Introduction
Colorectal cancer (CRC) is one of the most common
types of malignant tumor, with an increasing rate of incidence
worldwide (1). According to cancer
statistics for China, the incidence and mortality rates of CRC are
third and fifth, respectively, among all types of cancer, which
indicates that CRC is a major cause of cancer-associated deaths in
China (2). At present, surgical
resection followed by oxaliplatin-based chemotherapy is among the
most frequently used therapeutic strategies (3,4).
However, the long-term administration of oxaliplatin is associated
with drug resistance, which has been a major barrier to the
efficacy of CRC treatment (5,6). Thus,
improving chemosensitivity to oxaliplatin is an urgent requirement
for successful CRC therapeutic intervention.
Chemoresistance frequently compromises the function
of antitumor drug transporters (7,8).
Studies have revealed that the level of the transporter organic
cation transporter member 2 (OCT2) is critical for determining the
uptake of platinum compounds, particularly oxaliplatin (9,10).
OCT2 is N-glycosylated, and although previous studies have
demonstrated that N-linked glycosylation is important for the
transport function of OCT2(11),
systemic and complete information is not yet available on the
association of N-glycans with chemosensitivity.
The enzyme N-acetylglucosaminyltransferase V
(GnT-V), encoded by the gene MGAT5 (12), catalyzes the formation of β-1,6
branched complex N-glycans (13).
Previous studies have shown that aberrant N-glycans expressed by
tumor cells are associated with chemotherapeutic drug sensitivity
(14,15). Despite research advances regarding
the effects of glycosylation on chemosensitivity, the role of GnT-V
in CRC development and chemoresistance remains poorly
understood.
Our previous study demonstrated that GnT-V promotes
the chemosensitivity of bladder cancer cells to gemcitabine
(16); however, the mechanism
underlying the effects of β-1,6-N-glycosylation on chemosensitivity
was not defined. In the present study, in order to further
investigate the potential effects of GnT-V on chemosensitivity in
CRC cells, shRNA-mediated GnT-V silence was accomplished in CW-2
and CW-2/R (oxaliplatin-resistant) cells, through which the
alteration of oxaliplatin cytotoxicity was systematically explored.
Furthermore, N-glycan branches, distribution and transport activity
of OCT2 were observed to identify the target substrate of GnT-V.
The present results are expected to provide insights into the
molecular mechanisms underlying the oxaliplatin chemosensitivity in
CRC.
Materials and methods
Reagents and antibodies
Oxaliplatin and cimetidine were purchased from
Sigma-Aldrich (Merck KGaA). Zosuquidar was obtained from
MedChemExpress. DMSO and DAPI were purchased from Beyotime
Institute of Biotechnology. Rabbit anti-OCT2 monoclonal antibody
(cat. no. ab179808), mouse anti-MGAT5 (GnT-V) monoclonal antibody
(cat. no. ab87977), rabbit anti-β-actin polyclonal antibody (cat.
no. ab119716) and goat polyclonal secondary antibody to rabbit IgG
(Alexa Fluor® 555; cat. no. ab150078) were purchased
from Abcam. Goat anti-rabbit IgG horseradish peroxidase
(HRP)-conjugated (cat. no. 7074) and goat anti-mouse IgG
HRP-conjugated (cat. no. 7076) antibodies were obtained from Cell
Signaling Technology, Inc.
Cell culture
Human CRC cell lines were purchased from The Cell
Bank of Type Culture Collection of the Chinese Academy of Sciences.
CW-2 cells were cultured in RPMI-1640 medium (Thermo Fisher
Scientific, Inc.), and HT29 and HCT 116 cells were cultured in
McCoy's 5A medium (Biological Industries). Each growth medium was
supplemented with 10% fetal bovine serum (Beijing Solarbio Science
& Technology Co., Ltd.) and 1% penicillin/streptomycin, and all
cells were grown at 37˚C with 5% CO2. The
oxaliplatin-resistant CW-2 (CW-2/R) and HT29 (HT29/R) cell lines
were developed by exposure to increasing concentrations of
oxaliplatin, as previously described (17).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from cultured cells using
RNAiso Plus (Takara Biotechnology Co., Ltd.) according to the
manufacturer's protocol. Then, 1 µg total RNA was used for reverse
transcription with HiScript II Q RT SuperMix (Vazyme Biotech Co.,
Ltd.) according to the manufacturer's protocol. The ChamQ Universal
SYBR® qPCR Master Mix (Vazyme Biotech Co., Ltd.) was
used for qPCR. Primer sequences were as follows: MGAT5,
5'-ATCATGCAAATTATGCCCAATC-3' (forward) and
5'-GGTGCTGCTCAACCACAAAC-3' (reverse); GAPDH,
5'-CATGAGAAGTATGACAACAGCCT-3' (forward) and
5'-AGTCCTTCCACGATACCAAAGT-3' (reverse). PCR conditions were: 10 min
at 95˚C, followed by 40 cycles of 10 sec at 95˚C and 30 sec at
60˚C. Fold change was calculated by determining the ratio of mRNA
levels to control values using the Δ threshold cycle (Cq) method
(2−ΔΔCq) (18).
Western blot and lectin blot
analysis
Total protein was isolated from cultured cells with
a Qproteome Mammalia Protein Prep Kit (Qiagen China Co., Ltd.)
according to the manufacturer's protocol. Protein concentration was
measured using BCA Protein Assay reagent (Beyotime Institute of
Biotechnology). Then, 10-30 µg protein/lane was separated using 10%
SDS-PAGE and blotted onto nitrocellulose membranes. The blots were
then blocked for 2 h at room temperature with 5% non-fat dried
milk. The membranes were then incubated with anti-OCT2 (dilution
1:1,000), anti-GnT-V (dilution 1:200) and anti-β-actin antibodies
overnight at 4˚C. For lectin blot assay, blocked membranes were
incubated with biotinylated phytohemagglutin-L (PHA-L) lectin
(dilution 1:400; Vector Laboratories, Inc.) for 1 h at room
temperature. Subsequent to washing three times (15 min per wash)
with Tris-buffered saline and 0.05% Tween-20, the blots were
incubated with HRP-conjugated secondary antibodies (1:5,000) for 2
h at room temperature. Results were visualized using an ECL Kit
(Cytiva) and the signal intensity was measured using a VersaDoc™
Imaging system (version 4.0; Bio-Rad Laboratories, Inc.).
