Introduction
Diabetes, whose global prevalence in adults has
risen from 4.7% in 1980 to 8.5% in 2014, is currently one of the
most prevalent non-communicable diseases threatening global human
health (1). At present, the
etiology and pathogenesis of type 2 diabetes mellitus (T2DM) are
not completely understood. T2DM is characterized by insulin
resistance and pancreatic β-cell hypofunction (2), and is often accompanied by a variety
of complications, such as kidney disease, fundus disease and
neuropathy, that cause damage to various organs and tissues in the
body (3,4).
MicroRNAs (miRNAs/miRs) are single-stranded,
endogenous non-coding RNAs that are 18-22 nucleotides in length,
which regulate gene expression at the post-transcriptional level to
exert their biological effects (5,6).
miRNAs serve important roles in physiological alterations in the
human body, and during the occurrence and development of various
diseases, such as cancer, cardiovascular disease and diabetes
(7).
Alterations in serum miRNA expression levels occur
earlier compared with target genes, they are relatively stable in
body fluids, and they display intercellular shuttle ability and
tissue-specific specificity; therefore, miRNAs are well suited as
biomarkers for the early detection and diagnosis of diseases
(8). It has been reported that
miRNAs serve important roles in the development of islet cell
failure in patients with T2DM (9).
miR-770-5p is a form of mature miR-770and the high
evolutionary conservation of miR-770-5p suggests that it may be of
particular biological significance (10). Several studies have demonstrated
that miR-770 is clinically significant and serves a crucial role in
the carcinogenesis and progression of different types of cancer,
such as non-small cell lung, breast, ovarian and gastric cancer, as
well as hepatocellular carcinoma (11-15).
Moreover, previous studies have indicated the significant role of
miR-770-5p in diabetic nephropathy and gestational diabetes
mellitus (16-18),
suggesting that miR-770-5p serves a key role in the regulation of
cell function (11-18).
It has also been reported that compared with healthy volunteers,
miR-770-5p expression was significantly increased in patients with
T2DM (9); however, the mechanism
underlying miR-770-5p in patients with T2DM is not completely
understood. As the function of islet cells serves critical roles in
T2DM development (19,20), the aim of the present study was to
investigate the expression of miR-770-5p in patients with T2DM, as
well as its role in pancreatic β-cells to provide insight into
potential therapeutic targets for T2DM.
Materials and methods
Clinical samples
Peripheral blood samples from 20 patients with T2DM
(14 male patients and 8 female patients; age, 32-61 years) and 20
healthy subjects (14 male patients and 8 female patients; age,
29-64 years) were collected from the First Affiliated Huai'an
People's Hospital of Nanjing Medical University (Huai'an, China)
between May 2017 and September 2018. T2DM was diagnosed according
to the World Health Organization criteria (21) and was defined as a fasting plasma
glucose of ≥7 mmol/l (126 mg/dl), a 2-h glucose of ≥11.1 mmol/l
(200 mg/dl) in the 75-g Oral Glucose Tolerance Test and/or HbA1c
≥6.5%. The inclusion criteria were as follows: body mass index
<40 and no history of diagnosis of diabetes. The exclusion
criteria were as follows: i) Type 1 diabetes; ii) other types of
diabetes; iii) severe infections; iv) acute cerebrovascular
disease; or iv) those who had recently undergone surgery. There
were no significant differences in age and sex between patients
with T2DM and healthy volunteers. The present study was approved by
the Ethics Committee of The First Affiliated Huai'an People's
Hospital of Nanjing Medical University (approval no.
KY-P-2017-009-01). Written informed consent was obtained from each
patient prior to sample collection. Blood samples were immediately
frozen and stored at -80˚C. To detect the expression levels of
miR-770-5p and BAG5 mRNA in patients with T2DM, serum was isolated
from the peripheral blood samples by centrifugation at 1,500 x g
for 10 min at 4˚C.
Cell culture and treatment
Min6 cells (a mouse insulinoma cell line; American
Type Culture Collection) were cultured in 5 mM glucose DMEM
(Hyclone; Cytiva) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.), 50 mmol/l β-mercaptoethanol (Sigma-Aldrich;
Merck KGaA), 100 U/ml penicillin and 0.1 mg/ml streptomycin
(Sigma-Aldrich; Merck KGaA) at 37˚C with 5% CO2.
Uric acid (UA) was prepared as previously reported
(22). Briefly, UA sodium salt
(10-100 g/ml; Sigma-Aldrich; Merck KGaA) was added to prewarmed
EBM-2 medium (Lonza Group, Ltd.; 37˚C). The mixture was agitated at
37˚C for 30 min and passed through a sterile 0.22-µm filter.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from cells or serum samples
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. Total
RNA (1 µg) was reverse transcribed into cDNA using the PrimeScript
RT reagent kit (Takara Bio, Inc.) according the manufacturer's
protocol. Subsequently, qPCR was performed using SYBR®
Premix Ex Taq™ (Takara Bio, Inc.). The primer sequences used for
qPCR were as follows: U6 forward, 5'-GCT TCGGCAGCACATATACTAAAAT-3'
and reverse, 5'-CGCT TCACGAATTTGCGTGTCAT-3'; GAPDH forward, 5'-CTTT
GGTATCGTGGAAGGACTC-3' and reverse, 5'-GTAGAGGC AGGGATGATGTTCT-3';
miR-770-5p forward, 5'-CCAGTAC CACGTGTCAG-3' and reverse,
5'-GAACATGTCTGCGTAT CTC-3'; BAG5 forward, 5'-TGTCCCCGGGTTTAGGGGTG
TTC-3' and reverse, 5'-TTCACAAGCACTGTCCCGCCC-3'; Bcl-2 forward,
5'-TTGGATCAGGGAGTTGGAAG-3' and reverse, 5'-TGTCCCTACCAACCAGAAGG-3';
and Bax forward, 5'-CGTCCACCAAGAAGCTGAGCG-3 and reverse
5'-CGTCCACCAAGAAGCTGAGCG-3'. The following thermocycling conditions
were used for qPCR: Initial denaturation at 95˚C for 10 min;
followed by 35 cycles of 95˚C for 15 sec and 55˚C for 40 sec. miRNA
and mRNA expression levels were quantified using the
2-ΔΔCq method (23) and normalized to the internal
reference genes U6 and GAPDH, respectively.
Dual luciferase reporter assay
TargetScan (version 7.2; targetscan.org/vert_72) was used to predict the
potential targets of miR-770-5p, and the binding sites between
miR-770-5p and BAG5 were identified. To investigate the association
between miR-770-5p and BAG5, a dual luciferase reporter assay was
performed. The binding site for miR-770-5p in the 3'-untraslated
region (UTR) of BAG5 was constructed using synthetic
oligonucleotides (Beijing Augct DNA-Syn Biotechnology Co., Ltd.)
and cloned into the pmirGLO Dual-Luciferase expression vector
(Promega Corporation) to generate wild-type (WT)-BAG5
(5'-UAGUUGGAGGAUGA AUACUGGAG-3'). The mutated 3'-UTR sequence of
BAG5 was cloned into the pmirGLO Dual-Luciferase expression vector
to generate mutated (MUT)-BAG5 (5'-UAGUUGGAG GAUGAAACUACCAG-3').
