Introduction
A strong link between allergen exposure,
sensitization and asthma development has been reported (1). Epithelial permeability is indicated to
be markedly increased in allergic asthma, allowing allergens and
irritants to penetrate into the subepithelial area, thus
stimulating type 2 immune responses and constituting an important
process in the immunopathology of allergy (2). Various mediators, such as transforming
growth factor β (TGF-β), epidermal growth factor (EGF), fibroblast
growth factor (FGF), platelet-derived growth factor (PDGF),
insulin-like growth factor (IGF), tumor necrosis factor-α (TNF-α)
and angiogenic factors are released by airway epithelium,
inflammatory cells and structural cells (3-6).
The signaling pathways activated by these growth factors trigger
epithelial-mesenchymal transition (EMT), which contributes to
fibrosis and subsequent downregulation of E-cadherin (6). Published studies suggest that EMT
commonly occurs in epithelial cells in asthma and may contribute to
airway remodeling (7,8). Remodeling comprises structural
alterations to the airway wall, including epithelial cell loss,
goblet cell hyperplasia, hypertrophy of the airway smooth muscle,
basement membrane thickening and increased angiogenesis (9).
Protection of the integrity of the barrier function
of the airway epithelium depends on adherens junctional (AJ) and
tight junctional (TJ) proteins that link cells together (10,11).
AJ proteins are part of two main complexes, the E-cadherin-
β-catenin complex and the nectin-afadin complex, that have
important roles in arranging cell-cell adhesion and intercellular
motility (12). It has been
reported that the expression of E-cadherin, β-catenin and zonula
occludens-1 are reduced and that their functions are impaired in
asthmatic epithelial cells in comparison with healthy cells
(13-17).
Inhaled or oral corticosteroids are the most common
drugs used for asthma therapy in the majority of patients with
asthma (18). Despite providing
clinical and symptomatic improvement, existing therapies are
insufficient to prevent the onset of airway remodeling (9). The influence of various therapeutic
agents on epithelial barrier dysfunction, inhibition of airway
remodeling and controlling adaptive immunity guide the development
of further therapeutic strategies, targets and rational treatment
(19).
Vascular endothelial growth factor (VEGF) is a
signaling protein and the main regulator of endothelial cell
growth. It is released by many cells that stimulate the formation
of blood vessels and is a classic profibrotic growth factor
(20). The overexpression of VEGF
in the lung can lead to significant airway remodeling (21). Therefore, antagonizing VEGF is
important in the prevention of remodeling and in preserving
epithelial barrier function (3,22).
An example of a proinflammatory cytokine that plays
a role in the pathogenesis of asthma is TNF-α (4,23).
TNF-α is usually secreted by macrophages, lymphocytes and mast
cells in the lungs (24,25). However, lung epithelial cells may
also release TNF-α (25,26). It has been reported that patients
with severe asthma have a high amount of TNF-α in the airways in
comparison to healthy individuals (24,25).
Tissue damage occurs via a TNF-α driven effect in the airways and
remodeling may develop as a result (24). Therefore, anti-TNF therapy may be
useful to treat severe asthma. Co-prevention of remodeling and
tissue injury may be possible by antagonism of TNF-α.
The present study aimed to use an experimental
asthma mouse model, to investigate the effects of anti-VEGF,
anti-TNF and corticosteroid therapies on growth factors and
E-cadherin-β-catenin expression.
Materials and methods
Study animals
Male BALB/c mice (n=38; age, 6-8 weeks; weight,
18-20 g) were used to simulate chronic asthma. The mice were kept
in a pathogen-free animal facility at a controlled temperature of
20-24˚C with 60±10% relative humidity and had free access to food
and water on a 12-h light-dark cycle. The study was performed in
accordance with the suggestions specified in the Guide for the Care
and Use of Experimental Animals (27) and ethics approval was obtained from
the Ethics Committee of Dokuz Eylül University.
Sensitization protocol
Mice were divided into five groups: i) Control group
(n=6); ii) untreated asthma group (n=8); iii) TNF-α receptor
blocker group (n=8); iv) anti-VEGF group (n=8); and v)
corticosteroid group (n=8). Mice in the control group were not
administered medication. The chronic asthma model was applied
according to a previously described protocol in all groups except
for the control group (28). The
duration of the experiment was 10 weeks (2 weeks of ovalbumin
injection and 8 weeks of nebulization). Mice were sensitized by
injecting 10 µg (0.1 ml) ovalbumin (grade V; Sigma-Aldrich; Merck
KGaA) intraperitoneally on the 1st and 14th day of the experiment.
After the 3rd week of the experiment, mice were nebulized with 2.5%
OVA aerosol in sterile saline for 30 min, 3 days per week for 8
weeks. Nebulized saline inhalation was applied to the mice in the
control group. The mice were sacrificed after ketamine
hydrochloride (50 mg/ml and 200 mg/kg) anesthesia was applied via
the IP route on the first day after nebulized treatment was
completed.
Study design
Chronic asthma developed in 32 mice. The treatments
given to the study group were as follows: i) Control group,
medication was not applied; ii) untreated asthma group, IP saline
was administered once per week for 2 weeks; iii) TNF-α receptor
blocker group, IP etanercept (Wyeth Europa Ltd.) was administered
at a dose of 0.01 mg/dose (0.5 mg/kg) twice per week for two weeks;
iv) anti-VEGF group, IP bevacizumab (Avastin®; Roche
Diagnostics) was administered at a dose of 0.15 mg/dose 5 mg/kg
once per week for 2 weeks and v) corticosteroid group, IP
dexamethasone (Dekort®; Deva Holding A.S.) was
administered at a dose of 1 mg/kg for 7 days. The treatments
commenced at the same stage as the nebulization treatment, but in
the case of the corticosteroid group only, the treatments commenced
in the second week of nebulization treatment.
Histopathological and
immunohistochemical evaluations
Murine lung tissue was fixed at room temperature
with 10 % buffered formalin for three days, and a 5 mm thick
horizontal slice was obtained from the middle zone of the left
lung. Formalin-fixed lung tissue was embedded in paraffin for use
in histopathological evaluation. Tissue blocks were serially cut
into 5 µm sections. The sections were stained with hematoxylin and
eosin (H&E) for histological evaluation (29). Immunostaining for E-cadherin (cat.
no. sc-8426; mouse monoclonal IgG1; Santa Cruz Biotechnology,
Inc.), β-catenin (cat. no. E-5: sc-7963; mouse monoclonal IgG1;
Santa Cruz Biotechnology, Inc.), TGF-β1 (cat. no. bs-0086R; rabbit
polyclonal IgG1; BIOSS), FGF-1 (cat. no. C-19: sc-1884; goat
polyclonal IgG; Santa Cruz Biotechnology, Inc.), PDGF-A (cat. no.
