Introduction
Although cutaneous melanoma represents only 10% of
the total cutaneous malignant tumours, it is responsible for over
90% of the deaths caused by these tumours (1). Incidence rates of cutaneous melanoma
are increasing worldwide in the fair-skinned population (2). However, early detection and the
development of new drugs for metastatic melanoma lead to an
increased survival rate and, therefore, an increasing population at
risk of developing other neoplasms, especially cutaneous malignant
tumours (3-5).
It is well known that patients with melanoma have an increased risk
of having other neoplasms, and particularly other melanomas and
non-melanoma skin cancers (6-16).
A personal history of melanoma proved to be a potent risk factor
for the development of a subsequent primary melanoma (6-16).
Multiple lesions can be detected as synchronous or asynchronous
lesions. Synchronous lesions are defined as subsequent primary
melanomas diagnosed within three months of the incident primary
melanoma (7). Studies show that the
percentage of patients who develop multiple primaries ranges from
0.2 to 8.6%, out of which 26-40% develop as synchronous lesions
(7,8). The risk of a subsequent primary
melanoma seems to be higher in the first year following the first
diagnosis, but it remains increased for at least 20 years (7,8).
The present study aimed to describe multiple primary
melanomas in a large medical university centre from Romania
(Cluj-Napoca) from 2004 to 2020, focusing on the number of lesions
detected, tumour and patient characteristics, as well as time to
subsequent diagnosis.
Materials and methods
Setting
This observational, retrospective, cohort study was
carried out in Cluj County, in the North-Western Region of Romania.
The present study was approved by the Ethics Committee of ‘Iuliu
Hatieganu’ University of Medicine and Pharmacy (Cluj-Napoca,
Romania).
Participants
All patients diagnosed with melanoma and followed-up
in the Dermatology Department of the Cluj-Napoca Emergency County
Hospital were eligible for analysis. Patients identified as having
more than one invasive or in situ melanoma, followed up for
at least one year, were included in the study. Data on patient
characteristics included age, sex, family history of melanoma,
presence of atypical nevi. The tumour was characterised according
to the site (head and neck, trunk, upper limbs and lower limbs),
histological subtype [superficial spreading melanoma (SSM), lentigo
maligna melanoma (LMM), nodular melanoma (NM) and acral lentiginous
melanoma (ALM) other], other histological characteristics (Breslow
index, presence of mitosis, ulceration, vascular and neural
invasion) and melanoma stage. The time to diagnosis was defined as
the time from the first melanoma diagnosis to subsequent melanoma
diagnosis. Subsequent melanomas were divided into synchronous and
asynchronous melanomas. Synchronous melanomas were defined as those
diagnosed simultaneously or within the first three months after the
diagnosis of the first melanoma.
Statistical analysis was carried out using the
MedCalc Statistical Software version 19.2.1 (MedCalc Software Ltd.;
https://www.medcalc.org; 2020). Quantitative
variables were expressed as mean and standard deviation, or median
and 25-75 percentiles, depending on the normality of distribution.
Qualitative data were characterised as frequency and percentage.
Comparisons between groups were performed with Man-Whitney or
chi-square test. The agreement between several variables (location
and histology of melanoma) was established with Cohen's kappa
coefficient. A P-value <0.05 was considered statistically
significant.
Results
During the 16 years of the study period, 699
patients developed 732 melanomas. Out of the study population, 318
(45.4%) were men, and 381 (54.5%) were women. Of all patients, 673
(96.28%) developed only one primary melanoma, whereas 26 (3.71%),
11 men and 15 women, developed multiple tumours. The median age of
the patients having multiple primaries at the diagnosis of the
first melanoma was 55.34 (25-75th percentile: 40.37-64.29) and the
mean 52.47 years (SD 14.129). There were no statistically
significant differences regarding gender and age between patients
with one or multiple primary melanomas.