Chemosensitivity assay
To evaluate chemosensitivity to oxaliplatin, cell
death and cell viability assays were performed following protocols
previously described (16). In
brief, 3,000 to 4,000 cells per well were seeded in 96-well plates
and treated with oxaliplatin (ranging from 0.375-24 µg/ml). For the
functional detection of OCT2 and P-gp, cells were incubated at 37˚C
in the medium with cimetidine (100 µM) and zosuquidar (5 µM) for 1
h before exposure to oxaliplatin (0.2 µg/ml for CW-2 group and 2
µg/ml for CW-2/R group). Plates were incubated for 72 h at 37˚C.
The percentage of dead cells was measured using the trypan blue
method and cell viability was assessed using a Cell Counting Kit-8
(CCK-8; Beyotime Institute of Biotechnology).
Stable transfection and cell line
selection
Short hairpin RNAs (shRNAs) for the knockdown of
GnT-V (shRNA#1 and 2) were designed and inserted into the
pGPU6/GFP/Neo vector by Suzhou GenePharma Co., Ltd. Non-targeting
shRNA sequences were used as a negative control (NC). For
transfection, cells (5x105 cells/well) were seeded into
6-well culture plates at 60-70% confluence. After 24 h, vectors
(1.2 µg) were mixed with 4.5 µl Attractene Transfection Reagent
(Qiagen China Co., Ltd.) in 100 µl serum-free RPMI-1640 medium at
room temperature for 15 min. Then, the transfection complexes were
added to each well with fresh medium (containing serum and
antibiotics) and incubated at 37˚C with 5% CO2 for 48 h.
Polyclonal stable cell lines were isolated following
fluorescence-activated cell sorting using a FACSCalibur instrument
(BD Biosciences).
Colony formation assay
To analyze the sensitivity of cells to oxaliplatin,
a colony formation assay was performed as described previously
(19). Cells were plated at 50%
confluence (1.0x106 cells in a 10-cm dish) and treated
with oxaliplatin (0.2 µg/ml for CW-2 group and 2 µg/ml for CW-2/R
group) for 12, 24 and 48 h. Cells were then trypsinized and diluted
in 6-well plates (1,000 cells/well). After plating, cells were
grown in oxaliplatin-free medium for 2 weeks. Colonies were stained
with 1% crystal violet at room temperature for 30 min. Photographic
images of the dishes were captured.
Lectin precipitation
Cells were harvested in RIPA lysis buffer (Beyotime
Institute of Biotechnology), containing protein inhibitor (1 mM
PMSF, 10 mg/ml leupeptin, 10 mg/ml aprotinin; Beyotime Institute of
Biotechnology). The total cell lysates were centrifuged at 12,000 x
g for 15 min at 4˚C. In total, 300 µg cell lysates were incubated
with 30 µl PHA-L conjugated agarose beads (Vector Laboratories,
Inc.) at 4˚C overnight. The resulting β-1,6-glycosylated
protein-lectin-agarose complexes were collected by centrifugation
(13,000 x g for 30 sec at 4˚C) and then washed with lysis buffer.
Next, the glycoprotein/lectin conjugates were resolved by 10%
SDS-PAGE and immunoblotted for OCT2 by the aforementioned western
blot analysis method.
Immunofluorescence staining
Cells (1x105) were seeded in 6-well
dishes and cultured for 48 h. Cells were then washed twice with PBS
and fixed with acetone for 10 min at room temperature. Next, the
cells were blocked with 3% BSA-PBS (Beyotime Institute of
Biotechnology) for 1 h at room temperature following
permeabilization with 0.1% Triton X-100 for 5 min. Cells were then
incubated with OCT2 (1:200) antibody for 2 h at room temperature,
followed by Goat Anti-Rabbit (Alexa Fluor® 647)
secondary antibody (1:200; Abcam; cat. no. ab150079) incubation for
1 h at room temperature. DAPI was used to stain the cell nuclei at
room temperature for 3 min. Fluorescent signals were detected using
a fluorescence confocal microscope (Olympus Corporation;
magnification, x400).
Drug transportation analysis by liquid
chromatography-tandem mass spectrometry (LC-MS/MS)
Cells and cultured medium from each cell group (CW-2
and CW-2/R cells transfected with shRNA#2 or NC) were harvested
after exposure to 1 µM oxaliplatin for 0.5, 1 and 1.5 h at 37˚C.
The cells were washed twice with PBS and then crushed in 120 µl ice
ddH2O by an ultrasonic crusher for 10 sec. 120 µl methyl
alcohol was added to the cell lysates. Supernatant was extracted
after the lysates were centrifuged at 15,000 x g at 4˚C for 15 min.
Then, 10 µl each sample was injected into an LC-MS/MS system
(Agilent HP1200; Agilent Technologies, Inc.). The chromatographic
separation was performed on a Hypersil BDS-C18 column 150 mm x 4.6
i.d., 5 µm (Dalian Elite Analytical Instruments Co., Ltd.) at room
temperature. The mobile phase consisted of acetonitrile and water
with 0.1% (v/v) formic acid (60:40, v/v) for bestatin, methyl
alcohol and water with 0.1% formic acid (70:30, v/v) for digoxin at
a flow rate of 0.5 ml/min. The ionization was conducted using a
TurboIonspray interface in positive ion mode for bestatin and in
negative ion mode for digoxin. Multiple reactions monitoring mode
was utilized to detect the compound of interest. The selected
transitions of m/z were m/z 779.0 to 649.0 for digoxin, m/z 309.1
to 120.3 for bestatin and m/z 370.4 to 288.1 for cilostazol. The
intracellular oxaliplatin concentration was measured in each sample
according to a standard protocol (20).
Statistical analysis
Data were analyzed using SPSS software 16.0 (SPSS,
Inc.). Results are expressed as the mean ± SEM. Each experiment was
repeated at least three times and analyzed by either a Student's
t-test or one-way ANOVA followed by Tukey's test. P<0.05 was
considered to indicate a statistically significant difference.