N-30: sc-128; rabbit polyclonal IgG; Santa Cruz Biotechnology,
Inc.), EGF (cat. no. EGF-10: sc-57088; mouse monoclonal IgG1; Santa
Cruz Biotechnology, Inc.) and IGF-1 (cat. no. H-70: sc-9013; rabbit
polyclonal IgG; Santa Cruz Biotechnology, Inc.) was performed
according to manufacturer's instructions (30). Sections were washed three times (5
min each) with PBS, followed by incubation at 4˚C for 1 h with
biotinylated IgG and then with streptavidin-peroxidase conjugate.
For anti-mouse and anti-rabbit primary antibodies, the Histostain
Plus IHC secondary antibody system was used (cat. no. 85-9643;
broad spectrum; Invitrogen; Thermo Fisher Scientific, Inc.). For
anti-goat primary antibodies, ImmunoCruz goat ABC Staining System
was used (cat. no. sc-2023; Santa Cruz Biotechnology, Inc.). The
staining process was performed according to the manufacturer's
instructions. After washing with PBS three times for 5 min, these
sections were colored with 3,3'-diaminobenzidine for signal
detection and then counterstained with Mayer's hematoxylin at room
temperature for 2 min. All antibodies were used at 1:100 dilution.
The sections were mounted using Entellan® mounting
medium (HX265767; Merck KGaA) and evaluated using a light
microscope (model BX40; Olympus Corporation). Control samples were
processed similarly except for the use of IgG instead of a primary
antibody (30).
The samples were evaluated by two experienced
pathologists blinded to the clinical and serological
characteristics of the rodents. Based on the nuclear and
intracytoplasmic density of immunostaining of the airway
epithelium, an H-SCORE [∑ Pi (I+1)] was calculated for all growth
factors, E-cadherin and β-catenin. Both the ratio of positive
immunostained cells to all cells in the selected fields and the
respective H-scores were calculated. I is the intensity score (0,
1, 2 or 3 corresponding to the presence of negative, weak,
intermediate or strong staining, respectively). Pi is the
percentage of epithelial cells stained with each intensity between
0 and 100% (30). There were 25
slides per animal. From each slide 10 different areas (x400
magnification) were evaluated under a microscope and the percentage
of cells with different staining densities was determined.
Statistical analysis
Statistical analysis was performed using SPSS 15.0
(SPSS, Inc.). The statistical significance between the 5 groups was
assessed by Kruskal-Wallis test followed by the Dunn-Bonferroni
post-hoc test. H-SCORES were compared between pairs of groups (two
by two) using the Mann-Whitney U test. P<0.05 was accepted as
statistically significant.
Results
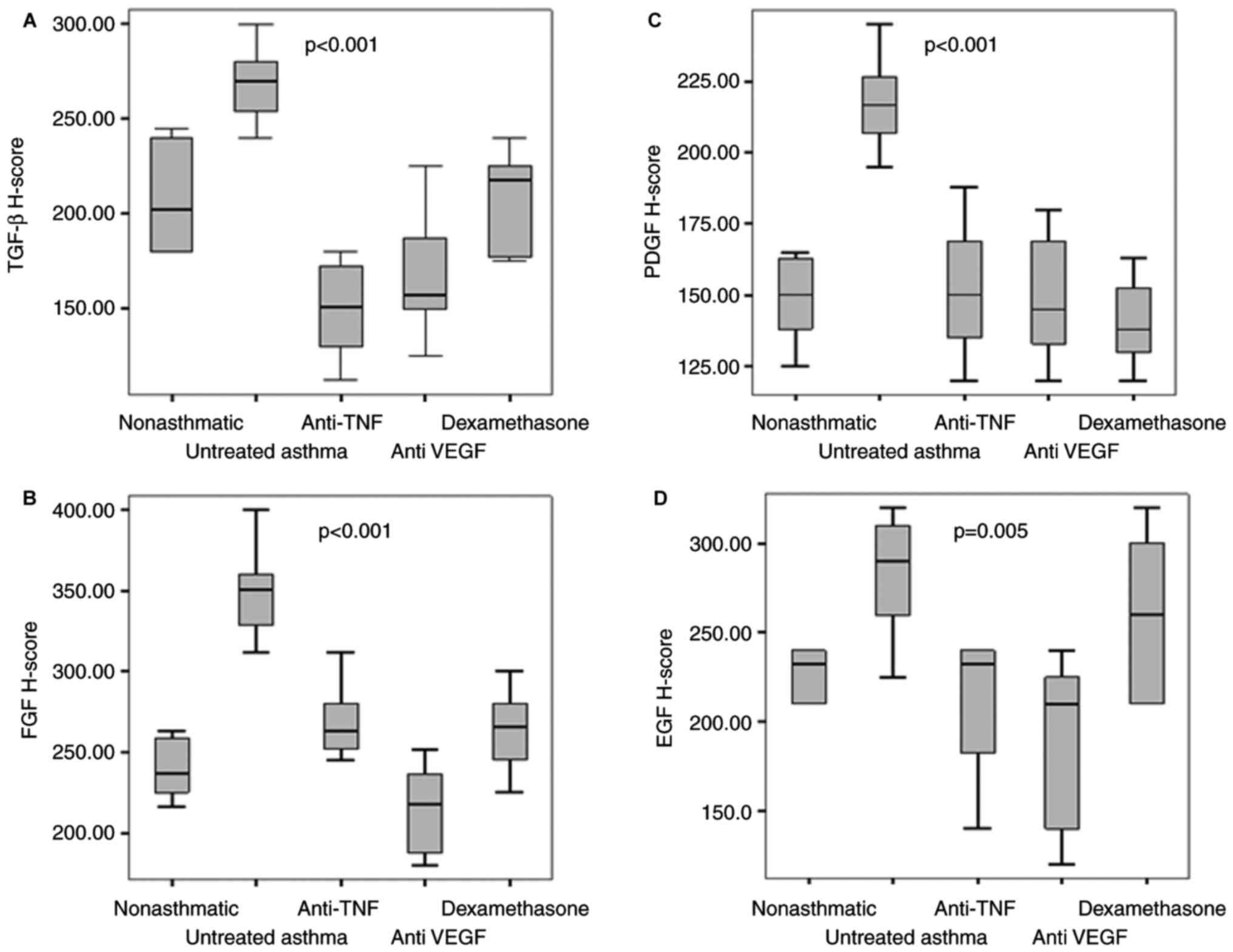
TGF-β1 H-scores
TGF-β1 H-scores were compared between the five
groups, and there was a statistically significant difference
(P<0.001). The highest TGF-β1 H-scores were observed in the
untreated asthma group and the lowest scores were observed in the
TNF-α blocker group (202.5 vs. 151). There were no statistically
significant differences among the TGF-β1 H-scores of the
etanercept, bevacizumab and dexamethasone treatment groups and the
non-asthmatic group (P>0.001, for all). The differences among
the TGF-β1 H-scores of the etanercept, bevacizumab and
dexamethasone treatment groups and the untreated asthma group were
statistically significant (P<0.001 for all; Table I; Figs.
