Introduction
In bone tissue engineering, the use of composite
carriers that encapsulate bioactive components is a key strategy
for drug delivery. Carriers may contain drugs, small molecules or
even nanomaterials (1,2). Electrospun nanofibers are effective
biocompatible drug carriers because of their ability to repair bone
tissue. These fibers can deliver significant amounts of
therapeutics and have, therefore, attracted attention as potential
drug delivering scaffolding materials (3,4). In
addition, nanofibers are highly porous, providing an artificial
milieu that is structurally comparable to the naturally occurring
extracellular matrix (ECM) (1,5). As
such, electrospun nanofibers are frequently utilized in tissue
engineering (1,6,7).
Electrospinning can also produce nanofibers with high bioactivity.
These are based on natural polymers, including gelatin (Gt),
chitosan and hyaluronic acid, and synthetic polymers, including
poly-D, L-lactide-coglycolide (PLGA), polycaprolactone (PCL) and
polyurethane. These polymers are all highly biocompatible and
biodegradable (5).
Guided bone regeneration (GBR) has been demonstrated
to be effective in periodontal therapy (8) and is an important strategy in bone
tissue engineering (9). In GBR, the
typical barrier membrane consists of two surfaces: The porous
surface that faces the osseous bone defect, to guide bone
formation, and the dense surface that faces the soft tissue, to
prevent non-osteogenic cells (such as fibroblasts) from interfering
with bone healing (10). Thus, the
barrier membrane plays a crucial role in bone regeneration
(8). In recent years,
co-electrospinning has been used to generate hybrid nanofibers with
specific features, such as its interconnected porous structures,
broad surface areas and capability of delivering drugs (11-13).
These nanofibers have the features of both naturally occurring and
synthetic polymers, which improve their ability to induce bone
tissue repair. Multi-layered scaffolds are also useful for vascular
tissue engineering (14-16).
The structural diversity of these scaffolds is more advantageous
than homogeneous structures due to their enhanced mechanical
features, biodegradability and biocompatibility (14,17,18).
Unfortunately, complications such as delamination (poor
biomechanics and operability) and difficulty in molding
three-dimensional structures (compact structure that can impede
cell migration) with many constituents have restricted the
widespread development of multi-layered scaffolds (19,20).
Simvastatin, a cholesterol-lowering drug, can
promote bone growth and this is hypothesized to be through
stimulation of BMP-2 expression (21,22).
Simvastatin has also been successfully integrated into drug
delivery vehicles consisting of a methylcellulose gel surrounded by
a polylactic acid (PLA) membrane. Using this system, a single
administration of 2.2 mg of simvastatin was demonstrated to induce
bone growth in vivo, however, soft-tissue inflammation was
observed (23).
In the present study, two multi-layered
co-electrospun nanofibers membranes made of natural (Gt) and
synthetic (PCL) polymers were designed. These membranes have both
porous and dense layers to improve their osteogenic efficacy and
barrier function. In addition, these membranes were loaded with
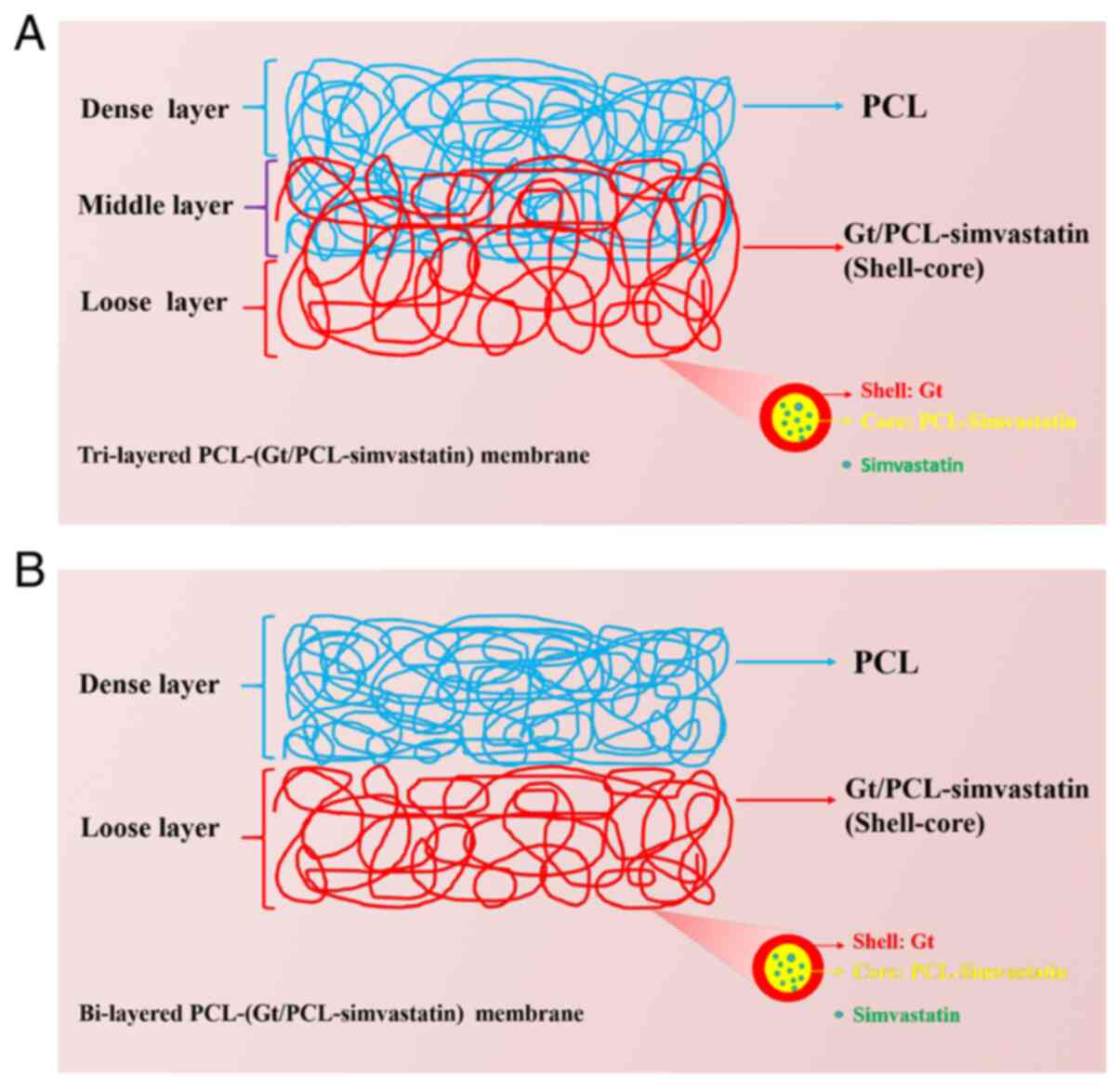
simvastatin to promote bone growth (Fig. 1). These membranes were designed to
promote osteoinduction and act as a barrier against cells but not
against water and molecules in order to promote guided bone
regeneration (GBR). The in vitro biological function of the
membranes was then evaluated. In order to verify the osteogenic and
barrier effects of the membranes, bone marrow mesenchymal stem
cells (BMMSCs) and human fibroblasts were seeded on the surface of
the porous and dense layers, respectively. The cell distribution on
the different surfaces was observed using a confocal laser scanning
microscope (CLSM). The osteogenic effects of simvastatin on
critically-sized calvarial defects in rabbits were evaluated to
assess the membrane's potential for GBR.
Materials and methods
Materials
PCL (85 kDa) and histopaque-1077 were obtained from
Sigma-Aldrich (Merck KGaA). 2,2,2-trifluroroethanol (TFE) was
obtained from the Weihai Newera Chemical Co., Ltd. Gelatin (Gt, 200
Bloom) was supplied by the Department of Polymer Science and
Engineering, Zhejiang University (China). Simvastatin was purchased
from BioSino Biotechnology & Science, Inc. 0.05% trypsin-5 Mm
EDTA was from Biochrom GmbH (Merck KGaA). Human foreskin
fibroblasts (HFFs-1) were obtained from the American Type Culture
Collection. Culture media and other cell culture medium supplements
were obtained from Thermo Fisher Scientific, Inc.
Preparation of the multilayered
nanofibers
Simvastatin was dissolved in a PCL (20% w/w in TFE)
solution to obtain a final concentration of 3.5%. Gt was dissolved
in TFE to obtain a 12% solution. Coaxial electrospinning was
conducted with reference to a previous study (24). The Gt/TFE solution was used to
create the outer shell, while the PCL/TFE solution containing
simvastatin was used to create the inner core. All feed rates were
set at 1 ml/h. The spinning electrode and the collector were
separated by 16 cm, with a voltage of 9-10 kV/cm. Collection was
carried out over 3 h to obtain the Gt/PCL-simvastatin membrane. A
single jet was used to electrospin the PCL mat on the
Gt/PCL-simvastatin membrane. The spinning electrode and the
collector were 17 cm apart, with a voltage of 11-12 kV/cm. The
duration of electrospinning was 4 h, and the feed rate was 1 ml/h.
Finally, a bi-layered PCL-Gt/PCL-simvastatin membrane (membrane A)
was obtained. The layers were 100-150 µm thick.
The tri-layered PCL-Gt/PCL-simvastatin membrane
(membrane B) was fabricated in a similar manner to membrane A.
First, the coaxial Gt/PCL-simvastatin fibers were electrospun for 2
h, followed by the introduction of PCL nanofibers onto the coaxial
membrane by simultaneous spinning using another electrode. The
position of the spray nozzle for the PCL nanofibers was adjusted to
ensure maximum overlap of the receiving area of the core-shell and
PCL fibers. The two nanofibers were collected for 1.5 h, before
coaxial electrospinning was stopped. The electrospinning of the PCL
nanofibers was continued for 2 h. The structure of the
multiple-layered nanofibrous membranes is shown in Fig. 1. The membranes were stored at room
temperature.
Scaffold characterization
A scanning electron microscope (SEM; model no.
JSM-5300; JEOL, Ltd.) was used to assess the surface and morphology
of the nanofibers under x1,000 magnification. Image-Pro Plus v6.0
(Media Cybernetics, Inc.) was used to measure the average fiber
diameter and pore size (n=100) of the materials. The samples used
for SEM were subjected to vacuum drying and were sputter-coated
with gold-palladium for 60 sec.
In order to evaluate nanofiber degradation, three
membrane pieces were immersed in 10 ml phosphate-buffered saline
(PBS, pH 7.2±0.1) and placed in a 37˚C water bath for 1 and 3
months, with PBS refreshed monthly. Sample degradation was examined
by SEM observation.
The membranes were cut into squares (10x10 mm; n=3)
and used to assess the in vitro release of simvastatin over
one month. After sterilization using Cobalt-60 (2 h), the specimens
were immersed in PBS (1 ml) and incubated at 37˚C under rotation
(150 RPM). Supernatants were obtained daily and stored at -20˚C,
with the addition of fresh PBS (1 ml). The amount of released
simvastatin was assessed by high-performance liquid chromatography
(HPLC), and the cumulative release of simvastatin was plotted.
Culture of bone marrow mesenchymal
stem cells (BMMSCs)
BMMSCs were isolated from iliac crest marrow
aspirates of human donors (aged 20-25 years) with no known disease
(n=4). Informed consent was obtained and signed by the donors and
the study was approved by the Research Ethics Committee of The
First Affiliated Hospital, College of Medicine, Zhejiang University
(Hangzhou, China; Reference number 2013-273). The human samples
were collected in the operating room of The First Affiliated
Hospital of Zhejiang University School of Medicine between November
2014 and October 2020.
