Introduction
Bone healing is a complex and long process which
involves the coordinated action of several cell types and a complex
cascade of mechanisms and signaling pathways (1,2). It
can be affected by various factors, such as the degree of the
fracture gap and comminution, soft tissue damage, age or nutrition
as well as the administration of several pharmacological agents
including steroids and antibiotics (3). Glucocorticoids are used at extremely
high doses for the treatment of acute situations such as spinal
cord injury or acute exacerbations of life-threatening immune
disorders (1,4). These drugs are potent
anti-inflammatory and immunosuppressive agents that inhibit the
expression of pro-inflammatory cytokines, adhesion molecules,
cyclooxygenases (COX) and the production of prostaglandins (PGs)
(4,5). Glucocorticoids also act directly on
bone cells and promote apoptosis of osteoblasts and osteocytes
leading to reduced bone formation and strength (6) or steroid-induced osteoporosis after
long treatment (7).
Non-steroidal anti-inflammatory drugs (NSAIDs) are
also widely used in the treatment of pain, including bone fracture
pain and orthopedic post-operative pain. Due to their analgesic
potency, anti-inflammatory properties and restricted side effects
as compared to opioids, there has been a tremendous expansion in
their use worldwide (3,8). NSAIDs exert anti-inflammatory effects
by inhibiting the synthesis of COX enzymes that catalyze the
production of PGs. PGs are lipid mediators playing an important
role in bone repair and were reported to regulate inflammation,
increase osteoblast proliferation and differentiation and enhance
osteoclast activity and bone resorption (9). Even though the exact mechanism of
action of PGs on bone cells is not clear, inhibition of PG
production retards bone formation, whereas local administration of
exogenous PGs can stimulate new bone formation on injured bone
(3). The effect of NSAIDs on
osteoblast growth has been attributed to the inhibition of PG
synthesis. However, the exact mechanism is still under debate. Some
NSAIDs (e.g., celecoxib, diclofenac, piroxicam and indomethacin)
suppress proliferation of osteoblasts by cell cycle arrest in phase
G0/G1, which may then suppress tissue formation and regeneration in
bone remodeling (10,11). In addition, changes in the
expression of antigenic molecules such as CD80, CD86, and HLA-DR
following treatment with NSAIDs have been reported in the MG-63
osteoblast-like cells (12,13), though not in osteoblasts as reported
in another publication investigating celecoxib (14). NSAIDs have been reported to affect
osteoblast differentiation by altering alkaline phosphatase
activity, type I collagen and the calcium mineralization, but there
is no consensus in the scientific publications that leads to a
general mode of action for the different NSAIDs (11). The use of NSAIDs in the clinical
setting of orthopedic surgery has long been a topic of controversy
(15-17).
Reports from animal studies and in vitro experiments have
shown that NSAIDs delayed fracture healing, caused non-union, and
decreased the mineral content and matrix of the callus (18-21),
whereas others studies failed to prove any inhibitory effect
(14,22,23).
NSAIDs exhibit differences in COX isoenzyme
inhibition and are classified as ‘conventional or non-selective’
acting as both COX-1 and COX-2 inhibitors, or ‘coxibs’ acting only
as COX-2 inhibitors (24). A
disputable issue is the superiority of coxibs over non-selective
COX inhibitors regarding bone metabolism and fracture repair
(25). While some authors claimed
that selective COX-2 inhibitors delayed fracture healing less than
non-selective COX inhibitors (19),
others point out that NSAID types compromise fracture healing and
should therefore be used with caution in the clinics (26). The controversy among different
studies, potentially deriving from the diversity of the
experimental studies, denotes that the effects of NSAIDs on bone
remain unclear. Hence, their use in fracture patients has been
suggested to be cautious (9,27) and
further evidence of potential effects on bone cells and their
precursors is imperative (8,28).
Here we investigated the effects of five different
COX-inhibitor NSAIDs, on the viability and osteogenic
differentiation of pre-osteoblasts using the MC3T3-E1 mouse
calvarial cell line. MC3T3-E1 cells were treated with the selective
COX-2 inhibitors parecoxib and meloxicam, the non-selective NSAIDs
lornoxicam and diclofenac, and paracetamol, a non-typical NSAID
with selectivity over COX-2(29).
The widely used steroidal drug, prednisolone, was used as a means
of comparison (4). Cell viability
was measured based on the redox potential of living cells, with the
PrestoBlue™ assay. The osteogenic differentiation was assessed with
the alkaline phosphatase (ALP) activity assay and quantification of
calcium deposits by means of alizarin Red S (ARS) staining.
Materials and methods
Materials and NSAIDs
Minimum essential Eagle's medium (α-MEM), ascorbic
acid, β-glycerophosphate, penicillin/streptomycin, fetal bovine
serum (FBS), trypsin/EDTA, ARS, cetylpyridinium chloride (CPC) and
p-nitrophenyl phosphate were purchased from Sigma-Aldrich; Merck
KGaA. PrestoBlue™ reagent was purchased from Invitrogen; Thermo
Fisher Scientific, Inc. Lornoxicam and prednisolone were purchased
from Nycomed; parecoxib from Pfizer; meloxicam from
Boehringer-Ingelheim; diclofenac sodium from Novartis; and
paracetamol from Uni-Pharma.
Cell culture and NSAID
concentrations
The MC3T3-E1 pre-osteoblasts are a non-transformed
cell line derived from newborn mouse calvaria which can
differentiate to osteoblasts (30).
MC3T3-E1 cells were obtained from DSMZ GmbH (DSMZ no. ACC 210).
Cells were grown in α-MEM medium supplemented with 10% fetal bovine
serum (FBS) and 1% penicillin/streptomycin and subcultured once a
week using trypsin/EDTA. Cultures were maintained at
37˚C in a humidified atmosphere of 5% CO2 in
air. Cells between passages 6 and 15 were used for all the
experiments.
Concentrations of NSAIDs in cell studies usually
range between 1-100 µM (10-4-10-6 M), but
higher or lower doses have also been reported. In this study, the
concentrations were selected based on previous in vitro
studies (9,22), and are consistent with the most
therapeutically relevant concentrations of NSAIDs (10-5
M for non-selective NSAIDs and 10-6 M for COX-2
inhibitors according to literature (10,13).
