Introduction
It is now well established that platelet-endothelial
interactions not only contribute to atherogenesis in the late stage
of atherosclerosis (AS), which is characterized by plaque
destabilization and thrombus formation, but also participate in
earlier stages of plaque development, through platelet-mediated
inflammatory pathways (1). Although
a growing body of literature indicates that contrast-enhanced
ultrasound (CEU) with targeted microbubbles allows for the
detection of molecular and cellular events related to AS
inflammation and plaque vulnerability (2), few studies have been performed to
explore the role of activated platelets in the procession of
AS.
At sites of vascular injury, the initial adhesion of
platelets to the endothelium is mediated through interaction
between the glycoprotein-Ibα (GPIbα) subunit of the platelet
GPIb-IX-V complex and von Willebrand factor (vWF) (3). After binding to exposed subendothelial
collagen, vWF multimers undergo conformational change with exposure
of the A1 binding domain to GPIbα. This is regarded as the primary
adhesive mechanism, helping the platelet attach itself to the
target endothelium in the situation of high shear stress generated
by blood flow (4,5).
As the binding of the vWF-A1 domain to the platelet
GPIbα receptor is the primary adhesive mechanism for
platelet-endothelial interactions (6), it was hypothesized that a recombinant
protein with an amino acid sequence corresponding to the vWF-A1
domain would be an ideal target ligand in the preparation of
targeted microbubbles to activated platelets. In the present study,
targeted CEU microbubbles bearing the recombinant vWF-A1 domain
were prepared and their use to image platelets-endothelium
interaction and assess platelet-mediated inflammation were assessed
in flow-chamber experiments and in a murine model of AS.
Materials and methods
Microbubble preparation
Targeted microbubbles were prepared by surface
conjugation of biotinylated ligands, as previously described, using
a streptavidin bridge (7). Soluble
recombinant vWF-A1 domain (amino acids 1238-1481; U-Protein Express
BV) was biotinylated at its N terminus for conjugation to the
microbubble shell. For this purpose, a molar excess of EZ-Link™
NHS-Biotin (Thermo Fisher Scientific, Inc.) was reacted overnight
at 4˚C with the recombinant vWF-A1 domain in phosphate-buffered
saline (PBS, pH 8.0). The resulting bioconjugate was purified using
Slide-A-Lyzer Dialysis Cassettes (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. The concentration of
the recombinant vWF-A1 domain was determined using an automatic
amino acid analyzer (model no. L8900; Hitachi, Ltd.) according to
the manufacturer's instructions.
Biotinylated, lipid-shelled decafluorobutane
microbubbles were prepared by sonication of a gas-saturated aqueous
suspension of distearoylphosphatidylcholine (Avanti Polar Lipids,
Inc.), polyoxyethylene-40-stearate (Sigma-Aldrich; Merck KGaA) and
distearoylphosphatidylethanolamine-PEG (2000) biotin (Avanti Polar
Lipids, Inc.). The size and concentration were assessed by an
electrozone sensing cell counter (Multisizer III;
Beckman-Coulter,Inc.). Control microbubbles (Mbctrl)
were prepared by conjugating biotinylated rat IgG (cat. no. 553880;
κ isotype control antibody; BD Biosciences) to microbubbles.
Microbubbles targeted to platelets GPIbα (MbA1) were
prepared by conjugating biotinylated vWF-A1 to the shell surface.
As described in a previous study (8), biotinylated ligands in excess amounts
(50 µg per 1x108 microbubbles) were added to occupy all
binding sites on the shell of the microbubbles.
In in vitro flow chamber studies,
MbA1 and Mbctrl were labeled with
dioctadecyloxacarbocyanine (DiO) and
dioctadecyltetramethylindocarbocyanine (DiI) perchlorate
(Sigma-Aldrich; Merck KGaA), separately.
Flow chamber activated platelets
attachment studies Preparation of platelet-rich plasma
Blood samples were obtained from 20 random healthy
human volunteers (age range, 20-50 years; mean age, 35±10 years; 11
males; 9 females) at the Department of Ultrasound, Tongji Hospital
from March 2015 to May 2015. A standardized technique of double
centrifugation (9) was used to
prepare platelet-rich plasma (PRP). Automatic hemocyte analyzer
(model no. XS-800i; Sysmex Corporation) was used to count platelets
in PRP. The platelet recovery rate was the total amount of
platelets obtained in PRP compared to the number in whole blood.
PRP smears were stained with Wright Giemsa (10) to reveal the purity of platelet
suspensions. The present study was approved by Tongji Hospital
Ethics Committee and written informed consent was obtained from all
volunteers before the study.
Coating and activation of platelets. Parallel
plate culture dishes with a diameter of 35 mm were incubated with
collagen type I (100 µg/ml; Beijing Solarbio Science &
Technology Co., Ltd.) at 4˚C overnight and then blocked with bovine
serum albumin (5 mg/ml; Wuhan Servicebio Technology Co., Ltd.) at
room temperature for 1 h. PRP (platelet concentration,
46x106/ml) and platelet activator was added and
incubated at room temperature for at least 15 min. To prepare the
platelet activator, 1 ml of 10% calcium chloride was mixed with 500
U of bovine thrombin (Beijing Solarbio Science & Technology
Co., Ltd). Indirect immunofluorescence was performed using a rat
monoclonal primary antibody against mouse P-selectin (cat. no.
CD62P; BD Biosciences) and goat anti-rat Alexa Fluor 488 secondary
antibody (Invitrogen; Thermo Fisher Scientific, Inc.) to identify
activated platelets.
