Introduction
Urticaria, also known as hives, is a common
condition estimated to affect up to 20% of the general population
during their lifetime (1). Frequent
episodes of urticaria can impact patients' lives directly, through
itching, and indirectly, by interfering with their sleep and daily
activities. While H1 antihistamines were used as first-line
treatment, certain reported adverse events and poorly responsive
cases have been associated with long-term use of the drugs,
particularly in patients with chronic urticarial (2), necessitating the development of novel
and efficient alternative therapies.
Paeoniflorin (PF) is a monoterpene glucoside
extracted from the root of Paeonia lactiflora Pall. In
China, ‘total glucosides of paeony’ capsule, of which PF is the
major component, was approved for the treatment of rheumatoid
arthritis by the National Medical Products Administration (approval
no. H20055058), and was recently recommended as a novel treatment
for psoriasis by the China Association of Chinese Medicine
(3). Previously, PF had been shown
to be a safe extraction used in clinical pharmacological research
(4). Experimentally, PF offered
protection against UV-induced DNA damage in human keratinocytes
(5), and could ameliorate several
skin disorders, such as allergic contact dermatitis (6) and psoriasis (7,8). In
addition, studies have also revealed that PF possess
anti-inflammatory (6),
antioxidative (9), antiapoptotic
(10) and immunoregulatory
bioactivities (11). It was
proposed herein that PF may serve as a promising candidate for
urticaria treatment.
Autophagy is a fundamental cellular pathway through
which cytoplasmic organelles and intracellular pathogens undergo
degradation. The autophagic process is closely associated with the
immune-inflammatory response (12):
Autophagy regulates both innate and adaptive immunity (13). Furthermore, cytokines produced
during the course of the immune response modulate various functions
of the autophagic cascade (14). An
essential role of autophagy is its involvement in the inflammatory
pathways in allergic disease, which was supported by recent data
showing that greater airway inflammation and autophagy activation,
marked by a higher Beclin-1 and ATG-5 expression and lowed P62
expression, was identified in human asthmatic tissues (15). Moreover, the induction of autophagy
in CX3Cr1+ mononuclear phagocytes inhibited the
expression of interleukin (IL)-23, leading to a reduced fibrotic
response (16). Conversely, the
inhibition of autophagy augmented IL-23 secretion in dendritic
cells and supernatants from these cells stimulated the innate
secretion of IL-17(17). A recent
study showed that a moisturizer with autophagy-stimulating
properties contributed to skin barrier restoration and control of
inflammation, thereby alleviating the symptoms and signs of atopic
dermatitis in patients (18). To
date, however, few studies have directly linked autophagy to
urticarial lesions.
In the present study, an ovalbumin (OVA)-induced
urticarial rat model was used to examine whether PF could
effectively protect against urticarial lesions. Subsequently, the
current study sought to determine whether and how PF affected the
levels of IL-23/IL-17 in the rats with urticaria. The possibility
of PF-induced regulatory effects on autophagic activity and the
potential underlying mechanism were also investigated.
Materials and methods
Animals
A total of 40 male Sprague-Dawley rats (8 weeks old)
were obtained from Chengdu Dashuo Experimental Animal Co., Ltd.
(license no. SCXK-2015-030). Animals were housed in a specific
pathogen-free animal room, kept under optimal conditions of 22±2˚C
and 40-60% humidity under a 12-h light/dark cycle with ad
libitum access to food and water. Animal health and behavior
were monitored on a daily basis by the research and animal care
staff. The animal experiment was performed at the Animal
Experimental Center, West China Hospital, Sichuan University
(Chengdu, China), all animal welfare considerations were taken, and
all procedures were approved by the Institutional Animal Care and
Use Committee of West China Hospital of Sichuan University
(approval no. 2019236A).
Drugs
PF (purity, ≥98%) was purchased from Beijing
Solarbio Science and Technology Co., Ltd. (cat. no. IP0030).
Loratadine (LOR) was supplied from Bayer Pharmaceutical (Shanghai)
Co., Ltd. (H10970410).
Animal model and treatments
After 1 week of acclimation, the animals were
randomly assigned to four groups as follows (n=10/group): i) Normal
group treated with saline; ii) model group treated with OVA/alum
(ChenDu Chron Chemicals Co., Ltd.; cat. no. 21645-51-2) and saline;
iii) model + LOR group treated with OVA/alum and 0.9 mg/kg LOR; and
iv) model + PF group treated with OVA/alum and 40 mg/kg PF. To
develop urticarial lesions, the animals, except the normal
controls, were sensitized intraperitoneally (i.p.) with 1 ml
aluminum hydroxide suspension (alum dissolved in 10 g/l 0.9% NaCl)
containing 1 mg OVA (cat. no. S7951; Sigma-Aldrich; Merck KGaA) on
days 0, 2 and 4, and challenged i.p. with 2 mg OVA in alum
suspension on day 14. Following the third sensitization, the normal
and model rats were treated with vehicle, while model + LOR and
model + PF rats were administered orally with LOR and PF,
respectively, on days 5-14, once daily. All animals were humanely
euthanatized with sodium pentobarbital (140 mg/kg i.p.) 3 h after
the final challenge, which was performed 1 h after oral
administration. Then, blood samples were collected by orbital
puncture, shaved dorsal skin tissues were harvested.
Pathological analysis
Shaved dorsal skin was fixed in 4% paraformaldehyde
overnight at 4˚C, embedded in paraffin and cut into 5-µm-thick
sections. The sections were stained at room temperature with
hematoxylin for 5 min and eosin (H&E) for 2 min to investigate
pathological changes. Three fields of view per slide were observed
under a light microscope (IX71; Olympus Corporation) and images
were captured. The magnifications used were x100 and x400. The
sections were evaluated by two independent pathologists in a
blinded manner.
Mast cell detection
Toluidine blue (TB) staining was performed for mast
cell detection (number and degranulation), which were clearly
recognizable due to the stained red-purple granules. Shaved dorsal
skin was fixed in 4% paraformaldehyde overnight at 4˚C, embedded in
paraffin and cut into 5-µm-thick sections. The sections were dipped
and stained with 0.5% toluidine blue solution for 30 min at room
temperature. Subsequently, the sections were washed with distilled
water and separated for 5 sec in 0.5% glacial acetic acid solution,
after which samples were dehydrated in a gradient alcohol series,
transparent xylene and neutral gum. Three random visual fields per
slide were selected and photographed under a light microscope
(IX71; Olympus Corporation). Mast cells in each field were counted,
and average number of the three field cells was presented as the
mast cell number per rat. The magnification used was x100.
Serum histamine levels
The blood samples were centrifuged at 3,500 x g for
10 min at 4˚C and then serum was collected and stored at
-80˚C for quantitative analysis. According to the manufacturer's
instructions, the serum concentrations of histamine were detected
using a rat histamine ELISA kit (cat. no. K4163; BioVision, Inc.).
Absorbance was measured at a wavelength of 450 nm.
Transmission electron microscopy
(TEM)
Dorsal skin tissues were fixed with 2.5%
glutaraldehyde in cacodylate buffer (pH 7.4) overnight at
4˚C, and then post-fixed in 2% osmium tetroxide in the same
buffer. Next, the specimens were conventionally dehydrated in
acetone, infiltrated and embedded in epoxy resin-filled capsules.
Finally, 70-nm ultrathin sections were prepared with a
ultramicrotome (LKB Instruments) and counterstained with 2% aqueous
uranyl acetate and 0.8% lead citrate at 25˚C for 3 h.
Ultrastructures were examined and imaged using a transmission
electron microscope (H-7650; Hitachi, Ltd.). The number of
autophagic vacuoles per unit field at a magnification of x12,000
was counted, and three randomly selected fields of view were
captured for each sample.
