Introduction
As a common type of skeletal disorder, osteoporosis
is characterized by abnormal bone architecture and low bone mineral
density, resulting in increased risk of fracture (1). Osteoporosis was once considered an
inevitable disorder in the elderly population. Recently, multiple
prevention and treatment approaches have been developed for
osteoporosis (2). However, with the
growth of the aging population, the incidence of osteoporosis is
increasing worldwide, alongside increased treatment costs (3). Therefore, novel therapeutic approaches
with higher efficiencies are required to improve the treatment
outcomes of osteoporosis. Osteoblasts and osteoclasts serve
critical roles in bone formation and resorption, respectively
(4,5). However, molecular mechanisms that are
associated with this disease remain poorly understood (4,5),
leading to difficulties in the development of novel therapeutic
regimens.
p53 signaling is a well-studied pathway that plays
pivotal roles in diverse cellular processes, such as cell cycle
progression, genomic stability and cell apoptosis (6-8).
p53 can regulate the apoptosis of osteoblastic cells (9), which plays a key role in the
pathogenesis of osteoporosis (10).
Inhibition of p53 suppresses the apoptosis of osteoblasts, thereby
contributing to recovery from osteoporosis (11). It has been reported that the
development and progression of osteoporosis also requires the
involvement of long (>200 nt) non-coding RNAs (lncRNAs), which
participates in diverse biological processes by regulating gene
expression (12). It has been
previously reported that WT1-AS can upregulate p53 in cervical
cancer to inhibit cancer progression (13). Therefore, WT1-antisense RNA (WT1-AS)
may also interact with p53 to participate in the development of
osteoporosis. The present study was performed to explore the
possible interaction between WT1-AS and p53 in osteoporosis.
Materials and methods
Research subjects
The present study included 60 patients with
osteoporosis (23 males and 37 females; age range 33-66 years; mean
age, 49.2±6.1 years) and 60 healthy volunteers (23 males and 37
females, 32-66 years; mean age, 49.6±6.3 years). All patients and
healthy volunteers were admitted to the First Affiliated Hospital
of Hainan Medical College between March 2015 and March 2018. The
inclusion criteria of patients were as follows: i) Newly diagnosed
cases; and ii) no initiated therapies. The exclusion criteria of
patient were as follows: i) Recurrent cases; and ii) complications
with other bone disorders or other types of diseases. All patients
were informed of the experimental principles, and gave their signed
informed consent. The Ethics Committee of the First Affiliated
Hospital of Hainan Medical College approved this study prior to the
admission of subjects. No significant differences in age, gender,
body mass index, or smoking and drinking history were indicated
between the two groups (data not shown). The T-score was calculated
using the following formula: T-score = (bone mineral
density-reference bone mineral density)/reference standard
deviation (14). The T-score of the
patients ranged from -2.5 to -4.7 (mean score, -3.3±0.4), while the
T-score for healthy volunteers ranged from -0.9 to 3.3 (mean score,
1.3±0.5). T-score was significantly lower in patients compared with
controls (data not shown). The disease duration of patients ranged
from 2.2 to 13.8 years, with a mean of 8.1±2.4 years.
Patient treatment and plasma sample
preparation
All 60 patients with osteoporosis were treated with
bisphosphonates, including alendronate, risedronate, zoledronic
acid and ibandronate. Estrogen was only used in females.
Bisphosphonates attenuate bone loss, and estrogen was used in
female patient to control postmenopausal symptoms (15). Drug doses were determined according
to patients' health conditions, disease severity and mid-term
treatment outcomes. Blood (5 ml) was extracted from each healthy
volunteer and patient under fasting conditions prior to therapy
initiation (1-3 days). The same amount of fasting blood was also
extracted from each patient with osteroporosis at 3 months after
therapy initiation. Blood samples were centrifuged at 1,200 x g
(room temperature) for 15 min to prepare plasma samples.
Transient transfection of
osteoblasts
In vitro experiments were performed using
primary osteoblasts purchased from Sigma-Aldrich; Merck KGaA.
Primary osteoblasts were cultivated in Osteoblast Growth Medium
(PromoCell GmbH) at 37˚C. All subsequent experiments were performed
using cells from passage 4 or 5. WT1-AS and p53 expression vectors
(pcDNA3.1) and empty pcDNA3.1 vector, as well as small interfering
RNA (siRNA) negative control and WT1-AS siRNA, were synthesized by
Guangzhou RiboBio Co., Ltd. Osteoblasts (1x106) were
transfected with 10 nM WT1-AS or p53 expression vector, 10 nM empty
pcDNA3.1 vector (negative control, NC group), 40 nM WT1-AS siRNA,
or 40 nM siRNA NC (NC group). Non-transfected osteoblasts acted as
the control (C) group. Osteoblast RNA and protein were harvested at
24 h post-transfection for use in subsequent experiments.