The 26 patients with multiple primaries developed a
total of 59 melanomas (26 for men and 33 for women), corresponding
to a mean of 2.3 melanomas per patient. Most patients (n=21,
80.76%) developed only 2 melanomas; 3 patients (11.53%) developed 3
melanomas and 2 patients (7.69%) developed 4 melanomas.
Table I describes
the tumour characteristics of the first melanoma, compared with the
characteristics of subsequent melanomas.
 | Table ITumour characteristics of the first
and subsequent melanomas. |
Table I
Tumour characteristics of the first
and subsequent melanomas.
| Characteristics | First melanoma n
(%) | Subsequent melanoma n
(%) | P-value |
|---|
| Histological
subtype | | | 0.025 |
|
SSM | 16 (61.5) | 26 (78.8) | |
|
NM | 8 (30.8) | 1 (3.0) | |
|
LMM | - | 3 (9.1) | |
|
ALM | - | 1 (3.0) | |
|
Not
specified | 2 (7.7) | 2 (6.1) | |
| Breslow index | | | 0.001 |
|
In
situ | 2 (7.7) | 17 (51.5) | |
|
<1
mm | 3 (11.5) | 8 (24.2) | |
|
1-2 mm | 6 (23.1) | 4 (12.1) | |
|
2-4 mm | 10 (38.5) | 3 (9.1) | |
|
>4
mm | 3 (11.5) | 1 (3.0) | |
|
Not
specified | 2 (7.7) | - | |
| Ulceration | | | 0.012 |
|
No | 14 (53.8) | 27 (81.81) | |
|
Yes | 9 (34.6) | 3 (9.09) | |
|
Not
specified | 3 (11.5) | 3 (9.09) | |
| Lymph node
involvement | | | 0.008 |
|
No | 19 (73.1) | 31 (93.93) | |
|
Yes | 5 (19.2) | - | |
|
Not
specified | 2 (7.7) | 2 (6.07) | |
| Stage | | | 0.001 |
|
MIS | 2 (7.7) | 17 (51.5) | |
|
IA | 3 (11.5) | 10 (30.3) | |
|
IB | 4 (15.4) | 4 (12.1) | |
|
IIA | 3 (11.5) | - | |
|
IIB | 5 (19.2) | 2 (6.1) | |
|
IIC | 1 (3.8) | - | |
|
IIIA | 1 (3.8) | - | |
|
IIIB | - | - | |
|
IIIC | 3 (11.5) | - | |
|
IV | 2 (7.7) | - | |
|
Not
specified | 2 (7.7) | - | |
| Total | 26(100) | 33(100) | |
We studied the concordance between the site of the
first and second melanoma, and we found a fair concordance
(P=0.007) (Table II). In 14 of the
second melanomas (53.84%) the site coincided with the first
melanoma, a fact that occurred more frequently on melanomas
occurring on the head and neck (80%), followed by those on the
trunk (61.5%).
 | Table IIConcordance between the site of the
first and second melanoma. |
Table II
Concordance between the site of the
first and second melanoma.
| | Second
melanoma |
|---|
| First melanoma | Head/neck (%) | Trunk (%) | Upper limbs
(%) | Lower limbs
(%) | Total (%) |
|---|
| Head/neck | 4(80) | 1 (7.7) | 0 (0) | 0 (0) | 5 (19.2) |
| Trunk | 1(20) | 8 (61.5) | 1(25) | 2(50) | 12 (46.2) |
| Upper limbs | 0 (0) | 2 (15.4) | 1(25) | 1(25) | 4 (15.4) |
| Lower limbs | 0 (0) | 2 (15.4) | 2(50) | 1(25) | 5 (19.2) |
| Total | 5(100) | 13(100) | 4(100) | 4(100) | 26(100) |
Regarding the histological subtype, the superficial
spreading melanoma was the most frequent type in both first and
subsequent melanomas, but nodular melanoma was more frequently seen
as the first primary. We studied the concordance between the
histological subtype of the first and second melanoma, but we found
no concordance (P=0.087) (Table
III).
