Introduction
Arterial dissection is defined as the cleavage of
the arterial wall by an intramural hematoma (1). Isolated visceral arterial dissection,
i.e., dissection that occurs in the absence of aortic dissection,
has been reported to involve the celiac artery and renal arteries;
however, the most frequent site of isolated dissection is the
superior mesenteric artery (SMA). SMA dissection was first
described by Bauersfeld (2-4)
in 1947 and may be categorized into: i) spontaneous isolated SMA
dissection (SISMAD) and ii) SMA dissection combined with aortic
dissection. The latter type is more common and is caused by the
extension of an aortic dissection flap into the SMA (5). By contrast, SISMAD is a rare but
potentially fatal condition.
Prior to 2012, no more than 270 cases of SISMAD were
reported in the PubMed database (3). The development of advanced imaging
technologies, such as multi-detector CT (CT), has likely led to an
increase in the detection of SISMAD (3). Since 2016, >622 cases of SISMAD
have been reported (6). CT imaging
with intravenous contrast administration is able to clearly
determine the location and extent of the dissection in patients
with SISMAD (6). However, in a
considerable subset of patients, SISMAD presents with atypical
clinical symptoms (e.g., acute or chronic abdominal pain) or is
entirely asymptomatic. Therefore, in numerous cases, plain CT is
the first imaging examination that such patients undergo (3,7-9).
Furthermore, plain CT remains an important examination in emergency
departments and hospitals with limited medical resources (6,10).
However, the characteristic imaging findings of SISMAD, namely an
intimal flap and a mural thrombus, may not be visible on plain CT
(8,11,12).
Suzuki et al (13) reported that increased perivascular
fat density around the SMA is a sign of arterial dissection or
thrombosis. The normal perivascular fat around the SMA appears as
homogeneous low attenuation signals on CT and on CT angiography
(CTA), the normal SMA walls have a distinct interface with the
perivascular fat (14).
Perivascular fat stranding (PFS) is the CT finding of abnormally
increased attenuation in fat tissue. PFS is a manifestation of
edema, inflammation and/or neoplastic infiltration and was first
detected on abdominopelvic CT (15).
The purpose of the present study was to determine
whether the detection of PFS on plain CT scans may serve as a
useful indicator of the requirement for further examination with
contrast-enhanced CT (CECT) or CTA to diagnose or rule out
SISMAD.
Materials and methods
Patient population
The medical and imaging data of consecutive patients
who were diagnosed with SISMAD on abdominal CECT or SMACTA at our
hospital between February 2015 and February 2018 were reviewed.
Certain patients initially underwent non-enhanced abdominal CT,
which was suggestive of SMA dissection; hence, these patients
subsequently underwent CTA for the diagnosis of SISMAD. Patients
who were admitted to the emergency department, as well as those who
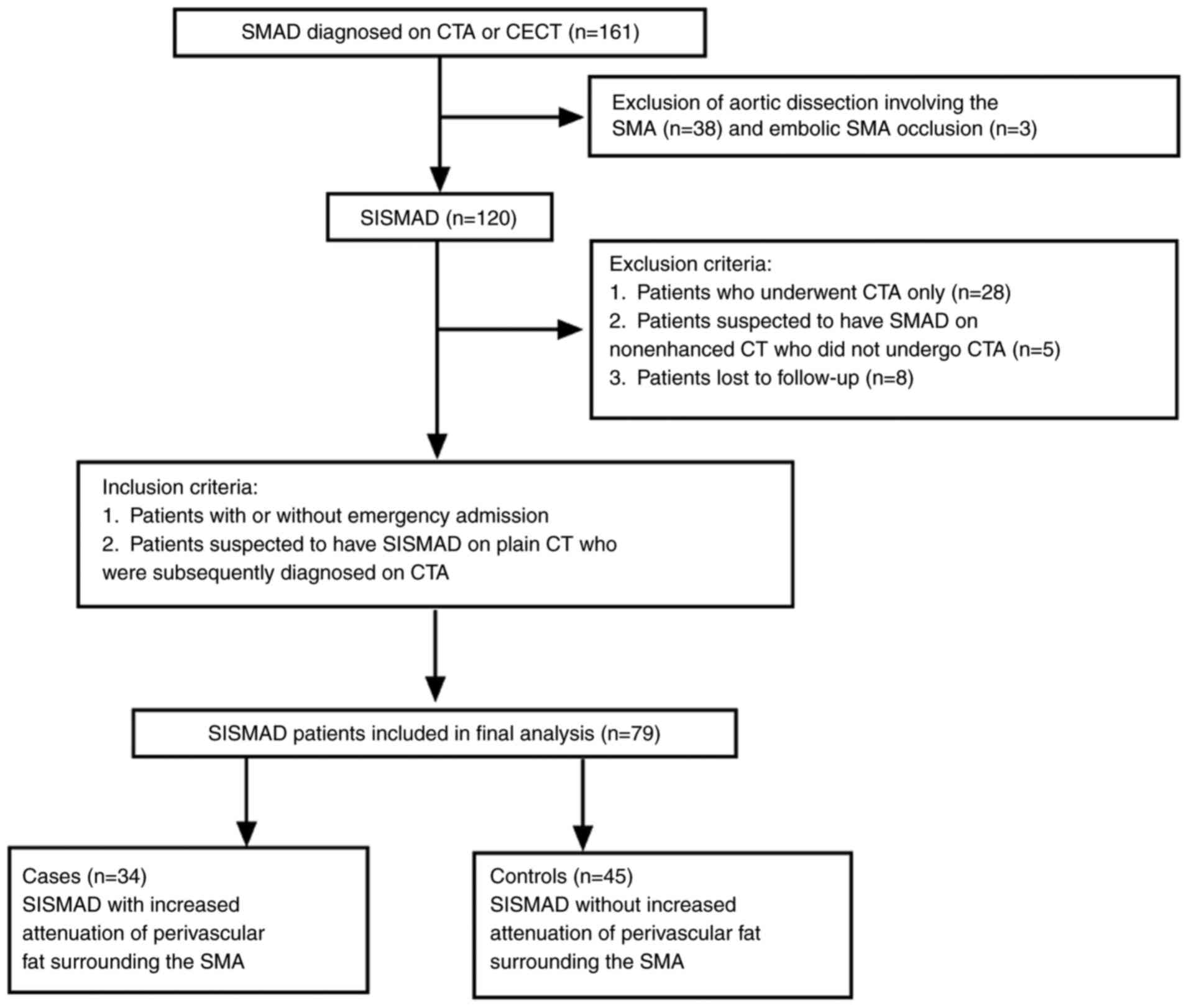
were not, were included in the study. A flow chart of patient
selection and the clinical inclusion and exclusion criteria for
patients with SISMAD are presented in Fig. 1. The demographic and clinical
characteristics of the patients with SISMAD are summarized in
Table I. The present retrospective
registry study was approved by the institutional review board of
our hospital.