Immunohistochemistry (IHC)
Skin tissues were fixed in 4% paraformaldehyde for
24 h at room temperature, embedded in paraffin and cut into 5
µm-thick sections. Sections were then deparaffinized in xylene for
10 min, hydrated through a series of graded alcohol (30, 50, 70, 95
and 100%), and microwaved in citrate buffer (pH 6.0) for 10 min at
95˚C. Following routine peroxidase blocking with 3% hydrogen
peroxide solution for 15 min and 5% bovine serum albumin blocking
(cat. no. G5001; Wuhan Servicebio Technology Co., Ltd.) for 30 min
at room temperature, the sections were incubated with antibodies
against IL-23 (dilution, 1:200; cat. no. orb184437; Biorbyt Ltd.),
IL-17 (dilution, 1:200; cat. no. NBP1-42746; Novus Biologicals,
LLC), Beclin-1 (dilution, 1:100; cat. no. PD017; MBL International
Co.), light chain 3B (LC3B; dilution, 1:200; cat. no. NB100-2220;
Novus Biologicals, LLC), P62 (dilution, 1:100; cat. no. ab56416;
Abcam), liver kinase B1 (LKB1; dilution, 1:150; cat. no. CPA3329;
Cohesion Biosciences, Ltd.) and AMP-activated protein kinase-α
(AMPK-α; dilution, 1:100; cat. no. 66536-1-lg; ProteinTech Group,
Inc.) overnight at 4˚C. The samples were then exposed to HRP/Fab
polymer-conjugated secondary antibodies (solution from the kit;
cat. no. PV-6000-D; OriGene Technologies, Inc.) at room temperature
for 30 min. Samples were then stained with 3,3'-diaminobenzidine
solution for 5 min and counterstained with hematoxylin for 20 sec
at room temperature.
Each slide was analysed using light microscopy and
three random fields of view were observed. Images were captured and
analyzed using Image Pro Plus 6.0 software (Media Cybernetics,
Inc.). The mean of integrated optical density was used to evaluate
the semi-quantitative levels of IL-23, IL-17, Beclin-1, LC3B, P62,
LKB1 and AMPK-α. The magnification used was x100.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
RNA from skin tissues was extracted using the
TRIzol® reagent (cat. no. 15596026; Thermo Fisher
Scientific, Inc.), and cDNA was synthesized using the RevertAid
First Strand cDNA Synthesis kit (cat. no. K1621; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. Real
Time PCR Easy™-SYBR Green I (cat. no. QP-01014; Foregene Co., Ltd.)
was used as fluorophore. Next, RT-qPCR of the samples was
determined using the real-time PCR detection system (PikoReal
96-well Thermal Cycler; Thermo Fisher Scientific, Inc.). The
following thermocycling conditions were used: Initial denaturation
for 30 sec at 95˚C; 45 cycles of 5 sec at 95˚C, 30 sec at 55˚C and
30 sec at 72˚C. The mRNA levels of IL-23, IL-17, Beclin-1, LC3B,
P62, LKB1 and AMPK-α were analyzed. β-actin was used as an
endogenous control to normalize the amounts of RNA between samples.
Differences in amplification were calculated using the
2-∆∆Cq method (19). The primer sequences used are listed
in Table I.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Primer name | Sequence
(5'-3') |
|---|
| β-actin | F:
GAAGATCAAGATCATTGCTCCT |
| | R:
TACTCCTGCTTGCTGATCCA |
| IL-23 | F:
ACCTGCTGGACTCGGACATCTTCACA |
| | R:
AAGGCTTGGAGGCTGCGAAGGATCT |
| IL-17 | F:
TGTGCCTGATGCTGTTGCTGCTACTG |
| | R:
GGTCCTCATTGCGGCTCAGAGTCCA |
| Beclin-1 | F:
GTCTAAGGCGTCCAGCAGCACCAT |
| | R:
GGTCACTCGGTCCAGGATCTTGAAGC |
| LC3B | F:
GCTGCCTGTCCTGGATAAGACCAAGT |
| | R:
CCTCCTCTTGACTCAGAAGCCGAAGG |
| P62 | F:
GCTGAGTCGGCTTCTGCTCCATCA |
| | R:
GCGGCTTCTCTTCCCTCCATGTTCC |
| LKB1 | F:
AGAGGAGGAGGAGGACGAGGACTTGT |
| | R:
CCTTCTGGCTTCACCTTGCTGCTGAG |
| AMPK-α | F:
CGATGATGAGGTGGTGGAGCAGAGGT |
| | R:
GTGAATGGTTCTCGGCTGTGCTGGAA |
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software (SPSS, Inc.). Differences between groups were evaluated
using one-way analysis of variance, followed by Tukey method for
data with equal variances and Dunnett's T3 method for data with
unequal variances. Data are expressed as the mean ± SD. P<0.05
was considered to indicate a statistically significant
difference.
Results
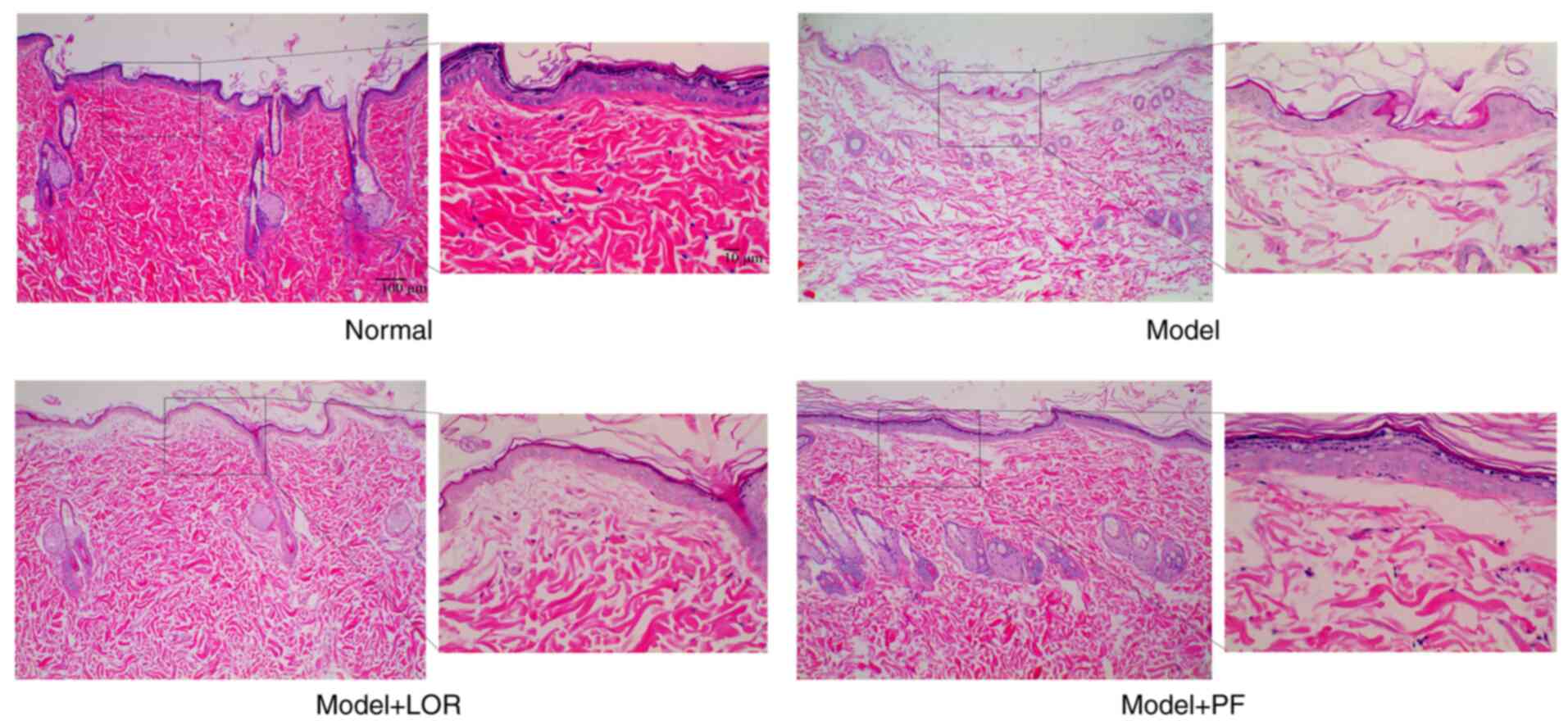
PF ameliorates urticarial
histopathology
H&E staining was first conducted to evaluate the
pathological features in dorsal skin from various groups.