 | Table IIIConcordance between the histological
subtype of the first and second melanoma. |
Table III
Concordance between the histological
subtype of the first and second melanoma.
| | Second
melanoma |
|---|
| First melanoma | SSM (%) | NM (%) | LMM (%) | ALM (%) | Not specified
(%) | Total (%) |
|---|
| SSM | 15 (68.2) | 1(100) | 0 (0) | 0 (0) | 0 (0) | 16 (61.5) |
| NM | 6 (27.3) | 0 (0) | 1(100) | 1(100) | 0 (0) | 8 (30.8) |
| LMM | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| ALM | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Not specified | 1 (4.5) | 0 (0) | 0 (0) | 0 (0) | 1(100) | 2 (7.7) |
| Total | 22(100) | 1(100) | 1(100) | 1(100) | 1(100) | 26(100) |
Analysis of the distribution of multiple primary
melanomas with respect to their Breslow index, showed that a higher
proportion of subsequent melanomas were in situ (51.5%) or
thin melanomas (<1 mm, 24.2%) compared with first melanomas
(7.7%, respectively 11.5%), the difference being statistically
significant (P<0.05). Except for two patients, in whom the
Breslow index for the first tumour was not specified, all the
others had proper registration of the tumour thickness both for the
first and subsequent melanomas. Twelve out of the last patients had
invasive melanoma in both the first and second melanoma; ten
(83.33%) patients developed thinner second melanoma, while thicker
second melanoma was documented in two (16.66%) patients. One of the
patients, who had in situ melanoma as the first primary,
developed an invasive second melanoma. All the patients with more
than two melanomas developed thinner subsequent melanomas.
Ulceration and lymph node involvement were more
common in the first primary tumour. Regarding stage at diagnosis,
most of the subsequent melanomas were in situ or stage I,
while first primary melanomas had a higher probability of being
stage II or more advanced.
The median time to diagnosis was 2.75 months
(25-75th percentile: 0-30.99), while the mean was 28.09 months (SD
58.511). From the subsequent melanomas, 13 (45.45%) developed as