Flow chamber studies. In vitro flow
chamber studies were performed at room temperature without specific
CO2 conditions to test the binding of microbubbles to
activated platelets under different flow conditions. The parallel
plate culture dishes coated with activated platelets were mounted
on a parallel plate flow chamber (GlycoTech Corporation) with a
gasket thickness of 0.01 in and a channel width of 2.5 mm. To
maintain the consistency of the activated platelets on culture
dishes, PRP of the same concentration (46x106/ml) was
used in each condition. An aqueous suspension of MbA1
labeled with DiO (6x106/ml) or Mbctrl labeled
with DiI (6x106/ml) was drawn through the flow chamber
with an adjustable withdrawal pump to generate a flow shear stress
of 2.0, 4.0, 6.0 or 8.0 dynes/cm2, respectively. After 5
min of continuous infusion, freely circulating microbubbles in the
chamber were removed by a 2 min PBS rinse at a low shear stress of
0.1 dynes/cm2. The number of microbubbles adhered to
platelets were averaged from 10-15 randomly selected nonoverlapping
optical fields under a fluorescent microscope with a 40x objective.
Experiments were performed at least in triplicate for each
condition.
CEU molecular imaging Animal
models
The study was approved by the Animal Care and Use
Committee of Tongji Medical College, Huazhong University of Science
and Technology. A total of 20 male wild-type C57Bl/6 mice and 31
apolipoprotein E deficient (ApoE-/-) mice with
age-dependent atherosclerosis produced on a C57Bl/6 background were
studied at 8, 16 and 32 weeks of age (n=8-12 for each strain at
each age; Beijing Vital River Laboratory Animal Technology Co.,
Ltd.). Upon purchase, the mice were 4 weeks of age and weighed ~9
g. As a control, C57Bl/6 mice were fed on a chow diet and from 5
weeks of age onwards, while ApoE-/- mice were fed on a
hypercholesterolemic diet (HCD) containing 21% fat by weight, 0.15%
cholesterol, and 19.5% casein without sodium cholate. All mice were
fed with free food and water access, and were raised in the Animal
Experimental Center of Tongji Medical College, Huazhong University
of Science and Technology, which had a specifical pathogen free
barrier environment. Animals were housed at a temperature of
20-26˚C and a relative humidity of 40-70%, under a 10/14 h
light/dark cycle.
CEU molecular imaging. Mice were anesthetized
with inhaled isoflurane (1.5%). The jugular vein was cannulated for
microbubble administration. Imaging of the proximal ascending aorta
was performed with an ultrahigh frequency (25 MHz) mechanical
sector imaging system. CEU was performed with Contrast Pulse
Sequencing, which detects the nonlinear fundamental signal
component for microbubbles. At each injection, images were acquired
(MI 0.16) 8 min after intravenous injection of targeted or control
microbubbles (3x106/ml; 2.2±1.7 µm) performed in random
order. Microbubbles in the sector were then destroyed by increasing
the mechanical index to 1.0 for 0.5 sec. Subsequent
post-destruction images were acquired at a mechanical index of
0.16. In order to test the specificity of MbA1 to
activated platelets on the endothelium, in vivo blocking
experiments were performed with an additional group of 3
ApoE-/- mice at 32 weeks of age. CEU molecular imaging
of platelets with MbA1 and Mbctrl was
performed in these mice before and after administration of 1.5 µg/g
GpIbα antibody (cat. no. R300, Emfret Analytics GmbH & Co. KG)
by intraperitoneal injection, which can result in 95% platelet
immune depletion.
Image analysis. Signals from microbubbles
were quantitatively estimated by commercially available software
(Vevo 2100 imaging analysis software; FUJIFILM Visual Sonics,
Inc.). During CEU imaging, 8 min after intravenous injection of
microbubbles, the first 20 pre-destruction contrast frames were
used to derive the total quantity of microbubbles, including
retained and freely circulating microbubbles. To determine the
signal from retained microbubbles alone, all microbubbles in the
region of interest were destroyed and the subsequent 20
post-destruction contrast frames represented the freely circulating
microbubbles. Therefore, the signal from targeted retained
microbubbles was calculated by digitally subtracting 20 averaged
post-destruction contrast frames from 20 averaged pre-destruction
frames. Intensity was measured from a region-of-interest placed on
the aorta arch.
Histology
After imaging, the 10% neutral formalin
perfusion-fixed short-axis sections from the ascending aorta in
ApoE-/- mice were evaluated in all study groups. Masson
trichrome staining (11) was
performed for assessment of plaque morphometry and the severity of
atherosclerotic lesions. Immunohistochemistry for platelet
expression was performed with a rabbit polyclonal primary antibody
against GPIIb (cat. no. Ab63983; Abcam) and species-appropriate
Alexa Fluor-594 secondary antibody (Invitrogen; Thermo Fisher
Scientific, Inc.) was used.
Statistical analysis
Data were analyzed using SPSS (version 17.0; SPSS,
Inc.), all parameter data were tested for normality and homogeneity
of variance. The Shapiro Wilk test was used to verify whether
continuous variables met the normal distribution, and all the data
were presented as the mean ± SD. Comparisons of in vitro
microbubble adhesion at different shear conditions were performed
using one-way ANOVA method followed by Bonferroni multiple
comparison tests. Independent t-tests were used to compare the
number of attached microbubbles and the signal enhancements between
MbA1 and Mbctrl. Comparisons of the signal
enhancements in the different age cohorts within the same animal
group were made with one-way ANOVA and Bonferroni for multiple
comparison tests. P<0.05 was considered to be statistically
significant.
Results
Flow chamber activated platelets
attachment studies Preparation of PRP
PRP was prepared with a concentration of
399x109/l. The platelet recovery rate was 167.65%, which
exceeded 100%. Under microscopy, platelets with a high purity were
seen on PRP smears stained with Wright Giemsa (Fig. S1A).
Platelet activation. Under the microscope,
activated platelets were seen in a state of aggregation on the
parallel plate culture dishes (Figs.
S1B and S2A). Indirect
immunofluorescence revealed a CD62 positive green fluorescent
signal from activated platelets (Fig.
S2B).
