The tumor necrosis factor superfamily (TNFSF)
consists of 19 ligands and 29 receptors (1,2). Death
receptor 3 (DR3) and tumor necrosis factor-like ligand 1A (TL1A)
are a TNFSF receptor-ligand pair that serve an essential role in
regulating immunity, inflammation, cell proliferation and death,
angiogenesis, and tumor metastasis (2,3). The
expression of DR3 and TL1A has been demonstrated in mice and
humans, and preclinical and clinical studies have shown that the
DR3/TL1A pathway serves a dual role in the development of
inflammatory and immune-mediated diseases (4). On one hand, interaction between DR3
and TL1A may trigger the proliferation of T effector cells and
cytokine production by these cells, which accelerate disease
progression (5-7).
On the other hand, activation of the DR3/TLA pathway serves an
anti-inflammatory role through the expansion of regulatory T cells
(Tregs) and alleviates diseases (8-10).
Based on the role of the DR3/TL1A pathway in
pro-inflammatory and anti-inflammatory processes, this review
summarizes DR3-associated signals and reviews therapeutic
strategies targeting the DR3/TL1A pathway in preclinical models,
intending to facilitate the development of attractive drug
candidates.
In the late 1990s, a clone was found to exhibit
identity to the death domains (DDs) of cluster of differentiation
95 (CD95; 23% identity) and tumor necrosis factor receptor-1
(TNFR-1; 47% identity), which are both members of the TNFSF
(11). Additionally, the homologies
of the cysteine-rich repeats between the clone and CD95 and TNFR-1
were 22 and 26%, respectively (11). Therefore, the clone was classified
as a member of the TNFSF and was designated as DR3 (TNFRSF25,
APO-3, TRAMP, WSL-1, TR3 or LARD) (11,12).
DR3 is encoded by the Tnfrsf25 gene located in the 4E1
region of the mouse chromosome (13) and 1p36.3 of the human chromosome
(4). As a type I transmembrane
protein with a calculated molecular weight of 45 kDa and 417-amino
acid (aa) sequence, full-length DR3 is composed of an N-terminal
signal sequence (aa 1-24), a repeat sequence consisting of four
cysteine residues, two potential N-linked glycosylation
sites (aa 25-198), a transmembrane domain (aa 199-244), and an
intracellular domain with a DD (aa 255-417) (14,15).
Notably, DR3 is exclusively expressed in lymphocyte-rich tissues,
including the thymus, spleen, colon and intestine (11); however, low levels of expression are
detected in the fetal lung (16),
kidney (17), hippocampus (18) and peritoneal tissue (12). At the cellular level, DR3 is mainly
expressed in immune cells, including naïve or resting
CD4+ T cells, CD8+ T cells, natural killer T
cells, innate lymphoid cells (ILCs), B cells and mononuclear cells
(10,19). Notably, sustained expression of DR3
is found in Tregs. However, DR3 also exists in non-immune cells,
including bone cells (20) and
endothelial cell colony-forming cells (21). Expression of DR3 is highly regulated
in multiple pathologies. The levels of DR3 are significantly
increased in inflammatory bowel disease (IBD) (22), pulmonary sarcoidosis (23) and psoriasis vulgaris (24). By contrast, DR3 expression in
mononuclear cells is downregulated in diseases, including sickle
cell anemia (25) and colon cancer
(26).
TL1A, also known as vascular endothelial growth
inhibitor (VEGI)-251 or TNFSF15, is the only proven ligand for DR3
to date (5). TL1A is a single-pass
type II transmembrane protein encoded by the Tnfsf15 gene on
chromosome 4 in mice and 9q32 in humans (4). Similar to other TNFSF cytokines,
membrane-bound TL1A can be cleaved by alternative splicing or
through TNF-α-converting enzyme, and is shed from the extracellular
domain to form soluble TL1A (sTL1A) (4,5,27).
Notably, sTL1A may continue to efficiently activate DR3.
Endothelial cells, dendritic cells (DCs), monocytes/macrophages,
activated T cells and human umbilical vein endothelial cells have
been identified as major sources of TL1A (5,28-30).
Additionally, the expression of TL1A on DCs and macrophages is
increased upon activation via the receptor for the Fc region of IgG
(FcγR) and Toll-like receptors (TLRs), including TLR2 and TLR4,
while expression of TL1A on endothelial cells may be enhanced by
interleukin-1 beta (IL-1β) and TNF-α stimulation (15). Nevertheless, chondrocytes, synovial
fibroblasts, tissue macrophages and lamina propria lymphocytes also
express TL1A at low levels under conditions of inflammation or upon
stimulation (5,6). Therefore, the binding of TL1A to DR3
triggers signal transduction and exerts pro- and anti-inflammatory
functions.
