Introduction
Tympanosclerosis is an irreversible pathological
change in the tympanic membrane and mucosa of the middle ear due to
prolonged chronic inflammation (1).
This condition can damage the auditory ossicles and block hearing
conduction. Patients may suffer from hearing loss, otorrhea,
tinnitus and other adverse symptoms that affect the quality of
their lives (2). The etiology of
tympanosclerosis merits further study. Chronic inflammation of the
middle ear has been widely acknowledged by otologists as the
etiology of tympanosclerosis (3).
In chronic suppurative otitis media, the incidence of
tympanosclerosis is 35.64% (4). In
addition, 35.7% of patients develop tympanosclerosis after being
treated for secretory otitis media with myringotomy and ventilation
tube insertion (5). High incidence
and severe clinical symptoms negatively affect the quality of life
of patients. Therefore, the pathogenesis of tympanosclerosis must
be explored to strengthen the early intervention and develop new
treatment methods.
TGF-β is the main regulator of inflammatory
responses. It can control the initiation and regression of
inflammation by regulating chemotaxis and activity of inflammatory
cells, such as macrophages and granulocytes (6). Previous studies have shown that TGF-β
can promote not only matrix deposition and tissue repair after
tissue injury but also the development of inflammation, and induce
abnormal collagen deposition and fibrosis under inflammatory
conditions (7,8). As one of the three subtypes of TGF-β,
TGF-β1 is an important cytokine and one of the essential
transmitters that induce inflammation and fibrosis (9). Melhus et al (10) found that high TGF-β1 expression in
secretory otitis media in rats can increase fibrinogen in the
middle ear mucosa and induce fibroid degeneration in the tympanic
membrane. Guo et al (11)
revealed that the expression of TGF-β1 was increased in middle ear
mucosa by tympanosclerosis model, and its expression was positively
correlated with the course of tympanosclerosis. The mechanisms and
signaling pathways of TGF-β1 involved in regulating the occurrence
and development of otitis media and tympanosclerosis caused by
nonhealing require further investigation.
TGF-β1 is also an important inducer of EMT (12). EMT refers to the process of
epithelial cells transforming into mesenchymal cells during normal
physiological development or in a pathological state. EMT is
divided into three subtypes, among which type II EMT is involved in
inflammation, tissue regeneration, wound healing, and organ
fibrosis (13,14). Existing literature recognized that
TGF-β1-mediated EMT plays an important role in the development of
chronic inflammation to fibrosis (15). A previous study has confirmed that
EMT regulates the progression of mastitis to mammary fibrosis
through the TGF-β1 signal pathway (16). Some scholars also revealed that
airway inflammation during asthma could induce increased secretion
of TGF-β1 and promote the occurrence of EMT in the airway,
eventually leading to airway remodeling (17). Numerous studies have been performed
to investigate the signaling pathways of TGF-β1 that may regulate
EMT. Some scholars reported that TGF-β1 mediates the snail
signaling pathway in EMT and snail is a key target of this pathway
(18,19). A previous study on liver fibrosis
has shown that using corresponding drugs to inhibit the gene
expression levels of TGF-β1 and snail could reduce the expression
of fibronectin in hepatocytes and block the process of EMT
(20). TGF-β1 is a key factor in
promoting tympanosclerosis; however, to the best of our knowledge,
TGF-β1-mediated EMT in the pathogenesis of tympanosclerosis has not
been previously investigated.
The aim of the present study was to detect the
expression levels of EMT-associated proteins in an animal model and
a primary cell culture, and to explore the mechanism of
TGF-β1-mediated EMT in tympanosclerosis.
Materials and methods
Materials
A total of 40 male Sprague Dawley rats (weight,
200-220 g; age, 8 weeks) were purchased from Jinan Pengyue
Experimental Animal Breeding Co., Ltd. All rats had a quality
certificate (license no. 1107261911003742) for experimental
animals. Animals were housed at a temperature of 23±2˚C, a humidity
of 50% and a 12/12 h light/dark cycle with free access to rat chow
and water. Adaptive feeding for 1 week was necessary before
modeling, and the absence of abnormalities of the external auditory
meatus and the tympanic membrane of the rats was confirmed. A total
of 30 rats were used to establish animal models and 10 were used to
harvest cells for the primary cell culture. Animal health and
behavior were monitored daily and animal welfare was in compliant
with requirements of the Animal Welfare Act (21). This study was approved by the
Medical Ethics Committee of the Affiliated Yuhuangding Hospital of
Qingdao University, [Yantai, China; approval no. 2018(135)], and
experiments were conducted according to the Guidelines for Animal
Experimentation of the Qingdao University.
A standard strain of Streptococcus pneumoniae
(49619™; American Type Culture Collection) was provided by the
microbiology laboratory of the Affiliated Yuhuangding Hospital of
Qingdao University. Recombinant human TGF-β1 protein (cat. no.
ab50036), and rabbit polyclonal β-actin (cat. no. ab8227), rabbit
polyclonal TGF-β1 (cat. no. ab92486), rabbit polyclonal fibronectin
(cat. no. ab2413), mouse monoclonal E-cadherin (cat. no. ab1416),
rabbit polyclonal N-cadherin (cat. no. ab18203) and goat polyclonal
snail (cat. no. ab53519) antibodies were purchased from Abcam.
Rabbit anti-mouse (cat. no. ab6728) and goat anti-rabbit (cat. no.
ab205718) HRP-conjugated secondary antibodies were obtained from
Abcam, and rabbit anti-goat HRP-conjugated secondary antibody (cat.
no. bs20005) was obtained from Absin Biotechnology, Inc.H&E
(cat. no. G1120-3) and von Kossa (cat. no. G3282-2) staining kits
were obtained from Beijing Solarbio Science & Technology Co.,
Ltd. The BCA protein assay kit (cat. no. PC0020), RIPA lysis buffer
(cat. no. R0020) and SDS-PAGE loading buffer (cat. no. P1040) used
for western blotting were also from Beijing Solarbio Science &
Technology Co., Ltd. Rabbit polyclonal vimentin antibody (cat. no.
AF7013), goat anti-rabbit IgG (H+L) Fluor594-conjugated secondary
antibody (cat. no. S0006) and enhanced chemiluminescence (ECL)
reagent (cat. no. K002) were from Affinity Biosciences. TGF-β
receptor type I/II inhibitor (LY2109761) was from
MedChemExpress.
Animal experiments
Before modeling, the standard strain of S.
pneumoniae was selected to 5% sheep blood agar plate
(BioMerieux SA) and cultured in a CO2 incubator at 35˚C
for 24 h. The concentration of S. pneumoniae was adjusted to
1x108 CFU/ml using a type 721 spectrophotometer.