1 and 2).
 | Figure 1Immunohistochemistry of lung tissue
sections from asthma model and control mice. E-cadherin, β-catenin,
TGF-β, PDGF, FGF staining of the five groups (original
magnification, x100; insert, x400). (A) Non-asthmatic control, (B)
untreated asthma, (C) TNF-α blocker, (D) corticosteroid and (E)
anti-VEGF groups. *Immunoreactivity. Ep, epithelium.
TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth
factor; TGF-β, transforming growth factor-β; PDGF, platelet derived
growth factor; FGF, fibroblast growth factor. |
 | Table IGrowth factors H-scores in the study
groups. |
Table I
Growth factors H-scores in the study
groups.
| | Study groups | |
|---|
| Protein | Non-asthmatic
control | Untreated
asthma | Etanercept | Bevacizumab | Dexamethasone |
P-valuea |
|---|
| TGF-β | 202.5
(180-240) | 270
(254-280)b | 151
(130-172.5)c | 157.5
(150-187.5)c | 217.5
(177.5-225)c | <0.001 |
| PDGF | 150 (138-163) | 216.5
(207-226.5)b | 150
(135-169)c | 145
(133-169)c | 138
(130-152.5)c | <0.001 |
| FGF | 237 (225-259) | 351
(329-360)b | 263
(252-280)c | 217
(187-236)c | 266
(245-280)c | <0.001 |
| EGF | 232.5
(210-240) | 290 (260-310) | 232.5
(182.5-240) | 210
(140-225)d | 260 (210-300) | 0.005 |
| IGF | 117.5 (90-130) | 97.5 (89-100) | 95 (90-100) | 80 (72.5-80) | 147 (130-165) | <0.001 |
PDGF-A H-scores
PDGF-A H-scores were compared between the five
groups, and there was a statistically significant difference
(P<0.001). The highest PDGF-A H-scores were observed in the
untreated asthma group and the lowest scores were seen in the
corticosteroid group (216.5 vs. 138). No statistically significant
difference was found among the PDGF-A H-scores of the etanercept,
bevacizumab and dexamethasone treatment groups in comparison with
the non-asthmatic group (P>0.005, for all). The comparison of
PDGF-A H-scores among the etanercept, bevacizumab, dexamethasone
treatment groups and the untreated asthma group were statistically
significantly (P<0.001 for all; Table I; Figs.
1 and 2).
FGF-1 H-scores
When FGF-1 H-scores were compared between the five
groups, there was a statistically significant difference
(P<0.001). The highest FGF-1 H-score was observed in the
untreated asthma group and the lowest score in the anti VEGF group
(351 vs. 217). When the FGF-1 H-scores of the etanercept,
bevacizumab and dexamethasone treatment groups were compared with
the non-asthmatic group no statistically significant difference was
found (P>0.005, for all). The differences among the FGF-1
H-scores of the etanercept, bevacizumab, dexamethasone treatment
groups and the untreated asthma group were statistically
significant (P<0.001 for all; Table
I; Figs. 1 and 2).
EGF H-scores
The EGF H-scores between the five groups were
significant (P=0.001). The highest EGF-H-score was observed in the
untreated asthma group and the lowest score in the anti-VEGF group
(290 vs. 210; P=0.002). The comparison of EGF H-scores between the
bevacizumab treatment group and the untreated asthma group
indicated a statistically significant difference (P=0.002). When
the EGF H-scores of the etanercept and dexamethasone treatment
groups were compared with the non-asthmatic group, no statistically
significant difference was observed (P>0.005; Table I; Figs.
1 and 2).
IGF H-scores
There was a statistically significant difference
when the IGF H-scores were compared between the five groups
(P<0.001). The highest IGF H-score was found in the
corticosteroid group, and the lowest score was in the anti-VEGF
group (147 vs. 80; P=0.001). The IGF H-scores of untreated asthma,
etanercept, bevacizumab and dexamethasone treatment groups were not
significantly different from those in healthy controls (P>0.005,
for all). The differences between the IGF H-score in the
corticosteroid group in comparison to the untreated asthma,
etanercept and anti-VEGF groups was statistically significant
(P=0.001 for all; Table I; Fig. 1).