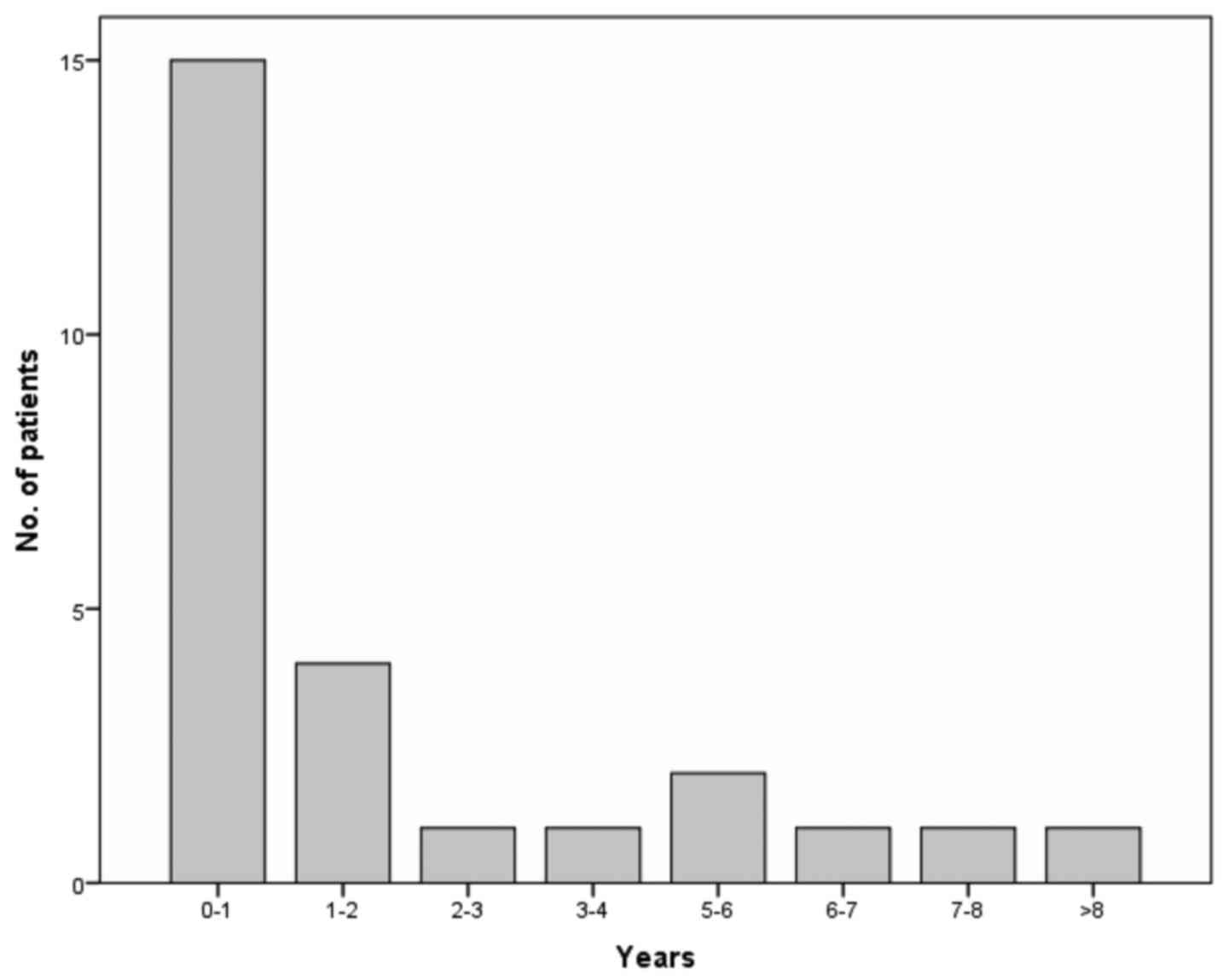
synchronous tumours. Out of the 15 (57.69%) patients with multiple
lesions, four (15.38%) had a subsequent melanoma within the first
and second year. Still, five patients (19.23%) were diagnosed with
a subsequent melanoma after >5 years of follow-up (Fig. 1).
Discussion
The present study was conducted to document multiple
primary melanomas in a large medical university centre from Romania
(Cluj-Napoca), in the period from 2004 to 2020. The study focused
on the number of lesions detected, tumour and patient
characteristics, as well as time to subsequent diagnosis, being the
first study performed in this country.
In our study comprising a series of 699 patients
with melanoma, 3.71% developed a second melanoma during the
follow-up, an incidence in the range reported in previous studies
and similar with the one reported by Pastor-Tomás et al in
Valencia, Spain (6). However, the
literature presents an incidence which varies from 0.2 to 8%,
variability caused by the lack of homogeneity in the studies, but
also by differences in ultraviolet radiation across geographical
regions (6-16).
Moreover, the above-reported incidence may underestimate the
lifetime incidence due to limited data capture and follow-up
periods (7).
Most patients in our study developed only two
primary melanomas, but two patients developed four primary
melanomas during follow-up. In the literature, we found a reported
case of as many as 48 melanomas in one patient (17).
In 53.84% of the cases, the site of the first and
second melanoma was the same, the correlation being fair. The
head/neck, followed by the trunk, was the site with the highest
correlation. In contrast, other studies failed to show a
correlation between the first and second site (6,18-21).
These findings emphasise the importance of full skin examination in
melanoma patients, not only at the first visit but also at
follow-ups. However, close surveillance of the same body region as
the initial primary melanoma in the follow-up is equally important
(6-8).