Min6 cells (5x104) cultured in 24-well plates were
co-transfected with 100 nM miR-770-5p mimic
(5'-UCCAGUACCACGUGUCAGGGCCA-3') or 100 nM mimic control
(5'-UCGCUUGGUGCAGGUCGG GAA-3') and 1 ng WT-BAG5 or 1 ng MUT-BAG5
reporter plasmids using the Lipofectamine® 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. At 48 h post-transfection, cells were
harvested, and a dual luciferase assay system (Promega Corporation)
was used to detect luciferase activities according to the
manufacturer's protocol. Luciferase activity was normalized to that
of Renilla luciferase activity.
Western blotting
Western blotting was used to measure protein
expression levels. Briefly, cells were lysed using RIPA lysis
buffer (Shanghai Yeasen Biotechnology Co., Ltd.) and centrifuged at
12,000 x g at 4˚C for 5 min. Protein concentrations were determined
using a bicinchoninic acid assay. Total protein (40 µg per lane)
was separated via 10% SDS-PAGE and transferred to PVDF membranes
(Invitrogen; Thermo Fisher Scientific, Inc.). Following blocking in
5% skimmed dry milk at room temperature for 1 h, the membranes were
incubated overnight at 4˚C with primary antibodies targeted against
the following: Caspase-3 (cat. no. 14220; 1:1,000; Cell Signaling
Technology, Inc.), cleaved caspase-3 (cat. no. 9664; 1:1,000; Cell
Signaling Technology, Inc.), Bcl-2 (cat. no. 3498; 1:1,000; Cell
Signaling Technology, Inc.), Bax (cat. no. 2772; 1:1,000; Cell
Signaling Technology, Inc.), BAG5 (cat. no. ab97660; 1:1,000;
Abcam) and β-actin (cat. no. 4970; 1:1,000; Cell Signaling
Technology, Inc.). Subsequently, the membranes were incubated with
horseradish peroxidase-conjugated goat anti-rabbit IgG secondary
antibodies (cat. no. ab205718; 1:2,000; Abcam) for 1 h at room
temperature. Protein bands were visualized using an ECL
chemiluminescence kit (Thermo Fisher Scientific, Inc.). Protein
expression was semi-quantified using Gel-Pro Analyzer densitometry
software (version 6.3; Media Cybernetics, Inc.) with β-actin as the
loading control.
Cell transfection and treatment
Min6 cells were transfected with 100 nM miR-770-5p
inhibitor (5'-UGGCCCUGACACG UGGUACUGGA-3'; Shanghai GenePharma Co.,
Ltd.), 100 nM inhibitor control (5'-CAGUACUUUUGUGUAGUACAA-3';
Shanghai GeneChem Co., Ltd.), 2 µM control-small interfering
(si)RNA (cat. no. sc-37007; Santa Cruz Biotechnology, Inc.), 2 µM
BAG5-siRNA (cat. no. sc-72605; Santa Cruz Biotechnology, Inc.) or
100 nM miR-770-5p inhibitor + 2 µM BAG5-siRNA using
Lipofectamine® 3000 reagent (Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. At 48 h
post-transfection, transfection efficiency was determined via
RT-qPCR.
After transfection, cells were treated with 5 mg/dl
UA at 37˚C for 24 h and then subjected to subsequent experiments.
Cells without any treatment were considered as the control.
MTT assay
Min6 cell viability was measured using an MTT assay
(Beyotime Institute of Biotechnology). Min6 cells were seeded
(5x103 cells/well) into 96-well plates and incubated
overnight in DMEM supplemented with 10% FBS. Following treatment
and transfection, 20 µl MTT solution (5 mg/ml in distilled water)
was added to each well and incubated for 4 h at 37˚C. The medium
was removed and 150 µl DMSO was added to dissolve purple formazan.
The optical density was measured at a wavelength of 490 nm using a
POLARstar OPTIMA multifunctional micro-plate reader (BMG Labtech
GmbH).
Apoptosis analysis
Min6 cell apoptosis was measured by performing flow
cytometry. Min6 cells (106) were digested using 0.2%
trypsin, followed by washing with pre-cooled PBS. Cells were
stained using an Annexin V-FITC/PI kit (cat. no. 70-AP101-100;
Multisciences (Lianke) Biotech Co., Ltd.) according to the
manufacturer's protocol. In brief, Min6 cells were stained with 5
µl Annexin V-FITC and 10 µl PI at room temperature for 5 min in the
dark. Apoptotic cells (early and late apoptosis) were analyzed
using a FACSCalibur flow cytometer (BD Biosciences) and CellQuest
software version 5.1 (BD Biosciences).
Glucose-stimulated insulin secretion
assay
Min6 cells were incubated in glucose-free Krebs
Ringer bicarbonate (KRB) buffer (115 mM NaCl, 4.7 mM KCl, 1.2 mM
MgSO4, 1.2 mM KH2PO4, 20 mM
NaHCO3, 16 mM HEPES and 2.56 mM CaCl2;
Sigma-Aldrich; Merck KGaA) supplemented with 0.2% BSA
(Sigma-Aldrich; Merck KGaA) at 37˚C for 1 h. Subsequently, cells
were treated with KRB buffer with basal glucose (3.3 mM) or high
concentration glucose (16.7 mM; Sigma-Aldrich; Merck KGaA) at 37˚C
for 1 h. The supernatants were collected by centrifugation at 4˚C
for 10 min at 500 x g, and subsequently insulin concentrations were
determined using an Insulin ELISA kit (cat. no. PI602; Beyotime
Institute of Biotechnology) according to the manufacturer's
protocol.
Statistical analysis
Statistical analyses were performed using SPSS
software (version 20.0; IBM Corp.). Comparisons between two groups
were analyzed using a unpaired Student's t-test. Comparisons among
multiple groups were analyzed using one-way ANOVA followed by
Tukey's post hoc test. Data are presented as the mean ± SD of three
independent experiments. P<0.05 was considered to indicate a
statistically significant difference.