 | Table IDemographic and clinical
characteristics of patients with SISMAD (n=79). |
Table I
Demographic and clinical
characteristics of patients with SISMAD (n=79).
|
Characteristics | Cases (n=34) | Controls
(n=45) | P-value |
|---|
| Mean age
(years) | 55.06±6.958 | 55.98±9.538 | 0.136 |
| Male sex | 31 (91.2) | 42 (93.3) | 1.000 |
| Type of
admission | | | 0.030 |
|
Emergency | 22 (64.7) | 18 (40.0) | |
|
Non-emergency | 12 (35.3) | 27 (60.0) | |
| Clinical
presentation | | | 0.024 |
|
Abdominal
pain | 29 (85.3) | 28 (62.2) | |
|
No abdominal
paina | 5 (14.7) | 17 (37.7) | |
| Comorbidities | | | 0.888 |
|
Hypertension | 13 (38.2) | 21 (46.7) | |
|
Smoking
(current or ex-smoker) | 12 (35.3) | 17 (37.8) | |
|
Alcohol
consumption (current or ex-drinker) | 10 (29.4) | 17 (37.8) | |
|
Diabetes
mellitus | 2 (5.9) | 1 (2.2) | |
|
Hyperlipidemia | 3 (8.8) | 2 (4.4) | |
|
Liver
cirrhosis | 1 (2.9) | 3 (6.7) | |
|
Gastric
ulcer | 1 (2.9) | 1 (2.2) | |
| Malignancy | | | 0.906 |
|
Hepatocellular
carcinoma | 1 (2.9) | 1 (2.2) | |
|
Colorectal
carcinoma | 1 (2.9) | 1 (2.2) | |
|
Stomach
cancer | 2 (5.9) | 1 (2.2) | |
| Diagnostic
modality | | | 0.017 |
|
Contrast-enhanced
CT | 16 (47.1) | 33 (73.3) | |
|
CT
angiography | 18 (52.9) | 12 (26.7) | |
| Time interval
between plain CT and CT angiography (days) | 3.5 | 4.6 | |
Image acquisition and analysis
Due to the retrospective nature of the present
study, images were acquired using a variety of CT scanners (Somatom
Definition from Siemens AG; Aquilion One from Toshiba; and
Discovery CT750 HD from Cytiva). The scans included both CECT and
CTA following non-enhanced abdominal CT according to our
institution's protocol. The scanning parameters were as follows: i)
for CTA: 100 kV; auto mAs; slice thickness, 0.625-1.0 mm; ii)
non-enhanced CT or CECT: 100 or 120 kV; auto mAs; and slice
thickness, 1.25-2.0 mm. For vessel reconstruction, the axial
imaging data were transferred to an Advanced Workstation for
post-processing (Extended Brilliance Workspace, EBW). The image of
the SMA was reformatted using volume rendering, maximum intensity
projection, multiplanar reconstruction and curved planar
reconstruction.
The diagnosis of SISMAD was confirmed when one of
the following signs was seen in the SMA: i) Intimal flap and
contrast enhancement within the false lumen; and ii) a
crescent-shaped area along the wall of the SMA indicating no
contrast enhancement (2,16,17).
All images were jointly reviewed by two radiologists (Examiner 1
and Examiner 2, with 5 and 20 years of experience in abdominal
radiology, respectively) who were blinded to the patients' history.
Any disagreements were resolved through consensus. The presence or
absence of PFS was ascertained using reformatted multiplanar plain
abdominal CT and precontrast CT scans that were free of any motion
artifacts. The case group was defined as patients with PFS, while
the control group was defined as patients without PFS. Axial,
coronal, sagittal, as well as multiplanar reconstruction images
obtained using abdominal CECT or CTA were reviewed for the
diagnosis of SISMAD. Each of these images was analyzed for the
following categories: Diameters of the affected segment and the