Cell viability measurement
Cell viability was assessed using the PrestoBlue™
assay (Invitrogen; Thermo Fisher Scientific, Inc.). The assay is
based on the cellular reduction of a resazurin-based, non-toxic
metabolic indicator to a red product, which can be detected using
absorbance measurements and provides a measure of cell viability.
MC3T3-E1 pre-osteoblasts were seeded in 96-well plates (5,000
cells/well) in culture medium (α-MEM containing 10% FBS) and
cellular adhesion was allowed to take place without the influence
of drugs. After 24 h, culture media were replaced with
drug-containing media or drug-free media (control samples) and
changed every 2-3 days. Cells were treated with drugs for a total
of 15 days and cell growth was monitored at 5, 10 and 15 days. At
each time point, Presto Blue reagent was directly added to the
wells (1:10) and incubated at 37˚C for 30 min.
Absorbance was measured using a spectrophotometer (SpectraMax M2,
Molecular Devices Inc.) at 570 and 600 nm. Culture medium was used
as blank and results were expressed as % cell viability of control
(non-treated) cultures (100%). The same cell cultures were used for
sequential cell viability measurements at different time points.
After each measurement supernatants were replaced with fresh
culture medium with or without NSAIDs and the plates were returned
to the incubator for further incubation.
Measurement of ALP activity
Measurement of increased ALP expression either
enzymatically, histochemically or at the mRNA level is considered
as an early marker and reliable indication of the osteoblastic
phenotype as well as a good predictor of tissue mineralization
(31). MC3T3-E1 cells were plated
in 96-well plates at a density of 5x104 cells/well. On
day 1, culture medium was replaced by osteogenic medium (culture
medium supplemented with 10 mM sodium glycerophosphate and 50 µg/ml
ascorbic acid) and cells were treated with drugs (10-6
M) for 4 or 7 days. ALP activity was assayed using an enzymatic
activity assay during the first week of differentiation. Briefly,
cells were rinsed with phosphate buffered saline (PBS, pH 7.4),
lysed with 100 µl lysis buffer (0.1% Triton X-100, 50 mM Tris-HCl,
50 mM phenylmethylsulfonyl fluoride (PMSF), pH 10) and frozen at
-60˚C. After thawing, 100 µl of freshly prepared assay
buffer [2 mg/ml p-nitrophenyl-phosphate (pNPP) substrate in 50 mM
Tris-HCl, pH 10 and 2 mM MgCl2], was added to each well
and the plate was incubated at 37˚C for 30 min. The
reaction was stopped with the addition of 50 µl 1N NaOH. The
optical density (OD) of p-nitrophenol (pNP) at 405 nm was measured
and was correlated to equivalent amounts of pNP generated, using a
calibration curve constructed of known concentrations of pNP. ALP
activity was calculated using the equation [units/µl=(nmol
p-nitrophenol/hour) x dilution factor] and expressed as
U/µl. ALP activity was normalized to cell number. For this purpose,
the cell number in each well was determined with the PrestoBlue™
viability assay immediately prior to the ALP activity assay, using
a calibration curve of absorbance units vs. known numbers of cells
(32).
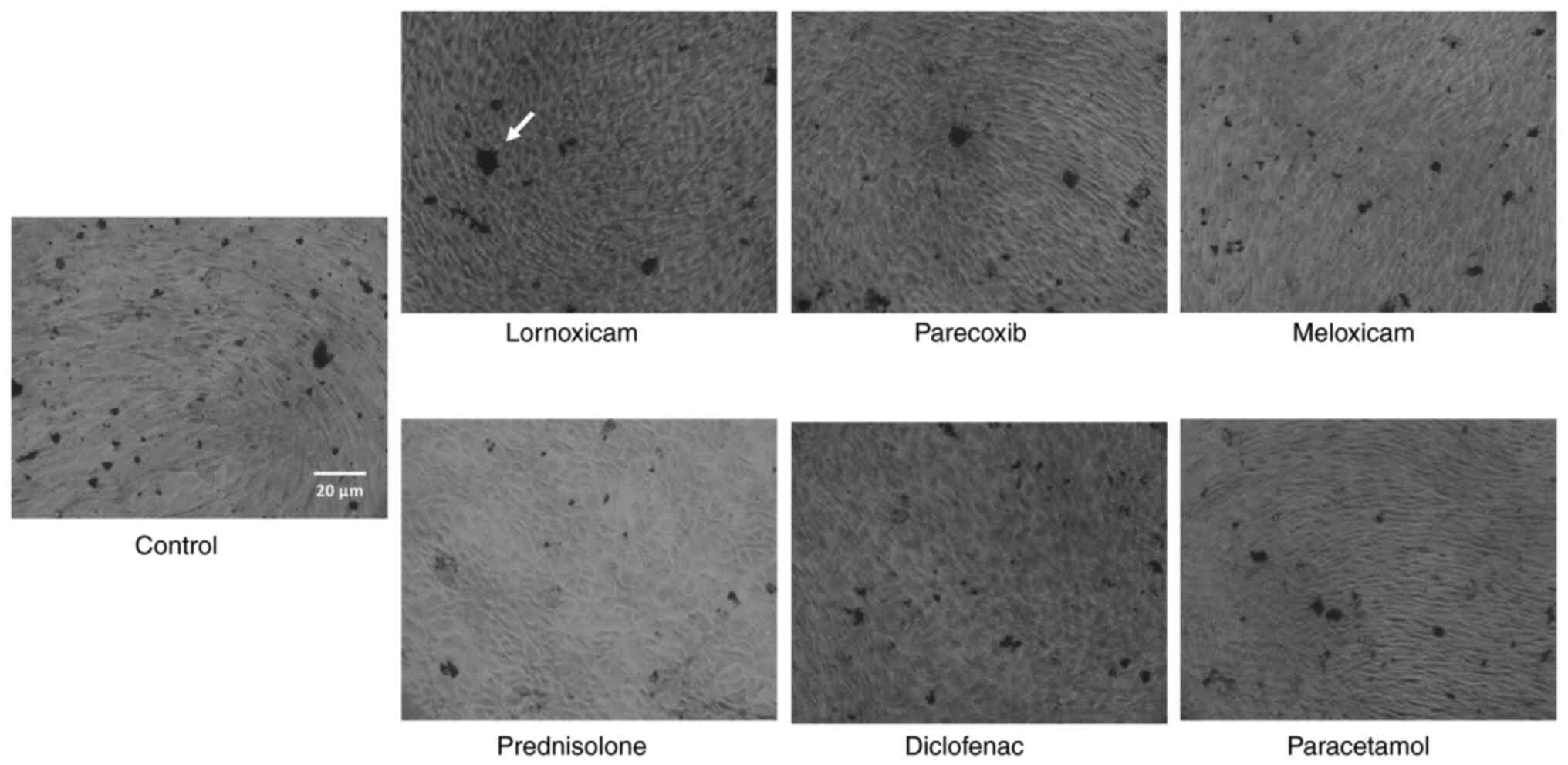
ARS staining of calcium deposits
Calcium-rich deposits representing the final stage
of mineralization were assessed in cultures undergoing
differentiation for 3 weeks (33).
