Introduction
Acute livfer failure (ALF) is a life-threatening
systemic disorder characterized by severe coagulopathy and
encephalopathy (1). Currently,
liver transplantation is the only therapeutic method proven to
improve the prognosis of ALF patients. However, the pathogeneses of
ALF is poorly understood (2,3). Rake
et al previously reported sinusoidal hypercoagulation in the
liver of ALF and demonstrated the usefulness of anticoagulation
therapy in ALF patients (4). Thus,
hepatic microcirculatory disturbance as well as excessive
activation of immune cells have attracted interest as the
pathogenesis of ALF (5). Massive
hepatic necrosis and scarce regeneration, which occur in acute
liver injury (ALI), result in acute depression of hepatic function
or ALF. The histological findings of ALF and its clinical features
including the sudden onset and aggressive expansion of liver damage
suggest the involvement of blood perfusion disorders. Indeed,
intravital microscopy analysis revealed a significant correlation
between the extent of sinusoidal flow disturbance and hepatic
tissue damage in animal studies of ALF (6-9),
and sinusoidal fibrin deposition as a result of impaired
coagulation system suggests the involvement of sinusoidal
hypercoagulation to induce hepatic blood flow disturbance (10,11).
However, the association between sinusoidal hypercoagulation and
intrahepatic hypoxia in ALF has not been addressed, because there
are no clinically applicable methods to assess sinusoidal
perfusion. On the other hand, there is no significant correlation
between the extent of liver damage and systemic coagulation
disorder, and heparin treatment is not effective in
paracetamol-induced ALF (10,12).
It is, therefore, unclear whether hepatic microcirculatory
disturbance and intrahepatic hypoxia occur in the liver of ALF and
if so, how they affect the clinical features of ALF is poorly
understood.
Lactate dehydrogenase (LDH) is a critical enzyme
which catalyzes the conversion of pyruvate to lactate under hypoxic
condition. Because LDH is transcriptionally upregulated when blood
supply is insufficient, it is likely that LDH serves as a marker
for tissue hypoxia (13-15).
Interestingly, immunostaining with liver biopsy samples showed
marked increase in LDH in ALF and cirrhosis but only slight
increase in chronic hepatitis, suggesting intrahepatic hypoxia in
the ALF liver (11). On the other
hand, alanine aminotransferase (ALT) is known to be released from
damaged hepatocytes, thereby serving as a maker of liver injury.
We, therefore, hypothesize that serum ALT/LDH ratio reflects the
hypoxia-induced liver damage, which can be clinically used for the
evaluation of intrahepatic hypoxia. Indeed, several studies have
reported serum ALT/LDH ratio reflects a hypoxic marker in the
prognosis of ALI patients (16,17),
but little has been reported on intrahepatic hypoxia classified
serum ALT/LDH ratio histologically.
In this study, we classified ALI patients based upon
serum ALT/LDH ratio and found increased expression of
hypoxia-related genes and fibrin deposition in the liver from ALI
patients with reduced ALT/LDH ratio. We also found intrahepatic
hypoxia secondary to sinusoidal hypercoagulation in a mouse model
of Concanavalin A (ConA)-induced ALI (18), where anticoagulation with
recombinant human soluble thrombomodulin (rhTM) effectively reduced
sinusoidal hypercoagulation and intrahepatic hypoxia, thereby
leading to the improvement of liver injury. This study provides
evidence that serum ALT/LDH ratio is clinically used as a biomarker
of intrahepatic hypoxia in ALI patients and suggests that
anticoagulation offers a novel therapeutic strategy for the
treatment of ALF, which arises from sinusoidal
hypercoagulation.
Materials and methods
Patients
This study was a retrospective single-center design.
Patients with ALI, who had been admitted to Kyushu University
Hospital between January 2005 and March 2018, were examined. ALI
was defined as any syndrome that causes elevation of liver function
tests for less than 6 months. Those who had serum ALT more than 200
U/l or serum total bilirubin (T. Bil.) more than 4 mg/dl or
prothrombin time-international normalized ratio (PT-INR) over 1.2
at admission were enrolled, reaching up to 309. Blood tests for
general hepatic function, coagulation ability, immunological
variables such as IgG, IgA, IgM, ANA, smooth muscle antibodies,
anti-mitochondrial antibody and liver-kidney microsomal antibodies
(if required), and viral markers for hepatitis A virus (HAV),
hepatitis B virus (HBV), hepatitis C virus (HCV), cytomegalovirus,
herpes simplex virus (type 1 and 2) and EB virus were assessed
using samples that had been obtained at admission. The diagnosis of
patients with autoimmune hepatitis (AIH) was confirmed at their
discharge according to the revised criteria of the International
Autoimmune Hepatitis Group. For all patients diagnosed with AIH,
pathological findings were required to fulfill the criteria.
Patients with malignant tumors (n=20) and those with liver
cirrhosis (n=10) were excluded. Ultrasound-guided percutaneous
liver biopsies were performed in 17 patients with ALI. This study
was approved by Kyushu University Hospital Ethics Committee (nos.
27-377 and 28-432). Informed consent of individual patients was not
obtained because this study is of retrospective nature.
Animals
Eight-week-old male C57BL/6J mice weighing 20-25 g
were obtained from Japan SLC (Shizuoka, Japan). Mice were
maintained under controlled conditions with free access to standard
chow and water. Mice were monitored via daily observations of
health and behavior. All studies were performed in accordance with
the Guide for the Care and Use of Laboratory Animals (National
Institutes of Health) and approved by the Animal Care Committee of
Kyushu University for three months starting in February 2020.
Totally 186 mice were used in this study including preliminary
experiments. All animals were euthanized by euthanasia under
isoflurane at concentrations of 4-5% for induction and 2-3% for
maintenance in accordance with the institutional guideline of the
Animal Care Committee of Kyushu University. The depth of anesthesia
was confirmed by loss of the postural reaction and righting reflex
(the pedal withdrawal reflex in the forelimbs and hind limbs, the
tail pinch reflex, and the eyelid reflex). Blood samples were drawn
from tail vein or inferior vena cava and the livers were collected.
Approximately 700-1,200 µl of blood was extracted by
exsanguination. A combination of lack of pulse, breathing, corneal
reflex, and presence of rigor mortis was used to confirm death. The
blood samples were centrifuged for 15 min at 3,000 rpm (1,500 x g)
at 4˚C, and serum samples were collected and stored at -80˚C. For
RNA isolation and western blot analysis, liver samples were
snap-frozen in liquid nitrogen and stored at -80˚C.
Experimental protocols
i) The ConA-induced ALI model mice (n=10). ConA
(Sigma-Aldrich; Merck KGaA) was injected at 15 mg/kg via the tail
vein (19). ii) The galactosamine
(GalN)/tumor necrosis factor-α (TNF-α) (G/T)-induced ALI model mice
(n=10). A total of 700 mg/kg of GalN (D-(+)-galactosamine
hydrochloride; Sigma-Aldrich; Merck KGaA) was initially injected
intraperitoneally. One hour after GalN injection, 15 µg/kg of TNFα
(Recombinant human TNFα; Peprotech) was injected intravenously via
the tail vein (20). iii) Control
mice (n=5). The control mice were received saline via the tail
vein. All animals were euthanized with isoflurane at 6 h after the
injection of ConA or TNFα. Liver tissue samples were collected at 6
h after the injection of ConA or TNFα.
Anticoagulant-treated mice (n=4). rhTM was purchased
from Asahi Kasei Pharma Co. Ltd. Upon ConA (15 mg/kg body weight)
injection, rhTM (5 mg/kg body weight) dissolved in saline was
injected intravenously via the tail vein (the ConA+TM group). The
control animals received saline treatment at the time of ConA
injection (the ConA group). The ConA group and ConA+TM group were
sacrificed at 6 h (n=4, each group). To detect the hypoxic lesions
in the liver, mice were injected intraperitoneally with
pimonidazole (120 mg/kg; Chemicon) dissolved in saline 1 h before
euthanasia.
Biochemical analysis
Normal ranges for human of ALT and LDH were 6-30 U/l
and 119-229 U/l, respectively. In this study, we calculated ALT/LDH
ratio with the following formula: ALT/LDH ratio=(serum
ALT-ULN)/(serum LDH-ULN), where ULN stands for the upper limit of
normal.
Blood samples of mice were withdrawn from the tail
vein 6 h after the injection of ConA or TNFα. Serum levels of ALT,
LDH, and fibrin degradation products (FDP) for mice were measured
using chemical analyzer Fuji DRI-CHEM (Fuji Film) and FDP-ELISA kit
(MyBioSource), respectively.