unaffected proximal segment, and the classification, complications,
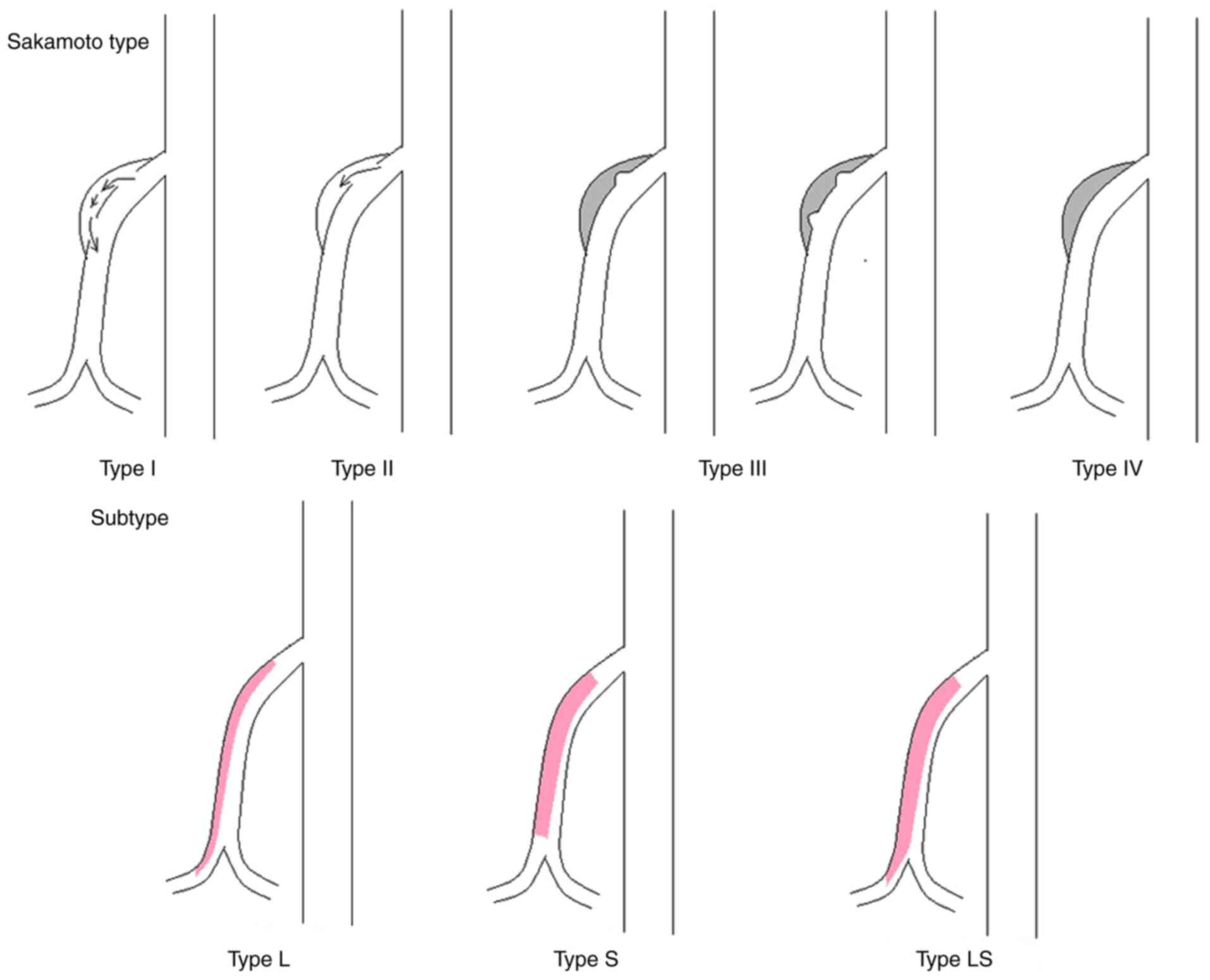
comorbidities and management of SISMAD. The SISMAD classifications
described by previous studies (3,9,10,12,16,18,19)
were used to divide SISMAD into types I to IV. In addition, each
type was further divided, if possible, into the following subtypes:
Subtype L, long segmental dissection (i.e., dissection extending
distally into the ileocolic or distal ileal artery); subtype S,
significant stenosis of the true lumen (i.e., >80%); and subtype
LS, a combination of subtypes L and S (Fig. 2).
Statistical analysis
Continuous variables are expressed as the mean ±
standard deviation. Categorical variables are presented as numbers
and percentages. Continuous variables were compared between the
case and control groups by using the Student's t-test or the
Mann-Whitney U-test. Categorical variables were compared between
the two groups by using the Pearson chi-square test, Fisher's exact
test or Wilcoxon test. All statistical analyses were performed
using SPSS (version 19; IBM, Corp.). P<0.05 was considered to
indicate a statistically significant difference.
Results
General information
A total of 161 patients (137 males, 24 females; mean
age, 54.7 years) were diagnosed with SMA dissection on CTA or CECT.
Of these patients, 38 patients with combined aortic and SMA
dissection and 3 patients with embolic SMA occlusion were excluded.
In addition, 28 patients with SISMAD who underwent only CTA
examination, 5 patients who were suspected to have SMA dissection
according to the non-enhanced CT findings but did not undergo CTA
examination and 8 patients who were lost to follow-up were
excluded. Thus, 79 patients with SISMAD were included in the final
analysis (Fig. 1).
Of these 79 patients, 34 (43.0%) patients with PFS
[including 31 (91.2%) males] were included in the case group, while
45 (57%) patients without PFS [including 42 (93.3%) males] were
assigned to the control group. The mean age of the patients in the
case and control groups was 55.06±6.958 years (range, 44-69 years)
and 55.98±9.538 years (range, 42-82 years), respectively. Age and
gender did not significantly differ between the case and control
groups (P>0.05; Table I).
Patient characteristics
The percentage of patients requiring emergency
admission was significantly higher in the case group than in the
control group (64.7 vs. 40.0%, P=0.030) and the incidence of PFS
was significantly higher in the emergency group than in the
non-emergency group (55.5 vs. 30.8%, P=0.030). The clinical
presentation of SISMAD ranged from asymptomatic to acute
peritonitis. The most common symptom was abdominal pain, which was
present in 29 (85.3%) patients in the case group and 28 (62.2%)
patients in the control group (P=0.024; Table I). The proportion of patients
admitted to the emergency and non-emergency departments did not
significantly differ between the two study groups (P=0.856 and
P=0.301, respectively; Table II).
However, the proportion of patients with abdominal and no abdominal
pain differed significantly between the emergency and non-emergency
groups (P<0.001; Table II).