The isolation and culture procedure of BMMSCs were
performed as described in our previous study (25). Histopaque-1077 density gradient
centrifugation was used for BMMSC isolation. The collected
mononuclear cells (MNCs) underwent PBS washes and resuspension in
Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal
bovine serum (FBS) and 100 U/ml penicillin/streptomycin and were
plated at a density of 2x106 MNCs/cm2.
Incubation was carried out at 37˚C in a humidified environment
containing 5% CO2, and the medium was replenished at
3-4-day intervals. At 80% confluency, BMMSCs were passaged after
trypsinization.
Seeding and culturing of cells on the
membranes
BMMSCs at passage 3 were seeded on the coaxial
surface of the membranes (10x10 mm) after sterilization (Cobalt-60,
2 h), placed in a 24-well culture plate under pressure (glass
column), and incubated overnight in a humid environment containing
5% CO2 at 37˚C, as previously described (25,26).
For seeding, cells at 5.4x106 cells/ml were added drop
by drop onto the membrane, with approximately 3.2x105
cells per membrane. Third-passage HFFs-1 were added to the PCL
surface of the membranes, as described above, at 5.4x106
cells/ml. Membrane A was used as the control group and membrane B
as the experimental group. Constructs were incubated for 2.5-3 h to
ensure cell adhesion to the membrane. Incubation was performed at
37˚C, as described above, with the medium refreshed at 3-4-day
intervals. At defined time points, triplicates per seeding group
were obtained for cell adhesion, distribution and differentiation
assessment.
Cell morphology, proliferation, and
distribution on membranes
To assess cell adhesion and morphology, cell-loaded
membranes at 10, 30 and 60 min post-seeding were submitted to two
PBS washes, glutaraldehyde fixation (2.5%; 2 h; 4˚C), and two
additional PBS washes. Graded ethanol solutions (30, 50, 70, 80,
90, 95 and 100%) were used for dehydration, followed by sputter
coating with gold and SEM analysis.
Cell proliferation was estimated with the Cell
Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.) after
the cells were cultured on the membranes for 1, 3, 5 and 7 days.
Cell proliferation was compared to cells seeded on Petri dishes as
control. CCK-8 solution (10 µl) was added to each well (three wells
for each group) and the cells were incubated at 37˚C for 4 h.
Absorbance at 450 nm was determined using a microplate reader
(Bio-Tek Instruments, Inc.).
For cell distribution assessment, PBS-washed
cell-loaded membranes were incubated with
4',6-diamidino-2-phenylindole (DAPI; Invitrogen; Thermo Fisher
Scientific, Inc.) for 30 min, washed, and further incubated in
fresh medium for 1 h. These procedures were performed at 37˚C in a
humidified 5% CO2 atmosphere. Laser confocal scanning
microscopy was performed and raw 3D images were analyzed using
NIS-Elements Basic Research v3.0 (Nikon Instruments Inc.) for cell
distribution.
Reverse transcription-quantitative PCR
(RT-qPCR) for the determination of osteogenic gene expression
Total RNA from cell-membrane samples was obtained
using the RNeasy micro kit (Qiagen GmbH). RNA amounts and purity
were assessed on a Bio-Photometer (Eppendorf). First-strand cDNA
(in 100-µl reaction volumes) synthesis was performed with a
QuantiTect Reverse Transcription kit (Qiagen GmbH) based on 100 ng
of total RNA, as recommended by the manufacturer (37˚C for 15 min
and 85˚C for 5 sec). qPCR was carried out on an Applied Biosystems
7500 Real-Time PCR System (Life Technologies; Thermo Fisher
Scientific, Inc.) with a QuantiTect SYBR Green PCR kit (Qiagen
GmbH). Reactions contained 1 µl of cDNA, 4.5 µl of Real Master
Mix/SYBR solution (Qiagen GmbH), 1 µl of each primer and 2.5 µl of
RNase-free water. The early-stage osteogenic differentiation gene
core-binding factor-α1 (Cbf-α1) (27,28)
and alkaline phosphatase (ALP) (29), the middle-to-late stage osteogenic
marker osteonectin (ON) (30), and
the late stage markers osteocalcin (OCN) and osteopontin (OPN)
(31) were assessed using
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) for normalization.
Cells cultured without a membrane constituted the control group for
assessing target gene expression. The primer sequences used are
listed in Table I. Amplification
was carried out for 40 cycles in a real-time PCR device, with a
program consisting of HotstarTaq DNA polymerase activation (2 min,
95˚C), denaturation (15 sec, 95˚C) and annealing for 15 sec at 62˚C
(ON, OCN, OPN), 63˚C (Cbf-α1) or 59˚C (GAPDH), with a final
extension (20 sec, 68˚C). Cycle threshold (Ct) values and melting
curves were assessed. The analysis was performed as described by
Pfaffl (32).
 | Table IPrimer sequences of target and
housekeeping genes that were utilized in the study. |
Table I
Primer sequences of target and
housekeeping genes that were utilized in the study.
| Gene | Primer Sequence
(5'-3') |
|---|
| Cbf-α1 | F:
TTCCAGACCAGCAGCACTC |
| | R:
CAGCGTCAACACCATCATT |
| ON | F:
CGAGCTGGATGAGAACAACA |
| | R:
AAGTGGCAGGAAGAGTCGAA |
| ALP | F:
GTCACTGCGGACCATTCC |
| | R:
GGCTGCATACGCCATCAC |
| OCN | F:
CAGCCACCGAGACACCATG |
| | R:
CAGAGCGACACCCTAGACC |
| OPN | F:
CAGTTGTCCCCACAGTAGACAC |
| | R:
GTGATGTCCTCGTCTGTAGCATC |
| GAPDH | F: GGAGCG
AGATCCCTCCAAAAT |
| | R:
GGCTGTTGTCATACTTCTCATGG |
Assessment of in vivo implants
Surgery
Critical-size defects were surgically created on the
calvaria of 8-month-old New Zealand white rabbits (male, 2.0-2.5
kg; n=24) (21,31-38)
purchased from the Laboratory Animal Center of Zhejiang Province.
The rabbits were assigned to three groups: Control group (no
membrane), group A (bi-layered PCL-Gt/PCL-simvastatin membrane) and
group B (tri-layered PCL-Gt/PCL-simvastatin membrane). The Animal
Experimental Ethical Committee of the First Affiliated Hospital,
College of Medicine, Zhejiang University (Reference no. 2013-273)
approved the protocols for animal experiments. The implantation
procedures in these rabbits were performed under general anesthesia
using fentanyl/fluanison (Hypnorm®, fentanyl citrate
0.315 mg/ml, fluanisone 10 mg/ml, Janssen Pharmaceuticals, Inc.;
Johnson + Johnson; 0.3 ml/kg intramuscular) combined with an
intravenous injection of 1 mg/kg diazepam. During surgery,
full-thickness flaps were made to reveal the cranial bone, and
critical size (15 mm) defects, as previously described (23,33-40),
were generated with trephines under irrigation with chilled saline.
For the membrane groups, the membranes were implanted and fixed
onto the defects at this stage. A resorbable suture was used for
closure of the soft tissues in layers. The rabbits underwent
euthanasia with an overdose (100 mg/kg) of intravenous
pentobarbital sodium at 4 or 12 weeks following implantation (n=4)
and were assessed for bone regeneration.
Micro-computed tomography (µ-CT)
At 4 or 12 weeks after implantation, the tissues
surrounding the membranes were harvested and fixed in 10% formalin
at room temperature for 24 h. Imaging was carried out on an animal
micro-CT scanner (SCANCO Medical AG) in high-resolution scanning
mode using 70 kV, 200 µA, a field of view of 15 mm and 34.4-µm
resolution. Data analysis was performed with the micro-CT image
analysis software (NRecon v.1.6.9; Bruker micro-CT; Bruker
Corporation) to determine the mean new bone volume (BV), bone
mineral density (BMD) and bone volume/total volume (BV/TV).
Histological analysis
Half of the samples underwent fixation with 10%
formalin at room temperature for 24 h, decalcification,
dehydration, paraffin embedding and hematoxylin and eosin (H&E)
staining (26). The remaining half
of the samples were fixed in 4% formalin (Formafix, Global
Technologies Ltd.) at room temperature for 7 days and dehydrated
for 14 days with increasing concentrations of alcohol (70, 80, 96
and 100%). Over a period of 28 days, the sections were
block-embedded in polymethyl methacrylate (PMMA;
Technovit® 7200 VLC; Kulzer GmbH), after which the
samples were ground in the sagittal direction and sliced into
250-µm thick sections with a microtome (EXAKT Technologies, Inc.).
The sections were further reduced to 15 µm, polished and stained
with Van Gieson and toluidine blue, as described previously
(41). Images were captured using
an IX 70 light microscope (Olympus Corporation; magnification,
x25). Three fields of view were observed for each section.
Statistical analysis
SPSS v11.5 (SPSS, Inc.) was used for the statistical
analyses. Values are presented as the mean ± standard deviation
(SD). Groups were compared by one-way analysis of variance (ANOVA)
with Tukey's post hoc test/P<0.05 was considered to indicate
statistical significance.
Results
Morphological and structural
features
The preparation process of the tri-layered
PCL-Gt/PCL-simvastatin membrane (membrane B) is shown in Fig. 1. The middle layer of membrane B was
generated by the simultaneous spinning of uniaxial PCL and coaxial
Gt/PCL-simvastatin nanofibers using two nozzles. As a control, the
bi-layered PCL-(Gt/PCL-simvastatin) membrane (membrane A) was made
with two separated fibrous mats by sequential electrospinning. As
shown in Fig. 1A, the
Gt/PCL-simvastatin and PCL nanofibers resulted in the porous and
dense layers, respectively, while the middle layer of membrane B
contained both types of fibers.