Results
High GnT-V is associated with a good
response to oxaliplatin in CRC cells
To begin the exploration of the oxaliplatin
sensitivity of CRC cells, the GnT-V expression levels of three
different human CRC cell lines were determined using RT-qPCR
(Fig. 1A) and western blotting
(Fig. 1B). Among the three CRC cell
lines, CW-2 cells exhibited the highest expression of GnT-V and the
highest cytotoxic response to oxaliplatin (Fig. 1C). We hypothesized that if GnT-V
activity is important for the chemosensitivity of CRC cells,
alteration of endogenous GnT-V expression levels should occur when
cells are treated with oxaliplatin. To investigate this hypothesis,
cells were exposed to 1.5 µM oxaliplatin for 24 or 48 h. As shown
in Fig. 1D, this short-term
oxaliplatin treatment did not lead to changes in the GnT-V level in
either CW-2 or HT29 cells. Furthermore, two stable
oxaliplatin-resistant cell lines (CW-2/R and HT29/R) were generated
by exposure to continuous oxaliplatin treatment. These stable
resistant cell lines were then subjected to RT-qPCR to quantify
MGAT5 gene expression. As shown in Fig. 1E, a marked and significant increase
of MGAT5 expression was observed in oxaliplatin-resistant
cells as compared with the respective wild-type CW-2 and HT29
cells. Consistent with this increased MGAT5 expression,
increased GnT-V protein levels and robust enrichment of
β-1,6-oligosaccharide levels were observed in the resistant cell
lines by western blotting and lectin blotting, respectively
(Fig. 1F). These results suggest
that GnT-V affects the chemosensitivity of CRC cells to
oxaliplatin.
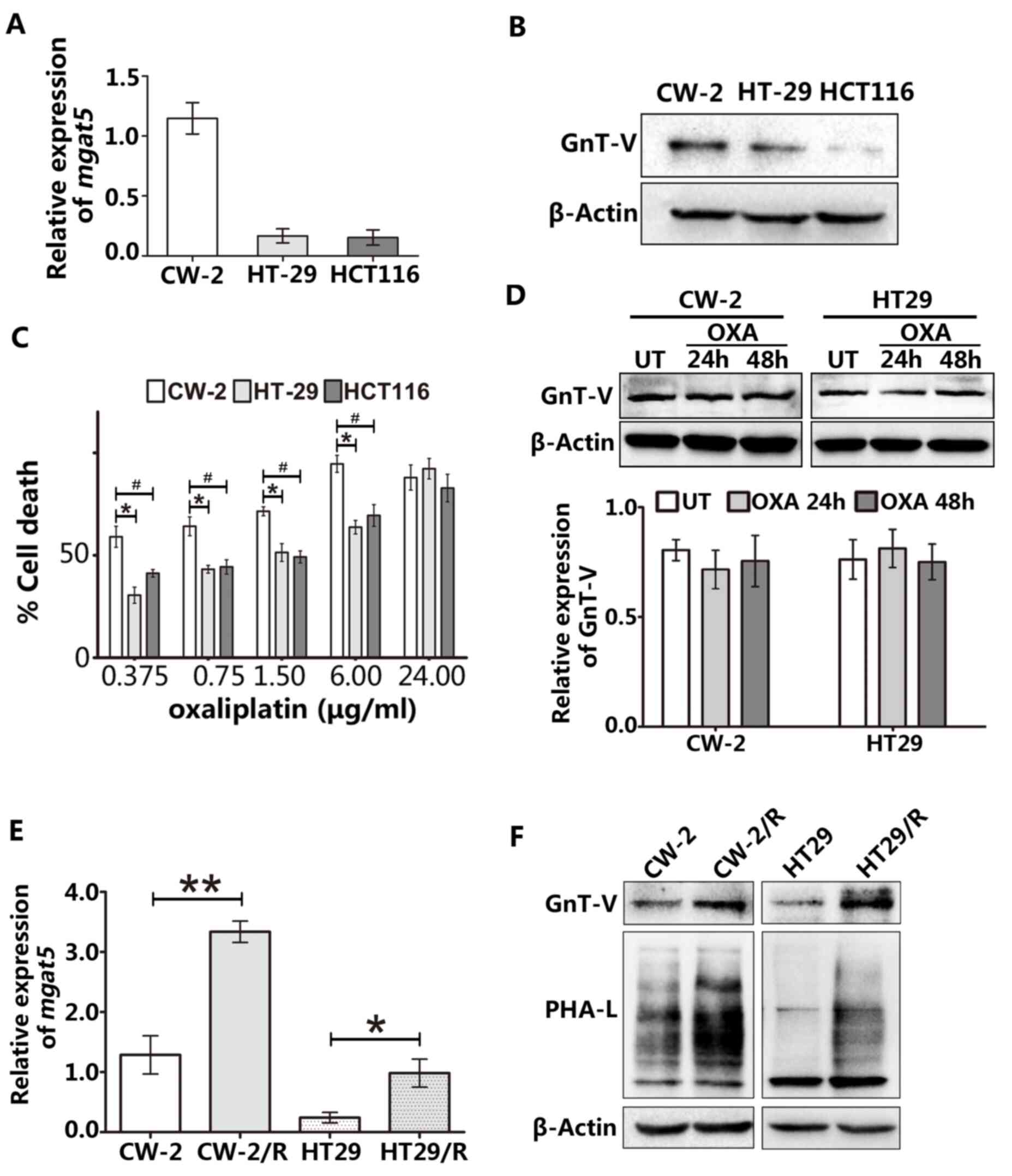 | Figure 1GnT-V expression is associated with
oxaliplatin chemosensitivity in CRC cells. (A) The mRNA expression
of MGAT5 was examined by reverse transcription-quantitative
PCR. (B) Protein levels of GnT-V in CRC cell lines were measured by
western blotting. (C) Cells were treated with the indicated
concentrations of oxaliplatin for 48 h, and oxaliplatin sensitivity
was determined based on cell death using the trypan blue method.
*P<0.05 vs. HT29 group and #P<0.05 vs.