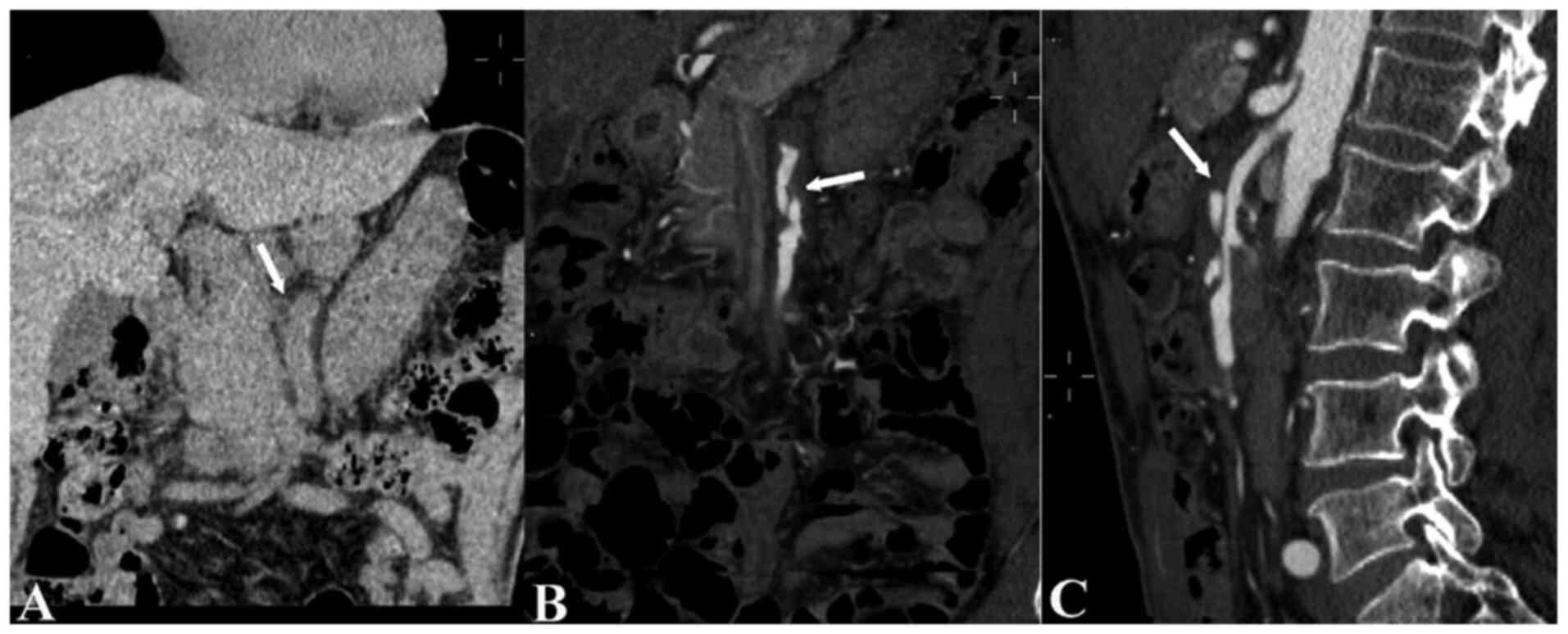
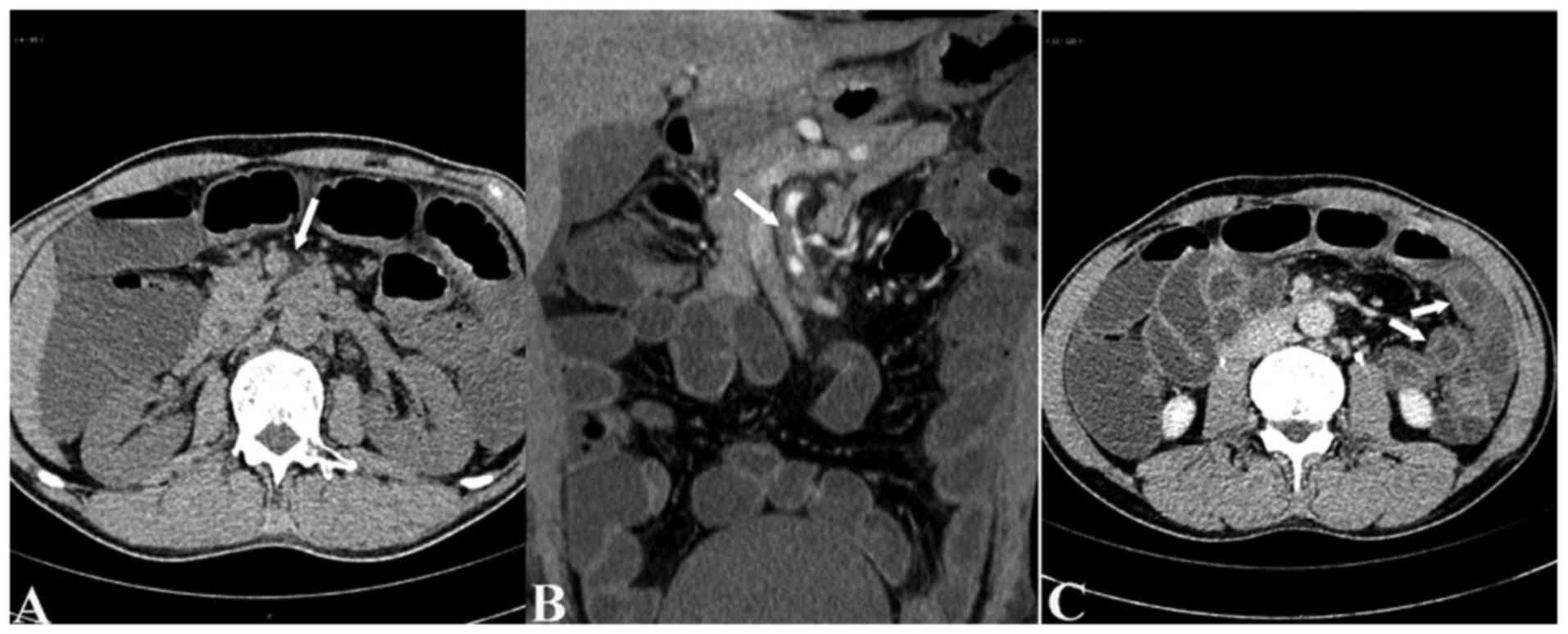
SISMAD was diagnosed using CTA in 18 patients in the case group
(Fig. 3) and 12 patients in the
control group (P=0.017; Table I).
