Introduction
Pulmonary fibrosis is a chronic interstitial lung
disease that can destroy the normal architecture of lung tissues
and lead to progressive respiratory dysfunction (1). Fibroblasts are the major effector
cells during pulmonary fibrosis due to their ability to
differentiate into myofibroblasts, which can synthesize excess
extracellular matrix (ECM) proteins, resulting in excessive ECM
deposition and pulmonary fibrosis (2). The key difference between quiescent
fibroblasts and activated fibroblasts (myofibroblasts) manifests as
aberrant proliferative capacity and increased production of
α-smooth muscle actin (α-SMA) and ECM proteins, such as type I
collagen (collagen I), type III collagen (collagen III), connective
tissue growth factor (CTGF) and fibronectin, when fibroblasts are
activated (3). Therefore, targeting
fibroblast activation may serve as a therapeutic strategy for
pulmonary fibrosis.
The transforming growth factor-β (TGF-β) family,
including TGF-β, bone morphogenetic proteins (BMPs) and activins,
is involved in the development of fibrotic diseases (4-6).
TGF-β1 is widely considered as the primary cytokine responsible for
the induction of fibroblast activation (7); however, the precise functions of other
BMPs, such as BMP9, in activating fibroblasts and fibrosis
development are not completely understood.
BMP9 was originally identified in liver tissues and
considered as an important participant of several biological
functions, including hepatocyte proliferation, chondrogenesis,
angiogenesis, glucose metabolism and neuronal differentiation
(8-12).
BMP9 primarily binds to its high affinity receptor activin
receptor-like kinase 1 (ALK1) to phosphorylate Smad1/5 and regulate
gene transcription (13,14). In addition, non-Smad signaling
proteins, such as mitogen-activated protein kinases, PI3K and
c-met, can also be activated by BMP9 (15,16).
The BMP9/ALK1 signaling pathway is involved in the angiogenesis and
metastasis of tumors (17-19).
For instance, exogenous BMP9 can significantly inhibit breast
cancer MDA-MB-231 cell invasion and migration in vitro and
in vivo (19). However, BMP9
is overexpressed in hepatocellular carcinoma tissues, and can
induce epithelial-mesenchymal transition and promote hepatocellular
carcinoma metastasis (20). BMP9
also induces proliferation in multiple types of endothelial cells
in vitro and promotes tumor angiogenesis and metastasis in a
xenograft model of pancreatic cancer (21).
It has been reported that BMP9 is a vital cytokine
that regulates the activation of fibroblasts and development of
fibrosis (9,22-26).
Increasing studies have indicated that BMP9 has a bidirectional
response to fibroblast activation and fibrotic diseases (9,22-26).
Breitkopf-Heinlein et al (9)
indicated that BMP9 serves as a profibrogenic cytokine in hepatic
tissues. Selective inhibition or deletion of BMP9 in mice results
in reduced liver fibrosis compared with the control group, and BMP9
overexpression promotes hepatic stellate cell proliferation and
migration, which are the major fibroblast-type cells involved in
hepatic fibrosis (9). Moreover, Li
et al (22) demonstrated a
positive correlation between serum levels of BMP9 and the stage of
fibrosis in 52 patients, suggesting that BMP9 might serve as an
early diagnostic marker for hepatic fibrosis (22). BMP9 also stimulates mouse embryo
fibroblast activation and increases the expression of fibronectin
and collagen I by triggering Smad1/5 signaling (23). By contrast, other studies have
identified an inhibitory role of BMP9 and its receptor ALK1 during
fibrogenesis. For instance, Morine et al (24) observed that BMP9 is expressed by
cardiac fibroblasts, but its expression is upregulated in the
circulation and left ventricle of human patients with heart failure
(24). Furthermore, BMP9 decreases
the synthesis of collagen I in cardiac fibroblasts, and loss of
BMP9 activity increases cardiac fibrosis by inhibiting Smad1
phosphorylation (24). Meanwhile,
ALK1 has been reported to suppress cardiac fibrosis by maintaining
Smad1 activity in a mouse model of heart failure (25). Moreover, reduced levels of ALK1 have
also been reported to accelerate heart failure without increasing
markers of cardiac fibrosis, such as collagen I, CTGF or
plasminogen activator inhibitor 1(26). Therefore, the contribution of BMP9
to fibroblast activation and fibrogenesis requires further
investigation. Certain strategies targeting BMP9 have been applied
in the preclinical treatment of fibrotic diseases and have
displayed positive effects (9,16,22,27).
For example, mice treated with BMP9-short hairpin RNA and
monoclonal antibodies display limited liver fibrosis (27).
At present, there is no direct evidence that BMP9
regulates pulmonary fibrosis. However, several studies have
indicated that BMP9 serves a pivotal role in several chronic lung
diseases related to fibrosis, such as bronchopulmonary dysplasia
and pulmonary arterial hypertension (28,29).
In the present study, the role of BMP9 in regulating human lung
fibroblast proliferation and differentiation, as well as underlying
signaling pathway, were investigated.
Materials and methods
Cell culture
Human fetal lung fibroblasts HFL-1 (The Cell Bank of
Type Culture Collection of the Chinese Academy of Sciences) were
cultured in DMEM (HyClone; Cytiva) supplemented with 6% FBS
(HyClone; Cytiva) and penicillin/streptomycin solution (Beijing
Solarbio Science & Technology Co., Ltd.) at 37˚C in a
humidified incubator with 5% CO2. Cells in the
exponential growth phase were used for subsequent experiments. For
the experiment of rBMP9 treatment, DMEM without rBMP9 is used as
control. For the experiment of rBMP9 combined with dorsomorphin-1
(Dor), DMEM with 0.1% dimethyl sulfoxide is used as control.
Cell viability assay
A Cell Counting Kit-8 (CCK-8) assay (Beijing
Transgen Biotech Co., Ltd.) was used to detect cell viability.
Briefly, HFL-1 cells (1x103) were cultured in a 96-well
plate for 24 h and starved in DMEM with 0.1% FBS at 37˚Covernight.
Subsequently, cells were treated with fresh DMEM containing: i)
Different concentrations of recombinant BMP9 (rBMP9; 0, 2, 10 or 50
ng/ml; BioLegend, Inc.) at 37˚C for 24, 48 or 72 h; or ii) 50 ng/ml
rBMP9 with different concentration of Dor (0, 0.1, 0.5 or 1 µM;
MedChemExpress LLC), a BMP type I receptor ALK1 inhibitor, at 37˚C
for 48 h. Subsequently, 10 µl CCK-8 solution was added to each
well. After incubation at 37˚C for 30 min, the absorbance of each
well was measured at a wavelength of 450 nm using a multi-mode
microplate reader (Molecular Devices, LLC). The absorbance value
recorded was directly proportional to the number of viable
cells.