Results
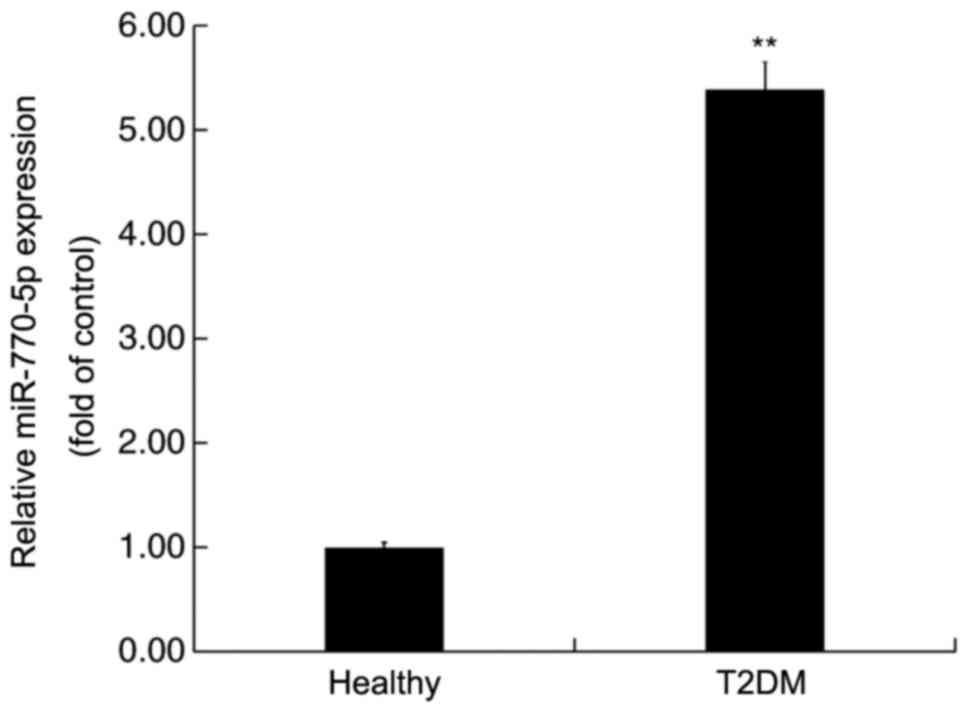
miR-770-5p is highly expressed in the
serum of patients with T2DM
RT-qPCR was performed to detect the expression
levels of miR-770-5p in the serum of patients with T2DM and healthy
volunteers. miR-770-5p expression levels in the serum of patients
with T2DM were significantly higher compared with healthy
volunteers (Fig. 1).
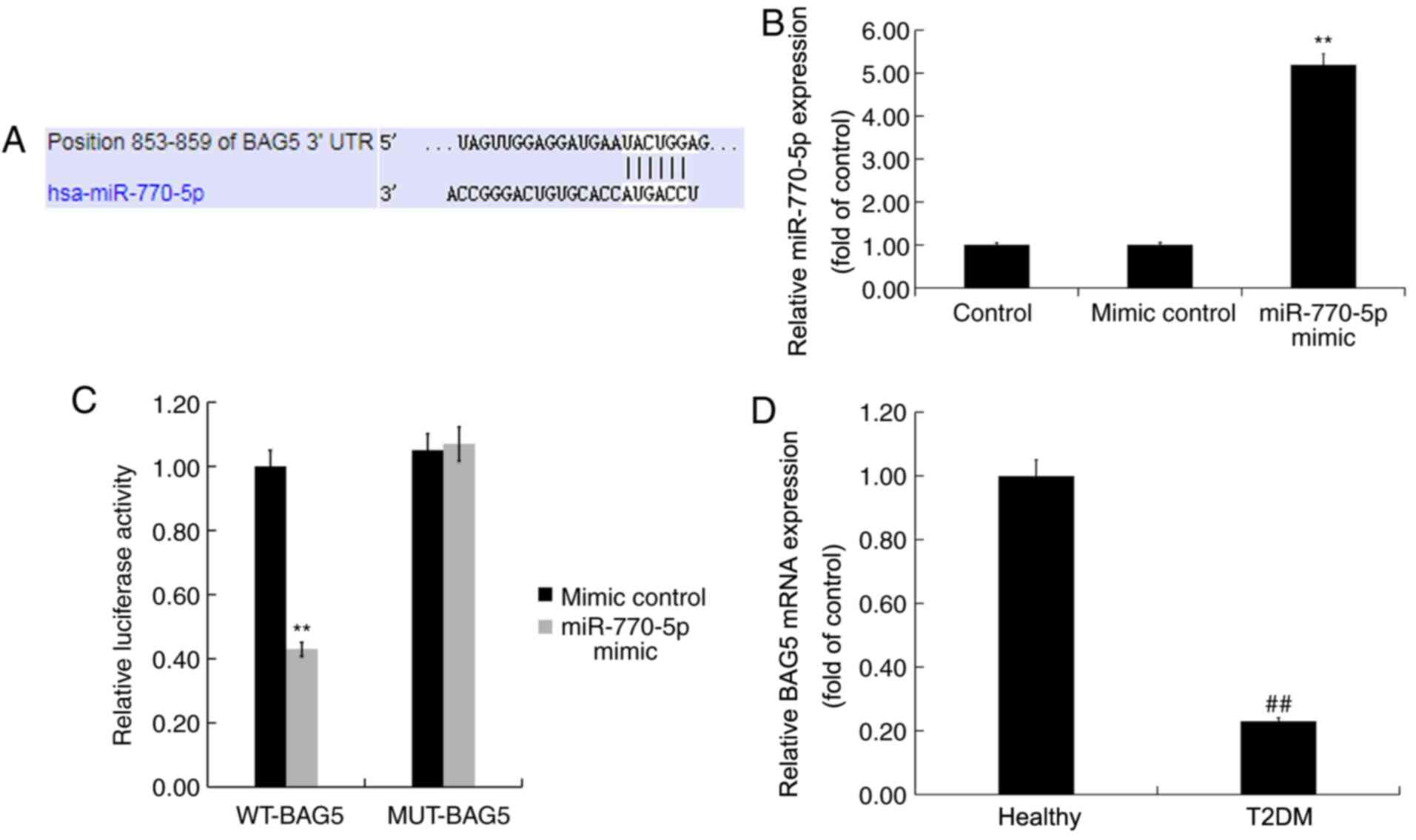
BAG5 is a target gene of
miR-770-5p
TargetScan was used to predict the possible targets
of miR-770-5p. miR-770-5p had numerous potential target genes,
including BAG5 (Fig. 2A). As a
member of the BAG protein family, which has been reported to
enhance cell proliferation and survival (24), BAG5 is involved in several diseases
via regulation of cell proliferation and gene expression (25-28).
Islet β-cell destruction and apoptosis serve an important role in
the development of DM (29).
However, the role of BAG5 in T2DM, and the relationship between
miR-770-5p and BAG5 are not completely understood. Thus, BAG5 was
selected for further examination in the present study. The dual
luciferase reporter assay is a well-established experimental method
for identifying whether a miRNA directly binds to its target gene
(30,31). Therefore, to determine whether
miR-770-5p directly targeted BAG5, a dual luciferase reporter assay
was used. Compared with the mimic control group, miR-770-5p mimic
significantly increased miR-770-5p expression levels in Min6 cells
(Fig. 2B). Subsequently, Min6 cells
were co-transfected with WT-BAG5 or MUT-BAG5 and miR-770-5p mimic
or mimic control. The results indicated that miR-770-5p mimic- +
WT-BAG5-transfected cells displayed significantly reduced levels of
luciferase activity compared with mimic control- +
WT-BAG5-transfected cells (Fig.
2C). By contrast, the luciferase activities of miR-770-5p
mimic- + MUT-BAG5-transfected cells were not significantly altered
compared with mimic control- + MUT-BAG5-transfected cells,
suggesting that miR-770-5p directly targeted the 3'-UTR of
BAG5.
RT-qPCR was performed to detect the mRNA expression
levels of BAG5 in the serum of patients with T2DM and healthy
volunteers. BAG5 mRNA expression levels in the serum of patients
with T2DM were significantly decreased compared with healthy
volunteers (Fig. 2D).
