Introduction
Myocardial infarction (MI) is known to be an
important causative element in congestive heart failure. Persistent
left ventricular (LV) remodeling after MI may lead to reduced LV
systolic function and increase the incidence and mortality rates of
cardiovascular events (1).
Oxidative stress is a major cause of MI and, as such, the use of
oxidants can effectively improve the disease progression (2). Kuro-o (2) was the first to successfully clone the
a-Klotho gene (also known as the Klotho gene) in an aged mouse and
found Klotho genes in both rats and humans that were highly
homologous (83%) with the mouse Klotho gene. The Klotho gene is
located on chromosome 13 in humans, whereas the rat Klotho gene is
on chromosome 12, which is 50 kb in length and contains five exons.
In the Klotho gene, its messenger RNA (mRNA) has an alternative
splice site and, as such, it can produce membrane and secretory
proteins. The human membrane Klotho protein is mainly expressed in
the kidneys, small intestine and placenta; in effect, the human
secretory Klotho protein essentially shares the same distribution
pattern as the membrane Klotho protein. However, the secretory
Klotho protein has no transmembrane or intracellular structure and
functions primarily in the free form. Also, Klotho proteins can be
found in the blood serum (3).
Klotho gene mutations in mice are associated with relevant symptoms
that resemble premature aging in humans and a shortened lifespan.
In contrast, Klotho gene overexpression has been shown to extend
the lifespan in mice (4). Relevant
studies (5-7)
demonstrated that downregulated Klotho expression under oxidative
stress can result in greater damage to organs and tissues. However,
the mechanism of action of the Klotho gene under MI-induced
oxidative stress remains unclear. As such, the present study
designed an MI model and injected Klotho gene-containing plasmids
into the LV-free wall to investigate the effects of the Klotho gene
on the MI model, and to explore its underlying mechanism by using
the Nrf2/ARE pathway inhibitor LY294002 which could inhibit Nrf2
and ARE gene and protein expressions.
Materials and methods
Materials
Superoxide dismutase (SOD), glutathione (GSH), and
malondialdehyde (MDA) assay kits (Nanjing Jiancheng Bioengineering
Institute); Klotho, Nrf2, caspase-9 and ARE rabbit anti-mouse
multiclonal antibodies (Abcam); recombinant adenovirus vector
(Shanghai Shinegene Molecular Biotechnology); horseradish
peroxidase (HRP)-labeled anti-mouse immunoglobulin G (ICL); a
bicinchoninic acid protein assay kit (Shanghai Generay Biotech); a
terminal deoxynucleotidyl transferase
2'-deoxyuridine-5'-triphosphate nick end-labeling (TUNEL) assay kit
(Abcam); and an Nrf2/ARE signaling pathway inhibitor (LY2994002;
Sigma-Aldrich, Merck KGaA) were employed. pDC316-NC and
pDC316-Klotho were obtained from Nanjing KeyGEN Biotech Co.,
Ltd.
Modeling and grouping
Except for those in the sham group, each rat was
injected with 45 mg/kg of pentobarbital, intubated, and connected
to ventilators for assisted respiration, with the tidal volume set
at 6.5 ml/kg and the respiratory frequency set at 100 breaths/min.
The left chest was shaved and the operative region was draped. An
incision was made over the top of the fourth rib to open up the
pleural cavity, expose the heart, and separate the pericardium. The
left anterior descending coronary artery was ligated between the
left atrium and the pulmonary artery with 5-0 Prolene (Ethicon,
Inc.). During the operation, the MI region turned pale white. At
the end of the operation, the chest walls were sutured layer by
layer. Subsequently, the tracheal cannula was removed after the
return of spontaneous respiration. The rat was not sent to a
laboratory animal environment until it awoke from anesthesia. Two
weeks after MI modeling, the study rats were divided into four
groups-that is, the model, pDC316-NC, LY294002 and pDC316-Klotho
groups. Intravenous tail injection was adopted, with the model,
pDC316-NC, LY294002 and pDC316-Klotho groups injected with 200 µl
of normal saline, 200 µl of pDC316 plasmids, 200 µl of Klotho
gene-containing plasmids, and 200 µl of LY294002 (15 mg/kg),
respectively. All groups were treated as mentioned above on a daily
basis. The rats were fed regularly for six weeks and killed for
histology after 12 h of fasting. The intra-abdominal injection of
3% pentobarbital (45 mg/kg) was performed before the rats were
killed. Subsequently, half of the hearts of each group were stored
at -80˚C and the other half were placed in 10% buffered formalin.
This study received ethical approval from Guangzhou 12th People's
Hospital.
Measurements of MDA, SOD, GSH and
cardiac function in myocardial tissue
The rat myocardial tissue samples stored at -80˚C
from each group were placed in a liquid nitrogen flash freezer and
then ground into powder to measure the MDA, SOD and GSH levels in
strict accordance with the instructions provided in the assay kit.
The heart function of each group was evaluated by color Doppler
echocardiography. The heart rate (HR), LV end-diastolic pressure
(LVEDP), and LV systolic pressure (LVSP) were recorded.
Hematoxylin and eosin staining
At this stage, the heart tissues were removed from
the 10% buffered formalin to dehydrate, clear and embed it into
paraffin blocks. Subsequently, paraffin-embedded sections were
prepared, measuring 3-4 µm in thickness, using a section cutter;
dewaxed with xylene and stained for 15 min with a hematoxylin
solution. Next, the sections were rinsed in running tap water;
stained using blue hematoxylin for 30 sec using Scott's Tap Water
Substitute and then rinsed again in running tap water for 15 min.
Finally, the sections were stained with eosin, dehydrated with
alcohol, cleared with xylene, mounted with neutral resin, analyzed
with a light microscope, and captured the resultant images.
Masson's trichrome staining
After the routine paraffin section of myocardial
tissue in the border area of MI was removed, Masson's trichrome
staining was performed according to the instructions of the kit.
The sections were stained with hematoxylin for 3 min, washed with
distilled water, differentiated with 1%
HCL-C2H5OH, returned to blue, soaked with
fuchsin acid for three mins, differentiated with phosphomolybdic
acid, redyed with aniline blue, and finally sealed by alcohol
dehydration. Ten fields of vision were randomly selected for each
section, and the myocardial collagen volume fraction (CVF) was
measured using the Proplus 6.0 image analysis software (Media
Cybernetics, Inc.).
