Introduction
Total joint arthroplasty (TJA) is considered as one
of the well-accepted surgeries to treat severe joint diseases,
including rheumatoid arthritis and osteoarthritis. It effectively
decreases patients' pain and improves their quality of life
(1,2). Approximately 1.5 million
arthroplasties are performed in the world every year, and the
demand for joint replacement is still increasing (3). However, revision is becoming a major
concern among the complications of arthroplasty surgeries.
Arthroplasty failure is mainly caused by aseptic loosening and
periprosthetic osteolysis due to particulate wear debris such as
titanium particles (4,5). However, there is no effective
clinically proven therapy or preventative drug for titanium
particle-induced inflammatory osteolysis.
The types of immune cells that participate in the
initiation and progression of inflammatory processes include
macrophages, dendritic cells (DCs), T-helper cells and B cells
(6,7). DCs are antigen-presenting cells that
transduce immune signals to initiate or terminate immune responses
(8,9). Tolerogenic dendritic cells (tolDCs)
are derived from DCs in the presence of anti-inflammatory
cytokines. In addition, tolDCs are thought to have the capacity to
prevent or even treat some autoimmune disorders by directly
interacting with antigen-specific T cells, resulting in autoimmune
tolerance (10-12).
For instance, tolDC has been reported to have a therapeutic effect
in encephalomyelitis and pulmonary inflammation (13,14).
It was also found that tolDCs showed a promising effect in
preventing and treating autoimmune disorders, such as inflammatory
arthritis and immune thrombocytopenia (15).
Given the importance of tolDCs in immune responses,
it was hypothesized that tolDCs might represent a novel therapeutic
effect for titanium-induced inflammatory osteolysis. In the present
study, the role of tolDCs following the induction of titanium
particle-induced inflammation was examined both in vitro and
in vivo. It was found that tolDCs protected against
titanium-induced inflammation by both decreasing the levels of
pro-inflammatory cytokines and increasing the levels of
anti-inflammatory cytokines.
Materials and methods
Media, reagents and cells
Dulbecco's Modified Eagle Medium (DMEM) and fetal
bovine serum (FBS) were obtained from Gibco (Thermo Fisher
Scientific, Inc.). Antibodies against nitric oxide synthase-2
(NOS-2) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were
purchased from Santa Cruz Biotechnology, Inc. Antibodies against
phosphorylated (p)-P38, P38, p-P65 and P65 were purchased from Cell
Signaling Technology, Inc. Enzyme-linked immunosorbent assay
(ELISA) kits for tumor necrosis factor (TNF)-α and interleukin
(IL)-1β were purchased from eBioscience (Thermo Fisher Scientific,
Inc.). Tris, glycine, sodium dodecyl sulfate (SDS) and other
reagents were obtained from Sigma-Aldrich (Merck KGaA), unless
stated otherwise. RAW264.7 cells were received as a gift from Dr.
Aijun Zhang's lab (Qilu Hospital, Jinan, China).
Particle preparation
Pure Ti particles were obtained from Johnson Matthey
Chemicals. Ti particles were prepared by autoclaving, followed by
washing in 100% alcohol 3 times. The endotoxin was then detected
and removed using the Limulus Amebocyte Lysate kit as described
previously (16,17).
Generation, cultivation and
identification of tolDCs
Bone marrow-derived dendritic cells (BmDCs) were
isolated and cultured in the presence of 10 ng/ml
granulocyte-macrophage colony-stimulating factor (GM-CSF) and 1
ng/ml IL-4, as previously described (18). TolDCs were generated by culturing
BmDCs in the presence of 20 ng/ml IL-10 and 20 ng/ml transforming
growth factor-β for 6 days. TolDCs were purified using anti-CD11c
microbeads. To determine the phenotype of generated tolDCs, flow
cytometry was performed by staining cell surface markers, including
FITC-labeled CD11c and major histocompatibility complex (MHC)-II
and phycoerythrin-conjugated CD80, as well as CD86 (Biolegend,
Inc.). DCs showed CD11 positivity.
Animals and titanium
particles-stimulated mouse air-pouch model
The Institutional Animal Care and Use Committee of
Jinan Central Hospital and Qilu Hospital approved all animal
studies. All procedures were performed in accordance with
institutional guidelines. BALB/c mice (8-10 weeks old) were
obtained from Shandong University. The animals were housed in a
clean facility under the controlled conditions of 22-24˚C, relative
humidity of 30-50%, and a 12-h light-dark cycle. A standard mouse
diet and filtered water were available ad libitum.
Titanium particle-induced (Ti-induced) air pouches
were generated according to a previously described method (19,20).
Mice were anesthetized with 3% pentobarbital (30 mg/kg, ip). The
dorsal skins of the mice were cleaned and shaved before surgery. A
sterilized air-pouch was prepared by subcutaneous injection of 3 ml
sterilized syringe. On the second day, pouches were initiated to
provoke inflammation by injecting 0.5 ml PBS diluted with 5 mg Ti
particles. In addition, the air pouches were maintained by
injecting 1 ml air every other day until day 5. The mice were
randomly divided into two groups, each group comprised 10 mice. For
the control group, 0.5 ml PBS was injected daily into pouches. For
the mice with treatment, tolDCs (1x106) were injected
into pouches daily. Mice were humanely sacrificed by cervical
dislocation on day 12. Cervical dislocation euthanasia was
preformed after the mouse was anesthetized with 3% pentobarbital
(30 mg/kg, ip). The thumb and index finger of one hand pressed the
head of the mouse, and the other hand grasped the tail, quickly and
forcefully pulled backward and upward to dislocate the cervical
vertebra. A slight sense of absence could be felt when the cervical
vertebra was dislocated. The mouse lost its vital signs in an
instant. No experimental animal died during the experiment. The
pouch membranes were harvested for further analysis.
RAW 264.7 cell culture and
stimulation
RAW 264.7 cells were cultured in DMEM containing 10%
FBS at 37˚C in a humidified incubator with 5% CO2. To
investigate the effects of particle stimulation on an array of mRNA
gene transcripts, RAW 264.7 cells were cultured in the absence or
presence of tolDC cultured medium with l% Ti particles dissolved in
the same medium for 6 h before RNA extraction. Protein was
extracted after 24 and 48 h of Ti particle stimulation.
