeletal tension, and
RhoA regulate stem cell lineage commitment. Dev Cell. 6:483–495.
2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ricci R, Pazos MC, Borges RE and
Pacheco-Soares C: Biomodulation with low-level laser radiation
induces changes in endothelial cell actin filaments and
cytoskeletal organization. J Photochem Photobiol B. 95:6–8.
2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tani A, Chellini F, Giannelli M, Nosi D,
Zecchi-Orlandini S and Sassoli C: Red (635 nm), Near-infrared (808
nm) and Violet-Blue (405 nm) photobiomodulation potentiality on
human osteoblasts and mesenchymal stromal cells: A morphological
and molecular in vitro study. Int J Mol Sci.
19(1946)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang S, Cheng J and Qin YX:
Mechanobiological modulation of cytoskeleton and calcium influx in
osteoblastic cells by short-term focused acoustic radiation force.
PLoS One. 7(e38343)2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Arnsdorf EJ, Tummala P, Kwon RY and Jacobs
CR: Mechanically induced osteogenic differentiation-the role of
RhoA, ROCKII and cytoskeletal dynamics. J Cell Sci. 122:546–553.
2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kempf SJ, Buratovic S, von Toerne C,
Moertl S, Stenerlöw B, Hauck SM, Atkinson MJ, Eriksson P and Tapio
S: Ionising radiation immediately impairs synaptic
plasticity-associated cytoskeletal signalling pathways in HT22
cells and in mouse brain: An in vitro/in vivo comparison study.
PLoS One. 9(e110464)2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Onoda JM, Kantak SS and Diglio CA:
Radiation induced endothelial cell retraction in vitro: Correlation
with acute pulmonary edema. Pathol Oncol Res. 5:49–55.
1999.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang S, Chen C, Su K, Zha D, Liang W,
Hillebrands JL, Goor Hv and Ding G: Angiotensin II induces
reorganization of the actin cytoskeleton and myosin light-chain
phosphorylation in podocytes through rho/ROCK-signaling pathway.
Ren Fail. 38:268–275. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Etienne-Manneville S and Hall A: Rho
GTPases in cell biology. Nature. 420:629–635. 2002.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Amano M, Nakayama M and Kaibuchi K:
Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell
polarity. Cytoskeleton (Hoboken, NJ). 67:545–554. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tang AT, Campbell WB and Nithipatikom K:
ROCK1 feedback regulation of the upstream small GTPase RhoA. Cell
Signal. 24:1375–1380. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Carlier MF and Pantaloni D: Control of
actin assembly dynamics in cell motility. J Biol Chem.
282:23005–23009. 2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Method. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Vardouli L, Moustakas A and Stournaras C:
LIM-kinase 2 and cofilin phosphorylation mediate actin cytoskeleton
reorganization induced by transforming growth factor-beta. J Biol
Chem. 280:11448–11457. 2005.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zeng X, Feng Q, Zhao F, Sun C, Zhou T,
Yang J and Zhan X: Puerarin inhibits TRPM3/miR-204 to promote
MC3T3-E1 cells proliferation, differentiation and mineralization.
Phytother Res. 32:996–1003. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Pollard TD and Goldman RD: Overview of the
cytoskeleton from an evolutionary perspective. Cold Spring Harb
Perspect Biol. 10(a030288)2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Blanquie O and Bradke F: Cytoskeleton
dynamics in axon regeneration. Curr Opin Neurobiol. 51:60–69.
2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang Y, Shan Q, Pan J and Yi S: Actin
cytoskeleton affects schwann cell migration and peripheral nerve
regeneration. Front Physiol. 9(23)2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Szymanski D and Staiger CJ: The actin
cytoskeleton: Functional arrays for cytoplasmic organization and
cell shape control. Plant Physiol. 176:106–118. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Galli C, Piemontese M, Lumetti S,
Ravanetti F, Macaluso GM and Passeri GJ: Actin cytoskeleton
controls activation of Wnt/β-catenin signaling in mesenchymal cells
on implant surfaces with different topographies. Acta Biomater.
8:2963–2968. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Panzetta V, De Menna M, Musella I,
Pugliese M, Quarto M, Netti PA and Fusco S: X-rays effects on
cytoskeleton mechanics of healthy and tumor cells. Cytoskeleton
(Hoboken, NJ). 74:40–52. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Mohammadkarim A, Tabatabaei M, Parandakh
A, Mokhtari-Dizaji M, Tafazzoli-Shadpour M and Khani MM: Radiation
therapy affects the mechanical behavior of human umbilical vein
endothelial cells. J Mech Behav Biomed Mater. 85:188–193.