TUNEL assay
The heart tissue was fixed in 10% buffered formalin,
dehydrated, cleared and embedded into paraffin blocks; and
subsequently adhered to poly-L-lysine-coated microscope slides.
Following dewaxing; each slide was incubated for 60 min at 37˚C in
50 µl proteinase K solution. Finally, the sections were mounted
with antifade mounting medium and the cells were counted under a
fluorescence microscope. Those observed in blue were considered as
normal myocardial cell nuclei, whereas apoptotic nuclei were
stained dark brown. Images of the different target areas were
captured with a light microscope in five vision fields
(magnification, x400), in order to calculate the apoptotic index
using the positive myocardial cells and the total myocardial cells
in each visual field; that is, the proportion of apoptotic cells to
the total myocardial cells in the same field of vision.
Assessing the expression of Klotho
proteins in rat myocardial tissue by immunohistochemistry (IHC)
assay
Antibodies in histiocytes were identified and
located using a labeling developer and performed qualitative and
quantitative analyses of the labeled antibodies, preparing and
staining with relevant reagents according to the instructions given
in the IHC assay kit. At this point, paraffin sections of muscular
tissue were dewaxed twice using xylene, hydrated with alcohol step
by step, and rinsed twice with phosphate-buffered saline (PBS). For
deactivating endogenous enzymes, we incubated samples in a wet box
with 3% H2O2 for 10 min at room temperature.
To complete antigen retrieval, a container filled with citrate was
placed in a microwave oven, which was brought to a boil, and the
slides were soaked in citrate for 3 min, cooled to room temperature
for renaturation, and rinsed twice with PBS. Subsequently, the
slides were placed in the wet box, and normal goat serum was added
and incubated for 10 min before removing it. Next, diluted primary
Klotho antibody (dilution ratio, 1:200) was added to the wet box
and incubated overnight at 4˚C. The following day, reagent 2 (a
biotin-labeled secondary antibody) was added to incubate for 30 min
at 37˚C, and then rinsed 3 times with PBS. HRP-labeled streptavidin
was added to incubate for 10 min at room temperature, followed by
three PBS rinses. Next, 3,3'-diaminobenzidine (DAB) was added and
incubated for 5 min at room temperature. When cells presented with
dark brown stain (positive) under observation, the development was
stopped. The sections were then counterstained with hematoxylin for
6 min, rinsed with running tap water, differentiated with
hydrochloric acid for 1 sec, and dehydrated with alcohol. The
slides were then cleared with xylene three times, mounted with gum,
and observed using a microscope. Images were captured using a
professional image acquisition and analysis system. Five different
fields of vision were selected to observe each slice and the
average greyscale of all positive results was analyzed.
Western blotting assay
Rat myocardial tissue from each experimental group
was stored at -80˚C. In order to obtain protein lysate, the tissue
was ground thoroughly and the concoction was centrifuged to obtain
the supernatant fluid. The protein concentration was measured using
a bicinchoninic acid protein assay kit. A total of 20 µl sample per
lane was separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE), and the proteins were transferred from
the gel onto a cellulose filter, incubated with 5% skim milk
solution for 1 h at room temperature. The membranes were then
incubated in primary antibodies [dilution ratio: Klotho=1:2,000;
Nrf2=1:2,000; ARE=1:2,000; caspase-9=1:2,000; glyceraldehyde
3-phosphate dehydrogenase (GAPDH)=1:10,000] for overnight
incubation at 4˚C. This was followed by incubation with secondary
antibody (dilution ratio=1:1,000) for incubation for 2-3 h at room
temperature. Finally, the membranes were treated with a developer
and developed onto films. GAPDH was used as an internal control
when the optical density of each band was quantified using a gel
imaging system. The ratio of the optical density of each band to
GAPDH was calculated, i.e., the result of the western blotting
semiquantitative analysis of protein expression.
Reverse transcription polymerase chain
reaction (RT-PCR) assay
The total RNA content was extracted from the tissues
using TRIzol and all RNA were reverse-transcribed to complementary
DNA (cDNA) using a reverse transcription kit (Takara Bio, Inc.).
The mixture was centrifuged to the bottom with an instantaneous
centrifuge and then put into a PCR apparatus. The reaction took
place at 70˚C for 10 min and the sample was quickly taken out and
inserted onto ice to stop the reaction. The PCR reaction system was
20 µl in total, consisting of 8 µl PCR-level water, 10 µl
SYBR-Green I Master Mix, 0.5 µl forward primer, 0.5 µl reverse
primer and 1 µl cDNA template. The aforementioned reagents were
mixed and were added into a 96-well plate that matched with the PCR
instrument. Each sample was set with three multiple holes,
centrifuged at 1,500 g for 3 min, and tested on the PCR instrument.
The primer sequence is shown in Table
I.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene name | Primer sequences |
|---|
| Klotho | F:
5'-CTAGCTAGCCACCATGCCAGCCCGCGCCCCTCCTCGCC-3' |
| | R:
5'-ATTTGCGGCCGCTTATTTATAACGTCTCCGGCCTTTCT-3' |
| Nrf2 | F:
5'-TGGACGGGACTATTGAAGGCT-3' |
| | R:
5'-GCCGCCTTTTCAGTAGATGGA-3' |
| ARE | F:
5'-CACGCATATACCCGCTACC-3' |
| | R:
5'-AAGGCGGTCTTAGCCTCTTC-3' |
| Caspase-9 | F:
5'-GAGAGACATGCAGATATGGCATACA-3' |
| | R:
5'-CAGAAGTTCACGTTGTTGATGATG-3' |
| β-actin | F:
5'-CTGAGCCAGATGCTGTCCCATA-3' |
| | R:
5'-GAGACCATCCAAGGTCTCGATGTA-3' |
Statistical analysis
All measurement data were expressed as means ±
standard deviations (SDs). An analysis of variance and Tukey's test
were applied to the intergroup comparison of those following normal
distribution and homogeneity of variance; in the case of non-normal
distribution and heterogeneity of variance, the Kruskal-Wallis test
by ranks was used, while the Nemenyi test was adopted for
intergroup comparisons. P<0.05 was used to indicate a
statistically significant difference.