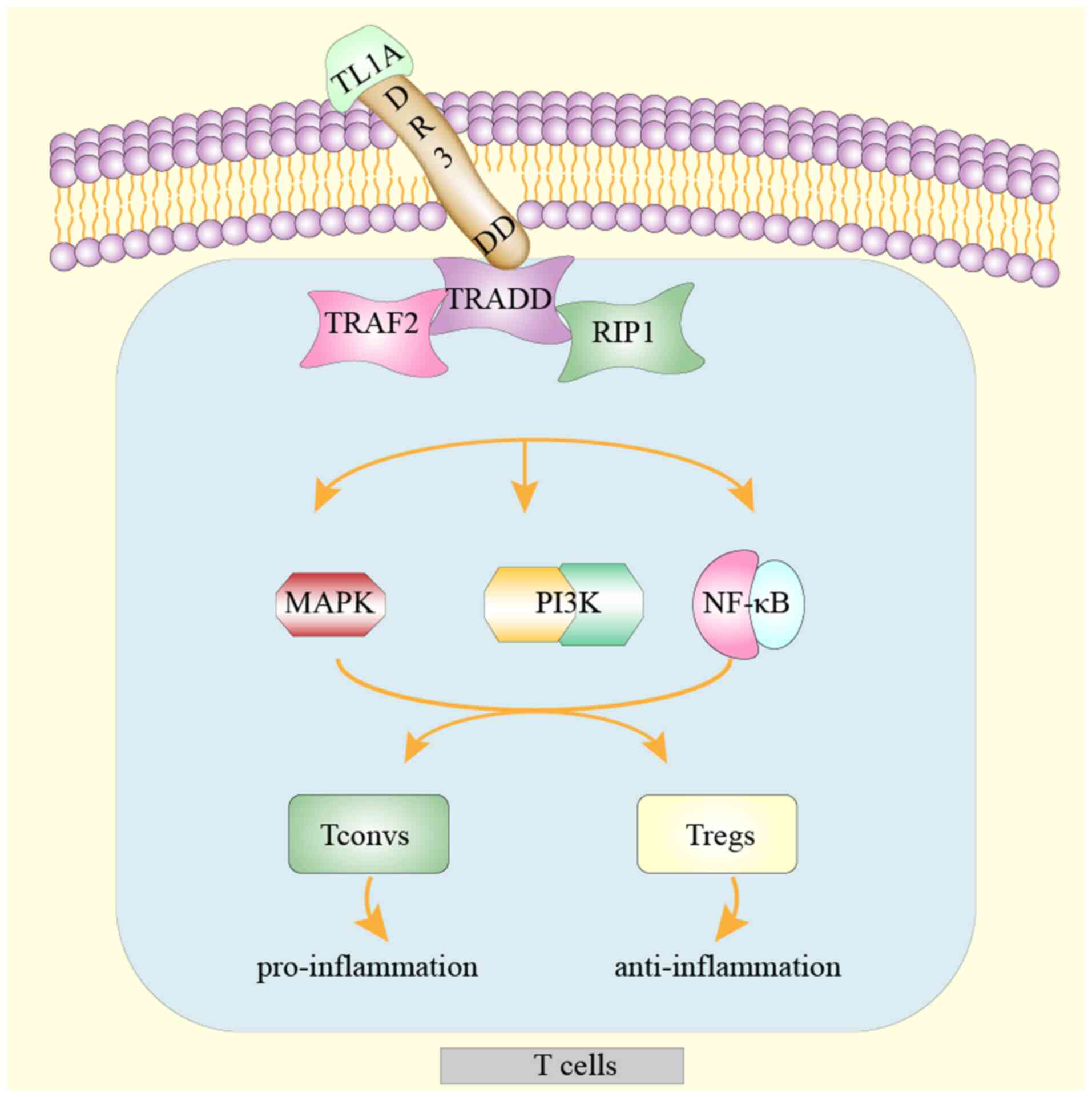
Upon binding to DR3 on immune cells, TL1A triggers
an interaction between the DD of DR3 and the adaptor protein,
TNFR-associated death domain (TRADD). Subsequently, TNFR-associated
factor 2 and receptor-interacting protein 1 bind to the DR3-TRADD
complex, leading to activation of mitogen-activated protein kinase
(MAPK), nuclear factor (NF)-κB, and phosphoinositide 3-kinase
(PI3K) signaling. Consequently, three distinct signaling cascades
induce immune cell activation, proliferation and cytokine secretion
(Fig. 1). The activation of DR3 on
immune cells by TL1A is an important prerequisite for its
pro-inflammatory and anti-inflammatory effects. Accumulating
evidence has revealed that pharmacological blockade or agonistic
activation of DR3/TL1A signaling is a novel and promising target
for inflammatory or immune-mediated diseases (6,8,10).
Co-stimulatory molecules expressed by
antigen-presenting cells and T cells appear to be indispensable for
the activation of T cells. The absence of co-stimulatory signals
leads to T cell incompetence (31).
The TNFSF is considered as one such group of co-stimulatory
molecules that activates T cells. Several studies have demonstrated
that, as a lymphocyte co-stimulatory signaling pathway, DR3/TL1A
amplifies effector CD4+ T cells in a non-specific manner
to aggravate the pathology of inflammatory diseases by triggering
the release of inflammatory cytokines. Furthermore, abundant
evidence also indicates that DR3 on ILCs affects the progression of
inflammatory diseases (32-34).
In addition to T cells, the DR3/TL1A pathway
co-stimulates ILCs. ILC2s and ILC3s are subsets of ILCs. DR3
signaling can expand ILC2s and ILC3s, driving IL-5 and IL-13
secretion from these cells. In vivo experimental evidence
has demonstrated that ILC2s cause deterioration in allergic lung
inflammation (34,35,47).
By contrast, ILC3s exert positive effects by increasing IL-22
production. Loss of DR3 signaling with decreased IL-22 production
has been associated with a higher histopathological score in a
DR3-deficient, dextran sulfate sodium (DSS)-induced colitis model
(33,48). Furthermore, an anti-DR3 antibody
triggered the loss of ILC3s from the intestine, ultimately
aggravating the colitis condition (32).