In our study, SSM and NM were, in decreasing order,
the most common histopathological subtypes, in line with the
results of other studies (8). While
SSM was the most frequent type both in first and subsequent
melanomas, NM was predominantly seen as the first melanoma. We did
not find a correlation between the histological subtype of the
first and second melanoma, SSM being the variant with the highest
agreement.
A significant finding, reported in most previous
studies, is the reduction in tumour thickness for subsequent
melanomas (6-8,21-23).
In our study, we observed a proportion of thin melanomas (<1 mm)
of 19.2, 69.3, 100 and 100% for the first, second, third and fourth
subsequent melanomas, respectively. This situation may be explained
by early detection due to medical surveillance and
self-examination. The early detection hypothesis is supported by
studies that demonstrated that patients undergoing rigorous
controls and adherent to regular follow-up had significantly
thinner subsequent primary melanomas than those who did not
(6,7,24).
Another possible explanation for the reduction of tumour thickness
would be the potential biological difference between the first and
second melanoma (6). Multiple
studies have suggested that melanoma is in fact, not a single
entity but a group of different neoplasms with variable
etiopathogenesis, biologic behaviour and prognosis (25-30).
This hypothesis needs to be tested in further studies in patients
with multiple melanomas.
Among patients with multiple primary melanomas,
synchronous lesions were reported in 26-40% of the cases, while the
remainder develops as asynchronous tumours (7). In our study, 45.45% of the lesions
presented as synchronous lesions, underscored the importance of
total body examination during the first visit in melanoma patients.
Previous studies have shown that the risk of a subsequent primary
melanoma is highest in the first year of the incident primary
melanoma and remains higher in the first five years (6-8).
However, cases reported up to 2 to 3 decades after the first
melanoma and some studies indicate that the risk remains stable in
time (6). In our study, almost 20%
of the patients developed a second melanoma after more than five
years of follow-up. Moreover, a patient who presented with a very
thin first melanoma (BI=0.25 mm), developed two synchronous in
situ melanomas after seven years of follow-up. All these data
reveal the importance of lifetime clinical follow-up in melanoma
patients, although some guidelines support a limited follow-up of
one year for stage IA melanoma (31).
The main strength of our study is the homogeneous
information collection in a single institution for a long period.
The main limitation consists in the relatively low number of cases,
but this can be explained by the fact that data was collected in a
single centre.
In conclusion, almost 4% of melanoma patients in our
centre developed subsequent melanomas during 2004 and 2020. Most
patients developed only two primary melanomas, and we could not
find a strong correlation between the sites and the
histopathological subtypes of the first and subsequent melanoma.
The most important findings of our study are the reduction in
tumour thickness in subsequent melanomas as well as the possibility
of diagnosing a subsequent melanoma more than five years after the
first diagnosis. However, the risk is highest in the first year.
Our findings highlight the need for lifetime clinical follow-up and
self-examination in melanoma patients regardless of the first
melanoma stage.
Acknowledgements
Not applicable.
Funding
The present study was partially supported by the
grant PN-III-P1-1.2-PCCDI-2017-0341.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LU and SS contributed to the research-creation and
design of the study, data acquisition, analysis and interpretation
of data, statistical analysis, manuscript drafting, and critical
revision of the manuscript for valuable intellectual content. IZ
contributed to the data acquisition, analysis and interpretation of
data, statistical analysis, manuscript drafting, and critical
review of the manuscript for valuable intellectual content. IC and
AV contributed to to the research-creation and design of the study,
data acquisition, and critical revision of the manuscript for
valuable intellectual content. SV performed the analysis and
interpretation of data, and the critical review of the manuscript
for valuable intellectual content. RC contributed to the
research-creation and design, and critical revision of the
manuscript for valuable intellectual content. All authors read and
approved the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the ‘Iuliu Hatieganu’ University of Medicine
and Pharmacy (Cluj-Napoca, Romania). All the participants gave
their consent to be included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Godar DE: Worldwide increasing incidence
of cutaneous malignant melanoma. J Skin Cancer.