Immunohistochemical analyses
(human)
Liver biopsy samples were fixed with 10% formalin
and embedded in paraffin. Serial sections (5 µm) were cut from the
blocks. Sinusoidal fibrin deposition was detected by
phosphotungstic acid-hematoxylin (PTAH) staining. Paraffin-embedded
liver sections were deparaffinized and rehydrated. Antigen
retrieval was performed with Proteinase K (Dako) treatment.
Endogenous peroxidase activity was blocked for 20 min with 3%
hydrogen peroxide (Sigma-Aldrich; Merck KGaA). After blocking with
diluted serum from the secondary antibodies host, the slides were
incubated overnight (4˚C) with the following antibodies: Tissue
factor (TF) antibodies (ab151748, Abcam, Cambridge, MA),
hypoxia-inducible factor-1α (HIF-1α) antibody (NB100-105; Novus
Biologicals), HIF-2α antibody (NB100-132; Novus Biologicals),
lactate dehydrogenase-V (LDH-V) antibodies (ab9002; Abcam) and
vascular endothelial growth factor A (VEGFA) antibodies (ab183100;
Abcam). Secondary goat anti-rabbit or anti-mouse antibodies
(Histofine Simple Stain kit; Nichirei Bioscience) was applied for
60 min at room temperature and stained with diaminobenzidine
tetrahydrochloride (Nichirei Bioscience). The sections were
counterstained with hematoxylin (Thermo Fisher Scientific, Inc.),
dehydrated, and mounted. Positive areas in five randomly selected
microscopic fields (magnification, x40) per section were measured
using analysis software (BZ-X analyzer; Keyence) and the mean
percentage of the positive area was calculated.
Histological examinations (Mice)
Liver tissue samples were collected at 6 h after the
injection of TNF-α or ConA, fixed in 10% formalin and embedded in
paraffin. Immunostaining was performed in the same way as human
pathological examination. TF antibody (ab151748; Abcam),
hypoxia-inducible factor (HIF)-1α antibodies (NB100-479; Novus
Biologicals), HIF-2α antibodies (NB100-122; Novus Biologicals),
LDH-V antibody (ab85472; Abcam) and vascular endothelial growth
factor (VEGF)-A antibody (ab183100; Abcam) were used as the first
antibody. Secondary goat anti-rabbit antibodies (Histofine Simple
Stain kit; Nichirei Bioscience) was applied. The sections were
visualized under a Keyence BZ-X700 microscope (Keyence). Positive
areas in five randomly selected microscopic fields (x40
magnification) per section were measured using analysis software
(BZ-X analyzer, Keyence, Osaka, Japan). For pimonidazole staining,
Hypoxyprobe Omni kit (Abcam) was used according to the
manufacturer's protocol (21).
Positive areas in five randomly selected microscopic fields
(magnification, x40) per section were measured using analysis
software (BZ-X analyzer; Keyence) and the mean percentage of
positive area was calculated.
Reverse transcription-quantitative
PCR
Total RNA from liver tissue was isolated using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and
cDNA was synthesized from 500 µg RNA by GeneAmp RNA polymerase
chain reaction (PCR) (Applied Biosystems). Quantitative polymerase
chain reaction (qPCR) was performed using SYBR-Green on the ABI
7500 real-time PCR System (Applied Biosystems). The PCR reaction
was carried out with a denaturation step at 95˚C for 30 sec, then
40 cycles at 95˚C for 5 sec and finally at 60˚C for 34 sec. To
control for variations in the reactions, all data were normalized
to GAPDH expression. Relative expression was presented using the
2-ΔCt method. The primer sequences
are listed in Table I.
 | Table IReverse-transcription quantitative
PCR primer sequences. |
Table I
Reverse-transcription quantitative
PCR primer sequences.
| | Primer
sequences |
|---|
| Gene | Forward
(5'-3') | Reverse
(5'-3') |
|---|
| GAPDH |
TACCCCCAATGTGTCCGTC |
GGTCCTCAGTGTAGCCCAAG |
| TF |
TGCTTCTCGACCACAGACAC |
TAAAAACTTTGGGGCGTTTG |
| PAI-1 |
TCTGGGAAAGGGTTCACTTTACC |
GACACGCCATAGGGAGAGAAG |
| HIF1-α |
GTATTATTCAGCACGACTT |
GACATTGCCAGGTTTAT |
| HIF2-α |
CTGAGGAAGGAGAAATCCCGT |
TGTGTCCGAAGGAAGCTGATG |
| LDHV |
TGGCGACTCCAGTGTGCCTG |
AGGCACTGTCCACCACCTGCT |
| VEGFA |
CTGTGCAGGCTGCTGTAACG |
GTTCCCGAAACCCTGAGGAG |
Western blot analysis
Aliquots of liver homogenate (30 µg) were separated
by sodium dodecyl sulfate (SDS) polyacrylamide gels and transferred
to polyvinylidene difluoride (PVDF) membranes. Nonspecific binding
was blocked with 5% nonfat milk for one hour and incubated
overnight at 4˚C with primary antibodies: HIF-1α antibodies
(NB100-479; Novus Biologicals), HIF-2α antibodies (NB100-102; Novus
Biologicals) and anti-β-actin antibodies (ab16039; Abcam).
Membranes were washed with PBS with Tween-20 (PBST) three times for
10 min and then incubated with a secondary goat anti-rabbit
antibodies (1:5,000) for one hour at 37˚C. Finally, the membranes
were washed with PBST three times for 10 min and developed with the
ECL system (GE Healthcare).
Statistical analysis
Data were analyzed using JMP Pro Version 11
statistical software (SAS Institute Inc.). The results were
expressed as the means and standard deviation (SD) or standard
error of the means (SEM), or Median and inter-Quartile range.
Significant differences between groups were assessed using the
χ2-square test and unpaired Student's t-test. The
differences of means among multiple groups were analyzed by using
one-way ANOVA and Tukey's post hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Characteristics of ALI patients
classified by serum ALT/LDH ratio
An ALT/LDH ratio of 1.5 was used to diagnose hypoxic
hepatitis, which is characterized by intrahepatic hypoxia and
massive liver damage as a result of cardiac failure-induced
reduction of oxygen delivery (22).