Colony formation assay
Briefly, HFL-1 cells (1x103) were
subcultured in 6-cm dishes at 37˚C for 24 h. Subsequently, cells
were treated with fresh DMEM containing different concentrations of
rBMP9 (0, 2, 10 or 50 ng/ml) in the presence or absence of 0.1 µM
Dor at 37˚C. The culture medium was replaced every 3 days.
Following incubation for 10 days, cells were fixed with 4%
paraformaldehyde at room temperature for 20 min and stained with 1%
crystal violet (Beijing Solarbio Science & Technology Co.,
Ltd.) at room temperature for 15 min. The images of 6-cm dish were
obtained by Scanner. The number of colonies (the cell clusters
>0.5 mm is defined as a colony) in each 6-cm dish was calculated
using ImageJ software (version 1.80; National Institutes of
Health).
Reverse transcription-quantitative PCR
(RT-qPCR)
Following incubation with 50 ng/ml rBMP9 and/or 0.1
µM Dor for 48 h, HFL-1 cells (5x106) in a 6-cm dish were
washed twice with ice-cold PBS. Total RNA was extracted from cells
using TRIzol® reagent (Beijing Transgen Biotech Co.,
Ltd.) according to the manufacturer's protocol. Total RNA was
reverse transcribed to cDNA using the Reverse Transcription System
(cat. no. A3500; Promega Corporation). The temperature protocol for
reverse transcription was 42˚C for 15 min, 95˚C for 5 min and 4˚C
for 5 min. Subsequently, qPCR was performed using the SYBR Green
kit (Beijing Transgen Biotech Co., Ltd.) and an ABI Prism 7500
Sequence Detection System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The following thermocycling conditions were used
for qPCR: Initial denaturation for 10 min at 95˚C; followed by 35
cycles of denaturation at 95˚C for 15 sec, annealing at 60˚C for 30
sec and extension at 72˚C for 10 sec. The sequences of the primers
used for qPCR are listed in Table
I. mRNA expression levels were quantified using the
2-∆∆Cq method (30) and
normalized to the internal reference gene GAPDH.
 | Table ISequences of the primers used for
reverse transcription-quantitative PCR. |
Table I
Sequences of the primers used for
reverse transcription-quantitative PCR.
| Gene | Sequence
(5'-3') |
|---|
| α-SMA | F:
AGCGTGGCTATTCCTTCGT |
| | R:
CTCATTTTCAAAGTCCAGAGCTACA |
| Collagen I | F:
AACCAAGGCTGCAACCTGGA |
| | R:
GGCTGAGTAGGGTACACGCAGG |
| Collagen III | F:
CTCCTGGGATTAATGGTAGT |
| | R:
CCAGGAGCTCCAGGAAT |
| GAPDH | F:
AGAAGGCTGGGGCTCATTTG |
| | R:
AGGGGCCATCCACAGTCTTC |
Western blotting
Briefly, HFL-1 cells (5x106) were
cultured in 6-cm dish with different concentrations of rBMP9 (0, 2,
10 or 50 ng/ml) or 0.5 µM Dor at 37˚C for 24 or 48 h. Cells were
then washed twice with ice-cold PBS. Total protein was extracted
from cells using RIPA lysis buffer (cat. no. P0013B; Beyotime
Institute of Biotechnology) on ice. Cell debris was discarded by
centrifugation at 12,000 x g at 4˚C for 5 min. Total protein was
quantified using a Bicinchoninic Acid Protein Assay kit (cat. no.
P0010; Beyotime Institute of Biotechnology). Proteins (40 µg/lane)
were separated via 8-10% SDS-PAGE and transferred to nitrocellulose
membranes (EMD Millipore). The membranes were blocked with 5%
non-fat milk for 1 h at room temperature. Subsequently, the
membranes were incubated overnight at 4˚C with primary antibodies
targeted against: ALK1 (1:500; cat. no. DF3170; Affinity
Biosciences), collagen I (1:500; cat. no. AF7001; Affinity
Biosciences), collagen III (1:500; cat. no. AF5457; Affinity
Biosciences), α-SMA (1:1,000; cat. no. ab124964; Abcam),
phosphorylated (p)-Smad1/5 (1:1,000; cat. no. CST9516; Cell
Signaling Technology, Inc.), total-Smad1/5 (1:1,000; cat. no.
CST6944; Cell Signaling Technology, Inc.) and GAPDH (1:1,000; cat.
no. T0004; Affinity Biosciences). Following primary incubation, the
membranes were incubated with horseradish peroxidase-conjugated
secondary antibodies (1:5,000; cat. nos. S0001 and S0002; Affinity
Biosciences) for 2 h at room temperature. Protein bands were
visualized using an enhanced chemiluminescence kit (EMD Millipore).
Protein expression was quantified using ImageJ software (version
1.80; National Institutes of Health) with GAPDH as the loading
control (31).