Results
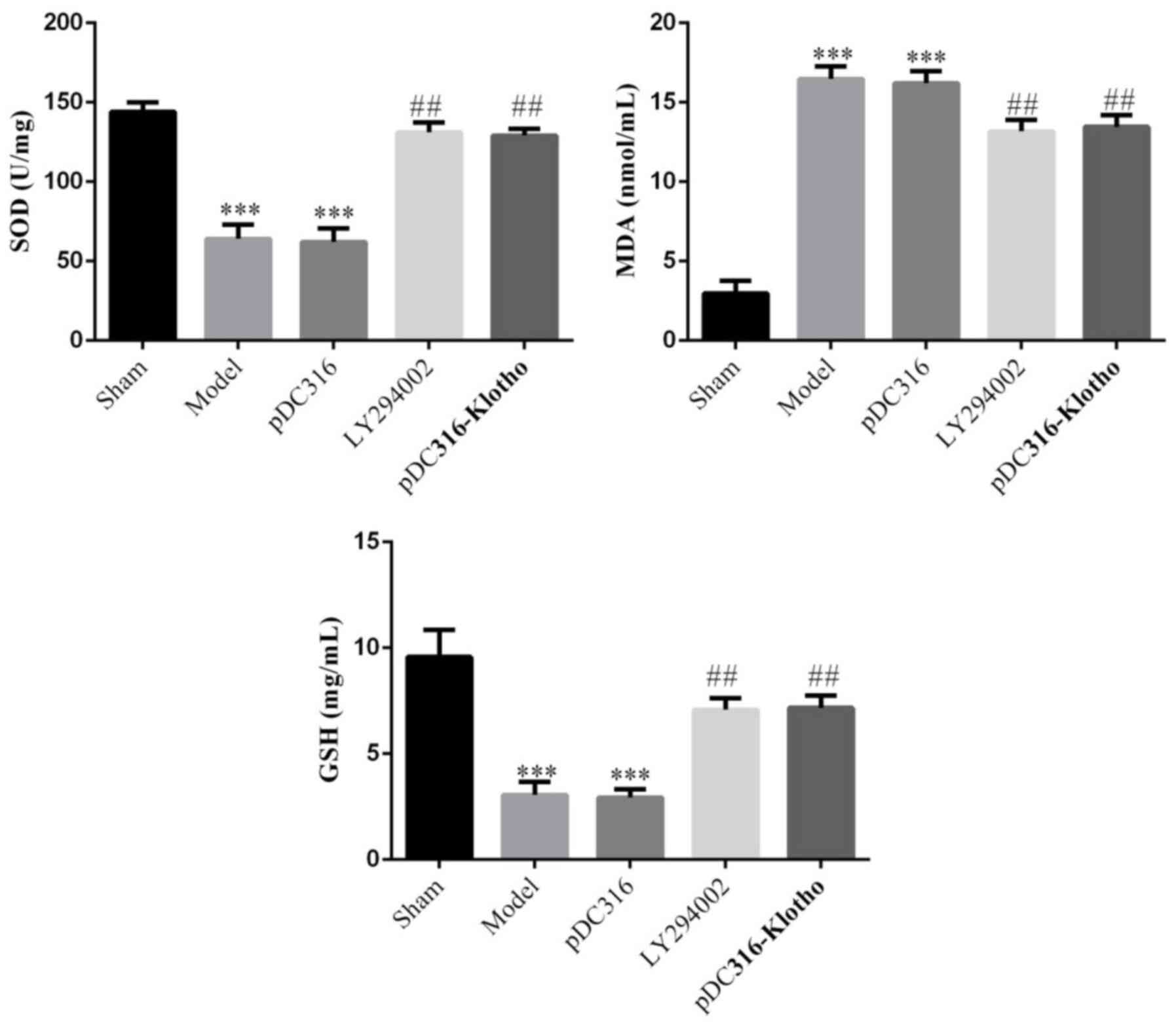
MDA, SOD and GSH concentrations in the
myocardial tissue
Compared with the sham group, the model group and
the pDC316 group's SOD and GSH concentrations were significantly
lower (P<0.001, respectively), whereas the MDA concentrations
were markedly increased (P<0.001). On the hand, following
treatment with LY294002 or pDC316-Klotho, the LY294002 and
pDC316-Klotho groups exhibited a sharp increase in their SOD and
GSH concentrations and a notable decline in MDA concentrations
compared with the model group (P<0.001, respectively). There was
no significant difference noted in the SOD, MDA, or GSH
concentrations between the model group and the pDC316 group
(P>0.05). Likewise, the differences between the LY294002 group
and the pDC316-Klotho group in the SOD, MDA and GSH concentrations
also lacked statistical significance (P>0.05). The data are
shown in Fig. 1.
Cardiac function
Compared with the sham group, the LVSP was
significantly downregulated and the HR and LVEDP were significantly
upregulated in the model and pDC316 groups (P<0.05,
respectively; Table II); however,
with LY294002 and Klotho overexpression, the LVSP was significantly
upregulated and the HR and LVEDP were significantly downregulated
in the LY294002 and pDC316-Klotho groups (P<0.05, respectively;
Table II).
 | Table IIComparison of cardiac function indexes
of rats in each group (mean ± SD). |
Table II
Comparison of cardiac function indexes
of rats in each group (mean ± SD).