In this study, when coagulopathy was defined as FDP >10 µ/ml,
ROC analysis showed the cut-off value of serum ALT/LDH to be 1.48
(Fig. S1). We, therefore, used 1.5
as the cut-off value to identify ALI patients with sinusoidal
hypercoagulation. It is likely that patients with ALT/LDH ratio
≤1.5 (i.e., the low ALT/LDH ratio group) were complicated with
microcirculatory disturbance relative to those with ALT/LDH ratio
>1.5 (i.e., the high ALT/LDH ratio group). Accordingly, we
classified our ALI patients into the low ALT/LDH ratio and high
ALT/LDH ratio groups, based upon ALT/LDH ratio of 1.5 (Table II). There were substantial numbers
of HBV and AIH patients in the high ALT/LDH ratio group (12 HAV, 55
HBV, 35 AIH, 9 drugs, 5 alcoholic, 36 undetermined etiologies, and
12 others). On the other hand, HAV patients and those with
undetermined etiologies were mostly in the low ALT/LDH ratio group
(22 HAV, 16 HBV, 4 AIH, 6 drugs, 13 alcoholic, 40 undetermined
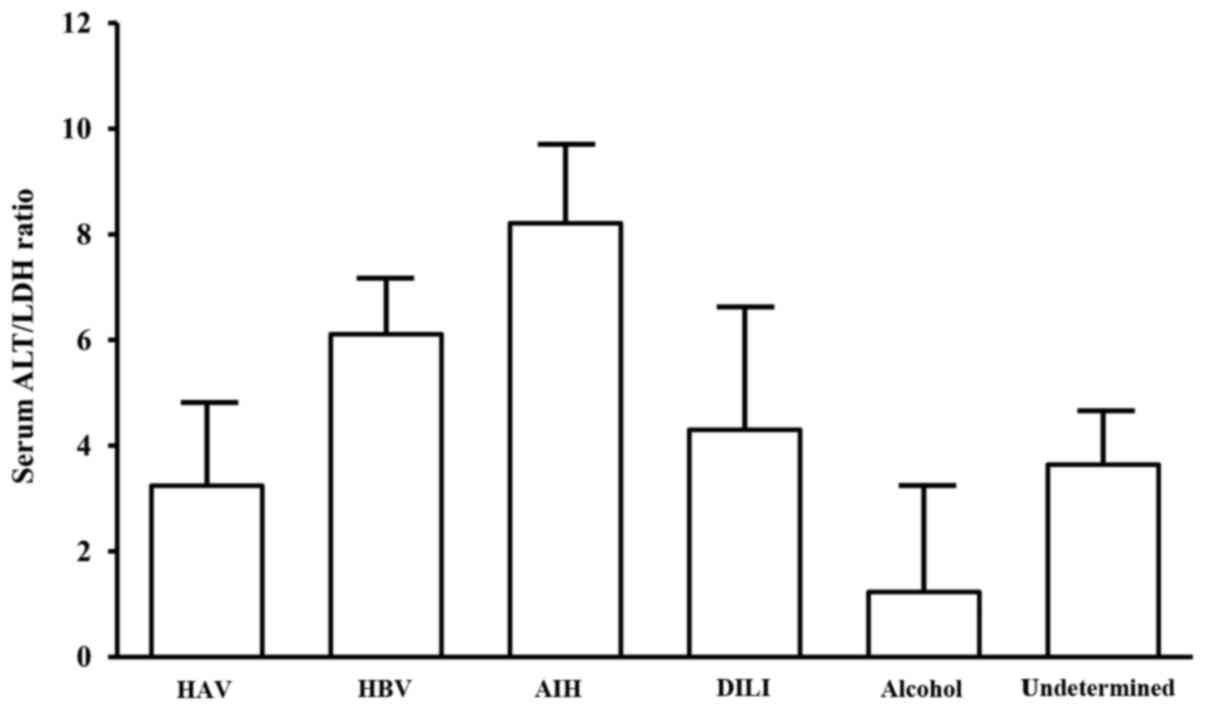
etiologies, and 14 others). The AIH patients showed the highest
ALT/LDH ratio, whereas HAV patients exhibited lower ALT/LDH ratio
(Fig. 1). Moreover, ALT
(P<0.0001), LDH (P<0.0001), ferritin (P<0.0001), and MELD
(P=0.0002) score were significantly higher in the low ALT/LDH ratio
group than those in the high ALT/LDH ratio group, while platelet
count (P=0.0096) and T. Bil. (P=0.0037) were significantly higher
in the high ALT/LDH ratio group. Importantly, the low ALT/LDH ratio
group showed significantly increased levels of PT-INR (P=0.0191)
and FDP (P<0.0001) relative to the high ALT/LDH ratio group
(Table II).
 | Table IICharacteristics of patients with ALI
upon admission, classified according to ALT/LDH ratio. |
Table II
Characteristics of patients with ALI
upon admission, classified according to ALT/LDH ratio.
| Characteristic | Total | High ALT/LDH ratio
(ALT/LDH>1.5) | Low ALT/LDH ratio
(ALT/LDH≤1.5) | P-value |
|---|
| N | 279 | 164 | 115 | |
| Age | 45 (34-58) | 45 (34-60) | 45 (35-55) | 0.5890 |
| Sex (M/F) | | | | 0.7180 |
|
Male | 154 | 92 | 62 | |
|
Female | 125 | 72 | 53 | |
| Etiology (%) | | | | <0.0001 |
|
HAV | 34 (12.2) | 12 (7.3) | 22 (19.1) | |
|
HBV | 71 (25.4) | 55 (33.5) | 16 (13.9) | |
|
AIH | 39 (13.9) | 35 (21.3) | 4 (3.5) | |
|
DILI | 15 (5.3) | 9 (5.4) | 6 (5.2) | |
|
Alcohol | 18 (6.4) | 5 (3.1) | 13 (11.3) | |
|
Undetermined | 76 (27.2) | 30 (18.3) | 40 (34.8) | |
|
Others | 24 (8.6) | 15 (9.1) | 9 (7.8) | |
| Platelet
(x103/µl) | 14.2
(9.6-19.1) | 15.6
(10.7-19.98) | 12.4
(8.6-16.6) | 0.0096 |
| FDP (µg/ml) | 10.8
(4.4-24.4) | 6.25
(2.6-11.58) | 19.3 (11.4-44) | <0.0001 |
| PT-INR | 1.78
(1.41-2.37) | 1.57
(1.28-2.17) | 1.97
(1.63-2.76) | 0.0191 |
| Alb (g/dl) | 3.5 (3.1-3.9) | 3.5 (3.1-3.9) | 3.5 (3.1-3.9) | 0.9353 |
| Cre (mg/dl) | 0.73
(0.56-1.02) | 0.675
(0.54-0.82) | 0.84
(0.61-1.61) | 0.0023 |
| TB (mg/dl) | 4.8 (2.6-11.1) | 6.2
(3.33-12.78) | 3.8 (2-7.3) | 0.0037 |
| AST (IU/l) | 1848
(517-5227) | 1216.5
(515.75-2696.5) | 4793
(517-9507) | <0.0001 |
| ALT (IU/l) | 2349
(680-4365) | 1670
(725.5-3338) | 3232
(324-6109) | <0.0001 |
| LDH (IU/l) | 783 (386-3310) | 526
(358.75-977.25) | 3669
(879-8350) | <0.0001 |
| Ferritin
(ng/ml) | 3890.6
(914.7-16955) | 2676.1
(562.75-7130.5) | 11980.5
(1846.1-49762) | <0.0001 |
| NH3 (µg/dl) | 63 (49-96) | 61 (49-86.5) | 66 (47.5-105) | 0.1734 |
| MELD | 16.78
(11.33-23.71) | 15.52
(10.15-20) | 18.79 (13-28) | 0.0002 |
| Survive/death and
LT | 224/55 | 134/30 | 90/25 | 0.4700 |
Sinusoidal hypercoagulation and
intrahepatic hypoxia in liver biopsy samples
Liver biopsy samples were obtained from 17 patients
(2 HAV, 6 AIH, 2 drugs, 7 undetermined etiologies) (Table III). Fibrin deposition, a hallmark
of sinusoidal hypercoagulation (10,23),
was diffusely distributed in the low ALT/LDH ratio group but barely
detected in the high ALT/LDH ratio group (Fig. 2A). We also found increased protein
expression of TF, a cell surface glycoprotein that plays a role in
the initiation of sinusoidal coagulopathy (24), in the low ALT/LDH ratio group
relative to high ALT/LDH ratio group; the immune-positive area was
significantly higher in the low ALT/LDH ratio group (47±13%) than
in the high ALT/LDH ratio group (25±17%) (P=0.01). We also
performed immunohistochemical analysis of hypoxia-related proteins.
Both HIF-1α and HIF-2α, key transcriptional factors for hypoxic
response, were strongly positive in the low ALT/LDH ratio group
relative to the high ALT/LDH ratio group, with immune-positive area
being significantly extended (Fig.
2B and C). There was increased
protein expression of LDH-V and VEGFA in the low ALT/LDH ratio
group relative to high ALT/LDH ratio group (Fig. 2B and C). Increased expression of hypoxia-related
proteins in the liver from the low ALT/LDH ratio group is
consistent with the notion that intrahepatic hypoxia develops in
the liver with low ALT/LDH ratio group, which suggests that serum
ALT/LDH ratio is useful to evaluate intrahepatic hypoxia in the
liver.