Thereafter, an Eppendorf tube containing S. pneumoniae was
heated using a 65˚C metal bath (GT120S; Leopard Scientific
Instruments (Beijing) Co., Ltd.) for 30 min to deactivate the
bacteria. The heated bacterial suspension was inoculated on blood
agar medium. When no bacterial growth was found via culture at 37˚C
for 24 h, the suspension was kept for modeling use.
Under sterile conditions, all rats were anesthetized
intraperitoneally with pentobarbital (30 mg/kg; NAF Apotek, Oslo).
Subsequently, a syringe needle was inserted into the anterior
inferior quadrant of the tympanic membrane of the right ear using
an otomicroscope (Haag-Streit; Moller-Wedel Optical GmbH), and 50
µl of the inactivated S. pneumoniae suspension was injected.
The left ear was not treated. Otomicroscope observation was
performed every three days within two weeks after injection of
S. pneumoniae, and every seven days after two weeks. The
duration of the animal experiment was 8 weeks. A total of 8 weeks
after modeling, the tympanic membrane of all rats was observed
under the otomicroscope. Tympanosclerosis is defined as the
formation of obvious turbid or white plaques on the tympanic
membrane (22).
After otomicroscopic observation, the rats with
sclerotic plaques on the tympanic membrane of the right ear were
euthanized and the tympanic bullae in the left and right ears were
removed. Rats were euthanized when they survived until 8 weeks
after modeling or if they reached a humane endpoint. Euthanasia was
performed via an overdose of sodium pentobarbital, as per the
recommendations established by the American Veterinary Medical
Association Panel on Euthanasia (23). Death was verified by
cardiorespiratory arrest and disappearance of various reflexes. The
middle ear mucosae of the right and left ears were used as
experimental and control groups, respectively. In total, 4/5 of the
middle-ear mucosa of all model rats was collected under the
otomicroscope and immediately frozen at -80˚C for western blot
analysis. The remaining 1/5 was fixed overnight in 5%
paraformaldehyde solution at room temperature and decalcified with
10% EDTA solution for histological evaluation. After
decalcification, the samples were dehydrated with an ascending
alcohol series, embedded in paraffin wax, cut into thin sections
(5-µm thick), and sequentially mounted on glass slides. Finally,
the sections were stained in accordance with the manufacturer's
instructions for H&E staining kit and von Kossa staining kit,
respectively.
Cell culture
Primary culture of middle ear epithelial cells was
performed, as previously described (24,25).
Cells were maintained in RPMI-1640 media supplemented with 5% fetal
bovine serum and 1% penicillin/streptomycin. After counting, the
cell concentration was adjusted to 5x105/ml, seeded into
a 12-well plate and cultured at 37˚C with 5% CO2 for 48
h. The culture medium was subsequently replaced with or without 20
ng/ml TGF-β1, a dose based on previous research (26). After 48 h treatment with or without
TGF-β1 at 37˚C, the cells were collected for subsequent
experiments.
For inhibition of the TGF-β1 pathway, primary cells
were inoculated on the 12-well plate (5x105/ml) again.
After culture at 37˚C with 5% CO2 for 48 h, cells were
treated with 10 µmol/ml LY2109761 at 37˚C for 6 h. Subsequently,
the medium with LY2109761 was removed, and the new medium was
replaced and treated with TGF-β1 at 37˚C for 48 h, then the cells
were collected for sbubsequent experiments.
Morphological observation and calcium
evaluation
The paraffin-embedded sections were heated at 65˚C
for 1 h and then deparaffinized in xylene and rehydrated in a
descending alcohol series. Subsequently, the sections were stained
using the aforementioned H&E and von Kossa staining kits. In
the process of H&E staining, the sections were stained with
hematoxylin for 1 min, soaked with distilled water for 15 min, and
then stained with eosin for 1 min. When von Kossa staining was
performed, the sections were incubated with von Kossa silver
solution, placed under ultraviolet light for 15 min, washed with
distilled water for 1 min, then treated with 5% sodium thiosulfate
for 2 min, and then re-stained with 0.1% nuclear fast red solution
staining for 1 min. The above staining processes were all carried
out at room temperature. The sections stained by H&E and von
Kossa were dehydrated and transparent, sealed with neutral gum and
observed under fluorescence microscopy (BX53; Olympus Soft Imaging
Solutions GmbH). The characteristics of tympanosclerosis, including
mucosa thickening, fibrosis and calcium deposition, were
recorded.
Western blot analysis
RIPA and PMSF were added to the Eppendorf tubes to
lyse the mucosa and primary cells. After sufficient grinding and
centrifugation (4˚C, 14,000 x g, 20 min), the protein was
extracted, and the concentration was measured using a BCA kit.
SDS-PAGE loading buffer (50 µl) was added to 200 µl protein
supernatant, and the mix was heated in a metal bath at 100˚C for 5
min to obtain the protein samples required for electrophoresis.
Equivalent amounts of protein (25 µg/lane) were separated by
SDS-PAGE (8% gel) and then electrotransferred onto nitrocellulose
membranes for 90 min at 260 mA. Subsequently, the membranes were
blocked with skimmed milk at a concentration of 5% for 1 h at room
temperature. Then, the membranes incubated overnight at 4˚C with
the aforementioned β-actin (1:2,000) TGF-β1 (1:1,000), fibronectin
(1:1,000), E-cadherin (1:1,000), N-cadherin (1:1,000) and snail
(1:500 dilution) antibodies. The membranes were washed three times
with TBS with 0.1% Tween-20 (TBST; 10 min each) and incubated with
the aforementioned HRP-conjugated goat anti-rabbit (1:10,000
dilution), rabbit anti-mouse (1:5,000 dilution) or rabbit anti-goat
(1:5,000 dilution) IgG antibodies at room temperature for 1 h. The
membranes were washed three times with TBST again and the ECL
reagent was used. The membranes were exposed for development using
a fluorescence and chemiluminescence imaging system (ChemiScope
6200 Touch; Clinx Science Instruments Co., Ltd.). The grayscale
value of each band was measured using ImageJ software (version
1.8.0; National Institutes of Health).
Immunofluorescence
Primary cells were fixed in 4% paraformaldehyde for
20 min at room temperature and then permeabilized with 0.5%
TritonX-100 for 20 min. After blocking with 5% BSA for 2 h at room
temperature, the cells were incubated with the aforementioned
anti-vimentin antibody (1:100) at 4˚C overnight. The next day, the
cells were incubated with the aforementioned goat anti-rabbit IgG
(H+L) Fluor594-conjugated secondary antibody (1:200) at 37˚C for 1
h. The cells were washed with PBS 3 times and then stained with
DAPI for 5 min at room temperature. The cells were observed using
fluorescence microscopy.