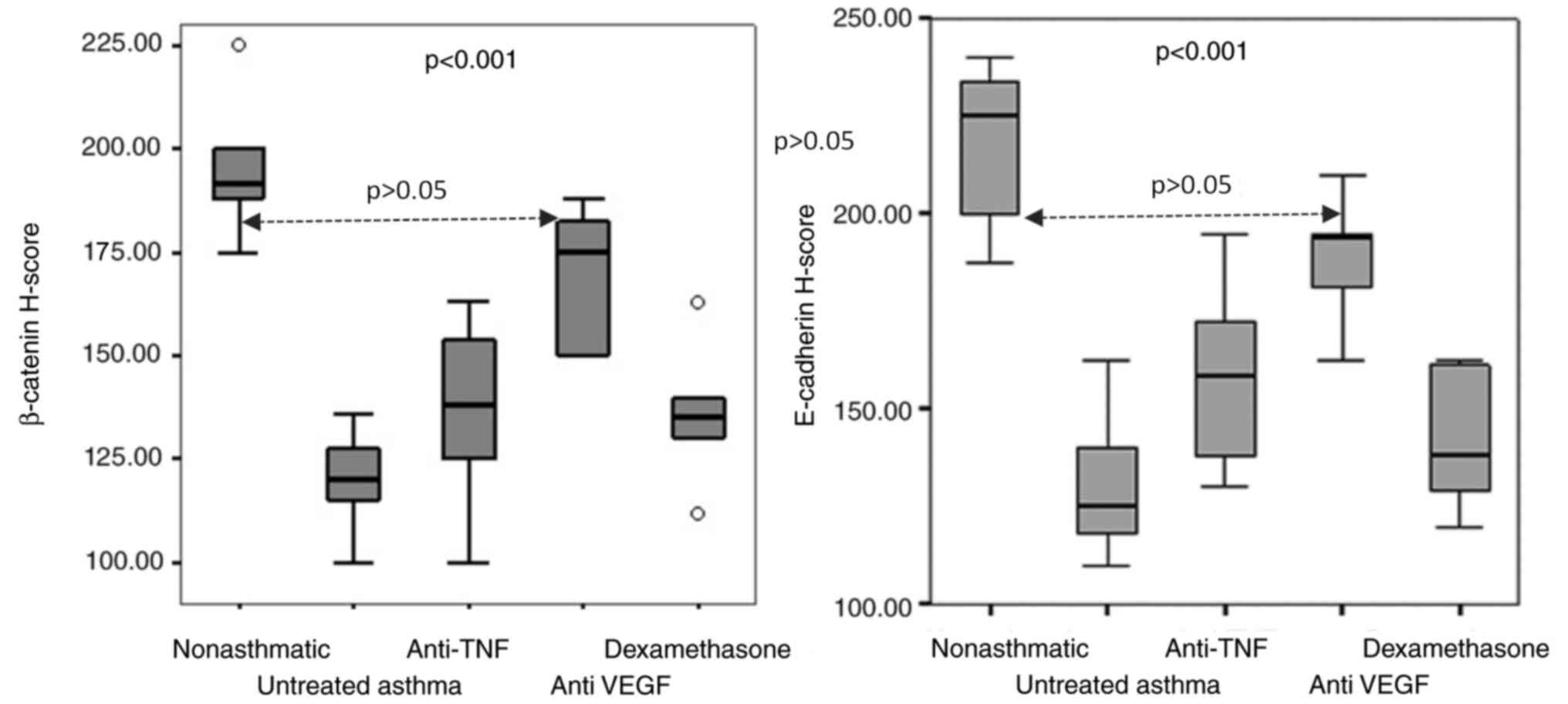
E-cadherin H-scores
The H-scores of E-cadherin between the five groups
were significantly different (P<0.001). The highest E-cadherin
H-score was observed in the non-asthmatic control group and the
lowest score was in the untreated asthma group (225 vs. 125;
P=0.002). The E-cadherin H-scores of the anti-VEGF group were
closest to the non-asthmatic control groups (193.5 vs. 225;
P=0.023; Table II). The H-scores
of the TNF-α receptor blocker and corticosteroid treatment groups
were significantly lower than the control group (P=0.003 and
P=0.002, respectively). Although the H-scores of the TNF-α receptor
blocker and corticosteroid treatment groups were higher than the
untreated asthma group, the differences were not significant (158.5
vs. 125 and 138 vs. 125; P=0.013 and P=0.138, respectively;
Table II; Figs. 1 and 3).
 | Table IIE-cadherin and β-catenin H-scores in
the study groups. |
Table II
E-cadherin and β-catenin H-scores in
the study groups.
| | Study groups | |
|---|
| Protein | Non-asthmatic
control | Untreated
asthma | Etanercept | Bevacizumab | Dexamethasone |
P-valuea |
|---|
| E-cadherin | 225
(200-234)b | 125 (118-40) | 158.5
(138-172.5)c | 193.5
(181-195)d | 138
(129-161)c | <0.001 |
| β-catenin | 191.5
(188-200)b | 120
(115-127.5) | 138
(125-154)c | 175
(150-182.5)d | 135
(130-140)c | <0.001 |
β-catenin H-scores
There was a statistically significant difference in
β-catenin H-scores (P<0.001) between the five groups. The
highest β-catenin H-score was found in the non-asthmatic control
group and lowest score was in the untreated asthma group (191.5 vs.
120; P=0.002). Among the etanercept, bevacizumab and dexamethasone
treatment groups, the β-catenin H-scores of the bevacizumab group
were closest to the non-asthmatic control groups (175 vs. 191.5;
P=0.002). The TNF-α receptor blocker and corticosteroid treatment
groups had lower β-catenin H-scores than the control group (P=0.002
and P=0.002, respectively). Although the β-catenin H-scores of the
etanercept and the corticosteroid treatment groups were higher than
those in the untreated asthma group, the differences were not
significant (138 vs. 120 and 135 vs. 120; P=0.056 and P=0.023,
respectively; Table II; Figs. 1 and 3).
Discussion
The results suggested that the levels of TGF-β, PDGF
and FGF were increased in the untreated asthma group compared with
non-asthmatic controls. All treatment groups had reduced TGF-β,
PDGF and FGF H-scores in comparison with the untreated asthma
group. It was determined that E-cadherin and β-catenin levels were
reduced in the untreated asthma group compared to the non-asthmatic
control and that their levels increased in non-asthmatic controls
following treatment with anti-VEGF treatment.
In asthma, growth factors can trigger inflammation
and structural changes in the airways (e.g., epithelial barrier
dysfunction and remodeling). This is a process in which EMT causes
epithelial cells to dedifferentiate into more mobile mesenchymal
cells, such as fibroblasts (6,31,32).
EMT can be stimulated by various signals and molecules, including
tyrosine kinase receptors, including FGF, IGF, EGF, PDGF and VEGF,
and the TGF-β and Wnt/β-catenin pathways (5,6). The
studies conducted to date have indicated that the asthmatic airway
epithelium is an important source of profibrogenic growth factors,
including FGF, IGF, EGF, TGF-β, PDGF and VEGF (6,33-35).
Similar to these studies, the present study determined that TGF-β,
PDGF and FGF levels were higher in asthmatic mice than control
mice; however, IGF and EGF levels were not different from the
control group in the present study.
The airway epithelium creates a structural and
immunological barrier against environmental stimuli, such as
aeroallergens, microorganisms and particulate matter (36). Changes in the asthmatic epithelium
include epithelial shedding and the destruction of ciliary cells
(37). E-cadherin is important in
maintenance of the cytoskeleton and TJ functional integrity
(38). Reduced E-cadherin activity
causes TJ barrier integrity to deteriorate (38,39).
In addition, E-cadherin plays a role in the repression of
intracellular signaling pathways, the arrangement of the epithelium
and cell proliferation and differentiation (37).