Statistical analysis
Data are presented as the mean ± standard deviation
from at least three independent experiments. Statistical analyses
were performed using GraphPad Prism software (version 5; GraphPad
Software, Inc.). Comparisons between two groups were analyzed using
the unpaired Student's t-test. Comparisons among multiple groups
were analyzed using one-way ANOVA followed by Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
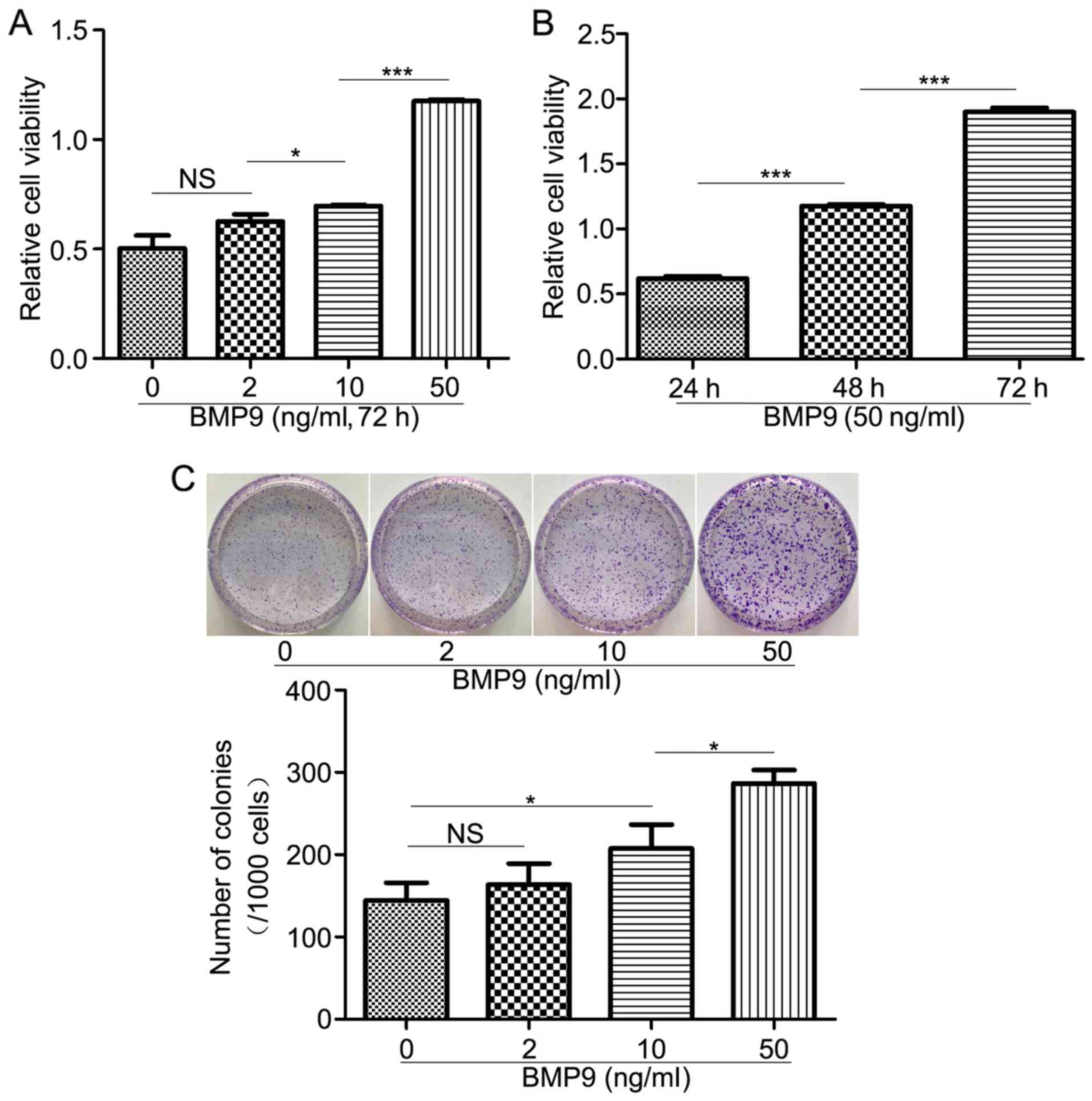
BMP9 increases HFL-1 cell
proliferation
Abnormal proliferation is a characteristic of lung
fibroblast activation (32).
Therefore, to determine whether BMP9 altered the proliferation of
HFL-1 cells, the CCK-8 assay was performed to assess HFL-1 cell
proliferation following treatment with BMP9. BMP9 enhanced HFL-1
cell proliferation in a concentration- and time-dependent manner
(Fig. 1A and B). Similarly, the colony formation assay
suggested that BMP9 increased colony formation in a dose-dependent
manner (Fig. 1C). The results
indicated that BMP9 stimulation promoted HFL-1 cell
proliferation.
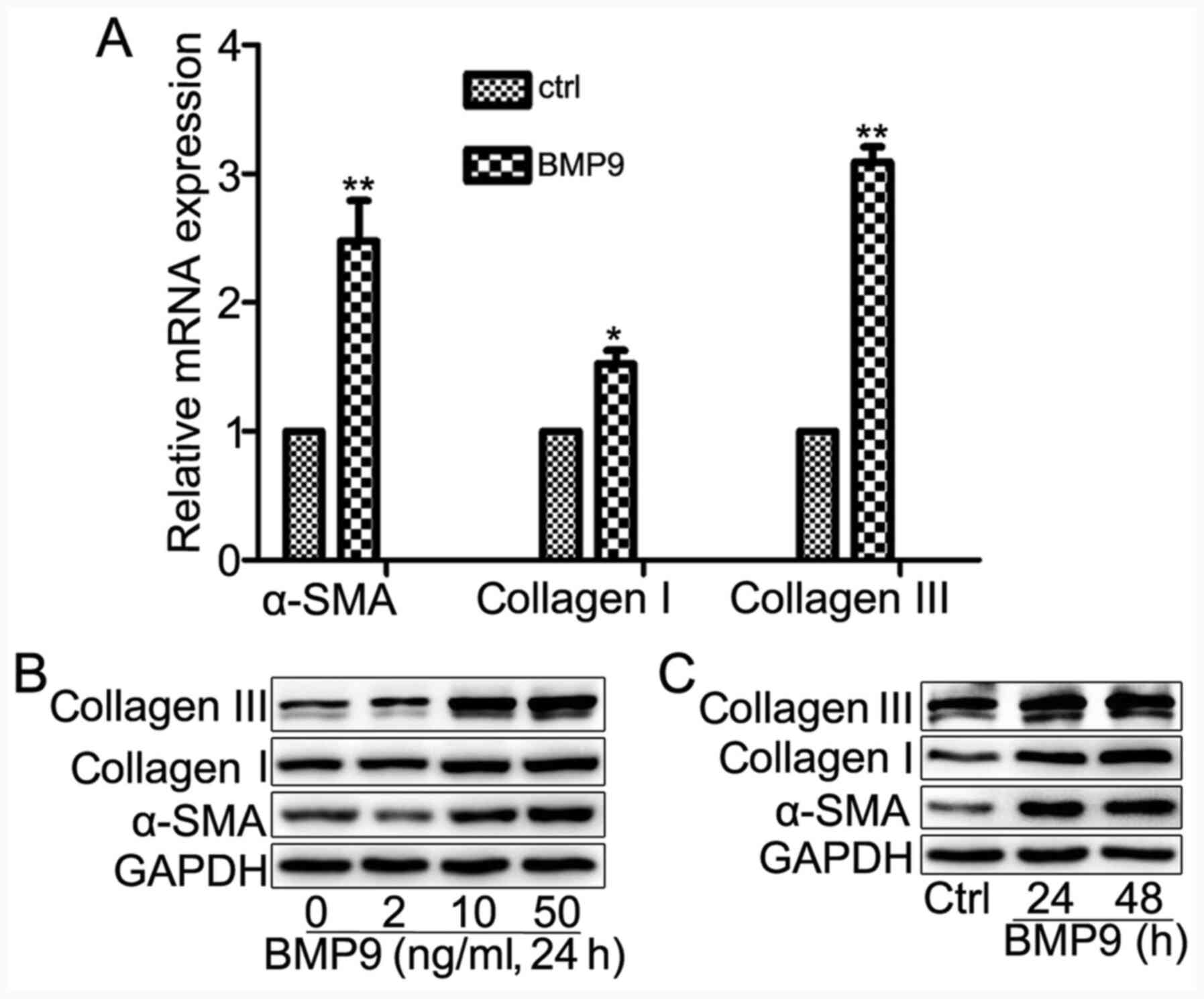
BMP9 induces HFL-1 cell
differentiation
To investigate whether BMP9 served an important role
in lung fibroblast activation, the effect of BMP9 on HFL-1 cell
transdifferentiation was investigated. Firstly, the mRNA expression
levels of myofibroblast marker α-SMA, and ECM markers collagen I
and collagen III were assessed. The results indicated that BMP9
significantly increased the expression levels of α-SMA, collagen I
and collagen III in HFL-1 cells compared with the control group
(Fig. 2A). Furthermore, the protein
expression levels of collagen I, collagen III and α-SMA were
increased in a dose- and time-dependent manner by BMP9 treatment
(Fig. 2B and C). The results indicated that BMP9 induced
HFL-1 cell transdifferentiation and increased HFL-1 cell ECM
protein synthesis.