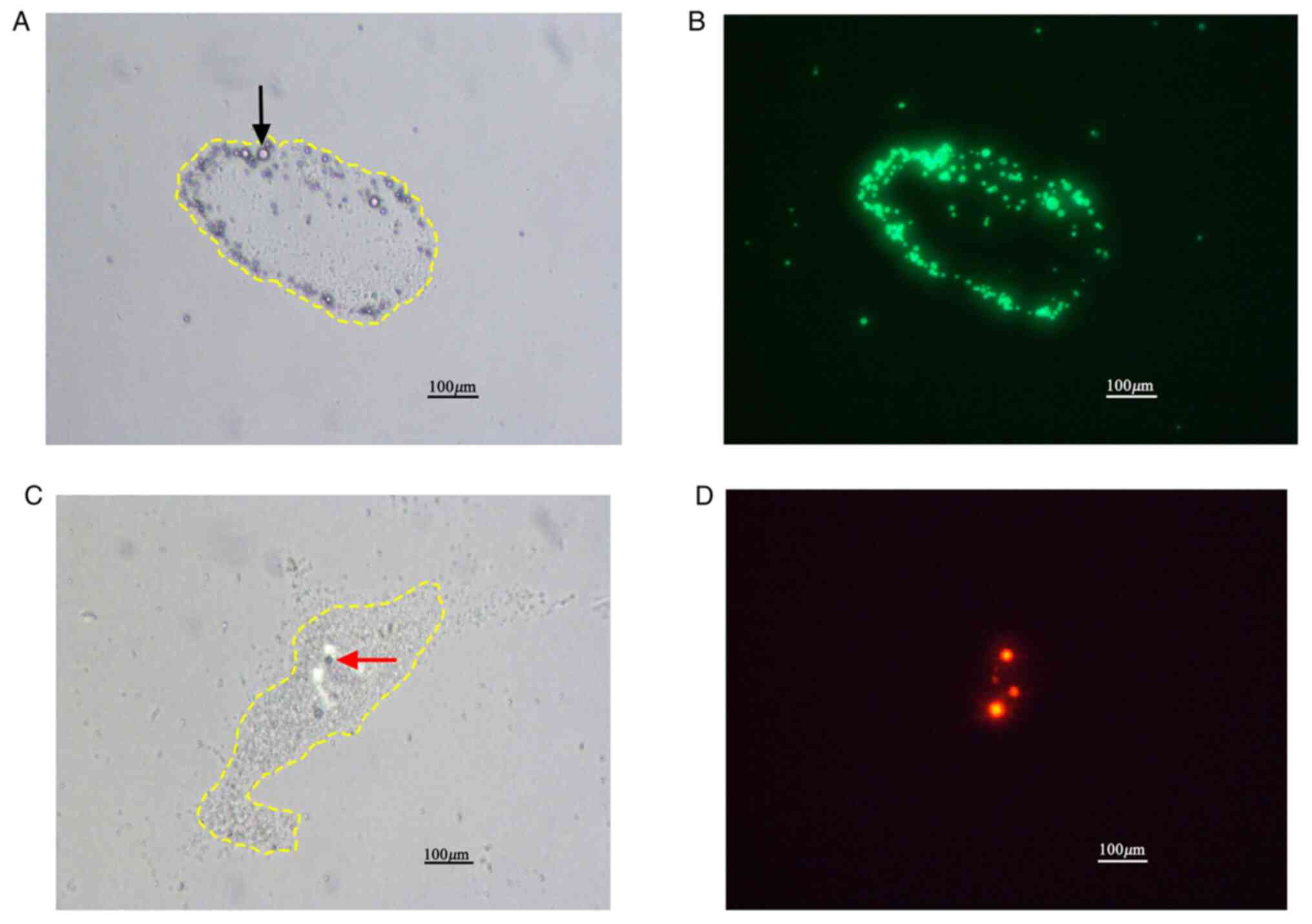
Flow chamber studies. Under light microscopy,
activated platelets coating the parallel plate culture dishes were
seen to be aggregated into irregular clusters (Fig. 1A and C). Under fluorescence microscopy, DiO
labeled MbA1 appeared selectively attached to activated
platelet aggregates (Fig. 1B; green
areas). However, DiI labeled Mbctrl (red areas), were
sparse and not related to the aggregated platelets (Fig. 1D), which indicated nonspecific
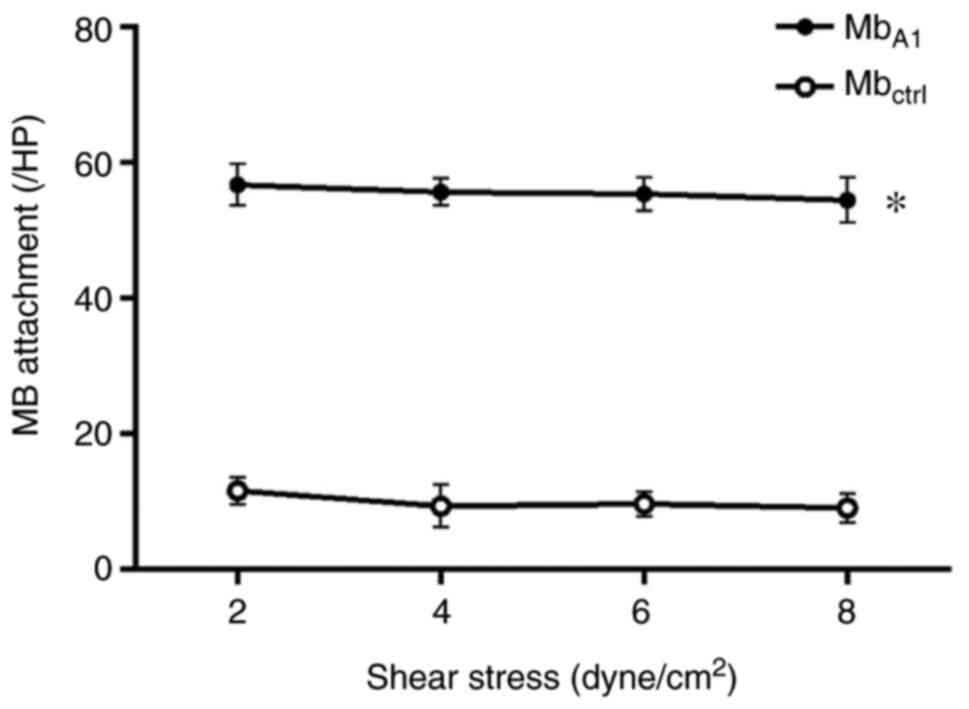
binding to the platelets. Across a range of shear stresses,
attachment of MbA1 to activated platelet aggregates was
significantly higher than that of Mbctrl lacking a
targeting ligand (P<0.05 at each shear stress; Fig. 2). With the shear stress increased
from 2.0 to 8.0 dynes/cm2, the number of attached
MbA1 remained constant (P>0.05; Fig. 2). There was no statistical
difference (P>0.05) in the quantity of attached
Mbctrl from 2.0 to 8.0 dynes/cm2.