ELISA
Human p53 ELISA kit (cat. no. ab46067; Abcam) was
used to measure levels of p53 in plasma samples. All steps were
performed following manufacturer's instructions. All plasma levels
of p53 were expressed as pg/ml.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
A total of 1x106 osteoblasts and 2 ml of
plasma was mixed with 1 ml of RNAzol (Sigma-Aldrich; Merck KGaA) to
extract total RNAs. DNase I (Invitrogen; Thermo Fisher Scientific,
Inc.) was used to digest genomic DNA in all RNA samples at 37˚C for
1 h. Following that, a qScript cDNA Synthesis kit (Quantabio) was
used to perform RT (25˚C for 10 min, 55˚C for 20 min and 85˚C for
10 min). qPCR reaction mixtures were prepared using a KAPA SYBR
FAST qPCR Master Mix kit (Roche Diagnostics). GAPDH was used as an
endogenous control. Primer sequences were: WT1-AS, 5'-GCC
TCTCTGTCCTCTTCTTTG-3' (forward), 5'-GCTGT GAGTC CTGGTGCTTA-3'
(reverse); GAPDH, 5'-TTGGCATCGTTG AGGGTC-3' (forward),
5'-AGTGGGAACACGGAAAGC-3' (reverse); P53, 5'-AGAGTCTATAGGCCCACCCC-3'
(forward), 5'-GCTCGACGCTAGGATCTGAC-3' (reverse). PCR cycling
conditions were: 95˚C for 1 min, followed by 40 cycles of 95˚C for
10 sec and 58˚C for 50 sec. The expression levels of WT1-AS and p53
mRNA were normalized using the 2-ΔΔCq method (16).
Western blot analysis
Osteoblasts were lysed with 1 ml RIPA buffer
(Guangzhou RiboBio Co., Ltd.) to extract total protein. Protein
samples were quantified using a bicinchoninic acid kit (Sangon
Biotech Co., Ltd.), followed by denaturation in boiling water for 5
min. Electrophoresis was performed using 12% SDS-PAGE to separate
proteins (30 µg per well) according to their molecular weights.
Proteins were transferred to a PVDF membrane and blocking was
carried out in 5% non-fat milk for 2 h at room temperature. Primary
antibodies of rabbit GAPDH (1:1,200; cat. no. ab181602; Abcam) and
p53 (1:1,200; cat. no. ab131442; Abcam) were used to incubate the
membranes for 15 h at 4˚C. Horseradish peroxidase goat anti-rabbit
(immunoglobulin G; 1:1,100; cat. no. ab6721; Abcam) secondary
antibody was then used to blot the membranes further at room
temperature for 2 h. The ECL Chemiluminescence Detection kit
(Sangon Biotech Co., Ltd.) was used to develop protein signals.
Gray values were normalized using ImageJ version 1.46 (National
Institutes of Health).
Cell apoptosis analysis
A total of 6x104 osteoblasts were mixed
with 1 ml serum-free aforementioned cell culture medium to prepare
single-cell suspensions. Osteoblasts were cultivated at 37˚C and 5%
CO2 in a 6-well plate with 2 ml of cell suspension in
each well. Cells were incubated at 37˚C for 48 h before being
harvested and digested with 0.25% trypsin. Cells were subsequently
stained using Annexin V-FITC (Thermo Fisher Scientific, Inc.) and
propidium iodide (Thermo Fisher Scientific, Inc.) at 4˚C for 20 min
in dark. Apoptotic cells were analyzed using a flow cytometer. Data
were processed using FCSalyzer Version 0.9.18 (SourceForge; DHI
Group, Inc.).
Statistical analysis
The mean ± SD values of three biological replicates
of each experiment were calculated and used for all comparisons.
Statistical power was calculated using Origin software version 10
(OriginLab Corp.) and a statistical power of ~0.9 was obtained.
Differences between patients and controls were measured using an
unpaired t-test. Differences between two time points in the patient
group were analyzed using a paired t-test. Differences among
different cell transfection groups were investigated using a
one-way ANOVA followed by Tukey's test. Correlations were analyzed
using Pearson's correlation coefficient. A receiver operating
characteristic (ROC) curve was plotted for diagnostic analysis.