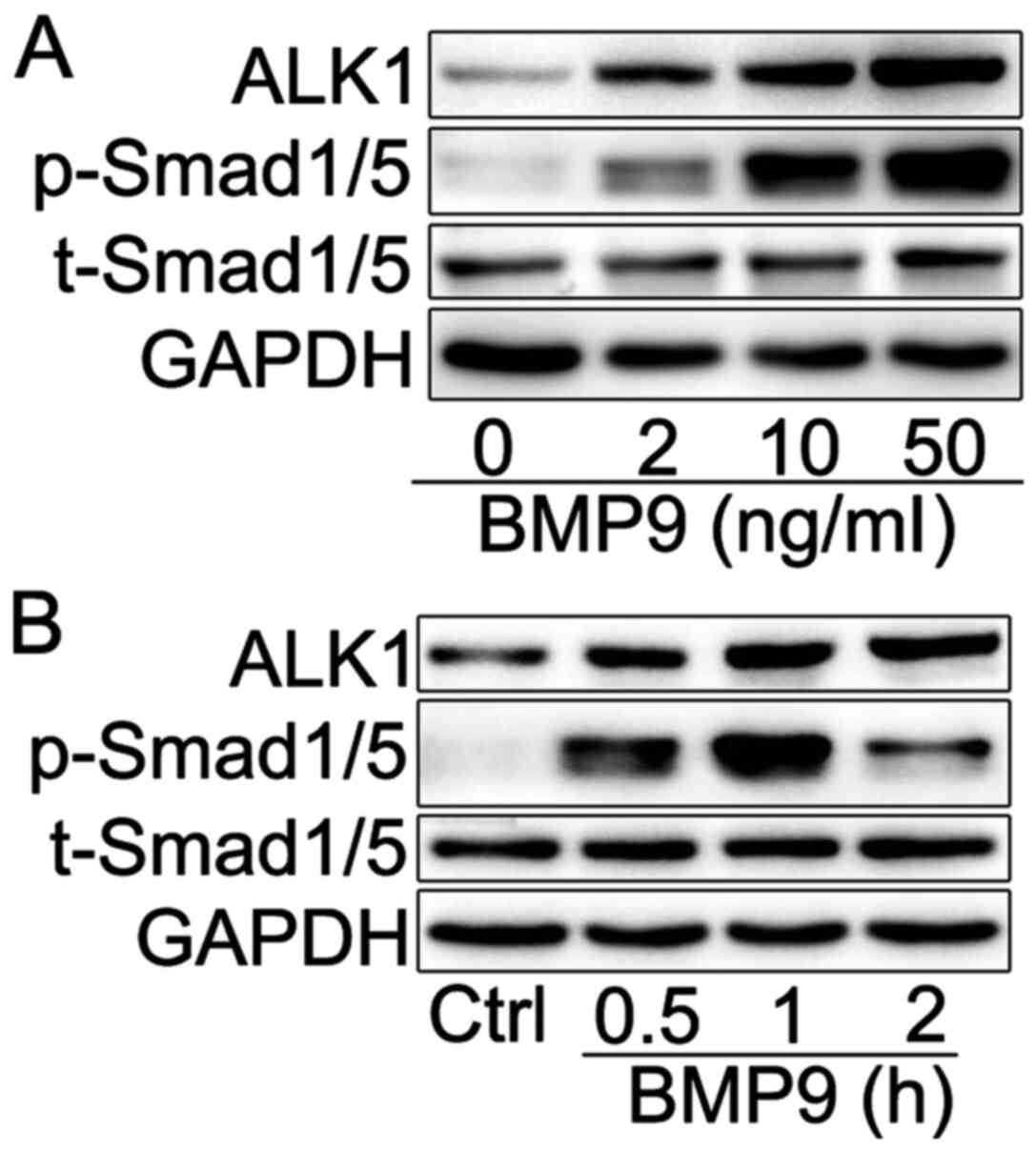
BMP9 activates Smad1/5 signaling in
HFL-1 cells
In the majority of cell types, BMP9 activates
Smad1/5 to regulate its biological function via ALK1, a receptor
with high affinity to BMP9 (13,14).
To assess whether BMP9 promoted HFL-1 cell proliferation and
differentiation via ALK1/Smad1/5 signaling, the expression levels
of ALK1 expression and p-Smad1/5 were assessed via western
blotting. BMP9 increased the expression levels of ALK1 and
p-Smad1/5 in a dose-dependent manner. At 10 ng/ml, BMP9 increased
the expression levels of ALK1 and p-Smad1/5 in a time-dependent
manner (Fig. 3B), reaching a
maximal level at the 1 h time point. In addition, the expression
level of p-Smad1/5 decreased when HFL-1 cells were exposed to BMP9
for 2 h compared with 1 h, which may be associated with the rapid
turnover of p-Smad1/5 in HFL-1 cells. Lo et al (33) suggested that once Smad proteins
enter the nucleus, they might be degraded by ubiquitination.