Statistical analysis
Statistical analyses were conducted using SPSS
version 22.0 (IBM Corp.) and GraphPad Prism version 7.0 (GraphPad
Software, Inc.). For western blotting results, paired samples were
compared using a paired samples t-test and multiple comparisons
were performed using one-way ANOVA followed by the Least
Significant Difference test. Relative vimentin intensity data were
compared using an unpaired samples t-test. P<0.05 was considered
to indicate a statistically significant difference.
Results
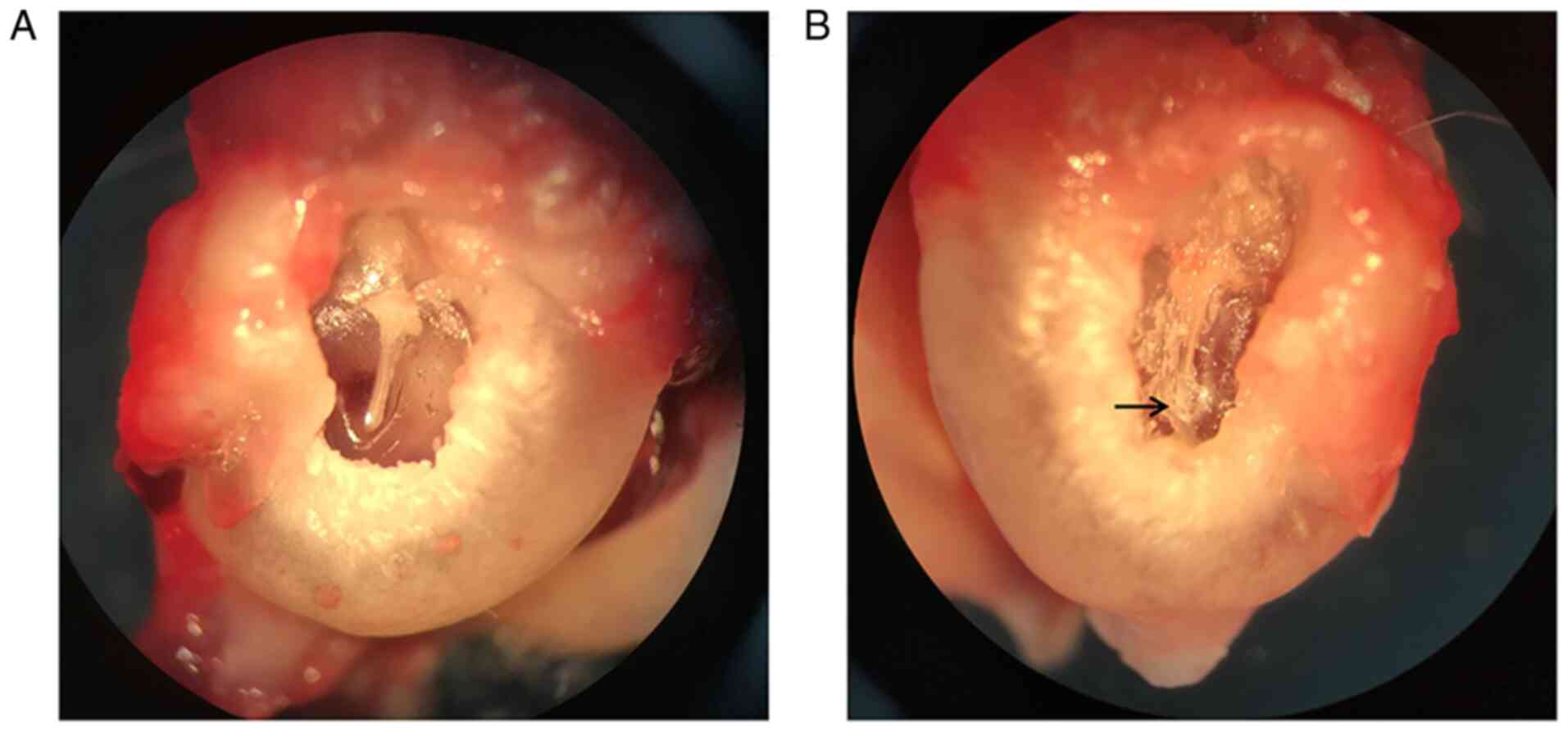
Otomicroscopic observations
Among the 30 rats, one died of digestive system
infection 6 days after modeling, while the remaining animals
survived until 8 weeks after modeling. Three days after the
establishment of the model, yellowish serous exudation could be
seen in the middle ear cavity of the rats. Two weeks later, the
inflammatory exudation was absorbed, and the tympanic membrane
perforation caused by puncture healed. The otomicroscopic results
showed that a tympanosclerosis model was successfully established
in 21 out of 29 right ears at 8 weeks, and no obvious abnormalities
in the tympanic membrane were found among the 29 left ears and 8
right ears (Fig. 1).
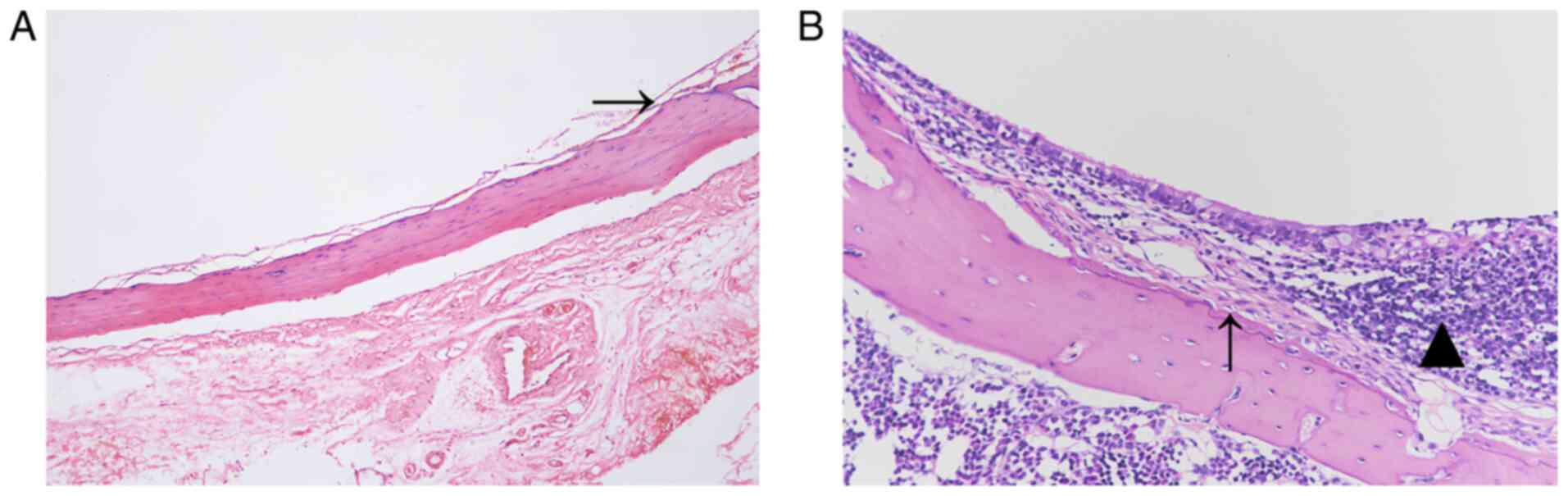
Histopathological observations
The H&E staining results are shown in Fig. 2. The mucosal structure of the
control group was intact, and no inflammation or fibroplasia was
observed (Fig. 2A). The mucosa in
the experimental group was markedly thicker than that in the
control group. In addition, inflammatory cell infiltration was
observed, and the fibrous tissue was hyperplastic (Fig. 2B).