Patients with disease are grouped in the true positive class and
healthy controls are grouped in the true negative class. Patients
were divided into high and low WT1-AS/p53 level groups (n=30),
using the mean expression levels of WT1-AS/p53 in osteoporosis as a
cutoff score. Any association between patients' clinical data and
WT1-AS/p53 expression was analyzed using a χ2 test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
WT1-AS and p53 levels are positively
correlated in patients with osteoporosis
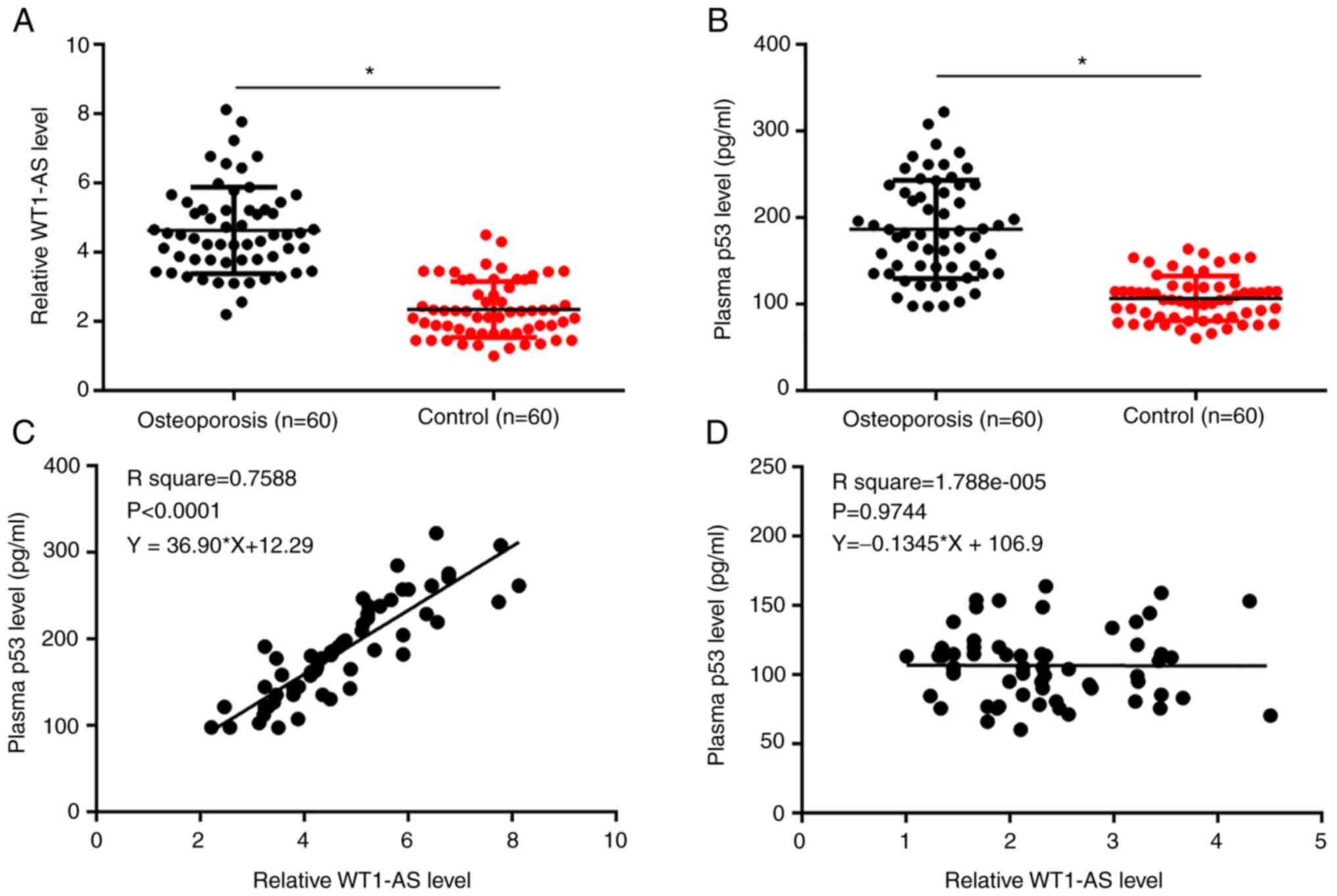
WT1-AS and p53 plasma levels were measured. Plasma
levels of WT1-AS (Fig. 1A) and p53
(Fig. 1B) were significantly higher
in patients with osteoporosis compared with the control group
(P<0.05). Correlations between WT1-AS and p53 were analyzed
using Pearson's correlation coefficient. WT1-AS and p53 levels were
significantly and positively correlated in patients with
osteoporosis (Fig. 1C,
P<0.0001), but not in healthy controls (Fig. 1D, P=0.9744). The T-score of the
patients ranged from -2.5 to -4.7. χ2 test revealed that
expression levels of WT1-AS and p53 were not associated with
patients' age, sex and smoking habits, but were closely associated
with T-score and disease duration (Table I).
 | Table IAssociation between patients' clinical
data and WT1-AS and p53 expression. |
Table I
Association between patients' clinical
data and WT1-AS and p53 expression.