β-catenin is a membrane-bound protein and forms a
key AJ component, interacting with E-cadherins and connecting them
to the cytoskeleton. Disruption of this epithelial barrier function
allows easier access of inhalant allergens to the
antigen-presenting cells in the submucosa, causing stimulation of
the Th2 type mediated immune response and EMT (13,14,38). A
gradual loss of E-cadherin is considered a hallmark of EMT
(5,6). Membrane levels of E-cadherin-β-
catenin have been found to be reduced in the asthmatic epithelial
cell in comparison with healthy cells (13-17).
When the E-cadherin-β-catenin structure is disrupted, β-catenin is
released into the cytosol and translocates to the nucleus, where it
activates the Wnt/β-catenin signaling pathway and contributes to
the EMT; this process is involved in tissue remodeling (7,14,40-42).
Studies on mice have shown that repetitive antigen
load causes extensive E-cadherin loss and that there is a
correlation between the number of allergen exposures and E-cadherin
levels (39,43). In biopsies of the bronchial mucosa
of patients with atopic asthma, E-cadherin levels in the
respiratory epithelia were shown to be lower than non-atopic and
non-asthmatic cases (17,44,45).
Similarly, a study published by our group showed that patients with
asthma had lower levels of E-cadherin in their airways (46). Similar to previous studies, the
present study suggested that E-cadherin and β-catenin levels were
lower in asthmatic model mice.
VEGF has significant roles in inflammation,
angiogenesis and the vascular permeability of airways and in
subepithelial collagen deposition, airway smooth muscle hyperplasia
and the development of physiological abnormalities of the airway
(33). Bevacizumab is a humanized
monoclonal antibody that inhibits the activation of VEGF-R and
tumor growth (20). TGF-β, IGF, FGF
and PDGF were shown to stimulate the secretion of VEGF (33,47).
Similar to these studies, the correlation of increased TGF-β levels
and the decrease in E-cadherin and β-catenin levels was shown in
the present study as well (11,14,32,40,45,48-50).
TGF-β has also caused a significant increase in transcriptional
activation of β-catenin (14,40).
The available knowledge suggests that VEGF potentially affects the
signal pathway, and thus triggers myofibroblast transformation
(51). In the present study of
asthmatic model mice, TGF-β, PDGF and FGF levels decreased with
treatment to show no difference from non-asthmatic controls. These
results suggest that EMT can be controlled by treatment approaches
via VEGF.
Although the role of VEGF in the pathogenesis in
asthma and remodeling is known, the effect of antagonizing VEGF on
epithelial barrier integrity and AJ structure is not fully
understood (33). Most knowledge on
the effects of VEGF on epithelial barrier integrity was obtained
from studies of the retinal endothelium (30,52).
VEGF exposure causes decreased transepithelial resistance and
increased vascular permeability. Permeability-enhancing agents also
change AJ (53). After exposure to
VEGF, the expression of VE-cadherin, β-catenin, occludin, claudin
and ZO-1 proteins in the endothelium decreased in vitro
(54-57).
Furthermore, in diabetic retinopathy, VEGF-R1 antagonism has been
shown to prevent vascular leakage and retinal leukostasis,
degeneration and disorganization of ZO-1 and VE-cadherin (55,57).
Similarly, in the present study, E-cadherin and β-catenin levels of
asthmatic mice treated with anti-VEGF increased to levels of
control mice without asthma. Overall, in the experimental asthma
mouse model, anti-VEGF therapy appeared to cause an elevation in
epithelial barrier proteins, E-cadherin and β-catenin levels.
Therefore, the use of anti-VEGF therapy in asthma may be a
therapeutic option for the restoration of epithelial barrier.
TNF-α is released from the airways in asthma and may
play a role in the pathogenesis of allergic inflammation through
the activation of transcription factors (4). TNF-α was shown to upregulate TGF-β
expression in mice lung fibroblasts (58). TNF-α stimulates TGF-β expression in
severe asthma, causing fibroblast growth and maturation into
myofibroblasts (59). TNF-α
enhances the effect of TGF-β1 on EMT induction in human bronchial
epithelial cells (HBECs) (60). The
effectiveness of a TNF-α receptor antagonist in severe asthma
therapy has recently been studied (25). In the present study of asthmatic
mice models, etanercept was shown to downregulate the expression of
PDGF and FGF as well as TGF-β. Despite the apparent efficacy in
treatment, the mechanism of action of anti-TNF-α is not fully
understood, which is limitation of the present study. Further
cellular and molecular mechanistic studies are needed to determine
how these changes occur in the future. TNF-α causes epithelial
barrier dysfunction in other tissues, and previous studies have
shown that TNF-α in combination with IFN-γ disrupted the TJ in
HBECs (61-63).
Therefore, the inhibition of TNF-α can be considered as an option
in maintaining epithelial integrity.
In another study, TNF-α stimulation in bronchial
epithelial cell culture has been shown to reduce E-cadherin and
β-catenin expression; however, improvements were observed with
TNF-α inhibition and corticosteroid therapy (48). In contrast to these studies,
E-cadherin and β-catenin expression partially increased with
corticosteroid treatment and TNF-α inhibition in the present study,
but did reach the levels of healthy controls. This may have been
caused by the difference between inflammatory processes of Th2 type
inflammation caused by the OVA-induced asthma model and Th1 type
inflammation caused by TNF-α.
In experimental asthma model studies,
corticosteroids were shown to decrease the release of TGF-β while
dexamethasone was shown to decline the release of TGF-β, IGF, and
FGF. ICS was shown to inhibit IGF expression (23,64,65).
All glucocorticosteroids have been shown to inhibit PDGF and
collagen I-induced proliferation and hypocontractility in airway
smooth muscle cells (66). The
anti-angiogenic effects of dexamethasone on VEGF release in
FGF-treated human airway smooth muscle cells via the p38MAPK
pathway were also demonstrated in vitro (47). Similar to these studies, the present
study suggested that dexamethasone decreased TGF-β, PDGF and FGF
levels.
By inducing tight junction formation and increasing
transepithelial resistance, corticosteroids play a crucial role in
the function and maintenance of cell-cell contact. However, the
potential effects of returning to the original state of AJs
(E-cadherin, β-catenin) and healing the epithelial barrier are not
fully clear (18,48,67).
