1. Introduction
Currently, diabetes is one of the most common
chronic diseases. Lifestyle changes, such as overeating and
increasingly sedentary lifestyles, continue to increase the number
of diabetic cases, particularly type 2 diabetes mellitus (T2DM)
(1). T2DM results from insulin
resistance, and the gradual loss of mass and function of pancreatic
β-cells (2). Inhibition of the
excessive apoptosis of islet β-cells is essential for the treatment
of diabetes (3). Increasing
evidence suggest that islet β-cell apoptosis, cell viability and
insulin secretion play essential roles in the pathogenesis of T2DM
(2-6).
In addition, insulin resistance plays a key role in T2DM and is
associated with metabolic syndrome (7). Autophagy, oxidative stress,
endoplasmic reticulum stress (ERS) and inflammation can moderate
islet β-cell dysfunction and insulin resistance and contribute to
the progression of T2DM (2,7). Although multiple signaling pathways
such as adenosine monophosphate-activated protein kinase
(AMPK)/sirtuin 1 (SIRT1), AMPK-mammalian target of rapamycin (mTOR)
are involved in the pathogenesis (8,9) of
T2DM, due to the complexity of T2DM, its overall pathogenesis
remains unclear.
Autophagy has three major subtypes, macroautophagy,
microautophagy and chaperone-mediated autophagy. The present review
focuses on macroautophagy, which is commonly referred to as
autophagy (10). Autophagy is a
highly conserved intracellular recycling degradation pathway that
targets cytosolic components for lysosomal degradation, and also
acts as a cell survival mechanism that promotes cellular
homeostasis (2,10). The degradation of cytosolic contents
via autophagy involves several intricate signaling pathways,
including inositol requiring kinase 1 (IRE1)-tumor necrosis factor
receptor associated factor 2 (TRAF2) and the AKT-TSC-mTOR pathway
(7,10). Autophagy is a complex process with
multiple steps, including initiation, nucleation,
elongation/completion and fusion/degradation (10). Furthermore, autophagy is
characterized by the lysosomal degradation of cellular material,
which is a process of subcellular membrane rearrangement that
sequesters the cytoplasm, proteins (or other cellular materials)
and organelles, forming the autophagosome (10). Under physiological conditions,
autophagy protects cellular homeostasis and prevent cells from
undergoing oxidative stress and inflammation (10). Autophagy can also respond to a
series of stresses, including the deprivation of nutrients or
growth factors, hypoxia and reactive oxygen species (ROS) (2,10).
Furthermore, autophagy is extensively involved in several diseases,
such as cancer, metabolic diseases and nervous system diseases
(2,4,11,12).
Autophagy has also been implicated in the pathophysiology of T2DM
(2-5,7),
including the regulation of pancreatic β-cell mass and function
(2-5)
and peripheral insulin resistance (7). In addition, multiple transcription
factors contribute to T2DM by regulating autophagy, such as
transcription factor Forkhead box O1 (FoxO1), SIRT1 and pancreatic
and duodenal homeobox 1 (Pdx1) (8,9).
However, the role of autophagy in the pathophysiology of T2DM
remains controversial.
As a member of the forkhead box protein family,
FoxO1 regulates fundamental cellular processes, including cell
differentiation, metabolism and cell cycle arrest (13-15).
FoxO1 activity is regulated by phosphorylation, acetylation and
ubiquitination (13,14,16).
FoxO1 is extensively expressed in several tissues, including the
pancreas (13), skeletal muscle
(17), adipose tissue (18) and the liver (19). Furthermore, FoxO1 is highly
expressed in pancreatic β-cells, and increasing evidence suggest
that FoxO1 regulates β-cell replication and differentiation
(13,20-23).
Several studies have suggested that FoxO1 is closely associated
with T2DM (9,14,16,24,25).
In addition, FoxO1 is a multifunctional protein that regulates the
insulin sensitivity of target tissues (19,26,27).
The present review summarizes the pathophysiological
pathways associated with autophagy and FoxO1 in T2DM. Furthermore,
the participation of FoxO1 in the development and occurrence of
T2DM via autophagy is discussed.
2. Autophagy and T2DM
Autophagy and T2DM. The characteristics of
T2DM are insulin resistance and the dysfunction of pancreatic
β-cells (2,7,10).
Multiple mechanisms contribute to pancreatic β-cell dysfunction and
mass loss, and insulin resistance, including oxidative stress, ERS,
upregulation of apoptosis and the uncontrolled autophagy of β-cells
(2,4,13,28).
Previous studies have reported that autophagy regulates β-cell
viability and apoptosis, and participates in the development and
occurrence of T2DM (2-4,7).
Dysregulation of autophagy can lead to the dysfunction of β-cells
or abnormal insulin secretion in pancreatic β-cells (2-4).
The present review summarizes current literature on
the role of autophagy in the survival and insulin secretion of
β-cells, and insulin resistance, focusing on autophagy during the
progression of T2DM.
Autophagy regulates β-cell viability and
apoptosis. The regulation of pancreatic β-cell mass and
function is vital for the pathogeny of T2DM. Furthermore, the
viability of β-cells is crucial in the occurrence and development
of T2DM. The number of β-cells decreases with an increase in the
apoptotic rate (2-5).
In addition, the extracellular accumulation of islet amyloid,
derived from human islet amyloid polypeptide (hIAPP), also
contributes to T2DM (29). Previous
studies have demonstrated that the inhibitor, 3-methyladenine
(3-MA), pretreated with autophagy decreases the viability of INS-1
cells and promotes cell apoptosis via ROS-mediated inflammation
(3,4). Furthermore, increasing evidence
suggest that certain drugs protect pancreatic β-cells by enhancing
autophagy (3,5,8). For
example, glucagon-like peptide-1 (GLP-1) analogs, liraglutide and
metformin, can suppress apoptosis by inducing autophagy in islet
β-cells in a high-glucose environment (3,8). In an
in vivo study, knockdown of autophagy-related gene (ATG)7 in
ob/ob mice increased the rate of apoptotic β-cells and decreased
the β-cell mass, which resulted in severe DM (30). In addition, the treatment of female
diabetic Akita mice with rapamycin-induced autophagy improved
ER-induced diabetes and inhibited β-cell apoptosis (31). The autophagy deficiency in mice
caused the accumulation of hIAPP and exacerbated the hIAPP-induced
β-cell toxicity and apoptosis (29). Fan et al (5) suggested that liraglutide can promote
pancreatic β-cell proliferation in a high-fat fed and
streptozotocin-induced mouse model of T2DM by enhancing autophagy.