Molecular imaging of platelet
adhesion
In ApoE-/- mice, CEU molecular imaging of
the proximal ascending aorta detected selective signal enhancement
from MbA1 compared to Mbctrl at 8, 16 and 32
weeks of age (Fig. 3). Imaging
signals from MbA1 increased from 8 to 32 weeks of age
(P<0.05). Signals from Mbctrl were low and similar
between groups. In C57Bl/6 mice, there was no statistical
difference in imaging signal between Mbctrl and
MbA1 (Fig. 3). After
in vivo platelet immune depletion in 3 ApoE-/-
mice at 32 weeks of age, the selective signal of MbA1
decreased significantly (P<0.05), from 10.71 before blocking to
2.2 after blocking.
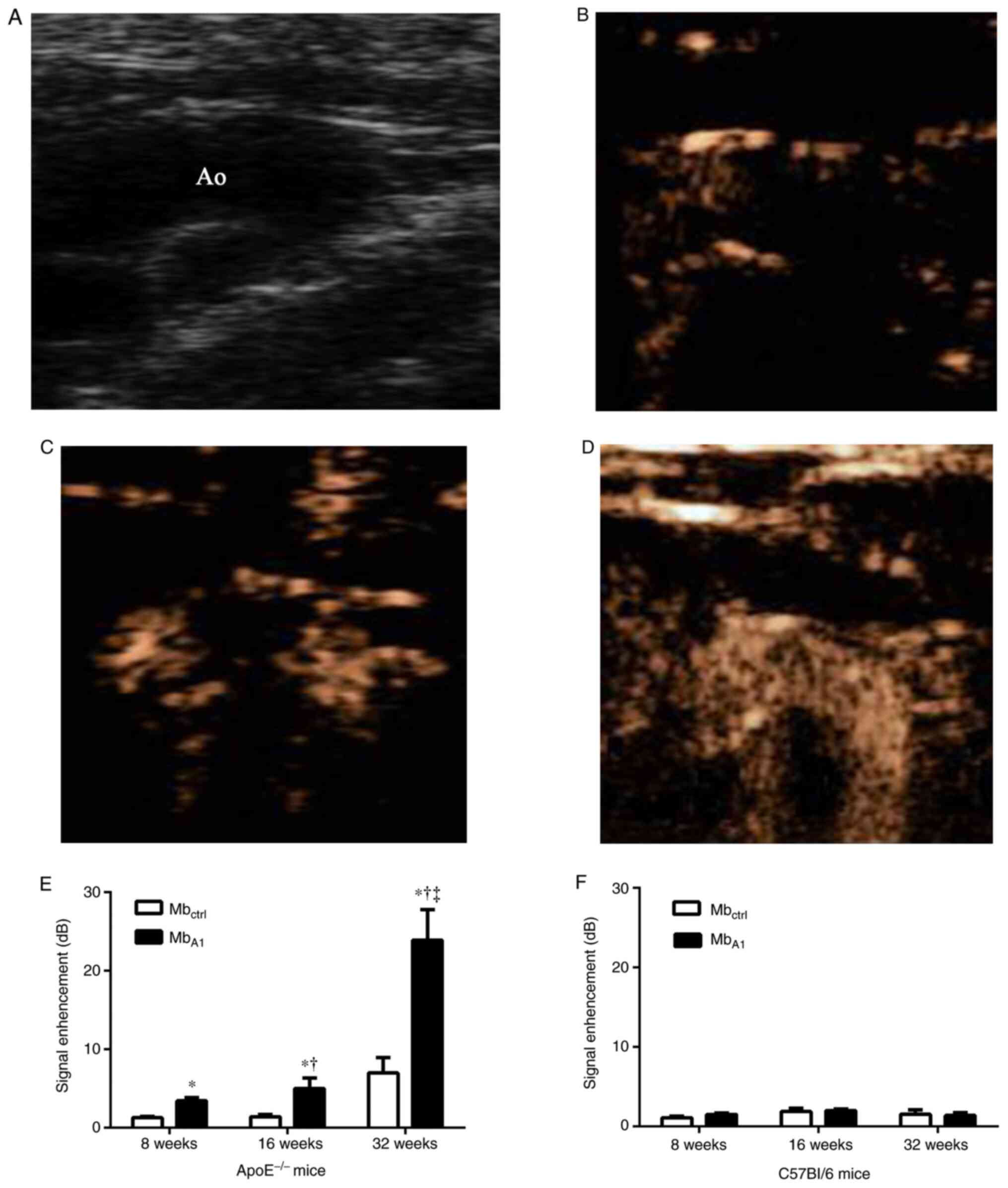 | Figure 3Examples of CEU molecular imaging of
the proximal aortic arch at various ages with microbubbles targeted
to platelets. (A) The proximal ascending aorta from
ApoE-/- mice. Selective signal enhancements from
MbA1 at (B) 8, (C) 16 and (D) 32 weeks of age,
separately. (E) In ApoE-/- mice, signal intensity from
MbA1 was stronger than that of Mbctrl at 8,
16, and 32 weeks of age, separately. Selective signal enhancement
from MbA1 can be detected at the early age of 8 weeks
and increased from 8 to 32 weeks of age in ApoE-/- mice.
MbA1 signals in ApoE-/- mice were greater
than that in C57Bl/6 mice at all time points. (F) In C57Bl/6 mice,
there was no statistical difference in CEU molecular imaging signal
between Mbctrl and MbA1. CEU,
contrast-enhanced ultrasound; ApoE, apolipoprotein E;
MbA1, microbubbles with the vWF-A1 domain conjugated to
the shell; Mbctrl; microbubbles with an isotype control
conjugated to the shell *P<0.05 vs.