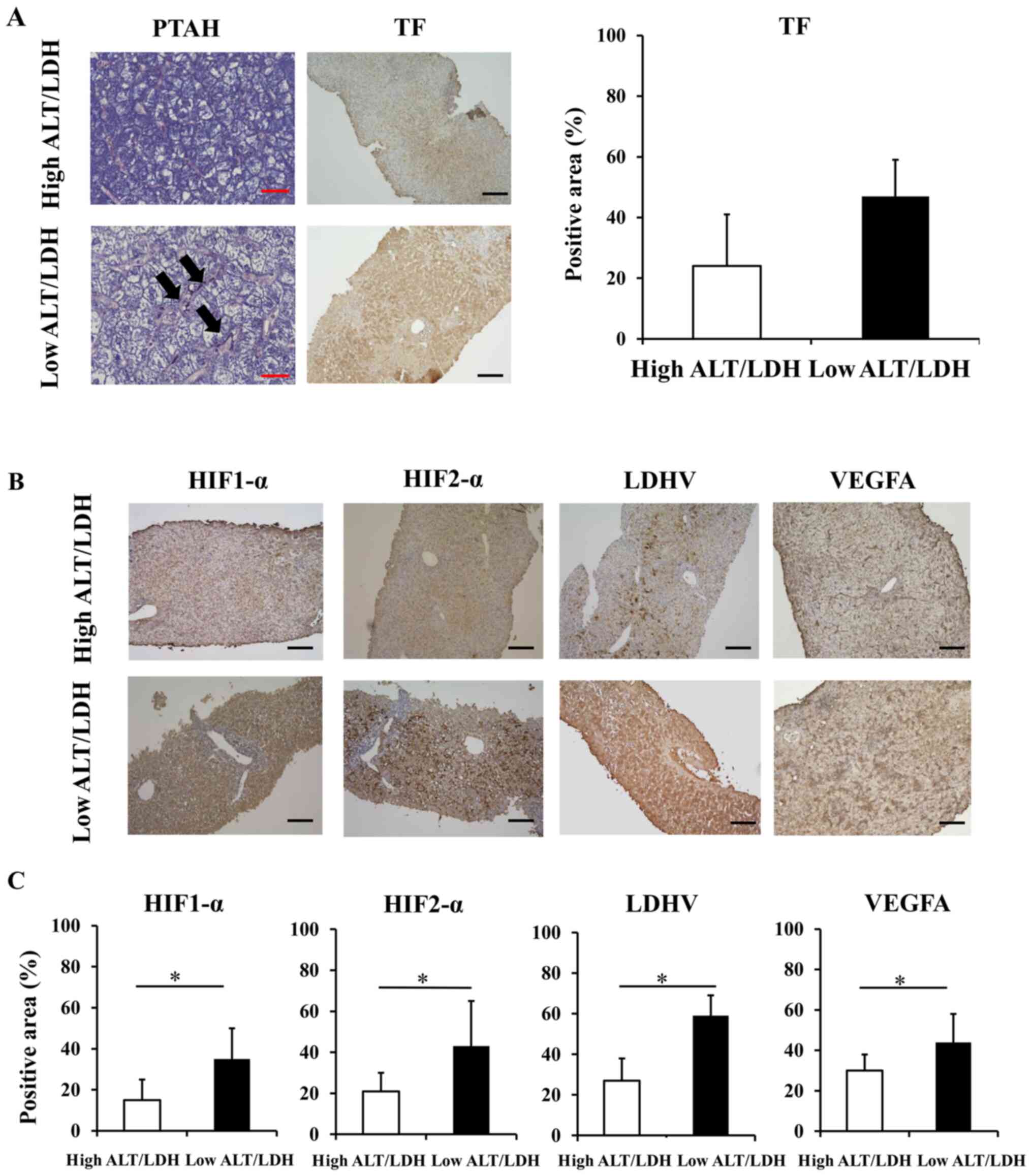 | Figure 2Histological evaluation of
intrahepatic hyper-coagulation and hypoxia related proteins of 17
patients with ALI. (A) TF staining (magnification, x100) and
phosphotungstic acid-hematoxylin staining (magnification, x400) of
liver sections in a patient with low ALT/LDH ratio and a patient
with high ALT/LDH ratio were performed to evaluate sinusoidal
coagulopathy. The arrows indicate fibrin depositions in sinusoids.
Black Scale bar=100 µm. Red Scale bar=25 µm. (B) HIF-1α, HIF-2α,
LDH-V and VEGFA staining of liver sections in a patient with low
ALT/LDH ratio and a patient with high ALT/LDH ratio were performed
to evaluate intrahepatic hypoxia (magnification, x100). Black Scale
bar=100 µm. (C) The percentage of positive area in HIF-1α, HIF-2α,
LDH-V and VEGFA staining of liver sections. *P<0.05.
ALI, acute liver injury; ALT/LDH, aminotransferase/lactate
dehydrogenase ratio; HIF, hypoxia-inducible factor; LDH-V, lactate
dehydrogenase-V; TF, tissue factor. |
 | Table IIIEtiology and laboratory findings for
17 patients with ALI in which liver biopsy was performed. |
Table III
Etiology and laboratory findings for
17 patients with ALI in which liver biopsy was performed.
| | Blood
chemistry | Positive area
(%) |
|---|
| Case | Etiology | ALT (IU/l) | LDH (IU/l) | ALT/LDH ratio | PT-INR | FDP (µg/ml) | TF | HIF1-α | HIF2-α | LDHV | VEGFA |
|---|
|
1 | Undetermined | 7,711 | 9,172 | 0.86 | 1.3 | 39.8 | 45 | 39 | 38 | 55 | 60 |
|
2 | Undetermined | 4,315 | 8,800 | 0.50 | 1.67 | 10 | 60 | 57 | 70 | 72 | 62 |
|
3 | HAV | 4,409 | 4,501 | 1.03 | 1.24 | 24.4 | 51 | 48 | 63 | 52 | 44 |
|
4 | Undetermined | 4,495 | 4,105 | 1.15 | 1.76 | 52.3 | 60 | 30 | 17 | 62 | 24 |
|
5 | Undetermined | 4,973 | 3,201 | 1.66 | 1.41 | 12 | 40 | 26 | 30 | 42 | 40 |
|
6 | Undetermined | 5,769 | 2,257 | 2.83 | 2.32 | 25.5 | 60 | 28 | 23 | 43 | 25 |
|
7 | HAV | 1,020 | 1,253 | 0.97 | 1.42 | 48.9 | 42 | 25 | 56 | 70 | 40 |
|
8 | Undetermined | 5,344 | 825 | 8.92 | 2.05 | 16 | 35 | 38 | 35 | 40 | 32 |
|
9 | AIH | 2,780 | 632 | 6.82 | 1.31 | 5.9 | 5 | 10 | 13 | 25 | 41 |
| 10 | AIH | 1,198 | 573 | 3.40 | 2.24 | 5.1 | 4 | 8 | 15 | 24 | 33 |
| 11 | DILI | 1,220 | 560 | 3.60 | 1.25 | 15.4 | 26 | 10 | 13 | 27 | 20 |
| 12 | AIH | 337 | 452 | 1.38 | 1.51 | 2.5 | 26 | 15 | 19 | 47 | 34 |
| 13 | DILI | 561 | 364 | 3.93 | 1.22 | 2.5 | 12 | 5 | 10 | 13 | 14 |
| 14 | AIH | 389 | 290 | 5.89 | 1.22 | 8 | 16 | 12 | 12 | 15 | 28 |
| 15 | Undetermined | 2,621 | 281 | 49.83 | 1.49 | 2.5 | 38 | 17 | 32 | 36 | 36 |
| 16 | AIH | 680 | 197 | 20.31 | 1.26 | 2.5 | 26 | 8 | 30 | 23 | 29 |
| 17 | AIH | 680 | 190 | 16.67 | 1.48 | 2.5 | 10 | 8 | 22 | 16 | 33 |
Sinusoidal hypercoagulation and
intrahepatic hypoxia in mouse models of ALI
We next examined sinusoidal hypercoagulation and
intrahepatic hypoxia in 2 different murine models of human ALI. The
ConA-induced ALI is a T-cell-driven liver injury model, where
cytotoxic effector molecules are thought to play a key role in the
development of cell death (25,26),
whereas the G/T-induced ALI represents an apoptotic model of liver
injury, thus resembling human acute viral hepatitis (27). Histological analysis of liver
sections revealed inflammation and piecemeal necrosis with robust
sinusoidal congestion in ConA mice and spotty hemorrhagic legions
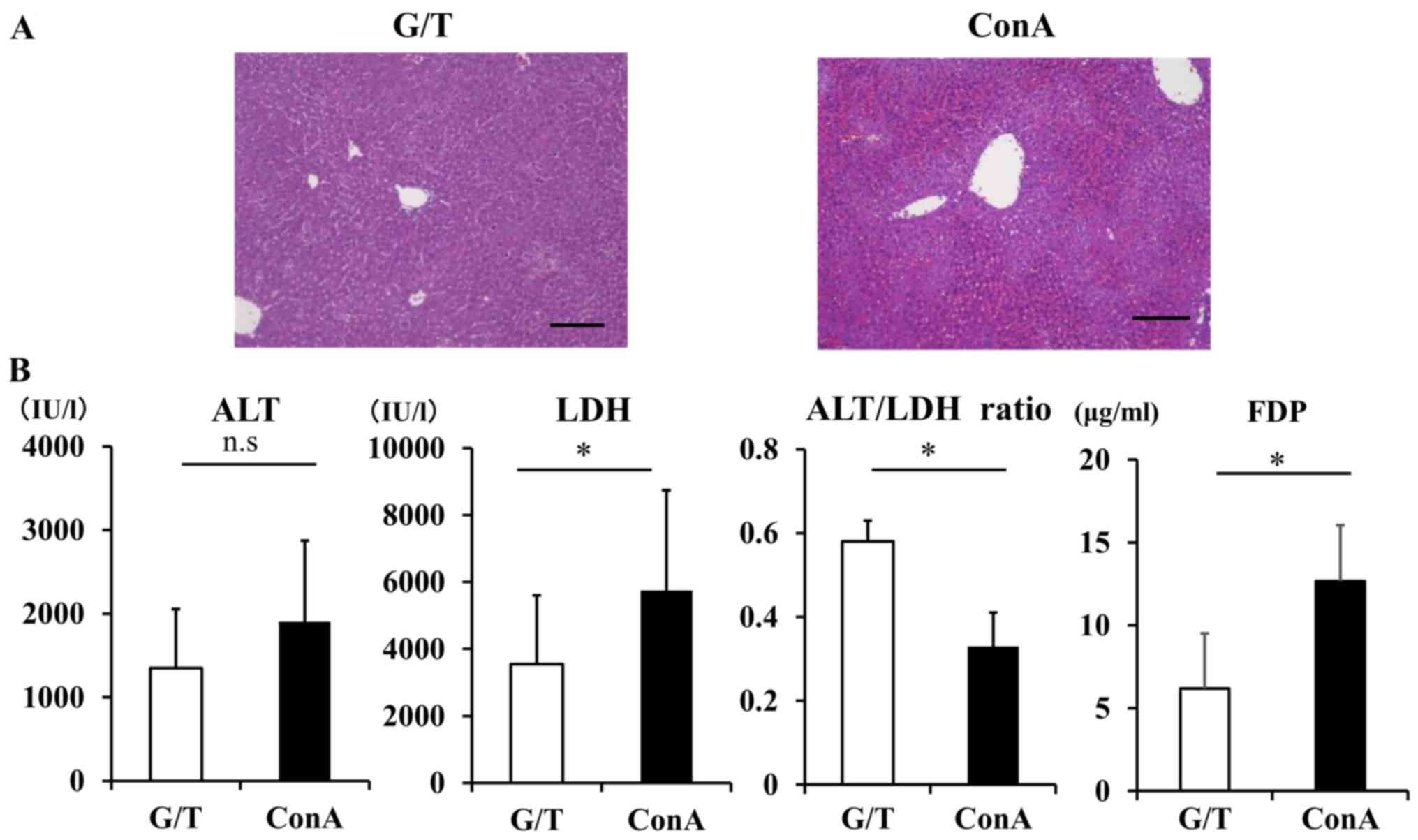