The deposits were visualized by ARS staining of cell monolayers and
quantified by dye extraction (34).
Initially, MC3T3-E1 cells were plated in 24-well plates at a
density of 105/well. On day 1, the culture medium was
replaced by osteogenic medium (as described in section 2.3) and
cells were treated with NSAIDs (10-6 M) or prednisolone
(10-6 M) for 21 days. Cells were washed with PBS and
fixed with 300 µl 4% (v/v) paraformaldehyde (Sigma-Aldrich) for 20
min at room temperature. Then, they were washed three times with
excess dH2O prior to staining with 300 µl/well of 2%
(w/v) ARS (pH 4.1) for 30 min. Excess dye was aspirated and the
cells were washed four times with 500 µl dH2O. Stained
monolayers were visualized by optical microscopy. For staining
quantification, 300 µl 10% (w/v) cetylpyridinium chloride (CPC) in
10 mM sodium phosphate (pH 7.0) was added to the wells for 1 h
under mild shaking. Aliquots (200 µl) of the extract were
transferred to a 96-well plate and read in duplicates at 550 nm.
Data were expressed as units of alizarin released (1 unit is
equivalent to 1 absorbance unit at 550 nm).
Statistical analysis
Statistical analysis was performed with ANOVA
(GraphPad Prism 8.0.2 software; GraphPad Software, Inc.) followed
by Bonferroni's post-hoc test for multiple comparisons. One-way
ANOVA with Bonferroni's post-hoc test was used with ARS
experimental data, whereas two-way ANOVA with Bonferroni's post-hoc
test was applied for analysis of the alkaline phosphatase activity
and cell viability data. P<0.05 was considered to indicate a
statistically significant difference. Results are expressed as mean
values ± standard error to the mean (SEM).
Results
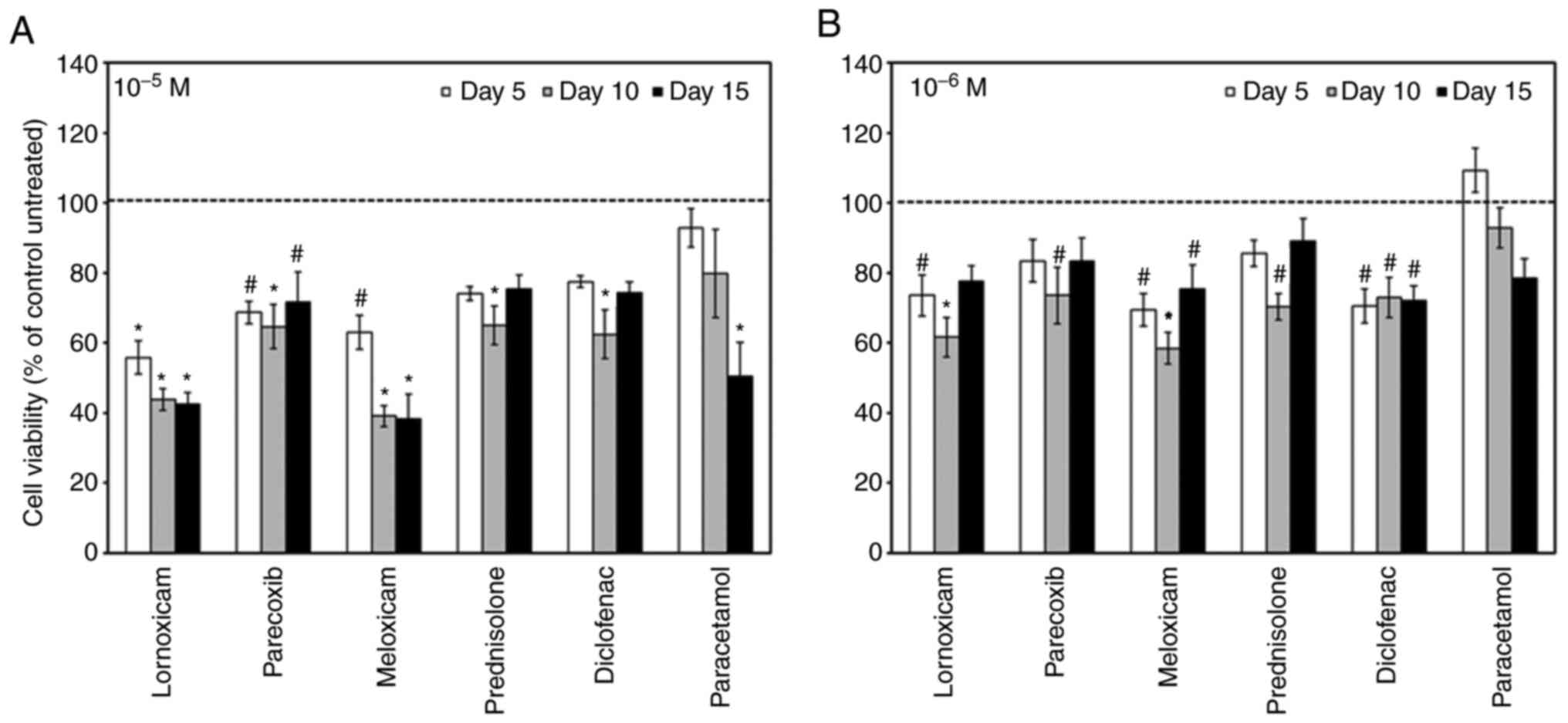
Effects of anti-inflammatory drugs on
the cell viability
First, we asked whether NSAIDs affect the cell
growth of MC3T3-E1 cells. To this end we treated MC3T3-E1 cells
with NSAIDs at 10-6 M and 10-5 M and assessed
cell viability after 5, 10 and 15 days of treatment. Regardless of
concentration, all NSAIDs significantly decreased the cell
viability compared to untreated cells, with the exception of
paracetamol at 10-6 M (Fig.