The DR3/TL1A pathway has been clearly implicated in
several diseases, including allergic lung inflammation (35), psoriasis (5), IBD (4)
and rheumatoid arthritis (43). The
aforementioned diseases share a common molecular mechanism in which
DR3 signaling exerts pleiotropic effects on the activation,
differentiation, proliferation and cytokine release of ILC2s and T
helper cells (including Th1, Th2, Th9 and Th17 cells). Therefore,
these findings suggested that pharmacological blockade of the
DR3/TL1A axis may have therapeutic value for human inflammatory and
immune-mediated diseases. An anti-TL1A monoclonal antibody
(anti-TL1A mAb) targeting the DR3/TL1A pathway has emerged as a
promising anti-inflammatory therapeutic agent in preclinical
studies (5,6,31,49).
Furthermore, unlike anti-TNF, anti-TL1A mAb may be relatively safe
and effective because it does not induce immunodeficiency (50).
It is evident that TL1A/DR3 signaling dampens gut
immune homeostasis and accelerates disease progression (4). Thus, blocking the TL1A/DR3 pathway may
be a useful strategy for relieving IBD. Studies in animal models
have demonstrated that anti-TL1A mAb exerts a protective function
in DSS-induced chronic colitis by suppressing Th1 and Th17 cell
activation (51). Additionally, in
acute 2,4,6-trinitrobenzene-sulfonic acid (TNBS)-induced colitis
involving increased production of Th1 cells and IFN-γ, blocking the
DR3/TL1A axis with anti-TL1A mAb almost completely prevented weight
loss and alleviated the histological score of inflammation
(52). However, administration of
anti-TL1A mAb, a neutralizing agent, mitigated the early phase, but
not the late stage of TNFΔARE/+ ileitis, thereby
suggesting the existence of other DR3 ligands aside from TL1A
(31). Of note, the aforementioned
studies have only demonstrated a decreased in inflammation caused
by anti-TL1A mAb rather than alleviation of intestinal fibrosis, a
hallmark of IBD. In DSS, as well as in the adoptive T cell transfer
chronic colitis models, treatment with anti-TL1A mAb resulted in a
decrease in the number of myofibroblasts and fibroblasts, leading
to a reversal of colonic fibrosis (53). Furthermore, Li et al
(54) discovered that intestinal
inflammation and fibrosis were attenuated in T cell transfer
chronic colitis models. Clarke et al (6) also confirmed that anti-TL1A mAb
significantly alleviated the clinical pathology of IBD by
decreasing inflammatory cell filtration and colonic fibrosis. In
general, therapeutic blockade of the DR3/TL1A pathway by anti-TL1A
mAb has numerous advantages, including amelioration of intestinal
inflammation and reversal of colonic fibrosis, compared with
traditional treatment for IBD, which only controls intestinal
inflammation.
In addition to IBD, blocking the DR3/TL1A pathway
with anti-TL1A mAb relieves collagen-induced arthritis (49), strongly reduces inflammatory cell
infiltration in OVA-induced asthma (6), and effectively alleviates the
histopathological changes in a psoriasis-like mouse model (5). Taken together, these results suggested
that anti-TL1A mAb has therapeutic potential for treating
inflammatory and immune-mediated diseases (Table I).
Activation of DR3 using an anti-DR3 agonistic
antibody or sTL1A has been shown to mediate Treg cell activation
and expansion because of the constitutive expression of DR3 on Treg
cells. DR3-mediated activation of Tregs mainly triggers the
activation of MAPK, NF-κB and PI3K signaling via DR3/TL1A signal
transduction (15,58,59),
thereby exerting anti-inflammatory effects and preventing
inflammatory and immune-mediated diseases in mice.
Due to the strong immunosuppressive function of
Tregs, adoptive transfer of exogenously expanded Tregs or IL-2/IL-2
complexes expanded Tregs in vivo can alleviate a variety of
immune-mediated diseases such as experimental autoimmune neuritis
(60), headache disorders (61), systemic lupus erythematosus
(62), IBD (63) and allograft tolerance (64-66),
as well as inflammatory diseases such as transfusion-related acute
lung injury (67,68) in preclinical models. Moreover,
adoptive Treg transfer and low-dose IL-2 therapy have been
associated with disease improvement and unconspicuous adverse
effects in patients (66). However,
the ability to obtain an adequate number of Tregs through adoptive
transfer limits their clinical use, while the cytotoxicity,
off-target effects and short half-life of IL-2 therapy in
vivo necessitate the use of approaches employing expanded Tregs
(64).
Clone 4C12 is an anti-DR3 agonistic antibody that
activates and expands Tregs and thus has been useful for preventing
or treating diseases in preclinical models (8,10,69).
To begin with, the stimulation of DR3 using 4C12 leads to rapid and
selective expansion of Tregs without influencing Tconvs (8,9,70,71).
Furthermore, 4C12 retains its capacity to expand Tregs ex
vivo (8). Additionally, the
half-life of 4C12 is 5 days (9),
ensuring prolonged contraction of expanded Tregs following 4C12
treatment compared with treatment with IL-2 or IL-2 complexes
(8). Prolonged contraction of
expanded Tregs is important for ameliorating inflammatory and
immune-mediated diseases.
Preclinical models have demonstrated that Tregs
expanded by 4C12 may alleviate disease. Schreiber et al
(8) first discovered that lung
inflammation was alleviated following treatment with 4C12 compared
with the control group in OVA-induced acute allergic pneumonia.
Madierddi et al (69) also
demonstrated that the administration of 4C12 relieved allergic
pneumonia and suppressed EAE through the expansion of Tregs. At
present, 4C12 is widely applied in the field of transplants,
including organ transplant, tissue transplant and cell transplant,
aiming to alleviate graft-versus-host disease (GVHD). For example,
Tregs expanded in vivo by 4C12 may provide tolerance to
cardiac allografts (71).