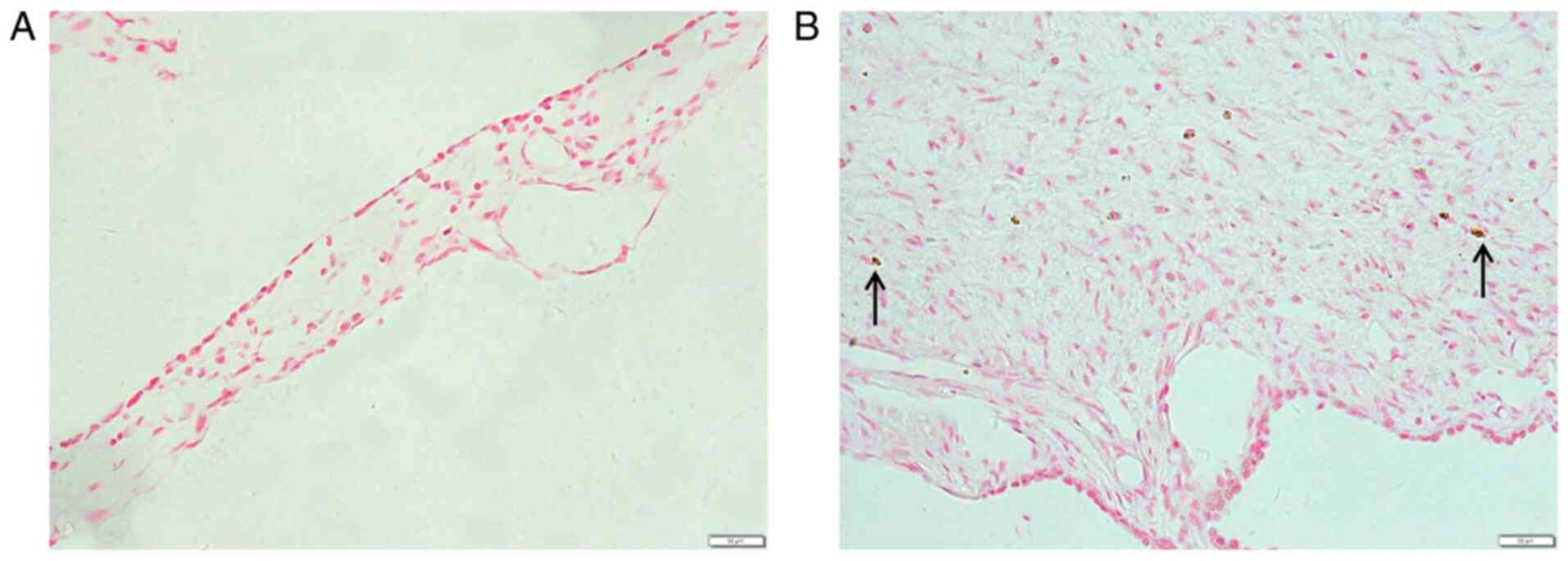
The von Kossa staining results are shown in Fig. 3. The mucosal structure of the
control group was normal with no calcium deposition (Fig. 3A). The mucosa of the experimental
group was markedly thickened and scattered calcium deposits were
found in the tissue (Fig. 3B).
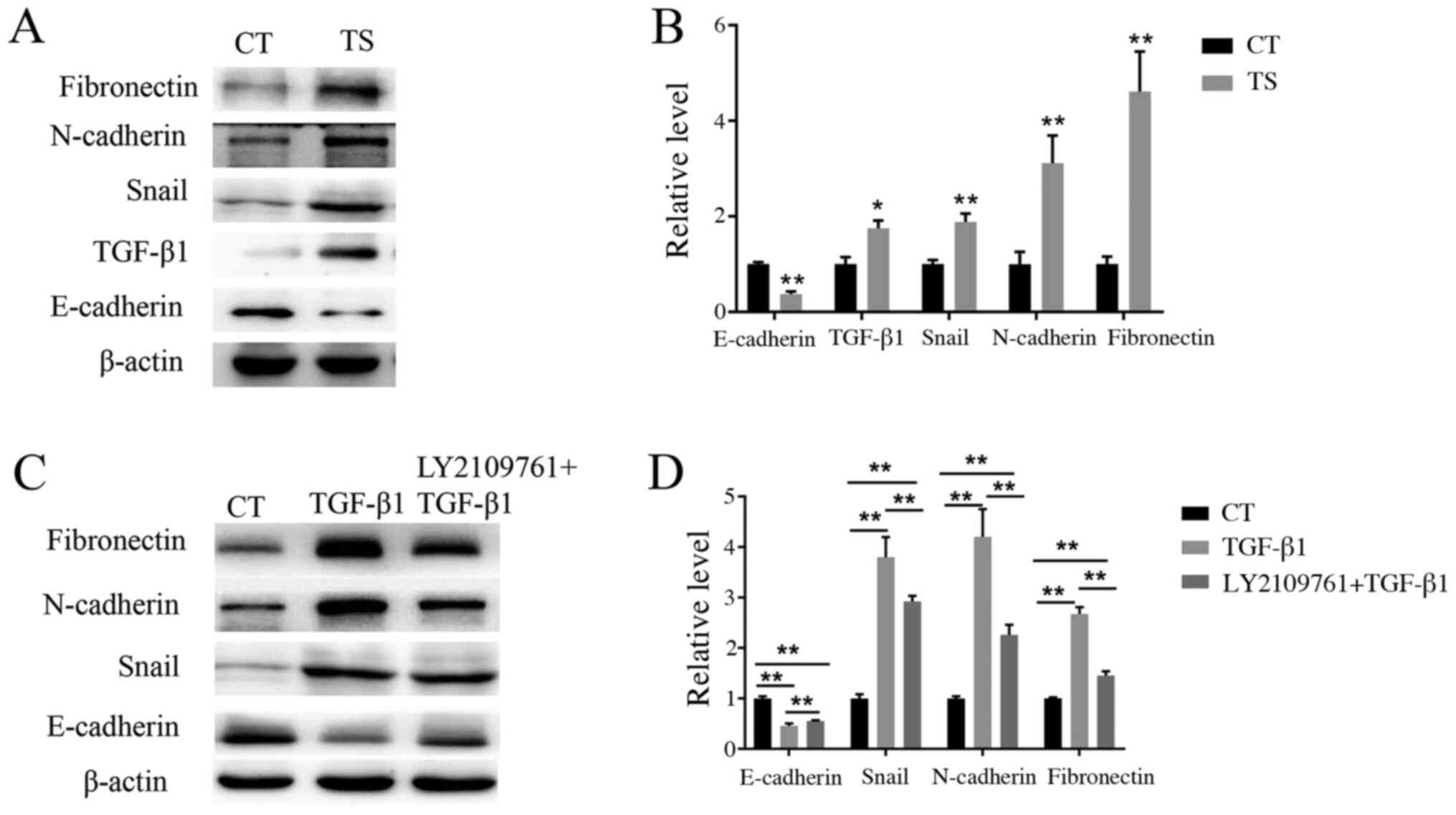
Western blot analysis
To confirm whether EMT was associated with
tympanosclerosis, western blotting was used to detect the
expression levels of E-cadherin, TGF-β1, snail, N-cadherin and
fibronectin in tympanosclerotic middle ear and normal middle ear
mucosa. As shown in Fig. 4A and
B, the expression levels of TGF-β1,
snail, N-cadherin and fibronectin in the experimental group were
increased, whereas E-cadherin expression level decreased, compared
with that in the control group. In order to further determine the
role of the EMT-associated pathway in the pathogenesis of
tympanosclerosis, middle ear mucosal cells were treated with
TGF-β1. Western blotting results showed that when stimulated with
TGF-β1, the expression of E-cadherin decreased, while the