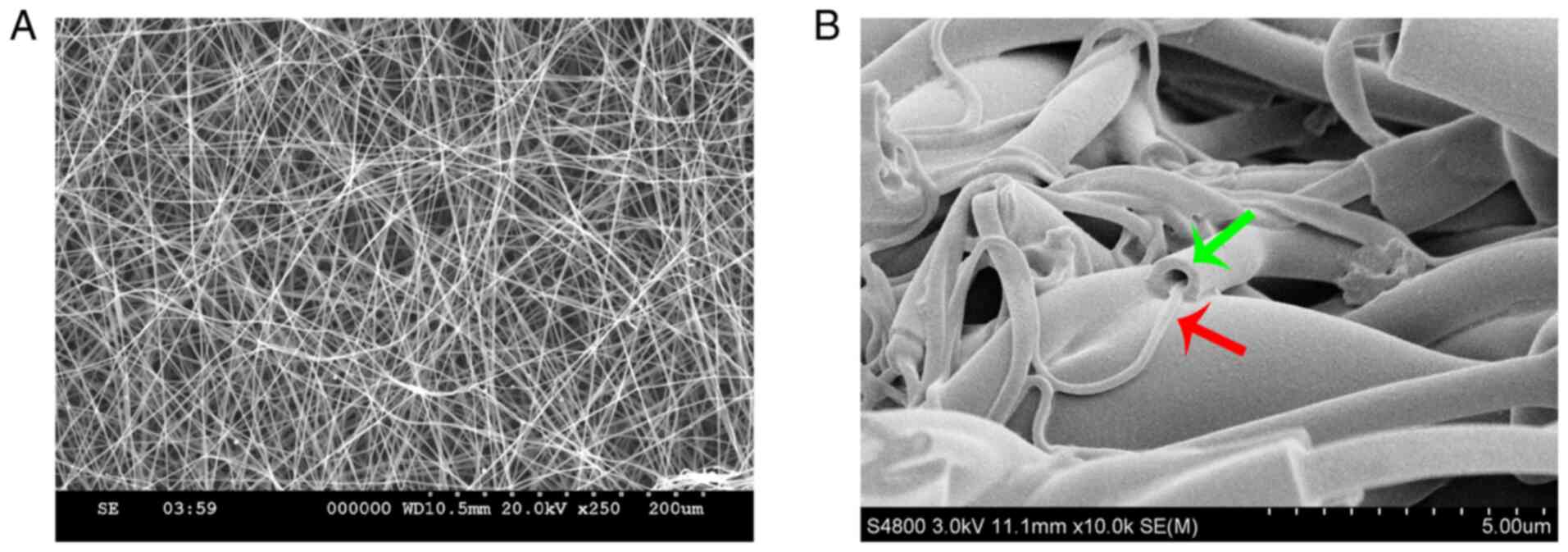
As indicated by the SEM images, electrospun
nanofibers were smooth. This indicates that both PCL and
Gt/PCL-simvastatin fibers could be generated under all flow rates
and methods used (Fig. 2A). The two
types of nanofibers had different morphologies. The pore sizes of
the Gt/PCL-simvastatin nanofibers (porous layer) were 30.27±4.23 µm
(membrane A) and 31.84±4.43 µm (membrane B), while the fiber
diameters were 419.28±59.23 nm (membrane A) and 444.62±96.53 nm
(membrane B). The pore sizes of the PCL nanofibers (dense layer)
were 13.88±4.38 µm (membrane A) and 14.75±2.96 µm (membrane B),
while the fiber diameters were 228.58±98.12 nm (membrane A) and
254.73± 0.68 nm (membrane B). Fig.
2B demonstrates the general structure of the Gt/PCL fibers. The
shell was composed of the natural polymer Gt, and the core was
composed of the synthetic polymer PCL containing simvastatin.
Fig. 2B does not represent the
final structure of the membrane, nor its evolution in time.
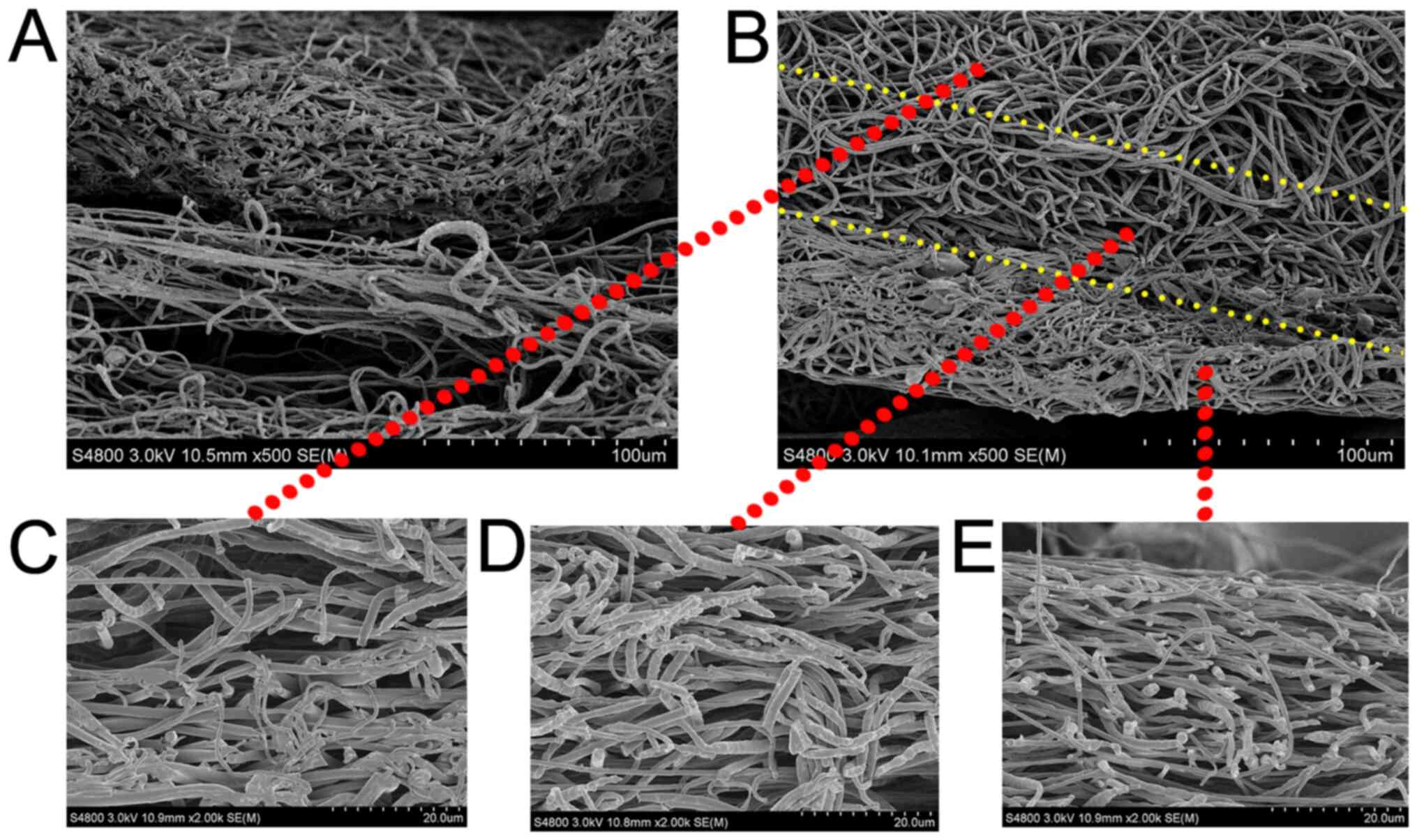
The structural details of the membranes are shown in
Fig. 3. Obvious delamination
appeared in membrane A with the PCL mat on the top and
Gt/PCL-simvastatin membrane on the bottom (Fig. 3A), while no delamination was
observed in membrane B (Fig. 3B).
SEM images demonstrating the morphology in membrane B are also
shown. The changes in structure and morphology in the different
layers indicated a distribution of multiple layers in the membrane.
The upper layer was composed of coaxial electrospun fibers with a
smooth surface (Fig. 3C). The layer
between the dense and porous layers is the middle layer (Fig. 3D), while the lower PCL layer
comprised greater corrugation nanofibers (Fig. 3E).
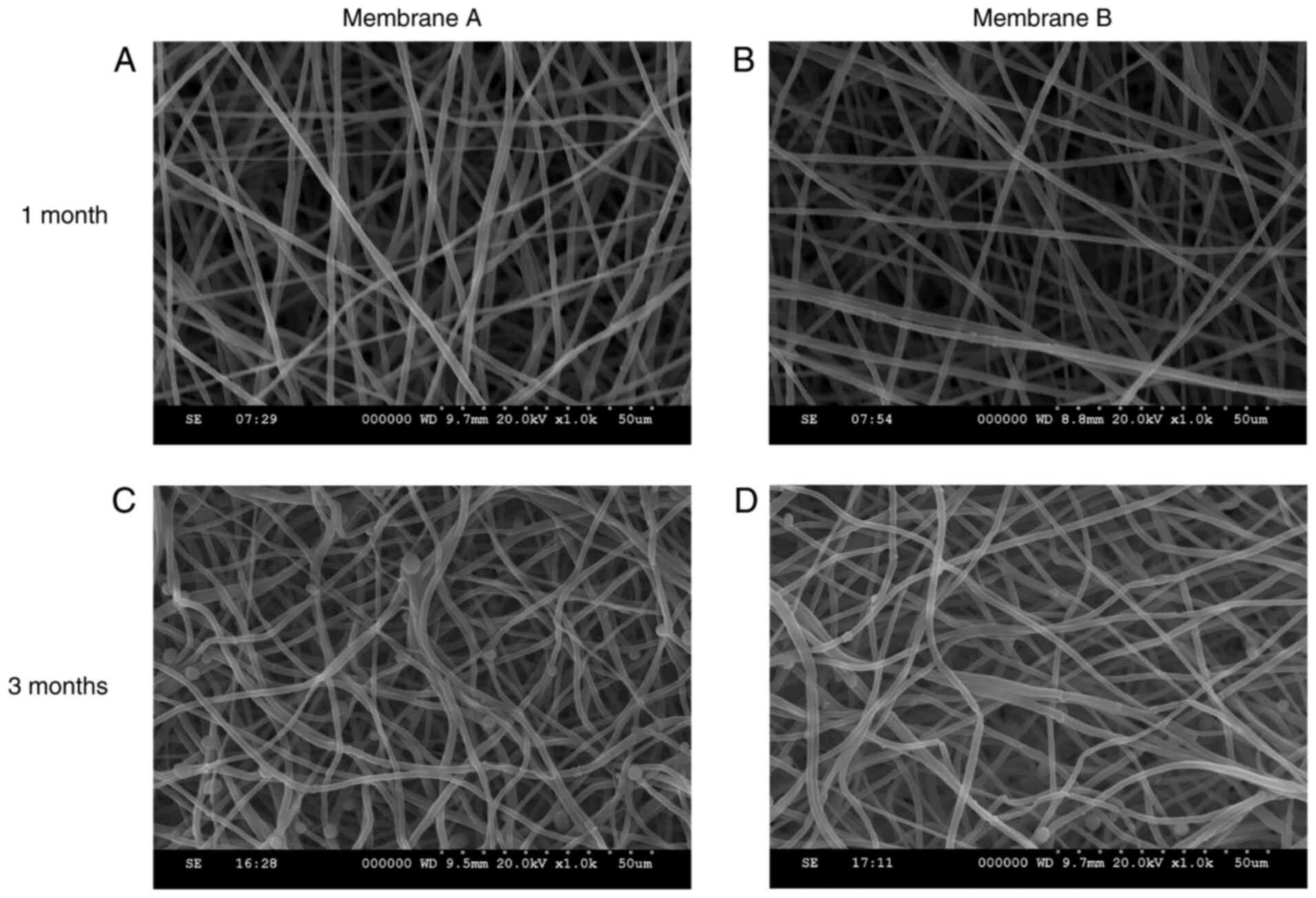
Biodegradability in vitro
SEM micrographs of the electrospun membranes during
degradation are shown in Fig. 4.
The porous layer of the nanofibrous membranes was morphologically
intact during the first month. After 3 months, the coaxial
nanofibers were partially dissolved, but the overall integrity of
the fiber structure was maintained.