1).
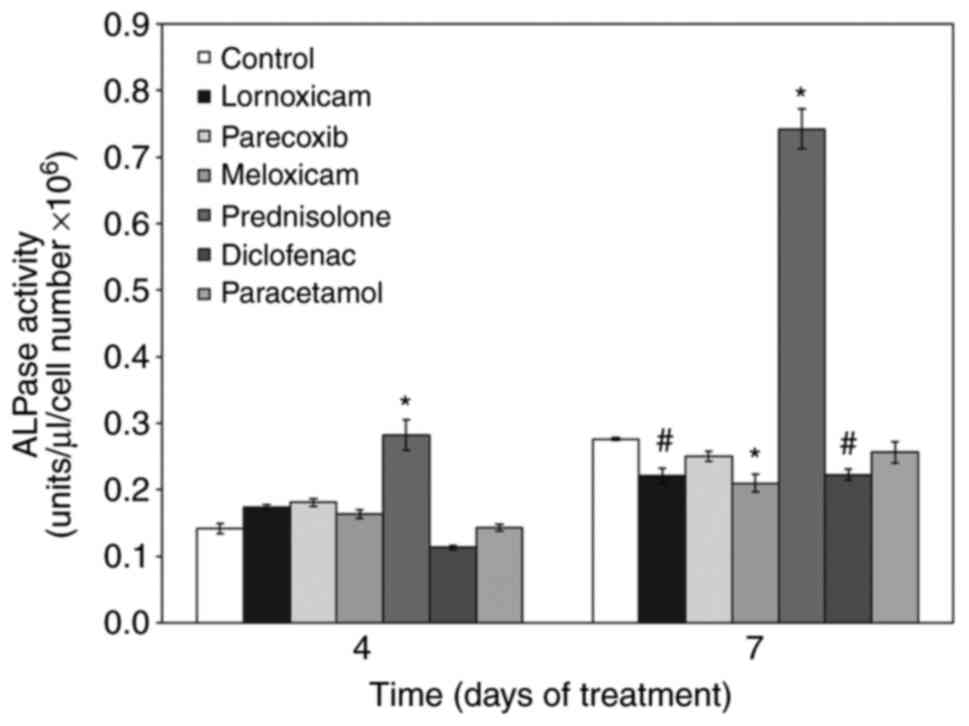
NSAIDs alter ALP activity
Next, we examined the effect of NSAIDs on the
osteogenic capacity of MC3T3-E1 cells. We first tested their effect
on ALP activity under osteogenic culture conditions. With time, ALP
activity increased in cells cultured under osteogenic conditions
(control). Addition of the non-selective COX inhibitors lornoxicam
and diclofenac as well as the selective COX-2 inhibitor meloxicam
during differentiation, reduced ALP activity. Parecoxib and
paracetamol did not affect ALP activity. In stark contrast,
treatment of cells with prednisolone strongly increased ALP
activity (Fig. 2).
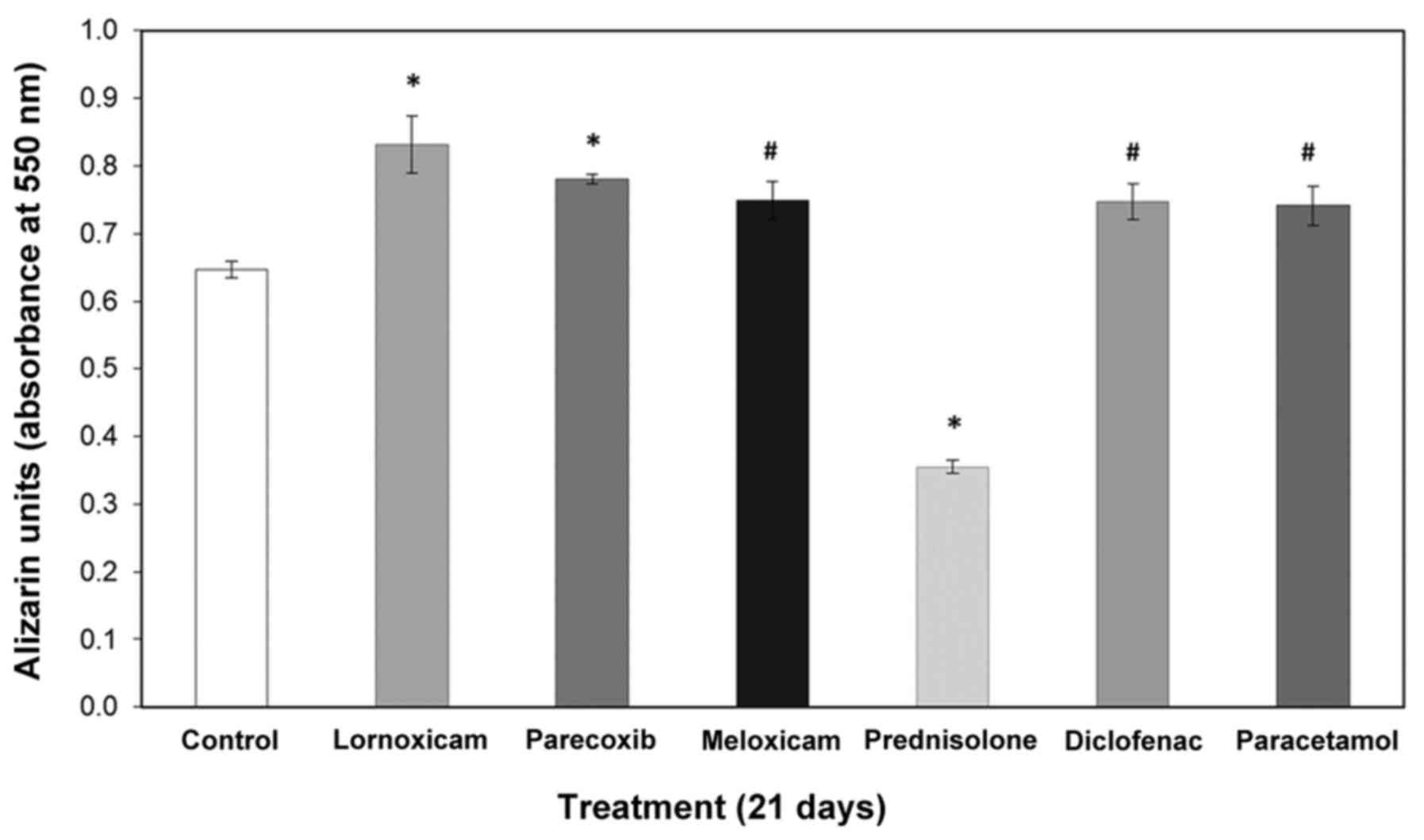
NSAIDs alter calcium deposition
Along the same line, we examined how calcium
deposition is altered by NSAIDs in pre-osteoblasts. Therefore, we
cultured MC3T3-E1 cells for 21 days in the presence or absence of
drugs and performed ARS staining. Extraction and quantitative
analysis of the deposits revealed that all NSAIDs increased calcium
deposition (Figs. 3 and 4) in contrast to prednisolone, which
significantly reduced the amount of calcium in the extracellular
matrix. Table I summarizes the
results on the effects of the tested NSAIDs on cell viability and
osteogenic differentiation potential.
 | Table ISummary of the effect of NSAIDs
(10-6 M) and prednisolone (10-6 M) in
MC3T3-E1 cells. |
Table I
Summary of the effect of NSAIDs
(10-6 M) and prednisolone (10-6 M) in
MC3T3-E1 cells.
| Drug treatment
(10-6 M) | Cell viability | ALP activity (Day
7) | Calcium Deposits
(Day 21) |
|---|
| Lornoxicam | Decreased | Decreased | Increased |
| Parecoxib | Decreased | No change | Increased |
| Meloxicam | Decreased | Decreased | Increased |
| Diclofenac | Decreased | Decreased | Increased |
| Paracetamol | No change | No change | Increased |
| Prednisolone
(steroid) | Decreased | Increased | Decreased |
Discussion
In the current study, we investigated the in
vitro effects of various non-selective or selective COX-2
inhibitor NSAIDs as well as prednisolone on the viability and
osteogenic differentiation of osteoblast precursor cells.
Currently, there is little evidence on the effects of NSAIDs
specifically on MC3T3-E1 cells. To our knowledge, our study is the
first to simultaneously investigate five different NSAIDs for their
effects on this cell line. Regarding cellular viability, we found
the effects of NSAIDs on mouse pre-osteoblasts to be dose and in
certain conditions (meloxicam and paracetamol at 10-5
M), time dependent. All NSAIDs suppressed the proliferation of
MC3T3-E1 cells at the concentrations tested, except paracetamol at