| Group | n | LVSP (mmHg) | LVEDP (mmHg) | HR (time/min) |
|---|
| Sham | 10 | 137.17±8.90 | 7.88±0.93 | 483.62±8.59 |
| Model | 10 |
105.95±7.88a |
14.86±0.76a |
518.74±9.74a |
| PDC316 | 10 |
103.10±7.41a |
14.94±0.73a |
543.21±11.71a |
| LY294002 | 10 |
127.56±8.34b |
8.31±1.09b |
497.44±12.14b |
| pcDC316-Klotho | 10 |
128.07±8.92b |
8.86±1.31b |
500.57±14.93b |
Myocardial pathology
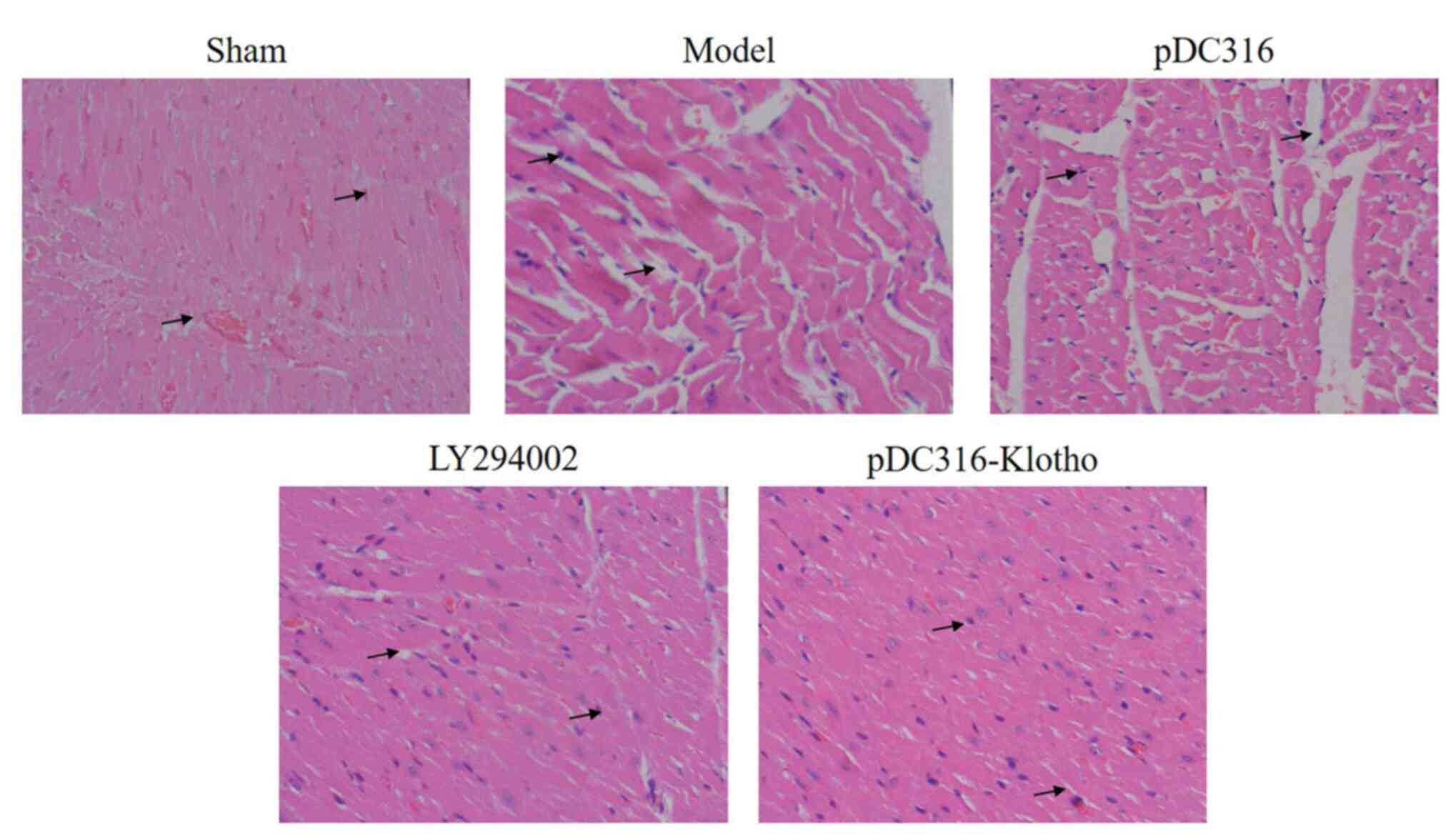
Upon comparing the hematoxylin and eosin staining
results of the sham group, myocardial necrosis, cytolysis,
fragmentation. vascular engorgement, interstitial edema, increased
infiltration of neutrophils, and stained cytoplasm were notable in
the model and pDC316 groups (Fig.
2). Furthermore, compared with the model group, the LY294002
group and the pDC316-Klotho group both expressed reduced findings