Recipients transplanted with donor-expanded Treg by 4C12 exhibited
enhanced skin allograft survival (72). Furthermore, the infusion of 4C12 in
recipient mice enhances skin graft survival associated with boosted
graft-infiltrating Tregs (73).
Administration of 4C12 also efficiently ameliorated GVHD in
hematopoietic stem cell transplantation (HSCT) mismatch mouse
models. Several studies have suggested that donor-derived Tregs are
more effective than recipient-derived Tregs for GVHD treatment
(74,75). Transfer of donor-derived Tregs
expanded by 4C12 markedly protected mice from GVHD (10,72,76).
Notably, Nishikii et al (29) demonstrated that treatment of
recipients with 4C12-induced Treg activation and proliferation was
sufficient to dampen GVHD lethality prior to exposure to HSCT. By
contrast, mice transplanted with Tregs that were expanded by 4C12
following HSCT exposure led to donor T cell proliferation and
ultimately fueled GVHD (29). These
findings demonstrated that activation of DR3 mitigated the
lethality of GVHD, depending on the timing of triggering DR3. Due
to the significance of IL-2 signals in DR3-mediated Tregs expansion
(8), low-dose IL-2 combined with
4C12 for donors resulted in decreased GVHD morbidity and prolonged
survival (10). Additionally, the
absolute number of Tregs induced by that combination was larger
than that observed following the treatment with 4C12 alone
(10). Therefore, this therapeutic
strategy may be an improvement for delaying allogeneic
rejection.
In addition to the anti-DR3 agonistic antibody,
which may induce rapid and selective expansion of Tregs, sTL1A is
sufficient as a soluble ligand for DR3 to trigger its signaling and
to expand Tregs in vivo (9).
Due to the shorter half-life of sTL1A (13.5 h), daily injection is
required to maintain the desired serum levels. Khan et al
(9) reported that sTL1A-meditated
Treg expansion alleviated allergic lung inflammation through
repeated administration, by blocking eosinophil infiltration into
the alveolar fluid and reversing the ratio of Tconvs to Tregs in
the lung without inducing any inflammatory changes in the liver,
kidney and myocardium. Additionally, sTL1A, alone or combined with
IL-2-expanded and IL-2-activated Tregs in donor mice, led to
decreased GVHD morbidity and mortality in HSCT models (10). Consistent with this observation,
low-dose IL-2 combined with sTL1A improved the clinical score,
dampened weight loss and increased survival prior to HSCT exposure
in animal models (77,78). Low-dose IL-2 combined with sTL1A
expanded a higher proportion of Tregs in the peripheral blood,
lymph nodes and spleen, compared with sTL1A alone (10). Whether the activation of Tregs
through sTL1A/IL-2 may prevent GVHD manifestations more effectively
than the treatment regimen of sTL1A requires further
investigation.
In conclusion, activation of the DR3/TL1A pathway
has been reported to alleviate allergic lung inflammation, improve
EAE symptoms and abrogate GVHD (Table
II). Physiologically, DR3 triggers pro-inflammatory and
anti-inflammatory effects. Blockade of DR3 signaling through
anti-TL1A mAb exerts anti-inflammatory effects by suppressing
effector immune cell activation. As anti-TL1A mAb is a neutralizing
antibody, the receptor-binding epitope and binding avidity may
affect antibody activity. As DR3 and Decoy receptor 3 (DcR3) are
receptors for TL1A, whether an antibody preferentially inhibits
TL1A binding to DR3 or to DcR3 should be determined during antibody
discovery campaigns. Additionally, activation of the DR3/TL1A axis
via 4C12, sTL1A or in combination with IL-2 shows distinct
immunosuppressive functions and controls aberrant immune reactions.
Due to the dose-dependent, DR3-mediated expansion of Tregs, it is
necessary to determine the optimal dose for Treg-based therapies.
During the development of an anti-DR3 agonistic antibody, attention
should be paid to FcγR-induced cellular cytotoxicity so as to
realize the full agonistic potential, while limiting binding to
FcγR. Therapeutically, interfering with the DR3/TL1A pathway in
preclinical models has shown great promise. Neutralizing antibodies
and agonists activating the DR3/TL1A pathway are required to target
inflammatory and immune-mediated diseases.
Not applicable.
Funding: The present study was funded by the National Natural
Science Foundation of China (grant no. 31801192) and CAMS
Innovation Fund for Medical Sciences (grant no.
2016-12M-3-024).
Not applicable.
YY and PJ designed the review. PS and NS analyzed
the relevant literature. YY drafted the manuscript and constructed
the figure. PJ edited the manuscript and constructed the tables. FL
provided financial resources and edited the manuscript. All authors
read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Dostert C, Grusdat M, Letellier E and
Brenner D: The TNF Family of Ligands and Receptors: Communication
Modules in the Immune System and Beyond. Physiol Rev. 99:115–160.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Aggarwal BB, Gupta SC and Kim JH:
Historical perspectives on tumor necrosis factor and its
superfamily: 25 years later, a golden journey. Blood. 119:651–665.
2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yang Y, Yeh SH, Madireddi S, Matochko WL,
Gu C, Pacheco Sanchez P, Ultsch M, De Leon Boenig G, Harris SF,
Leonard B, et al: Tetravalent biepitopic targeting enables
intrinsic antibody agonism of tumor necrosis factor receptor
superfamily members. MAbs. 11:996–1011. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Valatas V, Kolios G and Bamias G: TL1A
(TNFSF15) and DR3 (TNFRSF25): A Co-stimulatory System of Cytokines
With Diverse Functions in Gut Mucosal Immunity. Front Immunol.