Pathological abnormalities were clearly visible in urticarial
lesion biopsies in model rats, compared with skin in healthy
controls. Dorsal skin from model rats exhibited apparent edema in
the upper and mid dermis, forming separated collagenous fibers that
had faded in color, as well as widened spaces between collagen
bundles. Prominent dilated post-capillary venules surrounded with
infiltration of some inflammatory cells were also observed. PF
treatments could notably attenuate these pathological abnormalities
observed in model rats (Fig. 1).
Marked pathological mitigation was also observed in LOR-treated
rats compared with the model group. These findings indicated that
PF intervention allowed for a remission of urticarial
histopathology.
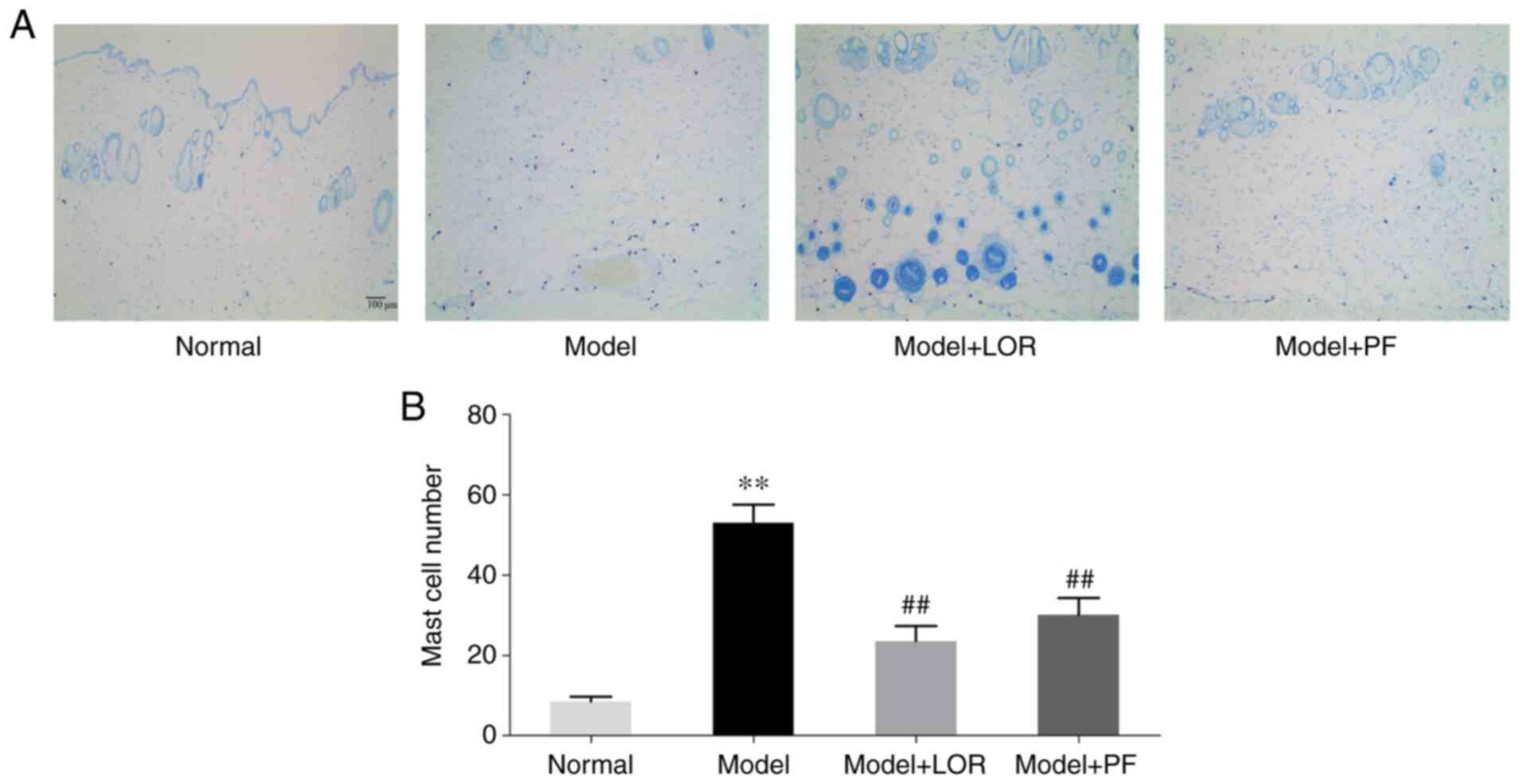
PF inhibits mast cell infiltration and
degranulation
Mast cell infiltration and degranulation are the
central events in type I allergic responses (20), which are responsible for active
urticaria. TB staining was therefore performed to examine whether
PF could be effective in ameliorating mast cell activation. In the
present study, the number of mast cells and granules released
(Fig. 2A) was significantly higher
in model (53.10±4.45) than in normal rats (8.57±1.16), suggesting
that an apparent type I allergic response had occurred in the rats
with urticaria. By contrast, the number of mast cells and granules
decreased significantly in rats following LOR or PF treatment
(Fig. 2). The administration of PF
was able to markedly attenuate the infiltration of mast cells and
suppress the elevated degranulation responses.
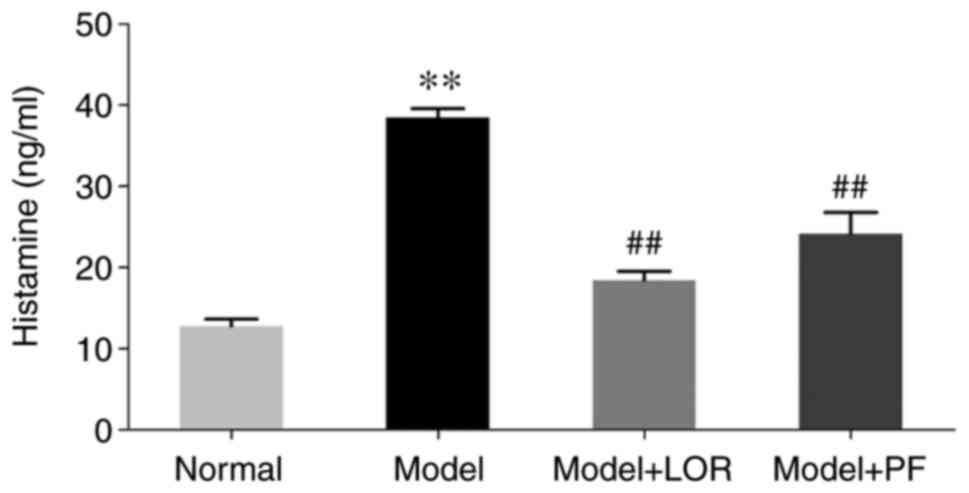
PF decreases serum histamine
levels
Histamine is a key mediator in the inflammatory
response, largely due to mast cell activation (21,22).