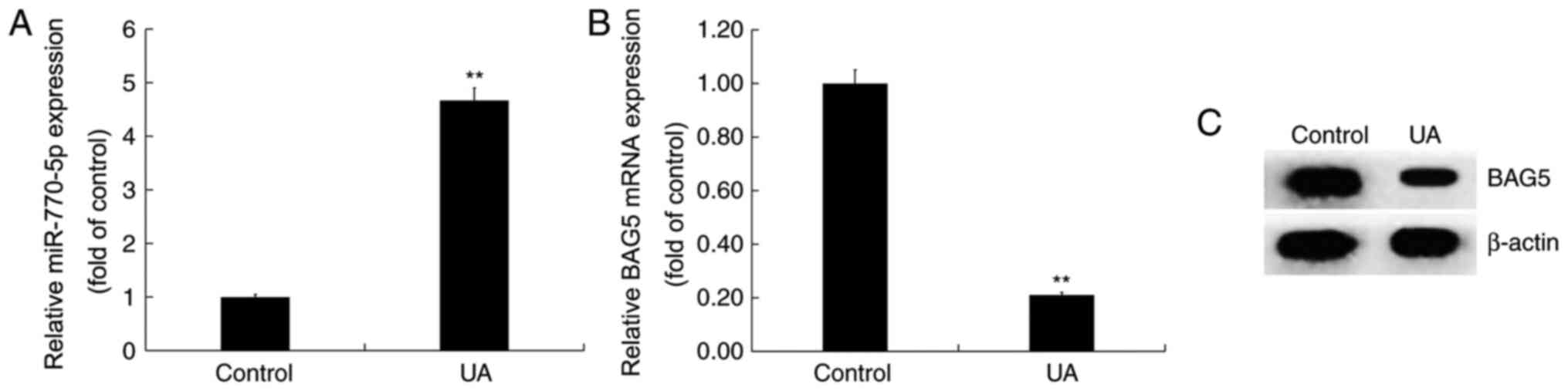
UA treatment upregulates miR-770-5p
expression and downregulates BAG5 expression in Min6 cells
Min6 cells were treated with UA (5 mg/dl) for 24 h.
Compared with the control group, miR-770-5p expression was
significantly increased in the UA treatment group (Fig. 3A). By contrast, the mRNA and protein
expression levels of BAG5 were decreased in UA-treated Min6 cells
compared with control cells (Fig.
3B and C).
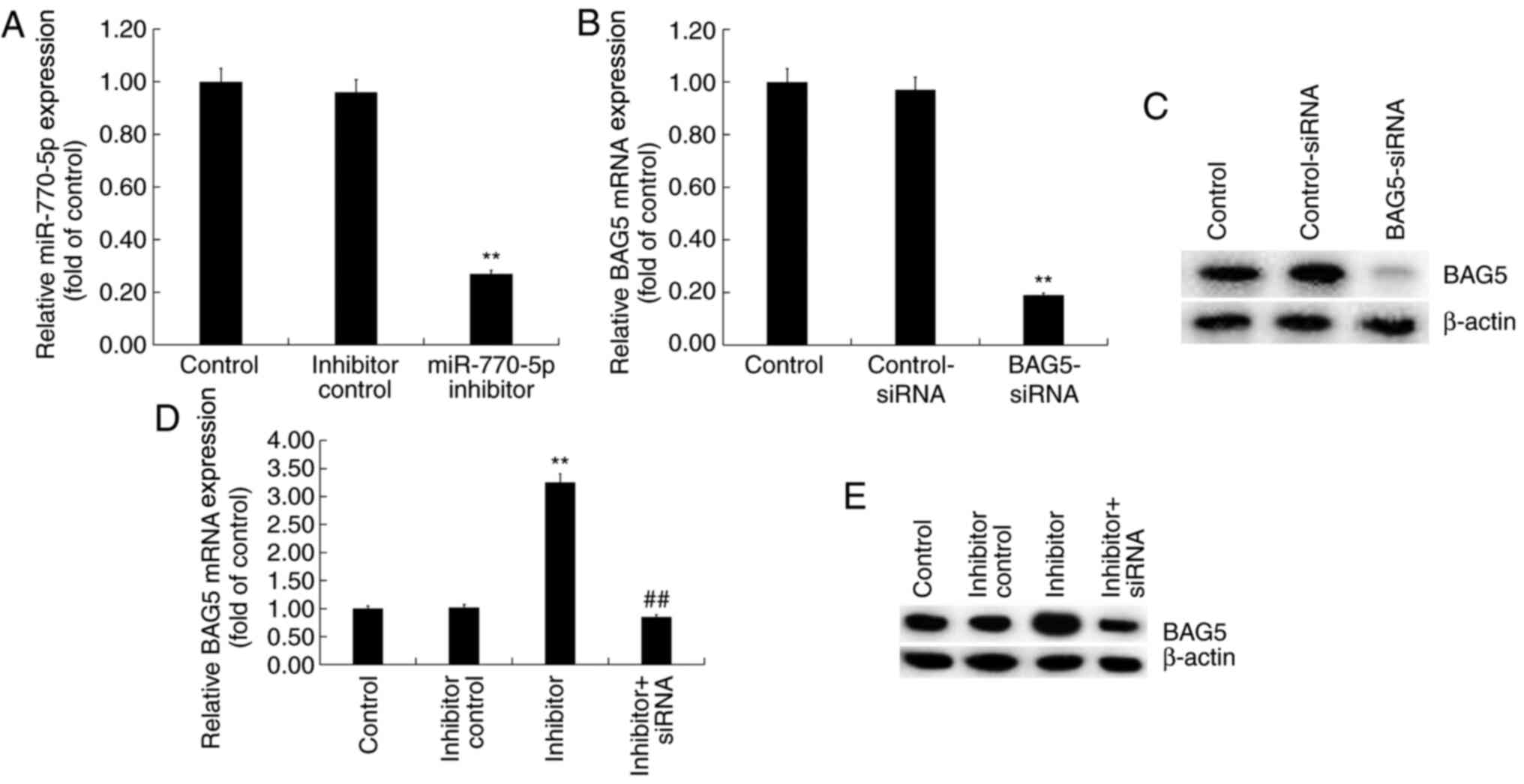
miR-770-5p negatively regulates BAG5
expression in Min6 cells
Min6 cells were transfected with control-siRNA,
BAG5-siRNA, miR-770-5p inhibitor, inhibitor control or BAG5-siRNA +
miR-770-5p inhibitor for 48 h. The RT-qPCR results demonstrated
that miR-770-5p inhibitor significantly decreased miR-770-5p
expression levels in Min6 cells compared with inhibitor control
(Fig. 4A). BAG5-siRNA markedly
reduced BAG5 mRNA and protein expression levels in Min6 cells
compared with control-siRNA (Fig.
4B and C). Moreover, compared
with the inhibitor control group, miR-770-5p inhibitor increased
the mRNA (Fig. 4D) and protein
(Fig. 4E) expression levels of BAG5
in Min6 cells, which was reversed by co-transfection with
BAG5-siRNA.