10-6 M, which had no effect. The dose-dependent effect
was more evident for lornoxicam, diclofenac and meloxicam. Our
findings are in agreement with previous studies which showed that
both non-selective and selective COX-2 inhibitor NSAIDs inhibited
the growth of osteoblasts: The cell number of cultured human
osteoblasts was reported to decrease following treatment with
indomethacin for 5 days (35) and
in another study, MC3T3-E1 cells treated with indomethacin (0.1 µM)
and celecoxib (1.5, 3.0, and 9.0 µM) displayed slower growth than
the control group (36). Both
studies reported that when more than one drug concentrations were
tested, the inhibitory effect was dose-dependent. However, our
group has previously demonstrated that at 10-6 M, NSAIDs
enhanced the viability of human adipose derived stem cells (hADSC).
At 10-5 M, only the non-selective COX-2 inhibitors
displayed cell growth-suppressive effects on hADSC (22). Our current study suggests that the
specific effects of NSAIDs greatly depend on the cell type and
species, in addition to dose and time of treatment and in agreement
with other reports (37,38). Secondly, regarding the effects on
osteogenic differentiation, we found that NSAIDs do not alter the
ALP activity of mouse MC3T3-E1 cells, with the exception of
lornoxicam, meloxicam and diclofenac (10-6 M), which
reduce ALP activity only after 7 days of treatment. Our results
also show, that the mineralization ability of mouse pre-osteoblasts
determined as calcium deposits, is enhanced by all NSAIDs at
10-6 M. For lornoxicam, meloxicam and diclofenac, this
finding does not correlate well to the small inhibitory effect
exerted on ALP activity (Fig. 2),
as an increase in both ALP and calcium deposits are considered
markers of osteogenic differentiation. However, previous studies on
different cell types demonstrated that calcium deposition or ALP
may not be influenced by NSAIDs (10,35,39).
For example, diclofenac was reported to have no effect on the
mineralization of mouse BMSCs and human MSCs (10,39).
We have previously reported, that meloxicam and paracetamol
enhanced the mineralization of hADSC, while lornoxicam, diclofenac
and parecoxib, had no effect (22).
Paracetamol was also investigated in this study; it
is not a typical NSAID, but it appears to inhibit COX-2 to a degree
similar to that of selective COX2 inhibitors and other NSAIDs
(40). However, the underlying
mechanism is believed to be more complex with several factors
involved, including inhibition of PG synthesis, induction of
apoptosis by different pathways and cell cycle arrest (41,42).