of myocardial necrosis, cytolysis, fragmentation, vascular
engorgement, interstitial edema, increased infiltration of
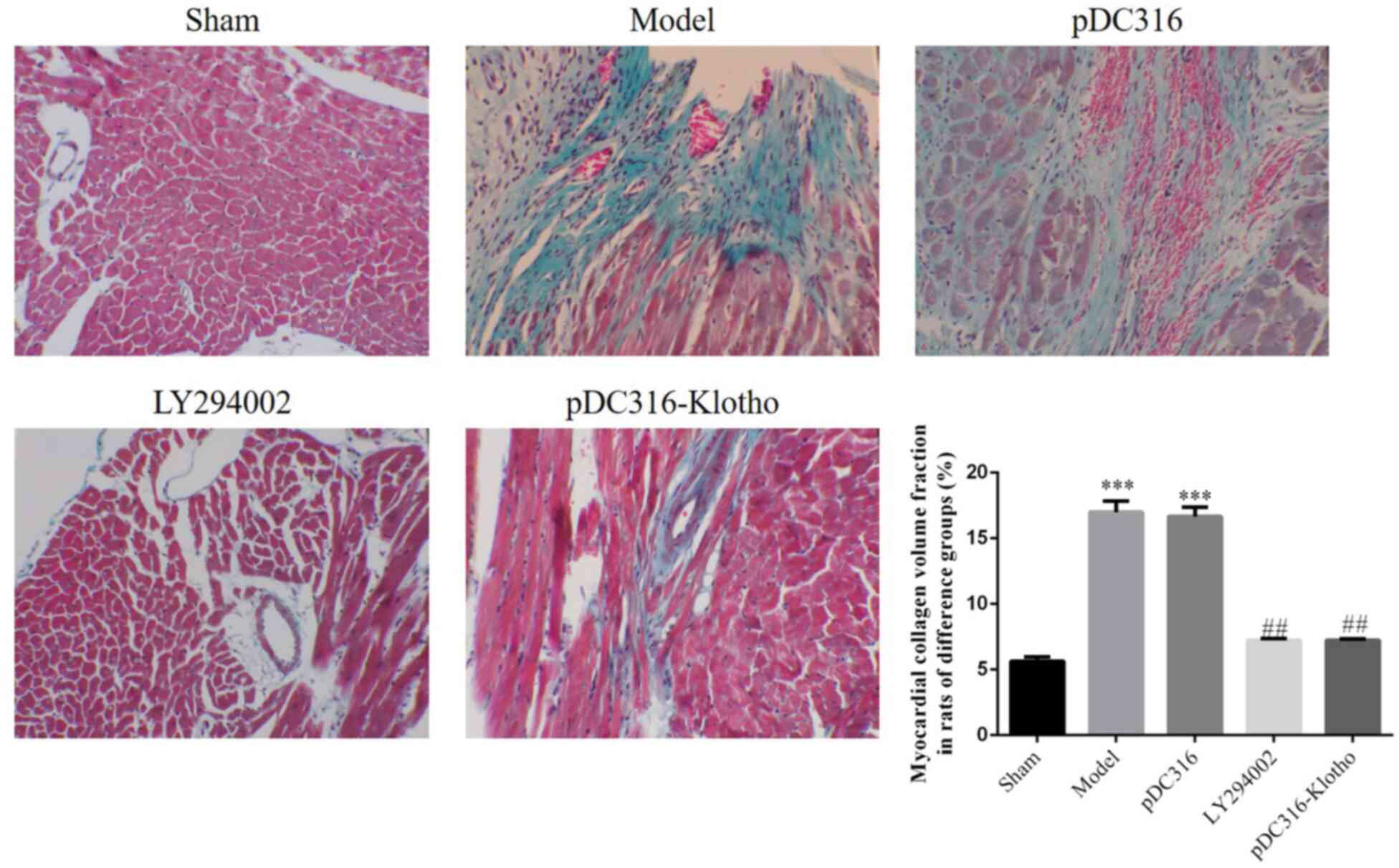
neutrophils, and stained cytoplasm (Fig. 2). Compared with the sham group, the
CVF% results of the model and pDC-316 groups were significantly
increased; however, with LY294002, which inhibited the Nrf2/ARE
pathway, and with Klotho overexpression, the CVF% outcomes of the
LY294002 and pDC316-Klotho groups were significantly decreased
(P<0.001, respectively; Fig. 3).
These findings indicate that LY294002 and pDC316-Klotho can
effectively resist pathological changes in rat hearts.
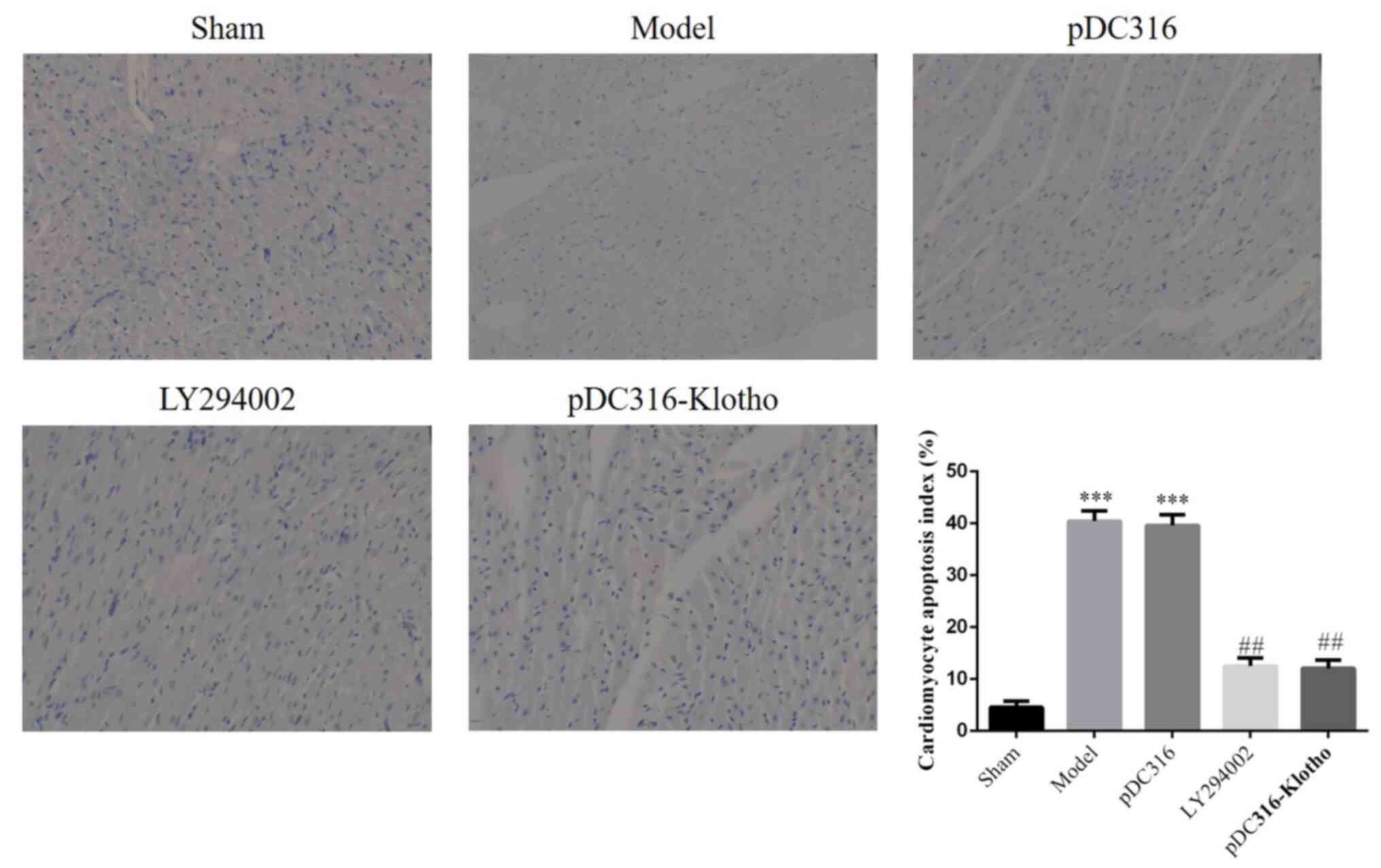
Apoptosis myocardial tissue by TUNEL
assay
Regarding microscopic findings, apoptotic nuclei
were stained dark brown (Fig. 3).