Histology
After harvesting the skin membrane, it was fixed in
4% paraformaldehyde for 24 h at room temperature and then embedded
it in olefin. At least 5 consecutive 6-µm sagittal sections were
collected for staining. Sections were stained using hematoxylin and
eosin (HE; cat. no. C0105S Beyotime Institute of Biotechnology) and
Masson's Trichrome (cat. no. G1340; Beijing Solarbio Science &
Technology Co., Ltd.). Images were acquired with an inverted
optical microscope (magnification, x10; Olympus, Corporation).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from RAW 264.7 cells or skin
using an RNeasy kit (Qiagen, Inc.). Reverse transcription was
performed using an RT-for-PCR kit (Qiagen, Inc.). The expression
levels of mRNAs were measured by qPCR with QuantiTect SYBR Green
RT-PCR Kit (Qiagen, Inc.) using a 7500 RT-PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling
conditions were as follows: Initial denaturation at 95˚C for 10
min, followed by 40 cycles of denaturation at 95˚C for 15 sec,
annealing at 60˚C for 30 sec and elongation at 72˚C for 1 min,
followed by final elongation at 72˚C for 2 min. All these
procedures were performed according to the manufacturer's protocol.
The primers for qPCR used in the present study are listed as
follows: IL-1β forward, 5'-AATCTCACAGCAGCACATCA-3'; IL-1β reverse,
5'-AAGGTGCTCATGTCCTCATC-3'; IL-6 forward,
5'-ATGAAGTTCCTCTCTGCAAGAGACT-3'; IL-6 reverse,
5'-CACTAGGTTTGCCGAGTAGATCTC-3'; cyclooxygenase (COX)-2 forward,
5'-AATGCTGACTATGGCTACAAAA-3'; COX-2 reverse,
5'-AAAACTGATGCGTGAAGTGCTG-3'; NOS-2 forward,
5'-CAGCCTCTGTCTCTCAGGCTCTT-3'; NOS-2 reverse,
5'-CTCTCTAAGTGAACAACTGGCCTGTGA-3'; IL-10 forward,
5'-ACCTGGTAGAAGTGATGCC-3'; IL-10 reverse,
5'-CAAGGAGTTGTTTCCGTTA-3'; GAPDH forward,
5'-ACCCAGAAGACTGTGGATGG-3'; GAPDH reverse,
5'-CACATTGGGGGTAGGAACAC-3'. The mRNA expression was normalized to
that of GAPDH. The value 2-ΔΔCq was used for comparative
quantitation (15). Each experiment
was repeated at least three times.
Western blotting
Total air-pouch membranes and RAW 264.7 cell
extracts were homogenized and proteins were collected using protein
extraction buffer (cat. no. P0013; Beyotime Institute of
Biotechnology). Protein concentrations in the supernatant were
detected using the enhanced BCA Protein Assay kit (Beyotime
Institute of Biotechnology). The supernatant samples containing 1
mg protein/500 µl were resolved on 8% SDS-polyacrylamide gel and
electroblotted onto a nitrocellulose membrane. BSA (3%) was diluted
in Tris-buffered saline-Tween 20 (TBST; 10 mM Tris-HCl, pH 8.0; 150
mM NaCl; and 0.5% Tween 20) and incubated with the membrane for 1 h
at room temperature. The membrane was then incubated at 4˚C with
anti-NOS-2 (1:1,000; cat. no. sc-649, 120 kDa; Santa Cruz
Biotechnology, Inc.), anti-p-P38 (1:1,000, cat. no. 4511, 43 kDa;
Cell Signaling Technology, Inc.), anti-P38 (1:1,000; cat. no. 8690;
40 kDa, Cell Signaling Technology, Inc.), anti-p-P65 (1:1,000, cat.
no. 3033, 65 kDa; Cell Signaling Technology, Inc.),
anti-P65(1:1,000, cat. no. 8242; 65 kDa; Cell Signaling Technology,
Inc.), or anti-GAPDH (1:1,000, cat. no. 25778; 36 kDa; Santa Cruz
Biotechnology, Inc.) primary antibodies overnight. After washing 3
times with TBST (each 10 min), the secondary antibody
(HRP-conjugated anti-rabbit immunoglobulin; GB23303, 1:5,000, Wuhan
Servicebio Technology Co., Ltd.) was added and incubated at room
temperature for 1 h, and the bound antibody was visualized using
West Pico typically enhanced chemiluminescent Substrate (Beijing
Solarbio Science & Technology Co., Ltd.).
ELISA
The levels of pro-inflammatory cytokines in the sera
or cultured medium were assessed using ELISA kits (IL-6, cat. no.
88-7064; IL-10, cat. no. 88-7105; TNF-α, cat. no. 88-7324; IL-1β,
cat. no. 88-7013; eBioscience; Thermo Fisher Scientific, Inc.), as
previously described (21). An
ELISA reader (Molecular Devices, LLC) at 450 nm was used to
determine the optical intensity. In addition, the concentration of
the examined cytokines were analyzed by regression against a
generated standard curve.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism software 8.0.2 (GraphPad Software, Inc.). Data are expressed
as means ± deviation (SD). Unpaired-samples t-test was used to
assess statistical significance. P<0.05 was considered to
indicate a statistically significant difference.
Results
TolDCs sustain immune tolerogenic
phenotype
To confirm the phenotype and function of the
generated tolDCs, cell surface markers were identified by cytometry
assay, including CD11c, CD80, MHC-II and CD86. As demonstrated in
Fig. 1A-D, compared with naïve DCs,
the expression of these markers in tolDCs was dramatically
decreased. To further confirm this finding, mRNA was extracted and
RT-qPCR analysis was performed. As shown in Fig. 1E and F, the transcriptional levels of IL-12 and
C-C chemokine receptor type 7 (CCR7) were significantly decreased
in tolDCs. In addition, as shown in Fig. 1G, the expression of the
anti-inflammatory cytokine IL-10 was significantly increased in
tolDCs. Collectively, tolDCs presented not only a tolerogenic
phenotype but also an anti-inflammatory effect.