expression of snail, N-cadherin and fibronectin increased (Fig. 4C and D). These results suggested that TGF-β1 can
activate the EMT, of middle ear mucosa cells, resulting in a
decrease in the expression of epithelial markers and an increase in
the expression of mesenchymal markers. A total of 6 h before TGF-β1
stimulation, LY2109761 was used to treat cells. It was found that
compared with the TGF-β1 treatment group, the expression of
E-cadherin increased but the expression levels of snail, N-cadherin
and fibronectin decreased in the group co-treated with LY2109761
(Fig. 4C and D), and the differences were statistically
significant (P<0.01), which indicated that LY210976 could
inhibit the occurrence of EMT in middle ear mucosa.
Immunofluorescence analysis
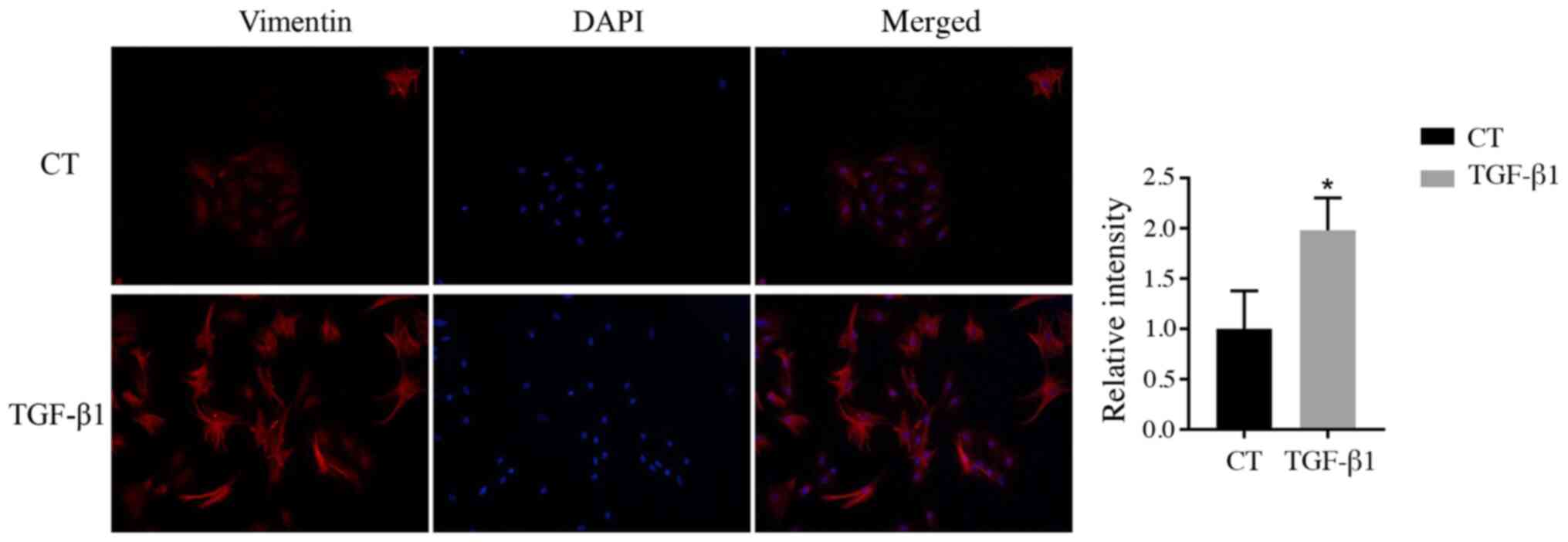
Vimentin is a hallmark of EMT (27). Vimentin was visualized in middle ear
epithelial cells at 48 h after TGF-β1 treatment using
immunofluorescence. The results showed that the relative
intensities of vimentin in the group treated with TGF-β1 were
significantly higher than those in the control group (Fig. 5).