Simvastatin release from the
electrospun fibers
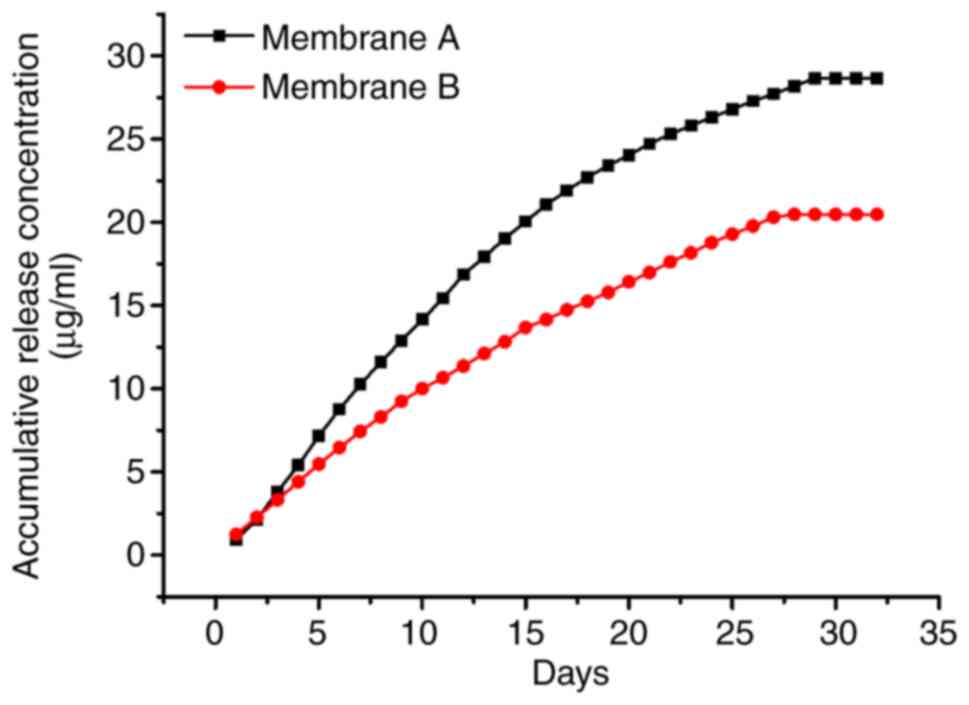
The release profile of simvastatin from the
core-shell structured fibers is shown in Fig. 5. Samples for assessing simvastatin
release into the solution were obtained every day for 32 days, and
the total simvastatin amounts were evaluated by HPLC. Approximately
28.66 µg of simvastatin was released per membrane within the first
25 days. The cumulative release of simvastatin was linearly
correlated with the incubation time at the early stage (about 15
days) and approached a plateau after 25 days.
Adhesion of BMMSCs to electrospun
membranes
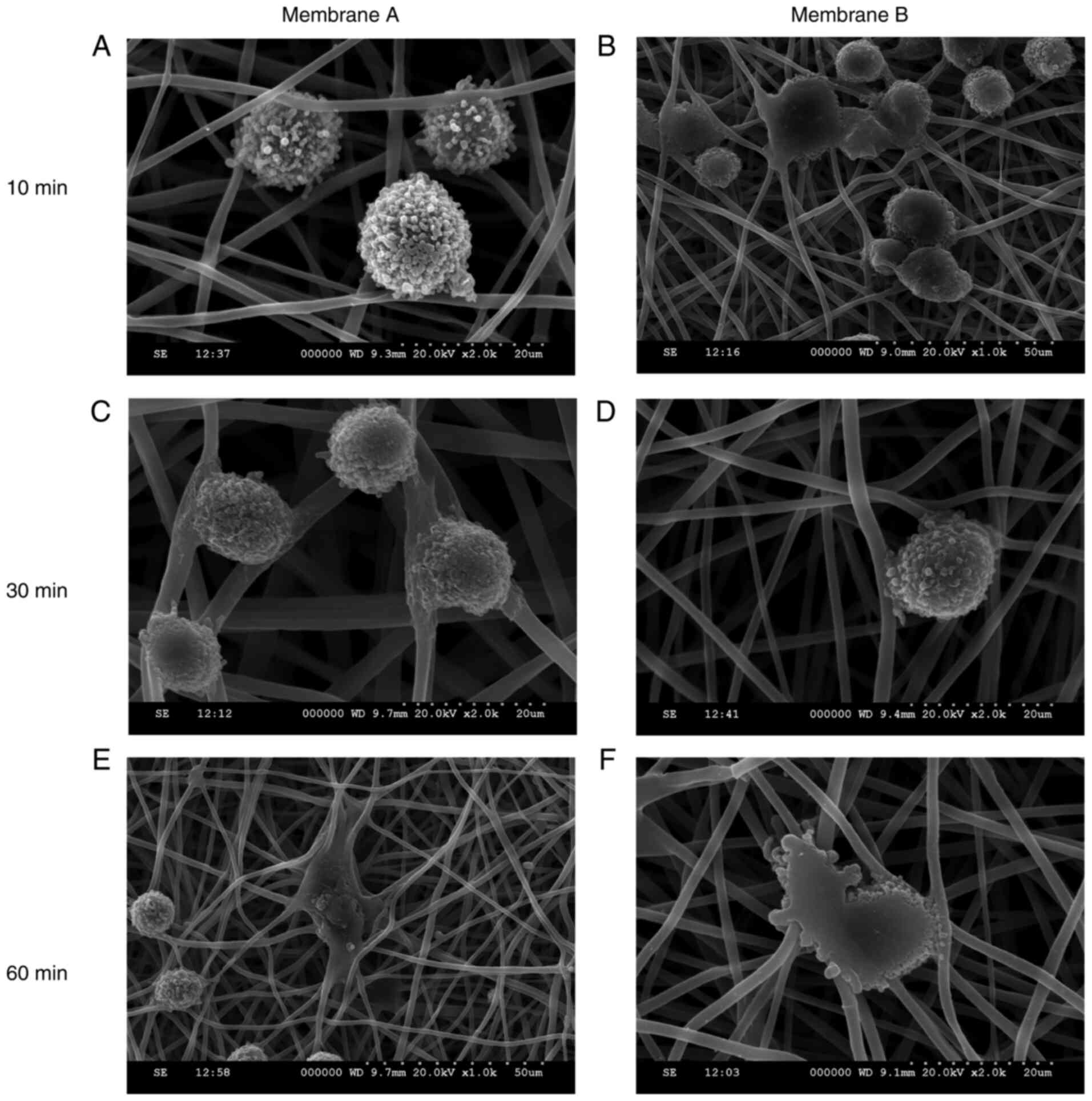
The adhesion of BMMSCs to the porous layer of the
membranes is shown in Fig. 6. BMMSC
adhesion was highly prominent at 10 min post-seeding. The cells
stretched out along the fiber processes on the membrane in all
directions, particularly at 60 min after seeding. No obvious
differences were found between membranes A and B.
Cell proliferation on the
membranes
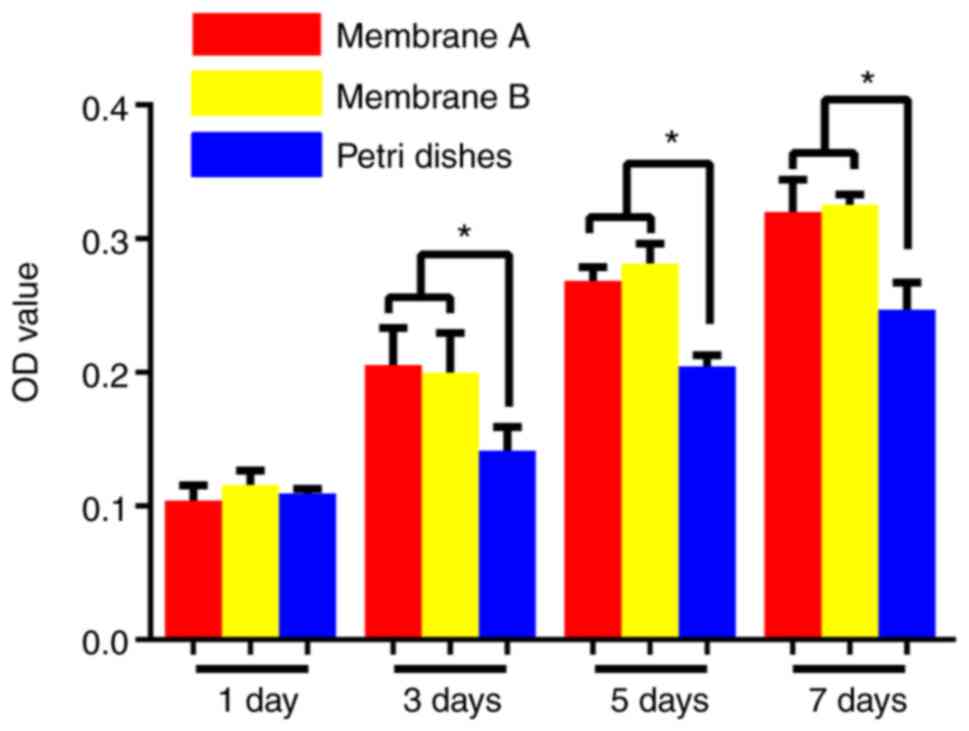
The cell proliferative activity on the membranes was
evaluated over 7 days (Fig. 7).
Compared to the control group, the numbers of BMMSCs from the
membrane groups were significantly higher than the numbers in the
control group throughout the 7 days (P<0.05). However, there
were no significant differences between the two membranes
(P>0.05).
Cell distribution on the electrospun
fibers
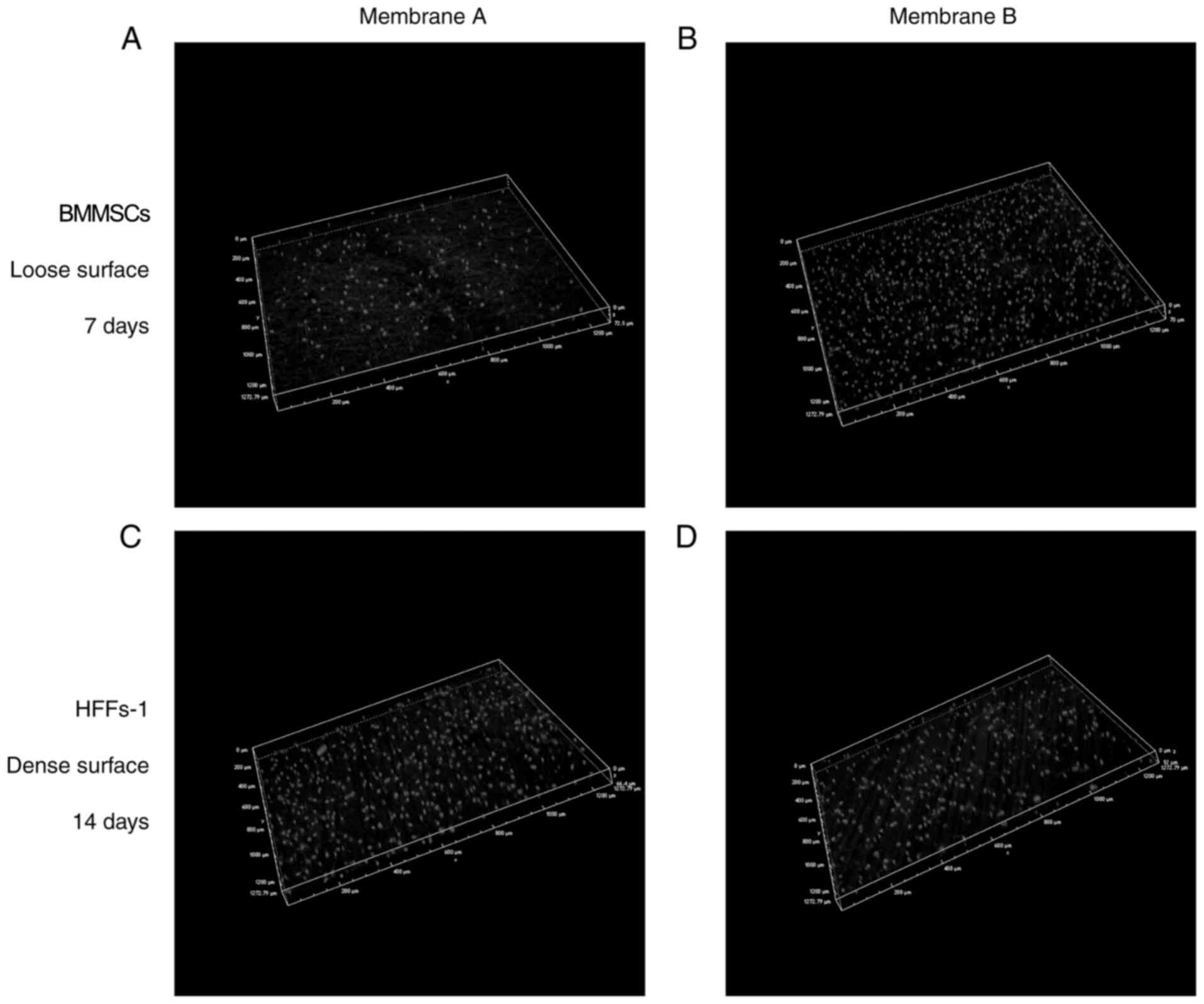
Confocal images showing cells on the fibers after
culturing for 7 and 14 days are shown in Fig. 8. At 7 days, the porous surface of
the membrane was covered by a continuous and structured MSC
monolayer. The dense surface was covered by HFFs-1 at 14 days. The
3D image shows that the MSCs seeded on the porous surface of the
membrane penetrated as deeply as 70 µm after 7 days, while HFFs-1
seeded on the dense surface of membrane penetrated as deeply as 60
µm after 14 days, indicating that the dense layer could act as an
effective barrier to prevent cell invasion. No cells were observed
to cross the membranes completely.