The mean time interval between the initial non-enhanced CT and the
subsequent CTA examination was slightly shorter in the case group
than in the control group, but the difference was not
significant.
 | Table IIClinical presentation of the patients
with SISMAD (n=79). |
Table II
Clinical presentation of the patients
with SISMAD (n=79).
| Setting/abdominal
pain | Total | Cases | Controls | P-value |
|---|
| Emergency | | | | 0.856 |
|
Abdominal
pain | 37
(92.5)a | 21 (56.8) | 16 | |
|
No abdominal
painb | 3 | 1 (33.3) | 2 | |
| Non-emergency | | | | 0.301 |
|
Abdominal
pain | 20 (51.3) | 8 (40.0) | 12 | |
|
No abdominal
painb | 19 | 4 (21.1) | 15 | |
Comorbidities
All 79 patients had various coexisting medical
conditions, including malignancies (Table I). The most common comorbidity was
hypertension (n=34), followed by smoking (n=29), alcohol intake
(n=27) and hyperlipidemia (n=5), although the percentages of these
comorbidities were low. All asymptomatic patients were diagnosed
through the incidental detection of SMA dissection on CT during an
examination for other conditions, including gastrointestinal or
liver tumor, obstructive jaundice, acute pancreatitis,
gastrointestinal hemorrhage, general health examination, neurogenic
bladder, hematuria or infective endocarditis. There were no
significant differences in coexisting medical conditions and
malignancies between the two groups.
SISMAD classification
As presented in Table
III, the diameters of the affected and unaffected segments of
the SMA differed significantly from each other in both the case
group (P<0.001) and the control group (P<0.001); however,
these diameters did not differ between the case and control groups
(affected segment, P=0.363; unaffected segment, P=0.307). According
to the CT findings, SISMAD was classified as follows: Type I,
24.05% of patients; type II, 8.86% of patients; type III, 33.97% of
patients; and type IV, 29.11% of patients. The proportions of these
types did not significantly differ between the case and control
groups (P=0.058). Type III and I was the most common type in the
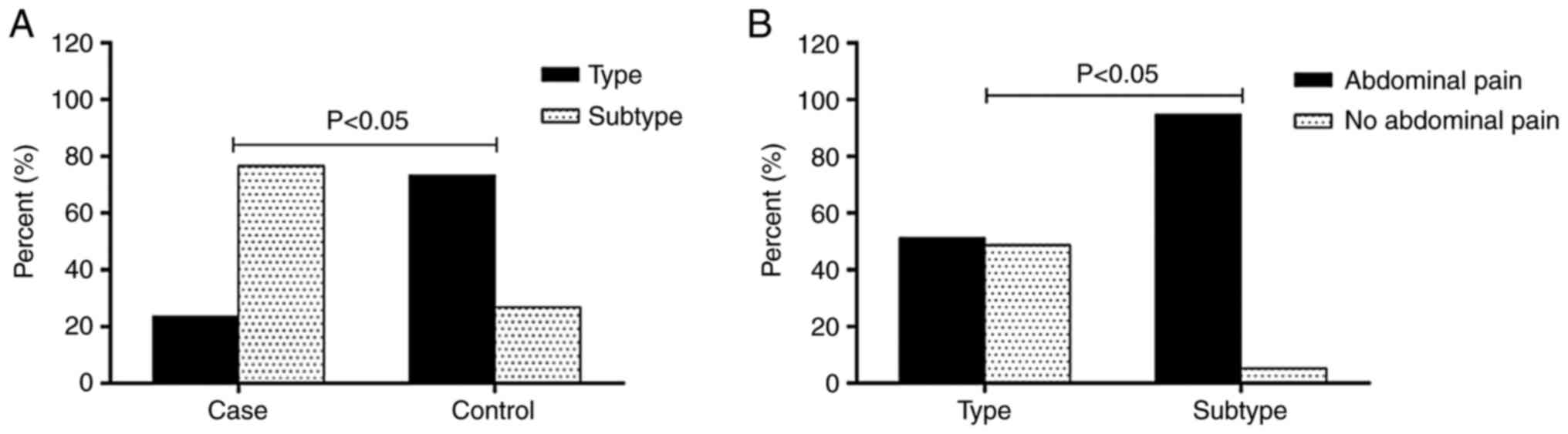
case and control group, respectively (Table III). A total of 38 patients had a
subtype L, S or LS dissection and these subtypes were significantly
more common in the case group than in the control group [26
(76.47%) vs. 12 (26.27%) patients; P<0.001; Fig. 4A]. None of the type I lesions could
be classified into subtypes L, S or LS. Of the 3 type II lesions, 1
was classified as subtype II-L and 2 as subtype II-S. Among the 22
type III lesions, there were 15 subtype III-L, 2 subtype III-S and
5 subtype III-LS lesions. The 13 type IV lesions were divided into
3 subtype IV-L, 1 subtype IV-S and 9 subtype IV-LS lesions.
Subtypes I and IV were significantly less common in the case group
than in the control group, while subtypes III-S (Fig. 5) and IV-L were more common in the
case group than in the control group. Of the 34 patients in the
case group, 29 (85.3%) were symptomatic. Abdominal pain was
significantly more common in patients with subtype L, S and LS
lesions (particularly in the case of type III and IV lesions) than
in patients without subtypes (Fig.
4B).
 | Table IIICT findings and management of the
patients with SISMAD (n=79). |
Table III
CT findings and management of the
patients with SISMAD (n=79).
|
Characteristics | Cases (n=34) | Controls
(n=45) | P-value |
|---|
| Diameter (mm) | | | |
|
Affected
segment | 9.79±1.452 | 10.24±2.577 | 0.363 |
|
Unaffected
proximal segment | 5.88±0.844 | 5.67±0.977 | 0.307 |
|
Classificationa | | | 0.058 |
|
Type Ⅰ | 4 (11.8) | 15 (33.3) | |
|
Type Ⅱ | 0 (0) | 4 (8.9) | |
|
Type
Ⅱ-L | 1 (2.9) | 0 (0) | |
|
Type
Ⅱ-S | 2 (5.9) | 0 (0) | |
|
Type Ⅲ | 2 (5.9) | 6 (13.3) | |
|
Type
Ⅲ-L | 11 (32.4) | 4 (8.9) | |
|
Type
Ⅲ-S | 2 (5.9) | 0 (0) | |
|
Type
Ⅲ-LS | 2 (5.9) | 3 (6.7) | |
|
Type Ⅳ | 2 (5.9) | 8 (17.8) | |
|
Type
Ⅳ-L | 3 (8.8) | 0 (0) | |
|
Type
Ⅳ-S | 0 (0) | 1 (2.2) | |
|
Type
Ⅳ-LS | 5 (14.7) | 4 (8.9) | |
| Concomitant
findings | | | |
|
Celiac trunk
dissection | 5 (14.7) | 10 (22.2) | 0.399 |
|
Renal artery
dissection | 1 (2.9) | 0 (0) | 0.887 |
| Intestinal
necrosis | 1 (2.9) | 4 (8.9) | 0.543 |
| Treatment | | | 0.126 |
|
Conservative | 27 (79.4) | 29 (64.4) | |
|
Endovascular | 5 (14.7) | 9 (0.2) | |
|
Interventional
thrombolysis | 1 (2.9) | 3 (6.7) | |
|
Surgery | 1 (2.9) | 4 (8.9) | |
Complications and outcomes
In the overall study cohort, SISMAD was accompanied
by celiac trunk dissection in 15 patients and with renal artery
dissection in 1 patient. In addition, 5 patients developed
intestinal necrosis as a complication. The incidence of these
complications did not significantly differ between the case and
control groups. In total, 56 patients underwent conservative
treatment, including antiplatelet and anticoagulation therapy (27
cases and 29 controls). Surgical intervention was eventually
required in 5 of these 56 patients due to suspicion of severe
intestinal ischemia or necrosis on CT scan and blood test results
(Table III).