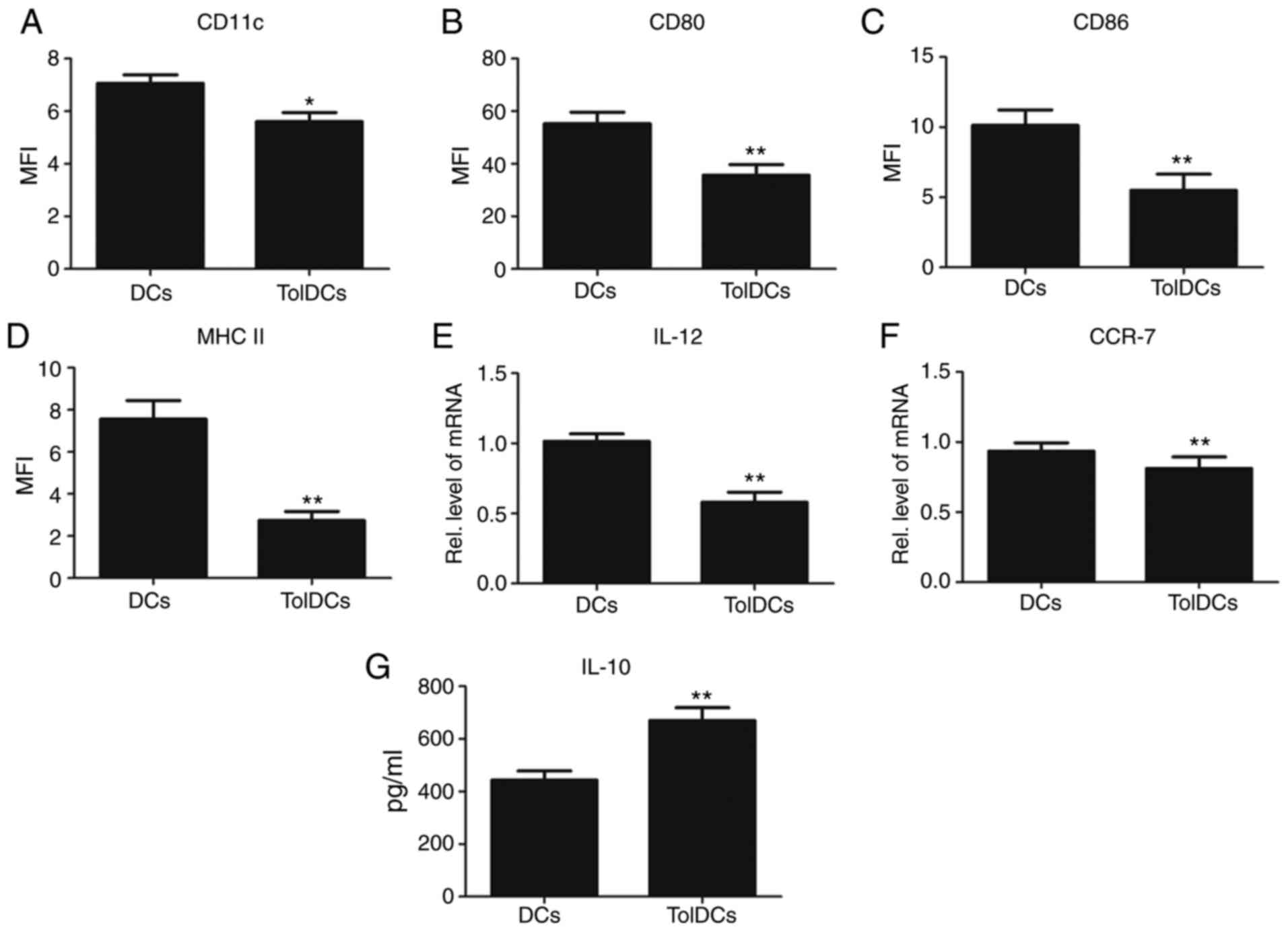 | Figure 1TolDCs sustain immune tolerogenic
phenotype. (A-D) Comparison of cell surface markers such as CD11c,
MHC-II, CD80 and CD86 in DCs and tolDCs, analyzed by flow cytometry
assay. (E and F) Transcriptional level of IL-12 and CCR7 in DCs and
tolDCs, analyzed by reverse transcription-quantitative PCR assay.
(G) Enzyme linked immunosorbent assay for determining IL-10 protein
levels in DC culture media. *P<0.05;
**P<0.01. MFI, median fluorescence intensity; DCs,
dendritic cells; TolDCs, tolerogenic DCs; MHC, major
histocompatibility complex; IL, interleukin; CCR7, C-C chemokine
receptor type 7. |
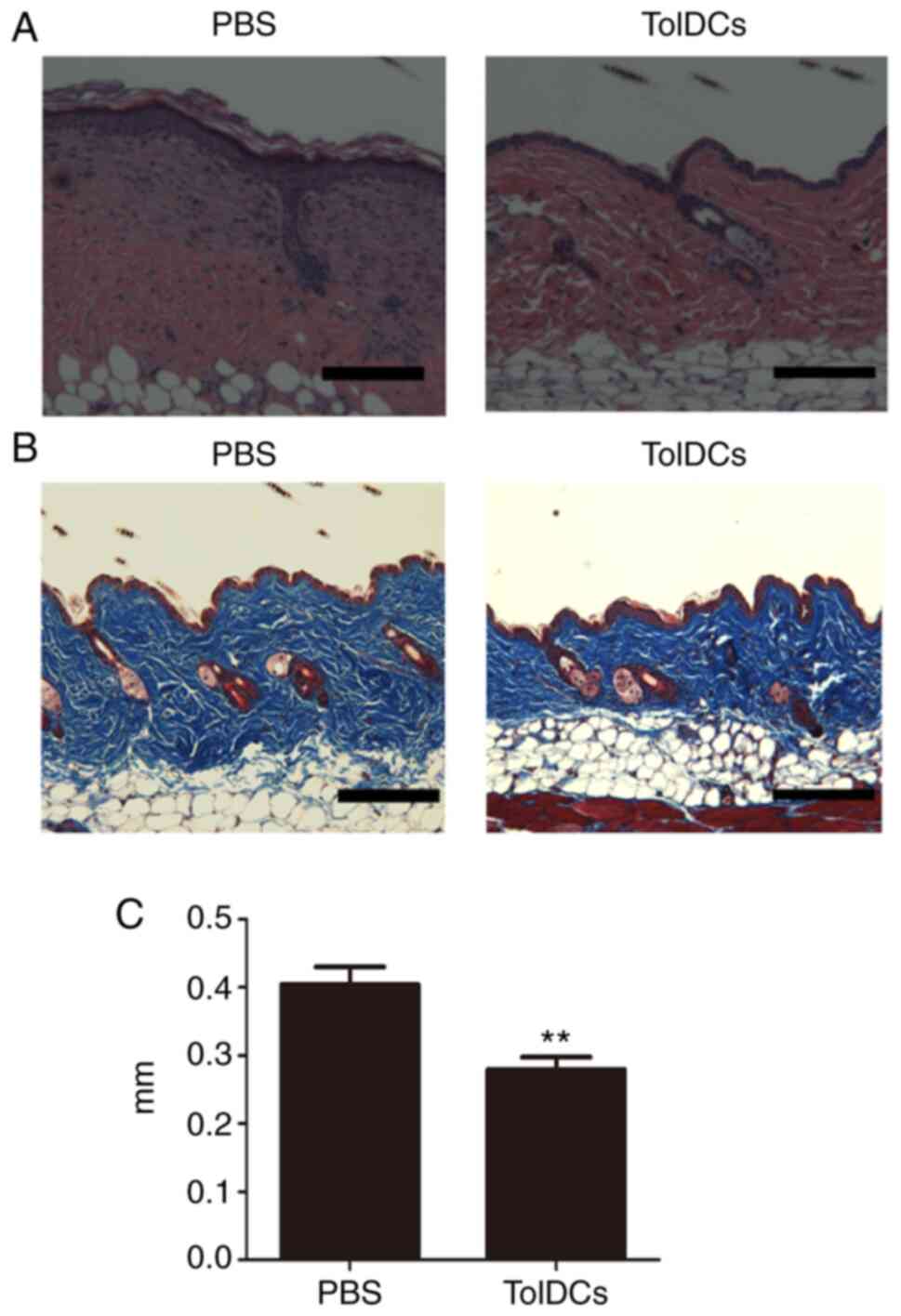
TolDCs suppress the Ti-induced
inflammatory phenotype in the mouse air-pouch model
To determine whether tolDCs could prevent Ti-induced
inflammation in vivo an air-pouch model was established in
BALB/c mice and PBS or tolDCs were injected daily until the mice
were sacrificed. As shown in Fig.