Compared with the sham group, the model group and the pDC316 group
presented with much higher apoptotic indexes (P<0.001,
respectively; Fig. 4). Following
intervention with LY294002 or pDC316-Klotho, the LY294002 group and
the pDC316-Klotho group's apoptotic indexes were significantly
lower than those of the model group (P<0.01, respectively;
Fig. 4). However, the intergroup
difference (model vs. pDC316; LY294002 vs. pDC316-Klotho) in terms
of apoptotic index was not statistically significant
(P>0.05).
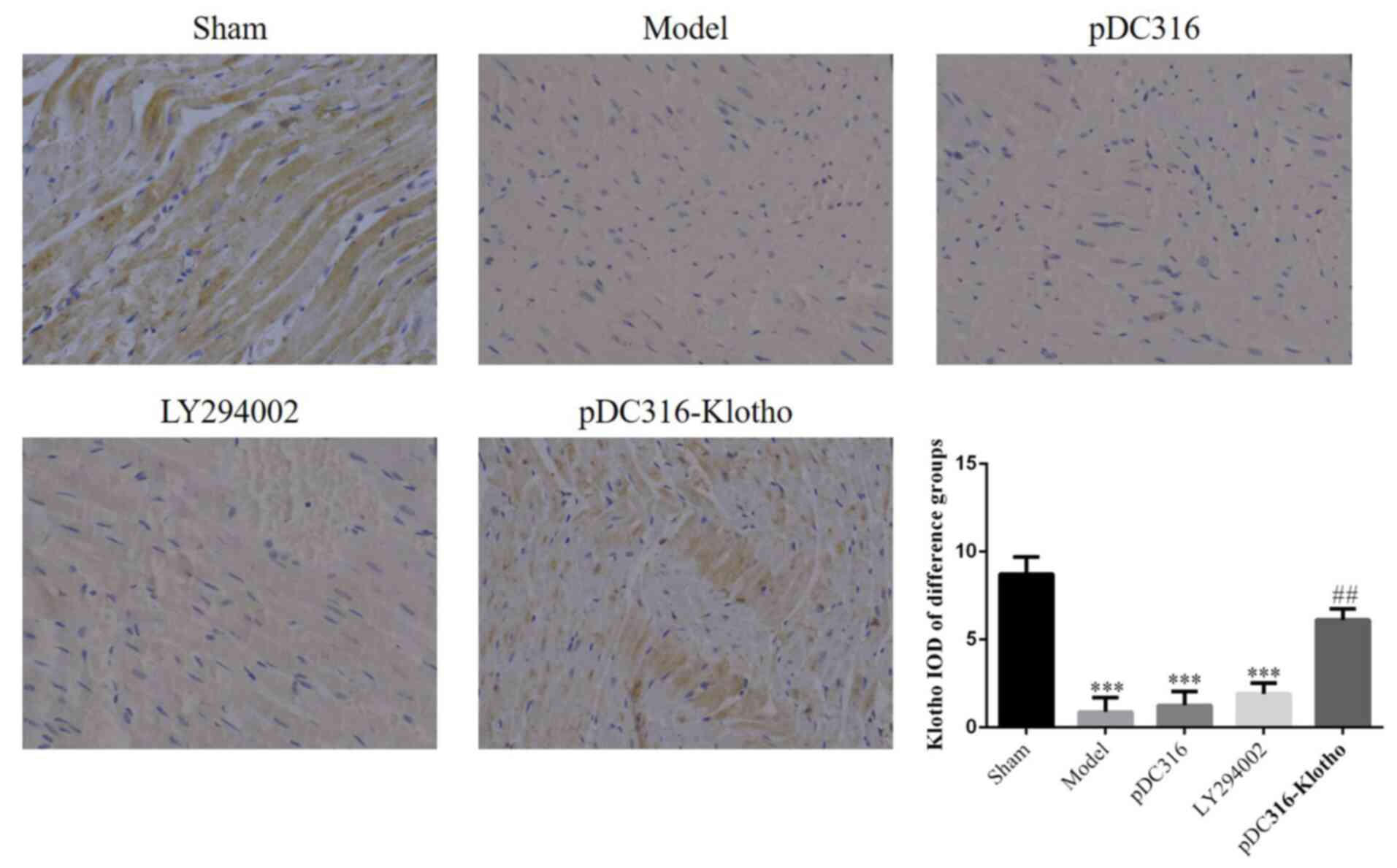
Expression of Klotho proteins in
myocardial tissue
The IHC assay indicated that the expression of
Klotho proteins of the model, pDC316, and LY294002 groups were
significantly lower than in the sham group (P<0.001,
respectively; Fig. 4). However,
these three groups showed no significant inter-group differences
(P>0.05). Compared with the model group, the pDC316-Klotho group
had a significantly elevated level of Klotho expression
(P<0.01). The relative data are shown in Fig. 5.