Glucocorticoid administration in Doerner and Zuraw (68) did not substantially change
E-cadherin mRNA downregulation mediated by TGF-β1. Song et
al (67) showed that E-cadherin
distribution could be partially salvaged following pretreatment
with dexamethasone in experimental asthma models. While the
downregulation of hypoxia-induced loss of ZO-1 expression was
inhibited by dexamethasone in the human corneal epithelia, it did
not affect the E-cadherin vs. β-catenin (69). In the present study, E-cadherin and
β-catenin expression did not reach the level of healthy controls
with dexamethasone treatment similar to the aforementioned
studies.
The limitations of our study are the inability to
look at the molecular level of β-catenin in terms of quantity, only
the adherens proteins, and the inability to show a barrier function
such as transepithelial resistance as physical data. Further
cellular and molecular mechanistic studies are needed to determine
how these changes occur in the future.
In the present study, the effects of conventional
corticosteroid treatment, VEGF inhibition treatments and TNF-α
antagonistic treatment on growth factor and E-cadherin-β-catenin
expression were compared. These proteins play roles in the
pathogenesis of asthma (3-6).
Growth factors TGF-β, PDGF and FGF expression levels were high in
asthmatic mice models and E-cadherin and β-catenin expressions were
low. Anti-VEGF and TNF-α inhibition treatments are effective in
decreasing growth factors similar to conventional corticosteroid
treatments. However, corticosteroid and TNF-α inhibition treatment
were not effective in increasing E-cadherin and β-catenin levels.
This leads to epithelial barrier function disturbance. Anti-VEGF
treatment increased the expression of AJ proteins in bronchial
epithelium, and VEGF and TNF inhibition may provide an alternative
therapeutic option to steroid-sparing agents. They are also a more
effective treatment of epithelial barrier restoration and
remodeling in asthma. However, before making firm recommendations,
further clinical studies are needed to investigate the in
vivo efficacy and safety profile of anti-VEGF treatment in
asthma.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HY and OY were involved in the conception,
hypotheses delineation and design of the study. AT wrote and HY
revised the manuscript. FF, AT and ETK took part in the acquisition
of the data. SI and MK participated in the analysis and
interpretation of the data. HY and OY confirmed the authenticity of
the raw data. All authors confirmed that they have read and
approved the final manuscript.
Ethics approval and consent to
participate
Ethics approval was obtained from the Ethics
Committee of Dokuz Eylül University and the Animal Experiment Unit
for the implementation of all experimental procedures.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Leynaert B, Le Moual N, Neukirch C, Siroux
V and Varraso R: Environmental risk factors for asthma
developement. Presse Med. 48:262–273. 2019.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
2
|
Goleva E, Berdyshev E and Leung DY:
Epithelial barrier repair and prevention of allergy. J Clin Invest.
129:1463–1474. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lee HY, Hur J, Kim IK, Kang JY, Yoon HK,
Lee SY, Kwon SS, Kim YK and Rhee CK: Effect of nintedanib on airway
inflammation and remodeling in a murine chronic asthma model. Exp
Lung Res. 43:187–196. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kim J and Remick DG: Tumor necrosis factor
inhibitors for the treatment of asthma. Curr Allergy Asthma Rep.
7:151–156. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Guntur VP and Reinero CR: The potential
use of tyrosine kinase inhibitors in severe asthma. Curr Opin
Allergy Clin Immunol. 12:68–75. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hasan NAHM, Harith HH, Israf DA and Tham
CL: The differential effects of commercial specialized media on
cell growth and transforming growth factor beta 1-induced
epithelial-mesenchymal transition in bronchial epithelial cells.
Mol Biol Rep. 47:3511–3519. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yuksel H and Türkeli A: Airway epithelial
barrier dysfunction in the pathogenesis and prognosis of
respiratory tract diseases in childhood and adulthood. Tissue
Barriers. 5(e1367458)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chu S, Zhang X, Sun Y, Liang Y, Sun J, Lu
M, Huang J, Jiang M and Ma L: Atrial natriuretic peptide inhibits
epithelial-mesenchymal transition (EMT) of bronchial epithelial
cells through cGMP/PKG signaling by targeting Smad3 in a murine
model of allergic asthma. Exp Lung Res. 45:245–254. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang J and Dong L: Status and prospects:
Personalized treatment and biomarker for airway remodeling in
asthma. J Thorac Dis. 12:6090–6101. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hellings PW and Steelant B: Epithelial
barriers in allergy and asthma. J Allergy Clin Immunol.
145:1499–1509. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Post S, Nawijn MC, Jonker MR, Kliphuis N,
van den Berge M, van Oosterhout AJ and Heijink IH: House dust
mite-induced calcium signaling instigates epithelial barrier
dysfunction and CCL20 production. Allergy. 68:1117–1125.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Niessen CM: Tight junctions/adherens
junctions: Basic structure and function. J Invest Dermatol.
127:2525–2532. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Knight DA, Stick SM and Hackett TL:
Defective function at the epithelial junction: A novel therapeutic
frontier in asthma? J Allergy Clin Immunol. 128:557–558.
2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Heijink IH, Postma DS, Noordhoek JA,
Broekema M and Kapus A: House dust mite-promoted
epithelial-to-mesenchymal transition in human bronchial epithelium.
Am J Respir Cell Mol Biol. 42:69–79. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cao N, Wang J, Xu X, Xiang M and Dou J:
PACAP38 improves airway epithelial barrier destruction induced by
house dust mites allergen. Immunobiology. 224:758–764.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yao L, Chen S, Tang H, Huang P, Wei S,
Liang Z, Chen X, Yang H, Tao A, Chen R, et al: Transient receptor
potential ion channels mediate adherens junctions dysfunction in a
toluene diisocyanate-induced murine asthma model. Toxicol Sci.
168:160–170. 2019.PubMed/NCBI View Article : Google Scholar : Erratum in Toxicol
Sci 170, 247, 2019.
|
|
17
|
de Boer WI, Sharma HS, Baelemans SM,
Hoogsteden HC, Lambrecht BN and Braunstahl GJ: Altered expression
of epithelial junctional proteins in atopic asthma: Possible role
in inflammation. Can J Physiol Pharmacol. 86:105–112.
2008.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Sekiyama A, Gon Y, Terakado M, Takeshita
I, Kozu Y, Maruoka S, Matsumoto K and Hashimoto S: Glucocorticoids
enhance airway epithelial barrier integrity. Int Immunopharmacol.
12:350–357. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
McLellan K, Shields M, Power U and Turner
S: Primary airway epithelial cell culture and asthma in
children-lessons learnt and yet to come. Pediatr Pulmonol.
50:1393–1405. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Takahashi S: Vascular endothelial growth
factor (VEGF), VEGF receptors and their inhibitors for
antiangiogenic tumor therapy. Biol Pharm Bull.