Mbctrl, †P<0.05 vs. 8 weeks,
‡P<0.05 vs. 16 weeks. |
Immunohistochemistry
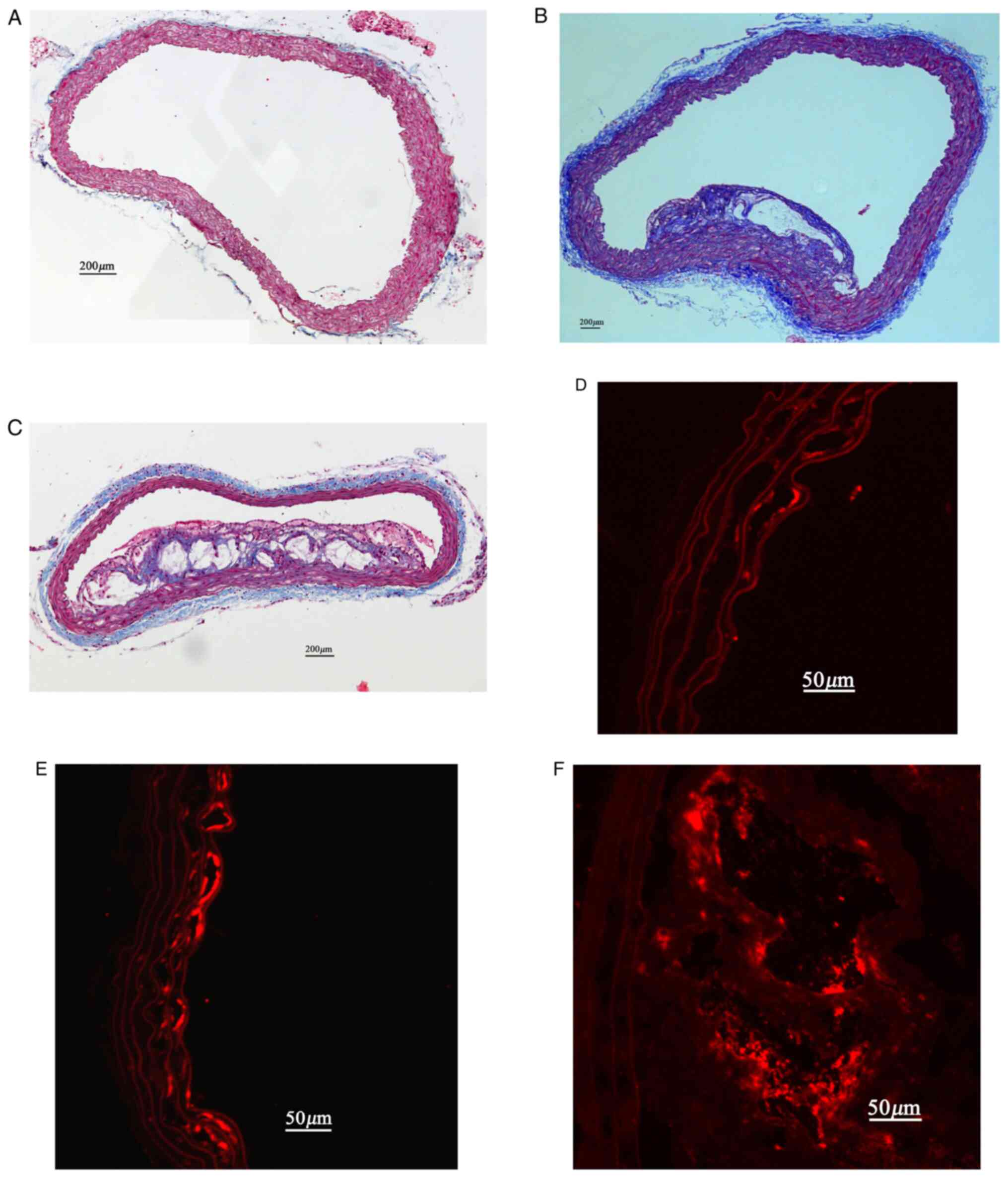
In ApoE-/- mice, the plaque lesions
progressed from 8-32 weeks. There was mild intimal thickening in
aorta sections at 8 weeks of age. Small but discrete fibrous
plaques were seen at 16 weeks of age. At 32 weeks of age, large
plaques with a lipid-rich core and a necros region were seen in all
the sections, and these lesions tended to protrude into the aortic
lumen (Fig. 4).
Immunohistochemistry for GPIIb revealed the presence of platelets
on the endothelial cell surface. In ApoE-/- mice,
minimal and local GPIIb expression were present on the intimal
surface of the aorta at 8 and 16 weeks of age (Fig. 4). With plaque progression, abundant
GPIIb expression was detected on the intimal surface and in
atherosclerotic lesions at 32 weeks of age (Fig. 4).
Discussion
Molecular imaging of inflammation with
ultrasonography has been achieved by surface modification of
microbubble contrast agents with ligands targeted to specific
molecular and cellular events (12). Kaufmann et al (13). successfully applied vascular cell
adhesion molecule-1 targeted microbubbles to detect vascular
inflammatory responses in apolipoprotein E deficiency mice with
atherosclerotic lesions. McCarty et al (14) used GPIbα antibody as a targeting
moiety and noninvasive molecular imaging could detect activated vWF
on the vascular endothelium, which further contributed to reveal an
advanced prothrombotic and inflammatory phenotype in
atherosclerotic disease.
It is widely accepted that platelets play a
significant role in thromboembolic complications of advanced
atherosclerotic lesions (15) and
research attention is focused om platelet involvement in the
formation of atherosclerotic lesions through platelet-endothelial
interactions (16). In the present
study, targeted CEU microbubbles bearing a recombinant vWF-A1
domain were prepared and their specific adhesion to activated
platelets in a model flow chamber system and in a murine model of
AS demonstrated. With these targeted microbubbles, the potent role
of activated platelets in the process of AS can be further explored
with CEU imaging.