10(583)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Li L, Fu L, Zhou P, Lu Y, Zhang L, Wang W,
Nie J, Zhang D, Liu Y, Wu B, et al: Effects of tumor necrosis
factor-like ligand 1A (TL1A) on imiquimod-induced psoriasiform skin
inflammation in mice. Arch Dermatol Res. 312:481–490.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Clarke AW, Poulton L, Shim D, Mabon D,
Butt D, Pollard M, Pande V, Husten J, Lyons J, Tian C, et al: An
anti-TL1A antibody for the treatment of asthma and inflammatory
bowel disease. MAbs. 10:664–677. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Richard AC, Tan C, Hawley ET,
Gomez-Rodriguez J, Goswami R, Yang XP, Cruz AC, Penumetcha P, Hayes
ET, Pelletier M, et al: The TNF-family ligand TL1A and its receptor
DR3 promote T cell-mediated allergic immunopathology by enhancing
differentiation and pathogenicity of IL-9-producing T cells. J
Immunol. 194:3567–3582. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Schreiber TH, Wolf D, Tsai MS, Chirinos J,
Deyev VV, Gonzalez L, Malek TR, Levy RB and Podack ER: Therapeutic
Treg expansion in mice by TNFRSF25 prevents allergic lung
inflammation. J Clin Invest. 120:3629–3640. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Khan SQ, Tsai MS, Schreiber TH, Wolf D,
Deyev VV and Podack ER: Cloning, expression, and functional
characterization of TL1A-Ig. J Immunol. 190:1540–1550.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mavers M, Simonetta F, Nishikii H, Ribado
JV, Maas-Bauer K, Alvarez M, Hirai T, Turkoz M, Baker J and Negrin
RS: Activation of the DR3-TL1A Axis in Donor Mice Leads to
Regulatory T Cell Expansion and Activation With Reduction in
Graft-Versus-Host Disease. Front Immunol. 10(1624)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chinnaiyan AM, O'Rourke K, Yu GL, Lyons
RH, Garg M, Duan DR, Xing L, Gentz R, Ni J and Dixit VM: Signal
transduction by DR3, a death domain-containing receptor related to
TNFR-1 and CD95. Science. 274:990–992. 1996.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Perks WV, Singh RK, Jones GW, Twohig JP,
Williams AS, Humphreys IR, Taylor PR, Jones SA and Wang ECY: Death
Receptor 3 Promotes Chemokine-Directed Leukocyte Recruitment in
Acute Resolving Inflammation and Is Essential for Pathological
Development of Mesothelial Fibrosis in Chronic Disease. Am J
Pathol. 186:2813–2823. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang EC, Kitson J, Thern A, Williamson J,
Farrow SN and Owen MJ: Genomic structure, expression, and
chromosome mapping of the mouse homologue for the WSL-1 (DR3, Apo3,
TRAMP, LARD, TR3, TNFRSF12) gene. Immunogenetics. 53:59–63.
2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Screaton GR, Xu XN, Olsen AL, Cowper AE,
Tan R, McMichael AJ and Bell JI: LARD: A new lymphoid-specific
death domain containing receptor regulated by alternative pre-mRNA
splicing. Proc Natl Acad Sci USA. 94:4615–4619. 1997.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Schreiber TH and Podack ER: Immunobiology
of TNFSF15 and TNFRSF25. Immunol Res. 57:3–11. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gout S, Morin C, Houle F and Huot J: Death
receptor-3, a new E-Selectin counter-receptor that confers
migration and survival advantages to colon carcinoma cells by
triggering p38 and ERK MAPK activation. Cancer Res. 66:9117–9124.
2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Al-Lamki RS, Wang J, Thiru S, Pritchard
NR, Bradley JA, Pober JS and Bradley JR: Expression of silencer of
death domains and death-receptor-3 in normal human kidney and in
rejecting renal transplants. Am J Pathol. 163:401–411.
2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu W, Vetreno RP and Crews FT:
Hippocampal TNF-death receptors, caspase cell death cascades, and
IL-8 in alcohol use disorder. Mol Psychiatry: Mar 5, 2020 (Epub
ahead of print). doi: 10.1038/s41380-020-0698-4.
|
|
19
|
Bittner S and Ehrenschwender M:
Multifaceted death receptor 3 signaling-promoting survival and
triggering death. FEBS Lett. 591:2543–2555. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Collins FL, Stone MD, Turton J, McCabe LR,
Wang ECY and Williams AS: Oestrogen-deficiency induces bone loss by
modulating CD14+ monocyte and CD4+ T cell DR3
expression and serum TL1A levels. BMC Musculoskelet Disord.
20(326)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Della Bella S, Calcaterra F, Bacci M,
Carenza C, Pandolfo C, Ferrazzi P, Uva P, Pagani M, Lodigiani C and
Mavilio D: Pathologic up-regulation of TNFSF15-TNFRSF25 axis
sustains endothelial dysfunction in unprovoked venous
thromboembolism. Cardiovasc Res. 116:698–707. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Slebioda TJ, Bojarska-Junak A, Cyman M,
Landowski P, Kaminska B, Celinski K and Kmiec Z: Expression of
death receptor 3 on peripheral blood mononuclear cells differes in
adult IBD patients and children with newly diagnosed IBD. Cytometry
B Clin Cytom. 92:165–169. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Facco M, Cabrelle A, Calabrese F, Teramo
A, Cinetto F, Carraro S, Martini V, Calzetti F, Tamassia N,
Cassatella MA, et al: TL1A/DR3 axis involvement in the inflammatory
cytokine network during pulmonary sarcoidosis. Clin Mol Allergy.