2A-C, HE staining showed that the thickness of the membrane in
the tolDCs-treated group was significantly decreased compared with
that of the PBS control group. In addition, Fig. 2B revealed that Masson's trichrome
staining also validated this finding, showing that tolDCs prevented
inflammatory swelling; which was further confirmed by statistical
analysis of the membrane thickness, as illustrated in Fig. 2C. These in vivo data
indicated that tolDCs effectively inhibited the Ti-induced
inflammatory phenotype.
TolDCs decreases inflammatory and
enhances anti-inflammatory gene expression in the air-pouch
membrane
To further determine whether gene expression changes
in the Ti-induced skin membrane, mice were sacrificed on day 6 and
collected the skin membrane, followed by RNA extraction. TNF-α is
known to play an important role in the Ti-induced inflammatory
process. To determine the anti-inflammatory effect of tolDCs, the
levels of pro-inflammatory markers such as TNF-α, IL-1β, IL-6,
NOS-2 and COX-2 were measured. As shown in Fig. 3A-E, all these pro-inflammatory
markers were dramatically decreased by the additional use of tolDCs
compared with those in the PBS-treated group. In addition, it is
well accepted that tolDCs secrete IL-10, which plays a critical
anti-inflammatory role. As illustrated in Fig. 3F, IL-10 gene expression was
significantly increased. Collectively, tolDCs effectively lowered
pro-inflammatory gene expression and enhanced anti-inflammatory
gene expression in the Ti particle-induced skin membrane.
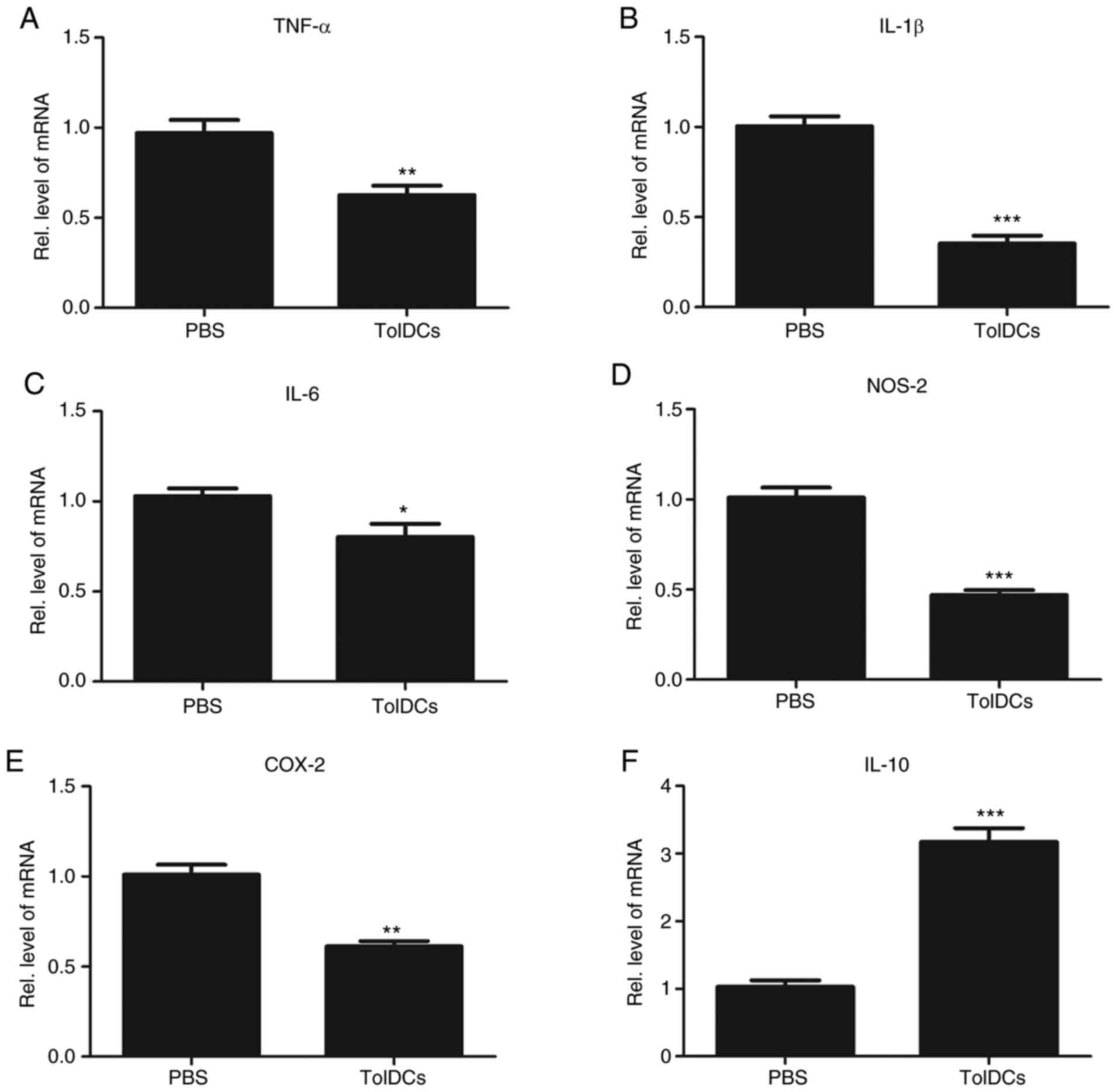 | Figure 3TolDCs decreases inflammatory gene
expression and enhances anti-inflammatory gene expression in
air-pouch membrane. Transcriptional levels of pro-inflammatory
mediators including (A) TNFα, (B) IL-1β, (C) IL-6, (D) NOS-2 and
(E) COX-2, and anti-inflammatory mediators (F) IL-10 in PBS- and
tolDCs-treated mice skin measured by RT-qPCR. The units were
arbitrary, and the normalized values were calibrated against the
PBS control, and each RT-qPCR was performed at least in triplicate.
