Introduction
Thyroid cancer is the most prevalent malignant tumor
of the endocrine system (1).
Globally, there has been an increase by 20% in the age-standardized
incidence rate of thyroid cancer, with a minimal alteration in the
mortality rates (2,3). Understanding the pathogenesis of
thyroid cancer is crucial for improving and appropriately tailoring
diagnostic and therapeutic strategies. MicroRNAs (miRNAs/miRs) act
by blocking mRNA translation or inducing mRNA degradation through
interactions with complementary sequences in the 3'-untranslated
regions (3'-UTRs) of their target mRNA transcripts (4,5). It
has been reported that several miRNAs, such as, miR-214 and
miR-451a, can act upstream of different proteins in papillary
thyroid carcinoma (PTC), thereby regulating PTC cell growth
(6-8).
A recent study has demonstrated that the differential expression of
mRNAs and miRNAs was closely associated with metastasis in PTC
(9). Additionally, another study
has revealed that miR-184 could regulate nasopharyngeal cancer cell
invasion and epithelial-mesenchymal transition (EMT) (10). In addition, a previous study has
suggested that oxidized miR-184 was involved in facilitating cell
apoptosis (11). EMT serves a key
role in the metastasis and invasion of malignant tumors, whereby
epithelial cells lose their adhesive ability and acquire the
migratory ability of mesenchymal cells (12-14).
The results of the UALCAN database (http://ualcan.path.uab.edu/index.html)
(15) revealed that the expression
level of thymosin β10 (TMSB10) was upregulated in thyroid cancer
tissues compared with that in normal tissues (data not shown). It
has been previously demonstrated that the mRNA expression level of
TMSB10 differed significantly between normal thyroid tissues and
PTC, with or without lymph node metastasis (LNM), and the increased
expression level of TMSB10 was associated with LNM in PTC (16), suggesting that TMSB10 may serve a
key role in PTC. Furthermore, TMSB10 knockout has been indicated to
inhibit the migratory and invasive ability of renal carcinoma cells
in vitro (17). TMSB10 has
also been revealed to be upregulated in breast cancer cells and
tissues, while TMSB10 silencing attenuates the proliferation,
invasion and migration of breast cancer cells in vitro and
in vivo (18). However, the
upstream targets and the mechanism underlying the role of TMSB10 in
PTC remain largely unknown. Therefore, further elucidation of the
effects of TMSB10 in thyroid cancer may enable the development of
novel targeted drugs for this type of cancer.
Materials and methods
Cells
The human PTC cell line TPC-1, the poorly
differentiated thyroid gland carcinoma cell line BCPAP, which has
been verified by short tandem repeat profiling, and the normal
thyroid epithelial cell line Nthy-ori 3-1 were all purchased from
American Type Culture Collection. Cells were cultured in RPMI-1640
medium containing penicillin (100 U/ml), streptomycin (0.1 mg/ml)
and supplemented with 10% FBS (both from Gibco; Thermo Fisher
Scientific, Inc.) at 37˚C in a 5% CO2 incubator.
Cell transfection
BCPAP cells were harvested using trypsin and then
seeded into 12-well plates at a density of 1x106
cells/well. Cells were transfected with 50 nM miR-184 mimics
(5'-UGGACGGAGAACUGAUAAGGGU-3') or negative control (NC) mimics
(mimic-NC; 5'-UGACGGCAUGUGAUAGAGAAGG-3'), (Guangzhou RiboBio Co.,
Ltd.) TMSB10 overexpression plasmid (pcDNA-TMSB10; 0.2 µg; Miaoling
Biological Technology Co., Ltd.) or pcDNA (empty vector, 0.2 µg)
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) for 24 h at 37˚C, according to the manufacturer's
instructions. The cells were then used for further experiments.
Reverse transcription-quantitative
(RT-q)PCR analysis
Total RNA was extracted from cells with
TRIzol® reagent (Thermo Fisher Scientific, Inc.) and
cDNA was synthesized using a Transcriptor Hi-Fi cDNA Synthesis kit
(Sigma-Aldrich; Merck KGaA). The temperature protocol was 25˚C for
5 min, 42˚C for 30 min and 85˚C for 5 min. Following PCR
amplification, RNA was quantitatively analyzed according to the
instructions of the TaqMan One Step PCR kit (Beijing Solarbio
Science & Technology Co., Ltd.). The cycling conditions of PCR
were pre-denaturation at 95˚C for 2 min, followed by a total of 40
cycles of 95˚C for 30 sec, 60˚C for 35 sec, 72˚C for 1 min. The
relative levels of miR-184 or TMSB10 mRNA were calculated using
2-ΔΔCq method (19).