Therefore, serum histamine levels were determined herein using
ELISA. Histamine levels in rats with urticaria were found to be
evidently elevated (38.45±1.15) compared with the control rats
(12.77±0.86), along with the aforementioned elevation in mast cell
number and degranulation. By contrast, PF treatment, which was akin
to the effectiveness of LOR, significantly lowered the increased
histamine levels (Fig. 3). The
application of PF was able to inhibit the overproduction of
mast-cell-released histamine.
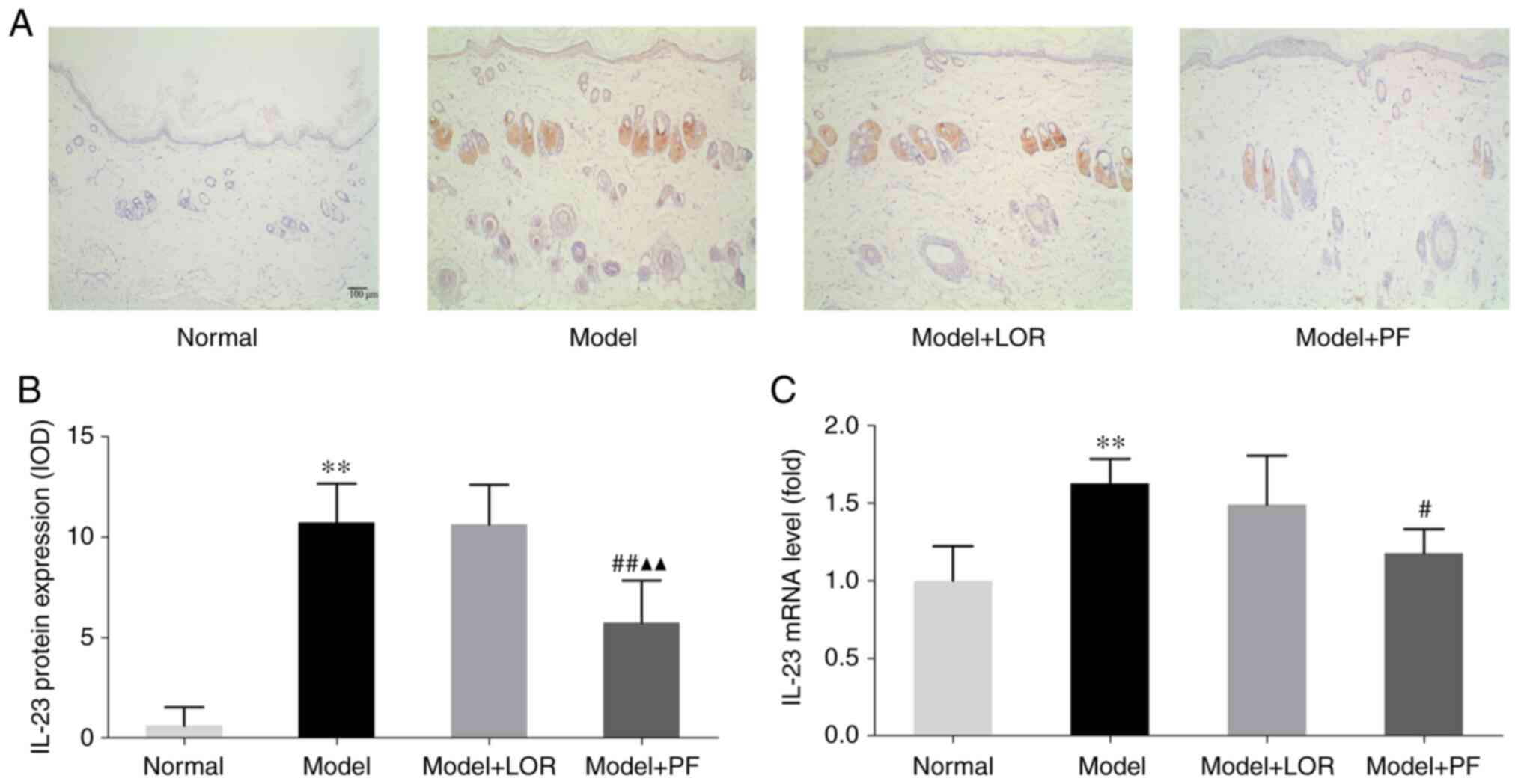
PF diminishes IL-23 production in rats
with urticaria
Several studies have revealed the key role that
IL-23/IL-17 play in promoting the development of skin lesions
infiltrated with inflammatory cells in several diseases, such as
psoriasis (23), atopic dermatitis
(24) and allergic contact
dermatitis (25). Whether IL-23-
and IL-17-producing cells could also be observed in urticarial
lesions was investigated in the current study. Detection of IL-23
and IL-17 protein by IHC confirmed a significant increase in the
number of IL-23-positive cells in dorsal skin sections from rats
with urticaria, while there were no noticeable changes in the
number of IL-17-positive cells (data not shown). PF treatment
markedly reduced the number of IL-23-positive cells and PF
outperformed LOR in diminishing the production of IL-23 (Fig. 4A and B). Subsequently, RT-qPCR analysis was
performed, and it was found that rats with urticaria displayed a
higher amount of IL-23 mRNA than normal rats, which was also
significantly lower following PF or LOR treatment (Fig. 4C). However, PF demonstrated lower
levels of mRNA and protein IL-23 expression compared with LOR
treatment, further supporting the effectiveness of PF in
attenuating the inflammatory response partly conferred by IL-23
inhibition.