34(1785e1798)2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hur GY and Broide DH: Genes and pathways
regulating decline in lung function and airway remodeling in
asthma. Allergy Asthma Immunol Res. 11:604–621. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Huang C, Dong H, Zou M, Luo L, Hu Y, Xie
Z, Le Y, Liu L, Zou F and Cai S: Bevacizumab reduced
auto-phosphorylation of VEGFR2 to protect HDM-induced
asthma mice. Biochem Biophys Res Commun. 478:181–186.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Herbert C, Hettiaratchi A, Webb DC, Thomas
PS, Foster PS and Kumar RK: Suppression of cytokine expression by
roflumilast and dexamethasone in a model of chronic asthma. Clin
Exp Allergy. 38:847–856. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ghebre MA, Pang PH, Desai D, Hargadon B,
Newby C, Woods J, Rapley L, Cohen SE, Herath A, Gaillard EA, et al:
Severe exacerbations in moderate-to-severe asthmatics are
associated with increased pro-inflammatory and type 1 mediators in
sputum and serum. BMC Pulm Med. 19(144)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Malaviya R, Laskin JD and Laskin DL:
Anti-TNFα therapy in inflammatory lung diseases. Pharmacol Ther.
180:90–98. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mukhopadhyay S, Hoidal JR and Mukherjee
TK: Role of TNFalpha in pulmonary pathophysiology. Respir Res.
7(125)2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animal: Guide for the Care and Use of Laboratory Animals, 8th
edition. National Academies Press (US), Washington, DC, 2011.
|
|
28
|
Temelkovski J, Hogan SP, Shepherd DP,
Foster PS and Kumar RK: An improved murine model of asthma:
Selective airway inflammation, epithelial lesions and increased
methacholine responsiveness following chronic exposure to
aerosolised allergen. Thorax. 53:849–856. 1998.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Cardiff RD, Miller CH and Munn RJ: Manual
hematoxylin and eosin staining of mouse tissue sections. Cold
Spring Harb Protoc. 2014:655–658. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yuksel H, Yilmaz O, Baytur YB and Ozbilgin
K: Prenatal administration of granulocyte-macrophage
colony-stimulating factor increases mesenchymal vascular
endothelial growth factor expression and maturation in fetal rat
lung. Exp Lung Res. 34:550–558. 2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang T, Zhou Q and Shang Y: MiRNA-451a
inhibits airway remodeling by targeting Cadherin 11 in an allergic
asthma model of neonatal mice. Int Immunopharmacol.
83(106440)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gong JH, Cho IH, Shin D, Han SY, Park SH
and Kang YH: Inhibition of airway epithelial-to-mesenchymal
transition and fibrosis by kaempferol in endotoxin-induced
epithelial cells and ovalbumin-sensitized mice. Lab Invest.
94:297–308. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Makinde T, Murphy RF and Agrawal DK:
Immunomodulatory role of vascular endothelial growth factor and
angiopoietin-1 in airway remodeling. Curr Mol Med. 6:831–841.
2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hough KP, Curtiss ML, Blain TJ, Liu RM,
Trevor J, Deshane JS and Thannickal VJ: Airway remodeling in
asthma. Front Med (Lausanne). 7(191)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hasan NAHM, Harith HH, Israf DA and Tham
CL: The differential effects of commercial specialized media on
cell growth and transforming growth factor beta 1-induced
epithelial-mesenchymal transition in bronchial epithelial cells.
Mol Biol Rep. 47:3511–3519. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Frey A, Lunding LP, Ehlers JC, Weckmann M,
Zissler UM and Wegmann M: More than just a barrier: The immune
functions of the airway epithelium in asthma pathogenesis. Front
Immunol. 11(761)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Post S, Heijink IH, Hesse L, Koo HK,
Shaheen F, Fouadi M, Kuchibhotla VNS, Lambrecht BN, Van Oosterhout
AJM, Hackett TL, et al: Characterization of a lung epithelium
specific E-cadherin knock-out model: Implications for obstructive
lung pathology. Sci Rep. 8(13275)2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cai J, Culley MK, Zhao Y and Zhao J: The
role of ubiquitination and deubiquitination in the regulation of
cell junctions. Protein Cell. 9:754–769. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Goto Y, Uchida Y, Nomura A, Sakamoto T,
Ishii Y, Morishima Y, Masuyama K and Sekizawa K: Dislocation of
E-cadherin in the airway epithelium during an antigen-induced
asthmatic response. Am J Respir Cell Mol Biol. 23:712–718.
2000.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Baarsma HA, Menzen MH, Halayko AJ, Meurs
H, Kerstjens HA and Gosens R: β-catenin signaling is required for
TGF-β1-induced extracellular matrix production by airway smooth
muscle cells. Am J Physiol Lung Cell Mol Physiol. 301:L956–L965.
2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kim HT, Yin W, Nakamichi Y, Panza P,
Grohmann B, Buettner C, Guenther S, Ruppert C, Kobayashi Y,
Guenther A, et al: WNT/RYK signaling restricts goblet cell
differentiation during lung development and repair. Proc Natl Acad
Sci USA. 116:25697–25706. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Jia XX, Zhu TT, Huang Y, Zeng XX, Zhang H
and Zhang WX: Wnt/β-catenin signaling pathway regulates asthma
airway remodeling by influencing the expression of c-Myc and cyclin
D1 via the p38 MAPK-dependent pathway. Exp Ther Med. 18:3431–3438.
2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Evans SM, Blyth DI, Wong T, Sanjar S and
West MR: Decreased distribution of lung epithelial junction
proteins after intratracheal antigen or lipopolysaccharide
challenge: Correlation with neutrophil influx and levels of BALF
sE-cadherin. Am J Respir Cell Mol Biol. 27:446–454. 2002.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Xiao C, Puddicombe SM, Field S, Haywood J,
Broughton-Head V, Puxeddu I, Haitchi HM, Vernon-Wilson E, Sammut D,
Bedke N, et al: Defective epithelial barrier function in asthma. J
Allergy Clin Immunol. 128:549–56.e1-12. 2011.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Trautmann A, Kruger K, Akdis M,
Muller-Wening D, Akkaya A, Brocker EB, Blaser K and Akdis CA:
Apoptosis and loss of adhesion of bronchial epithelial cells in
asthma. Int Arch Allergy Immunol. 138:142–150. 2005.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Yuksel H, Türkeli A, Taneli F, Horasan GD,
Kanik ET, Kizilkaya M, Gözükara C and Yilmaz O: E-cadherin as an
epithelial barrier protein in exhaled breath condensate. J Breath
Res. 8(046006)2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Willems-Widyastuti A, Vanaudenaerde BM,
Vos R, Dilisen E, Verleden SE, De Vleeschauwer SI, Vaneylen A, Mooi
WJ, de Boer WI, Sharma HS, et al: Azithromycin attenuates
fibroblast growth factors induced vascular endothelial growth
factor via p38(MAPK) signaling in human airway smooth muscle cells.