13(16)2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li L, Lu Y, Fu L, Zhou P, Zhang L, Wang W,
Nie J, Zhang D, Liu Y, Wu B, et al: Expression of death receptor 3
(DR3) on peripheral blood mononuclear cells of patients with
psoriasis vulgaris. Postgrad Med J. 94:551–555. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Safaya S, Alfarhan M, Sulaiman A,
Alsulaiman A and Al-Ali A: TNFSF/TNFRSF cytokine gene expression in
sickle cell anemia: Up-regulated TNF-like cytokine 1A (TL1A) and
its decoy receptor (DcR3) in peripheral blood mononuclear cells and
plasma. Cytokine. 123(154744)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ślebioda TJ, Stanisławowski M, Cyman M,
Wierzbicki PM, Żurawa-Janicka D, Kobiela J, Makarewicz W, Guzek M
and Kmieć Z: Distinct Expression Patterns of Two Tumor Necrosis
Factor Superfamily Member 15 Gene Isoforms in Human Colon Cancer.
Dig Dis Sci. 64:1857–1867. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bittner S, Knoll G, Füllsack S, Kurz M,
Wajant H and Ehrenschwender M: Soluble TL1A is sufficient for
activation of death receptor 3. FEBS J. 283:323–336.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li Z, Buttó LF, Buela KA, Jia LG, Lam M,
Ward JD, Pizarro TT and Cominelli F: Death Receptor 3 Signaling
Controls the Balance between Regulatory and Effector Lymphocytes in
SAMP1/YitFc Mice with Crohn's Disease-Like Ileitis. Front Immunol.
9(362)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Nishikii H, Kim BS, Yokoyama Y, Chen Y,
Baker J, Pierini A, Alvarez M, Mavers M, Maas-Bauer K, Pan Y, et
al: DR3 signaling modulates the function of Foxp3+
regulatory T cells and the severity of acute graft-versus-host
disease. Blood. 128:2846–2858. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Xu LX, Grimaldo S, Qi JW, Yang GL, Qin TT,
Xiao HY, Xiang R, Xiao Z, Li LY and Zhang ZS: Death receptor 3
mediates TNFSF15- and TNFα-induced endothelial cell apoptosis. Int
J Biochem Cell Biol. 55:109–118. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Buttó LF, Jia LG, Arseneau KO, Tamagawa H,
Rodriguez-Palacios A, Li Z, De Salvo C, Pizarro TT, Bamias G and
Cominelli F: Death-Domain-Receptor 3 Deletion Normalizes
Inflammatory Gene Expression and Prevents Ileitis in Experimental
Crohn's Disease. Inflamm Bowel Dis. 25:14–26. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li J, Shi W, Sun H, Ji Y, Chen Y, Guo X,
Sheng H, Shu J, Zhou L, Cai T, et al: Activation of DR3 signaling
causes loss of ILC3s and exacerbates intestinal inflammation. Nat
Commun. 10(3371)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Castellanos JG, Woo V, Viladomiu M, Putzel
G, Lima S, Diehl GE, Marderstein AR, Gandara J, Perez AR, Withers
DR, et al: Microbiota-Induced TNF-like Ligand 1A Drives Group 3
Innate Lymphoid Cell-Mediated Barrier Protection and Intestinal T
Cell Activation during Colitis. Immunity. 49:1077–1089.e5.
2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Karta MR, Broide DH and Doherty TA:
Insights into Group 2 Innate Lymphoid Cells in Human Airway
Disease. Curr Allergy Asthma Rep. 16(8)2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Singh RK, Perks WV, Twohig JP, Kidd EJ,
Broadley K, Farrow SN, Williams AS, Taylor PR and Wang ECY: Death
Receptor 3 regulates distinct pathological attributes of acute
versus chronic murine allergic lung inflammation. Cell Immunol.
320:62–70. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Jin S, Chin J, Seeber S, Niewoehner J,
Weiser B, Beaucamp N, Woods J, Murphy C, Fanning A, Shanahan F, et
al: TL1A/TNFSF15 directly induces proinflammatory cytokines,
including TNFα, from CD3+CD161+ T cells to
exacerbate gut inflammation. Mucosal Immunol. 6:886–899.
2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Papadakis KA, Zhu D, Prehn JL, Landers C,
Avanesyan A, Lafkas G and Targan SR: Dominant role for TL1A/DR3
pathway in IL-12 plus IL-18-induced IFN-gamma production by
peripheral blood and mucosal CCR9+ T lymphocytes. J
Immunol. 174:4985–4990. 2005.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Siakavellas SI and Bamias G: Tumor
Necrosis Factor-like Cytokine TL1A and Its Receptors DR3 and DcR3:
Important New Factors in Mucosal Homeostasis and Inflammation.
Inflamm Bowel Dis. 21:2441–2452. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Fang L, Adkins B, Deyev V and Podack ER:
Essential role of TNF receptor superfamily 25 (TNFRSF25) in the
development of allergic lung inflammation. J Exp Med.
205:1037–1048. 2008.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Meylan F, Davidson TS, Kahle E, Kinder M,
Acharya K, Jankovic D, Bundoc V, Hodges M, Shevach EM, Keane-Myers
A, et al: The TNF-family receptor DR3 is essential for diverse T
cell-mediated inflammatory diseases. Immunity. 29:79–89.