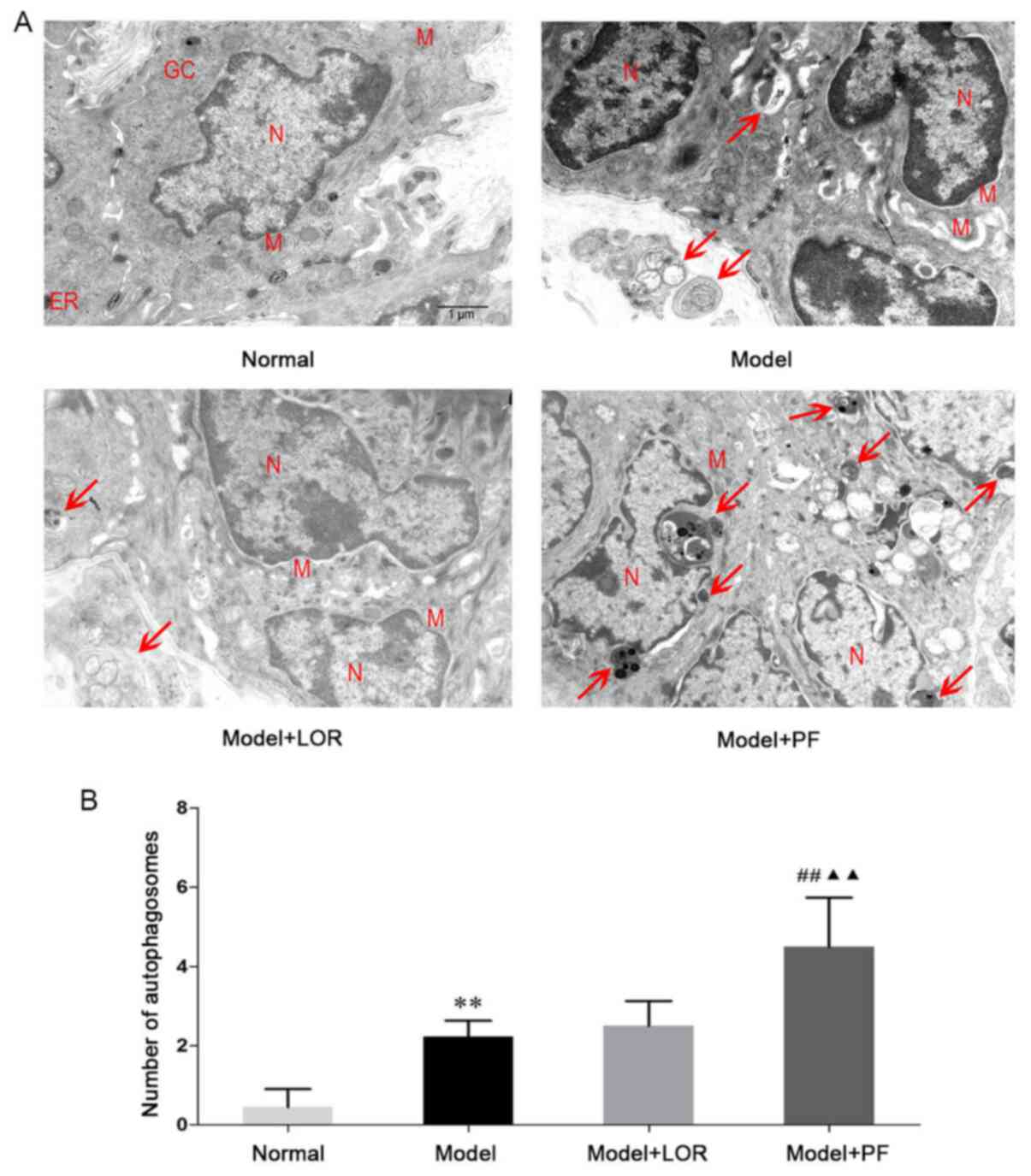
PF enhances autophagic activity in
rats with urticaria
To test whether a disturbed process of autophagy was
associated with urticarial activity, and whether PF had a
regulatory effect on the autophagic activity, autophagic morphology
was first examined in dorsal skin using TEM. As presented in
Fig. 5A, the nucleus, mitochondria,
endoplasmic reticulum and other organelles appeared morphologically
intact in normal rats. In model rats with urticaria, by contrast,
cell shrinkage, chromatin condensation and nuclear fragmentation
were observed. The presence of swollen mitochondria with broken
cristae, or mitochondrial vacuolization and expanded endoplasmic
reticulum were also noted. In addition, an increased number of
autophagic vacuoles engulfing structure-clear organelles or
degraded electron-dense material were found, indicating the
presence of elevated autophagic activity in model rats with
urticaria. Of note, the autophagic activity was further enhanced by
the co-administration of antigenic stimulation with PF, ascertained
by the observation that PF-treated rats had ~2-fold more
autophagosomes than non-treated model rats (Fig. 5B). Of note, aberrant morphological
ultrastructures, including nuclear fragmentation, chromatin
condensation, vacuolated cytoplasm and degenerated cell organelles,
were simultaneously improved in the majority of rats treated with
PF. However, there was no statistical difference between the LOR
treatment group and the model group. Accordingly, PF intervention
could, in effect, promote autophagic activity and also help restore
cellular ultrastructure in rats with urticaria.
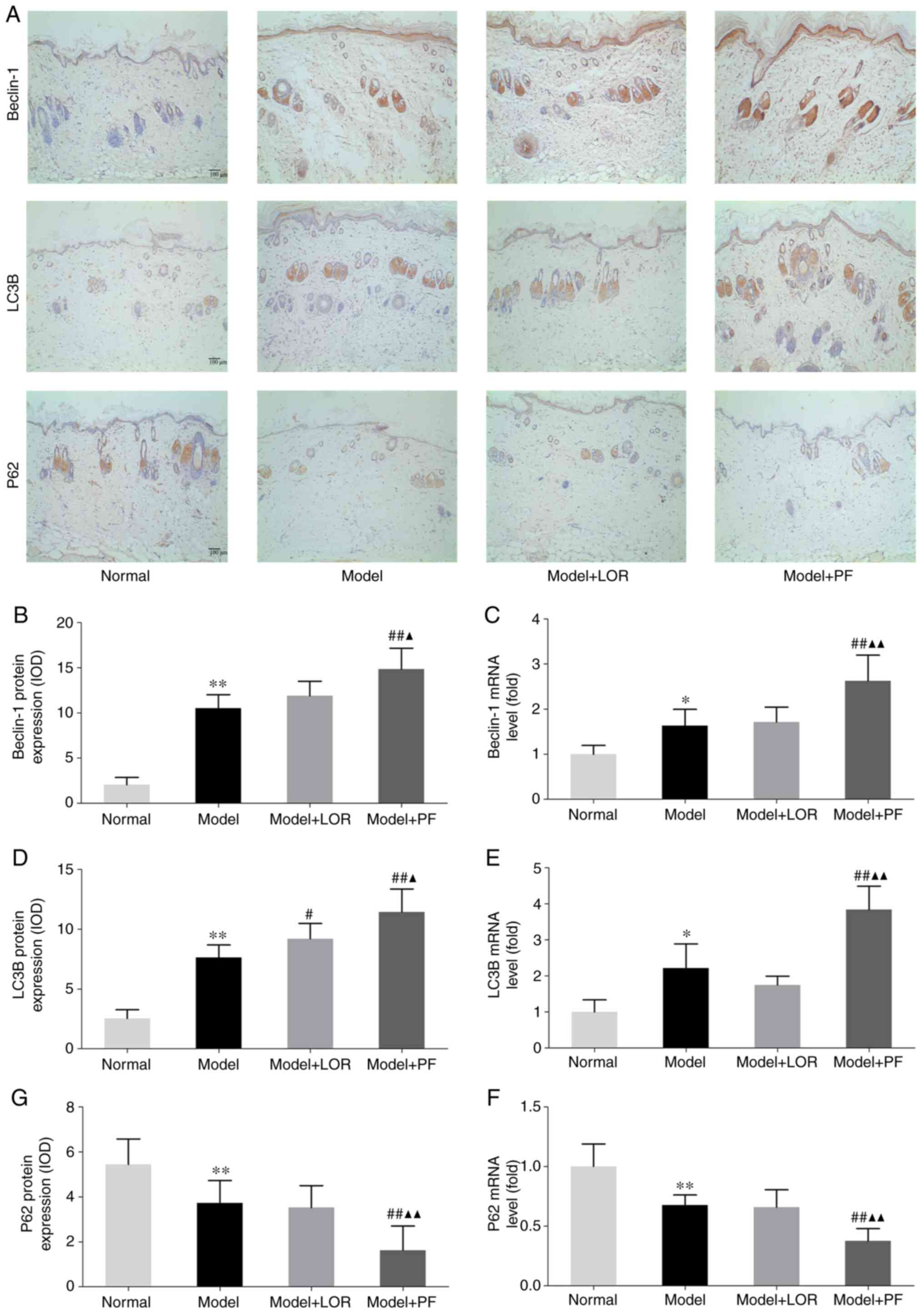
PF increases LC3B and Beclin-1 levels
and reduces P62 levels in rats with urticaria
Autophagic activity at the molecular level was
further examined by detecting the expression of autophagy markers
LC3B, Beclin-1 and P62 in dorsal skin tissues. Beclin-1 is an
essential autophagy effector, which forms a complex with PI3K,
accounting for autophagic vesicle nucleation in mammals (26). Antigen induction promoted an
increase in Beclin-1 levels in rats with urticaria. In addition,
treatment with PF had an additive effect in enhancing Beclin-1
levels, as confirmed by RT-qPCR and IHC (Fig. 6A-C). PF may function to promote the
production of Beclin-1, contributing to an enhancement in
autophagic vacuole formation.