2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Savla U and Waters CM: Barrier function of
airway epithelium: Effects of radiation and protection by
keratinocyte growth factor. Radiat Res. 150:195–203.
1998.PubMed/NCBI
|
|
45
|
Rousseau M, Gaugler MH, Rodallec A,
Bonnaud S, Paris F and Corre I: RhoA GTPase regulates
radiation-induced alterations in endothelial cell adhesion and
migration. Biochem Biophys Res Commun. 414:750–755. 2011.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Gabryś D, Greco O, Patel G, Prise KM,
Tozer GM and Kanthou C: Radiation effects on the cytoskeleton of
endothelial cells and endothelial monolayer permeability. Int J
Radiat Oncol Biol Phys. 69:1553–1562. 2007.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Speidel MT, Holmquist B, Kassis AI, Humm
JL, Berman RM, Atcher RW, Hines JJ and Macklis RM: Morphological,
biochemical, and molecular changes in endothelial cells after
alpha-particle irradiation. Radiat Res. 136:373–381.
1993.PubMed/NCBI
|
|
48
|
Kantak SS, Diglio CA and Onoda JM: Low
dose radiation-induced endothelial cell retraction. Int J Radiat
Biol. 64:319–328. 1993.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Seo CH, Jeong H, Feng Y, Montagne K,
Ushida T, Suzuki Y and Furukawa KS: Micropit surfaces designed for
accelerating osteogenic differentiation of murine mesenchymal stem
cells via enhancing focal adhesion and actin polymerization.
Biomaterials. 35:2245–2252. 2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Cao X, Wu X, Frassica D, Yu B, Pang L,
Xian L, Wan M, Lei W, Armour M, Tryggestad E, et al: Irradiation
induces bone injury by damaging bone marrow microenvironment for
stem cells. Proc Natl Acad Sci USA. 108:1609–1614. 2011.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Rodrigues-Moreira S, Moreno SG, Ghinatti
G, Lewandowski D, Hoffschir F, Ferri F, Gallouet AS, Gay D,
Motohashi H, Yamamoto M, et al: Low-dose irradiation promotes
persistent oxidative stress and decreases self-renewal in
hematopoietic stem cells. Cell Rep. 20:3199–3211. 2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Wang C, Blough E, Dai X, Olajide O,
Driscoll H, Leidy JW, July M, Triest WE and Wu M: Protective
effects of cerium oxide nanoparticles on MC3T3-E1 osteoblastic
cells exposed to X-ray irradiation. Cell Physiol Biochem.
38:1510–1519. 2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Chu S, Mao X, Guo H, Wang L, Li Z, Zhang
Y, Wang Y, Wang H, Zhang X and Peng W: Indoxyl sulfate potentiates
endothelial dysfunction via reciprocal role for reactive oxygen
species and RhoA/ROCK signaling in 5/6 nephrectomized rats. Free
Radic Res. 51:237–252. 2017.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Luo J, Li D, Wei D, Wang X, Wang L and
Zeng X: RhoA and RhoC are involved in stromal cell-derived
factor-1-induced cell migration by regulating F-actin
redistribution and assembly. Mol Cell Biochem. 436:13–21.
2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Aghajanian A, Wittchen ES, Campbell SL and
Burridge K: Direct activation of RhoA by reactive oxygen species
requires a redox-sensitive motif. PLoS One. 4(e8045)2009.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Truman JP, García-Barros M, Kaag M,
Hambardzumyan D, Stancevic B, Chan M, Fuks Z, Kolesnick R and
Haimovitz-Friedman A: Endothelial membrane remodeling is obligate
for anti-angiogenic radiosensitization during tumor radiosurgery.
PLoS One. 5(e12310)2010.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Shi F, Wang YC, Hu ZB, Xu HY, Sun J, Gao
Y, Li XT, Yang CB, Xie C, Li CF, et al: Simulated microgravity
promotes angiogenesis through RhoA-dependent rearrangement of the
actin cytoskeleton. Cell Physiol Biochem. 41:227–238.
2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Torroba B, Herrera A, Menendez A and Pons
S: PI3K regulates intraepithelial cell positioning through Rho
GTP-ases in the developing neural tube. Dev Biol. 436:42–54.
2018.PubMed/NCBI View Article : Google Scholar
|