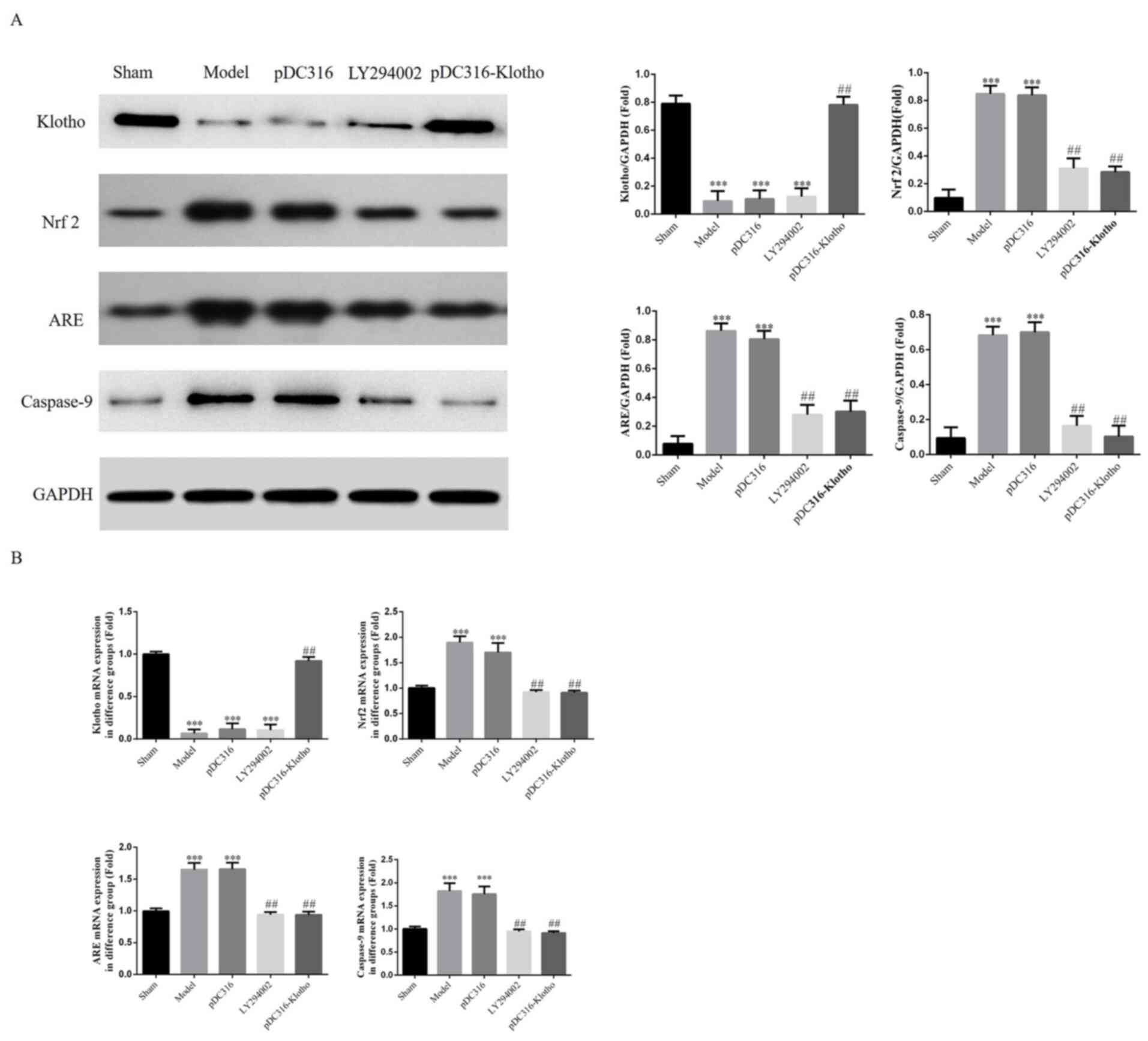
Klotho, Nrf2, ARE and caspase-9 mRNA
and protein expressions in each group's myocardial tissue by RT-PCR
and western blotting assay
According to the western blotting and RT-PCR assay
results, the model group and the pDC316 group showed higher
expression of Nrf2, ARE and caspase-9 compared with the sham group
(P<0.001, respectively; Fig. 6A
and B); however, there was no
significant difference between the model group and the pDC316 group
(P>0.05). Following LY294002 and pDC316-Klotho treatment, the
Nrf2, ARE and caspase-9 expression levels of the LY294002 group and
the pDC316-Klotho group were clearly inhibited in comparison with
those of the model group (P<0.01, respectively; Fig. 6A and B). Furthermore, compared with in the sham
group, the Klotho gene and protein expression levels of the model,
pDC316 and LY294002 groups were significantly decreased
(P<0.001, respectively; Fig. 6A
and B). Finally, with Klotho
overexpression, the Klotho gene and protein expression levels of
the pcDC316-Klotho group were significantly increased compared with
the model group.
Discussion
MI can cause ischemia, hypoxia and
ischemia-reperfusion injury of local tissue, and local or systemic
oxidative stress when a large number of oxygen free radicals are
produced by NADPH oxidase, xanthine oxidase, and respiratory chains
in neutrophils through oxygen bursts. Further, oxygen free radicals
may result in damage to myocardial cells and induce apoptosis and
necrosis using the nuclear factor kappa B signaling pathway that
c-Jun N-terminal kinase heavily depends upon, or cause
MI-associated pathological damage to the myocardial tissue through
other pathways (8-10).
Antioxidants can effectively inhibit post-MI oxidative stress,
thereby improving cardiac function and promoting heart repair. Zhou
et al (11) found that
probucol, an antioxidant, could effectively inhibit the level of
post-MI oxidative stress and collagen remodeling and improve the
diastolic function of the left ventricle. Khanna et al
(12) reported that smoking was
associated with a larger MI-related zone and LV hypofunction,
accompanied by increased proinflammatory factors, tissue repair
factors, and oxidative stress markers in the myocardial tissue; the
use of another antioxidant N-acetylcysteine resulted in a smaller
MI-related zone, improved cardiac function, and a significantly
lower level of proinflammatory factors and oxidative stress markers
in the myocardial tissue, which is likely correlated with the
substantial increase in the transcription levels of SOD,
thioredoxin, Nrf2 and the circulating GSH. In the present study,
the SOD and GSH concentrations of the model group increased
significantly, whereas this group's MDA concentration dropped
sharply. This outcome suggests the activation of MI-induced
oxidative stress. However, following intervention with LY294002
(Nrf2/ARE signaling pathway inhibitor) and pDC316-Klotho, the
oxidative stress level of the myocardial tissue was markedly
inhibited. Also, hematoxylin and eosin and TUNEL staining provided
evidence for the myocardial damage at a remarkably and increasingly
severe level, which was improved substantially by LY294002 Nrf2/ARE
signaling pathway inhibition and pDC316-Klotho.
Despite the regulation by multiple factors, recent
studies have noticed Klotho's extensive physiological and
pathophysiological functions and its presence in the development
and progression of many diseases. The Klotho gene is primarily
expressed in the kidneys, small intestine, lungs, prostate, blood
and brain tissue. The Klotho gene acts as an antioxidant agent by
improving the expression of such antioxidant genes, such as
catalases and SOD (13). Based on
the aforementioned studies, this study observed that the Klotho
expression of the model group was significantly lower than that of
the high-fat-fed control group. When the Klotho proteins play a
role of antioxidation, oxidative stress can downregulate the Klotho
expression through a range of pathways. Some studies (14,15)
have suggested that Klotho proteins were dramatically reduced in
the presence of diabetes, hypertension, and renal diseases, whereas
oxidative stress had an important impact on the development and
progression of these diseases. The expression of Klotho proteins in
the myocardial tissue was significantly increased following
pDC316-Klotho treatment, which may explain the post-MI alleviation
of apoptosis. On the other hand, LY294002, an inhibitor of the
Nrf2/ARE signaling pathway, also demonstrated the ability to repair
damage to the myocardial tissue.