| A, WTI-AS |
|---|
| | Expression | |
|---|
| Index | High (n=30) | Low (n=30) | P-value |
|---|
| Age | | | >0.05 |
|
≥50
years | 14 | 15 | |
|
<50
years | 16 | 15 | |
| Sex | | | >0.05 |
|
Male | 13 | 10 | |
|
Female | 17 | 20 | |
| Smoking | | | >0.05 |
|
Yes | 11 | 10 | |
|
No | 19 | 20 | |
| Disease duration | | | <0.001 |
|
≥8
years | 22 | 6 | |
|
<8
years | 8 | 24 | |
| T score | | | |
|
≥-3.5 | 19 | 6 |
| B, p53 |
| | Expression | |
| Index | High (n=30) | Low (n=30) | P-value |
| Age | | | >0.05 |
|
≥50
years | 14 | 15 | |
|
<50
years | 16 | 15 | |
| Sex | | | >0.05 |
|
Male | 13 | 10 | |
|
Female | 17 | 20 | |
| Smoking | | | >0.05 |
|
Yes | 11 | 10 | |
|
No | 19 | 20 | |
| Disease
duration | | | <0.001 |
|
≥8
years | 22 | 6 | |
|
<8
years | 8 | 24 | |
| T score | | | |
|
≥-3.5 | 19 | 6 | 0.001 |
Altered expression of WT1-AS and p53
separates patients with osteoporosis from healthy controls
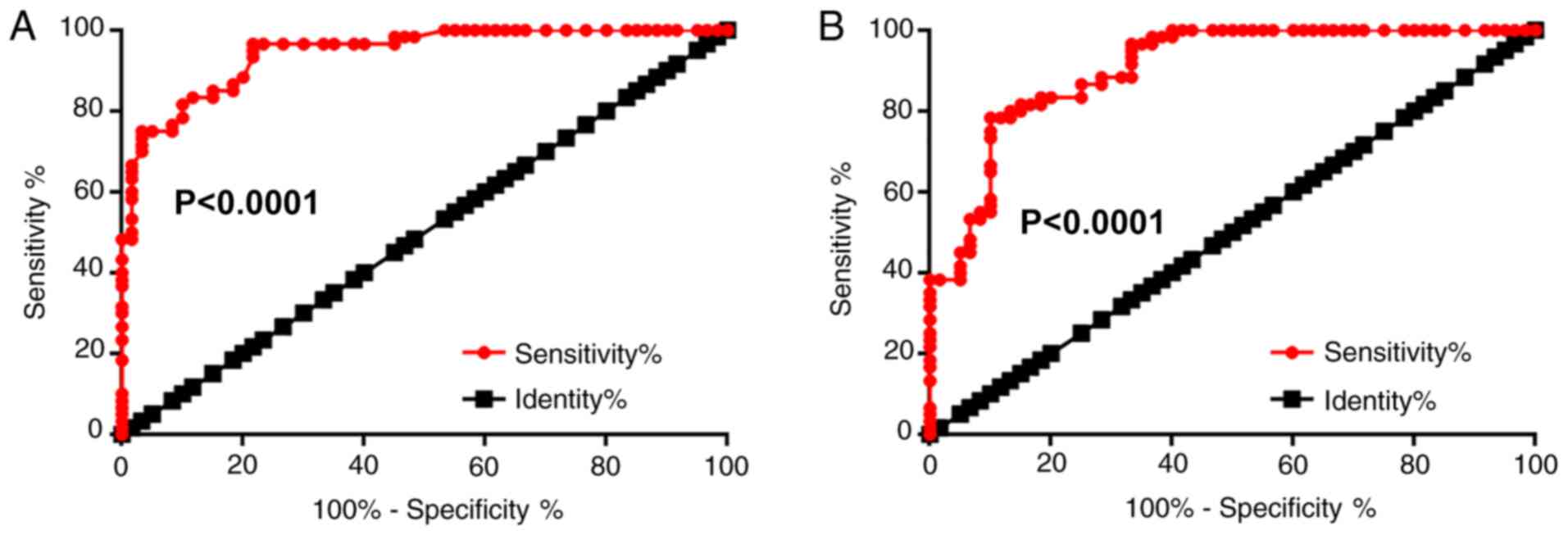
The potential application of plasma WT1-AS and p53
for the diagnosis of osteoporosis was explored by performing a ROC
curve analysis. The area under the curve (AUC) of plasma WT1-AS was
0.94 (95% confidence interval, 0.91-0.98; standard error, 0.019;
P<0.0001; Fig. 2A), while the
AUC of plasma p53 was 0.91 (95% confidence interval, 0.85-0.96;
standard error, 0.026; P<0.0001; Fig. 2B).