Therefore, the results suggested that BMP9 might trigger Smad1/5
activation via ALK1 in HFL-1 cells, which may serve as a mechanism
underlying BMP9-induced HFL-1 cell activation.
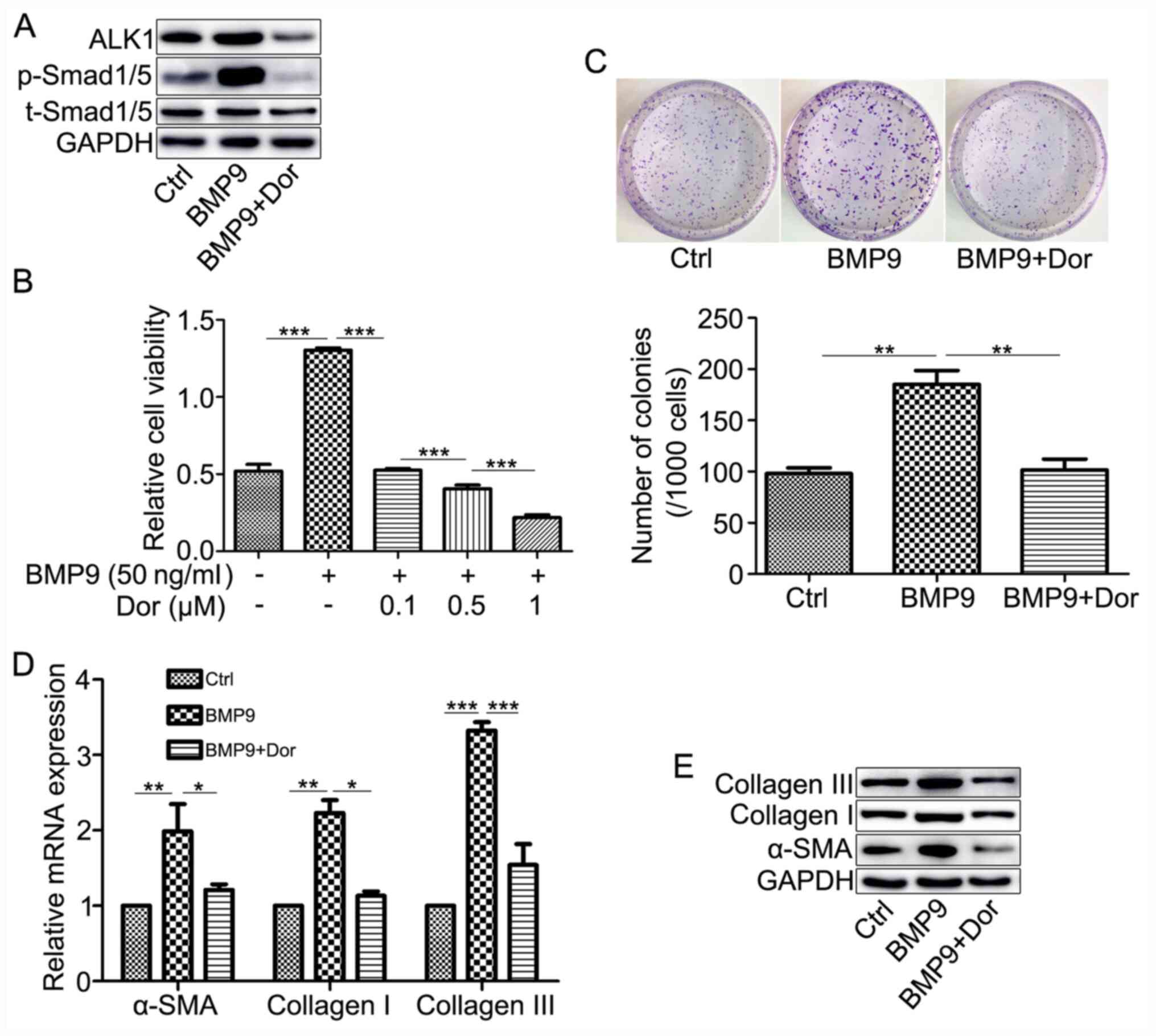
ALK1 inhibitor suppresses the effects
of BMP9 in HFL-1 cells
To investigate the effect of BMP9 on HFL-1 cells via
ALK1/Smad1/5 signaling, the inhibitory role of Dor, an ALK1
inhibitor, on BMP9-mediated HFL-1 cell responses was assessed. Dor
notably reduced BMP9-induced ALK1 and p-Smad1/5 expression levels
(Fig. 4A). Dor also significantly
inhibited BMP9-induced cell viability and colony formation
(Fig. 4B and C). Similarly, Dor decreased BMP9-mediated
upregulation of α-SMA, collagen I and collagen III expression
(Fig. 4D and E). The results further suggested that BMP9
increased HFL-1 cell proliferation and differentiation via
activating ALK1/Smad1/5 signaling.
Discussion
Fibroblast activation is a major mechanism
underlying the fibrotic process that occurs during pulmonary
fibrosis, in which a variety of cytokines have been reported to be
associated with, such as TGF-β1(34), BMP4 and BMP7(35). TGF-β1 is a profibrotic cytokine
(34), whereas BMP4 displays
antifibrotic properties (35).
However, the effect of BMP9 on lung fibroblast activation is not
completely understood. The present study suggested that BMP9 was a
profibrotic regulator that displayed a key role in inducing HFL-1
cell proliferation and differentiation via Smad1/5 activation.
Therefore, the present study suggested a potential mechanism
underlying pulmonary fibrosis and indicated that BMP9 may serve as
a potential therapeutic target for fibroblast activation-associated
lung diseases, such as lung fibrosis and cancer.
BMP9 is primarily synthesized in hepatic tissues,
but actively circulates in the bloodstream (36), performing physiological and
pathophysiological functions, such as angiogenesis (11), neuronal differentiation (12), osteogenic development (37) and carcinogenesis (38). Previous studies have indicated that
BMP9 is a key regulator of fibrogenesis (22-26).
For example, BMP9 promotes liver fibrosis as evidenced by rBMP9
increasing human hepatic stellate cell proliferation and migration,
whereas BMP9 inhibition or knockout reduces liver fibrosis
(22). By contrast, BMP9 decreases
the synthesis of collagen I in cardiac fibroblasts, and loss of
BMP9 activity increases cardiac fibrosis (24). Similarly to previous studies, the
present study indicated that rBMP9 significantly promoted cell
proliferation and induced differentiation into myofibroblasts, as
evidenced by increased α-SMA and ECM protein (collagen I and
collagen III) expression levels, which were suppressed by BMP type
I receptor inhibitor. Therefore, the present study supported the
hypothesis that BMP9 may induce lung fibroblast activation and
serve as a profibrotic cytokine during lung fibrosis. The role of
BMP9 in lung fibrosis requires further investigation in an animal
model of pulmonary fibrosis or in patients with pulmonary
fibrosis.