HCT116 group. (D) Acute treatment with 1.5 µM oxaliplatin for 24 or
48 h did not lead to changes in GnT-V expression levels in CW-2 and
HT29 cells. (E) CW-2 and HT29 cell lines were exposed to long-term
oxaliplatin treatment to obtain stably resistant lines, named
CW-2/R and HT29/R, respectively. Endogenous MGAT5 expression
was increased in the stably resistant cell lines as compared with
the parental cells at the mRNA level. (F) GnT-V expression and
β-1,6-oligosaccharide branches were detected by western blot and
lectin blot analyses, respectively, in wild-type and
oxaliplatin-resistant cell lines. The graphs depict results from
three independent experiments each performed in triplicate. Results
are presented as the mean ± SEM. *P<0.05,
#P<0.05 and **P<0.01. GnT-V,
N-acetylglucosaminyltransferase V; CRC, colorectal cancer; MGAT5,
the gene encoding GnT-V; OXA, oxaliplatin; UT, untreated; PHA-L,
phytohemagglutin-L. |
Downregulation of GnT-V reduces the
sensitivity of CW-2 cells to oxaliplatin
GnT-V expression was stably knocked down in the CW-2
and oxaliplatin-resistant CW-2/R cell lines using shRNA. Successful
knockdown was verified by western blotting, which showed decreased
GnT-V levels in the cells transfected with shRNA#1 and 2 as
compared with the wild-type and negative control (NC) cells.
Correspondingly, reduced GnT-V activity in the shRNA#1 and 2 cells
compared with the NC cells was confirmed by oligosaccharide
analyses in cells stained with PHA-L, a lectin that specifically
binds to surface β-1,6-N-acetylglucosamine (GlcNAc) branches
(Fig. 2A and B). To evaluate the role of GnT-V in
chemosensitivity, NC and shRNA#2 transfected cells were exposed to
different concentrations of oxaliplatin for 48 h, and then cell
viability was determined by CCK-8 assay. As shown in Fig. 2C, the knockdown of GnT-V increased
the viability of oxaliplatin-treated CW-2 cells, even at the
highest concentration of oxaliplatin (16 µg/ml) compared with the
NC group, and a similar result was observed in the CW-2/R group.
Moreover, a colony formation assay was performed (Fig. 2D and E). Notably, oxaliplatin-treated shRNA#2
transfected cells exhibited increased survival and clonogenic
potential compared with the NC controls. These results suggest that
GnT-V knockdown attenuates the chemosensitivity of cells to
oxaliplatin.
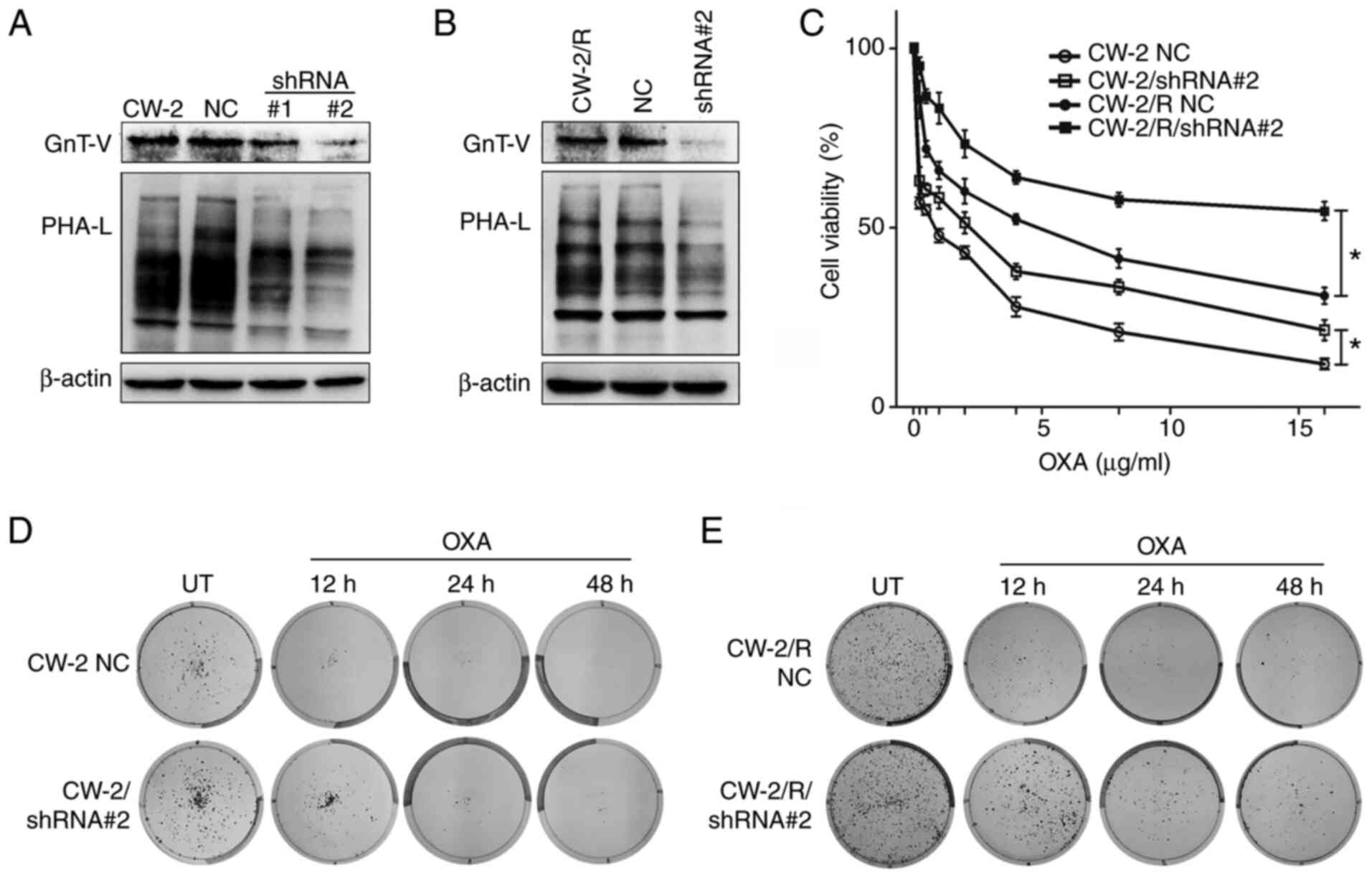 | Figure 2Cells with GnT-V knockdown exhibit
enhanced survival and cell viability upon exposure to oxaliplatin.