Bisphosphonate therapy downregulates
WT1-AS and p53 plasma levels in patients with osteoporosis
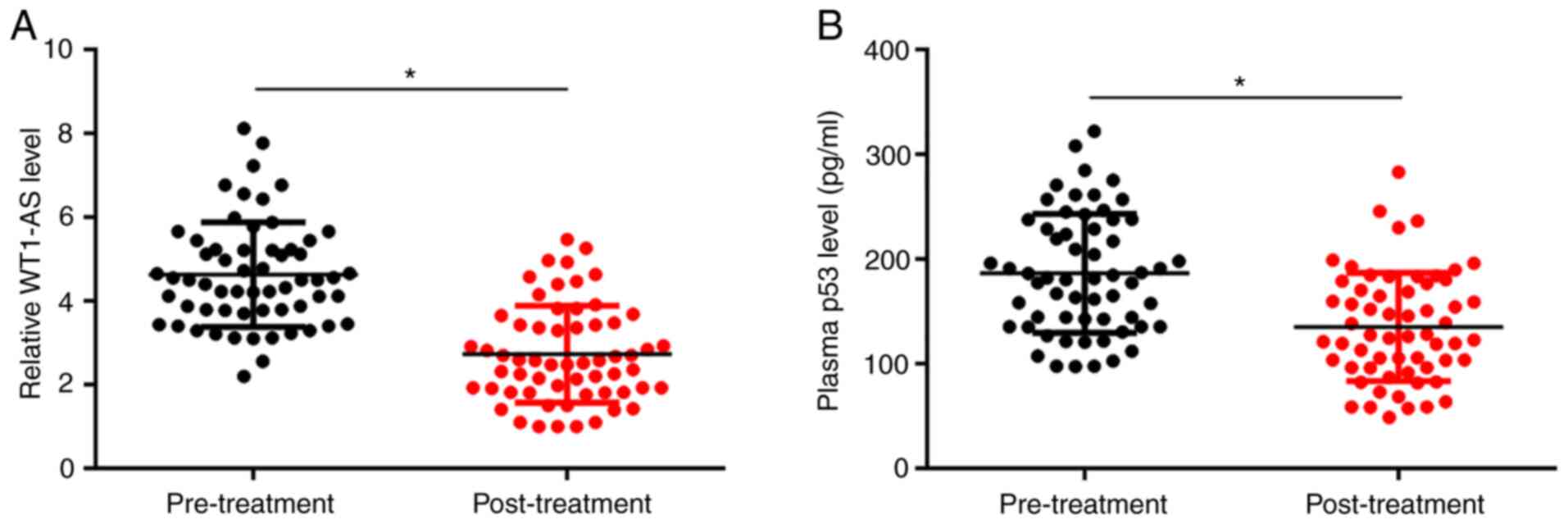
Plasma levels of WT1-AS and p53 were measured during
pre-treatment and at 3 months post-treatment. Levels of WT1-AS
(Fig. 3A) and p53 (Fig. 3B) significantly decreased at 3
months post-treatment compared with pre-treatment levels
(P<0.05).
WT1-AS positively regulates p53
expression in osteoblasts
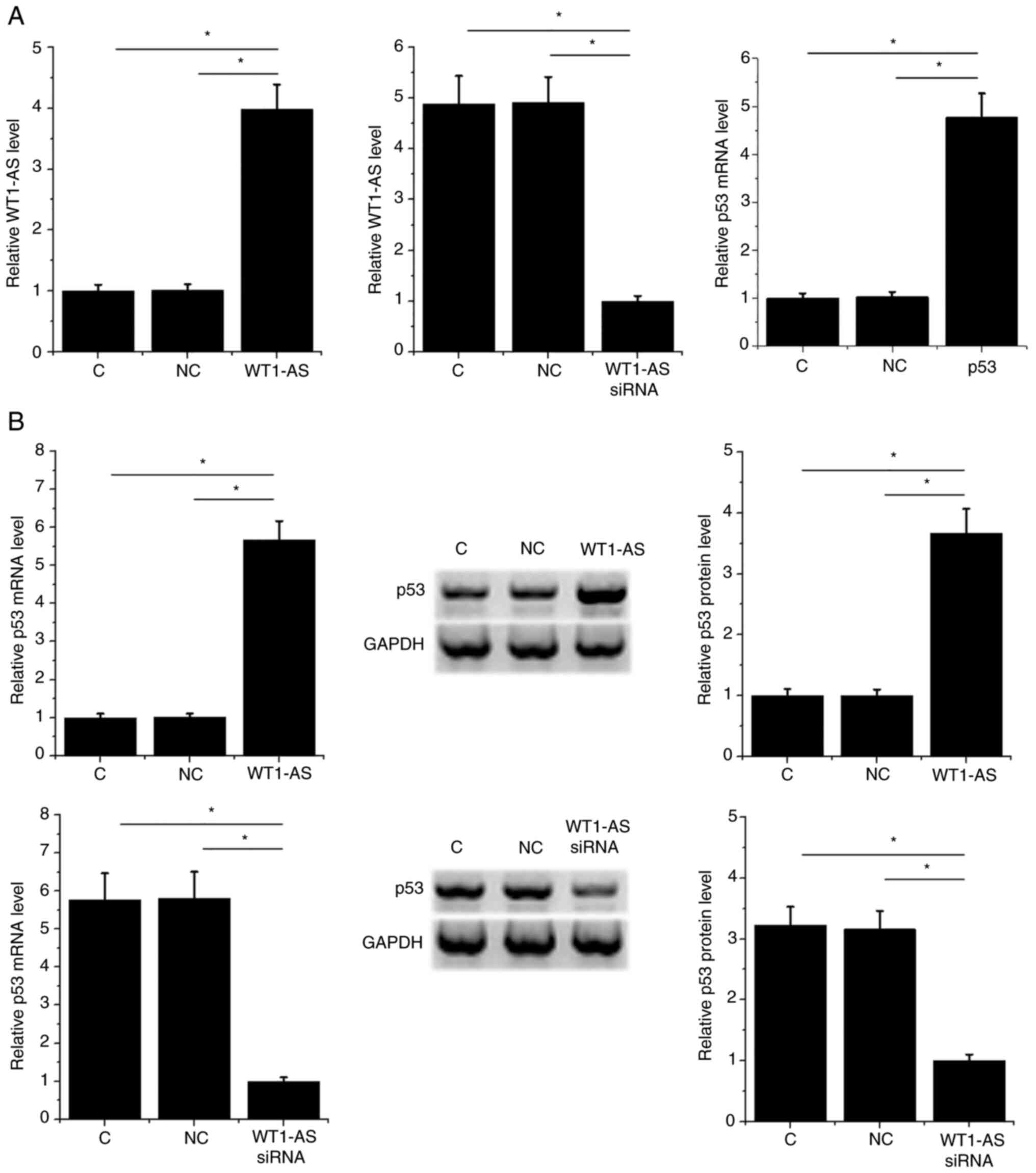
Osteoblasts were transfected with WT1-AS and p53
expression vectors and WT1-AS siRNA. WT1-AS and p53 expression was
significantly altered compared with NC and C groups at 24 h
post-transfection (P<0.05; Fig.