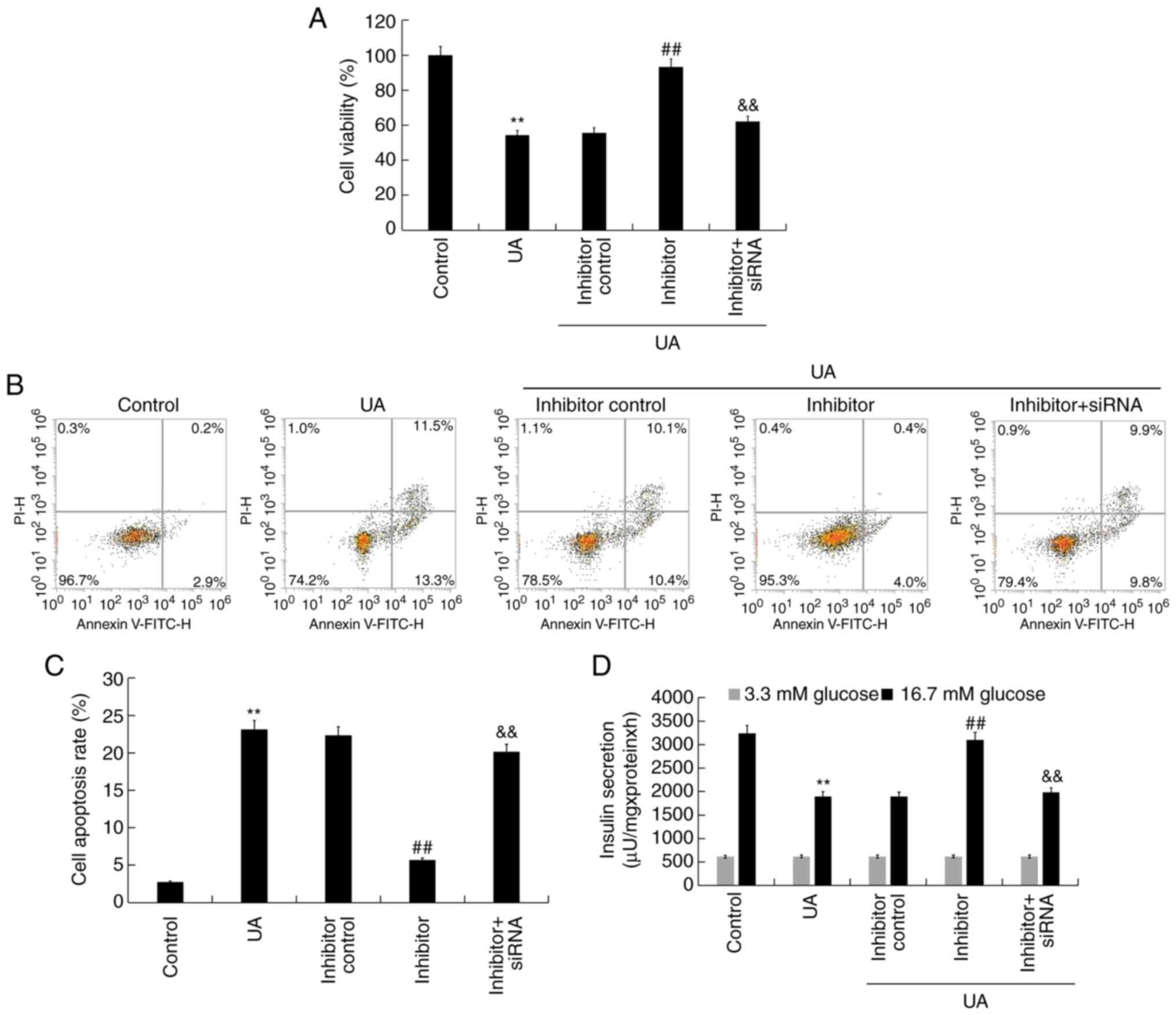
miR-770-5p inhibitor alleviates
UA-induced injury and dysfunction in Min6 cells
To explore the effect of miR-770-5p knockdown on
UA-induced Min6 cell injury and dysfunction, Min6 cells were
transfected with inhibitor control, miR-770-5p inhibitor, or
miR-770-5p inhibitor + BAG5-siRNA for 48 h, and subsequently
treated with UA (5 mg/dl) for 24 h. Cell viability, apoptosis and
insulin secretion were assessed. Compared with UA treatment alone,
miR-770-5p inhibitor significantly increased cell viability
(Fig. 5A) and decreased cell
apoptosis (Fig. 5B and C) in UA-treated Min6 cells. Similarly,
miR-770-5p inhibitor significantly increased insulin secretion from
UA- and glucose-treated Min6 cells compared with UA treatment alone
(Fig. 5D). Compared with the
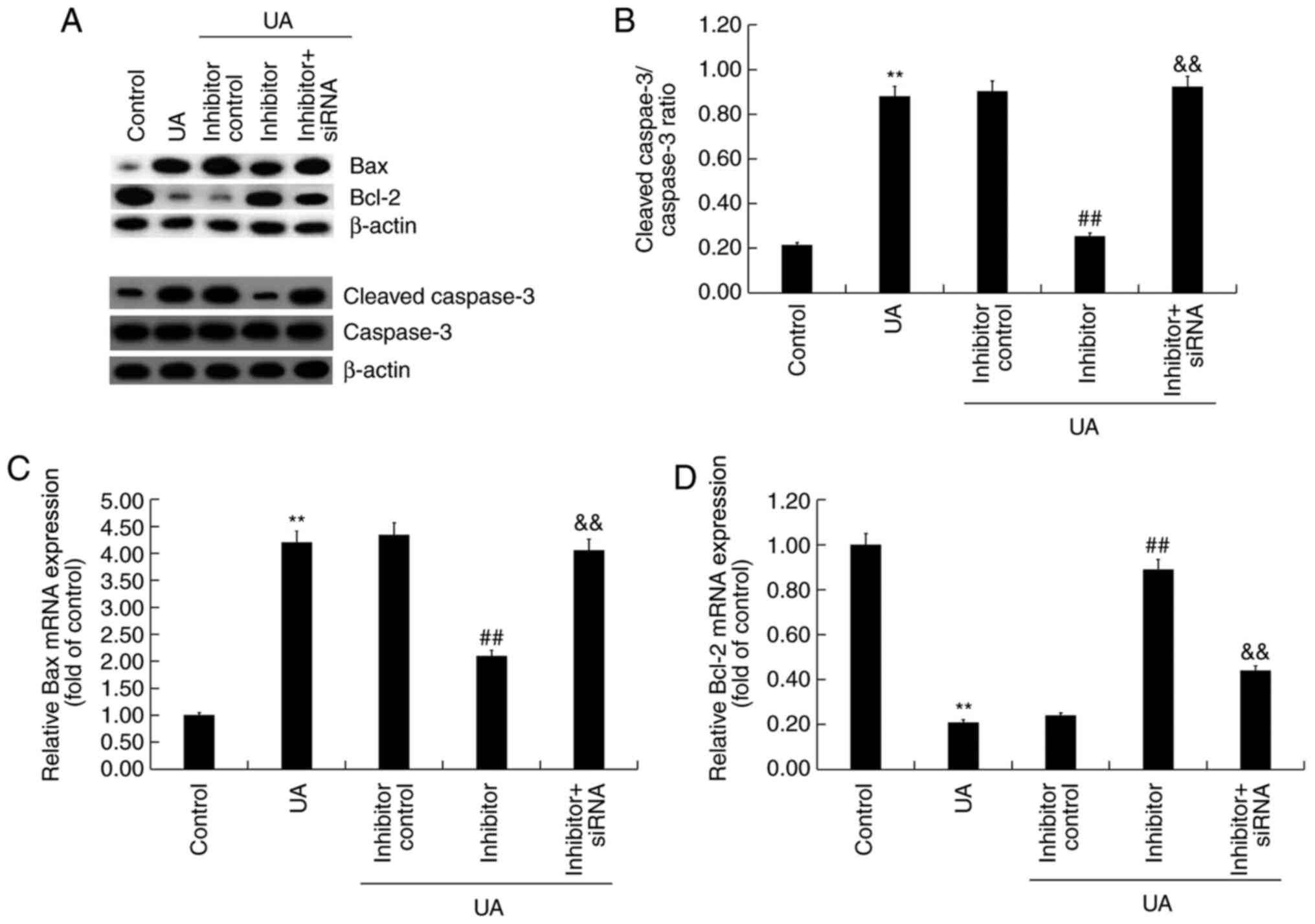
control group, UA treatment markedly increased Bax and
cleaved-caspase-3 protein expression levels, reduced Bcl-2 protein
expression levels and upregulated the ratio of
cleaved-caspase-3/caspase-3 in Min6 cells (Fig. 6A and B). miR-770-5p inhibitor markedly decreased
Bax and cleaved-caspase-3 protein expression levels, increased
Bcl-2 protein expression levels, and downregulated the ratio of
cleaved-caspase-3/caspase-3 in UA-treated Min6 cells compared with
the UA + inhibitor control treatment group. Moreover, compared with
the control group, UA treatment significantly increased Bax mRNA
expression levels and decreased Bcl-2 mRNA expression levels in
Min6 cells (Fig. 6C and D). However, miR-770-5p inhibitor
significantly decreased Bax expression levels and increased Bcl-2
mRNA expression levels in UA-treated Min6 cells compared with UA
treatment alone. Furthermore, miR-770-5p inhibitor-mediated effects
on UA-treated Min6 cells were significantly reversed by
co-transfection with BAG5-siRNA.