LC3B sensitively reflects autophagic activity and is
considered as a specific reporter of autophagic flux (27). Therefore, LC3B mRNA and protein
levels were assayed in skin tissues. Consistent with the varying
numbers of autophagic vacuoles visualized under TEM, RT-qPCR
results suggested that antigen induction led to a significant
increase in LC3B content, which could be further, markedly boosted
by PF treatment (Fig. 6E). This
result was also confirmed by similar trends in LC3B protein
expression evidenced by IHC (Fig.
6A and D). Moreover, LOR
treatment led to increased LC3B protein expression in rats with
urticaria (Fig. 6A and D).
The impact of PF on P62, an adaptor protein playing
a crucial role in the proteasome or lysosome degradation system,
was subsequently explored. In the present study, following
antigenic stimulation, rats with urticaria demonstrated a reduction
of P62 mRNA and protein levels in the dorsal skin, and rats with
urticaria treated with PF achieved a greater decrease in the amount
of P62 (Fig. 6A, F and G).
Collectively, these findings further supported the TEM observation
that PF may have a beneficial effect, given the pathological
remission yielded by PF intervention, through promoting autophagic
activity in rats with urticaria.
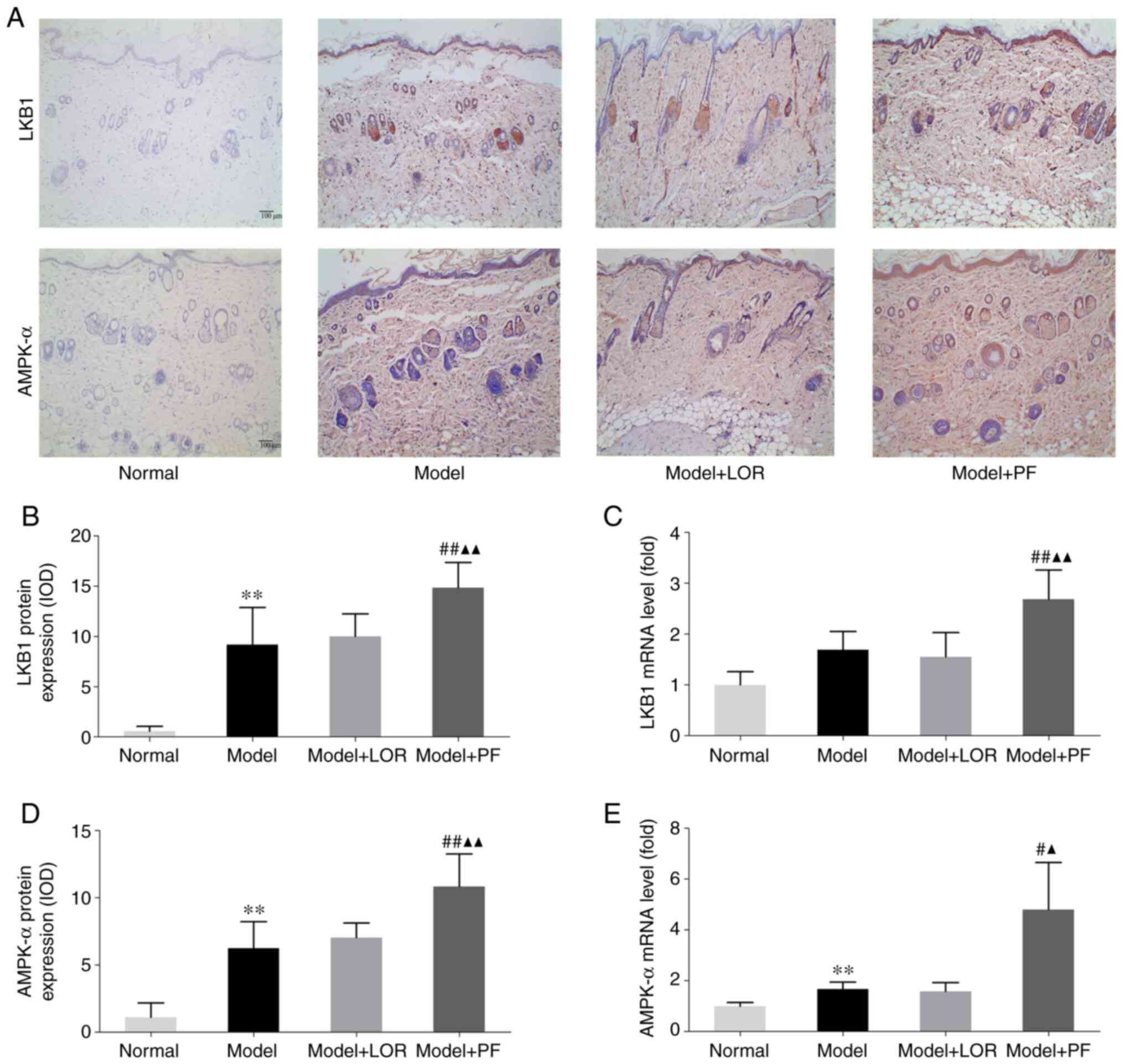
PF activates the LKB1/AMPK signaling
pathway in rats with urticaria
LKB1/AMPK is a canonical upstream signaling pathway
that modulates autophagic activity (28,29).
Whether LKB1/AMPK was involved in PF-enhanced autophagy in rats
with urticaria was therefore investigated. The RT-qPCR results
indicated that antigenic stimulation led to an increase in the LKB1
and AMPK-α mRNA expression (although no statistical significance
was showed for LKB1, P=0.053), which could be further,
significantly boosted by PF treatment (Fig. 7C and E). IHC also showed an increased protein
expression of LKB1 and AMPK-α in dorsal skin sections of rats with
urticaria, compared with normal rats, and PF treatment further
boosted the LKB1 and AMPK-α expression (Fig. 7A, B
and D). Accordingly, the activation
of LKB1/AMPK-α may be implicated in the role of PF-enhanced
autophagic activity in mitigating urticarial lesions.
Discussion
Urticaria is generally considered to be a mast
cell-driven disease. During a urticaria episode, activated skin
mast cells release histamine and other mediators, including
leukotriene, platelet-activating factor and cytokines (1), thereby leading to the dilatation and
augmentation of the permeability of vessels, plasma extravasation,
as well as inflammatory cell recruitment to the urticarial lesions.
In the present study, histopathological examination of the affected
skin showed apparent edema, dilated capillary and post-capillary
venules, as well as a perivascular infiltration of inflammatory
cells, including neutrophils, eosinophils and, in some cases,
macrophages. All of the above were evidence of salient features of
urticaria occurrence. An increased number of mast cells in the skin
and elevated concentrations of mast-cell-released histamine were
also observed. PF, similar to the effectiveness of LOR, could
notably improve urticarial histopathology and ameliorate the
overproduction of histamine. Considering that a positive
association was noted between the histamine-releasing activity and
severity of histopathological features, it may be hypothesized that
PF possess anti-allergic properties partly by alleviating mast cell
infiltration and degranulation and partly by normalizing histamine
release.
IL-23 is a heterodimeric cytokine that plays a
pivotal role in the inflammatory autoimmune pathogenesis of
multiple skin diseases. IL-23 is one of the essential mediators for
driving and maintaining the differentiation of T helper 17
lymphocytes (30,31), which is the resource for producing
another effector cytokine IL-17. Using a genetic mouse model,
researchers revealed that keratinocyte-produced IL-23 was
sufficient to cause chronic skin inflammation (32). Treatment with anti-IL-23p19 and
anti-IL-17A neutralizing antibodies improved diabetic wounds
harboring excessive inflammation (33). In addition, evidence has emerged
that specific inhibition or blockage of IL-23 or IL-17 could
improve inflammatory skin disorders, such as psoriasis (34), palmoplantar pustulosis (35) and pityriasis rubra pilaris (36). In the present study, the elevated
protein and mRNA expression of IL-23 was noted in OVA-challenged
rats with urticaria, which might be a major mechanism underlying
inflammatory cell recruitment and infiltration in urticarial
lesions. This increased secretion of IL-23 was diminished
significantly following PF intervention, thus contributing to the
remission of urticarial lesions.