In this study, we did not observe any significant reduction in the
proliferation capacity (except for 10-5 M and 15-day
treatment) or osteogenic maturation of the paracetamol-treated
MC3T3-E1 cells at 10-6 M. A previous study has reported
inhibition of ALP activity of MC3T3-E1 but a higher doses of
paracetamol (33-330 µM) (43).
The effect of prednisolone on MC3T3-E1 cells was
found to differ from that of NSAIDs in this study: The steroid drug
had a comparable effect to that of NSAIDs' on the viability of
growing cultures of MC3T3 pre-osteoblasts, but markedly increased
the ALP activity in confluent cultures undergoing osteogenic
differentiation. Simultaneously, prednisolone reduced calcium
deposition. We found that prednisolone (10-6 M) induced
a stimulatory effect on the ALP activity of MC3T3-E1 cells when
normalized to live cells. Notably, over the course of the
experiments in osteogenic media, we observed that prednisolone
(10-6 M) dramatically reduced the viability of confluent
differentiating cells (to about 50% as compared to the control
group-data not shown). This suggests that prednisolone may initiate
apoptosis of confluent osteoblast cultures, leading to fewer
osteoblasts in the culture. In fact, the negative effects of
corticosteroids on bone physiology are well established and
corticosteroid treatment is accepted as a cause of drug-induced
(secondary) osteoporosis (44).
Previous studies have shown that glucocorticoids act directly on
osteoblasts and osteocytes and induce apoptosis, but are also
essential in the regulation of osteoblast differentiation (45). However, reported data have shown
different or even contradictive outcomes on whether glucocorticoids
inhibit or increase biological activity of skeletal cells,
including osteoblasts, osteoclasts, osteocytes and their
precursors. Their activities on osteoblasts depend on
concentration, different developing stages of osteoblasts or
osteoblast precursors, different species and kinds of
glucocorticoids (46).
Future studies concerning changes in the osteogenic
marker gene expression as well as the antigenic profile of the
pre-osteoblastic cells will provide more insight into the mechanism
of the action of NSAIDs on these cells. Some gene expression
studies have already been conducted on human osteoblasts and the
osteosarcoma osteoblast-like cell line MG63(42). Regarding MC3T3-E1, Nagano et
al found that celecoxib inhibited the mRNA expression of both
Runx2 and Alp (47).
While further investigation is needed, we currently have
preliminary data (Data S1) to
suggest that none of the NSAIDs investigated in this study inhibits
the gene expression of either Runx2 or Bsp genes
(Fig. S1).
In conclusion, the present in vitro study
examined the interference of NSAIDs and prednisolone with the
proliferation and the osteogenic differentiation of mouse
pre-osteoblasts, a process that directly affects osteoblast
physiology and ultimately bone healing. Through the present study
as well as our previous study on ADMSCs, we have shown that NSAIDs
can modulate the behaviour of osteoblasts but not all NSAIDs share
specific effects, in agreement with previous reports (11). Certainly, prednisolone, a synthetic
glucocorticoid appears as the outlier in the osteogenic
differentiation experiments we performed. Its behaviour does not
correlate well to that of the NSAID group nor does its structure as
a steroid lipid molecule belonging to the class of organic
compounds known as 21-hydroxysteroids. It has been reported that
high glucocorticoid levels therapies impair bone growth leading to
glucocorticoid-induced osteoporosis (48). Elucidating structure-activity
relationships for NSAIDs requires data sets that provide
experimental measurements of activity for a group of chemicals
defined by some selection criteria in terms of structure. A
statistical analysis method can then be applied to relate structure
to activity. A recent review study on quantitative structure
activity relationships (QSAR) of NSAIDs has identified a link
between functional groups which enhance the lipophilicity and an
increase in the anti-inflammatory activity of the drugs (49). Structural characteristics of the
NSAIDs have been reported to govern anti-inflammatory activity,
including steric factors, physicochemical parameters expressing the
overall volume/size of the molecules, molar refractivity, the role
of heteroatoms such as nitrogen or sulfur in the heteroatomic
system bearing one or two phenyl rings and at least one carbonyl
group, and finally electronic parameters, indicative of
dipole-dipole or charge-dipole interactions, charge-transfer
phenomena and hydrogen bond formation (50). It would be certainly helpful to
explore derivatives with a wider spread in substituents to study
the effect of these physicochemical structural features. Overall,
it can be stated that there is a similarity in terms of the
requirement for hydrophobicity and size of the molecules for both
COX-1 and COX-2 receptors (50). In
the same direction, data sets from our study as well as similar
studies on NSAIDs are important for modelling NSAID
structure-activity relations in relation to their effects on
osteogenic response and cell proliferation. Even though concrete
conclusions on structure-activity cannot be drawn from a single
study, these data may be useful in future QSAR studies of NSAIDs
(49,50). Given the potential clinical
implications, our findings are also expected to be beneficial to
further investigation through in vivo studies.
Supplementary Material
Supplementary material is provided
below on preliminary experiments concerning the effect of NSAIDs
and prednisolone on the expression of Runx2 and Bsp genes in
MC3T3-E1 cells.
mRNA expression levels of (A)
Runx2 and (B) Bsp in cells treated with NSAIDs or
prednisolone for 1 or 7 days in osteogenic medium, respectively.
Expression levels were normalized to the 18S housekeeping
gene. Cells in non-osteogenic medium (control) and cells in
osteogenic medium (osteogen. M) are shown for comparison. Each bar
represents the mean ± SE, of triplicate technical and duplicate
biological samples in two independent experiments (n=12);
statistical analysis was performed using a t-test.
*P<0.05 vs. untreated control. NSAIDs, Nonsteroidal
anti-inflammatory drugs.
Acknowledgements
The authors would like to thank Dr Vasileia Ismini
Alexaki (Institute of Clinical Chemistry and Laboratory Medicine,
Faculty of Medicine and University Clinic Carl Gustav Carus,
Technische Universität Dresden, Germany) for aiding in useful
discussion regarding this manuscript.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CH, KA and MC made substantial contributions to
conception and design of the study and the experiments, CH
performed the experiments, analyzed and interpreted the data, CH
and KA wrote the manuscript, MC revised the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Barry S: Non-steroidal anti-inflammatory
drugs inhibit bone healing: A review. Vet Comp Orthop Traumatol.