(A) and (B) shRNA mediated GnT-V knockdown and
β-1,6-oligosaccharide reduction in (A) CW-2 and (B) CW-2/R cells as
depicted by western blotting and lectin blotting, respectively,
compared with the respective parental cell lines and NC cells. (C)
Cells were exposed to indicated concentrations of oxaliplatin
(0.25-16 µg/ml) for 48 h, and cell viabilities were determined by
Cell Counting Kit-8 assay. Representative images of (D) wild-type
and (E) drug-resistant cells showing that oxaliplatin-treated GnT-V
knockdown cells had reduced chemosensitivity, resulting in an
increased colony-forming potential compared with NC cells. Results
are presented as the means ± SEM from three independent
experiments. *P<0.05. GnT-V,
N-acetylglucosaminyltransferase V; shRNA, short hairpin RNA;
shRNA#1 and #2, shRNAs for knockdown of GnT-V; NC, negative
control; OXA, oxaliplatin; PHA-L, phytohemagglutin-L; UT,
untreated. |
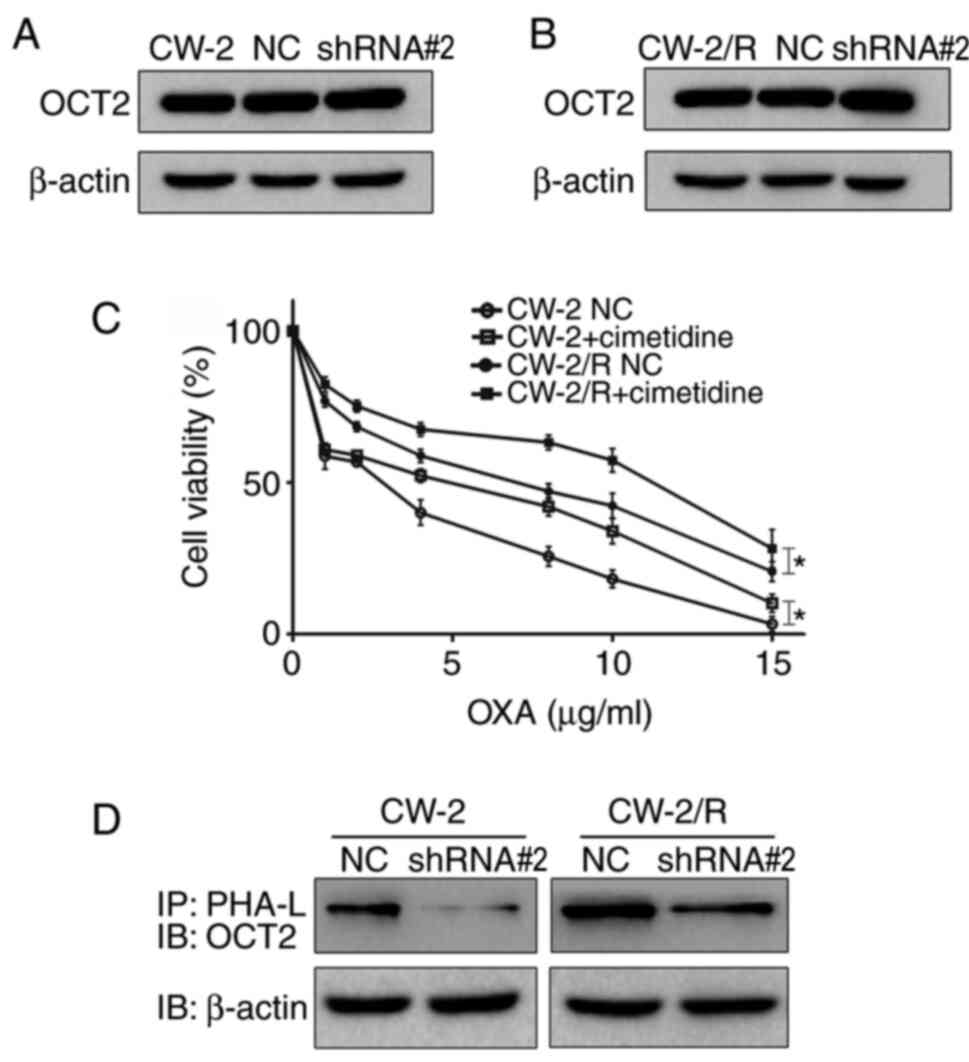
β-1,6-N-glycan branches on OCT2 may
affect cytotoxic response to oxaliplatin
OCT2 has been indicated to be an independent factor
that affects the success of oxaliplatin-based chemotherapy in CRC
(10). We hypothesized that the
N-glycans of OCT2 may play an important role in the
chemosensitivity of CRC cells to oxaliplatin. To investigate this
hypothesis, first, OCT2 expression levels were determined by
western blotting. As shown in Fig.
3A and B, the expression level
of OCT2 protein was almost unchanged in CW-2 and CW-2/R cells
following GnT-V knockdown. To confirm the role of OCT2 in
chemosensitivity to oxaliplatin, transfected cells were treated
with cimetidine, an inhibitor of OCT2 activity, before exposure to
oxaliplatin (21). Notably, the
pretreatment of CW-2 and CW-2/R cells with cimetidine significantly
increased cell viability, suggesting that the reduction of OCT2
activity reduced the cytotoxic effects of oxaliplatin (Fig. 3C). To further investigate the
association between GnT-V activity and OCT2 N-glycosylation, a
PHA-L precipitation experiment was performed. The results indicate
that the presence of β-1,6-N-glycan branches on OCT2 was decreased
in the GnT-V knockdown group compared with the NC group (Fig. 3D). This suggests that N-glycosylated
OCT2 may be a potential substrate of GnT-V and could play a part in
the modulation of the chemosensitivity of CRC cells to
oxaliplatin.
The possible involvement of another substrate,
P-glycoprotein (P-gp), a membrane glycoprotein known to regulate
drug sensitivity in cancer cells was also evaluated (22,23).
Cells were treated for 24 h with the P-gp inhibitor zosuquidar in
combination with oxaliplatin. However, in contrast to OCT2,
zosuquidar had no effect on oxaliplatin sensitivity (data not
shown).