in G/T mice (Fig. 3A). In this
study, peak value of serum ALT in G/T-treated mice were slightly
lower than those in ConA-treated mice, with no statistically
significant difference (Fig. 3B).
On the other hand, serum LDH was significantly elevated in
ConA-treated mice relative to G/T-treated mice (G/T mice vs. ConA
mice; 3541.2±2069.1 vs. 5740.0±3005.1 IU/l, P=0.04). Consequently,
serum ALT/LDH ratio was significantly lower in ConA-treated mice
than G/T-treated mice (G/T mice vs. ConA mice; 0.48±0.05 vs.
0.33±0.08, P=0.04). Serum FDP in ConA-treated mice was
significantly higher than those in G/T-treated mice (G/T mice vs.
ConA mice: 6.19±3.33 vs. 12.67±3.38 µg/ml, P=0.01) (Fig. 3B).
Hepatic mRNA expression of TF and plasminogen
activation inhibitor-1 (PAI-1) were markedly higher in ConA mice
than those in G/T mice (Fig. 4A).
Histological examination showed strong lobular expression of TF and
diffuse distribution of fibrin in ConA mice, but scarcely detected
in G/T mice (Fig. 4B). Hepatic mRNA
expression of HIF-1/2α, LDH-V, and VEGFA was also significantly
upregulated in ConA mice relative to G/T mice (Fig. 4C). Immunohistochemical analysis
revealed their strong expression in the liver from ConA-treated
mice (Fig. 4D, E). The hypoxic area was diffusely
distributed in ConA mice, but faintly detected in G/T mice as
revealed by pimonidazole immunostaining (Fig. 4D). These observations, taken
together, suggest the development of intrahepatic hypoxia and
sinusoidal hypercoagulation in ConA mice relative to G/T mice.
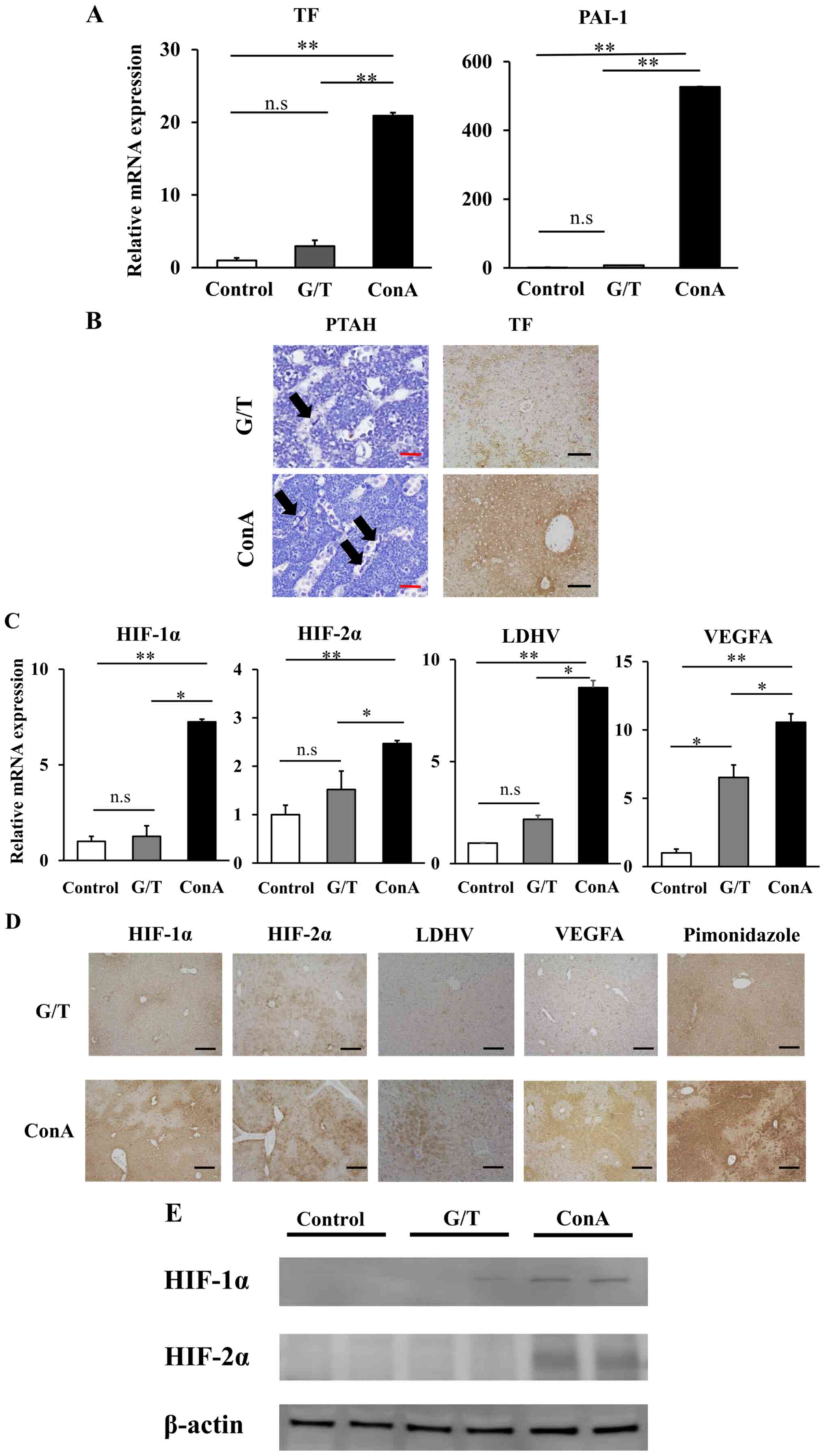 | Figure 4Intrahepatic hyper-coagulation and
hypoxia related proteins expressions of ALI model mice. (A) Hepatic
mRNA expression of TF and PAI-1 were quantified by RT-q PCR. Data
are expressed as the mean ± SE (control mice, n=5; G/T induced ALI
model mice, n=5; ConA induced ALI model mice, n=5). (B) TF staining
(magnification, x100) and PTAH staining (magnification, x400) of GT
and ConA induced ALI model mice liver. The arrows indicate fibrin
depositions in sinusoids. Black Scale bar=100 µm. Red Scale bar=5
µm. (C) Hepatic mRNA expression of HIF-1α, HIF-2α, LDHA and VEGFA
were quantified by RT-qPCR. Data are expressed as the mean ± SE
(control mice, n=5; G/T induced ALI model mice, n=5; ConA induced
ALI model mice, n=5). (D) HIF-1α, HIF-2α, LDH-V, VEGFA and
pimonidazole staining of GT and ConA induced ALI model mice liver
were performed to evaluate intrahepatic hypoxia (magnification,
x100). Black Scale bar=100 µm. (E) Western blot analysis showing
the levels of expression of HIF-1α and HIF-2α in GT and ConA
induced ALI model mice liver. *P<0.05,
**P<0.01. ns, non-significant. ALI, acute liver
injury, RT-q, reverse-transcription quantitative; PAI-1,
plasminogen activation inhibitor-1; ConA, Concanavalin A; PTAH,
phosphotungstic acid-hematoxylin; HIF, hypoxia-inducible factor;
LDH, lactate dehydrogenase; TF, tissue factor. |
Therapeutic effect of anticoagulant in
the ConA-induced mouse model of ALI
Given that ConA mice develop sinusoidal
hypercoagulation and intrahepatic hypoxia during the progression of
ALF, we examined the therapeutic effect of anticoagulant rhTM in
the ConA-induced mouse model of ALI. Surprisingly, the ConA+TM
group showed significantly decreased serum ALT and LDH relative to
the vehicle-treated ConA group (Fig.