Additionally, accumulating studies have demonstrated
that fibroblast-type cells are a key host cell type in tumor
metastasis via microenvironmental modulation, and fibroblast
activation is a critical event in tumor progression and metastasis
(39-42).
A number of ECM proteins have been identified, including an
elevated deposition of fibroblast-derived fibronectin, in the tumor
microenvironment (43-45).
Moreover, previous studies have indicated that the transformation
of normal lung fibroblasts to a myofibroblast phenotype and the
formation of cancer-associated fibroblasts promoted tumor
progression in non-small cell lung cancer (46-48).
Therefore, targeting cancer-associated fibroblasts is an important
alternative treatment strategy for lung cancer. The present study
further suggested that exogenous BMP9 activated lung fibroblasts,
which indicated that BMP9 might be involved in the progression and
metastasis of lung cancer via regulating cancer-associated
fibroblasts, a process that is similar to lung fibroblast
activation during pulmonary fibrosis. Meanwhile, several studies
have reported that BMP signaling, including BMP receptor type II,
endoglin, growth differentiation factor 15 and Smad4, is involved
in tumor progression and metastasis via regulating
cancer-associated fibroblasts (49-51).
For example, Paauwe et al (49) demonstrated that endoglin, a
coreceptor for TGF-β and BMP9, is expressed in cancer-associated
fibroblasts, and endoglin-expressing cancer-associated fibroblasts
contribute to colorectal cancer progression and metastasis.
Therefore, whether BMP9 is implicated in cancer-associated
fibroblast metastasis requires further investigation.
As a member of the TGF-β superfamily, BMP9 binds to
type I and type II transmembrane receptors, including ALK1, ALK2,
BMP receptor type II and activin receptor type IIB, to exert its
effects (17,18,27).
Type I BMP receptor ALK1 has been identified as displaying the
highest affinity with BMP9 in the majority of cell types (52). Typically, when BMP9 binds to the
ALK1 receptor, it primarily activates the expression of downstream
p-Smad1/5 (17,18). Numerous studies have reported that
Smad1/5 is activated in fibrotic diseases (20,53-55).
During hepatic fibrosis, BMP9 induced the expression of inhibitor
of differentiation 1 via the Smad1 signaling pathway, thereby
triggering hepatic stellate cells to differentiate into
myofibroblasts (52,53). In addition, Li et al
(20) demonstrated that the
BMP9/ALK1/Smad1 signaling pathway is involved in the
epithelial-mesenchymal transition of liver cancer cells, which is
an important mechanism underlying hepatic fibrosis. In the cellular
context of scleroderma fibroblasts, ALK1/Smad1/5 signaling promotes
ECM proteins, such as collagen I and CTGF (54). Consistent with the aforementioned
studies, the present study indicated that rBMP9 increased ALK1
expression and Smad1/5 phosphorylation, and promoted cell
viability, colony formation and the expression of α-SMA, collagen I
and collagen III in HFL-1 cells. Moreover, the present study
suggested that Dor partly rescued BMP9-induced effects. Therefore,
the results supported the hypothesis that the ALK1/Smad1/5
signaling pathway might be implicated in BMP9-mediated HFL-1 cell
proliferation and differentiation.
Collectively, the present study indicated that BMP9
directly induced HFL-1 cell proliferation. In addition, the results
suggested that the activated ALK1/Smad1/5 signaling pathway may
mediate BMP9-induced HFL-1 cell proliferation and differentiation.
Moreover, the results indicated that BMP9 may serve as an activator
of lung fibroblast proliferation and differentiation and may also
serve as a profibrotic cytokine in pulmonary fibrosis, supporting
its potential role in the treatment of lung fibrosis. Furthermore,
BMP9-mediated lung fibroblast activation indicated that the
potential role of BMP9-regulated cancer-associated fibroblasts
during the progression and metastasis of lung cancer requires
further investigation. However, the present study had a number of
limitations. For example, the study focused on in vitro
experiments and only HFL-1 cells were used in the study. Therefore,
future studies should explore the role of BMP9 in other cell lines,
in vivo or by using clinical tissue samples to identify the
exact role of BMP9 in pulmonary fibrosis and evaluate the possible
effect of BMP9-regulating cancer-associated fibroblasts on lung
cancer.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant nos. 81660163 and 81560299) and
the National Natural Science Foundation of Jiangxi Province (grant
nos. 20151BAB205002 and 20161BAB205205).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW designed and performed the main experiments and
analyzed the data. XS analyzed the data. YY performed the
experiments and analyzed the data. YH contributed to designing the
study. YW and YH can authenticate the raw data in this study. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
King TE Jr, Pardo A and Selman M:
Idiopathic pulmonary fibrosis. Lancet. 378:1949–1961.
2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tsubouchi K, Araya J, Minagawa S, Hara H,
Ichikawa A, Saito N, Kadota T, Sato N, Yoshida M, Kurita Y, et al:
Azithromycin attenuates myofibroblast differentiation and lung
fibrosis development through proteasomal degradation of NOX4.
Autophagy. 13:1420–1434. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Shu DY and Lovicu FJ: Myofibroblast
transdifferentiation: The dark force in ocular wound healing and
fibrosis. Prog Retin Eye Res. 60:44–65. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Meng XM, Nikolic-Paterson DJ and Lan HY:
TGF-β: The master regulator of fibrosis. Nat Rev Nephrol.
12:325–338. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mody AA, Wordinger RJ and Clark AF: Role
of ID proteins in BMP4 inhibition of profibrotic effects of TGF-β2
in human TM cells. Invest Ophthalmol Vis Sci. 58:849–859.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liu L, Wang Y, Yan R, Liang L, Zhou X, Liu
H, Zhang X, Mao Y, Peng W, Xiao Y, et al: BMP-7 inhibits renal
fibrosis in diabetic nephropathy via miR-21 downregulation. Life
Sci. 238:116957–116967. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Xiao L, Du Y, Shen Y, He Y, Zhao H and Li
Z: TGF-beta 1 induced fibroblast proliferation is mediated by the
FGF-2/ERK pathway. Front Biosci. 17:2667–2674. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Miller AF, Harvey SA, Thies RS and Olson
MS: Bone morphogenetic protein-9. An autocrine/paracrine cytokine
in the liver. J Biol Chem. 275:17937–17945. 2000.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Breitkopf-Heinlein K, Meyer C, König C,
Gaitantzi H, Addante A, Thomas M, Wiercinska E, Cai C, Li Q, Wan F,
et al: BMP-9 interferes with liver regeneration and promotes liver
fibrosis. Gut. 66:939–954. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen C, Grzegorzewski KJ, Barash S, Zhao
Q, Schneider H, Wang Q, Singh M, Pukac L, Bell AC, Duan R, et al:
An integrated functional genomics screening program reveals a role
for BMP-9 in glucose homeostasis. Nat Biotechnol. 21:294–301.