Discussion
According to relevant epidemiological statistics,
the number of new cases of diabetes mellitus (DM) worldwide is
increasing on a yearly basis (32).
Although multiple pathogenic mechanisms underlying DM complications
have been identified, the clinical results achieved are limited and
the exact pathogenesis is not completely understood. With
continuous advances in molecular biotechnology, the discovery of
miRNAs has provided a novel insight into the investigation of DM
(33). An increasing number of
studies have demonstrated that miRNAs serve important roles in the
pathological processes underlying DM (9,33,34).
In the present study, the expression of miR-770-5p and its target
gene BAG5 in patients with T2DM was assessed. miR-770-5p expression
was significantly increased in the serum of patients with T2DM
compared with healthy volunteers. By contrast, BAG5 expression was
significantly decreased in the serum of patients with T2DM compared
with healthy volunteers.
The relationship between T2DM and serum UA levels
has been investigated (35). A
number of studies have reported that the risk of T2DM was greatly
increased under high UA conditions (36,37).
Enhanced UA levels not only resulted in insulin resistance but also
negatively affected pancreatic β-cell survival and insulin release
(38). The Min6 cell line has been
used to investigate pancreatic β-cell function in vitro
(38,39). In the present study, UA-treated Min6
cells were used to explore the effect of miR-770-5p on UA-induced
pancreatic β-cell damage and dysfunction.
Islet β-cell destruction, apoptosis and
dedifferentiation can result in the loss of islet β-cells, which
decreases insulin secretion resulting in blood sugar level
disorders, ultimately leading to diabetes and the associated
complications (29). miRNAs
destruct multiple target genes, such as C-C chemokine receptor type
7 and CD247, in islet β-cells (40). Apoptosis refers to the autonomous
and orderly death of cells under the regulation of genes in order
to maintain the homeostasis of the internal environment (41). Islet β-cell apoptosis is a primary
cause of DM and islet β-cell function damage is also closely
associated with DM. Islet β-cell function damage mechanisms may be
associated with oxidative stress, glucose toxicity, lipotoxicity
and hypoxia. Inflammation caused by these mechanisms may initially
maintain and repair the function of pancreatic islet β-cell, but
persistent inflammatory reactions can cause damage and permanent
destruction to islet β-cells (42,43).
Several miRNAs have been reported to be involved in islet β-cell
apoptosis, including miR-23a-3p, miR-23b-3p and miR-149-5p. These
miRNAs are associated with type 1 diabetes mellitus via
downregulating Bcl-2 expression, which promotes islet β-cell
apoptosis (44). miR-770-5p, which
has been studied in several types of cancer, such as non-small cell
lung, breast, ovarian and gastric cancer, as well as hepatocellular
carcinoma (11-15),
was reported to serve key roles in diabetic nephropathy and
gestational diabetes mellitus (16-18).
A recent study indicated that compared with healthy volunteers,
miR-770-5p expression was upregulated in patients with gestational
diabetes mellitus, and it can regulate INS-1 insulinoma cell
proliferation, apoptosis and insulin secretion by targeting tumor
protein p53 regulated inhibitor of apoptosis 1(18). However, the roles and mechanism
underlying miR-770-5p in pancreatic β-cells are not completely
understood.
In the present study, BAG5 was identified as a
direct target of miR-770-5p. BAG5 expression was significantly
downregulated in patients with T2DM and UA-treated Min6 cells
compared with healthy volunteers and control cells, respectively.
BAG5 is a member of the BAG protein family, which enhance cell
proliferation and survival (24).
Previous studies have demonstrated that BAG5 is involved in several
diseases, including cancer and Alzheimer's disease, via regulation
of apoptosis and gene expression (25-28).
Therefore, it was hypothesized that miR-770-5p may serve an
important role in T2DM via regulating islet β-cell apoptosis by
targeting BAG5. The effects of miRNA-770-5p on islet β-cell damage
and dysfunction were determined by assessing cell viability using
an MTT assay, detecting apoptosis via flow cytometry, and measuring
the expression levels of apoptosis-associated genes and proteins
via RT-qPCR and western blotting, respectively. The results
demonstrated that UA treatment reduced cell viability, promoted
apoptosis, increased the cleaved-caspase-3/caspase-3 ratio and Bax
expression levels, and decreased Bcl-2 expression levels in Min6
cells compared with the control group. However, UA
treatment-mediated effects were significantly attenuated by
transfection with miR-770-5p inhibitor. Moreover,
miR-770-5p-mediated effects on UA-treated Min6 cells were
significantly reversed by co-transfection with BAG5-siRNA. The
aforementioned results were consistent with a previous study that
reported that miRNAs are involved in islet β-cell destruction and
apoptosis (44).
Insulin is a protein hormone secreted by islet
β-cells that are stimulated by endogenous or exogenous substances
(45). Insulin serves an important
role in the balance of blood sugar levels in the human body,
whereby insufficiency results in hyperglycemia, DM and DM
complications (46). Previous
studies have reported that miRNAs can regulate the synthesis and
secretion of insulin (47,48) and regulate the blood sugar balance
in the human body (49). miRNAs not
only promote insulin synthesis and secretion, but also serve a
negative regulatory role in insulin synthesis and secretion
(50-52).