Taken together, these findings confirm that autophagy is
indispensable for the survival of islet β-cells.
Autophagy and insulin secretion. Dysfunction
of insulin secretion is due to the abnormalities in insulin
biosynthesis or degradation (32).
During the early stages of T2DM, islet β-cells exhibit a
compensated higher insulin release. However, as T2DM progresses,
islet β-cells gradually exhibit impaired glucose-stimulated insulin
secretion (32,33). The deletion of free fatty acid
receptor 3 enhances insulin secretion in mice; however, this is not
due to the increase in β-cell mass (34). Thus, the underlying molecular
mechanisms of impaired insulin secretion remain unclear.
Previous studies have demonstrated the essential
roles of autophagy in T2DM development (3-5).
Furthermore, autophagy plays an essential role in insulin
homeostasis (32). Riahi et
al (32) revealed that the
lysosomal degradation of proinsulin through autophagy. Autophagy
deficiency does not affect basal insulin secretion but increases
glucose or KCl-stimulated insulin secretion (32). A recent study reported that
pretreatment with rapamycin, an mTOR inhibitor, induces autophagy
and significantly decreases the insulin content in NIT-1 cells
(35). Another study reported
similar results, indicating that the level of insulin significantly
increases due to glibenclamide, and further increases with 3-MA
(36). Collectively, these findings
suggest that suppressing autophagy increases β-cell insulin
secretion. In an in vivo study, autophagy hyperactive mice
exhibited impaired insulin secretion compared with mice with normal
autophagy, the effects of which were reversed following transient
treatment with autophagy inhibitors (37). This may be due to the theory that
overexpression of autophagy increases the degradation of insulin
granules in insulin-secreting β-cells (32,37).
Conversely, several studies have suggested that
deficiency of autophagy decreases the insulin secretion in
pancreatic β-cells (30,38,39).
Mice with the β-cell specific deletion of ATG7, which is a
necessary for autophagy (10),
developed glucose intolerance that resulted from decreased insulin
secretion (30,38,39).
Due to the insufficient unfolded protein response machinery,
autophagy-deficient β-cells fail to adapt to the lipotoxicity
environment, which decreases insulin secretion and diabetes in ATG7
deficiency mice (30). Jung et
al (39) revealed that fasting
serum insulin concentration is notably lower in the β-cell specific
deletion of ATG7 mice compared with the control mice; however, the
levels of ubiquitinated proteins increase.
Taken together, these findings suggest that
autophagy has a dual effect with the insulin secretion of β-cells,
but the underlying mechanisms still need further research.
Autophagy and insulin resistance. Insulin
resistance has an important effect on the occurrence of T2DM and is
closely associated with metabolic syndrome (40). Insulin resistance refers to the
effect of insulin, which decreases in insulin-responsive tissues,
including adipocytes, the liver and skeletal muscles (7,40). In
the liver, failure of insulin action decreases glycogen synthesis
and increases gluconeogenesis. For adipose tissues,
insulin-mediated glucose utilization markedly decreases, which
results in high levels of free fatty acids in insulin-resistant
states. The skeletal muscle shares 80% of the total
insulin-stimulated glucose uptake in humans (7). Thus, insulin resistance in the
skeletal muscle plays a significant role in the development of T2DM
(7,41).
Multiple factors cause insulin resistance in target
tissues, such as mitochondrial dysfunction, ERS and inflammation
(2,7). However, the underlying molecular
mechanism of insulin resistance remains unclear. Increasing
evidence suggest an association between autophagy and insulin
resistance (41-44).
Progranulin may mediate adipose insulin resistance, at least in
part, by inducing autophagy through activation of oxidative stress
and ERS (43).
Autophagy-hyperactive mice have improved insulin sensitivity in
insulin target tissues by decreasing ERS (5). The suppression of autophagy results in
insulin resistance in the liver tissues of mice. Furthermore,
restoration of autophagy in obese mice induces insulin action in
liver tissues (28). Insulin
receptor substrate-1 (IRS1) is the direct substrate of the
insulin-sensing insulin receptor, which is closely associated with
insulin resistance (7). ATG16L1
deficiency in mouse embryonic fibroblasts results in insulin
resistance, which is mediated by IRS1 degradation (41). Collectively, these studies indicate
that autophagy suppresses insulin resistance in insulin target
tissues. Furthermore, these studies demonstrate that autophagy has
a beneficial effect in regulating insulin resistance. Thus, the
level of autophagy is important for tissue insulin sensitivity.
3. FoxO1 and T2DM
Increasing evidence suggest that FoxO1 regulates
cell proliferation, apoptosis, differentiation, antioxidant stress
and insulin secretion in pancreatic β-cells, and is closely
associated with T2DM (13,24,25,45-48).
Furthermore, FoxO1 plays a crucial role in regulating the insulin
sensitivity of insulin target tissues (17,19,27,49).
FoxO1 regulates the survival of β cells. The
regulation of pancreatic β-cell survival is vital for the β-cell
mass in T2DM (3,4). FoxO1 plays a crucial role in β-cell
survival (50,51). Nak et al (18) reported that protein kinase C delta
induces the nuclear accumulation of FoxO1 but does not increase
β-cell death. Furthermore, FoxO1 does not induce pro-apoptotic
genes but may exert beneficial effects when phosphorylated under
non-stress environments (22).
Inhibition of FoxO1 dephosphorylation is necessary for the
induction of pro-apoptotic gene Bcl-2, which may explain why
nuclear FoxO1 is not pro-apoptotic on pancreatic β-cells (22). However, whether downregulation or
upregulation of FoxO1 affect the glucocorticoid receptor-induced
β-cell apoptosis remains to be investigated (23).
Chronic oxidative stress is associated with cellular
apoptosis (47). Furthermore, it
has been demonstrated that oxidative stress is one of the
mechanisms for β-cell dysfunction and failure by chronic exposure
to elevate glucose concentrations (50). Zhang et al (51) reported that FoxO1 protects β-cells
from oxidative stress by inducing the expression of β-cell
antioxidant enzymes, such as superoxide dismutase, catalase and
glutathione peroxidase 1. Furthermore, FoxO1 protects pancreatic
β-cells against oxidative stress by suppressing the expression of
the thioredoxin-interacting protein, which is a cytosolic factor
that regulates β-cell apoptosis (52). Taken together, these findings
suggest that FoxO1 plays a protective role in β-cell survival.