2003.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Scharpfenecker M, van Dinther M, Liu Z,
van Bezooijen RL, Zhao Q, Pukac L, Löwik CWGM and ten Dijke P:
BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell
proliferation and VEGF-stimulated angiogenesis. J Cell Sci.
120:964–972. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Schnitzler AC, Mellott TJ, Lopez-Coviella
I, Tallini YN, Kotlikoff MI, Follettie MT and Blusztajn JK: BMP9
(bone morphogenetic protein 9) induces NGF as an
autocrine/paracrine cholinergic trophic factor in developing basal
forebrain neurons. J Neurosci. 30:8221–8228. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
David L, Mallet C, Keramidas M, Lamandé N,
Gasc JM, Dupuis-Girod S, Plauchu H, Feige JJ and Bailly S: Bone
morphogenetic protein-9 is a circulating vascular quiescence
factor. Circ Res. 102:914–922. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fong D, Bisson M, Laberge G, McManus S,
Grenier G, Faucheux N and Roux S: Bone morphogenetic protein-9
activates Smad and ERK pathways and supports human osteoclast
function and survival in vitro. Cell Signal. 25:717–728.
2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang J, Weng Y, Zhang M, Li Y, Fan M, Guo
Y, Sun Y, Li W and Shi Q: BMP9 inhibits the growth and migration of
lung adenocarcinoma A549 cells in a bone marrow stromal
cell-derived microenvironment through the MAPK/ERK and NF-κB
pathways. Oncol Rep. 36:410–418. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Addante A, Roncero C, Almalé L,
Lazcanoiturburu N, García-Álvaro M, Fernández M, Sanz J, Hammad S,
Nwosu ZC, Lee SJ, et al: Bone morphogenetic protein 9 as a key
regulator of liver progenitor cells in DDC-induced cholestatic
liver injury. Liver Int. 38:1664–1675. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mostafa S, Pakvasa M, Coalson E, Zhu A,
Alverdy A, Castillo H, Fan J, Li A, Feng Y, Wu D, et al: The
wonders of BMP9: From mesenchymal stem cell differentiation,
angiogenesis, neurogenesis, tumorigenesis, and metabolism to
regenerative medicine. Genes Dis. 6:201–223. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cunha SI and Pietras K: ALK1 as an
emerging target for antiangiogenic therapy of cancer. Blood.
117:6999–7006. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang T, Zhang Z, Wang K, Wang J, Jiang Y,
Xia J, Gou L, Liu M, Zhou L, He T, et al: Inhibitory effects of
BMP9 on breast cancer cells by regulating their interaction with
pre-adipocytes/adipocytes. Oncotarget. 8:35890–35901.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li Q, Gu X, Weng H, Ghafoory S, Liu Y,
Feng T, Dzieran J, Li L, Ilkavets I, Kruithof-de Julio M, et al:
Bone morphogenetic protein-9 induces epithelial to mesenchymal
transition in hepatocellular carcinoma cells. Cancer Sci.
104:398–408. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Suzuki Y, Ohga N, Morishita Y, Hida K,
Miyazono K and Watabe T: BMP-9 induces proliferation of multiple
types of endothelial cells in vitro and in vivo. J Cell Sci.
123:1684–1692. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li P, Li Y, Zhu L, Yang Z, He J, Wang L,
Shang Q, Pan H, Wang H, Ma X, et al: Targeting secreted cytokine
BMP9 gates the attenuation of hepatic fibrosis. Biochim Biophys
Acta Mol Basis Dis. 1864:709–720. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Muñoz-Félix JM, Cuesta C, Perretta-Tejedor
N, Subileau M, López-Hernández FJ, López-Novoa JM and
Martínez-Salgado C: Identification of bone morphogenetic protein 9
(BMP9) as a novel profibrotic factor in vitro. Cell Signal.
28:1252–1261. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Morine KJ, Qiao X, York S, Natov PS,
Paruchuri V, Zhang Y, Aronovitz MJ, Karas RH and Kapur NK: Bone
morphogenetic protein 9 reduces cardiac fibrosis and improves
cardiac function in heart failure. Circulation. 138:513–526.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Morine KJ, Qiao X, Paruchuri V, Aronovitz
MJ, Mackey EE, Buiten L, Levine J, Ughreja K, Nepali P, Blanton RM,
et al: Reduced activin receptor-like kinase 1 activity promotes
cardiac fibrosis in heart failure. Cardiovasc Pathol. 31:26–33.
2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Morine KJ, Qiao X, Paruchuri V, Aronovitz
MJ, Mackey EE, Buiten L, Levine J, Ughreja K, Nepali P, Blanton RM,
et al: Conditional knockout of activin like kinase-1 (ALK-1) leads
to heart failure without maladaptive remodeling. Heart Vessels.
32:628–636. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bi J and Ge S: Potential roles of BMP9 in
liver fibrosis. Int J Mol Sci. 15:20656–20667. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chen X, Orriols M, Walther FJ, Laghmani
EH, Hoogeboom AM, Hogen-Esch ACB, Hiemstra PS, Folkerts G, Goumans
MTH, Ten Dijke P, et al: Bone morphogenetic protein 9 protects
against neonatal hyperoxia-induced impairment of alveolarization
and pulmonary inflammation. Front Physiol. 8:486–502.
2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang XJ, Lian TY, Jiang X, Liu SF, Li SQ,
Jiang R, Wu WH, Ye J, Cheng CY, Du Y, et al: Germline BMP9 mutation
causes idiopathic pulmonary arterial hypertension. Eur Respir J.
53:1801609–1801618. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Huang YH, Zhou XY, Wang HM, Xu H, Chen J
and Lv NH: Aquaporin 5 promotes the proliferation and migration of
human gastric carcinoma cells. Tumour Biol. 34:1743–1751.