In the present study, the effects of miR-770-5p on insulin
secretion were determined. The results demonstrated that compared
with the control group, UA treatment significantly reduced insulin
secretion in Min6 cells, which was significantly reversed by
transfection with miR-770-5p inhibitor. However, miR-770-5p
inhibitor-mediated effects on insulin secretion in UA-treated Min6
cells were significantly reversed by co-transfection with
BAG5-siRNA. A key limitation of the present study was that a UA +
miR-770-5p inhibitor + control-siRNA group was not included.
In conclusion, the results of the present study
demonstrated that miR-770-5p expression was significantly
upregulated in patients with T2DM compared with healthy volunteers.
Compared with the inhibitor control group, miR-770-5p knockdown
increased cell viability, reduced cell apoptosis, regulated the
expression of apoptosis-related genes and increased insulin
secretion in UA-treated pancreatic β-cells by targeting BAG5, thus
suppressing the development and/or progression of T2DM. The results
of the present study provided novel insight into potential
prevention and treatment strategies for T2DM. However, the present
study was only a preliminary study of the role of miR-770-5p in
T2DM. Therefore, in vivo studies are required to confirm the
role of miR-770-5p in T2DM. Additionally, the relationship between
miR-770-5p and BAG5 expression levels and the clinicopathological
characteristics of patients with T2DM should be investigated in
future studies.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MW contributed to designing the study, collecting,
analyzing and interpreting the data, and preparing the manuscript.
JW, TJ and KZ contributed to collecting, analyzing and interpreting
the data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The First Affiliated Huai'an People's Hospital of
Nanjing Medical University (approval no. KY-P-2017-009-01). Written
informed consent was obtained from each patient prior to sample
collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kanter JE and Bornfeldt KE: Impact of
Diabetes Mellitus. Arterioscler Thromb Vasc Biol. 36:1049–1053.
2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ashcroft FM and Rorsman P: Diabetes
mellitus and the β cell: The last ten years. Cell. 148:1160–1171.
2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wright E Jr, Scism-Bacon JL and Glass LC:
Oxidative stress in type 2 diabetes: The role of fasting and
postprandial glycaemia. Int J Clin Pract. 60:308–314.
2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Oelze M, Schuhmacher S and Daiber A:
Organic nitrates and nitrate resistance in diabetes: The role of
vascular dysfunction and oxidative stress with emphasis on
antioxidant properties of pentaerithrityl tetranitrate. Exp
Diabetes Res. 2010(213176)2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lund E, Güttinger S, Calado A, Dahlberg JE
and Kutay U: Nuclear export of microRNA precursors. Science.
303:95–98. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Han J, Lee Y, Yeom KH, Nam JW, Heo I, Rhee
JK, Sohn SY, Cho Y, Zhang BT and Kim VN: Molecular basis for the
recognition of primary microRNAs by the Drosha-DGCR8 complex. Cell.
125:887–901. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Flynt AS and Lai EC: Biological principles
of microRNA-mediated regulation: Shared themes amid diversity. Nat
Rev Genet. 9:831–842. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Camussi G, Deregibus MC, Bruno S,
Cantaluppi V and Biancone L: Exosomes/microvesicles as a mechanism
of cell-to-cell communication. Kidney Int. 78:838–848.
2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang C, Wan S, Yang T, Niu D, Zhang A,
Yang C, Cai J, Wu J, Song J, Zhang CY, et al: Increased serum
microRNAs are closely associated with the presence of microvascular
complications in type 2 diabetes mellitus. Sci Rep.
6(20032)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Guo J, Han J, Liu J and Wang S:
MicroRNA-770-5p contributes to podocyte injury via targeting E2F3
in diabetic nephropathy. Braz J Med Biol Res.
53(e9360)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li Y, Liang Y, Sang Y, Song X, Zhang H,
Liu Y, Jiang L and Yang Q: miR-770 suppresses the chemo-resistance
and metastasis of triple negative breast cancer via direct
targeting of STMN1. Cell Death Dis. 9(14)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang Z, Yang Y and Zhang X: miR-770
inhibits tumorigenesis and EMT by targeting JMJD6 and regulating
WNT/β-catenin pathway in non-small cell lung cancer. Life Sci.
188:163–171. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wu WJ, Shi J, Hu G, Yu X, Lu H, Yang ML,
Liu B and Wu ZX: Wnt/β-catenin signaling inhibits FBXW7 expression
by upregulation of microRNA-770 in hepatocellular carcinoma. Tumour
Biol. 37:6045–6051. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Guo W, Dong Z, Liu S, Qiao Y, Kuang G, Guo
Y, Shen S and Liang J: Promoter hypermethylation-mediated
downregulation of miR-770 and its host gene MEG3, a long non-coding
RNA, in the development of gastric cardia adenocarcinoma. Mol
Carcinog. 56:1924–1934. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Zhao H, Yu X, Ding Y, Zhao J, Wang G, Wu
X, Jiang J, Peng C, Guo GZ and Cui S: miR-770-5p inhibits cisplatin
chemoresistance in human ovarian cancer by targeting ERCC2.
Oncotarget. 7:53254–53268. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang L and Li H: miR-770-5p facilitates
podocyte apoptosis and inflammation in diabetic nephropathy by
targeting TIMP3. Biosci Rep. 40(BSR20193653)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang SZ, Qiu XJ, Dong SS, Zhou LN, Zhu Y,
Wang MD and Jin LW: MicroRNA-770-5p is involved in the development
of diabetic nephropathy through regulating podocyte apoptosis by
targeting TP53 regulated inhibitor of apoptosis 1. Eur Rev Med
Pharmacol Sci. 23:1248–1256. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang YL and Chen XQ: Dysregulation of
microRNA-770-5p influences pancreatic-β-cell function by targeting
TP53 regulated inhibitor of apoptosis 1 in gestational diabetes
mellitus. Eur Rev Med Pharmacol Sci. 24:793–801. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Böni-Schnetzler M and Meier DT: Islet
inflammation in type 2 diabetes. Semin Immunopathol. 41:501–513.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mezza T, Cinti F, Cefalo CMA, Pontecorvi
A, Kulkarni RN and Giaccari A: β-Cell Fate in Human Insulin
Resistance and Type 2 Diabetes: A Perspective on Islet Plasticity.
Diabetes. 68:1121–1129. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Alberti KG and Zimmet PZ: Definition,
diagnosis and classification of diabetes mellitus and its
complications. Part 1: Diagnosis and classification of diabetes
mellitus provisional report of a WHO consultation. Diabet Med.
15:539–553. 1998.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kanellis J, Watanabe S, Li JH, Kang DH, Li
P, Nakagawa T, Wamsley A, Sheikh-Hamad D, Lan HY, Feng L, et al:
Uric acid stimulates monocyte chemoattractant protein-1 production
in vascular smooth muscle cells via mitogen-activated protein
kinase and cyclooxygenase-2. Hypertension. 41:1287–1293.