Molecular imaging with CEU relies on the selective
targeting and retention of acoustically active contrast agents at
sites of disease (17). In the
production of targeted microbubbles for molecular imaging, a
typical strategy is to attach disease-specific ligands, including
monoclonal antibodies, peptides, glycoproteins and other small
molecules to the microbubble shell surface (18). A number of molecular imaging studies
have focused on the attachment of monoclonal antibodies on the
microbubbles because of their easy availability (19,20).
However, there have been difficulties concerning the firm adhesion
of microbubbles combined with antibodies under shear flow (21). The slow association kinetic
properties and the high molecular weight of antibodies could be the
reasons that reduce the capture efficiency on the target of
interest. In previously studies on platelet targeting, targeted
microbubbles were outfitted with monoclonal antibodies against
GPIIb/IIIa (3,6). Unfortunately, the specific adhesion of
these platelet targeted microbubbles was only observed under static
conditions or dynamic flow conditions with low shear stress.
An increasing number of more studies have focused on
preparation of targeted microbubbles with smaller peptide or
peptide mimetic ligand molecules (22-24).
Peptides, including polymeric sialyl Lewis X oligosaccharide
derivatives (23) and short
glycosulfopeptides (24), have been
confirmed to provide efficient microbubble targeting even in fast
flow. In the process of AS, platelet GPIbα plays a critical role in
platelet adhesion and aggregation under high-shear conditions
(7). GPIbα is a component of the
GPIb-V-IX complex and initiates platelet adhesion by binding to
collagen bound vWF (5). vWF is
synthesized by endothelial cells and has multiple A, C and D type
domains (25). In human blood flow,
when the shear stress exceeds 400 dynes/cm2, active A1
domains become exposed and can bind to GPIbα, acting as a hook for
the capture of platelets and contributing to platelet adhesion
under high vascular shear stress (26,27).
The pivotal role of vWF-platelet interaction in mediating platelet
adhesion under high-shear conditions indicated that the A1 domain
of vWF could be a potential ligand used to prepare microbubbles
targeted to platelets.
In the present study, a recombinant protein with an
amino acid sequence corresponding to the A1 domain of vWF was used
as the specific targeting ligand attached to the microbubble
surface. The present study results confirmed the specific
attachment of MbA1 to platelet aggregates under dynamic
flow chamber conditions.
Differing from previous flow chamber studies, in
which the specific attachment of targeted microbubbles conjugated
to antibodies decreased with increasing shear stress, the present
flow chamber experiment revealed that the number of MbA1
binding to platelet aggregates remained constant when shear stress
increased from 2.0 to 8.0 dynes/cm2. The present results
may provide insight into the high-strength interactions between
platelets and vWF-A1 domain under dynamic flow conditions. In a
study on a molten globule intermediate of the von Willebrand
factor, the A1 domain firmly tethers platelets under shear flow.
Tischer et al (28)
suggested that the mean platelet pause times increased from ~0.75
sec at a shear stress of nearly 2.0 dynes/cm2 to 0.9 sec
at 8.0 dynes/cm2, and then decreased again upon further
increase of the shear stress. Pause times determined the average
length of time a platelet was immobile, which has a complex
dependence on the shear stress (28). With high shear stress exposing the
vWF-A1 domain expressed on the endothelium, the active A1 domains
become firmly bound to platelets. This behavior is referred to as a
catch-slip interaction where the bond initially becomes weaker at
low force, strengthens at intermediate forces and weakens again at
higher forces (29,30), which could be the reason for the
stable interactions between MbA1 and platelets in the
conditions of increasing shear stress from 2.0 to 8.0
dynes/cm2. To the best of our knowledge, the targeted
microbubbles bearing vWF-A1 domain prepared in the present study
have higher affinities to platelets, which may produce favorable
binding kinetics of microbubbles under high shear forces.
Additionally, the vWF-A1 domain as a small molecular peptide is
less immunogenic in humans, which may provide a better safety
profile in future clinical application (31).
AS is a chronic inflammatory disease and various
complex processes contribute to its pathophysiology and the
development of the atherosclerotic plaque over decades (32,33).
While it is now well established that platelets play a critical
role in thrombotic complications of advanced AS, such as rupture of
the vulnerable plaque, a growing experimental literature has
established that platelets participate in the initiation of the
atherogenic process through platelet-endothelium interaction
(34). In vivo study of
labeled platelets indicated that substantial numbers of platelets
adhered to the carotid endothelium before the development of
manifest atherosclerotic lesions in ApoE-/- mice
(35). Consistent with these
studies, using CEU molecular imaging in the present study, the
presence of activated platelets on the vascular endothelium at the
early lesion-prone stage of AS in in vivo experiments was
confirmed. Moreover, there was also an age-dependent increase in
selective signal enhancement from microbubbles bearing the A1
domain, indicating a relationship between the degree of platelet
adhesion and disease severity. Platelets interacting with the
endothelium may influence the development and progression of AS
through a variety of mechanisms (36,37).
The intact nonactivated endothelium represents a
natural barrier preventing platelet adhesion to the extracellular
matrix. However, platelets can adhere directly to the intact but
activated endothelial cell monolayer via GPIb/P-selectin or
P-selectin glycoprotein ligand (PSGL)1/P-selectin (38). After contact between platelets and
the endothelium has been established, platelets are activated and
release proinflammatory cytokines and chemoattractants and express
CD40 ligand to further induce the proatherogenic phenotype of
endothelial cells (39,40). In this manner, adherent platelets
enhance the recruitment of leukocytes, progenitor cells and
dendritic cells to the vascular wall and tissues in the process for
AS (41). Platelets not only
promote an inflammatory response in leukocytes and endothelial
cells, but may also themselves respond to inflammatory mediators
produced by these cells. Studies have demonstrated that the
bidirectional interaction between platelets and leukocytes and
endothelial cells involves both inflammatory and prothrombotic
pathways, contributing to a pathogenic loop in AS and plaque
destabilization (37,42). Our application of CEU molecular
imaging to evaluate the biological process of activated platelets
in AS not only contribute to understanding the inflammatory role of
platelets in AS, also can be used to explore the effects of new
anti-platelets therapy in preventing AS.