Values are the normalized mean ± SEM. *P<0.05;
**P<0.01; and ***P<0.001. Ten mice were
used in each group. TolDCs, tolerogenic dendritic cells; TNFα,
tumor necrosis factor-α; IL, interleukin; COX, cyclooxygenase;
NOS-2, nitric oxide synthase-2; RT-qPCR, reverse
transcription-quantitative PCR; Rel., relative. |
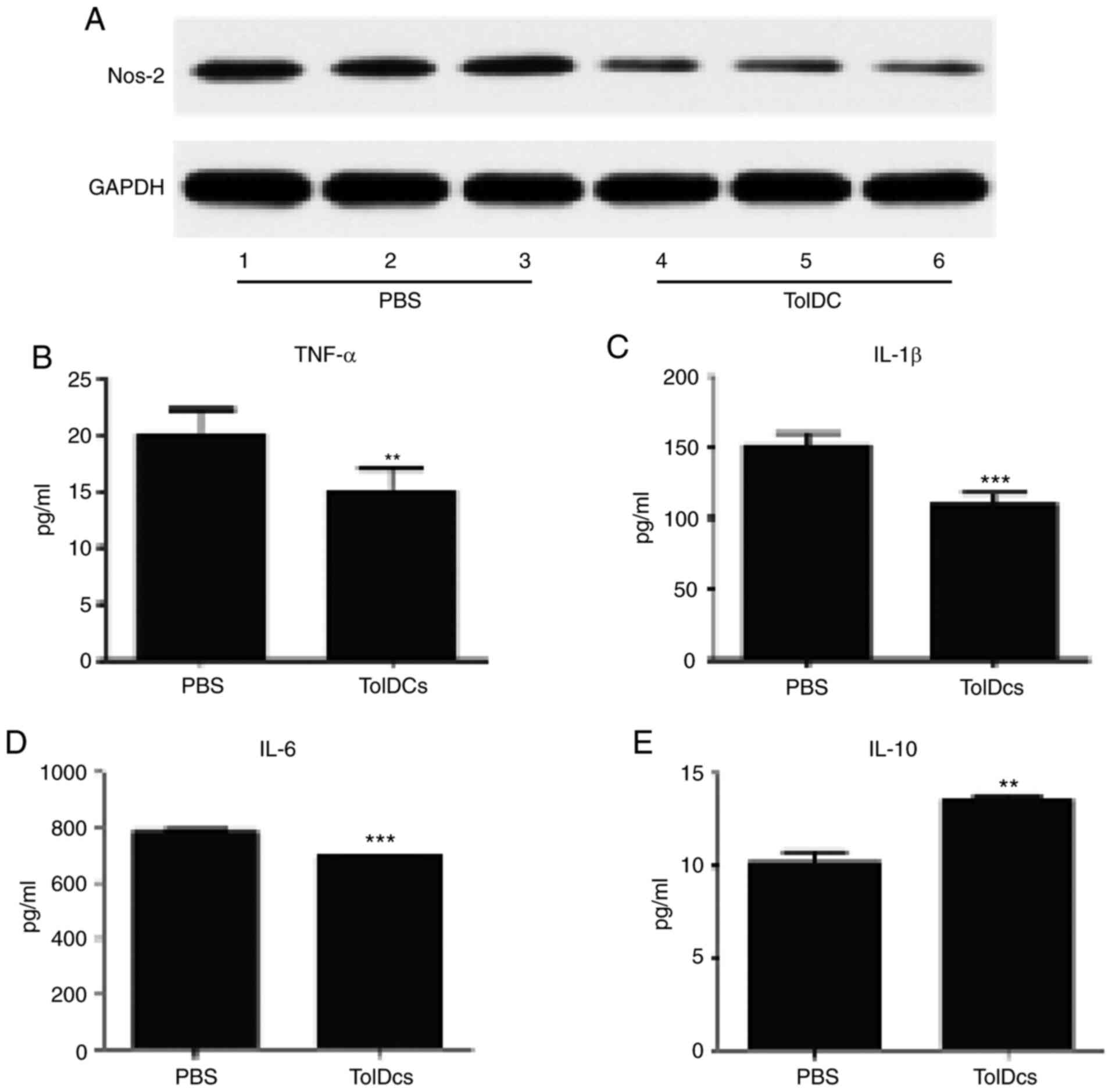
TolDCs decreased Ti-induced
inflammation
To further confirm the present findings, the skin
membranes were collected and western blotting was performed for the
expression of NOS-2, which is regarded as an indicator of
inflammatory severity. As shown in Fig.
4A, tolDCs dramatically decreased NOS-2 protein expression,
indicating that tolDCs decreased inflammatory severity. In
addition, sera was collected after sacrificing the mice and ELISA
was performed for TNF-α, IL-1β, and IL-6 expression levels. As
shown in Fig. 4B-D, all these
pro-inflammatory cytokines were significantly decreased in the
tolDCs-treated group. In contrast, the expression of the
anti-inflammatory cytokine IL-10 was significantly increased after
the additional use of tolDCs (Fig.
4E). Overall, tolDCs effectively treated Ti particle-induced
inflammation both locally and systematically.
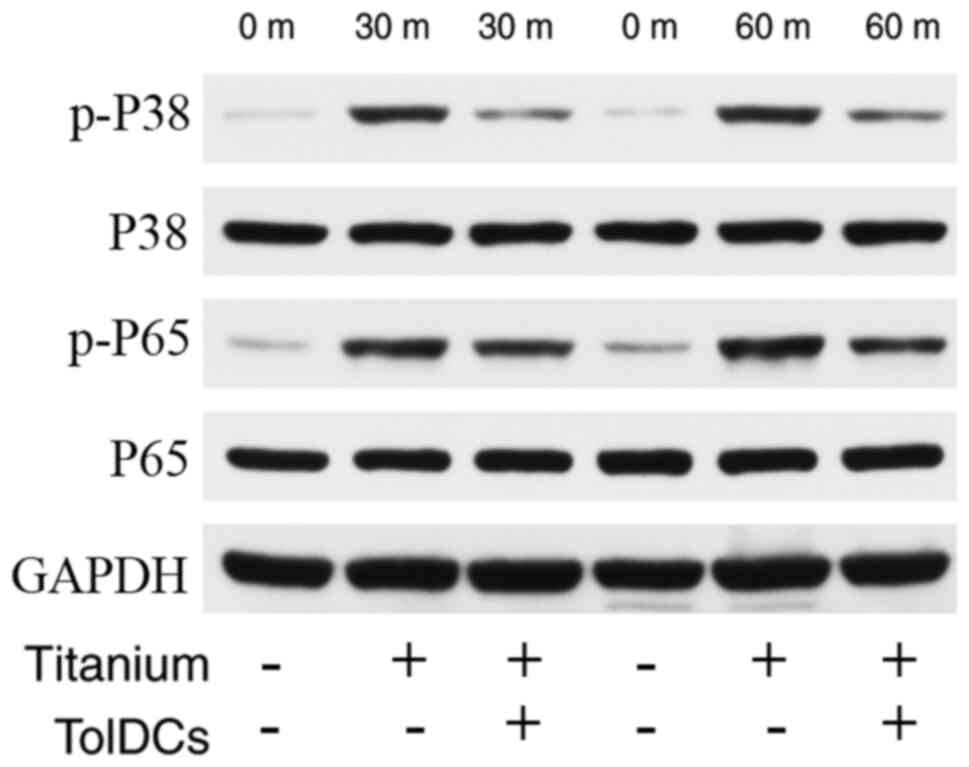
TolDCs suppress Ti-induced
inflammatory signaling in vitro
Given the importance of tolDCs-mediated
anti-titanium-induced inflammation in vivo, primary bone
marrow cells were cultured to determine the molecular mechanisms
involved. Briefly, tolDCs were cultured in regular medium for 3
days and then collected the supernatant for further study. The
collected medium was used to treat primary bone marrow cells in the
presence of 1% titanium particles for different time points. As
illustrated in Fig. 5, titanium
promotes the phosphorylation of P35 and P65, but tolDCs inhibit
this process.