Discussion
A tympanosclerosis model in rats can be established
in a number ways, including tympanotomy and S. pneumoniae
injection. The latter was chosen for the present study because the
course of tympanosclerosis induced by S. pneumoniae
injection is closer to that of humans than that in tympanotomy
(28). Tympanosclerosis is one of
the main reactions of the tympanic membrane and middle-ear mucosa
to long-term aseptic secretory otitis media, and the course of
tympanosclerosis is progressive (29). The main pathogenic substance of
inactivated S. pneumoniae is endotoxin, which can cause
connective tissue and goblet cell proliferation in the middle-ear
mucosa, and induce the accumulation of glandular secretions leading
to secretory otitis media. Three days after injection of
inactivated S. pneumoniae, yellowish serous fluid was found
in the middle ear cavity of most rats, but there was generally no
purulent fluid. Long-term exudation of inflammatory substances
stimulates tympanosclerosis in the middle ear mucosa; meanwhile,
perforation of the tympanic membrane during injection promotes the
formation of a high-oxygen environment in the tympanum, which in
turn induces accumulation of oxygen free radicals and promotes
tympanosclerosis (30). Perforation
of the tympanic membrane caused by puncture usually heals within 2
weeks. In the present study, the middle ear mucosa was obtained 8
weeks after modeling, as most studies revealed that the typical
histological changes in tympanosclerosis could be observed 8 weeks
after modeling (1,11,31).
The modeling method used in the present study was
simple, safe and reliable. One rat died on the sixth day after
S. pneumoniae injection due to digestive tract infection,
this was considered to be due to the poor resistance of the
individual., while the remaining 29 survived for 8 weeks. The
otomicroscopic observations showed that the tympanic membrane of
the left ear was transparent in all rats, and 21 rats (72.4% of the
total) had turbid or obvious sclerotic plaques in the tympanic
membrane of the right ears. It is possible that the inflammation in
the middle ear of some rats was self-cured before the development
of tympanosclerosis due to anti-infection ability. Thus, the
success rate of modeling did not reach 100%. In future studies,
changing some modeling conditions, such as increasing the frequency
of S. pneumoniae injections and combining two modeling
methods, could be explored to increase the rate of success for the
model and obtain a sufficient number of experimental specimens. In
the present study, H&E staining showed that the morphology of
the middle ear mucosa in the control group was normal, whereas that
in the experimental group was thickened. In addition, extensive
inflammatory cell infiltration was observed in certain
fibroblast-rich areas. This finding was consistent with the
histological changes in tympanosclerosis shown in a previous study
(1). Therefore, the results of the
present study indicated that intratympanic injection of inactivated
S. pneumoniae is an effective way to establish a model of
tympanosclerosis.
The progression of middle-ear inflammation to
tympanosclerosis mainly occurs in five stages: Inflammatory
exudation, granulation, fibrosis, hyalinization and calcification,
among which fibrosis is the last reversible stage (31). Thus, exploring the progression from
middle ear inflammation to fibrosis is important in preventing and
treating tympanosclerosis. To the best of our knowledge, the
present study is the first to suggest that TGF-β1-mediated EMT is
involved in the pathogenesis of tympanosclerosis. The current
findings provide a new theoretical basis for the prevention and
treatment of tympanosclerosis caused by otitis media.
TGF-β1 is a multifunctional cytokine involved in the
regulation of various biological processes, among which
pro-inflammatory and pro-fibrotic effects have been widely studied.
Numerous hypotheses have been proposed on the specific mechanism of
TGF-β1 in promoting the progression of chronic inflammation into
fibrosis (32,33). Most studies suggested that TGF-β1 is
associated with EMT, which is considered as an important process in
the progression of inflammation to degenerative fibrous disease
(34,35). TGF-β1 is a core factor that
regulates the procession of fibrosis (36). TGF-β1 promotes the synthesis of
extracellular matrix protein fibronectin by regulating the
transformation of epithelial cells associated with type II EMT into
fibroblasts, thereby resulting in the contraction of extracellular
matrix (37). A previous study has
confirmed that high expression of TGF-β1 is observed in secretory
otitis media (38). The present
study used western blotting to detect the expression levels of
TGF-β1 in the tympanosclerotic and normal middle ear mucosae of
rats. TGF-β1 expression was significantly increased in the
experimental group. Therefore, secretory otitis media induced by
S. pneumoniae could stimulate the secretion of TGF-β1, which
might subsequently promote fibrosis in the transition from otitis
media to tympanosclerosis.
Fibronectin and N-cadherin proteins are mesenchymal
markers of EMT (39). Fibronectin
is also the most abundant extracellular matrix protein in various
tissues. Its overexpression promotes the accumulation of
extracellular matrix and eventually leads to organ fibrosis by
changing the cell structure and behavioral characteristics. such as
promoting the recruitment and stimulation of inflammatory cells
(40,41). Kriegel et al (42) reported that the accumulation of
extracellular matrix proteins and the occurrence of interstitial
fibrosis are the main results of TGF-β1-induced EMT in renal
epithelial cells. N-cadherin is not only a typical EMT marker but
also an important intercellular adhesion molecule. A previous study
has shown that the process of fibronectin promoting the deposition
of extracellular matrix is associated with the increase in
cytoskeleton tension resulting from N-cadherin adhesion (43). In the present study, western blot
analysis confirmed that the expression levels of fibronectin and
N-cadherin, two main EMT markers, were significantly increased in
the experimental group compared with the control. Histological
analysis showed obvious fibrous adhesion and fibrous hyperplasia in
the middle-ear mucosa of the experimental group, which may be
associated with the deposition of the extracellular matrix caused
by the overexpression of fibronectin and N-cadherin. The
aforementioned results further demonstrated the role of
TGF-β1-induced EMT in tympanosclerosis, suggesting that may promote
the development of otitis media and participate in middle-ear
mucosal fibrosis.
Snail is a zinc finger transcription factor located
in the nucleus that promotes EMT by inhibiting the expression of
E-cadherin, a typical epithelial marker (44). TGF-β1 is the upstream pathway that
induces snail activation. Park et al (45) reported that EMT induced by the
TGF-β1/snail signal pathway was involved in the formation of
pulmonary fibrosis. The results of the present study suggested that
the expression level of snail in the experimental group was
significantly higher than that in the normal control group. At the
same time, the expression level of E-cadherin in the
tympanosclerosis mucosa was significantly decreased compared with
control. Thus, the results suggest that TGF-β1 may regulate EMT in
tympanosclerosis through the snail signal pathway.