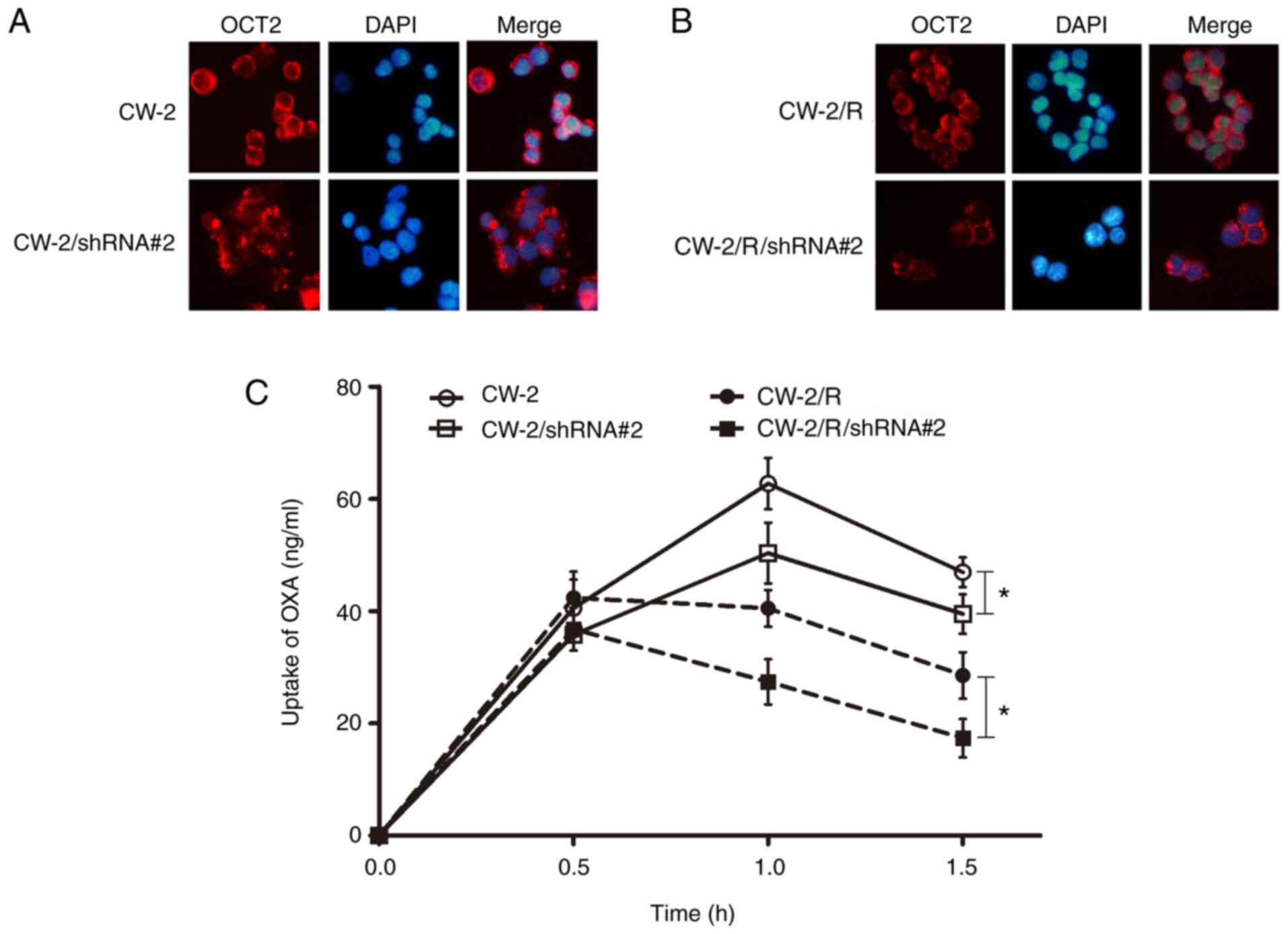
GnT-V knockdown changes the
distribution and function of OCT2
The abnormal N-glycosylation of transporters has
been shown to alter their localization and activity in cells
(24). Therefore, the intracellular
distribution of OCT2 in CRC cells was examined using confocal
immunofluorescence microscopy. A decreased localization of OCT2 was
observed in the cytomembrane of the CW-2 and CW-2/R/shRNA cells
with GnT-V knockdown compared with the respective NC groups
(Fig. 4A and B). Subsequently, the transport activity of
OCT2 was detected by LC-MS/MS. The uptake of oxaliplatin in the CRC
cells exhibited no significant difference between all groups at an
early stage (<0.5 h incubation). However, after another 1 h, the
concentration of oxaliplatin in the CW-2/R cells was lower than
that in the respective non-resistant CW-2 cells, and the
oxaliplatin concentration decreased significantly in the GnT-V
knockdown groups when compared with respective wild-type CW-2 and
CW-2/R groups (Fig. 4C), indicating
that OCT2 transport activity was reduced when GnT-V was knocked
down. Together, these data imply that GnT-V might affect
chemosensitivity to oxaliplatin via regulation of the
oligosaccharide branches on OCT2.
Discussion
Glycosylation participates in various biological
processes, including cell adhesion, signal transduction and
receptor activation (25-27).
Previous studies suggest that N-glycosylation serves an important
role in tumor chemosensitivity (28-30).
The present study identified GnT-V as a regulator of the
chemosensitivity of CRC cells. The results demonstrated that higher
endogenous GnT-V expression was positively associated with
increased sensitivity to oxaliplatin in CRC cells. Additionally,
oxaliplatin-resistant CRC cells exhibited elevated levels of GnT-V
expression relative to those in the parental oxaliplatin-naïve
cells, while short-term oxaliplatin treatment did not increase
GnT-V levels. Therefore, it is speculated that GnT-V might serve an
important role in the chemosensitivity of CRC cells.
CRC is a common malignant tumor of the digestive
tract, and its mortality rate is the second highest among
tumor-associated deaths worldwide (31). Oxaliplatin is one of the most
commonly used chemotherapeutics after surgical resection due to its
improved safety profile and lack of cross-resistance with cisplatin
(32). Unfortunately, intrinsic (or
de novo) and acquired oxaliplatin resistance are considered
to be major challenges in the treatment of CRC (33). Therefore, elucidating the underlying
mechanisms of the resistance and exploring biomolecules that can
reliably predict the response to oxaliplatin are clinical
priorities. The results of the present study demonstrated that the
downregulation of GnT-V reduced the sensitivity of CW-2 and
oxaliplatin-resistant CW-2/R cells to oxaliplatin.