5A). Histologically, extensive necrosis was observed in the
ConA group, while necrotic area was obviously smaller in the
ConA+TM group (Fig. 5B). Hepatic
mRNA expressions of TF and PAI-1 were downregulated in the ConA+TM
group relative to the ConA group (Fig.
5C). TF staining was lower in the ConA+TM group than that in
the ConA group, and diffuse sinusoidal fibrin deposition observed
in the ConA group was abolished by rhTM administration (the ConA+TM
group) (Fig. 5D). In addition, rhTM
treatment significantly reduced hepatic expression of LDHV and
VEGFA (Fig. 5E and F). Immunostaining positive area of HIF1-α
and HIF2-α were lower in the ConA+TM group than that in the ConA
group (Fig. 5F). Hepatic mRNA
expression of HIF1-α and HIF2-α in the ConA+TM group tended to be
reduced relative to the ConA group, with no statistical
significance. Importantly, anticoagulant treatment with rhTM
resulted in the reduction of hypoxic area detected by pimonidazole
staining relative to the ConA group (Fig. 5F).
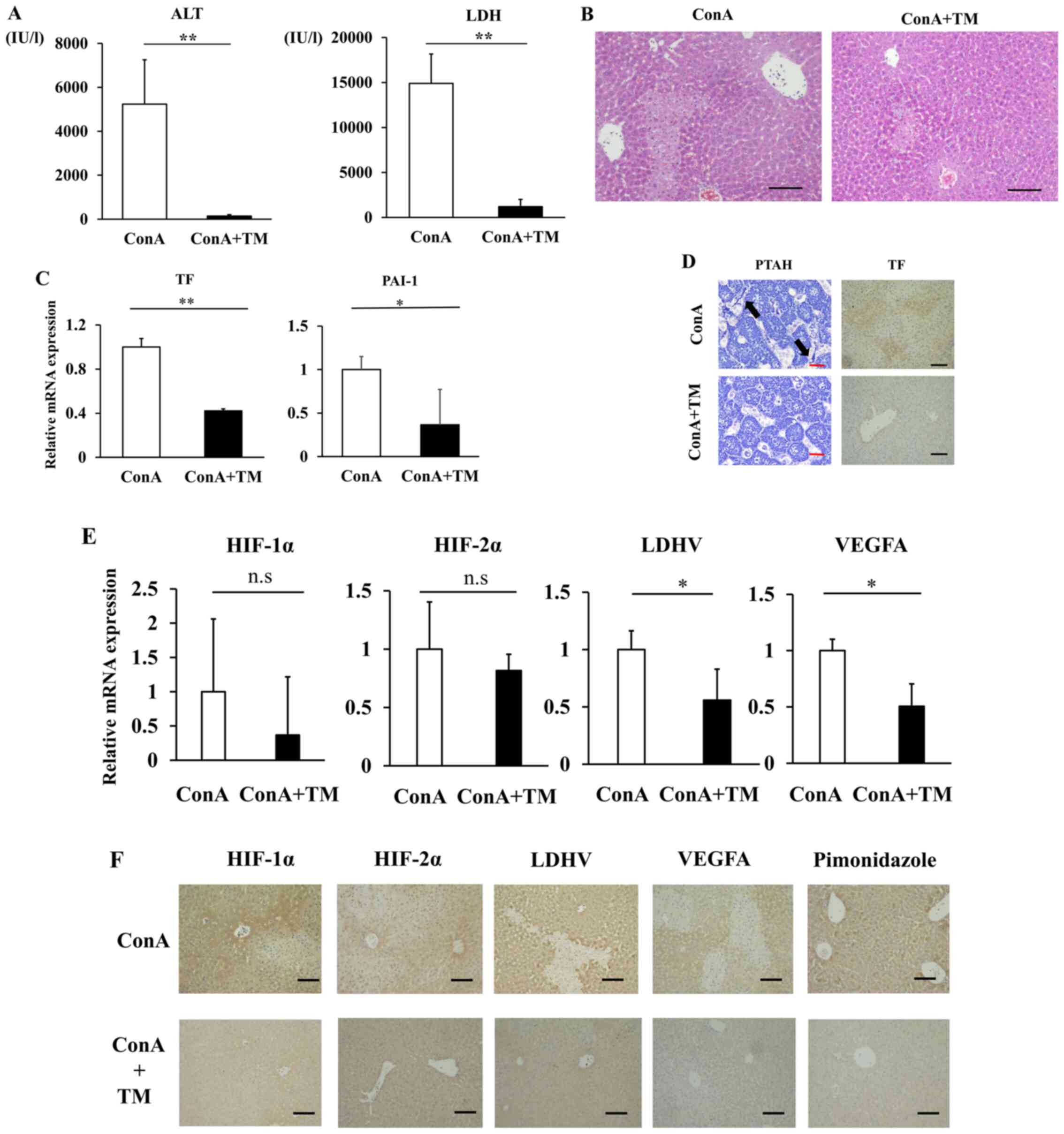 | Figure 5rhTM suppresses liver damage in
ConA-induced ALF model mice. (A) Serum ALT and LDH. Data are
expressed as the mean ± SD. (B) Hematoxylin and eosin staining of
liver sections (magnification, x100). Scale bar=100 µm. (C) Hepatic
mRNA expression levels of TF and PAI-1 were quantified by RT-qPCR.
Data are expressed as the mean ± SE. (D) TF staining
(magnification, x100) and phosphotungstic acid-hematoxylin staining
(magnification, x400) of liver. The arrows indicate fibrin
depositions in sinusoids. Black Scale bar=100 µm. Red Scale bar=5
µm. (E) Hepatic mRNA expression levels of HIF-1α, HIF2α, LDHA and
VEGFA were quantified by RT-qPCR. Data are expressed as the mean ±
SE. (F) HIF-1α, HIF-2α, LDH-V, VEGFA and pimonidazole staining of
liver were performed to evaluate intrahepatic hypoxia
(magnification, x100). Black Scale bar=100 µm.
*P<0.05, **P<0.01. ns, non-significant.
rhTM, human soluble thrombomodulin; ConA, Concanavalin A; ALF,
Acute liver failure; ALT, aminotransferase; LDH, lactate
dehydrogenase ratio; PAI-1, plasminogen activation inhibitor-1;
RT-q, reverse-transcription quantitative; HIF, hypoxia-inducible
factor; TF, tissue factor. |
Discussion
Sinusoidal microcirculatory disturbance has been
thought to be involved in the pathogenesis of ALF (5). However, it is currently unclear
whether it modulates the onset and progression of liver diseases
and if so, how it occurs in any etiologies. In this study, we
classified ALI patients into two groups by serum ALT/LDH ratio; the
low ALT/LDH ratio and high ALT/LDH ratio groups, and found
upregulated FDP and PT-INR and sinusoidal fibrin deposition in the
low ALT/LDH ratio group relative to the high ALT/LDH ratio group.