2011(858425)2011.
|
|
3
|
Tripp MK, Watson M, Balk SJ, Swetter SM
and Gershenwald JE: State of the science on prevention and
screening to reduce melanoma incidence and mortality: The time is
now. CA Cancer J Clin. 66:460–480. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ribas A, Hamid O, Daud A, Hodi FS, Wolchok
JD, Kefford R, Joshua AM, Patnaik A, Hwu WJ, Weber JS, et al:
Association of pembrolizumab with tumor response and survival among
patients with advanced melanoma. JAMA. 315:1600–1609.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Long GV, Stroyakovskiy D, Gogas H,
Levchenko E, de Braud F, Larkin J, Garbe C, Jouary T, Hauschild A,
Grob JJ, et al: Dabrafenib and trametinib versus dabrafenib and
placebo for Val600 BRAF-mutant melanoma: A multicentre,
double-blind, phase 3 randomised controlled trial. Lancet.
386:444–451. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pastor-Tomás N, Martínez-Franco A, Bañuls
J, Peñalver JC, Traves V, García-Casado Z, Requena C, Kumar R and
Nagore E: Risk factors for the development of a second melanoma in
patients with cutaneous melanoma. J Eur Acad Dermatol Venereol: Mar
12, 2020 (Online ahead of print).
|
|
7
|
Adler NR, Kelly JW, Haydon A, McLean CA
and Mar VJ: Clinicopathological characteristics and prognosis of
patients with multiple primary melanomas. Br J Dermatol.
178:e44–e45. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Claeson M, Holmström P, Hallberg S,
Gillstedt M, Gonzalez H, Wennberg AM and Paoli J: Multiple primary
melanomas: A common occurrence in Western Sweden. Acta Derm
Venereol. 97:715–719. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
van der Leest RJT, Flohil SC, Arends LR,
de Vries E and Nijsten T: Risk of subsequent cutaneous malignancy
in patients with prior melanoma: A systematic review and
meta-analysis. J Eur Acad Dermatology Venereol. 29:1053–1062.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Caini S, Boniol M, Botteri E, Tosti G,
Bazolli B, Russell-Edu W, Giusti F, Testori A and Gandini S: The
risk of developing a second primary cancer in melanoma patients: A
comprehensive review of the literature and meta-analysis. J
Dermatol Sci. 75:3–9. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bradford PT, Freedman DM, Goldstein AM and
Tucker MA: Increased risk of second primary cancers after a
diagnosis of melanoma. Arch Dermatol. 146:265–272. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Spanogle JP, Clarke CA, Aroner S and
Swetter SM: Risk of second primary malignancies following cutaneous
melanoma diagnosis: A population-based study. J Am Acad Dermatol.
62:757–767. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Crocetti E, Guzzinati S, Paci E, Falcini
F, Zanetti R, Vercelli M, Rashid I, De Lisi V, Russo A, Vitarelli
S, et al: The risk of developing a second, different, cancer among
14560 survivors of malignant cutaneous melanoma: A study by AIRTUM
(the Italian Network of Cancer Registries). Melanoma Res.
18:230–234. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bhatia S, Estrada-Batres L, Maryon T,
Bogue M and Chu D: Second primary tumors in patients with cutaneous
malignant melanoma. Cancer. 86:2014–2020. 1999.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Espinosa P, Pfeiffer RM, García-Casado Z,
Requena C, Landi MT, Kumar R and Nagore E: Risk factors for
keratinocyte skin cancer in patients diagnosed with melanoma, a
large retrospective study. Eur J Cancer. 53:115–124.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Moore MM, Geller AC, Warton EM, Schwalbe J
and Asgari MM: Multiple primary melanomas among 16,570 patients
with melanoma diagnosed at Kaiser Permanente Northern California,
1996 to 2011. J Am Acad Dermatol. 73:630–636. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Slingluff CL Jr, Vollmer RT and Seigler
HF: Multiple primary melanoma: Incidence and risk factors in 283
patients. Surgery. 113:330–339. 1993.PubMed/NCBI
|
|
18
|
Stam-Posthuma JJ, van Duinen C, Scheffer
E, Vink J and Bergman W: Multiple primary melanomas. J Am Acad
Dermatol. 44:22–27. 2011.