23:385–392. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cottrell J and O'Connor JP: Effect of
non-steroidal anti-inflammatory drugs on bone healing.
Pharmaceuticals (Basel). 3:1668–1693. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pountos I, Georgouli T, Blokhuis TJ, Pape
HC and Giannoudis PV: Pharmacological agents and impairment of
fracture healing: What is the evidence? Injury. 39:384–394.
2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Patschan D, Loddenkemper K and Buttgereit
F: Molecular mechanisms of glucocorticoid-induced osteoporosis.
Bone. 29:498–505. 2001.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Canalis E: Mechanisms of glucocorticoid
action in bone. Curr Osteoporos Rep. 3:98–102. 2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
O'Brien CA, Jia D, Plotkin LI, Bellido T,
Powers CC, Stewart SA, Manolagas SC and Weinstein RS:
Glucocorticoids act directly on osteoblasts and osteocytes to
induce their apoptosis and reduce bone formation and strength.
Endocrinology. 145:1835–1841. 2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Paget S: Steroids cause osteoporosis. Ann
Rheum Dis. 61:1–3. 2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Harder AT and An YH: The mechanisms of the
inhibitory effects of nonsteroidal anti-inflammatory drugs on bone
healing: A concise review. J Clin Pharmacol. 43:807–815.
2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pountos I, Georgouli T, Calori GM and
Giannoudis PV: Do nonsteroidal anti-inflammatory drugs affect bone
healing? A critical analysis. ScientificWorldJournal.
2012(606404)2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chang JK, Li CJ, Wu SC, Yeh CH, Chen CH,
Fu YC, Wang GJ and Ho ML: Effects of anti-inflammatory drugs on
proliferation, cytotoxicity and osteogenesis in bone marrow
mesenchymal stem cells. Biochem Pharmacol. 74:1371–1382.
2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
García-Martínez O, De Luna-Bertos E,
Ramos-Torrecillas J, Manzano-Moreno FJ and Ruiz C: Repercussions of
NSAIDS drugs on bone tissue: The osteoblast. Life Sci. 123:72–77.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Díaz-Rodríguez L, García-Martínez O, De
Luna-Bertos E, Ramos-Torrecillas J and Ruiz C: Effect of ibuprofen
on proliferation, differentiation, antigenic expression, and
phagocytic capacity of osteoblasts. J Bone Miner Metab. 30:554–560.
2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
De Luna-Bertos E, Ramos-Torrecillas J,
García-Martínez O, Guildford A, Santin M and Ruiz C: Therapeutic
doses of nonsteroidal anti-inflammatory drugs inhibit osteosarcoma
MG-63 osteoblast-like cells maturation, viability, and
biomineralization potential. ScientificWorldJournal.
2013(809891)2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Costela-Ruiz VJ, Melguizo-Rodríguez L,
Illescas-Montes R, Ramos-Torrecillas J, Manzano-Moreno FJ, Ruiz C
and Bertos EL: Effects of therapeutic doses of celecoxib on several
physiological parameters of cultured human osteoblasts. Int J Med
Sci. 16:1466–1472. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wheatley BM, Nappo KE, Christensen DL,
Holman AM, Brooks DI and Potter BK: Effect of NSAIDs on bone
healing rates: A meta-analysis. J Am Acad Orthop Surg.
27:e330–e336. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lisowska B, Kosson D and Domaracka K:
Positives and negatives of nonsteroidal anti-inflammatory drugs in
bone healing: The effects of these drugs on bone repair. Drug Des
Devel Ther. 12:1809–1814. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Geusens P, Emans PJ, de Jong JJ and van
den Bergh J: NSAIDs and fracture healing. Curr Opin Rheumatol.
25:524–531. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Beck A, Salem K, Krischak G, Kinzl L,
Bischoff M and Schmelz A: Nonsteroidal anti-inflammatory drugs
(NSAIDs) in the perioperative phase in traumatology and orthopedics
effects on bone healing. Oper Orthop Traumatol. 17:569–578.
2005.PubMed/NCBI View Article : Google Scholar : (In English,
German).
|
|
19
|
Gerstenfeld LC, Thiede M, Seibert K,
Mielke C, Phippard D, Svagr B, Cullinane D and Einhorn TA:
Differential inhibition of fracture healing by non-selective and
cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J
Orthop Res. 21:670–675. 2003.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Herbenick MA, Sprott D, Stills H and
Lawless M: Effects of a cyclooxygenase 2 inhibitor on fracture
healing in a rat model. Am J Orthop (Belle Mead NJ). 37:E133–E137.
2008.PubMed/NCBI
|
|
21
|
Manzano-Moreno FJ, Costela-Ruiz VJ,
Melguizo-Rodriguez L, Illescas-Montes R, García-Martínez O, Ruiz C
and Ramos-Torrecillas J: Inhibition of VEGF gene expression in
osteoblast cells by different NSAIDs. Arch Oral Biol. 92:75–78.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hadjicharalambous C, Alexaki VI, Alpantaki
K and Chatzinikolaidou M: Effects of NSAIDs on the osteogenic
differentiation of human adipose tissue-derived stromal cells. J
Pharm Pharmacol. 68:1403–1408. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jain NX, Barr-Gillespie AE, Clark BD,
Kietrys DM, Wade CK, Litvin J, Popoff SN and Barbe MF: Bone loss
from high repetitive high force loading is prevented by ibuprofen
treatment. J Musculoskelet Neuronal Interact. 14:78–94.
2014.PubMed/NCBI
|
|
24
|
Darnis D, Veyrac G and Jolliet P: The
special case of diclofenac. Enliven: Pharmacovigil Drug Saf.
1(3)2014.
|
|
25
|
Schwarting T, Pretzsch S, Debus F,
Ruchholtz S and Lechler P: The effect of cyclooxygenase inhibition
on tendon-bone healing in an in vitro coculture model. Med
Inflammation. 2015(926369)2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kidd LJ, Cowling NR, Wu AC, Kelly WL and
Forwood MR: Selective and non-selective cyclooxygenase inhibitors
delay stress fracture healing in the rat ulna. J Orthop Res.