Chemosensitivity to oxaliplatin depends, at least in
part, on the concentration of the drug inside cells. Therefore,
facilitating increases in intracellular oxaliplatin accumulation
through membrane transporters is an important mechanism for
enhancing the efficacy of oxaliplatin. An investigation into the
drug transporter expression of OCT2 was performed in the present
study. The results showed almost no change in OCT2 expression in
CW-2 and CW-2/R cells following GnT-V knockdown. Therefore, we
speculate that a possible mechanism by which OCT2 affects the
sensitivity of CRC cells to oxaliplatin in associated with its
N-glycosylation.
OCT2 is a drug transporter with three
N-glycosylation sites in the long extracellular loop between
transmembrane domains 1 and 2. Changes in N-glycosylation can
markedly affect the stabilization and localization of cell membrane
glycoproteins, thus regulating cell differentiation, signal
transduction and cell behaviors (34,35).
The enzyme GnT-V can affect the biological function of
glycoproteins via the modulation of β-1,6-GlcNAc branches (36,37).
To date, studies have shown that the expression and N-glycosylation
of OCT2 are able to increase oxaliplatin intake (38,39).
The present study explored the potential mechanisms underlying
OCT2-mediated chemosensitivity in CRC by focusing on the
β-1,6-GlcNAc branches of OCT2. OCT2 was identified as a functional
target of GnT-V in the regulation of chemosensitivity. It was found
that the number of β-1,6-N-glycan branches decreased on OCT2 by
silencing GnT-V expression, and led to an apparent translocation of
OCT2 from the cell membrane to the cytoplasm in both CW-2 and
CW-2/R cells.
It is likely that N-glycan modifications may have an
impact on other proteins that affect drug chemosensitivity. As a
well-known drug resistance-associated protein, P-gp is a heavy
N-glycosylated transmembrane transporter that can pump anticancer
drugs out of cells in a reverse concentration gradient, ultimately
mediating drug resistance (40,41).
Notably, the differential β-1,6-glycosylation of P-gp by GnT-V was
not observed in the present study (data not shown). Intriguingly,
preliminary experiments suggested that blocking the activity of
P-gp by zosuquidar (a specific P-gp inhibitor) increased the
survival of cells when treated with oxaliplatin and eliminated the
effect of P-gp on the oxaliplatin resistance of CRC cells (data not
shown). Therefore, further study is needed to investigate this.
In summary, the present study demonstrated for the
first time that OCT2 is a target substrate of GnT-V, and that its
distribution and function were affected by the downregulation of
GnT-V. The collective results presented in this study add to the
body of evidence pointing to an important function for
GnT-V-mediated β-1,6-glycosylation in the resistance of CRC to
oxaliplatin chemotherapy. Therefore, the present study may provide
an experimental basis for clinical individualized
chemotherapies.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 31400687
and 81702485), the Natural Science Foundation of Liaoning Province
of China (grant no. 20180550631) and the Dalian Young Star of
Science and Technology Project (grant no. 2018RQ64).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors contributed to study conception. The
study was designed by JF and ZS. Material preparation, data
collection and analysis were performed by XC, SF, XL and XD. The
first draft of the manuscript was written by XC and ZS and all
authors commented on previous versions of the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Feng RM, Zong YN, Cao SM and Xu RH:
Current cancer situation in China: Good or bad news from the 2018
global cancer statistics? Cancer Commun (Lond).
39(22)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yang AD, Fan F, Camp ER, van Buren G, Liu
W, Somcio R, Gray MJ, Cheng H, Hoff PM and Ellis LM: Chronic
oxaliplatin resistance induces epithelial-to-mesenchymal transition
in colorectal cancer cell lines. Clin Cancer Res. 12:4147–4153.
2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bano N, Najam R, Qazi F and Mateen A:
Clinical features of oxaliplatin induced hypersensitivity reactions
and therapeutic approaches. Asian Pac J Cancer Prev. 17:1637–1641.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Goldberg RM, Sargent DJ, Morton RF, Fuchs
CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC and Alberts
SR: A randomized controlled trial of fluorouracil plus leucovorin,
irinotecan, and oxaliplatin combinations in patients with
previously untreated metastatic colorectal cancer. J Clin Oncol.
22:23–30. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liu T, Zhang X, Du L, Wang Y, Liu X, Tian
H, Wang L, Li P, Zhao Y, Duan W, et al: Exosome-transmitted
miR-128-3p increase chemosensitivity of oxaliplatin-resistant
colorectal cancer. Mol Cancer. 18(43)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wongsirisin P, Limpakan Yamada S,
Yodkeeree S, Punfa W and Limtrakul P: Association of DNA repair and
drug transporter in relation to chemosensitivity in primary culture
of thai gastric cancer patients. Biol Pharm Bull. 41:360–367.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Herraez E, Sanchez-Vicente L, Macias RIR,
Briz O and Marin JJG: Usefulness of the MRP2 promoter to overcome
the chemoresistance of gastrointestinal and liver tumors by
enhancing the expression of the drug transporter OATP1B1.
Oncotarget. 8:34617–34629. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Burger H, Zoumaro-Djayoon A, Boersma AW,
Helleman J, Berns EM, Mathijssen RH, Loos WJ and Wiemer EA:
Differential transport of platinum compounds by the human organic
cation transporter hOCT2 (hSLC22A2). Br J Pharmacol. 159:898–908.
2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tatsumi S, Matsuoka H, Hashimoto Y, Hatta
K, Maeda K and Kamoshida S: Organic cation transporter 2 and tumor
budding as independent prognostic factors in metastatic colorectal
cancer patients treated with oxaliplatin-based chemotherapy. Int J
Clin Exp Pathol. 7:204–212. 2013.PubMed/NCBI
|
|
11
|
Pelis RM, Suhre WM and Wright SH:
Functional influence of N-glycosylation in OCT2-mediated
tetraethylammonium transport. Am J Physiol Renal Physiol.
290:F1118–F1126. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Taniguchi N and Kizuka Y: Glycans and
cancer: Role of N-glycans in cancer biomarker, progression and
metastasis, and therapeutics. Adv Cancer Res. 126:11–51.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kleene R and Berger EG: The molecular and
cell biology of glycosyltransferases. Biochim Biophys Acta.
1154:283–325. 1993.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kudo T, Nakagawa H, Takahashi M, Hamaguchi
J, Kamiyama N, Yokoo H, Nakanishi K, Nakagawa T, Kamiyama T,
Deguchi K, et al: N-glycan alterations are associated with drug
resistance in human hepatocellular carcinoma. Mol Cancer.