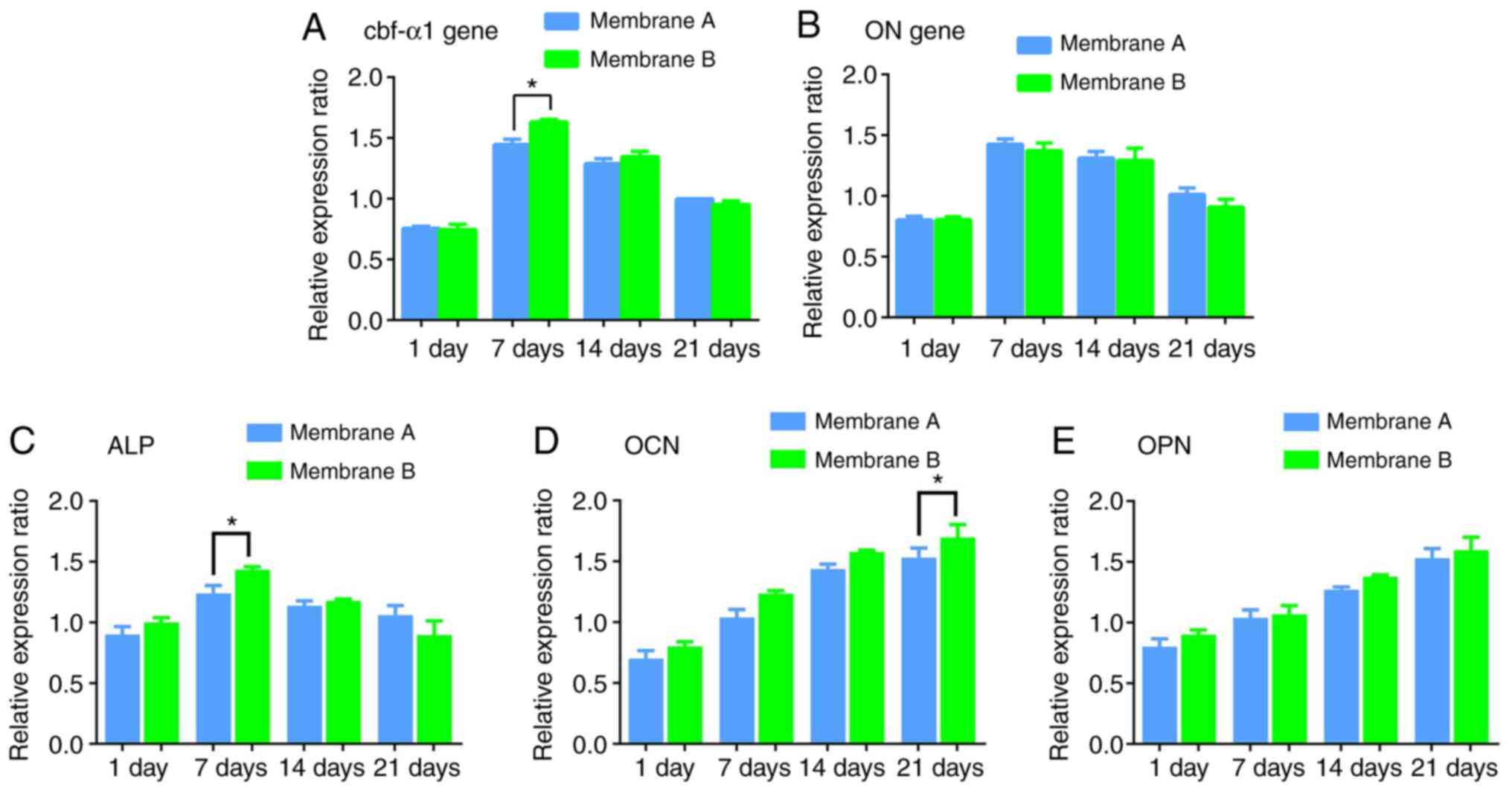
Osteogenic gene expression in BMMSCs
on the fibers
While continuously co-culturing the MSC-membrane
composite, osteogenic gene expression levels were comparable in
groups A and B after 1, 7, 14 or 21 days (all P>0.05; Fig. 9), with peaks at 7 days. An exception
was Cbf-α1 gene expression in MSCs grown on tri-layered
PCL-Gt/PCL-simvastatin fibers, which was significantly increased at
7 days (P<0.05). ALP was also higher in membrane B than in
membrane A at 7 days (P<0.05), while OCN was higher in membrane
B at 21 days (P<0.05). There were no differences in OPN
expression between the two membranes (P>0.05).
In vivo implantation
To assess the osteogenic defect of nanofibers in
vivo, 15-mm complete calvarial defects were generated in
rabbits. During the experiment, no necrosis was observed in the
animals.
Micro-CT analysis
Bone formation was increased in the nanofiber groups
compared with the control group at 4 and 12 weeks (Fig. 10A-F). Quantitation of µ-CT imaging
data was carried out by assessing BV, BMD and BV/TV of the defects
(Fig. 10G-I). Defects treated with
membrane B showed healing with BV, BMD and BV/TV of 25.13±2.50
mm3, 539.74±8.38 mg/mm3 and 4.17±0.51%,
respectively, at 4 weeks following implantation. New bone formation
was similar in groups A and B and almost inexistent in the control
group. At 12 weeks, new bone was found in the three groups. In
comparison with controls, group B had markedly increased healing,
with BV, BMD and BV/TV of 56.05±60.15 mm3, 767.83±29.10
mg/mm3 and 8.22±1.17%. In group B, bone formation
covered most of the defects. By contrast, healing was only observed
in the center or at the edges in controls. These findings
demonstrated that the released simvastatin had in vivo
activity.
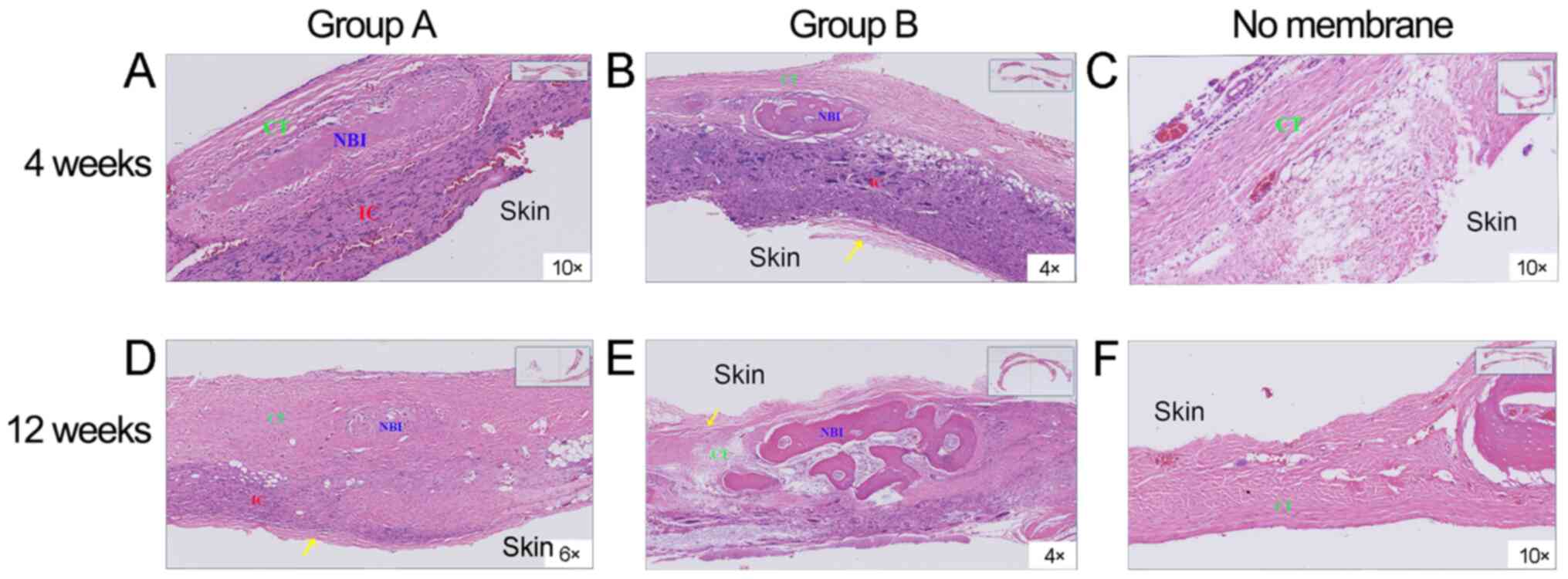
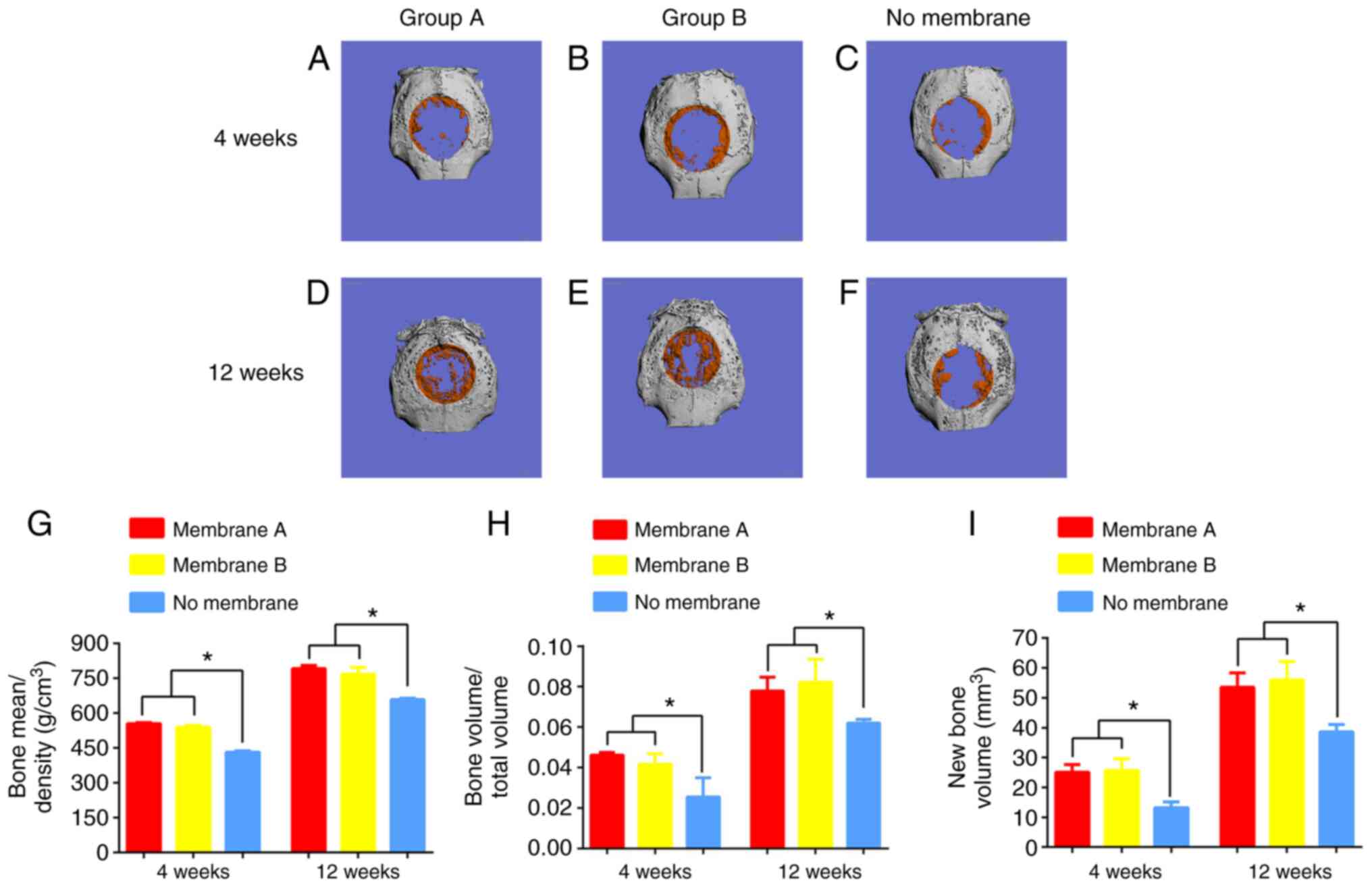 | Figure 10Micro-CT results of surgical defects
treated with membranes A or B, or a no membrane (control group) at
4 and 12 weeks. Osteogenesis effects were seen in (A) group A and
(B) group B in repair of the defect in comparison to (C) the
control at 4 weeks. A greater extent of defect repair was also seen
in (D) group A and (E) group B in repair of the defect in
comparison to (F) the control at 12 weeks. There were more blue
areas in the control group and more orange areas in groups A and B,
where blue represents fibrous tissue, and orange represents new
mineralized bone. At 12 weeks, the greatest extent of repair
appeared in group B, and the defect region was mainly covered by
orange. (G) BMD (mg/mm3), (H) BV/TV (%) and (I) BV
(mm3) within the defects were calculated. BV, BMD, and
BV/TV of groups A and B were significantly higher than in the
control group at both 4 and 12 weeks. Data are presented as the
mean ± SD; n=4; *P<0.05. BMD, bone mineral density;
BV/TV, bone volume/total volume; new bone volume. |
Histological properties
Using eosin ethanol staining, new bone generation
was observed in groups A and B at 4 weeks after surgery, whereas in
the control group, only soft tissues and limited new bone were
observed (Fig. 11). At 12 weeks,
the new bone area was markedly elevated in group B in comparison
with the two other groups. The control group only showed minimal
fibrous-connective tissue. At the initial stage (4 weeks) of bone
healing, an overt immunologic response with inflammatory signs were
recorded. At 12 weeks, there were slight inflammatory signs in both
membrane groups, indicating that the membranes were gradually
degraded. Over time, residual membrane materials were observed in
both membrane groups.
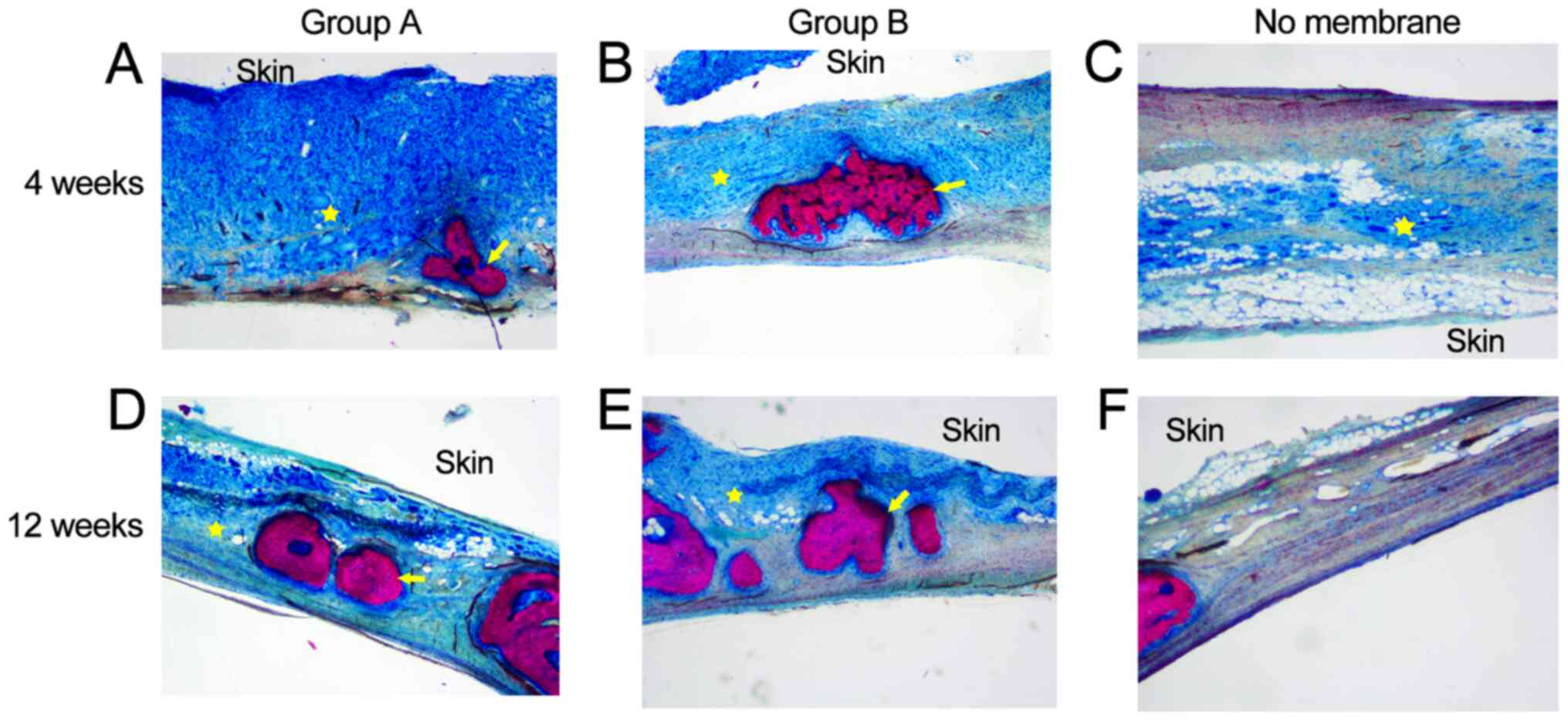
The results of Van Gieson and toluidine blue stain
were consistent with the H&E staining results (Fig. 12). New bone formation in groups A
and B occurred to a greater extent than in the control group at 4
and 12 weeks. In the control group, only a large amount of
connective tissue was observed.
Discussion
Our previous studies demonstrated that the diameter
of electrospinning nanofibers could be affected by flow rate and
receiving distance (42,43). The parameters for the present study
were optimized during our earlier studies (42,43).
SEM revealed that the membranes were nano-scaled and that the