2008.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Basnyat P, Sumelahti ML, Lehtimäki T,
Elovaara I and Hagman S: Gene expression profiles of TNF-like
cytokine 1A (TL1A) and its receptors death receptor 3 (DR3) and
decoy receptor 3 (DcR3) in multiple sclerosis. J Neuroimmunol.
335(577020)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Jones GW, Stumhofer JS, Foster T, Twohig
JP, Hertzog P, Topley N, Williams AS, Hunter CA, Jenkins BJ, Wang
EC, et al: Naive and activated T cells display differential
responsiveness to TL1A that affects Th17 generation, maintenance,
and proliferation. FASEB J. 25:409–419. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhou M, Liu R, Su D, Feng X and Li X: TL1A
increased the differentiation of peripheral Th17 in rheumatoid
arthritis. Cytokine. 69:125–130. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Pappu BP, Borodovsky A, Zheng TS, Yang X,
Wu P, Dong X, Weng S, Browning B, Scott ML, Ma L, et al: TL1A-DR3
interaction regulates Th17 cell function and Th17-mediated
autoimmune disease. J Exp Med. 205:1049–1062. 2008.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wang D, Li H, Duan YY, Han F, Luo YX, Wu
MY, Yang MY, Zhan RR, Song J, Zhang H, et al: TL1A modulates the
severity of colitis by promoting Th9 differentiation and IL-9
secretion. Life Sci. 231(116536)2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Tsuda M, Hamade H, Thomas LS, Salumbides
BC, Potdar AA, Wong MH, Nunnelee JS, Stamps JT, Neutzsky-Wulff AV,
Barrett RJ, et al: A role for BATF3 in TH9 differentiation and
T-cell-driven mucosal pathologies. Mucosal Immunol. 12:644–655.
2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Meylan F, Hawley ET, Barron L, Barlow JL,
Penumetcha P, Pelletier M, Sciumè G, Richard AC, Hayes ET,
Gomez-Rodriguez J, et al: The TNF-family cytokine TL1A promotes
allergic immunopathology through group 2 innate lymphoid cells.
Mucosal Immunol. 7:958–968. 2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Castellanos JG and Longman RS: Innate
lymphoid cells link gut microbes with mucosal T cell immunity. Gut
Microbes. 11:231–236. 2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Bull MJ, Williams AS, Mecklenburgh Z,
Calder CJ, Twohig JP, Elford C, Evans BA, Rowley TF, Slebioda TJ,
Taraban VY, et al: The Death Receptor 3-TNF-like protein 1A pathway
drives adverse bone pathology in inflammatory arthritis. J Exp Med.
205:2457–2464. 2008.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Tougaard P, Zervides KA, Skov S, Hansen AK
and Pedersen AE: Biologics beyond TNF-α inhibitors and the effect
of targeting the homologues TL1A-DR3 pathway in chronic
inflammatory disorders. Immunopharmacol Immunotoxicol. 38:29–38.
2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Takedatsu H, Michelsen KS, Wei B, Landers
CJ, Thomas LS, Dhall D, Braun J and Targan SR: TL1A (TNFSF15)
regulates the development of chronic colitis by modulating both
T-helper 1 and T-helper 17 activation. Gastroenterology.
135:552–567. 2008.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Meylan F, Song YJ, Fuss I, Villarreal S,
Kahle E, Malm IJ, Acharya K, Ramos HL, Lo L, Mentink-Kane MM, et
al: The TNF-family cytokine TL1A drives IL-13-dependent small
intestinal inflammation. Mucosal Immunol. 4:172–185.
2011.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Shih DQ, Zheng L, Zhang X, Zhang H,
Kanazawa Y, Ichikawa R, Wallace KL, Chen J, Pothoulakis C, Koon HW,
et al: Inhibition of a novel fibrogenic factor Tl1a reverses
established colonic fibrosis. Mucosal Immunol. 7:1492–1503.
2014.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Li H, Song J, Niu G, Zhang H, Guo J, Shih
DQ, Targan SR and Zhang X: TL1A blocking ameliorates intestinal
fibrosis in the T cell transfer model of chronic colitis in mice.
Pathol Res Pract. 214:217–227. 2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Deng G, Song X and Greene MI: FoxP3 in
Treg cell biology: A molecular and structural perspective. Clin Exp
Immunol. 199:255–262. 2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Williams LM and Rudensky AY: Maintenance
of the Foxp3-dependent developmental program in mature regulatory T
cells requires continued expression of Foxp3. Nat Immunol.
8:277–284. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
57
|
Allos H, Al Dulaijan BS, Choi J and Azzi
J: Regulatory T Cells for More Targeted Immunosuppressive
Therapies. Clin Lab Med. 39:1–13. 2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Lubrano di Ricco M, Ronin E, Collares D,
Divoux J, Grégoire S, Wajant H, Gomes T, Grinberg-Bleyer Y, Baud V,
Marodon G, et al: Tumor necrosis factor receptor family
costimulation increases regulatory T-cell activation and function
via NF-κB. Eur J Immunol. 50:972–985. 2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Bittner S, Knoll G and Ehrenschwender M:
Death receptor 3 signaling enhances proliferation of human
regulatory T cells. FEBS Lett. 591:1187–1195. 2017.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Tran GT, Hodgkinson SJ, Carter N, Verma
ND, Robinson CM, Plain KM, Nomura M and Hall BM: Autoantigen
specific IL-2 activated CD4+CD25+T regulatory
cells inhibit induction of experimental autoimmune neuritis. J
Neuroimmunol. 341(577186)2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Zhang J, Czerpaniak K, Huang L, Liu X,
Cloud ME, Unsinger J, Hotchkiss RS, Li D and Cao YQ: Low-dose
interleukin-2 reverses behavioral sensitization in multiple mouse
models of headache disorders. Pain. 161:1381–1398. 2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Scalapino KJ, Tang Q, Bluestone JA,
Bonyhadi ML and Daikh DI: Suppression of disease in New Zealand
Black/New Zealand White lupus-prone mice by adoptive transfer of ex
vivo expanded regulatory T cells. J Immunol. 177:1451–1459.