The protective mechanisms of PF against OVA-elicited
urticaria were also investigated in our study. Autophagy is crucial
to the degradation (37) and
recycling of cellular components, and occurs physiologically at a
low basal level, aiming at performing homeostatic functions. In the
present study, along with the intact nucleus, mitochondria,
endoplasmic reticulum and other organelles, autophagosomes were
occasionally present in normal skin cells, as determined by TEM.
This observation supports the notion that a normal biogenesis and
function of autophagic vacuoles is essential for protein and
organelle turnover in physiological states (38). P62 binds directly to autophagosomal
membrane protein LC3B and presents P62-containing protein
aggregates to the autophagy machinery, facilitating the clearance
of such aggregates and, thereby, contributing to the process of
autophagy (39,40). Lysosomal degradation of
autophagosomes has been shown to give rise to reduced P62 levels
during autophagy. Conversely, autophagy inhibition has been shown
to lead to an increase in P62 protein levels (39). Of note, rats with urticaria
exhibited an increased number of autophagosomes, also revealed by
the unregulated levels of LC3B, suggesting that skin cells may
undergo an increased autophagic process, and hence remove the
impairing cytoplasmic components caused by allergic reactions. In
the current study, administration of PF further promoted the
autophagic activity in model rats as demonstrated by a higher
number of autophagosomes together with higher LC3B and lower P62
levels. It was also found that the IL-23 reduction occurred in
parallel with the degree of pathological remission, as well as the
elevation of autophagic activity in PF-treated rats. In previous
studies, LKB1 signaling was found to trigger and modulate autophagy
by activating AMPK (28,29,41).
Pharmacological intervention with PF has been reported to relieve
certain diseases by activating the LKB1/AMPK signaling pathway. A
recent study found that PF protected against intestinal
ischemia/reperfusion by activating LKB1/AMPK and thereby promoting
autophagic activity (42). In
addition, the beneficial effects of PF in the treatment of insulin
resistance and hepatic steatosis were associated with the
activation of LKB1/AMPK-α (43). In
the present study, PF treatment could boost the expression of LKB1
and AMPK-α, contributing to the enhancement of autophagy.
Collectively, the results of the current study indicated that PF
may confer an augmented autophagic activity, which, in part, could
be linked to the activation of LKB1/AMPK-α in urticarial lesions,
so as to abrogate IL-23-associated skin inflammatory responses.
Overall, the present study demonstrated the benefits
of PF in the treatment of OVA-induced urticaria. However, there
were also certain limitations. For instance, only a single dose of
PF was administered during urticaria treatment and a detailed
understanding of the pharmacodynamics of PF, such as dose-dependent
effects, is further required. In addition, further studies that
focus on characterizing the association between the autophagic
activity and inflammatory disorders after PF treatment are
required.
In conclusion, the present study provided evidence
that PF serves as an effective antagonist normalizing the IL-23
secretion and ameliorating urticaria, and revealed the novel
finding that PF can increase autophagic activity that involves
LKB1/AMPK-α activation, thereby possibly accelerating the
elimination of the elicited inflammatory mediators. The present
study extended the understanding of the pharmaceutical functions of
PF and may provide experimental evidence for its potential clinical
use in urticaria treatment.
Acknowledgements
Not applicable.
Funding
Funding: This work was funded by the National Natural Science
Foundation of China (grant nos. 81573986 and 81873310), the Project
of Science and Technology Department of Sichuan Province (grant no.
2018JY0660), the Project of ‘Xing-lin Scholars’ of Chengdu
University of Traditional Chinese Medicine (grant nos. CGZH2018001
and QNXZ2019017), Science and Technology Developmental Foundation
of the Hospital of Chengdu University of Traditional Chinese
Medicine (grant no. 19TS03) and ‘Hundred Talents Program’ of the
Hospital of Chengdu University of Traditional Chinese Medicine
(grant nos. 20-B01, 20-Q03 and 20-Q05).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JG conceived and designed the research, and
interpreted the results of the experiments. LP and JHZ designed the
current study. LP, FX and MHZ performed the experiments. XTZ and QW
conducted the statistical analysis. JHZ and LP organized the
database prepared the manuscript. JG and JHZ confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Animal
Care and Use Committee of West China Hospital, Sichuan University,
Chengdu, China (approval no. 2019236A).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Saini SS: Chronic spontaneous urticaria:
Etiology and pathogenesis. Immunol Allergy Clin North Am. 34:33–52.
2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Maurer M, Staubach P, Raap U, Richter-Huhn
G, Bauer A, Ruëff F, Jakob T, Yazdi AS, Mahler V, Wagner N, et al:
H1-antihistamine-refractory chronic spontaneous urticaria: It's
worse than we thought - first results of the multicenter real-life
AWARE study. Clin Exp Allergy. 47:684–692. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zheng Q, Jiang W, Sun X, Ma T, Xu W, Shen
F, Li H, Xie S, Li B and Li X: Total glucosides of paeony for the
treatment of psoriasis: A systematic review and meta-analysis of
randomized controlled trials. Phytomedicine.
62(152940)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Li X, Shi F, Zhang R, Sun C, Gong C, Jian
L and Ding L: Pharmacokinetics, safety, and tolerability of
amygdalin and paeoniflorin after single and multiple intravenous
infusions of huoxue-tongluo lyophilized powder for injection in
healthy chinese volunteers. Clin Ther. 38:327–337. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lee S, Lim JM, Jin MH, Park HK, Lee EJ,
Kang S, Kim YS and Cho WG: Partially purified paeoniflorin exerts
protective effects on UV-induced DNA damage and reduces facial
wrinkles in human skin. J Cosmet Sci. 57:57–64. 2006.PubMed/NCBI
|
|
6
|
Wang C, Yuan J, Wu HX, Chang Y, Wang QT,
Wu YJ, Liu LH and Wei W: Paeoniflorin inhibits inflammatory
responses in mice with allergic contact dermatitis by regulating
the balance between inflammatory and anti-inflammatory cytokines.
Inflamm Res. 62:1035–1044. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sun Y, Zhang J, Huo R, Zhai T, Li H, Wu P,
Zhu X, Zhou Z, Shen B and Li N: Paeoniflorin inhibits skin lesions
in imiquimod-induced psoriasis-like mice by downregulating
inflammation. Int Immunopharmacol. 24:392–399. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chen T, Fu LX, Zhang LW, Yin B, Zhou PM,
Cao N and Lu YH: Paeoniflorin suppresses inflammatory response in
imiquimod-induced psoriasis-like mice and peripheral blood
mononuclear cells (PBMCs) from psoriasis patients. Can J Physiol
Pharmacol. 94:888–894. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Choi EM, Suh KS, Rhee SY and Kim YS:
Inhibitory effect of paeoniflorin on methylglyoxal-mediated
oxidative stress in osteoblastic MC3T3-E1 cells. Phytomedicine.
21:1170–1177. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang D, Wong HK, Feng YB and Zhang ZJ:
Paeoniflorin, a natural neuroprotective agent, modulates multiple
anti-apoptotic and pro-apoptotic pathways in differentiated PC12
cells. Cell Mol Neurobiol. 33:521–529. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang T, Yang Z, Yang S, Du J and Wang S:
Immunoregulatory effects of paeoniflorin exerts anti-asthmatic
effects via modulation of the Th1/Th2 equilibrium. Inflammation.