co-axial electrospun Gt/PCL-simvastatin fibers had a distinct
core-shell structure. Using a sequential quantity gradient
co-electrospinning approach, PCL markedly improved the mechanical
features of the nanofibers. The membrane structure was similar to
that of the ECM, and the pore size of the PCL surface was
significantly smaller than that of the coaxial surface. The present
study demonstrated that the designed membrane had good
morphological, physicochemical and mechanical features that enable
it to serve as a barrier membrane. Cross-sectional images of
membrane B showed that the Gt/PCL-simvastatin and PCL nanofibers
were well-mixed and integrated between the membrane layers. By
contrast, membrane A was observed to have two layers without
connection and could be easily separated.
An ideal drug delivery system should supply
effective product amounts, avoid systemic undesired reactions, and
convey products into target areas at fixed rates (44). The generation of a distinct boundary
could be theoretically assessed (45,46).
Studies by both our group (25) and
others (47) have indicated that
coaxial electrospinning can achieve effective product loading as
well as continuous and controlled local drug delivery. In the
present study, simvastatin was embedded in the core (PCL) of a
coaxial structure. The mechanism of simvastatin release at a
controlled rate can be explained by diffusion and polymer
degradation (48,49). Simvastatin is first released by
diffusion onto the fiber's surface. Subsequently, when the membrane
is incubated with the medium, the small pores on the shell (Gt) of
the fibers undergo gradual polymer degradation. This allows the
slow and constant release of the drug through the pores. Both
membranes in the present study demonstrated constant and continuous
drug release, with membrane B demonstrating a more controlled drug
release, due to its gradient structure.
Implantable materials should undergo in vivo
degradation at a controlled rate to maintain and provide space for
new bone formation (50). Material
degeneration speed is critical in tissue engineering (51-54).
If the membrane is degraded too fast, cell proliferation may be
hampered, with insufficient secretion of the new matrix (51). Conversely, if degradation is too
slow, residual materials could adversely affect new bone's
homogeneity and function (55).
Furthermore, the optimal time for degradation of materials in the
skull or maxillofacial bones is 3-6 months, whereas the scaffold
used in spinal fusion should be degraded after 9 months or longer
(50). In the present study, an
artificial synthetic material (PCL) was used to provide controlled
release kinetics (47) and an
appropriate degradation rate. The results of degradation testing
in vitro suggested that the fiber surface became
increasingly rough within 3 months, consistent with the optimal
time of 3-6 months. Nevertheless, the complete degradation time of
PCL is about 2 years, and future studies should examine for how
long a PCL-based membrane retains its support and barriers effects.
H&E staining also suggested that new bone islands and osteoid
tissue had replaced the degraded membrane material at this time
point. Furthermore, the timing coincides well with the release of
simvastatin.
Electrospun nanofibers have high surface-to-volume
ratios and are potential ECM substitutes that improve cell
attachment (56,57). Simultaneously, high porosity confers
optimal permeability for nutrient and gas exchange in the newly
formed tissue (58). SEM of BMMSCs
seeded membranes showed that cells were firmly attached to fibers,
with cytoplasmic processes spreading throughout. This suggests that
such membranes are an optimal scaffold for cell attachment. CCK-8
testing revealed that the membranes could promote cell adhesion and
proliferation, with no cytotoxicity in vitro. The confocal
microscopy results established that MSCs proliferated on the
coaxial fibrous surface and the superficial layer of the internal
part of the membrane. HFFs-1 with poorer adhesion and more
superficial invasive depth also showed an excellent barrier
function in membrane B. The same results were obtained from
histological assessment of in vivo samples, with group B
showing less fibrous tissue in the defect lending itself to ideal
barrier function.