FoxO1 regulates β-cell insulin secretion.
Previous studies have demonstrated that FoxO1 is an important
player in β-cell insulin secretion (8,9,13,53-55).
Pdx1 is a key regulator of β-cell differentiation, proliferation
and insulin synthesis (56,57). A previous study reported that FoxO1
inhibits Pdx1 expression, thereby offering a mechanism in which
FoxO1 may regulate β-cell insulin secretion (24). Chronic exposure to high glucose in
β-cells activates FoxO1 and increases its nuclear location, which
in turn decreases Pdx1 expression and reduces insulin secretion
(55). FoxO1 knockdown efficiently
decreases microRNA (miR)-802 expression in Min6 cells in mice
(58), while overexpression of
miR-802 in β-cells impairs insulin synthesis and secretion
(58). FoxO1 knock-in mice in the
pancreas develops glucose intolerance and impairs insulin secretion
due decreased Pdx1 expression (53). Inhibition of FoxO1 reverses the
dexamethasone-induced impairment on insulin secretion in rat islets
by inhibiting Pdx-1 expression (54).
Neurogenic differentiation factor (NeuroD) and Mus
musculus v-maf muscu-loaponeurotic fibrosarcoma oncogene family
protein A (MafA) are insulin gene transcription factors of β-cells
that promote insulin secretion (20,56).
In cultured βTC-3 cells, FoxO1 stimulates NeuroD and MafA
expression in response to oxidative damage induced by
H2O2 treatment, and preserves islet function
(20). This concept was confirmed
by a study that revealed that FoxO1-transgenic mice exhibit
significantly elevated insulin 1 gene expression levels,
accompanied by upregulation of pancreatic Pdx1 and NeuroD (51). However, db/db mice lack FoxO1,
particularly in pancreatic β-cells, which decreases insulin
secretion and exhibits severe glucose intolerance (21). This suggests that FoxO1 is necessary
for the maintenance of insulin secretion under metabolic stress
(21). Collectively, these findings
indicate that FoxO1 plays an essential role in insulin
secretion.
FoxO1 regulates insulin resistance. FoxO1 is
extensively expressed in insulin target tissues, such as
hepatocytes, adipocytes and skeletal muscles (17-19).
Previous studies have proposed that FoxO1 plays a significant role
in the occurrence and development of insulin resistance (18,59,60).
However, FoxO1 also promotes insulin resistance, which has been
demonstrated in several studies (17,19,27,49).
FoxO1 knockdown in HepG2-insulin resistance cells decreases the
glucose content and alleviates insulin resistance in primary
hepatocytes (22).
Phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase
(G6Pase) are the two key enzymes of gluconeogenesis (19,49). A
recent study revealed that inhibiting FoxO1 expression
significantly decreases the blood glucose content and mRNA level of
PEPCK/G6Pase in HepG2 cells (50).
Gu et al (19) supported
these results, suggesting that FoxO1 inhibits PEPCK and G6Pase
expression, and glucose output in hepatocytes. In addition, it has
been reported that FoxO1 expression decreases in the liver of T2DM
insulin resistance mice induced by high-fat diet (19). Inhibiting FoxO1 mRNA expression with
antisense oligonucleotides therapy in mice improves the clearance
of glucose following intraperitoneal glucose load, and improves the
hepatic insulin sensitivity and adipocyte insulin action (27). Mice specifically overexpressing
FoxO1 in the skeletal muscle exhibit impaired glucose tolerance and
insulin tolerance (17), suggesting
that FoxO1 promotes insulin resistance and muscle atrophy (17).
Some studies have indicated that FoxO1 is a
counter-regulator of target tissue insulin resistance (18,26,59-63).
Dysregulation of lipid metabolism in the liver contributes to
insulin resistance (63,64). Fasting triglyceride and cholesterol
levels, and postprandial triglyceride levels are substantially
suppressed in transgenic mice that express active FoxO1(63). Zhang et al (64) reported similar results, indicating
that hepatic deletion of FoxO1 increases cholesterol concentrations
and serum triglyceride and elevates hepatic lipid secretion.
Furthermore, overexpression of FoxO1 inhibits the expression levels
of lipogenesis genes, including Fasn and Hmgcr (64). The expression of pseudokinase
tribble 3 has been reported to negatively regulate insulin
sensitivity (60,65). FoxO1 suppresses tribble 3 expression
in an insulin-dependent manner in hepatocytes and promotes insulin
sensitivity during fasting (60).
Phosphorylated mammalian FoxO1 suppresses hepatic glucose
production (66). Adiponectin is a
hormone that plays a key role in regulating energy homeostasis and
metabolism (67,68). Furthermore, adiponectin plays a
crucial role in improving insulin sensitivity in adipose tissues,
the liver and muscles, and exerts antidiabetic functions (67,68).
GLP-1 agonist exendin-4 induces adiponectin expression (69). However, suppression of Foxo-1
impairs the effect of exendin-4 on the adiponectin expression in
3T3-L1 adipocytes (69).
Overexpression of mutant FoxO1 in brown adipose tissues increases
the number of brown adipocytes and the expression of the uncoupling
protein 1 (UCP1) gene in T37i cells (18). FoxO1 protects against insulin
resistance during adipocyte differentiation (26). FoxO1 transgenic mice increased
insulin sensitivity in adipose tissue (60). FoxO1 deletion decreases IRS2
expression, glucose uptake and insulin action in muscle tissues
(62).