Discussion
The present study demonstrated that SISMAD with PFS
was significantly associated with admission type (emergency),
clinical manifestations (abdominal pain), diagnostic modality and
dissection subtype. Thus, PFS is a potential cause of abdominal
pain in patients with SISMAD and the detection of PFS on plain CT
scans may be suggestive of a diagnosis of SISMAD and indicates the
requirement for further investigation.
To the best of our knowledge, ultrasound examination
has a limited value in the diagnosis of SMA disease due to the
influence of intestinal gas. Furthermore, emergency magnetic
resonance imaging is difficult and expensive. Therefore, CTA is
currently the most sensitive and reliable method for diagnosing
SISMAD. At our different hospitals, CTA is the diagnostic test of
choice for patients suspected to have SISMAD, while CECT is the
most commonly used test when SISMAD is diagnosed incidentally in
China (5,6,17).
However, in clinical practice, numerous patients cannot directly
undergo CTA and rather undergo plain CT first. This is because of
the influence of various factors such as atypical clinical
manifestations and a low clinical suspicion for this condition.
Therefore, abdominal plain CT is the most important examination in
routine radiological work-up, particularly in the emergency
radiological work-up for abdominal pain.
The specific etiology of SISMAD is unclear and
multiple risk factors have been suggested to be related to this
arteriopathy, including connective tissue diseases, cystic medial
necrosis, arterial wall dysfunction involving atherosclerosis,
fibromuscular dysplasia, vasculitis, tobacco use, atherosclerosis,
segmental arterial mediolysis, alcohol abuse, obesity, heavy weight
lifting, history of smoking or hypertension, pregnancy and
hemodynamic forces caused by the convex curvature of the SMA
(1,3,6,18,20-25).
Patients with SISMAD were predominantly males with an average age
of 55 years and this may be related to male smoking and drinking
behaviors (6,17). Perhaps, SISMAD can be prevented by
measures such as cessation of smoking and drinking alcohol and
keeping good dietary habits (6,22).
The imaging characteristics of SISMAD include an
intimal flap, a mural thrombus and an intramural hematoma, which
may not be visible on plain CT (11). Other CT findings suggestive of
dissection include an enlarged SMA diameter and PFS around the SMA.
Although non-specific, PFS is a critical clue for SMA dissection,
particularly when no aneurysmal dilatation or definitive findings
are present on the initial plain CT (1,8,12,13,15,22,26).
Therefore, identifying the sign of PFS on plain CT is helpful to
not miss the diagnosis of SISMAD. The rate of PFS among all the
SISMAD patients in the present study was only 43% (34/79); however,
among the 30 (38.0%) patients who initially underwent non-enhanced
CT prior to CTA examination, PFS was detected in 60% (18/30). In
clinical practice, most patients with SISMAD do not initially have
indications for CECT or CTA due to the atypical symptoms of SISMAD.
Therefore, the possibility of SISMAD is usually not considered in
these patients, who typically undergo plain CT. However, as this
disease may cause fatal intestinal ischemia or necrosis, a timely
and accurate diagnosis is important. Suzuki et al (13) reported on the case of a patient with
acute abdominal pain in whom the initial CT scan indicated PFS
around the SMA without any definite abnormalities in the SMA; a
second examination performed 1 month later confirmed SISMAD.
Patients with SISMAD cannot be screened out based on clinical
symptoms alone; therefore, indirect signs such as PFS that may be
sensitively detected on plain CT may be useful to indicate the
requirement for further CTA or CECT examination to diagnose or rule
out SISMAD.
PFS around the SMA is a pathological condition
(15,26-28)
and may be caused by edema or inflammation of the fat. Endovascular
injury, thinning of the vascular wall and increased permeability
cause the inflammatory cells in the vessels to migrate to the
perivascular fat, which leads to the augmented interaction of local
adipocytes and inflammatory cells within the periadventitial
adipose tissue. This interaction changes in response to
endovascular injury (29) and
induces periadventitial fat inflammation. The cause of abdominal
pain in SISMAD is unclear. Certain studies reported that the pain
is related to mesenteric ischemia caused by stenosis of the true
lumen or to the dissection itself (3,7,10,13,30).
In the present study, the proportion of patients with abdominal
pain was higher in the case group than in the control group,
possibly because perivascular inflammation stimulates visceral
neuroplexuses, causing abdominal pain (1,13,31).
This is also consistent with abdominal pain being more common in
patients requiring emergency treatment in the case group.
Inflammation beyond the intima has been described in patients with
acute coronary syndrome (14) and
arterial dissection (32,33). Therefore, PFS may also represent the
acute phase of intimal tearing during dissection (31,34).
Of all patients with abdominal pain, 64.9% (37/57) were initially
admitted to the emergency department, of which 56.8% (21/37) were
in the case group. Patients with SISMAD and PFS mainly complained
of abdominal pain at the time of presentation and were therefore
admitted to the emergency department.
Early classifications of SISMAD were based mainly on
morphological appearance, and disease severity was incorporated in
later classifications (3,7-9,12,20,35).