|
|
19
|
Titus-Ernstoff L, Perry AE, Spencer SK,
Gibson J, Ding J, Cole B and Ernstoff MS: Multiple primary
melanoma: Two-year results from a population-based study. Arch
Dermatol. 142:433–438. 2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Siskind V, Hughes MC, Palmer JM, Symmons
JM, Aitken JF, Martin NG, Hayward NK and Whiteman DC: Nevi, family
history, and fair skin increase the risk of second primary
melanoma. J Invest Dermatol. 131:461–467. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Johnson TM, Hamilton T and Lowe L:
Multiple primary melanomas. J Am Acad Dermatol. 39:422–427.
1998.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Vecchiato A, Pasquali S, Menin C, Montesco
MC, Alaibac M, Mocellin S, Campana LG, Nitti D and Rossi CR:
Histopathological characteristics of subsequent melanomas in
patients with multiple primary melanomas. J Eur Acad Dermatol
Venereol. 28:58–64. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
DiFronzo LA, Wanek LA and Morton DL:
Earlier diagnosis of second primary melanoma confirms the benefits
of patient education and routine postoperative follow-up. Cancer.
91:1520–1524. 2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
De Giorgi V, Rossari S, Papi F, Gori A,
Alfaioli B, Grazzini M, Crocetti E, Verdelli A, Foo CW and Lotti T:
Multiple primary melanoma: The impact of atypical naevi and
follow-up. Br J Dermatol. 163:1319–1322. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ilie MA, Caruntu C, Lupu M, Lixandru D,
Tampa M, Georgescu SR, Bastian A, Constantin C, Neagu M, Zurac SA
and Boda D: Current and future applications of confocal laser
scanning microscopy imaging in skin oncology. Oncol Lett.
17:4102–4111. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ancuceanu R, Dinu M, Neaga I, Laszlo FG
and Boda D: Development of QSAR machine learning-based models to
forecast the effect of substances on malignant melanoma cells.
Oncol Lett. 17:4188–4196. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Caruntu C, Boda D, Constantin C, Caruntu A
and Neagu M: Catecholamines increase in vitro proliferation of
murine B16F10 melanoma cells. Acta Endocrinol. 10:545–558.
2014.
|
|
28
|
Boda D: Cellomics as integrative omics for
cancer. Curr Proteomics. 10:237–245. 2013.
|
|
29
|
Stefan O, Tudor G, Constantinescu C, Luca
C, Boda D, Caruntu C, Cioplea M, Nichita L and Zurac SA: E-cadherin
and N-cadherin expression pattern in common melanocytic nevi.
Virchows Archiv. 475 (Suppl)(S28)2019.
|
|
30
|
Zurac S, Neagu M, Constantin C, Cioplea M,
Nedelcu R, Bastian A, Popp C, Nichita L, Andrei R, Tebeica T, et
al: Variations in the expression of TIMP1, TIMP2 and TIMP3 in
cutaneous melanoma with regression and their possible function as
prognostic predictors. Oncol Lett. 11:3354–3360. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Garbe C, Amaral T, Peris K, Hauschild A,
Arenberger P, Bastholt L, Bataille V, Del Marmol V, Dréno B,
Fargnoli MC, et al: European consensus-based interdisciplinary
guideline for melanoma. Part 1: Diagnostics-update 2019. Eur J
Cancer. 126:141–158. 2020.PubMed/NCBI View Article : Google Scholar
|