2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
.
|
|
33
|
Lo RS and Massagué J: Ubiquitin-dependent
degradation of TGF-β-activated smad2. Nat Cell Biol. 1:472–478.
1999.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Huang LS, Jiang P, Feghali-Bostwick C,
Reddy SP, Garcia JGN and Natarajan V: Lysocardiolipin
acyltransferase regulates TGF-β mediated lung fibroblast
differentiation. Free Radic Biol Med. 112:162–173. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Pegorier S, Campbell GA, Kay AB and Lloyd
CM: Bone morphogenetic protein (BMP)-4 and BMP-7 regulate
differentially transforming growth factor (TGF)-beta1 in normal
human lung fibroblasts (NHLF). Respir Res. 11:85–94.
2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tillet E, Ouarné M, Desroches-Castan A,
Mallet C, Subileau M, Didier R, Lioutsko A, Belthier G, Feige JJ
and Bailly S: A heterodimer formed by bone morphogenetic protein 9
(BMP9) and BMP10 provides most BMP biological activity in plasma. J
Biol Chem. 293:10963–10974. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liao J, Yu X, Hu X, Fan J, Wang J, Zhang
Z, Zhao C, Zeng Z, Shu Y, Zhang R, et al: lncRNA H19 mediates
BMP9-induced osteogenic differentiation of mesenchymal stem cells
(MSCs) through Notch signaling. Oncotarget. 8:53581–53601.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ouarné M, Bouvard C, Boneva G, Mallet C,
Ribeiro J, Desroches-Castan A, Soleilhac E, Tillet E, Peyruchaud O
and Bailly S: BMP9, but not BMP10, acts as a quiescence factor on
tumor growth, vessel normalization and metastasis in a mouse model
of breast cancer. J Exp Clin Cancer Res. 37:209–218.
2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Strell C, Rundqvist H and Ostman A:
Fibroblasts - a key host cell type in tumor initiation,
progression, and metastasis. Ups J Med Sci. 117:187–195.
2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chen X and Song E: Turning foes to
friends: Targeting cancer-associated fibroblasts. Nat Rev Drug
Discov. 18:99–115. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Affo S, Yu LX and Schwabe RF: The role of
cancer-associated fibroblasts and fibrosis in liver cancer. Annu
Rev Pathol. 12:153–186. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Richards KE, Zeleniak AE, Fishel ML, Wu J,
Littlepage LE and Hill R: Cancer-associated fibroblast exosomes
regulate survival and proliferation of pancreatic cancer cells.
Oncogene. 36:1770–1778. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Najafi M, Farhood B and Mortezaee K:
Extracellular matrix (ECM) stiffness and degradation as cancer
drivers. J Cell Biochem. 120:2782–2790. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Paolillo M and Schinelli S: Extracellular
matrix alterations in metastatic processes. Int J Mol Sci.
20:4947–4956. 2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Erdogan B, Ao M, White LM, Means AL,
Brewer BM, Yang L, Washington MK, Shi C, Franco OE, Weaver AM, et
al: Cancer-associated fibroblasts promote directional cancer cell
migration by aligning fibronectin. J Cell Biol. 216:3799–3816.
2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Li F, Zhao S, Cui Y, Guo T, Qiang J, Xie
Q, Yu W, Guo W, Deng W, Gu C, et al: α1,6-Fucosyltransferase (FUT8)
regulates the cancer-promoting capacity of cancer-associated
fibroblasts (CAFs) by modifying EGFR core fucosylation (CF) in
non-small cell lung cancer (NSCLC). Am J Cancer Res. 10:816–837.
2020.PubMed/NCBI
|
|
47
|
Luo M, Luo Y, Mao N, Huang G, Teng C, Wang
H, Wu J, Liao X and Yang J: Cancer-associated fibroblasts
accelerate malignant progression of non-small cell lung cancer via
connexin 43-formed unidirectional gap junctional intercellular
communication. Cell Physiol Biochem. 51:315–336. 2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Li H, Zhang Q, Wu Q, Cui Y, Zhu H, Fang M,
Zhou X, Sun Z and Yu J: Interleukin-22 secreted by
cancer-associated fibroblasts regulates the proliferation and
metastasis of lung cancer cells via the PI3K-Akt-mTOR signaling
pathway. Am J Transl Res. 11:4077–4088. 2019.PubMed/NCBI
|
|
49
|
Paauwe M, Schoonderwoerd MJA, Helderman
RFCP, Harryvan TJ, Groenewoud A, van Pelt GW, Bor R, Hemmer DM,
Versteeg HH, Snaar-Jagalska BE, et al: Endoglin expression on
cancer-associated fibroblasts regulates invasion and stimulates
colorectal cancer metastasis. Clin Cancer Res. 24:6331–6344.
2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Pickup MW, Hover LD, Polikowsky ER, Chytil
A, Gorska AE, Novitskiy SV, Moses HL and Owens P: BMPR2 loss in
fibroblasts promotes mammary carcinoma metastasis via increased
inflammation. Mol Oncol. 9:179–191. 2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Bruzzese F, Hägglöf C, Leone A, Sjöberg E,
Roca MS, Kiflemariam S, Sjöblom T, Hammarsten P, Egevad L, Bergh A,
et al: Local and systemic protumorigenic effects of
cancer-associated fibroblast-derived GDF15. Cancer Res.
74:3408–3417. 2014.PubMed/NCBI View Article : Google Scholar
|
|
52
|
David L, Mallet C, Mazerbourg S, Feige JJ
and Bailly S: Identification of BMP9 and BMP10 as functional
activators of the orphan activin receptor-like kinase 1 (ALK1) in
endothelial cells. Blood. 109:1953–1961. 2007.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Shen H, Fan J, Burczynski F, Minuk GY,
Cattini P and Gong Y: Increased Smad1 expression and
transcriptional activity enhances trans-differentiation of hepatic
stellate cells. J Cell Physiol. 212:764–770. 2007.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Wang CY, Xiao X, Bayer A, Xu Y, Dev S,
Canali S, Nair AV, Masia R and Babitt JL: Ablation of hepatocyte
Smad1, Smad5, and Smad8 causes severe tissue iron loading and liver
fibrosis in mice. Hepatology. 70:1986–2002. 2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Pannu J, Asano Y, Nakerakanti S, Smith E,
Jablonska S, Blaszczyk M, ten Dijke P and Trojanowska M: Smad1
pathway is activated in systemic sclerosis fibroblasts and is
targeted by imatinib mesylate. Arthritis Rheum. 58:2528–2537.
2008.PubMed/NCBI View Article : Google Scholar
|