Despite significant advances in our understanding and diagnosis of
this condition, no consensus has emerged regarding which
classification and management strategy are optimal. The ideal
SISMAD classification should reflect both its morphological
features and the severity of the disease. In addition, it should be
as simple as possible. The more detailed classification systems
established by Yoo et al (3), Sakamoto et al (9) and Luan and Li (12), which are all based on morphological
appearance, clinical symptoms and management, were adopted in the
present study. The results indicated that none of the dissection
types significantly differed between the case and control groups.
This may be attributable to a type of selection bias, as only
patients who were admitted to hospital were studied; asymptomatic
patients may not be admitted to a hospital and the diagnosis may be
missed. In addition, the present study was limited to a single
institution. However, the prevalence of subtypes III-L and IV-L was
high in patients with PFS and type-III lesions were more common
than other lesion types in the case group, which is consistent with
previous studies (35). The
proportion of patients with abdominal pain was significantly
greater in the case of lesions that were able to be assigned a
subtype than in lesions that were not. It may be speculated that
PFS leads to clinical symptoms owing to the involvement of a longer
segment of the SMA and the aggravation of stenosis (12,18).
Patients with type-III or -IV lesions are likely to present with
acute abdominal symptoms (6). It
appears logical that longer dissections and/or more severe true
lumen stenosis would cause more inflammation and thus pain.
In the present study, abnormalities of the celiac
trunk and renal arteries were observed in 16 patients. This
suggests that radiological evaluation of SISMAD should not be
limited to the SMA and attempts should be made to evaluate the
morphological characteristics of the rest of the abdominal arterial
system. A total of 5 patients developed intestinal ischemia, only 1
of whom was in the case group; the remaining patients with severe
stenosis in the case group did not develop intestinal ischemia. It
may be postulated that collateral flow from the celiac artery and
inferior mesenteric artery or patent branches of the proximal SMA
may have a critical role in preventing the development of bowel
infarction during the early stage of dissection (7). The goals of SISMAD treatment are
symptom relief, as well as prevention of intestinal necrosis and
SMA rupture. Therefore, the management of patients with SISMAD
tends to depend on the presence or absence of symptoms (7,8,10,12,19).
The present study had certain limitations. First,
the number of cases with PFS was small, which may be associated
with the pain endurance of certain patients, who may not have
noticed their own symptoms, as well as with the retrospective study
design, due to which selection bias cannot be excluded.
Furthermore, motion artifacts were common distractors while
evaluating images for the presence of fat stranding. However, the
images were evaluated by two readers to estimate the inter-observer
consistency in detecting the sign of PFS and reduce this error. In
addition, patients were included in the study based on whether
there was SISMAD and patients with embolic occlusion of the SMA who
may have had PFS were excluded. As another limitation, SISMAD was
not able to be distinguished from an intramural hematoma of the
SMA, as the density of hematoma and dissection are not distinctly
different on plain CT (13).
Therefore, it is possible that certain cases of SISMAD were missed.
In addition, symptom severity was not assessed in detail in
patients with mild abdominal pain who were admitted to the
emergency department in accordance with China's national
regulations.
In conclusion, although PFS surrounding the SMA on
CT has been reported in the literature, to our knowledge, the
frequency and clinical implications of PFS surrounding SISMAD on CT
have not been previously described. To the best of our knowledge,
the present study was the first to focus on PFS in patients with
SISMAD. The present results indicated that PFS was significantly
associated with abdominal pain, type of admission (emergency) and
SISMAD classification. The results suggest that when PFS
surrounding the SMA is detected on plain CT in a patient presenting
with abdominal pain, particularly in an emergency setting, further
CTA examination should be performed to diagnose or rule out SISMAD.
It is esteemed that this CT marker may help in the timely detection
and treatment of SISMAD and thereby prevent the development of
life-threatening complications, such as intestinal necrosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81701673) and the
Natural Science Foundation of Hubei Province.
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL designed the study. XL, QJ and ZT collected the
data. XL, ZT, QJ and PH reviewed the imaging data. ZT and WF
analyzed the data, reviewed the charts and interpreted the data. ZT
and QJ performed a literature search and selected the studies to be
included. XL and ZT wrote the manuscript. PH and QJ edited the
manuscript. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present retrospective registry study was
approved by the institutional review board of our hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
D'Ambrosio N, Friedman B, Siegel D, Katz
D, Newatia A and Hines J: Spontaneous isolated dissection of the
celiac artery: CT findings in adults. AJR Am J Roentgenol.
188:W506–W511. 2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Satokawa H, Takase S, Seto Y, Yokoyama H,
Gotoh M, Kogure M, Midorikawa H, Saito T and Maehara K: Management
strategy of isolated spontaneous dissection of the superior
mesenteric artery. Ann Vasc Dis. 7:232–238. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yoo J, Lee JB, Park HJ, Lee ES, Park SB,
Kim YS and Choi BI: Classification of spontaneous isolated superior
mesenteric artery dissection: Correlation with multi detector CT
features and clinical presentation. Abdom Radiol (NY).
43:3157–3165. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bauersfeld SR: Dissecting aneurysm of the
aorta; a presentation of 15 cases and a review of the recent
literature. Ann Intern Med. 26:873–889. 1947.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ghodasara N, Liddell R, Fishman EK and
Johnson PT: High-value multidetector CT angiography of the superior
mesenteric artery: What emergency medicine physicians and
interventional radiologists need to know. Radiographics.
39:559–577. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Luan JY, Guan X, Li X, Wang CM, Li TR,
Zhang L and Han JT: Isolated superior mesenteric artery dissection
in China. J Vasc Surg. 63:530–536. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kim HK, Jung HK, Cho J, Lee JM and Huh S:
Clinical and radiologic course of symptomatic spontaneous isolated
dissection of the superior mesenteric artery treated with
conservative management. J Vasc Surg. 59:465–472. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Park WJ and Seo JW: Follow-up with
computed tomography after spontaneous isolated dissection of the
splanchnic artery. Clin Imaging. 52:1–7. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sakamoto I, Ogawa Y, Sueyoshi E, Fukui K,
Murakami T and Uetani M: Imaging appearances and management of
isolated spontaneous dissection of the superior mesenteric artery.