In order to assess the clinical usefulness of
membrane B, a critical-sized defect in rabbit calvaria was created,
based on previous studies (23,33-40).
Micro-CT revealed an elevated BV/TV ratio, suggesting new bone
generation, for both membrane groups compared with controls. Both
membrane groups showed comparable values. In vivo
histological data demonstrated that both membranes enhanced bone
regeneration in comparison with controls. Meanwhile, in
vitro testing of osteoinduction of simvastatin released from
the membrane fibers revealed that osteogenic genes were upregulated
in culture. Thus, simvastatin released from the membrane increases
new bone generation and accelerates bone healing in the
calvarial-defect model, but osteoinductive effects were comparable
in both membrane groups.
The present study demonstrated that a novel
multi-layered PCL-Gt/PCL-simvastatin membrane promoted osteogenesis
in cultured BMMSCs and repaired bone defects in rabbits. As the
sole bioactive agent, simvastatin could be successfully delivered
and in a controlled manner. The role of multi-layered structured
membranes in GBR technology remains unclear. These results suggest
that the novel gradient nanofibrous membrane could be a candidate
for an ideal barrier membrane, which is the primary end goal for
current GBR technologies.
Inflammation could be seen in the specimens from the
rabbits that received the membranes. Gelatin and PCL are
widely-used polymers in the field of tissue engineering and are
highly biocompatible and biodegradable (5). However, though simvastatin has been
shown to induce bone growth in vivo, pronounced soft-tissue
inflammation was observed (23). It
can be hypothesized that this inflammation is the result of
simvastatin, which was suggested by a previous study (59). Why simvastatin induces inflammation
and how it might participate in the osteogenesis process remain to
be examined.
The barrier membrane developed in this study is a
guide for tissue regeneration. It has a loose surface and a dense
surface. The loose surface faces the bone tissue to facilitate the
crawling of bone cells or stem cells and guide bone tissue
regeneration. The dense surface has a small pore size to prevent
fibrosis. Since the regenerative cells invade the bone defect area
while the fibrotic cells invade from the connective tissues, the
stem cells were inoculated on the loose surface and fibroblasts on
the dense surface to observe the barrier function of the barrier
membrane and guide the regeneration ability of totipotent stem
cells. The differential impact of different cell types will have to
be examined in a future study.
There are several limitations to this study.
Multilayered membranes without simvastatin as controls were not
included. Indeed, the main research focus of this article was on
the structural innovation of the barrier membrane, rather than the
bone-promoting function of simvastatin, which is relatively
well-known (21,22). In addition, it has previously been
shown that coaxial electrospinning can achieve effective product
loading as well as continuous and controlled local drug delivery
(26). The present study
demonstrates the potential application of novel multilayered
electrospun membranes for GBR with simvastatin in the treatment of
bone regeneration.
The characteristics of cell donor may influence the
results, however all donors in this study were 20-25 years of age
and healthy, and the effect of age difference on BMMSCs should be
minimal (60-62).
In a published Master's degree thesis (entitled Osteogenic ability
and differential gene expression profile of human bone marrow
mesenchymal stem cells of different ages), BMMSCs from the fetal
group (20-24 weeks of age), youth group (16-30 years), and elderly
group (60+ years) showed consistent characteristics within their
respective groups, but the osteogenic properties clearly decreased
with age (63). In addition, the
cell growth rate of donors over 50 years old was significantly
lower than that of young donors. Furthermore, Choudhery et
al (64) demonstrated that the
BMSC markers do not change with age, but that the number of cells
per gram, colony-forming units and the number of cells doubled per
unit time decreases with the increase of the age of mesenchymal
stem cells and donors. Fosset et al (65) also suggested that there is no
age-related change in the expression of cell surface markers,
however, Stolzing et al (66) reported that the expression levels of
the MSC cell surface markers CD90, CD105 and stro1 decrease and
CD44 increase, and that the differentiation potential is also
different with age. In addition, Aksoy et al (67) suggested that various surface markers
of MSCs are expressed at different ages, but compared with older
donor cells, MSCs isolated from younger human donors have a higher
cell metabolic activity and proliferation rate. Therefore,
including cells from a single age group should minimize the
variability due to age, however, some interindividual variability
might remain.
Limits in image resolution and difficulties in
accurately determining tissue regions may present a potential
limitation of the present study. The applicability and clinical
safety of the present study remain to be assessed in future
studies, including more appropriate controls and intravital
microscopy. Finally, the systemic concentrations of simvastatin
were not determined, and it is unknown whether simvastatin can be
released into the peripheral circulation and whether it could exert
systemic effects that could influence bone regeneration, either
directly or indirectly. This will have to be addressed in future
studies.
In conclusion, a novel multi-layered
PCL-Gt/PCL-simvastatin membrane was successfully established by
coaxial electrospinning. This membrane is suitable for use in GBR.
The membrane was able to deliver simvastatin continuously and
promoted new bone formation without overt cytotoxic effects. This
new coaxial electrospinning method might provide new means for
fabricating membranes with both multi-layered structure and
osteoinductive ability.
Acknowledgements
We are grateful to Dr. Zhengjian Chen at the
Department of Polymer Science and Engineering, Zhejiang University
and Prof. Jindan Wu at the MOE Key Laboratory of Advanced Textile
Materials & Manufacturing Technology, Zhejiang Sci-Tech
University for their help with the synthesis of the membranes used
in this study.
Funding
Funding: The present study was funded by grants from the Medical
and Health Major Science and Technology Plan of Zhejiang Province,
China (grant no. wsk2014-2-008), the National Natural Science
Foundation of China (grant no. 31570989), the Natural Science
Foundation of Zhejiang Province of China (grant no. LY15H140002),
the Young Talents Project of Zhejiang Provincial Health Department,
China (grant no. 2019RC151) and Zhejiang Province Welfare
Technology Research Project, China (grant no. LGF20H140007).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DY and CH characterized the morphology of the
nanofibers and their release of simvastatin, assessed their
biological function in vitro, analyzed and interpreted the
data and was a major contributor to writing the manuscript. CH and
HZ performed the histological examination in vivo. CJ and DY
analyzed and interpreted the data. HZ was responsible for
conceptualization, project administration, article review and
quality control. DY, CH, CJ and HZ confirmed the authenticity of
all raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The Animal Experimental Ethical Committee of the
First Affiliated Hospital, College of Medicine, Zhejiang University
(Reference no. 2013-273) approved the protocols for animal
experiments. Informed consent was obtained and signed by the BMMSC
donors and the human cell study was approved by the Research Ethics
Committee of the First Affiliated Hospital, College of Medicine,
Zhejiang University (Hangzhou, China; Reference number
2013-273).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rezvani Z, Venugopal JR, Urbanska AM,
Mills DK, Ramakrishna S and Mozafari M: A bird's eye view on the
use of electrospun nanofibrous scaffolds for bone tissue
engineering: Current state-of-the-art, emerging directions and
future trends. Nanomedicine. 12:2181–2200. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yang G, Li X, He Y, Ma J, Ni G and Zhou S:
From nano to micro to macro: Electrospun hierarchically structured
polymeric fibers for biomedical applications. Prog Polymer Sci.
81:80–113. 2018.
|
|
3
|
Jang JH, Castano O and Kim HW: Electrospun
materials as potential platforms for bone tissue engineering. Adv
Drug Deliv Rev. 61:1065–1083. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Holzwarth JM and Ma PX: Biomimetic
nanofibrous scaffolds for bone tissue engineering. Biomaterials.
32:9622–9629. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mata A, Geng Y, Henrikson KJ, Aparicio C,
Stock SR, Satcher RL and Stupp SI: Bone regeneration mediated by
biomimetic mineralization of a nanofiber matrix. Biomaterials.
31:6004–6012. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li C, Vepari C, Jin HJ, Kim HJ and Kaplan
DL: Electrospun silk-BMP-2 scaffolds for bone tissue engineering.
Biomaterials. 27:3115–3124. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sharifi F, Atyabi SM, Irani S and Bakhshi
H: Bone morphogenic protein-2 immobilization by cold atmospheric
plasma to enhance the osteoinductivity of carboxymethyl
chitosan-based nanofibers. Carbohydr Polym.
231(115681)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kostopoulos L and Karring T: Augmentation
of the rat mandible using guided tissue regeneration. Clin Oral
Implants Res. 5:75–82. 1994.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sedghi R, Shaabani A and Sayyari N:
Electrospun triazole-based chitosan nanofibers as a novel scaffolds
for bone tissue repair and regeneration. Carbohydr Polym.
230(115707)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Elgali I, Turri A, Xia W, Norlindh B,
Johansson A, Dahlin C, Thomsen P and Omar O: Guided bone
regeneration using resorbable membrane and different bone
substitutes: Early histological and molecular events. Acta
Biomater. 29:409–423. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Samavedi S, Olsen Horton C, Guelcher SA,
Goldstein AS and Whittington AR: Fabrication of a model
continuously graded co-electrospun mesh for regeneration of the
ligament-bone interface. Acta Biomater. 7:4131–4138.
2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dalgic AD, Atila D, Karatas A, Tezcaner A
and Keskin D: Diatom shell incorporated PHBV/PCL-pullulan
co-electrospun scaffold for bone tissue engineering. Mater Sci Eng
C Mater Biol Appl. 100:735–746. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang Y, Cui W, Zhao X, Wen S, Sun Y, Han J
and Zhang H: Bone remodeling-inspired dual delivery electrospun
nanofibers for promoting bone regeneration. Nanoscale. 11:60–71.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu S, Dong C, Lu G, Lu Q, Li Z, Kaplan DL
and Zhu H: Bilayered vascular grafts based on silk proteins. Acta
Biomater. 9:8991–9003. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wu T, Zhang J, Wang Y, Li D, Sun B,
El-Hamshary H, Yin M and Mo X: Fabrication and preliminary study of
a biomimetic tri-layer tubular graft based on fibers and fiber
yarns for vascular tissue engineering. Mater Sci Eng C Mater Biol
Appl. 82:121–129. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Han F, Jia X, Dai D, Yang X, Zhao J, Zhao
Y, Fan Y and Yuan X: Performance of a multilayered small-diameter
vascular scaffold dual-loaded with VEGF and PDGF. Biomaterials.
34:7302–7313. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
de Valence S, Tille JC, Giliberto JP,
Mrowczynski W, Gurny R, Walpoth BH and Möller M: Advantages of
bilayered vascular grafts for surgical applicability and tissue
regeneration. Acta Biomater. 8:3914–3920. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Attalla R, Puersten E, Jain N and
Selvaganapathy PR: 3D bioprinting of heterogeneous bi- and
tri-layered hollow channels within gel scaffolds using scalable
multi-axial microfluidic extrusion nozzle. Biofabrication.
11(015012)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Blakeney BA, Tambralli A, Anderson JM,
Andukuri A, Lim DJ, Dean DR and Jun HW: Cell infiltration and
growth in a low density, uncompressed three-dimensional electrospun
nanofibrous scaffold. Biomaterials. 32:1583–1590. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wu T, Huang C, Li D, Yin A, Liu W, Wang J,
Chen J, Ei-Hamshary H, Al-Deyab SS and Mo X: A multi-layered
vascular scaffold with symmetrical structure by bi-directional
gradient electrospinning. Colloids Surf B Biointerfaces.