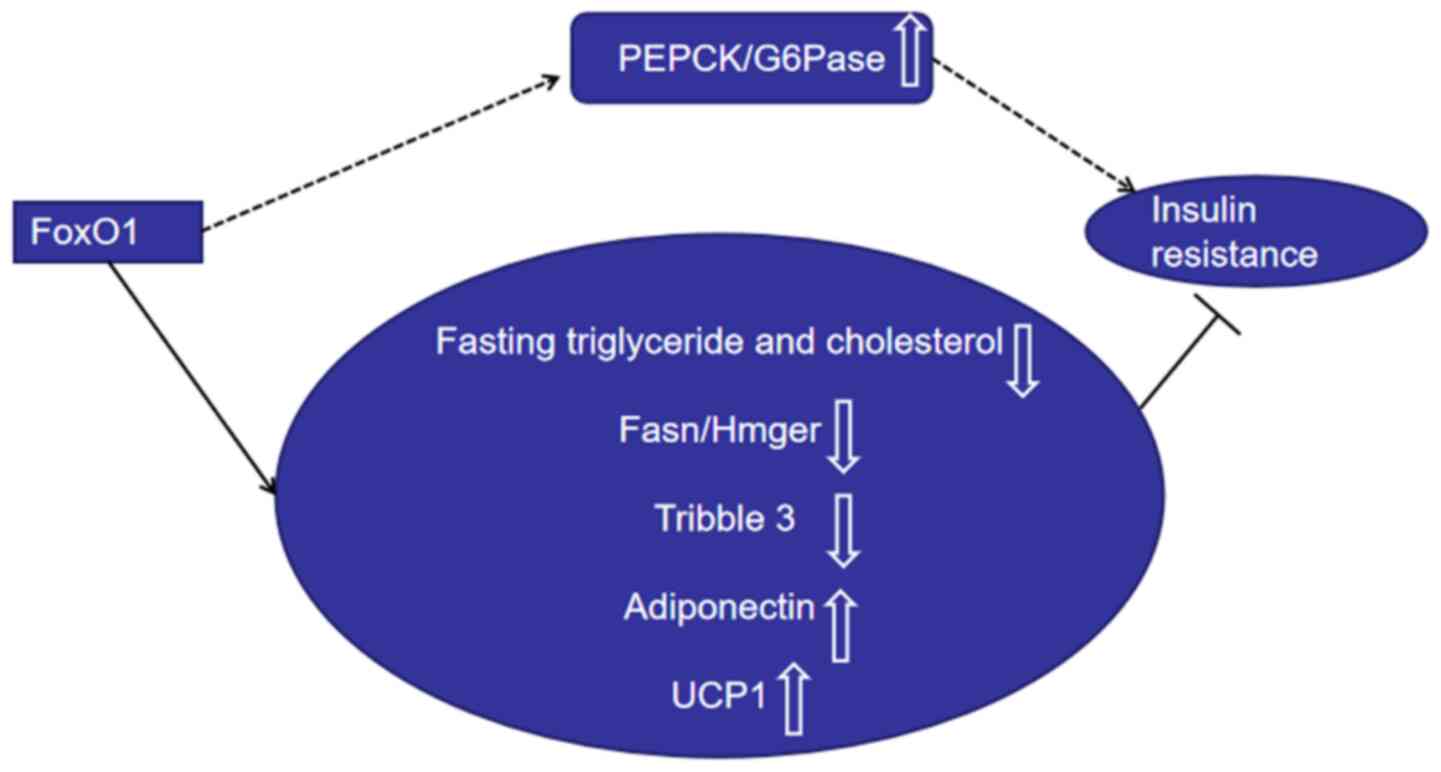
Collectively, these findings suggest that FoxO1 has
a dual role in regulating insulin resistance (Fig. 1). In addition to regulating β-cell
mass, FoxO1 also regulates β-cell function, and the level of FoxO1
is important for tissue insulin sensitivity.
4. Autophagy, FoxO1 and T2DM
FoxO1-autophagy axis. FoxO transcription
factors regulate two main proteolytic systems, the
ubiquitin-proteasome and autophagy-lysosome systems (70). Increasing evidence suggest that
FoxO1 upregulates the expression of autophagy (71-73).
FoxO1 elicits autophagy in a transcription-independent manner
(72). Acetylated FoxO1 interacts
with endogenous ATG7 and induces autophagy and suppresses cell
viability in cancer cells (72).
Liu et al (73) reported
that FoxO1 antagonists suppress autophagy during adipocyte
differentiation and decrease lipid droplet size in adipocytes.
FoxO1 enhances autophagy, and the FoxO1-autophagy axis regulates
lipid droplet growth in white adipose tissues (73). Notably, activation of FoxO1
increases Rab7 expression, while the accumulation of p62 decreases
in vascular endothelial cells treated with ox-LDL (11). Treatment with FoxO1 small
interfering RNA inhibits autophagic flux, resulting in oxidative
stress and apoptosis in human cholangiocarcinoma QBC939 cells
(12). A recent study reported that
metformin alleviates renal injury in diabetic rats by regulating
the adenosine monophosphate-activated protein kinase-SIRT1-FoxO1
pathway, thus promoting autophagy (74). The inhibitory effect of miR-21 on
the expression of the autophagy-related protein, LC3II/I, and the
facilitation of p62 is abrogated following upregulation of FoxO1 in
high glucose-cultured podocytes (75). The inhibitor of FoxO1
transcriptional activity, AS, decreases autophagic activity and
cell death of cardiomyocyte H9c2 cells. Both in vivo and
in vitro experiments have demonstrated that
1,25-dihydroxyvitamin D attenuates diabetic heart-related cardiac
autophagy and damage by activating vitamin D to suppress the
nuclear translocation of FoxO1(76). Activation of FoxO1 in cardiomyocytes
decreases the cell size and significantly increases the expression
levels of LC3 and ATG12(77). FoxO1
silencing markedly decreases autophagy induced by oxidative stress
and increases apoptosis in cardiomyocytes (78). Resveratrol enhances the autophagic
flux via the SIRT1/FoxO1/Rab7 axis, and ameliorates myocardial
oxidative stress injury in diabetic mice (79). Taken together, these findings
suggest that FoxO1 promotes autophagy in cardiomyocytes.
Platelet-rich plasma decreases apoptosis in human osteoarthritic
cartilage by increasing FoxO1 and autophagy (80). Autophagy inhibitor, 3-MA,
significantly aggravates high-glucose-induced apoptosis and
decreases cell viability in cardiac myocytes (81). In addition to decreasing the
autophagy-related protein, LC3II/I and cell viability, FoxO1
silencing also enhances the high-glucose-mediated apoptosis-related
protein caspase-3 activation (81).
Collectively, these results suggest that FoxO1 inhibits apoptosis
and enhances cell survival by promoting autophagy.
FoxO1-autophagy axis and T2DM. Autophagy and
FoxO1 are both involved in the development of T2DM (2,10,13,14).
Autophagy has a crucial effect on β-cell mass and function, and
target tissue insulin resistance (2,7,10,44).
Previous studies have reported that moderate autophagy can promote
β-cell survival, insulin secretion and target tissue insulin
sensitivity (2,3,7,10).
Furthermore, it has been demonstrated that antidiabetic agents,
liraglutide and metformin, promote islet β-cell proliferation and
suppress β-cell apoptosis by enhancing autophagy (5,8).