The present study had several limitations. Firstly,
the selective attachment of targeted microbubbles to activated
platelets was explored in a parallel plate chamber. However, the
flow in parallel plate chamber may not accurately simulate
hemodynamics in the vessel to assess the binding efficiency of
microbubbles in vivo. Secondly, the specific attachment to
activated platelets was not directly compared between microbubbles
bearing the vWF-A1 domain and those bearing antibodies. However, in
the flow chamber studies with targeted microbubbles conjugated to
monoclonal antibodies, the specific attachment of the targeted
microbubbles decreased with increasing shear stress. Thirdly,
though CEU molecular imaging was used to assess the extent of
activated platelets in the process of atherogenesis, the underlying
mechanisms contributing to the inflammatory progress were not
explored in the present study.
Supplementary Material
PRP smears with Wright Giemsa
staining. (A) A large number of platelets presenting normal
morphology (black arrow) were observed on PRP smears. Several red
blood cells (white arrowhead) and neutrophils (black arrowhead)
were occasionally seen surrounding the platelets. (B) After coating
and activation of PRP, aggregated platelets (yellow arrow) and
collagen (red arrowhead) were observed on parallel plate culture
dishes. PRP, platelet rich plasma.
Identification of platelet activation.
(A) Aggregated platelets (black arrow) were observed on the
parallel plate culture dishes. (B) CD62 positive green fluorescent
signals were detected from activated platelets (white arrow).
Acknowledgements
Not applicable.
Funding
Funding: This research was supported by grants from the National
Science Foundation of China (grant no. 81371581) and from the
Natural Science Foundation of Hubei Province in China (grant no.
2019CFB691).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL, JT and HL designed the experiments and wrote the
manuscript. JT, YW, RS, YZ and JZ performed experiments and
analyzed data. YL, HL and YZ assessed all the raw data and
confirmed its authenticity and legitimacy. All authors discussed
the results and reviewed and approved the final manuscript.
Ethics approval and consent to
participate
All animal experimental procedures were approved by
the Animal Care and Use Committee of Tongji Medical College,
Huazhong University of Science and Technology (Wuhan, China).
Experiments involving human tissues were approved by Tongji
Hospital Ethics Committee and volunteers provided their informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lievens D and von Hundelshausen P:
Platelets in atherosclerosis. Thromb Haemost. 106:827–838.
2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Feinstein SB, Coll B, Staub D, Adam D,
Schinkel AF, ten Cate FJ and Thomenius K: Contrast enhanced
ultrasound imaging. J Nucl Cardiol. 17:106–115. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mendolicchio GL and Ruggeri ZM: New
perspectives on von Willebrand factor functions in hemostasis and
thrombosis. Semin Hematol. 42:5–14. 2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ruggeri ZM: Platelets in atherothrombosis.
Nat Med. 8:1227–1234. 2002.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Theilmeier G, Michiels C, Spaepen E, Vreys
I, Collen D, Vermylen J and Hoylaerts MF: Endothelial von
Willebrand factor recruits platelets to atherosclerosis-prone sites
in response to hypercholesterolemia. Blood. 99:4486–4493.
2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kumar RA, Dong JF, Thaggard JA, Cruz MA,
López JA and McIntire LV: Kinetics of GPIbalpha-vWF-A1 tether bond
under flow: Effect of GPIbalpha mutations on the association and
dissociation rates. Biophys J. 85:4099–4109. 2003.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sun R, Tian J, Zhang J, Wang L, Guo J and
Liu Y: Monitoring inflammation injuries in the progression of
atherosclerosis with contrast enhanced ultrasound molecular
imaging. PLoS One. 12(e0186155)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shim CY, Liu YN, Atkinson T, Xie A, Foster
T, Davidson BP, Treible M, Qi Y, López JA, Munday A, et al:
Molecular imaging of platelet-endothelial interactions and
endothelial von Willebrand factor in early and mid-stage
atherosclerosis. Circ Cardiovasc Imaging. 8(e002765)2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dhurat R and Sukesh M: Principles and
methods of preparation of platelet-rich plasma: A review and
author's perspective. J Cutan Aesthet Surg. 7:189–197.
2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dunning K and Safo AO: The ultimate
Wright-Giemsa stain: 60 years in the making. Biotech Histochem.
86:69–75. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gao M, Xin G, Qiu X, Wang Y and Liu G:
Establishment of a rat model with diet-induced coronary
atherosclerosis. J Biomed Res. 31:47–55. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Moccetti F, Weinkauf CC, Davidson BP,
Belcik JT, Marinelli ER, Unger E and Lindner JR: Ultrasound
molecular imaging of atherosclerosis using small-peptide targeting
ligands against endothelial markers of inflammation and oxidative
stress. Ultrasound Med Biol. 44:1155–1163. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kaufmann BA, Sanders JM, Davis C, Xie A,
Aldred P, Sarembock IJ and Lindner JR: Molecular imaging of
inflammation in atherosclerosis with targeted ultrasound detection
of vascular cell adhesion molecule-1. Circulation. 116:276–284.
2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
McCarty OJ, Conley RB, Shentu W, Tormoen
GW, Zha D, Xie A, Qi Y, Zhao Y, Carr C, Belcik T, et al: Molecular
imaging of activated von Willebrand factor to detect high-risk
atherosclerotic phenotype. JACC Cardiovasc Imaging. 3:947–955.
2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Coenen DM, Mastenbroek TG and Cosemans
JMEM: Platelet interaction with activated endothelium: Mechanistic
insights from microfluidics. Blood. 130:2819–2828. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Behm CZ, Kaufmann BA, Carr C, Lankford M,
Sanders JM, Rose CE, Kaul S and Lindner JR: Molecular imaging of
endothelial vascular cell adhesion molecule-1 expression and
inflammatory cell recruitment during vasculogenesis and
ischemia-mediated arteriogenesis. Circulation. 117:2902–2911.