TolDCs suppress Ti-induced
inflammation by decreasing the expression of pro-inflammatory
cytokines and enhancing the expression of anti-inflammatory
cytokines in vitro
To further determine the role of tolDCs in
Ti-induced inflammation, tolDCs were cultured in regular medium for
3 days and the supernatant was then collected for further study.
The tolDC medium was diluted with regular medium (RM) at a ratio of
1:3 to make the conditional medium (CM). The CM or RM was used to
culture RAW264.7 cells in the presence of 1% titanium particles for
6 h. Next, mRNA was extracted and a RT-qPCR assay was performed. As
indicated in Fig. 6A-D and F, the gene expression of pro-inflammatory
cytokines such as TNF-α, IL-1β, IL-6, NOS-2 and COX-2 was notably
decreased in the CM treated group, indicating that tolDCs strongly
inhibit Ti-induced inflammation. To further extend this finding,
RAW 264.7 cells were cultured with CM or RM in the presence of 1%
titanium particles for 3 days. Then, supernatant was collected for
ELISA analysis. As shown in Fig.
6G-I, the release of pro-inflammatory cytokines, including
TNF-α, IL-1β and IL-6, was dramatically decreased in the CM. In
contrast, as shown in Fig. 6E and
J, the expression of the
anti-inflammatory cytokine IL-10 was significantly increased in the
CM-treated group. Collectively, tolDCs could suppress Ti-induced
inflammation by decreasing the levels of pro-inflammatory cytokines
and increasing the levels of anti-inflammatory cytokines.
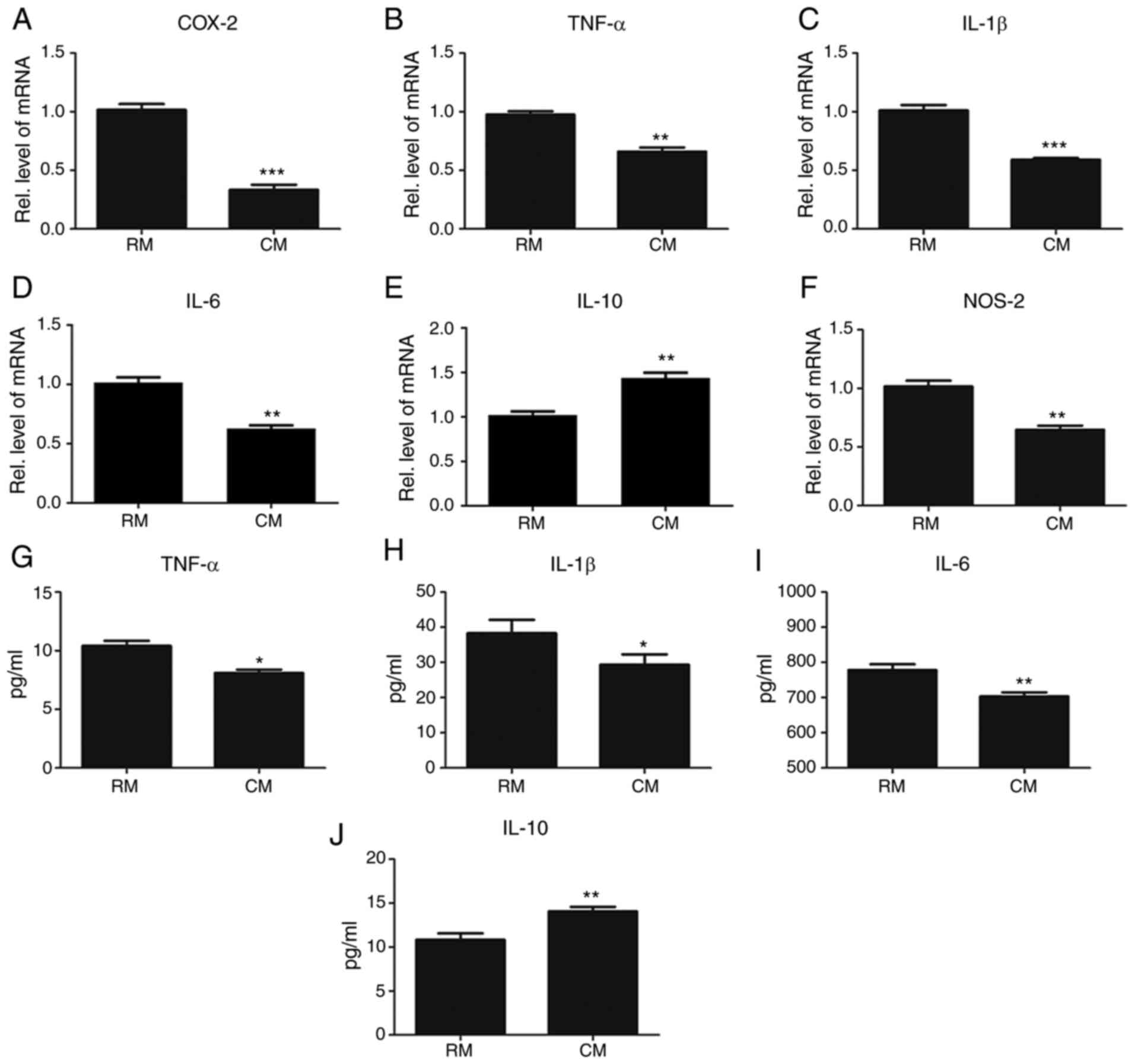 | Figure 6TolDCs suppress titanium
particle-induced inflammation in vitro. (A) COX-2, (B)
TNF-α, (C) IL-1β, (D) IL-6 and (F) NOS-2 from RAW264.7 cells with
RM or CM in the presence of titanium, measured by RT-qPCR assay.