4A). Moreover, WT1-AS overexpression resulted in upregulated
p53 expression, while WT1-AS siRNA silencing resulted in
downregulated p53 expression at mRNA and protein levels compared
with the control and NC groups (P<0.05; Fig. 4B).
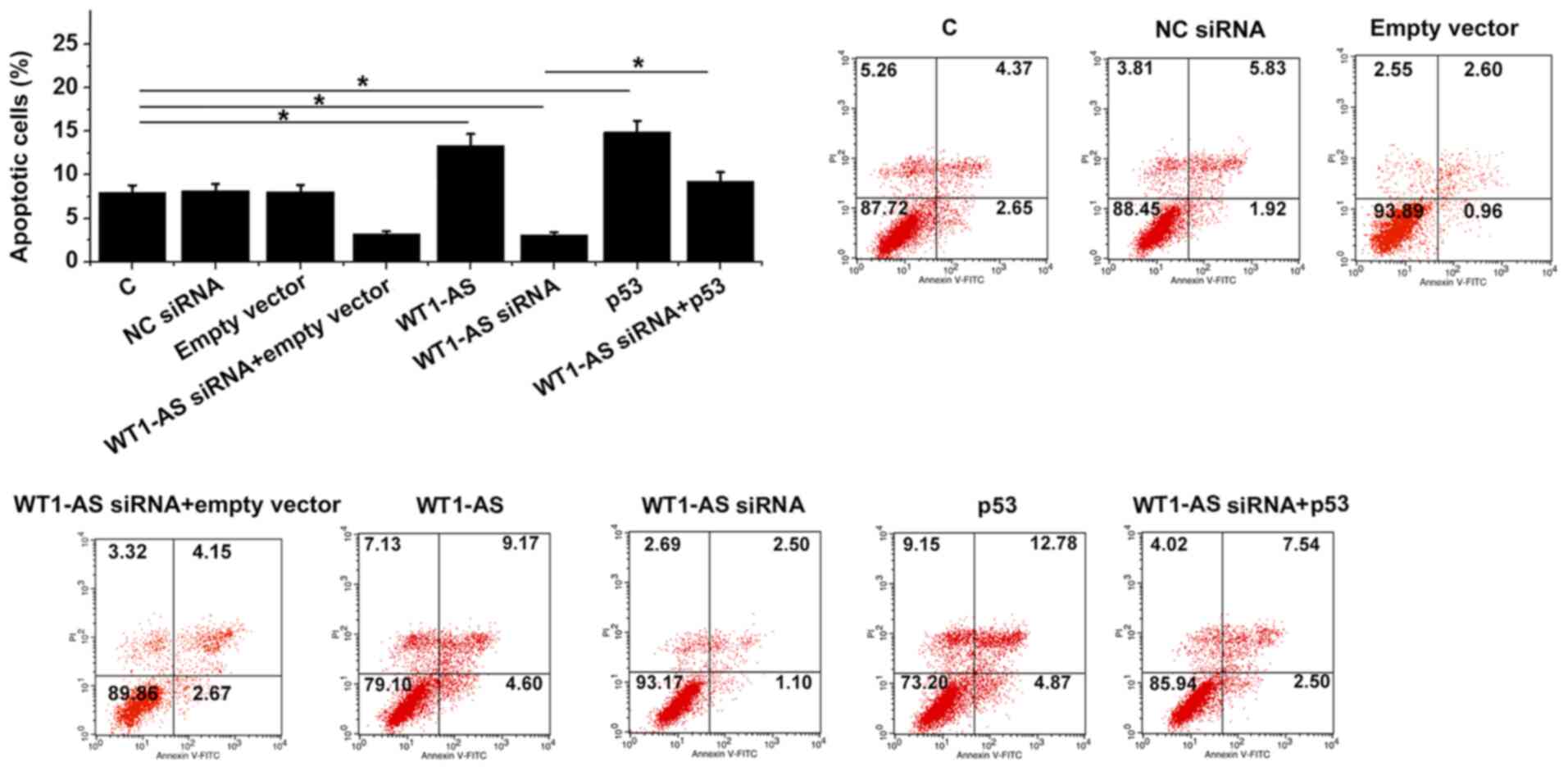
WT1-AS promotes osteoblast apoptosis
through p53
WT1-AS and p53 overexpression resulted in
significantly increased apoptosis rates, while WT1-AS siRNA
silencing resulted in significantly decreased rates of osteoblast
apoptosis compared with NC and C groups. In addition, p53
overexpression was indicated to attenuate the effect of WT1-AS
siRNA silencing on cell apoptosis in comparison to cells with
WT1-AS siRNA transfection alone (P<0.05; Fig. 5).