31:235–242. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Vuolteenaho K, Moilanen T and Moilanen E:
Non-steroidal anti-inflammatory drugs, cyclooxygenase-2 and the
bone healing process. Basic Clin Pharmacol Toxicol. 102:10–14.
2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Marquez-Lara A, Hutchinson ID, Nunez F Jr,
Smith TL and Miller AN: Nonsteroidal anti-inflammatory drugs and
bone-healing: A systematic review of research quality. JBJS Rev.
4(01874474-201603000-00005)2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Graham GG, Davies MJ, Day RO, Mohamudally
A and Scott KF: The modern pharmacology of paracetamol: Therapeutic
actions, mechanism of action, metabolism, toxicity and recent
pharmacological findings. Inflammopharmacology. 21:201–232.
2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kodama H, Amagai Y, Sudo H and Yamamoto S:
Establishment of a clonal osteogenic cell line from newborn mouse
calvaria. Jpn J Oral Biol. 23:899–901. 1981.
|
|
31
|
Golub E and Boesze-Battaglia KJ: The role
of alkaline phosphatase in mineralization. Curr Opin Orthopaedics.
18:444–448. 2007.
|
|
32
|
Hadjicharalambous C, Buyakov A, Buyakova
S, Kulkov S and Chatzinikolaidou M: Porous alumina, zirconia and
alumina/zirconia for bone repair: fabrication, mechanical and in
vitro biological response. Biomed Mater. 10(025012)2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Feng Y, Su L, Zhong X, Guohong W, Xiao H,
Li Y and Xiu L: Exendin-4 promotes proliferation and
differentiation of MC3T3-E1 osteoblasts by MAPKs activation. J Mol
Endocrinol. 56:189–199. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hadjicharalambous C, Kozlova D, Sokolova
V, Epple M and Chatzinikolaidou M: Calcium phosphate nanoparticles
carrying BMP-7 plasmid DNA induce an osteogenic response in
MC3T3-E1 pre-osteoblasts. J Biomed Mater Res A. 103:3834–3842.
2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Evans CE and Butcher C: The influence on
human osteoblasts in vitro of non-steroidal anti-inflammatory drugs
which act on different cyclooxygenase enzymes. J Bone Joint Surg
Br. 86:444–449. 2004.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Arpornmaeklong P, Akarawatcharangura B and
Pripatnanont P: Factors influencing effects of specific COX-2
inhibitor NSAIDs on growth and differentiation of mouse osteoblasts
on titanium surfaces. Int J Oral Maxillofac Implants. 23:1071–1081.
2008.PubMed/NCBI
|
|
37
|
Gunaydin C and Bilge SS: Effects of
nonsteroidal anti-inflammatory drugs at the molecular level.
Eurasian J Med. 50:116–121. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kotake S, Yago T, Kawamoto M and Nanke Y:
Effects of NSAIDs on differentiation and function of human and
murine osteoclasts-crucial ‘Human osteoclastology’. Pharmaceuticals
(Basel). 3:1394–1410. 2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Pountos I, Giannoudis PV, Jones E, English
A, Churchman S, Field S, Ponchel F, Bird H, Emery P and McGonagle
D: NSAIDS inhibit in vitro MSC chondrogenesis but not
osteogenesis: Implications for mechanism of bone formation
inhibition in man. J Cell Mol Med. 15:525–534. 2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hinz B, Cheremina O and Brune K:
Acetaminophen (paracetamol) is a selective cyclooxygenase-2
inhibitor in man. FASEB J. 22:383–390. 2008.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Díaz-Rodríguez L, García-Martínez O,
Arroyo-Morales M, Rubio-Ruiz B and Ruiz C: Effect of acetaminophen
(paracetamol) on human osteosarcoma cell line MG63. Acta Pharmacol
Sin. 31:1495–1499. 2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Melguizo-Rodriguez L, Costela-Ruiz VJ,
Manzano-Moreno FJ, Illescas-Montes R, Ramos-Torrecillas J,
García-Martínez O and Ruiz C: Repercussion of nonsteroidal
anti-inflammatory drugs on the gene expression of human
osteoblasts. PeerJ. 6(e5415)2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Nakatsu Y, Nakagawa F, Higashi S, Ohsumi
T, Shiiba S, Watanabe S and Takeuchi H: Effect of acetaminophen on
osteoblastic differentiation and migration of MC3T3-E1 cells.
Pharmacol Rep. 70:29–36. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Sivagurunathan S, Muir MM, Brennan TC,
Seale JP and Mason RS: Influence of glucocorticoids on human
osteoclast generation and activity. J Bone Miner Res. 20:390–398.
2005.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Mitra R: Adverse effects of
corticosteroids on bone metabolism: A review. PM R. 3:466–471.
2011.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Hong D, Chen HX, Ge RS and Li JC: The
biological roles of extracellular and intracytoplasmic
glucocorticoids in skeletal cells. J Steroid Biochem Mol Biol.
111:164–170. 2008.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Nagano A, Arioka M, Takahashi-Yanaga F,
Matsuzaki E and Sasaguri T: Celecoxib inhibits osteoblast
maturation by suppressing the expression of Wnt target genes. J
Pharmacol Sci. 133:18–24. 2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Hachemi Y, Rapp AE, Picke AK, Weidinger G,
Ignatius A and Tuckermann J: Molecular mechanisms of
glucocorticoids on skeleton and bone regeneration after fracture. J
Mol Endocrinol. 61:R75–R90. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Asirvatham S, Dhokchawle BV and Tauro SJ:
Quantitative structure activity relationships studies of
non-steroidal anti-inflammatory drugs: A review. Arabian J Chem.
12:3948–3962. 2019.
|
|
50
|
Michaelidou AS and Hadjipavlou-Litina D:
Nonsteroidal anti-inflammatory drugs (NSAIDs): A comparative QSAR
study. Chem Rev. 105:3235–3271. 2005.PubMed/NCBI View Article : Google Scholar
|