38:2017–2025. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Levine B, Mizushima N and Virgin HW:
Autophagy in immunity and inflammation. Nature. 469:323–335.
2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Orosz L, Papanicolaou EG, Seprenyi G and
Megyeri K: IL-17A and IL-17F induce autophagy in RAW 264.7
macrophages. Biomed Pharmacother. 77:129–134. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Racanelli AC, Kikkers SA, Choi AMK and
Cloonan SM: Autophagy and inflammation in chronic respiratory
disease. Autophagy. 14:221–232. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mathur R, Alam MM, Zhao XF, Liao Y, Shen
J, Morgan S, Huang T, Lee H, Lee E and Huang Y and Huang Y:
Induction of autophagy in Cx3cr1+ mononuclear cells
limits IL-23/IL-22 axis-mediated intestinal fibrosis. Mucosal
Immunol. 12:612–623. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Peral de Castro C, Jones SA, Ni Cheallaigh
C, Hearnden CA, Williams L, Winter J, Lavelle EC, Mills KH and
Harris J: Autophagy regulates IL-23 secretion and innate T cell
responses through effects on IL-1 secretion. J immunol.
189:4144–4153. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kwon SH, Lim CJ, Jung J, Kim HJ, Park K,
Shin JW, Huh CH, Park KC and Na JI: The effect autophagy-enhancing
peptide in moisturizer on atopic dermatitis: A randomized
controlled trial. J Dermatolog Treat. 30:558–564. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ying S, Kikuchi Y, Meng Q, Kay AB and
Kaplan AP: TH1/TH2 cytokines and inflammatory cells in skin biopsy
specimens from patients with chronic idiopathic urticaria:
Comparison with the allergen-induced late-phase cutaneous reaction.
J Allergy Clin Immunol. 109:694–700. 2002.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sun B, Wang B and Xu M: Esculetin inhibits
histamine-induced expression of inflammatory cytokines and mucin in
nasal epithelial cells. Clin Exp Pharmacol Physiol. 46:821–827.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Metz M and Maurer M: Mast cells-key
effector cells in immune responses. Trends Immunol. 28:234–241.
2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hawkes JE, Yan BY, Chan TC and Krueger JG:
Discovery of the IL-23/IL-17 signaling pathway and the treatment of
psoriasis. J Immunol. 201:1605–1613. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mizutani N, Sae-Wong C, Kangsanant S, Nabe
T and Yoshino S: Thymic stromal lymphopoietin-induced
interleukin-17A is involved in the development of IgE-mediated
atopic dermatitis-like skin lesions in mice. Immunology.
146:568–581. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Larsen JM, Bonefeld CM, Poulsen SS,
Geisler C and Skov L: IL-23 and T(H)17-mediated inflammation in
human allergic contact dermatitis. J Allergy Clin Immunol.
123:486–492. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kihara A, Kabeya Y, Ohsumi Y and Yoshimori
T: Beclin-phosphatidylinositol 3-kinase complex functions at the
trans-Golgi network. EMBO Rep. 2:330–335. 2001.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kabeya Y, Mizushima N, Ueno T, Yamamoto A,
Kirisako T, Noda T, Kominami E, Ohsumi Y and Yoshimori T: LC3, a
mammalian homologue of yeast Apg8p, is localized in autophagosome
membranes after processing. EMBO J. 19:5720–5728. 2000.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jin P, Jiang J, Xie N, Zhou L, Huang Z,
Zhang L, Qin S, Fu S, Peng L, Gao W, et al: MCT1 relieves
osimertinib-induced CRC suppression by promoting autophagy through
the LKB1/AMPK signaling. Cell Death Dis. 10(615)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang M, Deng YN, Zhang JY, Liu J, Li YB,
Su H and Qu QM: SIRT3 Protects rotenone-induced Injury in SH-SY5Y
cells by promoting autophagy through the LKB1-AMPK-mTOR pathway.
Aging Dis. 9:273–286. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Schön MP and Erpenbeck L: The
interleukin-23/Interleukin-17 axis links adaptive and innate
immunity in psoriasis. Front Immunol. 9(1323)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Langrish CL, Chen Y, Blumenschein WM,
Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA and
Cua DJ: IL-23 drives a pathogenic T cell population that induces
autoimmune inflammation. J Exp Me. 201:233–240. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li H, Yao Q, Mariscal AG, Wu X, Hülse J,
Pedersen E, Helin K, Waisman A, Vinkel C, Thomsen SF, et al:
Epigenetic control of IL-23 expression in keratinocytes is
important for chronic skin inflammation. Nat Commun.
9(1420)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lee J, Rodero MP, Patel J, Moi D, Mazzieri
R and Khosrotehrani K: Interleukin-23 regulates interleukin-17
expression in wounds, and its inhibition accelerates diabetic wound
healing through the alteration of macrophage polarization. FASEB J.
32:2086–2094. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kopp T, Riedl E, Bangert C, Bowman EP,
Greisenegger E, Horowitz A, Kittler H, Blumenschein WM, McClanahan
TK, Marbury T, et al: Clinical improvement in psoriasis with
specific targeting of interleukin-23. Nature. 521:222–226.
2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Terui T, Kobayashi S, Okubo Y, Murakami M,
Hirose K and Kubo H: Efficacy and safety of guselkumab, an
anti-interleukin 23 monoclonal antibody, for palmoplantar
pustulosis: A randomized clinical trial. JAMA Dermatol.
154:309–316. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Feldmeyer L, Mylonas A, Demaria O,
Mennella A, Yawalkar N, Laffitte E, Hohl D, Gilliet M and Conrad C:
Interleukin 23-helper T cell 17 axis as a treatment target for
pityriasis rubra pilaris. JAMA Dermatol. 153:304–308.
2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Rai S, Arasteh M, Jefferson M, Pearson T,
Wang Y, Zhang W, Bicsak B, Divekar D, Powell PP, Naumann P, et al:
The ATG5-binding and coiled coil domains of ATG16L1 maintain
autophagy and tissue homeostasis in mice independently of the WD
domain required for LC3-associated phagocytosis. Autophagy.
15:599–612. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cecconi F and Levine B: The role of
autophagy in mammalian development: Cell makeover rather than cell
death. Dev Cell. 15:344–357. 2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Bjørkøy G, Lamark T, Brech A, Outzen H,
Perander M, Overvatn A, Stenmark H and Johansen T: p62/SQSTM1 forms
protein aggregates degraded by autophagy and has a protective
effect on huntingtin-induced cell death. J Cell Biol. 171:603–614.
2005.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Pankiv S, Clausen TH, Lamark T, Brech A,
Bruun JA, Outzen H, Øvervatn A, Bjørkøy G and Johansen T:
p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of
ubiquitinated protein aggregates by autophagy. J Biol Chem.
282:24131–24145. 2007.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zimmermann K, Baldinger J, Mayerhofer B,
Atanasov AG and Dirsch VM: Activated AMPK boosts the Nrf2/HO-1
signaling axis-A role for the unfolded protein response. Free Radic
Biol Med. 88:417–426. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wen J, Xu B, Sun Y, Lian M, Li Y, Lin Y,
Chen D and Diao Y: Paeoniflorin protects against intestinal
ischemia/reperfusion by activating LKB1/AMPK and promoting
autophagy. Pharmacol Res. 146(104308)2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Li YC, Qiao JY, Wang BY, Bai M, Shen JD
and Cheng YX: Paeoniflorin ameliorates fructose-induced insulin
resistance and hepatic steatosis by activating LKB1/AMPK and AKT
pathways. Nutrients. 10(1024)2018.PubMed/NCBI View Article : Google Scholar
|