Discussion
To date, the functions of WT1-AS have only been
investigated in a few types of cancer (13,17,18).
The participation of WT1-AS in cancer biology is mainly mediated by
its roles in regulating cell behaviors such as proliferation,
apoptosis and invasion (3,17,16).
It is known that cell death in osteoblasts contributes to the
pathogenesis of osteoporosis (19).
The present study investigated the roles of WT1-AS in osteoporosis.
WT1-AS was upregulated in osteoporosis and exhibited diagnostic
values. In addition, WT1-AS was revealed to serve a role in
osteoblast apoptosis and indicated an association with p53, which
may indicate an interaction between the genes. Therefore,
overexpression of WT1-AS may promote the progression of
osteoporosis by promoting osteoblast apoptosis.
The diagnosis of osteoporosis mainly relies on the
measurement of bone mineral density (BMD) (20). However, the threshold of BMD to
diagnose osteoporosis is debated, and there is no way to use BMD to
screen people with high risk of osteoporosis (20). In the present study, ROC curve
analysis indicated that WT1-AS and p53 expression could be used to
distinguish patients with osteoporosis from healthy controls. In
addition, increased levels of WT1-AS and p53 were observed after
treatment with bisphosphonates or estrogen. Therefore, plasma
WT1-AS and p53 may be used as a marker to predict osteoporosis.
However, clinical trials are required to test sensitivity and
specificity.
The results of the current study revealed that
WT1-AS positively regulated the expression of p53 in osteoblasts.
The lack of significant correlation between WT1-AS and p53 across
the healthy controls suggested that the interaction between them
was indirect. It is known that lncRNAs can sponge miRNAs to
upregulate the expression of their downstream genes (21). Future studies should assess the
involvement of miRNAs and its interaction with lncRNAs. WT1-AS can
sponge miR-330-5p to regulate p53 in cervical cancer (13). Future studies should try to explore
the involvement of miRNAs in this process.
The present study only investigated the expression
of WT1-AS in plasma. Future studies should include patient
osteoclasts and animal model experiments to further verify the
results of the current study. The pathogenesis of osteoporosis is
complicated and requires the involvement of multiple factors, such
as epigenetic and hormonal factors (22-27).
The interactions between WT1-AS and these factors are needed to be
further analyzed.
However, the present study has limitations,
including the small sample size. Future studies with bigger sampler
sizes are required to further confirm the results of the current
study. The involvement of p53-related apoptotic factors, including
Bax and Bcl-2, was not explored and p53 knockdown experiments were
not included. The current study did not include osteoblast
functionality and proliferation assays and the analysis of changes
in the expression of marker genes. Therefore, future studies are
needed to examine these factors.
In conclusion, WT1-AS was demonstrated to be
upregulated in osteoporosis and promoted the apoptosis of
osteoblasts by upregulating p53.
Acknowledgements
Not applicable.
Funding
Funding: This research was supported by the Youth Foundation
Project of the First Affiliated Hospital of Hainan Medical College
in 2018 (grant no. HYFYPY201813) and Key R&D Program Projects
of Hainan Province in 2018 (grant no. ZDYF2018158).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CW, QX and YZ designed experiments. CW and QX
performed experiments. WS and YL analysed data. YZ drafted the
manuscript. All authors read and approved the manuscript.
Ethics approval and consent to
participate
The Ethics Committee of the First Affiliated
Hospital of Hainan Medical College approved this study prior to the
admission of subjects.
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Siris ES, Adler R, Bilezikian J, Bolognese
M, Dawson-Hughes B, Favus MJ, Harris ST, Jan de Beur SM, Khosla S,
Lane NE, et al: The clinical diagnosis of osteoporosis: A position
statement from the National Bone Health Alliance Working Group.
Osteoporos Int. 25:1439–1443. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Khosla S and Hofbauer LC: Osteoporosis
treatment: Recent developments and ongoing challenges. Lancet
Diabetes Endocrinol. 5:898–907. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lötters FJB, van den Bergh JP, de Vries F
and Rutten-van Mölken MP: Current and future incidence and costs of
osteoporosis-related fractures in the Netherlands: Combining claims
data with BMD measurements. Calcif Tissue Int. 98:235–243.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Curtis EM, Moon RJ, Dennison EM, Harvey NC
and Cooper C: Recent advances in the pathogenesis and treatment of
osteoporosis. Clin Med (Lond). 15 (Suppl 6):S92–S96.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Raisz LG: Pathogenesis of osteoporosis:
Concepts, conflicts, and prospects. J Clin Invest. 115:3318–3325.