Eur J Radiol. 64:103–110. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zerbib P, Perot C, Lambert M, Seblini M,
Pruvot FR and Chambon JP: Management of isolated spontaneous
dissection of superior mesenteric artery. Langenbecks Arch Surg.
395:437–443. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Schwartz SA, Taljanovic MS, Smyth S,
O'Brien MJ and Rogers LF: CT findings of rupture, impending
rupture, and contained rupture of abdominal aortic aneurysms. AJR
Am J Roentgenol. 188:W57–W62. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Luan JY and Li X: Computed tomography
imaging features and classification of isolated dissection of the
superior mesenteric artery. Eur J Vasc Endovasc Surg. 46:232–235.
2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Suzuki S, Furui S, Kohtake H, Sakamoto T,
Yamasaki M, Furukawa A, Murata K and Takei R: Isolated dissection
of the superior mesenteric artery: CT findings in six cases. Abdom
Imaging. 29:153–157. 2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hedgire S, Baliyan V, Zucker EJ, Bittner
DO, Staziaki PV, Takx RAP, Scholtz JE, Meyersohn N, Hoffmann U and
Ghoshhajra B: Perivascular epicardial fat stranding at coronary CT
angiography: A marker of acute plaque rupture and spontaneous
coronary artery dissection. Radiology. 287:808–815. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Thornton E, Mendiratta-Lala M, Siewert B
and Eisenberg RL: Patterns of fat stranding. AJR Am J Roentgenol.
197:W1–W14. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li DL, He YY, Alkalei AM, Chen XD, Jin W,
Li M, Zhang HK and Liang TB: Management strategy for spontaneous
isolated dissection of the superior mesenteric artery based on
morphologic classification. J Vasc Surg. 59:165–172.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li T, Zhao S, Li J, Huang Z, Luo C and
Yang L: Value of multi-detector CT in detection of isolated
spontaneous superior mesenteric artery dissection. Chin Med Sci J.
32:28–33. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yun WS, Kim YW, Park KB, Cho SK, Do YS,
Lee KB, Kim DI and Kim DK: Clinical and angiographic follow-up of
spontaneous isolated superior mesenteric artery dissection. Eur J
Vasc Endovasc Surg. 37:572–577. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jia Z, Tu J and Jiang G: The
classification and management strategy of spontaneous isolated
superior mesenteric artery dissection. Korean Circ J. 47:425–431.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li S, Gu X, Jiang G and Tian F: Comment on
‘The value of a new image classification system for planning
treatment and prognosis of spontaneous isolated superior mesenteric
artery dissection’. Vascular. 23(558)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kimura Y, Kato T, Nagao K, Izumi T, Haruna
T, Ueyama K, Inada T and Inoko M: Outcomes and radiographic
findings of isolated spontaneous superior mesenteric artery
dissection. Eur J Vasc Endovasc Surg. 53:276–281. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tomita K, Obara H, Sekimoto Y, Matsubara
K, Watada S, Fujimura N, Shibutani S, Nagasaki K, Hayashi S, Harada
H, et al: Evolution of computed tomographic characteristics of
spontaneous isolated superior mesenteric artery dissection during
conservative management. Circ J. 80:1452–1459. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Nath A, Yewale S and Kousha M: Spontaneous
isolated superior mesenteric artery dissection. Case Rep
Gastroenterol. 10:775–780. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
El-Zein RS, Sobecki J, Greenberg R,
Keleher M and Palma RA: A spontaneous isolated superior mesenteric
artery dissection associated with cocaine abuse: A pathomechanistic
association. Case Rep Vasc Med. 2020(2514687)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Park YJ, Park CW, Park KB, Roh YN, Kim DI
and Kim YW: Inference from clinical and fluid dynamic studies about
underlying cause of spontaneous isolated superior mesenteric artery
dissection. J Vasc Surg. 53:80–86. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mindelzun RE, Jeffrey RB Jr, Lane MJ and
Silverman PM: The misty mesentery on CT: Differential diagnosis.
AJR Am J Roentgenol. 167:61–65. 1996.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Filippone A, Cianci R, Di Fabio F and
Storto ML: Misty mesentery: A pictorial review of multidetector-row
CT findings. Radiol Med. 116:351–365. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Okino Y, Kiyosue H, Mori H, Komatsu E,
Matsumoto S, Yamada Y, Suzuki K and Tomonari K: Root of the
small-bowel mesentery: Correlative anatomy and CT features of
pathologic conditions. Radiographics. 21:1475–1490. 2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Antonopoulos AS, Sanna F, Sabharwal N,
Thomas S, Oikonomou EK, Herdman L, Margaritis M, Shirodaria C,
Kampoli AM, Akoumianakis I, et al: Detecting human coronary
inflammation by imaging perivascular fat. Sci Transl Med.
9(eaal2658)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Furukawa H and Moriyama N: Spontaneous
dissection of the superior mesenteric artery diagnosed on
multidetector helical CT. J Comput Assist Tomogr. 26:143–144.
2002.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Takaoka M, Suzuki H, Shioda S, Sekikawa K,
Saito Y, Nagai R and Sata M: Endovascular injury induces rapid
phenotypic changes in perivascular adipose. Arterioscler Thromb
Vasc Biol. 30:1576–1582. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Thanvi B, Munshi SK, Dawson SL and
Robinson TG: Carotid and vertebral artery dissection syndromes.
Postgrad Med J. 81:383–388. 2005.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Park KW, Park JS, Hwang SC, Im SB, Shin WH
and Kim BT: Vertebral artery dissection: Natural history, clinical
features and therapeutic considerations. J Korean Neurosurg Soc.
44:109–115. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Vela D, Buja LM, Madjid M, Burke A,
Naghavi M, Willerson JT, Casscells SW and Litovsky S: The role of
periadventitial fat in atherosclerosis. Arch Pathol Lab Med.
131:481–487. 2007.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Xiong J, Wu Z, Guo W, Liu X, Wang L, Zhang
H, Jia X and Ma X: The value of a new image classification system
for planning treatment and prognosis of spontaneous isolated
superior mesenteric artery dissection. Vascular. 23:504–512.
2015.PubMed/NCBI View Article : Google Scholar
|