2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Δ Δ C(T)) Method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Townsend PA, Cutress RI, Sharp A, Brimmell
M and Packham G: BAG-1: A multifunctional regulator of cell growth
and survival. Biochim Biophys Acta. 1603:83–98. 2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bruchmann A, Roller C, Walther TV, Schäfer
G, Lehmusvaara S, Visakorpi T, Klocker H, Cato AC and Maddalo D:
Bcl-2 associated athanogene 5 (Bag5) is overexpressed in prostate
cancer and inhibits ER-stress induced apoptosis. BMC Cancer.
13(96)2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Guo K, Li L, Yin G, Zi X and Liu L: Bag5
protects neuronal cells from amyloid β-induced cell death. J Mol
Neurosci. 55:815–820. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gupta MK, Tahrir FG, Knezevic T, White MK,
Gordon J, Cheung JY, Khalili K and Feldman AM: GRP78 Interacting
Partner Bag5 Responds to ER Stress and Protects Cardiomyocytes From
ER Stress-Induced Apoptosis. J Cell Biochem. 117:1813–1821.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang X, Guo J, Fei E, Mu Y, He S, Che X,
Tan J, Xia K, Zhang Z, Wang G, et al: BAG5 protects against
mitochondrial oxidative damage through regulating PINK1
degradation. PLoS One. 9(e86276)2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Xue M and Jackson CJ: Activated protein C
and its potential applications in prevention of islet β-cell damage
and diabetes. Vitam Horm. 95:323–363. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liu Q and Axtell MJ: Quantitating plant
microRNA-mediated target repression using a dual-luciferase
transient expression system. Methods Mol Biol. 1284:287–303.
2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang Z, Zhu Z, Lin Z, Luo Y, Liang Z,
Zhang C, Chen J and Peng P: miR-429 suppresses cell proliferation,
migration and invasion in nasopharyngeal carcinoma by
downregulation of TLN1. Cancer Cell Int. 19(115)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wild S, Roglic G, Green A, Sicree R and
King H: Global prevalence of diabetes: Estimates for the year 2000
and projections for 2030. Diabetes Care. 27:1047–1053.
2004.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Tiwari J, Gupta G, de Jesus Andreoli Pinto
T, Sharma R, Pabreja K, Matta Y, Arora N, Mishra A, Sharma R and
Dua K: Role of microRNAs (miRNAs) in the pathophysiology of
diabetes mellitus. Panminerva Med. 60:25–28. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhang Y, Sun X, Icli B and Feinberg MW:
Emerging Roles for MicroRNAs in Diabetic Microvascular Disease:
Novel Targets for Therapy. Endocr Rev. 38:145–168. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Behradmanesh S, Horestani MK, Baradaran A
and Nasri H: Association of serum uric acid with proteinuria in
type 2 diabetic patients. J Res Med Sci. 18:44–46. 2013.PubMed/NCBI
|
|
36
|
Ye X, Cao Y, Gao F, Yang Q, Zhang Q, Fu X,
Li J and Xue Y: Elevated serum uric acid levels are independent
risk factors for diabetic foot ulcer in female Chinese patients
with type 2 diabetes. J Diabetes. 6:42–47. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Viazzi F, Piscitelli P, Giorda C, Ceriello
A, Genovese S, Russo G, Guida P, Fioretto P, De Cosmo S and
Pontremoli R: AMD-Annals Study Group. Metabolic syndrome, serum
uric acid and renal risk in patients with T2D. PLoS One.
12(e0176058)2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ding Y, Shi X, Shuai X, Xu Y, Liu Y, Liang
X, Wei D and Su D: Luteolin prevents uric acid-induced pancreatic
β-cell dysfunction. J Biomed Res. 28:292–298. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Adeyemo AA, Zaghloul NA, Chen G, Doumatey
AP, Leitch CC, Hostelley TL, Nesmith JE, Zhou J, Bentley AR,
Shriner D, et al: South Africa Zulu Type 2 Diabetes Case-Control
Study: ZRANB3 is an African-specific type 2 diabetes locus
associated with beta-cell mass and insulin response. Nat Commun.
10(3195)2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Fornari TA, Donate PB, Assis AF, Macedo C,
Sakamoto-Hojo ET, Donadi EA and Passos GA: Comprehensive Survey of
miRNA-mRNA Interactions Reveals That Ccr7 and Cd247 (CD3 zeta) are
Posttranscriptionally Controlled in Pancreas Infiltrating T
Lymphocytes of Non-Obese Diabetic (NOD) Mice. PLoS One.
10(e0142688)2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gerber PA and Rutter GA: The Role of
Oxidative Stress and Hypoxia in Pancreatic Beta-Cell Dysfunction in
Diabetes Mellitus. Antioxid Redox Signal. 26:501–518.
2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Fu Z, Gilbert ER and Liu D: Regulation of
insulin synthesis and secretion and pancreatic Beta-cell
dysfunction in diabetes. Curr Diabetes Rev. 9:25–53.
2013.PubMed/NCBI
|
|
44
|
Grieco FA, Sebastiani G, Juan-Mateu J,
Villate O, Marroqui L, Ladrière L, Tugay K, Regazzi R, Bugliani M,
Marchetti P, et al: MicroRNAs miR-23a-3p, miR-23b-3p, and
miR-149-5p Regulate the Expression of Proapoptotic BH3-Only
Proteins DP5 and PUMA in Human Pancreatic β-Cells. Diabetes.
66:100–112. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhao X, Mohan R, Özcan S and Tang X:
MicroRNA-30d induces insulin transcription factor MafA and insulin
production by targeting mitogen-activated protein 4 kinase 4
(MAP4K4) in pancreatic β-cells. J Biol Chem. 287:31155–31164.
2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Schroeder IS, Kania G, Blyszczuk P and
Wobus AM: Insulin-producing cells. Methods Enzymol. 418:315–333.
2006.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Ramzy A, Mojibian M and Kieffer TJ:
Insulin-Deficient Mouse β-Cells Do Not Fully Maturebut Can Be
Remedied Through Insulin Replacementby Islet Transplantation.
Endocrinology. 159:83–102. 2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Melkman-Zehavi T, Oren R, Kredo-Russo S,
Shapira T, Mandelbaum AD, Rivkin N, Nir T, Lennox KA, Behlke MA,
Dor Y, et al: miRNAs control insulin content in pancreatic β-cells
via downregulation of transcriptional repressors. EMBO J.
30:835–845. 2011.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Guay C and Regazzi R: MicroRNAs and the
functional β cell mass: For better or worse. Diabetes Metab.
41:369–377. 2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Poy MN, Eliasson L, Krutzfeldt J, Kuwajima
S, Ma X, Macdonald PE, Pfeffer S, Tuschl T, Rajewsky N, Rorsman P,
et al: A pancreatic islet-specific microRNA regulates insulin
secretion. Nature. 432:226–230. 2004.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Fred RG, Mehrabi S, Adams CM and Welsh N:
PTB and TIAR binding to insulin mRNA 3'- and 5'UTRs; implications
for insulin biosynthesis and messenger stability. Heliyon.
2(e00159)2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Setyowati Karolina D, Sepramaniam S, Tan
HZ, Armugam A and Jeyaseelan K: miR-25 and miR-92a regulate insulin
I biosynthesis in rats. RNA Biol. 10:1365–1378. 2013.PubMed/NCBI View Article : Google Scholar
|