Increasing evidence suggest that the FoxO1-autophagy
axis is involved in several diseases, including diabetes,
cardiovascular disease and cancer (11,12,15,74,77).
FoxO1 has been demonstrated to upregulate cell autophagy in
different types of cells, including cancer cells (72), adipocytes (73), podocytes (75) and cardiomyocytes (78). FoxO1 is essential for the
progression of T2DM, although the role of FoxO1 in β-cell function
and mass remains controversial. Increasing evidence suggest that
FoxO1 improves insulin resistance via autophagy in T2DM (82,83).
Inflammatory factors, such as interleukin-6 (IL)-6, tumor necrosis
factor-α (TNF-α) and nod-like receptor 3 (NLRP3), contribute to the
insulin resistance of pancreatic islets in T2DM (4). Upregulation of FoxO1 enhances
autophagy activity and decreases the expression levels of the
inflammatory cytokines, IL-6, TNF-α and NLRP3, in the liver
(82). The FoxO1-mediated
transcription of key autophagy genes is an intricate part of
insulin suppression of autophagy in cultured hepatocytes (82). FoxO1 deficiency impairs autophagy in
the liver, and overexpression of FoxO1 stimulates ATG14 gene
expression in mouse primary hepatocytes and lowers triglyceride
concentrations in mice (83).
miR-378 inhibits apoptosis by promoting autophagy in skeletal
muscles and sustains autophagy through FoxO1-mediated
transcriptional reinforcement by targeting
phosphoinositide-dependent protein kinase 1(80). FoxO1 activation induces lipid
droplet degradation via autophagy and improves metabolic stress in
adipocytes (84).
Increasing evidence suggest that FoxO1-mediated
autophagy enhances cell viability and inhibits cell apoptosis in
different types of cells (12,79,80,81).
However, the effect of FoxO1 on autophagy in pancreatic β-cells
requires further investigation.
Taken together, current literature suggest that
FoxO1 silencing inhibits autophagy of INS-1 cells and decreases the
survival of INS-1 cells (unpublished data). In conclusion, the
FoxO1-autophagy axis is closely associated with T2DM.
5. Conclusions and future direction
T2DM poses a serious public-health threat to those
affected. Autophagy and FoxO1 are involved in pancreatic β-cell
dysfunction and insulin resistance. Thus, regulation of FoxO1 and
autophagy activity in islet β-cells has the potential to serve as a
novel therapeutic strategy against T2DM. In addition, some
hypoglycemic drugs, such as metformin, GLP-1 receptor agonists and
GLP-1 analogs, have been demonstrated to exert anti-T2DM effects
via autophagy and FoxO1. However, further studies are required to
determine whether these drugs can achieve the effect of improving
β-cell function and mass, and insulin resistance via the
FoxO1-autophagy axis.
Acknowledgements
Not applicable.
Funding
Funding: The present review was supported by the National
Natural Science Foundation of China (grant no. 81770820).
Availability of data and materials
Not applicable.
Authors' contributions
All authors contributed equally to the conception
and design of the present review, and drafted and revised the
initial manuscript for important intellectual content. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Harris SR, Carrillo M and Fujioka K:
Binge-eating disorder and type 2 diabetes: A Review. Endocr Pract:
December 20, 2020. doi: 10.1016/j.eprac.2020.10.005.
|
|
2
|
Wang Y, Li YB, Yin JJ, Wang Y, Zhu LB, Xie
GY and Pan SH: Autophagy regulates inflammation following oxidative
injury in diabetes. Autophagy. 9:272–277. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chen ZF, Li YB, Han JY, Yin JJ, Wang Y,
Zhu LB and Xie GY: Liraglutide prevents high glucose level induced
insulinoma cells apoptosis by targeting autophagy. Chin Med J
(Engl). 126:937–941. 2013.PubMed/NCBI
|
|
4
|
Zhu LB, Cao MM, Wang J, Su Y, Jiang W, Liu
GD and Li YB: Role of autophagy in LPS-induced inflammation in
INS-1 cells. Mol Med Rep. 19:5211–5218. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fan M, Jiang H, Zhang Y, Ma Y, Li L and Wu
J: Liraglutide enhances autophagy and promotes pancreatic β cell
proliferation to ameliorate type 2 diabetes in high-fat-fed and
streptozotocin-treated mice. Med Sci Monit. 24:2310–2316.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Melmed L, Polonsky KS, Reed Larsen P and
Kronenberg H: Chapter on pathogenesis of type 2 diabetes-Williams
Textbook of Endocrinology. 13th edition. Elsevier, 2016.
|
|
7
|
Zhang N, Cao MM, Liu H, Xie GY and Li YB:
Autophagy regulates insulin resistance following endoplasmic
reticulum stress in diabetes. J Physiol Biochem. 71:319–327.
2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li Q, Jia S, Xu L, Li B and Chen N:
Metformin-induced autophagy and irisin improves INS-1 cell function
and survival in high-glucose environment via AMPK/SIRT1/PGC-1α
signal pathway. Food Sci Nutr. 7:1695–1703. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cheng STW, Li SYT and Leung PS: Fibroblast
growth factor 21 stimulates pancreatic islet autophagy via
inhibition of AMPK-mTOR signaling. Int J Mol Sci 20: 2517. doi:
10.3390/ijms20102517.
|
|
10
|
Lee YH, Kim J, Park K and Lee MS: β-cell
autophagy: Mechanism and role in β-cell dysfunction. Mol Metab.
27S:S92–S103. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wu Q, Hu Y, Jiang M, Wang F and Gong G:
Effect of autophagy regulated by Sirt1/FoxO1 pathway on the release
of factors promoting thrombosis from vascular endothelial cells.
Int J Mol Sci. 20(4132)2019.PubMed/NCBI View Article : Google Scholar : doi:
10.3390/ijms20174132.
|
|
12
|
He W, Zhang A, Qi L, Na C, Jiang R, Fan Z
and Chen J: FOXO1, a potential therapeutic target, regulates
autophagic Flux, oxidative stress, mitochondrial dysfunction, and
apoptosis in human cholangiocarcinoma QBC939 cells. Cell Physiol
Biochem. 45:1506–1514. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kitamura T: The role of FOXO1 in β-cell
failure and type 2 diabetes mellitus. Nat Rev Endocrinol.