Cell Biochem Biophys. 67:331–339. 2013.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Carayol N, Campbell A, Vachier I,
Mainprice B, Bousquet J, Godard P and Chanez P: Modulation of
cadherin and catenins expression by tumor necrosis factor-alpha and
dexamethasone in human bronchial epithelial cells. Am J Respir Cell
Mol Biol. 26:341–347. 2002.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Moheimani F, Roth HM, Cross J, Reid AT,
Shaheen F, Warner SM, Hirota JA, Kicic A, Hallstrand TS, Kahn M, et
al: Disruption of β-catenin/CBP signaling inhibits human airway
epithelial-mesenchymal transition and repair. Int J Biochem Cell
Biol. 68:59–69. 2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Winton HL, Wan H, Cannell MB, Thompson PJ,
Garrod DR, Stewart GA and Robinson C: Class specific inhibition of
house dust mite proteinases which cleave cell adhesion, induce cell
death and which increase the permeability of lung epithelium. Br J
Pharmacol. 124:1048–1059. 1998.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Park HY, Kim JH and Park CK: VEGF induces
TGF-β1 expression and myofibroblast transformation after glaucoma
surgery. Am J Pathol. 182:2147–2154. 2013.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Chatterjee S, Wang Y, Duncan MK and Naik
UP: Junctional adhesion molecule-A regulates vascular endothelial
growth factor receptor-2 signaling-dependent mouse corneal wound
healing. PLoS One. 8(e63674)2013.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wallez Y and Huber P: Endothelial adherens
and tight junctions in vascular homeostasis, inflammation and
angiogenesis. Biochim Biophys Acta. 1778:794–809. 2008.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Díaz-Coránguez M, Lin CM, Liebner S and
Antonetti DA: Norrin restores blood-retinal barrier properties
after vascular endothelial growth factor-induced permeability. J
Biol Chem. 295:4647–4660. 2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
He J, Wang H, Liu Y, Li W, Kim D and Huang
H: Blockade of vascular endothelial growth factor receptor 1
prevents inflammation and vascular leakage in diabetic retinopathy.
J Ophthalmol. 2015(605946)2015.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Wisniewska-Kruk J, Hoeben KA, Vogels IM,
Gaillard PJ, Van Noorden CJ, Schlingemann RO and Klaassen I: A
novel co-culture model of the blood-retinal barrier based on
primary retinal endothelial cells, pericytes and astrocytes. Exp
Eye Res. 96:181–190. 2012.PubMed/NCBI View Article : Google Scholar : Erratum in Exp Eye
Res 138, 167, 2015.
|
|
57
|
Harhaj NS, Felinski EA, Wolpert EB,
Sundstrom JM, Gardner TW and Antonetti DA: VEGF activation of
protein kinase C stimulates occludin phosphorylation and
contributes to endothelial permeability. Invest Ophthalmol Vis Sci.
47:5106–5115. 2006.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Sullivan DE, Ferris M, Nguyen H, Abboud E
and Brody AR: TNF-alpha induces TGF-beta1 expression in lung
fibroblasts at the transcriptional level via AP-1 activation. J
Cell Mol Med. 13:1866–1876. 2009.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Brightling C, Berry M and Amrani Y:
Targeting TNF-alpha: A novel therapeutic approach for asthma. J
Allergy Clin Immunol. 121:5–10; quiz 11-12. 2008.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Câmara J and Jarai G: .
Epithelial-mesenchymal transition in primary human bronchial
epithelial cells is Smad-dependent and enhanced by fibronectin and
TNF-alpha. Fibrogenesis Tissue Repair. 3(2)2010.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Hardyman MA, Wilkinson E, Martin E,
Jayasekera NP, Blume C, Swindle EJ, Gozzard N, Holgate ST, Howarth
PH, Davies DE, et al: TNF-α-mediated bronchial barrier disruption
and regulation by src-family kinase activation. J Allergy Clin
Immunol. 132:665–675.e8. 2013.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Coyne CB, Vanhook MK, Gambling TM, Carson
JL, Boucher RC and Johnson LG: Regulation of airway tight junctions
by proinflammatory cytokines. Mol Biol Cell. 13:3218–3234.
2002.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Pohl C, Hermanns MI, Uboldi C, Bock M,
Fuchs S, Dei-Anang J, Mayer E, Kehe K, Kummer W and Kirkpatrick CJ:
Barrier functions and paracellular integrity in human cell culture
models of the proximal respiratory unit. Eur J Pharm Biopharm.
72:339–349. 2009.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Doherty T and Broide D: Cytokines and
growth factors in airway remodeling in asthma. Curr Opin Immunol.
19:676–680. 2007.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Wang Z, Li W, Guo Q, Wang Y, Ma L and
Zhang X: Insulin-like growth factor-1 signaling in lung development
and inflammatory lung diseases. BioMed Res Int.
2018(6057589)2018.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Dekkers BG, Pehlic A, Mariani R, Bos IS,
Meurs H and Zaagsma J: Glucocorticosteroids and
β2-adrenoceptor agonists synergize to inhibit airway
smooth muscle remodeling. J Pharmacol Exp Ther. 342:780–787.
2012.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Song J, Zhao H, Dong H, Zhang D, Zou M,
Tang H, Liu L, Liang Z, Lv Y, Zou F, et al: Mechanism of E-cadherin
redistribution in bronchial airway epithelial cells in a
TDI-induced asthma model. Toxicol Lett. 220:8–14. 2013.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Doerner AM and Zuraw BL: TGF-β1 induced
epithelial to mesenchymal transition (EMT) in human bronchial
epithelial cells is enhanced by IL-1β but not abrogated by
corticosteroids. Respir Res. 10(100)2009.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Kimura K, Teranishi S, Kawamoto K and
Nishida T: Protective effect of dexamethasone against
hypoxia-induced disruption of barrier function in human corneal
epithelial cells. Exp Eye Res. 92:388–393. 2011.PubMed/NCBI View Article : Google Scholar
|