Because these findings were also accompanied with enhanced hepatic
expression of hypoxia-related genes, it is likely that intrahepatic
hypoxia develops as a result of sinusoidal microcirculatory
disturbance in the livers in the low ALT/LDH ratio group.
Complication with systemic coagulopathies such as disseminated
intravascular coagulation could also increase FDP and PT-INR,
however, fibrin deposition observed locally in the liver suggests
that sinusoidal hypercoagulation is responsible for the apparent
increase in systemic coagulation. Because LDH is known to be
induced under the hypoxic condition, serum ALT/LDH ratio can be a
noninvasive surrogate to evaluate the involvement of intrahepatic
hypoxia in ALI. However, there is no direct evidence for
intrahepatic hypoxia in humans. Cassidy and Reynolds previously
showed that the cut-off value of 1.5 for ALT/LDH ratio can be used
to diagnose the hypoxic hepatitis, which is characterized by
massive liver damage in patients with severe heart failure
(22). In this study, using the
cut-off value of 1.5 for serum ALT/LDH ratio, we successfully
classified ALI patients with and without sinusoidal
hypercoagulation.
To further investigate the correlation between
sinusoidal hypercoagulation and intrahepatic hypoxia, we analyzed
two different animal models of ALI: ConA mice and G/T mice.
Concanavalin A is known to stimulate T-lymphocyte mediated immune
activation and thus promote massive liver injury enhanced by
sinusoidal hypercoagulation (18,25,28).
G/T mice represents parenchymal cell apoptosis model of liver
injury (27). Many therapeutic
approaches have been tested in these models; however, little was
examined for sinusoidal hypercoagulation and intrahepatic hypoxia.
In this study, we found increased expressions of TF and PAI-1 and
histological fibrin deposition in ConA mice but barely observed in
G/T mice. The pimonidazole-stained hypoxic area as well as
upregulation of hypoxia-related genes were markedly extended in
ConA mice relative to G/T mice. Moreover, histological findings in
ConA mice are similar to ALI patients in the low ALT/LDH ratio
group. These observations, taken together, suggest that liver
damages in the low ALT/LDH ratio group as well as ConA mice are
largely enhanced by intrahepatic hypoxia as a result of sinusoidal
hypercoagulation.
In this study, we demonstrated that ALI patients in
the low ALT/LDH ratio show sinusoidal hypercoagulation and
intrahepatic hypoxia. MELD score was higher in the low ALT/LDH
ratio groups than the high ALT/LDH ratio group, but there was no
significant difference in prognosis between the high and low
ALT/LDH ratio groups (P=0.47). Interestingly, HAV and undetermined
etiologies were mostly classified in the low ALT/LDH ratio group,
while AIH and HBV were in the high ALT/LDH ratio group. We analyzed
the history of the patients with undetermined etiologies, but there
was nothing of note. While ALI patients with HAV barely progress to
liver failure, those with HBV and AIH show a poor prognosis and
occasionally require liver transplantation (29-31).
Hepatocyte cell death occurs in the livers from ALI, and there is
evidence that skewing cell death toward apoptosis is correlated to
poor outcome (32-35).
Because apoptosis is an energy-consuming process, hypoxia impairs
ATP production and thus shifts cell death toward necrosis (36). It is conceivable that sinusoidal
hypercoagulation and intrahepatic hypoxia in the low ALT/LDH ratio
group might induce necrosis-dominant cell death, which is
correlated with a favorable prognosis of the low ALT/LDH ratio
group (HAV) relative to high ALT/LDH ratio group (AIH and HBV).
Kato et al previously reported that proinflammatory signals
elicited by IFN-γ and TNFα in both hepatic macrophages and
sinusoidal endothelial cells are important for the development of
sinusoidal hyper coagulation in ConA mice (18). The immune reactions including
specific set of the etiology-dependent proinflammatory cytokines
might provide particular pathology and prognosis of ALI (37-40).
In this study, anticoagulation therapy using rhTM
reduced liver damages in ConA mice, which is consistent with our
previous report of the beneficial effect of rhTM on
acetaminophen-induced ALI mice (41). Given that acetaminophen-induced ALI
is known to trigger sinusoidal hypercoagulation, the data of this
study suggest that sinusoidal hypercoagulation is responsible for
the impaired hepatic microcirculation, and anticoagulation therapy
can attenuate liver damage probably by blood reperfusion.
Therefore, anticoagulation therapy might be useful in ALI patients
with intrahepatic hypoxia as a result of sinusoidal
hypercoagulation, who are classified into the low ALT/LDH ratio
group.
The limitation of this study is the small number of
liver biopsy samples and wide spectrum of background for ALI
patients. For that reason it is not completely certified that serum
ALT/LDH ratio reflect sinusoidal hyper coagulation and intrahepatic
hypoxia, however correlation of serum marker and histological
examination for human and mouse samples might complement the
finding.
In conclusion, we demonstrate that serum ALT/LDH
ratio helps to identify ALI patients with intrahepatic hypoxia as a
result of sinusoidal hypercoagulation. Our data also provide
evidence that sinusoidal hypercoagulation precedes intrahepatic
hypoxia during the course of ALI and thus offer a novel therapeutic
strategy, which might produce appropriate treatment selection and
better prognostic implication.
Supplementary Material
ROC Curves for Coagulopathy Defined as
FDP >10 mg/ml based upon serum ALT/LDH Ratio. ROC was performed
for coagulopathy defined as FDP >10 mg/ml based on ALT/LDH
ratio. Area under the curve (AUC) was 0.77. ROC, Receiver Operating
Characteristic; FDP, fibrin degradation products; ALT,
aminotransferase; LDH, lactate dehydrogenase ratio.
Acknowledgements
Not applicable.
Funding
Funding: The current study was supported in part by Takeda
Science Foundation, Grant-in-Aid for Scientific Research on
Innovative Areas 18H05039 and JSPS KAKENHI (grant nos. JP17K09430
and 19H01054).
Availability of data and materials
The dataset used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AK, MKo, and MKa designed the study. AK performed
experiments. MKu, HS, ST, and KI assisted experiments and data
analyses. AK wrote the initial draft of the manuscript. MKo, MT,
SO, MKa and YO contributed to analysis and interpretation of data.
MKo, MT, MKa and YO assisted in the preparation of the manuscript
and critically reviewed the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Ethics Committee approval was obtained. The study
was conducted in accordance with Declaration of Helsinki ethical
principles. The current study was also performed in accordance with
the Guide for the Care and Use of Laboratory Animals (National
Institutes of Health) and approved by the Animal Care Committee of
Kyushu University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fujiwara K, Mochida S, Matsui A, Nakayama
N, Nagoshi S and Toda G: Intractable Liver Diseases Study Group of
Japan. Fulminant hepatitis and late onset hepatic failure in Japan.
Hepatol Res. 38:646–657. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Starzl TE, Iwatsuki S, Van Thiel DH,
Gartner JC, Zitelli BJ, Malatack JJ, Schade RR, Shaw BW Jr, Hakala
TR, Rosenthal JT and Porter KA: Evolution of liver transplantation.
Hepatology. 2:614–636. 1982.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Polson J and Lee WM: American Association
for the Study of Liver Disease. AASLD position paper: The
management of acute liver failure. Hepatology. 41:1179–1197.
2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rake MO, Flute PT, Pannell G and Williams
R: Intravascular coagulation in acute hepatic necrosis. Lancet.
1:533–537. 1970.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mochida S and Fujiwara K: Symposium on
clinical aspects in hepatitis virus infection. 2. Recent advances
in acute and fulminant hepatitis in Japan. Int Med. 40:175–177.
2001.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hirata K, Ogata I, Ohta Y and Fujiwara K:
Hepatic sinusoidal cell destruction in the development of
intravascular coagulation in acute liver failure of rats. J Pathol.