The units were arbitrary, and the normalized values were calibrated
against the PBS control group, and each RT-qPCR was performed at
least in triplicate. (E) Levels of anti-inflammatory cytokine IL-10
in RM or CM in titanium particle-stimulated RAW 264.7 cells assayed
by RT-qPCR. (G-I) Levels of the pro-inflammatory cytokines (G)
TNF-α, (H) IL-1β, and (I) IL-6 in RM or CM in titanium
particle-stimulated RAW 264.7 cells assayed by ELISA. (J) Levels of
the anti-inflammatory cytokine IL-10 in RM or CM in titanium
particle-stimulated RAW 264.7 cells assayed by ELISA. Values are
the normalized mean ± SEM. *P<0.05;
**P<0.01; and ***P<0.001. TolDCs,
tolerogenic dendritic cells; RM, regular medium; CM, conditional
medium; RT-qPCR, reverse transcription-quantitative PCR; ELISA,
enzyme-linked immunosorbent assay; TNFα, tumor necrosis factor-α;
IL, interleukin; COX, cyclooxygenase; NOS-2, nitric oxide
synthase-2. |
Discussion
Aseptic loosening may cause periprosthetic
osteolysis, resulting in TJA failure and revision surgery. It is
known that prosthetic loosening is primarily driven by wear
debris-mediated inflammatory osteolysis and has been shown to
involve titanium particles (22-24).
The air-pouch model exhibits cellular infiltration and mediators of
inflammation that appear to closely resemble the pseudo synovium
associated with aseptic loosening (19,20).
Marked responses to titanium particles were observed, the particles
deeply embedded within the tissue and surrounded by macrophages.
This model accurately simulates the physiological state of
peri-prosthetic tissue.
Dendritic cells (DCs) are a type of well-known
immune cells that present antigens for the induction of immune
response. Based on the properties of DCs, many worldwide labs
generated tolDCs independently and they confirmed the autoimmune
tolerance effect (6). Given the
importance of tolDCs in the immune system, more and more findings
indicated that tolDCs would be a promising cellular
immunotherapeutic agent for some inflammatory diseases.
Furthermore, it was shown that tolDCs could induce
platelet-specific immune tolerance (25). In addition, antigen-specific tolDCs
that were generated showed an effective therapeutic effect in
inflammatory arthritis. The present study extended the previous
findings that tolDCs were therapeutic in Ti-induced
inflammation.
TolDCs are known to have anti-inflammatory activity
under various conditions. In the present study, it was found that
tolDCs could significantly suppress Ti-induced inflammation in a
mouse air-pouch model, which implied that tolDCs might also play an
anti-inflammatory role in wear debris-mediated pathological
processes. Indeed, tolDCs decreased the Ti-induced inflammatory
response, as indicated by the phenotype and decreased levels of
pro-inflammatory cytokines such as TNFα, IL-1β and IL-6, both at
the mRNA expression and protein synthesis levels. In addition,
tolDCs effectively prevent Ti-induced inflammation. COX-2 has been
recognized as an inducible or pathological enzyme and participates
in the inflammatory response by facilitating prostaglandin
synthesis (26). The NOS-2 level
was elevated in the joint interface membrane tissues of arthritis,
and suppression of NOS-2 indicated alleviation of inflammation
(27). In the present study, it was
found that COX-2 and NOS-2 levels were enhanced by titanium
particles, a finding that is consistent with a previous publication
(28). This Ti-induced COX-2 and
NOS-2 expression was markedly inhibited by tolDCs. From the
aforementioned, various inflammatory factors are regulated by
TolDCs, and the present study preliminarily detected and verified
the associated signaling pathways. The nuclear factor (NF)-κB and
MAPK signaling pathways regulate numerous cellular activities,
particularly in arthritis-associated inflammation (24,27).
The results showed that titanium dramatically activated P38 MAPK
and NF-κB P65 signaling. In contrast, the CM from tolDCs
effectively prevented the activation of these pro-inflammatory
pathways. Thus, tolDCs could inhibit Ti-induced pro-inflammatory
pathways.
To better understand the role of tolDCs in
Ti-induced inflammation, tolDCs were cultured in RM for 3 days.
Subsequently, the medium was collected and mixed with the RM at a
ratio of 1:3 to make the CM. It was found that the CM strongly
suppressed Ti-induced pro-inflammatory cytokine expression at both
the gene and protein levels. This finding suggests that tolDCs
might secrete anti-inflammatory cytokines, such as IL-10, to
protect against Ti-induced inflammation. To identify these possible
cytokines, further studies need to be performed. The present study
also has the following limitations: Histological staining for
animal modeling was lacking in this study; this study has
preliminarily verified that tolDCs regulated the expression of
COX-2, NOS-2, TNFα, IL-1β, IL-6 and IL-10 through the NF-κB and
MAPK signaling pathways. However, the specific target is still
unknown. In future studies, the specific target and mechanism of
Ti-induced inflammation should be explored.
Based on the findings in the present study, as well
as previous reports, it was found that titanium particles lead to
the release of pro-inflammatory cytokines such as TNF-α, IL-1β and
IL-6. However, local injection of tolDCs prevents Ti-induced
inflammatory pathogenesis by decreasing pro-inflammatory cytokines
and increasing anti-inflammatory cytokines. Collectively, the
findings reported in the present study not only provide new
insights into the molecular mechanisms underlying Ti-induced
inflammation but may also present tolDCs as a new therapeutic
strategy for the prevention of aseptic loosening.
Acknowledgements
The authors wish to acknowledge Ms Yutian Huang
(Department of Education, University of Sheffield) for her guidance
on language.
Funding
Funding: This study was funded by Shandong Medical and Health
Science and Technology Development Programs (grant no. 2016WS0618);
and The Innovation and Entrepreneurship Project of Sichuan
Technology Gallery, Sichuan Science and Technology Program, China
(grant no. 2020JDRC0054).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WW, BH and JC carried out the animal model design
and the molecular study. HT and MS participated in the data
analysis. YJ and WX conceived the study, participated in its design
and coordination, and helped to draft the manuscript. YJ and WX
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The Institutional Animal Care and Use Committee of
Qilu Hospital approved all animal studies (approval no.