TGF-β1 is an extracellular signaling molecule that
transmits the extracellular signal to the nucleus by binding to its
receptor on the cell membrane (46). In the case of EMT, TGF-β1 activates
snail, which inhibits the expression of epithelial genes and
promotes the transcription of mesenchymal genes (47). The present study showed that the
expression levels of snail, N-cadherin and fibronectin were
upregulated and E-cadherin was downregulated following TGF-β1
stimulation in middle ear epithelial cells. In addition, when
compared with TGF-β1 treatment group, the current study also found
that the expression levels of mesenchymal markers such as snail,
N-cadherin and fibronectin decreased, while the expression level of
epithelial marker E-cadherin increased in epithelial cells treated
with LY2109761. Notably, immunofluorescence results revealed that
the expression of vimentin, a hallmark of EMT, was significantly
increased after middle ear epithelial cells were stimulated by
TGF-β1. The current results provide evidence for the involvement of
EMT in tympanosclerosis formation.
The present study represents an advancement in the
research on the pathogenesis of tympanosclerosis; however, it has
certain limitations. Although the pathogenesis of tympanosclerosis
regulated by TGF-β1-mediated EMT was studied in animal models, the
mechanism in humans remains unclear. In the future, human
tympanosclerosis specimens will be collected to further explore the
pathogenic factors associated with tympanosclerosis.
In conclusion, high expression of TGF-β1 is a key
factor in promoting the formation of tympanosclerosis via the EMT
process. The present study provides strong evidence for the
involvement of TGF-β1-induced EMT in the formation of
tympanosclerosis and provides a new direction for the treatment and
prevention of tympanosclerosis. The pathogenesis of
tympanosclerosis is complex and, to the best of our knowledge, has
not been fully elucidated. The majority of patients report to the
clinic for consultation only when they have reached the
irreversible stage of this condition. Thus, exploring the
pathogenesis of tympanosclerosis is necessary for prevention and
further treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology Plan Project of Yantai (grant no. 2018SFGY107).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WG, LC, YM, YS and FH were responsible for the
conception of this study and designed the experiments. JQ, YW and
LX performed the experiments and analyzed the data. YS and FH
contributed to reviewing and proofreading the manuscript. All
authors contributed to the interpretation of data and writing of
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of the Affiliated Yuhuangding Hospital of Qingdao
University (Yantai, China) and experiments were conducted according
to the Guidelines for Animal Experimentation of the Qingdao
University (Qingdao, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang Y, Wang S, Zheng Y and Liu A:
Possible role of dickkopf-1 protein in the pathogenesis of
tympanosclerosis in a rat model. J Laryngol Otol. 131:860–865.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Qiu JJ, Wang JX and Sun Y: Advances in
research on etiology of tympanosclerosis and related factors. Chin
J Otol. 18:792–796. 2020.
|
|
3
|
Asiri S, Hasham A, Al Anazy F, Zakzouk S
and Banjar A: Tympanosclerosis: Review of literature and incidence
among patients with middle-ear infection. J Laryngol Otol.
113:1076–1080. 1999.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wu Y, Yin S, Zhu H and Zhang S:
Tympanosclerosis incidence among patients with chronic suppurative
otitis media. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 20:1016–1017.
2006.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
5
|
Branco C, Monteiro D and Paco J:
Predictive factors for the appearance of myringosclerosis after
myringotomy with ventilation tube placement: Randomized study. Eur
Arch Otorhinolaryngol. 274:79–84. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wrzesinski SH, Wan YY and Flavell RA:
Transforming growth factor-beta and the immune response:
Implications for anticancer therapy. Clin Cancer Res. 13:5262–5270.
2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fabregat I, Moreno-Caceres J, Sanchez A,
Dooley S, Dewidar B, Giannelli G and Ten Dijke P: IT-LIVER
Consortium. TGF-β signalling and liver disease. FEBS J.
283:2219–2232. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Berksoy Hayta S, Durmus K, Altuntas EE,
Yildiz E, Hisarciklio M and Akyol M: The reduction in inflammation
and impairment in wound healing by using strontium chloride
hexahydrate. Cutan Ocul Toxicol. 37:24–28. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang X, Zhang MC and Wang CT: Loss of
LRRC25 accelerates pathological cardiac hypertrophy through
promoting fibrosis and inflammation regulated by TGF-β1. Biochem
Biophys Res Commun. 506:137–144. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Melhus A and Ryan AF: Expression of
cytokine genes during pneumococcal and nontypeable haemophilus
influenzae acute otitis media in the rat. Infect Immun.
68:4024–4031. 2000.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Guo W, Bai X, Han Y, Xu L, Liu W, Zhang G,
Li J, Fan Z and Wang H: Expressions of TGF-β1 and MMP-9 in a guinea
pig model of tympanosclerosis: Possible role in the pathogenesis of
this disorder. Laryngoscope. 122:2037–2042. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang X, Gao JL, Zhao MM, Zhu HX, Tian YX,
Li R, Jiang XH, Yu L, Tian JR and Cui JZ: Therapeutic effects of
conditioned medium from bone marrow-derived mesenchymal stem cells
on epithelial-mesenchymal transition in A549 cells. Int J Mol Med.
41:659–668. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Stone RC, Pastar I, Ojeh N, Chen V, Liu S,
Garzon KI and Tomic-Canic M: Epithelial-mesenchymal transition in
tissue repair and fibrosis. Cell Tissue Res. 365:495–506.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Guarino M, Tosoni A and Nebuloni M: Direct
contribution of epithelium to organ fibrosis:
Epithelial-mesenchymal transition. Hum Pathol. 40:1365–1376.
2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sisto M, Lorusso L, Ingravallo G, Tamma R,
Ribatti D and Lisi S: The TGF-β1 signaling pathway as an attractive
target in the fibrosis pathogenesis of sjogren's syndrome.
Mediators Inflamm. 2018(1965935)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen Q, Yang W, Wang X, Li X, Qi S, Zhang
Y and Gao MQ: TGF-β1 induces EMT in bovine mammary epithelial cells
through the TGFβ1/smad signaling pathway. Cell Physiol Biochem.
43:82–93. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhu X, Li Q, Hu G, Wang J, Hu Q, Liu Z, Wu
G and Zhong Y: BMS345541 inhibits airway inflammation and
epithelialmesenchymal transition in airway remodeling of asthmatic
mice. Int J Mol Med. 42:1998–2008. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yang HW, Lee SA, Shin JM, Park IH and Lee
HM: Glucocorticoids ameliorate TGF-β1-mediated
epithelial-to-mesenchymal transition of airway epithelium through
MAPK and Snail/Slug signaling pathways. Sci Rep.
7(3486)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Peinado H, Quintanilla M and Cano A:
Transforming growth factor beta-1 induces snail transcription
factor in epithelial cell lines: Mechanisms for epithelial
mesenchymal transitions. J Biol Chem. 278:21113–21123.
2003.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wu T, Liu T, Xing L and Ji G: Baicalin and
puerarin reverse epithelial-mesenchymal transition via the
TGF-beta1/Smad3 pathway in vitro. Exp Ther Med.