9:615–623. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Xing YQ, Li A, Yang Y, Li XX, Zhang LN and
Guo HC: The regulation of FOXO1 and its role in disease
progression. Life Sci. 193:124–131. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cheng Z: The FoxO-autophagy axis in health
and disease. Trends Endocrinol Metab. 30:658–671. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen B, Zhou W, Zhao W, Yuan P, Tang C,
Wang G, Leng J, Ma J, Wang X, Hui Y, et al: Oxaliplatin reverses
the GLP-1R-mediated promotion of intrahepatic cholangiocarcinoma by
altering FoxO1 signaling. Oncol Lett. 18:1989–1998. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kamei Y, Miura S, Suzuki M, et al:
Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal
muscle mass, down-regulated Type I (slow twitch/red muscle) fiber
genes, and impaired glycemic control. J Biol Chem.
2004;279(39):41114‐41123. doi:10.1074/jbc.M400674200.
|
|
18
|
Nakae J, Cao Y, Oki M, et al: Forkhead
transcription factor FoxO1 in adipose tissue regulates energy
storage and expenditure. Diabetes. 2008;57(3):563‐576.
doi:10.2337/db07-0698.
|
|
19
|
Gu L, Ding X, Wang Y, et al: Spexin
alleviates insulin resistance and inhibits hepatic gluconeogenesis
via the FoxO1/PGC-1α pathway in high-fat-diet-induced rats and
insulin resistant cells. Int J Biol Sci. 2019;15(13):2815‐2829.
Published 2019 Nov 1. doi:10.7150/ijbs.31781.
|
|
20
|
Kitamura YI, Kitamura T, Kruse JP, Raum
JC, Stein R, Gu W and Accili D: FoxO1 protects against pancreatic
beta cell failure through NeuroD and MafA induction. Cell Metab.
2:153–163. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kobayashi M, Kikuchi O, Sasaki T, Kim HJ,
Yokota-Hashimoto H, Lee YS, Amano K, Kitazumi T, Susanti VY,
Kitamura YI, et al: FoxO1 as a double-edged sword in the pancreas:
Analysis of pancreas- and β-cell-specific FoxO1 knockout mice. Am J
Physiol Endocrinol Metab. 302:E603–E613. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gerst F, Kaiser G, Panse M, Sartorius T,
Pujol A, Hennige AM, Machicao F, Lammers R, Bosch F, Häring HU, et
al: Protein kinase Cδ regulates nuclear export of FOXO1 through
phosphorylation of the chaperone 14-3-3ζ. Diabetologia.
58:2819–2831. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kaiser G, Gerst F, Michael D, Berchtold S,
Friedrich B, Strutz-Seebohm N, Lang F, Häring HU and Ullrich S:
Regulation of forkhead box O1 (FOXO1) by protein kinase B and
glucocorticoids: Different mechanisms of induction of beta cell
death in vitro. Diabetologia. 56:1587–1595. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kitamura T, Nakae J, Kitamura Y, Kido Y,
Biggs WH III, Wright CV, White MF, Arden KC and Accili D: The
forkhead transcription factor Foxo1 links insulin signaling to Pdx1
regulation of pancreatic beta cell growth. J Clin Invest.
110:1839–1847. 2002.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mo X, Wang X, Ge Q and Bian F: The effects
of SIRT1/FoxO1 on LPS induced INS-1 cells dysfunction. Stress.
22:70–82. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Boughanem H, Cabrera-Mulero A,
Millán-Gómez M, Garrido-Sánchez L, Cardona F, Tinahones FJ,
Moreno-Santos I and Macías-González M: Transcriptional analysis of
FOXO1, C/EBP-α and PPAR-γ2 genes and their association with
obesity-related insulin resistance. Genes. 10(706)2019.PubMed/NCBI View Article : Google Scholar :
doi.org/10.3390/genes10090706.
|
|
27
|
Samuel VT, Choi CS, Phillips TG, Romanelli
AJ, Geisler JG, Bhanot S, McKay R, Monia B, Shutter JR, Lindberg
RA, et al: Targeting foxo1 in mice using antisense oligonucleotide
improves hepatic and peripheral insulin action. Diabetes.
55:2042–2050. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yang L, Li P, Fu S, Calay ES and
Hotamisligil GS: Defective hepatic autophagy in obesity promotes ER
stress and causes insulin resistance. Cell Metab. 11:467–478.
2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rivera JF, Costes S, Gurlo T, Glabe CG and
Butler PC: Autophagy defends pancreatic β cells from human islet
amyloid polypeptide-induced toxicity. J Clin Invest. 124:3489–3500.
2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Quan W, Hur KY, Lim Y, Oh SH, Lee JC, Kim
KH, Kim GH, Kim SW, Kim HL, Lee MK, et al: Autophagy deficiency in
beta cells leads to compromised unfolded protein response and
progression from obesity to diabetes in mice. Diabetologia.
55:392–403. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Bachar-Wikstrom E, Wikstrom JD, Ariav Y,
Tirosh B, Kaiser N, Cerasi E and Leibowitz G: Stimulation of
autophagy improves endoplasmic reticulum stress-induced diabetes.
Diabetes. 62:1227–1237. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Riahi Y, Wikstrom JD, Bachar-Wikstrom E,
Polin N, Zucker H, Lee MS, Quan W, Haataja L, Liu M, Arvan P, et
al: Erratum to: Autophagy is a major regulator of beta cell insulin
homeostasis. Diabetologia. 59:1575–1576. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yin JJ, Li YB, Wang Y, Liu GD, Wang J, Zhu
XO and Pan SH: The role of autophagy in endoplasmic reticulum
stress-induced pancreatic β cell death. Autophagy. 8:158–164.
2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Priyadarshini M, Cole C, Oroskar G, Ludvik
AE, Wicksteed B, He C and Layden BT: Free fatty acid receptor 3
differentially contributes to β-cell compensation under high-fat
diet and streptozotocin stress. Am J Physiol Regul Integr Comp
Physiol. 318:R691–R700. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Huang C, Wang HY, Wang ME, Hsu MC, Wu YS,
Jiang YF, Wu LS, Jong DS and Chiu CH: Kisspeptin-activated
autophagy independently suppresses non-glucose-stimulated insulin
secretion from pancreatic β-cells. Sci Rep. 9(17451)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhou J, Kang X, Luo Y, Yuan Y, Wu Y, Wang
M and Liu D: Glibenclamide-induced autophagy inhibits its insulin
secretion-improving function in β cells. Int J Endocrinol.