2006.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Canavan JB, Scottà C, Vossenkämper A,
Goldberg R, Elder MJ, Shoval I, Marks E, Stolarczyk E, Lo JW,
Powell N, et al: Developing in vitro expanded CD45RA+
regulatory T cells as an adoptive cell therapy for Crohn's disease.
Gut. 65:584–594. 2016.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Golshayan D, Jiang S, Tsang J, Garin MI,
Mottet C and Lechler RI: In vitro-expanded donor
alloantigen-specific CD4+CD25+ regulatory T
cells promote experimental transplantation tolerance. Blood.
109:827–835. 2007.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Xia G, He J and Leventhal JR: Ex
vivo-expanded natural CD4+CD25+ regulatory T
cells synergize with host T-cell depletion to promote long-term
survival of allografts. Am J Transplant. 8:298–306. 2008.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Matsuoka KI: Low-dose interleukin-2 as a
modulator of Treg homeostasis after HSCT: Current understanding and
future perspectives. Int J Hematol. 107:130–137. 2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Kapur R, Kim M, Aslam R, McVey MJ, Tabuchi
A, Luo A, Liu J, Li Y, Shanmugabhavananthan S, Speck ER, et al: T
regulatory cells and dendritic cells protect against
transfusion-related acute lung injury via IL-10. Blood.
129:2557–2569. 2017.PubMed/NCBI View Article : Google Scholar
|
|
68
|
He R, Li L, Kong Y, Tian L, Tian X, Fang
P, Bian M and Liu Z: Preventing murine transfusion-related acute
lung injury by expansion of CD4+ CD25+
FoxP3+ Tregs using IL-2/anti-IL-2 complexes.
Transfusion. 59:534–544. 2019.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Madireddi S, Eun SY, Mehta AK, Birta A,
Zajonc DM, Niki T, Hirashima M, Podack ER, Schreiber TH and Croft
M: Regulatory T Cell-Mediated Suppression of Inflammation Induced
by DR3 Signaling Is Dependent on Galectin-9. J Immunol.
199:2721–2728. 2017.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Schreiber TH, Wolf D, Bodero M, Gonzalez L
and Podack ER: T cell costimulation by TNFR superfamily (TNFRSF)4
and TNFRSF25 in the context of vaccination. J Immunol.
189:3311–3318. 2012.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Wolf D, Schreiber TH, Tryphonopoulos P, Li
S, Tzakis AG, Ruiz P and Podack ER: Tregs expanded in vivo by
TNFRSF25 agonists promote cardiac allograft survival.
Transplantation. 94:569–574. 2012.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Wolf D, Bader CS, Barreras H, Copsel S,
Pfeiffer BJ, Lightbourn CO, Altman NH, Komanduri KV and Levy RB:
Superior immune reconstitution using Treg-expanded donor cells
versus PTCy treatment in preclinical HSCT models. JCI Insight.
3(e121717)2018.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Gorczynski RM, Sadozai H, Zhu F and Khatri
I: Effect of infusion of monoclonal antibodies to tumour necrosis
factor-receptor super family 25 on graft rejection in allo-immune
mice receiving autologous marrow transplantation. Immunology.
150:418–431. 2017.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Pierini A, Colonna L, Alvarez M,
Schneidawind D, Nishikii H, Baker J, Pan Y, Florek M, Kim BS and
Negrin RS: Donor Requirements for Regulatory T Cell Suppression of
Murine Graft-versus-Host Disease. J Immunol. 195:347–355.
2015.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Hoffmann P, Ermann J, Edinger M, Fathman
CG and Strober S: Donor-type CD4(+)CD25(+) regulatory T cells
suppress lethal acute graft-versus-host disease after allogeneic
bone marrow transplantation. J Exp Med. 196:389–399.
2002.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Kim BS, Nishikii H, Baker J, Pierini A,
Schneidawind D, Pan Y, Beilhack A, Park CG and Negrin RS: Treatment
with agonistic DR3 antibody results in expansion of donor Tregs and
reduced graft-versus-host disease. Blood. 126:546–557.
2015.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Wolf D, Barreras H, Bader CS, Copsel S,
Lightbourn CO, Pfeiffer BJ, Altman NH, Podack ER, Komanduri KV and
Levy RB: Marked In Vivo Donor Regulatory T Cell Expansion via
Interleukin-2 and TL1A-Ig Stimulation Ameliorates Graft-versus-Host
Disease but Preserves Graft-versus-Leukemia in Recipients after
Hematopoietic Stem Cell Transplantation. Biol Blood Marrow
Transplant. 23:757–766. 2017.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Copsel S, Wolf D, Kale B, Barreras H,
Lightbourn CO, Bader CS, Alperstein W, Altman NH, Komanduri KV and
Levy RB: Very Low Numbers of CD4+ FoxP3+
Tregs Expanded in Donors via TL1A-Ig and Low-Dose IL-2 Exhibit a
Distinct Activation/Functional Profile and Suppress GVHD in a
Preclinical Model. Biol Blood Marrow Transplant. 24:1788–1794.
2018.PubMed/NCBI View Article : Google Scholar
|