133:179–188. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Garrett IR, Gutierrez G and Mundy GR:
Statins and bone formation. Curr Pharm Des. 7:715–736.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mundy G, Garrett R, Harris S, Chan J, Chen
D, Rossini G, Boyce B, Zhao M and Gutierrez G: Stimulation of bone
formation in vitro and in rodents by statins. Science.
286:1946–1949. 1999.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Thylin MR, McConnell JC, Schmid MJ,
Reckling RR, Ojha J, Bhattacharyya I, Marx DB and Reinhardt RA:
Effects of simvastatin gels on murine calvarial bone. J
Periodontol. 73:1141–1148. 2002.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Jiang H, Hu Y, Li Y, Zhao P, Zhu K and
Chen W: A facile technique to prepare biodegradable coaxial
electrospun nanofibers for controlled release of bioactive agents.
J Control Release. 108:237–243. 2005.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhu H, Yu D, Zhou Y, Wang C, Gao M, Jiang
H and Wang H: Biological activity of a nanofibrous barrier membrane
containing bone morphogenetic protein formed by core-shell
electrospinning as a sustained delivery vehicle. J Biomed Mater Res
B Appl Biomater. 101:541–552. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chen H, Malheiro A, van Blitterswijk C,
Mota C, Wieringa PA and Moroni L: Direct writing electrospinning of
scaffolds with multidimensional fiber architecture for hierarchical
tissue engineering. ACS Appl Mater Interfaces. 9:38187–38200.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang X, Aubin JE and Inman RD: Molecular
and cellular biology of new bone formation: Insights into the
ankylosis of ankylosing spondylitis. Curr Opin Rheumatol.
15:387–393. 2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lee JS, Lee JM and Im GI:
Electroporation-mediated transfer of Runx2 and Osterix genes to
enhance osteogenesis of adipose stem cells. Biomaterials.
32:760–768. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wrobel E, Leszczynska J and Brzoska E: The
characteristics of human bone-derived cells (HBDCS) during
osteogenesis in vitro. Cell Mol Biol Lett. 21(26)2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Termine JD, Kleinman HK, Whitson SW, Conn
KM, McGarvey ML and Martin GR: Osteonectin, a bone-specific protein
linking mineral to collagen. Cell. 26:99–105. 1981.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Graneli C, Thorfve A, Ruetschi U, Brisby
H, Thomsen P, Lindahl A and Karlsson C: Novel markers of osteogenic
and adipogenic differentiation of human bone marrow stromal cells
identified using a quantitative proteomics approach. Stem Cell Res.
12:153–165. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29(e45)2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bacevic M, Brkovic B, Lambert F, Djukic L,
Petrovic N and Roganovic J: Leukocyte- and platelet-rich fibrin as
graft material improves microRNA-21 expression and decreases
oxidative stress in the calvarial defects of diabetic rabbits. Arch
Oral Biol. 102:231–237. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chen P and Liu B: Study on repair of
critical calvarial defects with
nano-hydroxyapatite/collagen/polylactic acid material compounded
recombinant human bone morphogenetic protein 2 in rabbits. Zhongguo
Xiu Fu Chong Jian Wai Ke Za Zhi. 21:1191–1195. 2007.PubMed/NCBI(In Chinese).
|
|
35
|
Durmus E, Celik I, Aydin MF, Yildirim G
and Sur E: Evaluation of the biocompatibility and osteoproductive
activity of ostrich eggshell powder in experimentally induced
calvarial defects in rabbits. J Biomed Mater Res B Appl Biomater.
86:82–89. 2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li G, Wang X, Cao J, Ju Z, Ma D, Liu Y and
Zhang J: Coculture of peripheral blood CD34+ cell and mesenchymal
stem cell sheets increase the formation of bone in calvarial
critical-size defects in rabbits. Br J Oral Maxillofac Surg.
52:134–139. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Swain LD, Cornet DA, Manwaring ME, Collins
B, Singh VK, Beniker D and Carnes DL: Negative pressure therapy
stimulates healing of critical-size calvarial defects in rabbits.
Bonekey Rep. 2(299)2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Tuusa SM, Peltola MJ, Tirri T, Puska MA,
Roytta M, Aho H, Sandholm J, Lassila LVJ and Vallittu PK:
Reconstruction of critical size calvarial bone defects in rabbits
with glass-fiber-reinforced composite with bioactive glass granule
coating. J Biomed Mater Res B Appl Biomater. 84:510–519.
2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang Z, Han L, Sun T, Wang W, Li X and Wu
B: Osteogenic and angiogenic lineage differentiated adipose-derived
stem cells for bone regeneration of calvarial defects in rabbits. J
Biomed Mater Res A. 109:538–550. 2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wang Z, Hu H, Li Z, Weng Y, Dai T, Zong C,
Liu Y and Liu B: Sheet of osteoblastic cells combined with
platelet-rich fibrin improves the formation of bone in
critical-size calvarial defects in rabbits. Br J Oral Maxillofac
Surg. 54:316–321. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kammerer PW, Lehnert M, Al-Nawas B, Kumar
VV, Hagmann S, Alshihri A, Frerich B and Veith M: Osseoconductivity
of a specific streptavidin-biotin-fibronectin surface coating of
biotinylated titanium implants-a rabbit animal study. Clin Implant
Dent Relat Res. 17 (Suppl 2):e601–e612. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chen Z, Wang L and Jiang H: The effect of
procyanidine crosslinking on the properties of the electrospun
gelatin membranes. Biofabrication. 4(035007)2012.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chen Z, Cao L, Wang L, Zhu H and Jiang H:
Effect of fiber structure on the properties of the electrospun
hybrid membranes composed of poly(ε-caprolactone) and gelatin. J
Appl Polymer Sci. 127:4225–4232. 2013.
|
|
44
|
McHugh J: Promising drug delivery system.
Nat Rev Rheumatol. 15(64)2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Amschler K, Erpenbeck L, Kruss S and Schon
MP: Nanoscale integrin ligand patterns determine melanoma cell
behavior. ACS Nano. 8:9113–9125. 2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Qian C, Zhu C, Yu W, Jiang X and Zhang F:
High-Fat diet/low-dose streptozotocin-induced type 2 diabetes in
rats impacts osteogenesis and wnt signaling in bone marrow stromal
cells. PLoS One. 10(e0136390)2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Seif S, Planz V and Windbergs M: Delivery
of therapeutic proteins using electrospun fibers-recent
developments and current challenges. Arch Pharm (Weinheim).
350(1700077)2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Yin L, Wang K, Lv X, Sun R, Yang S, Yang
Y, Liu Y, Liu J, Zhou J and Yu Z: The fabrication of an ICA-SF/PLCL
nanofibrous membrane by coaxial electrospinning and its effect on
bone regeneration in vitro and in vivo. Sci Rep.
7(8616)2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Bigi A, Cojazzi G, Panzavolta S, Rubini K
and Roveri N: Mechanical and thermal properties of gelatin films at
different degrees of glutaraldehyde crosslinking. Biomaterials.
22:763–768. 2001.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Yi H, Ur Rehman F, Zhao C, Liu B and He N:
Recent advances in nano scaffolds for bone repair. Bone Res.
4(16050)2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Gilbert TW, Stewart-Akers AM and Badylak
SF: A quantitative method for evaluating the degradation of
biologic scaffold materials. Biomaterials. 28:147–150.
2007.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Sung HJ, Meredith C, Johnson C and Galis
ZS: The effect of scaffold degradation rate on three-dimensional
cell growth and angiogenesis. Biomaterials. 25:5735–5742.
2004.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Alsberg E, Kong HJ, Hirano Y, Smith MK,
Albeiruti A and Mooney DJ: Regulating bone formation via controlled
scaffold degradation. J Dent Res. 82:903–908. 2003.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zhang B, Zhang PB, Wang ZL, Lyu ZW and Wu
H: Tissue-engineered composite scaffold of
poly(lactide-co-glycolide) and hydroxyapatite nanoparticles seeded
with autologous mesenchymal stem cells for bone regeneration. J
Zhejiang Univ Sci B. 18:963–976. 2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Gong YY, Xue JX, Zhang WJ, Zhou GD, Liu W
and Cao Y: A sandwich model for engineering cartilage with
acellular cartilage sheets and chondrocytes. Biomaterials.
32:2265–2273. 2011.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Yin L, Yang S, He M, Chang Y, Wang K, Zhu
Y, Liu Y, Chang Y and Yu Z: Physicochemical and biological
characteristics of BMP-2/IGF-1-loaded three-dimensional coaxial
electrospun fibrous membranes for bone defect repair. J Mater Sci
Mater Med. 28(94)2017.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Mi R, Liu Y, Chen X and Shao Z: Structure
and properties of various hybrids fabricated by silk nanofibrils
and nanohydroxyapatite. Nanoscale. 8:20096–20102. 2016.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Teo BK, Wong ST, Lim CK, Kung TY, Yap CH,
Ramagopal Y, Romer LH and Yim EKF: Nanotopography modulates
mechanotransduction of stem cells and induces differentiation
through focal adhesion kinase. ACS Nano. 7:4785–4798.
2013.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Stein D, Lee Y, Schmid MJ, Killpack B,
Genrich MA, Narayana N, Marx DB, Cullen DM and Reinhardt RA: Local
simvastatin effects on mandibular bone growth and inflammation. J
Periodontol. 76:1861–1870. 2005.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Khan H, Mafi P, Mafi R and Khan W: The
effects of ageing on differentiation and characterisation of human
mesenchymal stem cells. Curr Stem Cell Res Ther. 13:378–383.
2018.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Prall WC, Saller MM, Scheumaier A,
Tucholski T, Taha S, Bocker W and Polzer H: Proliferative and
osteogenic differentiation capacity of mesenchymal stromal cells:
Influence of harvesting site and donor age. Injury. 49:1504–1512.
2018.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Mendes SC, Tibbe JM, Veenhof M, Bakker K,
Both S, Platenburg PP, Oner FC, de Bruijn JD and van Blitterswijk
CA: Bone tissue-engineered implants using human bone marrow stromal
cells: Effect of culture conditions and donor age. Tissue Eng.
8:911–920. 2002.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Hu ML: Osteogenic differentiation ability
and related gene profiles of bone marrow mesenchymal stem cells
derived from different ages (unpublished PhD thesis). Tianjin
Medical University, 2019.
|
|
64
|
Choudhery MS, Khan M, Mahmood R, Mehmood
A, Khan SN and Riazuddin S: Bone marrow derived mesenchymal stem
cells from aged mice have reduced wound healing, angiogenesis,
proliferation and anti-apoptosis capabilities. Cell Biol Int.
36:747–753. 2012.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Fossett E, Khan WS, Pastides P and Adesida
AB: The effects of ageing on proliferation potential,
differentiation potential and cell surface characterisation of
human mesenchymal stem cells. Curr Stem Cell Res Ther. 7:282–286.
2012.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Stolzing A, Jones E, McGonagle D and Scutt
A: Age-related changes in human bone marrow-derived mesenchymal
stem cells: Consequences for cell therapies. Mech Ageing Dev.
129:163–173. 2008.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Aksoy C, Kaya FA, Kuskonmaz BB, Uckan D
and Severcan F: Structural investigation of donor age effect on
human bone marrow mesenchymal stem cells: FTIR spectroscopy and
imaging. Age (Dordr). 36(9691)2014.PubMed/NCBI View Article : Google Scholar
|