2019(1265175)2019.PubMed/NCBI View Article : Google Scholar :
doi.org/10.1155/2019/1265175.
|
|
37
|
Yamamoto S, Kuramoto K, Wang N, Situ X,
Priyadarshini M, Zhang W, Cordoba-Chacon J, Layden BT and He C:
Autophagy differentially regulates insulin production and insulin
sensitivity. Cell Rep. 23:3286–3299. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ebato C, Uchida T, Arakawa M, Komatsu M,
Ueno T, Komiya K, Azuma K, Hirose T, Tanaka K, Kominami E, et al:
Autophagy is important in islet homeostasis and compensatory
increase of beta cell mass in response to high-fat diet. Cell
Metab. 8:325–332. 2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Jung HS, Chung KW, Won Kim J, Kim J,
Komatsu M, Tanaka K, Nguyen YH, Kang TM, Yoon KH, Kim JW, et al:
Loss of autophagy diminishes pancreatic beta cell mass and function
with resultant hyperglycemia. Cell Metab. 8:318–324.
2008.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Galicia-Garcia U, Benito-Vicente A, Jebari
S, Larrea-Sebal A, Siddiqi H, Uribe KB, Ostolaza H and Martín C:
Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci.
21(21)2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Frendo-Cumbo S, Jaldin-Fincati JR, Coyaud
E, Laurent EMN, Townsend LK, Tan JMJ, Xavier RJ, Pillon NJ, Raught
B, Wright DC, et al: Deficiency of the autophagy gene ATG16L1
induces insulin resistance through KLHL9/KLHL13/CUL3-mediated IRS1
degradation. J Biol Chem. 294:16172–16185. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chan YK, Sung HK, Jahng JW, Kim GH, Han M
and Sweeney G: Lipocalin-2 inhibits autophagy and induces insulin
resistance in H9c2 cells. Mol Cell Endocrinol. 430:68–76.
2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Guo Q, Xu L, Li H, Sun H, Liu J, Wu S and
Zhou B: Progranulin causes adipose insulin resistance via increased
autophagy resulting from activated oxidative stress and endoplasmic
reticulum stress. Lipids Health Dis. 16(25)2017.PubMed/NCBI View Article : Google Scholar : doi.org/10.1186/s12944-017-0425-6.
|
|
44
|
Barlow AD and Thomas DC: Autophagy in
diabetes: Β-cell dysfunction, insulin resistance, and
complications. DNA Cell Biol. 34:252–260. 2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Chen ZF, Li YB, Han JY, Wang J, Yin JJ, Li
JB and Tian H: The double-edged effect of autophagy in pancreatic
beta cells and diabetes. Autophagy. 7:12–16. 2011.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Buteau J and Accili D: Regulation of
pancreatic beta-cell function by the forkhead protein FoxO1.
Diabetes Obes Metab. 9 (Suppl 2):140–146. 2007.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Kitamura T and Ido Kitamura Y: Role of
FoxO proteins in pancreatic beta cells. Endocr J. 54:507–515.
2007.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Talchai SC and Accili D: Legacy effect of
Foxo1 in pancreatic endocrine progenitors on adult β-cell mass and
function. Diabetes. 64:2868–2879. 2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Cai H, Jiang Z, Yang X, Lin J, Cai Q and
Li X: Circular RNA HIPK3 contributes to hyperglycemia and insulin
homeostasis by sponging miR-192-5p and upregulating transcription
factor forkhead box O1. Endocr J. 67:397–408. 2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Robertson RP, Harmon J, Tran PO, Tanaka Y
and Takahashi H: Glucose toxicity in beta-cells: Type 2 diabetes,
good radicals gone bad, and the glutathione connection. Diabetes.
52:581–587. 2003.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Zhang T, Kim DH, Xiao X, Lee S, Gong Z,
Muzumdar R, Calabuig-Navarro V, Yamauchi J, Harashima H, Wang R, et
al: FoxO1 plays an important role in regulating β-cell compensation
for insulin resistance in male mice. Endocrinology. 157:1055–1070.
2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Kibbe C, Chen J, Xu G, Jing G and Shalev
A: FOXO1 competes with carbohydrate response element-binding
protein (ChREBP) and inhibits thioredoxin-interacting protein
(TXNIP) transcription in pancreatic beta cells. J Biol Chem.
288:23194–23202. 2013.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kim HJ, Kobayashi M, Sasaki T, Kikuchi O,
Amano K, Kitazumi T, Lee YS, Yokota-Hashimoto H, Susanti VY,
Kitamura YI, et al: Overexpression of FoxO1 in the hypothalamus and
pancreas causes obesity and glucose intolerance. Endocrinology.
153:659–671. 2012.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zhang X, Yong W, Lv J, Zhu Y, Zhang J,
Chen F, Zhang R, Yang T, Sun Y and Han X: Inhibition of forkhead
box O1 protects pancreatic beta-cells against dexamethasone-induced
dysfunction. Endocrinology. 150:4065–4073. 2009.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Kong X, Zhang L, Hua X and Ma X:
Squamosamide derivative FLZ protects pancreatic β-cells from
glucotoxicity by stimulating Akt-FOXO1 pathway. J Diabetes Res.
2015(803986)2015.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Liu XD, Ruan JX, Xia JH, Yang SL, Fan JH
and Li K: The study of regulatory effects of Pdx-1, MafA and
NeuroD1 on the activity of porcine insulin promoter and the
expression of human islet amyloid polypeptide. Mol Cell Biochem.
394:59–66. 2014.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Shao S, Liu Z, Yang Y, Zhang M and Yu X:
SREBP-1c, Pdx-1, and GLP-1R involved in palmitate-EPA regulated
glucose-stimulated insulin secretion in INS-1 cells. J Cell
Biochem. 111:634–642. 2010.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Zhang F, Ma D, Zhao W, Wang D, Liu T, Liu
Y, Yang Y, Liu Y, Mu J, Li B, et al: Obesity-induced overexpression
of miR-802 impairs insulin transcription and secretion. Nat Commun.
11(1822)2020.PubMed/NCBI View Article : Google Scholar : doi:
10.1038/s41467-020-15529-w.
|
|
59
|
Kim DH, Zhang T, Ringquist S and Dong HH:
Targeting FoxO1 for hypertriglyceridemia. Curr Drug Targets.
12:1245–1255. 2011.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Matsumoto M, Han S, Kitamura T and Accili
D: Dual role of transcription factor FoxO1 in controlling hepatic
insulin sensitivity and lipid metabolism. J Clin Invest.