With Nrf 2 being an important transcription factor
that reduces reactive oxygen in oxidative stress, the Nrf 2/ARE
signaling pathway plays a crucial role in oxidative stress.
Normally, Nrf2 remains deactivated in the cytoplasm and thus cannot
enter the cell nuclei as a transcription factor. However, Nrf2 will
be activated when exposed to oxidative stress. The activated Nrf2
enters the cell nuclei and forms heterodimers with small Maf
proteins. Together with ARE, the expression of downstream target
genes is promoted to mediate a series of reactions to produce MnSOD
(Manganese superoxide dismutase), HO-1 (heme oxygenase-1) and other
enzymes, thereby improving the systemic antioxidant ability
(16,17). When oxidation-reduction reactions
reach equilibrium, Nrf2 returns to the cytoplasm and maintains a
normal level through degradation or negative feedback, via the
ubiquitin-proteasome pathway (18).
The results of the present study indicate that both LY294002
(Nrf2/ARE signaling pathway inhibitor) and pDC316-Klotho can
effectively impede the Nrf2/ARE signaling pathway and reduce damage
to the myocardial tissue caused by post-MI oxidative stress.
In summary, overexpression of the Klotho gene, to a
certain degree, can repair damage to myocardial tissue caused by
post-MI oxidative stress through inhibiting the Nrf2/ARE signaling
pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZX conceptualized and developed the study design,
and performed the majority of the experiments. SZ and XF acquired
the data, which were analyzed by SZ, XF, CC, XY and PL. XY and PL
wrote the manuscript, and XF and CC suggested appropriate
modifications, which were corrected by SZ. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study received ethical approval from Guangzhou
12th People's Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu X, Hou L, Xu D, Chen A, Yang L, Zhuang
Y, Xu Y, Fassett JT and Chen Y: Effect of asymmetric
dimethylarginine (ADMA) on heart failture development. Nitric
Oxide. 54:73–81. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
LeBaron TW, Kura B, Kalocayova B,
Tribulova N and Slezak J: A new approach for the prevention and
treatment of cardiovascular disorders. Molecular hydrogen
significantly reduces the effects of oxidative stress. Molecules.
24: pii(E2076)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi
H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, et
al: Mutation of the mouse klotho gene leads to a syndrome
resembling ageing. Nature. 390:45–51. 1997.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Zhou HJ, Zeng CY, Yang TT, Long FY, Kuang
X and Du JR: Lentivirus-mediated klotho up-regulation improves
aging-related memory deficits and oxidative stress in
senescence-accelerated mouse prone-8 mice. Life Sci. 200:56–62.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mitobe M, Yoshida T, Sugiura H, Shirota S,
Tsuchiya K and Nihei H: Oxidative stress decreases klotho
expression in a mouse kidney cell line. Nephron Exp Nephrol.
101:e67–e74. 2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Padanilam BJ: Cell death induced by acute
renal injury: A perspective on the contributions of apoptosis and
necrosis. Am J Physiol Renal Physiol. 284:F608–F627.
2003.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yamamoto M, Clark JD, Pastor JV, Gurnani
P, Nandi A, Kurosu H, Miyoshi M, Ogawa Y, Castrillon DH, Rosenblatt
KP and Kuro-o M: Regulation of oxidative stress by the anti-aging
hormone klotho. J Biol Chem. 280:38029–38034. 2005.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bagatini MD, Martins CC, Battisti V,
Gasparetto D, da Rosa CS, Spanevello RM, Ahmed M, Schmatz R,
Schetinger MR and Morsch VM: Oxidative stress versus antioxidant
defenses in patients with acute myocardial infarction. Heart
Vessels. 26:55–63. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kuroda J, Ago T, Matsushima S, Zhai P,
Schneider MD and Sadoshima J: NADPH oxidase 4 (Nox4) is a major
source of oxidative stress in the failing heart. Proc Natl Acad Sci
USA. 107:15565–15570. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Williams AR and Hare JM: Mesenchymal stem
cells: Biology, pathophysiology, translational findings, and
therapeutic implications for cardiac disease. Circ Res.
109:923–940. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhou SX, Zhou Y, Zhang YL, Lei J and Wang
JF: Antioxidant probucol attenuates myocardial oxidative stress and
collagen expressions in post-myocardial infarction rats. J
Cardiovasc Pharmacol. 54:154–162. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Khanna AK, Xu J and Mehra MR: Antioxidant
N-acetyl cysteine reverses cigarette smoke-induced myocardial
infarction by inhibiting inflammation and oxidative stress in a rat
model. Lab Invest. 92:224–235. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Olejnik A, Franczak A, Krzywonos-Zawadzka
A, Kałużna-Oleksy M and Bil-Lula I: The biological role of Klotho
protein in the development of cardiovascular diseases. Biomed Res
Int. 2018(5171945)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hu MC, Kuro-o M and Moe OW: Renal and
extrarenal actions of Klotho. Semin Nephrol. 33:118–129.
2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liu JJ, Liu S, Morgenthaler NG, Wong MD,
Tavintharan S, Sum CF and Lim SC: Association of plasma soluble
α-klotho with pro-endothelin-1 in patients with type 2 diabetes.
Atherosclerosis. 233:415–418. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Menshchikova EB, Zenkov NK, Tkachev VO,
Lemza AE and Kandalintseva NV: Protective effect of ARE-inducing
phenol antioxidant TS-13 in chronic inflammation. Bull Exp Biol
Med. 155:330–334. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jing X, Ren D, Wei X, Shi H, Zhang X,
Perez RG and Lou H and Lou H: Eriodictyol-7-O-glucoside activates
Nrf2 and protects against cerebral ischemic injury. Toxicol Appl
Pharmacol. 273:672–679. 2013.PubMed/NCBI
|
|
18
|
Canning P, Cooper CD, Krojer T, Murray JW,
Pike AC, Chaikuad A, Keates T, Thangaratnarajah C, Hojzan V,
Ayinampudi V, et al: Structural basis for Cul3 protein assembly
with the BTB-Kelch family of E3 ubiquitin ligases. J Biol Chem.
288:7803–7814. 2013.PubMed/NCBI View Article : Google Scholar
|