16:1968–1974. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
U.S. Department of Agriculture 2001. Code
of Federal Regulations, Animal Welfare Act and Regulation chapter
1, subchapter A: Animals and animal products. U.S. Department of
Agriculture, Beltsville, MD.
|
|
22
|
Emir H, Kaptan ZK, Samim E, Sungu N,
Ceylan K and Ustun H: The preventive effect of ginkgo biloba
extract in myringosclerosis: Study in rats. Otolaryngol Head Neck
Surg. 140:171–176. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Anonymous AVMA Guidelines for the
Euthanasia of Animals. 2013 edition. American Veterinary Medical
Association, Schaumburg, IL, 2013.
|
|
24
|
Chen L, Huang YL, Wang J and You JM:
Effect of platelet activating factor on mucous glycoprotein
secretion in culture middle ear epithelium cells. Guangdong Med J
698-700, 2003.
|
|
25
|
Nakamura A, DeMaria TF, Lim DJ and van
Blitterswijk CA: Primary culture of chinchilla middle ear
epithelium. Ann Otol Rhinol Laryngol. 100:774–782. 1991.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Han F, Shu J, Wang S, Tang CE and Luo F:
Metformin inhibits the expression of biomarkers of fibrosis of EPCs
in vitro. Stem Cells Int. 2019(9019648)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li J, Peng W, Yang P, Chen R, Gu Q, Qian
W, Ji D, Wang Q, Zhang Z, Tang J and Sun Y: MicroRNA-1224-5p
inhibits metastasis and epithelial-mesenchymal transition in
colorectal cancer by targeting SP1-mediated NF-κB signaling
pathways. Front Oncol. 10(294)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang YF, Zheng YX, Wang SJ, Peng LY, He C
and Liu AG: Comparison of two methods for the modeling of
tympanosclerosis in rat. J Audiol Speech Pathol. 25:284–287.
2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Russell JD and Giles JJ: Tympanosclerosis
in the rat tympanic membrane: An experimental study. Laryngoscope.
112:1663–1666. 2002.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Koc S, Kiyici H, Toker A, Soyaliç H, Aslan
H, Kesici H and Karaca ZI: The effect of melatonin and vitamin C
treatment on the experimentally induced tympanosclerosis: Study in
rats. Braz J Otorhinolaryngol. 83:541–545. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Santos PF, Leal MC, Peixoto C, Caldas Neto
S and Rosas ST: Otomicroscopic and histologic findings of induced
myringosclerosis in rats: A critical study of an experimental
model. Braz J Otorhinolaryngol. 71:668–674. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mahmoud AM, Hozayen WG, Hasan IH, Shaban E
and Bin-Jumah M: Umbelliferone ameliorates CCl(4)-induced liver
fibrosis in rats by upregulating PPARγ and attenuating oxidative
stress, inflammation, and TGF-β1/Smad3 signaling. Inflammation.
42:1103–1116. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kania G, Blyszczuk P and Eriksson U:
Mechanisms of cardiac fibrosis in inflammatory heart disease.
Trends Cardiovasc Med. 19:247–252. 2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lopez-Novoa JM and Nieto MA: Inflammation
and EMT: An alliance towards organ fibrosis and cancer progression.
EMBO Mol Med. 1:303–314. 2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sato M, Muragaki Y, Saika S, Roberts AB
and Ooshima A: Targeted disruption of TGF-beta1/Smad3 signaling
protects against renal tubulointerstitial fibrosis induced by
unilateral ureteral obstruction. J Clin Invest. 112:1486–1494.
2003.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Loboda A, Sobczak M, Jozkowicz A and Dulak
J: TGF-β1/Smads and miR-21 in renal fibrosis and inflammation.
Mediators Inflamm. 2016(8319283)2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yang YC, Zhang N, Van Crombruggen K, Hu
GH, Hong SL and Bachert C: Transforming growth factor-beta1 in
inflammatory airway disease: A key for understanding inflammation
and remodeling. Allergy. 67:1193–1202. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cooter MS, Eisma RJ, Burleson JA, Leonard
G, Lafreniere D and Kreutzer DL: Transforming growth factor-beta
expression in otitis media with effusion. Laryngoscope.
108:1066–1070. 1998.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Rout-Pitt N, Farrow N, Parsons D and
Donnelley M: Epithelial mesenchymal transition (EMT): A universal
process in lung diseases with implications for cystic fibrosis
pathophysiology. Respir Res. 19(136)2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Altrock E, Sens C, Wuerfel C, Vasel M,
Kawelke N, Dooley S, Sottile J and Nakchbandi IA: Inhibition of
fibronectin deposition improves experimental liver fibrosis. J
Hepatol. 62:625–633. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Iredale JP: Models of liver fibrosis:
Exploring the dynamic nature of inflammation and repair in a solid
organ. J Clin Invest. 117:539–548. 2007.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kriegel AJ, Fang Y, Liu Y, Tian Z,
Mladinov D, Matus IR, Ding X, Greene AS and Liang M:
MicroRNA-target pairs in human renal epithelial cells treated with
transforming growth factor beta 1: A novel role of miR-382. Nucleic
Acids Res. 38:8338–8347. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Dzamba BJ, Jakab KR, Marsden M, Schwartz
MA and DeSimone DW: Cadherin adhesion, tissue tension, and
noncanonical Wnt signaling regulate fibronectin matrix
organization. Dev Cell. 16:421–432. 2009.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Chaffer CL, San Juan BP, Lim E and
Weinberg RA: EMT, cell plasticity and metastasis. Cancer Metastasis
Rev. 35:645–654. 2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Park YJ, Bang IJ, Jeong MH, Kim HR, Lee
DE, Kwak JH and Chung KH: Effects of β-sitosterol from corn silk on
TGF-β1-induced epithelial-mesenchymal transition in lung alveolar
epithelial cells. J Agric Food Chem. 67:9789–9795. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lee HY, Kim IK, Yoon HK, Kwon SS, Rhee CK
and Lee SY: Inhibitory effects of resveratrol on airway remodeling
by transforming growth factor-β/smad signaling pathway in chronic
asthma model. Allergy Asthma Immunol Res. 9:25–34. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Kee JY, Han YH, Mun JG, Park SH, Jeon HD
and Hong SH: Effect of Korean red ginseng extract on colorectal
lung metastasis through inhibiting the epithelial-mesenchymal
transition via transforming growth
factor-β1/Smad-signaling-mediated Snail/E-cadherin expression. J
Ginseng Res. 43:68–76. 2019.PubMed/NCBI View Article : Google Scholar
|