116:2464–2472. 2006.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Wang S, Ai H, Liu L, Zhang X, Gao F, Zheng
L, Yi J, Sun L, Yu C, Zhao H, et al: Micro-RNA-27a/b negatively
regulates hepatic gluconeogenesis by targeting FOXO1. Am J Physiol
Endocrinol Metab. 317:E911–E924. 2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Penniman CM, Suarez Beltran PA, Bhardwaj
G, Junck TL, Jena J, Poro K, Hirshman MF, Goodyear LJ and O'Neill
BT: Loss of FoxOs in muscle reveals sex-based differences in
insulin sensitivity but mitigates diet-induced obesity. Mol Metab.
30:203–220. 2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Zhang W, Patil S, Chauhan B, Guo S, Powell
DR, Le J, Klotsas A, Matika R, Xiao X, Franks R, et al: FoxO1
regulates multiple metabolic pathways in the liver: Effects on
gluconeogenic, glycolytic, and lipogenic gene expression. J Biol
Chem. 281:10105–10117. 2006.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Zhang K, Li L, Qi Y, Zhu X, Gan B, DePinho
RA, Averitt T and Guo S: Hepatic suppression of Foxo1 and Foxo3
causes hypoglycemia and hyperlipidemia in mice. Endocrinology.
153:631–646. 2012.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Du K, Herzig S, Kulkarni RN and Montminy
M: TRB3: A tribbles homolog that inhibits Akt/PKB activation by
insulin in liver. Science. 300:1574–1577. 2003.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Greer EL, Oskoui PR, Banko MR, Maniar JM,
Gygi MP, Gygi SP and Brunet A: The energy sensor AMP-activated
protein kinase directly regulates the mammalian FOXO3 transcription
factor. J Biol Chem. 282:30107–30119. 2007.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Caselli C: Role of adiponectin system in
insulin resistance. Mol Genet Metab. 113:155–160. 2014.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Yaghootkar H, Lamina C, Scott RA, Dastani
Z, Hivert MF, Warren LL, Stancáková A, Buxbaum SG, Lyytikäinen LP,
Henneman P, et al: GENESIS Consortium; RISC Consortium: Mendelian
randomization studies do not support a causal role for reduced
circulating adiponectin levels in insulin resistance and type 2
diabetes. Diabetes. 62:3589–3598. 2013.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Wang A, Li T, An P, Yan W, Zheng H, Wang B
and Mu Y: Exendin-4 upregulates adiponectin level in adipocytes via
Sirt1/Foxo-1 signaling pathway. PLoS One.
12(e0169469)2017.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Sanchez AM, Bernardi H, Py G and Candau
RB: Autophagy is essential to support skeletal muscle plasticity in
response to endurance exercise. Am J Physiol Regul Integr Comp
Physiol. 307:R956–R969. 2014.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Jash S and Puri V: FoxO1-autophagy axis
regulates lipid droplet growth via FSP27. Cell Cycle. 15:2856–2857.
2016.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Zhao Y, Yang J, Liao W, Liu X, Zhang H,
Wang S, Wang D, Feng J, Yu L and Zhu WG: Cytosolic FoxO1 is
essential for the induction of autophagy and tumour suppressor
activity. Nat Cell Biol. 12:665–675. 2010.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Liu L, Zheng LD, Zou P, Brooke J, Smith C,
Long YC, Almeida FA, Liu D and Cheng Z: FoxO1 antagonist suppresses
autophagy and lipid droplet growth in adipocytes. Cell Cycle.
15:2033–2041. 2016.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Ren H, Shao Y, Wu C, Ma X, Lv C and Wang
Q: Metformin alleviates oxidative stress and enhances autophagy in
diabetic kidney disease via AMPK/SIRT1-FoxO1 pathway. Mol Cell
Endocrinol. 500(110628)2020.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Wang J, Shen L, Hong H, Li J, Wang H and
Li X: Atrasentan alleviates high glucose-induced podocyte injury by
the microRNA-21/forkhead box O1 axis. Eur J Pharmacol. 852:142–150.
2019.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Guo X, Lin H, Liu J, Wang D, Li D, Jiang
C, Tang Y, Wang J, Zhang T, Li Y, et al: 1,25-Dihydroxyvitamin D
attenuates diabetic cardiac autophagy and damage by vitamin D
receptor-mediated suppression of FoxO1 translocation. J Nutr
Biochem. 80(108380)2020.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Sengupta A, Molkentin JD and Yutzey KE:
FoxO transcription factors promote autophagy in cardiomyocytes. J
Biol Chem. 284:28319–28331. 2009.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Ning Y, Li Z and Qiu Z: FOXO1 silence
aggravates oxidative stress-promoted apoptosis in cardiomyocytes by
reducing autophagy. J Toxicol Sci. 40:637–645. 2015.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Wang B, Yang Q, Sun YY, Xing YF, Wang YB,
Lu XT, Bai WW, Liu XQ and Zhao YX: Resveratrol-enhanced autophagic
flux ameliorates myocardial oxidative stress injury in diabetic
mice. J Cell Mol Med. 18:1599–1611. 2014.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Li Y, Jiang J, Liu W, Wang H, Zhao L, Liu
S, Li P, Zhang S, Sun C, Wu Y, et al: microRNA-378 promotes
autophagy and inhibits apoptosis in skeletal muscle. Proc Natl Acad
Sci USA. 115:E10849–E10858. 2018.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Yang M, Lin Y and Wang Y and Wang Y:
High-glucose induces cardiac myocytes apoptosis through Foxo1 /GRK2
signaling pathway. Biochem Biophys Res Commun. 513:154–158.
2019.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Liu HY, Han J, Cao SY, Hong T, Zhuo D, Shi
J, Liu Z and Cao W: Hepatic autophagy is suppressed in the presence
of insulin resistance and hyperinsulinemia: Inhibition of
FoxO1-dependent expression of key autophagy genes by insulin. J
Biol Chem. 284:31484–31492. 2009.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Xiong X, Tao R, DePinho RA and Dong XC:
The autophagy-related gene 14 (Atg14) is regulated by forkhead box
O transcription factors and circadian rhythms and plays a critical
role in hepatic autophagy and lipid metabolism. J Biol Chem.
287:39107–39114. 2012.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Lettieri Barbato D, Tatulli G, Aquilano K
and Ciriolo MR: FoxO1 controls lysosomal acid lipase in adipocytes:
implication of lipophagy during nutrient restriction and metformin
treatment. Cell Death Dis. 4(e861)2013.PubMed/NCBI View Article : Google Scholar : doi.org/10.1038/cddis.2013.404.
|