KYLL-2019(KS)-028). All procedures were performed in accordance
with institutional guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tian B, Jiang T, Shao Z, Zhai Z, Li H, Fan
Q, Liu X, Ouyang Z, Tang T, Jiang Q, et al: The prevention of
titanium-particle-induced osteolysis by OA-14 through the
suppression of the p38 signaling pathway and inhibition of
osteoclastogenesis. Biomaterials. 35:8937–8950. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wei J, Liu CJ and Li Z: ADAMTS-18: A
metalloproteinase with multiple functions. Front Biosci (Landmark
Ed). 19:1456–1467. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Teeny SM, York SC, Mesko JW and Rea RE:
Long-term follow-up care recommendations after total hip and knee
arthroplasty: Results of the American Association of Hip and Knee
Surgeons' member survey. J Arthroplasty. 18:954–962.
2003.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Harris WH: Wear and periprosthetic
osteolysis: The problem. Clin Orthop Relat Res. 393:66–70.
2001.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dumbleton JH, Manley MT and Edidin AA: A
literature review of the association between wear rate and
osteolysis in total hip arthroplasty. J Arthroplasty. 17:649–661.
2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhao Y, Zhang A, Du H, Guo S, Ning B and
Yang S: Tolerogenic dendritic cells and rheumatoid arthritis:
Current status and perspectives. Rheumatol Int. 32:837–844.
2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Landgraeber S, Jäger M, Jacobs JJ and
Hallab NJ: The Pathology of orthopedic implant failure is mediated
by innate immune system cytokines. Mediators Inflamm.
2014(185150)2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Piccioli D, Tavarini S, Borgogni E, Steri
V, Nuti S, Sammicheli C, Bardelli M, Montagna D, Locatelli F and
Wack A: Functional specialization of human circulating CD16 and
CD1c myeloid dendritic-cell subsets. Blood. 109:5371–5379.
2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Xie ZX, Zhang HL, Wu XJ, Zhu J, Ma DH and
Jin T: Role of the immunogenic and tolerogenic subsets of dendritic
cells in multiple sclerosis. Mediators Inflamm.
2015(513295)2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Oriss TB, Ostroukhova M, Seguin-Devaux C,
Dixon-McCarthy B, Stolz DB, Watkins SC, Pillemer B, Ray P and Ray
A: Dynamics of dendritic cell phenotype and interactions with CD4+
T cells in airway inflammation and tolerance. J Immunol.
174:854–863. 2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang L, Pino-Lagos K, de Vries VC, Guleria
I, Sayegh MH and Noelle RJ: Programmed death 1 ligand signaling
regulates the generation of adaptive Foxp3+CD4+ regulatory T cells.
Proc Natl Acad Sci USA. 105:9331–936. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Morelli AE and Thomson AW: Tolerogenic
dendritic cells and the quest for transplant tolerance. Nat Rev
Immunol. 7:610–621. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Torres-Aguilar H, Aguilar-Ruiz SR,
González-Pérez G, Munguía R, Bajaña S, Meraz-Ríos MA and
Sánchez-Torres C: Tolerogenic dendritic cells generated with
different immunosuppressive cytokines induce antigen-specific
anergy and regulatory properties in memory CD4+ T cells. J Immunol.
184:1765–1775. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fu J, Zhang A and Ju X: Tolerogenic
dendritic cells as a target for the therapy of immune
thrombocytopenia. Clin Appl Thromb Hemost. 18:469–475.
2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ning B, Wei J, Zhang A, Gong W, Fu J, Jia
T and Yang SY: Antigen-specific tolerogenic dendritic cells
ameliorate the severity of murine collagen-induced arthritis. PLoS
One. 10(e0131152)2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ragab AA, Van De Motter R, Lavish SA,
Goldberg VM, Ninomiya JT, Carlin CR and Greenfield EM: Measurement
and removal of adherent endotoxin from titanium particles and
implant surfaces. J Orthop Res. 17:803–809. 1999.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Vallés G, Gil-Garay E, Munuera L and
Vilaboa N: Modulation of the cross-talk between macrophages and
osteoblasts by titanium-based particles. Biomaterials.
29:2326–2335. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang M, Tang H, Guo Z, An H, Zhu X, Song
W, Guo J, Huang X, Chen T, Wang J and Cao X: Splenic stroma drives
mature dendritic cells to differentiate into regulatory dendritic
cells. Nat Immunol. 5:1124–1133. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Yang SY, Ren W, Park Y, Sieving A, Hsu S,
Nasser S and Wooley PH: Diverse cellular and apoptotic responses to
variant shapes of UHMWPE particles in a murine model of
inflammation. Biomaterials. 23:3535–3543. 2002.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wooley PH, Morren R, Andary J, Sud S, Yang
SY, Mayton L, Markel D, Sieving A and Nasser S: Inflammatory
responses to orthopaedic biomaterials in the murine air pouch.
Biomaterials. 23:517–526. 2002.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wong RH, Wei JC, Huang CH, Lee HS, Chiou
SY, Lin SH, Cai YW, Hung PH, Wang MF and Yang SF: Association of
IL-12B genetic polymorphism with the susceptibility and disease
severity of ankylosing spondylitis. J Rheumatol. 39:135–1340.
2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu S, Virdi AS, Sena K and Sumner DR:
Sclerostin antibody prevents particle-induced implant loosening by
stimulating bone formation and inhibiting bone resorption in a rat
model. Arthritis Rheum. 64:4012–4020. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
de Avila ED, Lima BP, Sekiya T, Torii Y,
Ogawa T, Shi W and Lux R: Effect of UV-photofunctionalization on
oral bacterial attachment and biofilm formation to titanium implant
material. Biomaterials. 67:84–92. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wei J, Richbourgh B, Jia T and Liu C:
ADAMTS-12: A multifaced metalloproteinase in arthritis and
inflammation. Mediators Inflamm. 2014(649718)2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang A, Fu J, Ning B, Li D, Sun N, Wei W,
Wei J and Ju X: Tolerogenic dendritic cells generated with
IL-10/TGFβ1 relieve immune thrombocytopenia in mice. Thromb Res.
132:63–68. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lee KH, Abas F, Mohamed Alitheen NB,
Shaari K, Lajis NH, Israf DA and Syahida A: Chemopreventive effects
of a curcumin-like diarylpentanoid
[2,6-bis(2,5-dimethoxybenzylidene)cyclohexanone] in cellular
targets of rheumatoid arthritis in vitro. Int J Rheum Dis.
18:616–627. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhou F, Mei J, Han X, Li H, Yang S, Wang
M, Chu L, Qiao H and Tang T: Kinsenoside attenuates osteoarthritis
by repolarizing macrophages through inactivating NF-κB/MAPK
signaling and protecting chondrocytes. Acta Pharm Sin B. 9:973–985.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wei X, Zhang X, Flick LM, Drissi H,
Schwarz EM and O'Keefe RJ: Titanium particles stimulate COX-2
expression in synovial fibroblasts through an oxidative
stress-induced, calpain-dependent, NF-kappaB pathway. Am J Physiol
Cell Physiol. 297:C310–C320. 2009.PubMed/NCBI View Article : Google Scholar
|