6(32)2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lattova E, Tomanek B, Bartusik D and
Perreault H: N-glycomic changes in human breast carcinoma MCF-7 and
T-lymphoblastoid cells after treatment with herceptin and
herceptin/Lipoplex. J Proteome Res. 9:1533–1540. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tang Y, Cong X, Wang S, Fang S, Dong X,
Yuan Y and Fan J: GnT-V promotes chemosensitivity to gemcitabine in
bladder cancer cells through β1,6 GlcNAc branch modification of
human equilibrative nucleoside transporter 1. Biochem Biophys Res
Commun. 503:3142–3148. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li P, Zhang X, Wang H, Wang L, Liu T, Du
L, Yang Y and Wang C: MALAT1 is associated with poor response to
oxaliplatin-based chemotherapy in colorectal cancer patients and
promotes chemoresistance through EZH2. Mol Cancer Ther. 16:739–751.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Franken NA, Rodermond HM, Stap J, Haveman
J and van Bree C: Clonogenic assay of cells in vitro. Nat Protoc.
1:2315–2319. 2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhu Y, Meng Q, Wang C, Liu Q, Sun H, Kaku
T and Liu K: Organic anion transporters involved in the excretion
of bestatin in the kidney. Peptides. 33:265–271. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sprowl JA, van Doorn L, Hu S, van Gerven
L, de Bruijn P, Li L, Gibson AA, Mathijssen RH and Sparreboom A:
Conjunctive therapy of cisplatin with the OCT2 inhibitor
cimetidine: Influence on antitumor efficacy and systemic clearance.
Clin Pharmacol Ther. 94:585–592. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pan G, Li T, Zeng Q, Wang X and Zhu Y:
Alisol F 24 acetate enhances chemosensitivity and apoptosis of
MCF-7/DOX cells by inhibiting P-glycoprotein-mediated drug efflux.
Molecules. 21(183)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Morad SAF, Davis TS, MacDougall MR, Tan
SF, Feith DJ, Desai DH, Amin SG, Kester M, Loughran TP Jr and Cabot
MC: Role of P-glycoprotein inhibitors in ceramide-based
therapeutics for treatment of cancer. Biochem Pharmacol. 130:21–33.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Uemura T, Ito S, Ohta Y, Tachikawa M, Wada
T, Terasaki T and Ohtsuki S: Abnormal N-glycosylation of a novel
missense creatine transporter mutant, G561R, associated with
cerebral creatine deficiency syndromes alters transporter activity
and localization. Biol Pharm Bull. 40:49–55. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kazuaki O and Marth JD: Glycosylation in
cellular mechanisms of health and disease. Cell. 126:855–867.
2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Eichler J: Protein glycosylation. Curr
Biol. 29:R229–R231. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Varki A: Biological roles of glycans.
Glycobiology. 27:3–49. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nakahara S, Miyoshi E, Noda K, Ihara S, Gu
J, Honke K, Inohara H, Kubo T and Taniguchi N: Involvement of
oligosaccharide changes in alpha5beta1 integrin in a
cisplatin-resistant human squamous cell carcinoma cell line. Mol
Cancer Ther. 2:1207–1214. 2003.PubMed/NCBI
|
|
29
|
Lattová E, Bartusik D, Spicer V, Jellusova
J, Perreault H and Tomanek B: Alterations in glycopeptides
associated with herceptin treatment of human breast carcinoma mcf-7
and T-lymphoblastoid cells. Mol Cell Proteomics.
10(M111.007765)2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wojtowicz K, Januchowski R, Nowicki M and
Zabel M: Inhibition of protein glycosylation reverses the MDR
phenotype of cancer cell lines. Biomed Pharmacother. 74:49–56.
2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Xue L, Williamson A, Gaines S, Andolfi C,
Paul-Olson T, Neerukonda A, Steinhagen E, Smith R, Cannon LM,
Polite B, et al: An update on colorectal cancer. Curr Probl Surg.
55:76–116. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kalayda GV, Kullmann M, Galanski M and
Gollos S: A fluorescent oxaliplatin derivative for investigation of
oxaliplatin resistance using imaging techniques. J Biol Inorg Chem.
22:1295–1304. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Meads MB, Gatenby RA and Dalton WS:
Environment-mediated drug resistance: A major contributor to
minimal residual disease. Nat Rev Cancer. 9:665–674.
2009.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Sanchez-Pupo RE, Johnston D and Penuela S:
N-glycosylation regulates pannexin 2 localization but is not
required for interacting with pannexin 1. Int J Mol Sci.
19(1837)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Srinivasan S, Romagnoli M, Bohm A and
Sonenshein GE: N-glycosylation regulates ADAM8 processing and
activation. J Biol Chem. 289:33676–33688. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Cui J, Huang W, Wu B, Jin J, Jing L, Shi
WP, Liu ZY, Yuan L, Luo D, Li L, et al: N-glycosylation by
N-acetylglucosaminyltrans-ferase V enhances the interaction of
CD147/basigin with integrin β1 and promotes HCC metastasis. J
Pathol. 245:41–52. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yang X, Li J and Geng M:
N-acetylglucosaminyltransferase V modifies TrKA protein, regulates
the receptor function. Cell Mol Neurobiol. 28:663–670.
2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhang S, Lovejoy KS, Shima JE, Lagpacan
LL, Shu Y, Lapuk A, Chen Y, Komori T, Gray JW, Chen X, et al:
Organic cation transporters are determinants of oxaliplatin
cytotoxicity. Cancer Res. 66:8847–8857. 2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Sprowl JA, Ciarimboli G, Lancaster CS,
Giovinazzo H, Gibson AA, Du G, Janke LJ, Cavaletti G, Shields AF
and Sparreboom A: Oxaliplatin-induced neurotoxicity is dependent on
the organic cation transporter OCT2. Proc Natl Acad Sci USA.
110:11199–11204. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
French JA: P-glycoprotein expression and
antiepileptic drug resistance. Lancet Neurol. 12:732–733.
2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Bergman AM, Pinedo HM, Talianidis I,
Veerman G, Loves WJ, van der Wilt CL and Peters GJ: Increased
sensitivity to gemcitabine of P-glycoprotein and multidrug
resistance-associated protein-overexpressing human cancer cell
lines. Br J Cancer. 88:1963–1970. 2003.PubMed/NCBI View Article : Google Scholar
|