158:157–165. 1989.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mochida S, Arai M, Ohno A, Yamanobe F,
Ishikawa K, Matsui A, Maruyama I, Kato H and Fujiwara K: Deranged
blood coagulation equilibrium as a factor of massive liver necrosis
following endotoxin administration in partially hepatectomized
rats. Hepatology. 29:1532–1540. 1999.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Vollmar B, Glasz J, Leiderer R, Post S and
Menger MD: Hepatic microcirculatory perfusion failure is a
determinant of liver dysfunction in warm ischemia-reperfusion. Am J
Pathol. 145:1421–1431. 1994.PubMed/NCBI
|
|
9
|
Palmes D, Skawran S, Stratmann U, Armann
B, Minin E, Herbst H and Spiegel HU: Amelioration of
microcirculatory damage by an endothelin a receptor antagonist in a
rat model of reversible acute liver failure. J Hepatol. 42:350–357.
2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hillenbrand P, Parbhoo SP, Jedrychowski A
and Sherlock S: Significance of intravascular coagulation and
fibrinolysis in acute hepatic failure. Gut. 15:83–88.
1974.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kotoh K, Kato M, Kohjima M, Tanaka M,
Miyazaki M, Nakamura K, Enjoji M, Nakamuta M and Takayanagi R:
Lactate dehydrogenase production in hepatocytes is increased at an
early stage of acute liver failure. Exp Ther Med. 2:195–199.
2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gazzard BG, Clark R, Borirakchanyavat V
and Williams R: A controlled trial of heparin therapy in the
coagulation defect of paracetamol-induced hepatic necrosis. Gut.
15:89–93. 1974.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lukacova S, Sørensen BS, Alsner J,
Overgaard J and Horsman MR: The impact of hypoxia on the activity
of lactate dehydrogenase in two different pre-clinical tumour
models. Acta Oncol. 47:941–947. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Selmeci L, Farkas A, Pósch E, Szelényi I
and Sós J: The effect of hypoxia on the lactic dehydrogenase (LDH)
activity of serum and heart muscle of rats. Life Sci. 6:649–653.
1967.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Firth JD, Ebert BL, Pugh CW and Ratcliffe
PJ: Oxygen-regulated control elements in the phosphoglycerate
kinase 1 and lactate dehydrogenase a genes: Similarities with the
erythropoietin 3'enhancer. Proc Natl Acad Sci USA. 91:6496–6500.
1994.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kotoh K, Enjoji M, Kato M, Kohjima M,
Nakamuta M and Takayanagi R: A new parameter using serum lactate
dehydrogenase and alanine aminotransferase level is useful for
predicting the prognosis of patients at an early stage of acute
liver injury: A retrospective study. Comp Hepatol.
7(6)2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Panda S, Jena SK, Nanda R, Mangaraj M and
Nayak P: Ischaemic markers in acute hepatic injury. J Clin Diagn
Res. 10:BC17–BC20. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kato J, Okamoto T, Motoyama H, Uchiyama R,
Kirchhofer D, Van Rooijen N, Enomoto H, Nishiguchi S, Kawada N,
Fujimoto J and Tsutsui H: Interferon-gamma-mediated tissue factor
expression contributes to T-cell-mediated hepatitis through
induction of hypercoagulation in mice. Hepatology. 57:362–372.
2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang Y, Feng D, Wang H, Xu MJ, Park O, Li
Y and Gao B: STAT4 knockout mice are more susceptible to
Concanavalin A-induced T-cell hepatitis. Am J Pathol.
184:1785–1794. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gezginci S and Bolkent S: The effect of
Z-FA.FMK on D-galactosamine/TNF-alpha-induced liver injury in mice.
Cell Biochem Funct. 25:277–286. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Caria CR, Moscato CH, Tomé RB, Pedrazzoli
J Jr, Ribeiro ML and Gambero A: Nitric oxide interferes with
hypoxia signaling during colonic inflammation. Arq Gastroenterol.
51:302–308. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cassidy WM and Reynolds TB: Serum lactic
dehydrogenase in the differential diagnosis of acute hepatocellular
injury. J Clin Gastroenterol. 19:118–121. 1994.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Qu A, Taylor M, Xue X, Matsubara T,
Metzger D, Chambon P, Gonzalez FJ and Shah YM: Hypoxia-inducible
transcription factor 2α promotes steatohepatitis through augmenting
lipid accumulation, inflammation, and fibrosis. Hepatology.
54:472–483. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Engelmann B, Luther T and Müller I:
Intravascular tissue factor pathway-a model for rapid initiation of
coagulation within the blood vessel. Thromb Haemost. 89:3–8.
2003.PubMed/NCBI
|
|
25
|
Tiegs G, Hentschel J and Wendel A: A T
cell-dependent experimental liver injury in mice inducible by
Concanavalin A. J Clin Invest. 90:196–203. 1992.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tagawa Y, Sekikawa K and Iwakura Y:
Suppression of Concanavalin A-induced hepatitis in IFN-gamma(-/-)
mice, but not in TNF-alpha(-/-) mice: Role for IFN-gamma in
activating apoptosis of hepatocytes. J Immunol. 159:1418–1428.
1997.PubMed/NCBI
|
|
27
|
Bradham CA, Plümpe J, Manns MP, Brenner DA
and Trautwein C: Mechanisms of hepatic toxicity. I. TNF-induced
liver injury. Am J Physiol. 275:G387–G392. 1998.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang HX, Liu M, Weng SY, Li JJ, Xie C, He
HL, Guan W, Yuan YS and Gao J: Immune mechanisms of Concanavalin A
model of autoimmune hepatitis. World J Gastroenterol. 18:119–125.
2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Suzuki H, Harada S, Takao S, Takahashi M,
Kato M and Kotoh K: Low-grade elevation of fibrinogen-degradation
products is an important parameter to identify acute presentation
of autoimmune hepatitis. Scand J Gastroenterol. 51:986–993.
2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kogiso T, Sagawa T, Oda M, Yoshiko S,
Kodama K, Taniai M and Tokushige K: Characteristics of acute
hepatitis A virus infection before and after 2001: A hospital-based
study in Tokyo, Japan. J Gastroenterol Hepatol. 34:1836–1842.
2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Manka P, Verheyen J, Gerken G and Canbay
A: Liver failure due to acute viral hepatitis (A-E). Visc Med.
32:80–85. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Rutherford A and Chung RT: Acute liver
failure: Mechanisms of hepatocyte injury and regeneration. Semin
Liver Dis. 28:167–174. 2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Guicciardi ME, Malhi H, Mott JL and Gores
GJ: Apoptosis and necrosis in the liver. Compr Physiol. 3:977–1010.
2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Rutherford AE, Hynan LS, Borges CB,
Forcione DG, Blackard JT, Lin W, Gorman AR, Shaikh OS, Reuben A,
Harrison E, et al: Serum apoptosis markers in acute liver failure:
A pilot study. Clin Gastroenterol Hepatol. 5:1477–1483.
2007.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Rutherford A, King LY, Hynan LS, Vedvyas
C, Lin W, Lee WM and Chung RT: ALF Study Group. Development of an
accurate index for predicting outcomes of patients with acute liver
failure. Gastroenterology. 143:1237–1243. 2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zamaraeva MV, Sabirov RZ, Maeno E,
Ando-Akatsuka Y, Bessonova SV and Okada Y: Cells die with increased
cytosolic ATP during apoptosis: A bioluminescence study with
intracellular luciferase. Cell Death Differ. 12:1390–1397.
2005.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kopec AK and Luyendyk JP: Role of
Fibrin(ogen) in progression of liver disease: Guilt by association?
Semin Thromb Hemost. 42:397–407. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lohse AW, Knolle PA, Bilo K, Uhrig A,
Waldmann C, Ibe M, Schmitt E, Gerken G and Zum Büschenfelde KH:
Antigen-presenting function and B7 expression of murine sinusoidal
endothelial cells and Kupffer cells. Gastroenterology.
110:1175–1181. 1996.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Groeneveld D, Cline-Fedewa H, Baker KS,
Williams KJ, Roth RA, Mittermeier K, Lisman T, Palumbo JS and
Luyendyk JP: Von Willebrand factor delays liver repair after
acetaminophen-induced acute liver injury in mice. J Hepatol.
72:146–155. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Rani R, Tandon A, Wang J, Kumar S and
Gandhi CR: Stellate cells orchestrate Concanavalin a-induced acute
liver damage. Am J Pathol. 187:2008–2019. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kuwano A, Kohjima M, Suzuki H, Yamasaki A,
Ohashi T, Imoto K, Miho Kurokawa M, Morita Y, Kato M and Ogawa Y:
Recombinant human soluble thrombomodulin ameliorates
acetaminophen-induced liver toxicity in mice. Exp Ther Med.
18:1323–1330. 2019.PubMed/NCBI View Article : Google Scholar
|