2005.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Reyes J, Chen JY, Stewart-Ornstein J,
Karhohs KW, Mock CS and Lahav G: Fluctuations in p53 signaling
allow escape from cell-cycle arrest. Mol Cell. 2018. 71:581–591.
e5. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Willms A, Schittek H, Rahn S, Sosna J,
Mert U, Adam D and Trauzold A: Impact of p53 status on
TRAIL-mediated apoptotic and non-apoptotic signaling in cancer
cells. PLoS One. 14(e0214847)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yeo CQX, Alexander I, Lin Z, Lim S, Aning
OA, Kumar R, Sangthongpitag K, Pendharkar V, Ho VHB and Cheok CF:
p53 maintains genomic stability by preventing interference between
transcription and replication. Cell Rep. 15:132–146.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hirasawa H, Tanaka S, Sakai A, Tsutsui M,
Shimokawa H, Miyata H, Moriwaki S, Niida S, Ito M and Nakamura T:
ApoE gene deficiency enhances the reduction of bone formation
induced by a high-fat diet through the stimulation of p53-mediated
apoptosis in osteoblastic cells. J Bone Miner Res. 22:1020–1030.
2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Weinstein RS and Manolagas SC: Apoptosis
and osteoporosis. Am J Med. 108:153–164. 2000.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhen YF, Wang GD, Zhu LQ, Tan SP, Zhang
FY, Zhou XZ and Wang XD: P53 dependent mitochondrial permeability
transition pore opening is required for dexamethasone-induced death
of osteoblasts. J Cell Physiol. 229:1475–1483. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hao L, Fu J, Tian Y and Wu J: Systematic
analysis of lncRNAs, miRNAs and mRNAs for the identification of
biomarkers for osteoporosis in the mandible of ovariectomized mice.
Int J Mol Med. 40:689–702. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cui L, Nai M, Zhang K, Li L and Li R:
lncRNA WT1-AS inhibits the aggressiveness of cervical cancer cell
via regulating p53 expression via sponging miR-330-5p. Cancer Manag
Res. 11:651–667. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Coulson KA, Reed G, Gilliam BE, Kremer JM
and Pepmueller PH: Factors influencing fracture risk, T score, and
management of osteoporosis in patients with rheumatoid arthritis in
the Consortium of Rheumatology Researchers of North America
(CORRONA) registry. J Clin Rheumatol. 15:155–160. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Al-Azzawi F: Prevention of postmenopausal
osteoporosis and associated fractures: Clinical evaluation of the
choice between estrogen and bisphosphonates. Gynecol Endocrinol.
24:601–609. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Du T, Zhang B, Zhang S, Jiang X, Zheng P,
Li J, Yan M, Zhu Z and Liu B: Decreased expression of long
non-coding RNA WT1-AS promotes cell proliferation and invasion in
gastric cancer. Biochim Biophys Acta. 1862:12–19. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dai SG, Guo LL, Xia X and Pan Y: Long
non-coding RNA WT1-AS inhibits cell aggressiveness via
miR-203a-5p/FOXN2 axis and is associated with prognosis in cervical
cancer. Eur Rev Med Pharmacol Sci. 23:486–495. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Komori T: Cell death in chondrocytes,
osteoblasts, and osteocytes. Int J Mol Sci. 17(2045)2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lorentzon M and Cummings SR: Osteoporosis:
The evolution of a diagnosis. J Intern Med. 277:650–661.
2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Thomson DW and Dinger ME: Endogenous
microRNA sponges: Evidence and controversy. Nat Rev Genet.
17:272–283. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yu Y, Newman H, Shen L, Sharma D, Hu G,
Mirando AJ, Zhang H, Knudsen E, Zhang GF, Hilton MJ, et al:
Glutamine metabolism regulates proliferation and lineage allocation
in skeletal stem cells. Cell Metab. 29:966–978. e4. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hayashi M, Nakashima T, Yoshimura N,
Okamoto K, Tanaka S and Takayanagi H: Autoregulation of osteocyte
Sema3A orchestrates estrogen action and counteracts bone aging.
Cell Metab. 29:627–637.e5. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fan Y, Hanai JI, Le PT, Bi R, Maridas D,
DeMambro V, Figueroa CA, Kir S, Zhou X, Mannstadt M, et al:
Parathyroid hormone directs bone marrow mesenchymal cell fate. Cell
Metab. 25:661–672. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Stegen S, van Gastel N, Eelen G,
Ghesquière B, D'Anna F, Thienpont B, Goveia J, Torrekens S, Van
Looveren R, Luyten FP, et al: HIF-1α promotes glutamine-mediated
redox homeostasis and glycogen-dependent bioenergetics to support
postimplantation bone cell survival. Cell Metab. 23:265–279.
2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liu S, Liu D, Chen C, Hamamura K,
Moshaverinia A, Yang R, Liu Y, Jin Y and Shi S: MSC transplantation
improves osteopenia via epigenetic regulation of notch signaling in
lupus. Cell Metab. 22:606–618. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mera P, Laue K, Ferron M, Confavreux C,
Wei J, Galán-Díez M, Lacampagne A, Mitchell SJ, Mattison JA, Chen
Y, et al: Osteocalcin signaling in myofibers is necessary and
sufficient for optimum adaptation to exercise. Cell Metab.
23:1078–1092. 2016.PubMed/NCBI View Article : Google Scholar
|