2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Myrset AH, Fjerdingstad HB, Bendiksen R,
Arbo BE, Bjerke RM, Johansen JH, Kulseth MA and Skurtveit R: Design
and characterization of targeted ultrasound microbubbles for
diagnostic use. Ultrasound Med Biol. 37:136–150. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Piedra M, Allroggen A and Lindner JR:
Molecular imaging with targeted contrast ultrasound. Cerebrovasc
Dis. 27 (Suppl 2):66–74. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
von Zur Muhlen C, von Elverfeldt D,
Choudhury RP, Ender J, Ahrens I, Schwarz M, Hennig J, Bode C and
Peter K: Functionalized magnetic resonance contrast agent
selectively binds to glycoprotein IIb/IIIa on activated human
platelets under flow conditions and is detectable at clinically
relevant field strengths. Mol Imaging. 7:59–67. 2008.PubMed/NCBI
|
|
20
|
Yan F, Sun Y, Mao Y, Wu M, Deng Z, Li S,
Liu X, Xue L and Zheng H: Ultrasound Molecular Imaging of
Atherosclerosis for Early Diagnosis and Therapeutic Evaluation
through Leucocyte-like Multiple Targeted Microbubbles.
Theranostics. 8:1879–1891. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Guenther F, von zur Muhlen C, Ferrante EA,
Grundmann S, Bode C and Klibanov AL: An ultrasound contrast agent
targeted to P-selectin detects activated platelets at
supra-arterial shear flow conditions. Invest Radiol. 45:586–591.
2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pochon S, Tardy I, Bussat P, Bettinger T,
Brochot J, von Wronski M, Passantino L and Schneider M: BR55: A
lipopeptide-based VEGFR2-targeted ultrasound contrast agent for
molecular imaging of angiogenesis. Invest Radiol. 45:89–95.
2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Klibanov AL, Rychak JJ, Yang WC, Alikhani
S, Li B, Acton S, Lindner JR, Ley K and Kaul S: Targeted ultrasound
contrast agent for molecular imaging of inflammation in high-shear
flow. Contrast Media Mol Imaging. 1:259–266. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rychak JJ, Li B, Acton ST, Leppänen A,
Cummings RD, Ley K and Klibanov AL: Selectin ligands promote
ultrasound contrast agent adhesion under shear flow. Mol Pharm.
3:516–524. 2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ju L, Chen Y, Zhou F, Lu H, Cruz MA and
Zhu C: Von Willebrand factor-A1 domain binds platelet glycoprotein
Ibα in multiple states with distinctive force-dependent
dissociation kinetics. Thromb Res. 136:606–612. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ruggeri ZM, Orje JN, Habermann R, Federici
AB and Reininger AJ: Activation-independent platelet adhesion and
aggregation under elevated shear stress. Blood. 108:1903–1910.
2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ruggeri ZM: Von Willebrand factor,
platelets and endothelial cell interactions. J Thromb Haemost.
1:1335–1342. 2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tischer A, Madde P, Blancas-Mejia LM and
Auton M: A molten globule intermediate of the von Willebrand factor
A1 domain firmly tethers platelets under shear flow. Proteins.
82:867–878. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Thomas W: Catch bonds in adhesion. Annu
Rev Biomed Eng. 10:39–57. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Thomas WE, Vogel V and Sokurenko E:
Biophysics of catch bonds. Annu Rev Biophys. 37:399–416.
2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Inaba Y and Lindner JR: Molecular imaging
of disease with targeted contrast ultrasound imaging. Transl Res.
159:140–148. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hansson GK: Atherosclerosis - an immune
disease: The Anitschkov Lecture 2007. Atherosclerosis. 202:2–10.
2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Davis NE: Atherosclerosis--an inflammatory
process. J Insur Med. 37:72–75. 2005.PubMed/NCBI
|
|
34
|
Hamilos M, Petousis S and Parthenakis F:
Interaction between platelets and endothelium: From pathophysiology
to new therapeutic options. Cardiovasc Diagn Ther. 8:568–580.
2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Massberg S, Brand K, Grüner S, Page S,
Müller E, Müller I, Bergmeier W, Richter T, Lorenz M, Konrad I, et
al: A critical role of platelet adhesion in the initiation of
atherosclerotic lesion formation. J Exp Med. 196:887–896.
2002.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kim H and Conway EM: Platelets and
Complement Cross-Talk in Early Atherogenesis. Front Cardiovasc Med.
6(131)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bakogiannis C, Sachse M, Stamatelopoulos K
and Stellos K: Platelet-derived chemokines in inflammation and
atherosclerosis. Cytokine. 122(154157)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Schulz C, Schäfer A, Stolla M, Kerstan S,
Lorenz M, von Brühl M-L, Schiemann M, Bauersachs J, Gloe T, Busch
DH, et al: Chemokine Fractalkine Mediates Leukocyte Recruitment to
Inflammatory Endothelial Cells in Flowing Whole Blood. Circulation.
116:764–773. 2007.PubMed/NCBI View Article : Google Scholar
|
|
39
|
King SM, McNamee RA, Houng AK, Patel R,
Brands M and Reed GL: Platelet dense-granule secretion plays a
critical role in thrombosis and subsequent vascular remodeling in
atherosclerotic mice. Circulation. 120:785–791. 2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Aidoudi S and Bikfalvi A: Interaction of
PF4 (CXCL4) with the vasculature: A role in atherosclerosis and
angiogenesis. Thromb Haemost. 104:941–948. 2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Alexandru N, Andrei E, Dragan E and
Georgescu A: Interaction of platelets with endothelial progenitor
cells in the experimental atherosclerosis: Role of transplanted
endothelial progenitor cells and platelet microparticles. Biol
Cell. 107:189–204. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Aukrust P, Halvorsen B, Ueland T,
Michelsen AE, Skjelland M, Gullestad L, Yndestad A and Otterdal K:
Activated platelets and atherosclerosis. Expert Rev Cardiovasc
Ther. 8:1297–1307. 2010.PubMed/NCBI View Article : Google Scholar
|