β-actin and U6 served as the internal references for mRNA and
miRNA, respectively. The primers used for PCR were designed using
Primer3 Input software (version 0.4.0; https://primer3.ut.ee) and synthesized by Sangon
Biotech Co., Ltd. The primers used were: miR-184 forward:
5'-TACGACTATGTGACCTGCCTG-3', reverse 5'-TGGTTCAACTCTCCTTTCCA-3'.
TMSB10 forward: 5'-GAAATCGCCAGCTTCGATAAGG-3', reverse
5'-TCAATGGTCTCTTTGGTCGGC-3' U6 forward,
5'-TGCTGGGGCTTTCCGGCAGCGC-3' and reverse,
5'-CCCAGTGAGGTCCGGAGGT-3'. β-actin: Forward:
5'-CTCGCCTTTGCCGATCC-3', 5'-GGGGTACTTCAGGGTGAGGA-3'.
MTT assay
BCPAP cells were cultured into a 96-well plate at a
cell density of 2x104 cells/well. Following
transfection, 10 µl MTT reagent was added into each well and the
cells were incubated for an additional 4 h at 37˚C. Subsequently,
150 µl DMSO was added to each well and mixed at a low speed on a
shaking bed to dissolve formazan crystals. The absorbance at 570 nm
was measured using a microplate reader.
Colony formation assay
BCPAP cells in the logarithmic growth phase were
cultured in 6-cm culture dishes at a density of 1x103
cells/dish. The culture medium was replaced every 2 days. Following
culture for 2 weeks, the formed colonies (>50 cells) visible to
the naked eye were observed under a microscope and the culture was
then terminated. Cells were rinsed thrice in D-Hank's Balanced Salt
Solution (D-HBSS; Thermo Scientific, Inc.), fixed in 4%
paraformaldehyde for 20 min at room temperature and stained with
0.1% crystal violet solution at room temperature for 20 min.
Subsequently, the cells were washed again three times with D-HBSS,
and then observed in five random fields under a light
microscope.
Immunofluorescence (IF) staining
Briefly, BCPAP cells in the logarithmic growth phase
were seeded into 24-well plates (5x105 cells), fixed in
4% paraformaldehyde at room temperature for 10 min, and were
subsequently incubated with 1% Triton X-100 for 10 min at room
temperature. Following blocking with 2% BSA (Beijing Solarbio
Science & Technology Co., Ltd.) for 30 min at room temperature,
the cells were incubated with primary antibodies (anti-E-cadherin;
cat. no. ab231303; 1:500 and anti-vimentin; cat. no. ab8978; 1:100;
Abcam) at 4˚C overnight. The primary antibodies were then
discarded, and cells were incubated with corresponding secondary
antibodies (goat polyclonal secondary antibody to rabbit IgG, cat.
no. ab150084; goat secondary antibody to Mouse IgG; cat. no.
ab150117; 1:1,000; Abcam) at 37˚C for 1 h. Subsequently, the cells
were treated with a DAPI staining solution at room temperature in
the dark for 10 min, followed by washing three times with PBS.
Finally, the cells were observed, and images were captured under an
inverted fluorescence microscope (Olympus Corporation,
magnification, x200).
Western blot analysis
Total proteins in each group were extracted with a
RIPA buffer (Beyotime Institute of Biotechnology) and the protein
concentration was measured using a BCA kit (Beyotime Institute of
Biotechnology). The protein concentration in each group was
adjusted, and protein samples (50 µg) were separated via 10%
SDS-PAGE and then transferred onto PVDF membranes. Following
blocking with 5% skimmed milk for 2 h at room temperature, the
membranes were incubated with the appropriate concentration of the
primary antibody (anti-E-cadherin; cat. no. ab231303; 1:500;
anti-vimentin, cat. no. ab8978; 1:500; ZEB1; cat. no. ab203829;
1:500; GAPDH; cat. no. ab8245; 1:1,000; all from Abcam) at 4˚C
overnight. On the following day, the membranes were washed with PBS
for 5 min and then incubated with the corresponding secondary
antibody (goat anti-mouse IgG; cat. no. ab97040; goat anti-rabbit
IgG; cat. no. ab7090; 1:10,000; Abcam) for 1 h at room temperature.
A color-developing solution (Super ECL Detection Reagent kit;
Shanghai Yeasen Biotechnology Co., Ltd.) was then added, followed
by exposure in a dark room. Images of protein strips were captured
using a gel imaging system (Bio-Rad Laboratories, Inc.). The grey
value of the protein bands was evaluated with ImageJ 1.46r software
(National Institutes of Health). GAPDH was used as an internal
reference.
Bioinformatics analysis
StarBase database (http://starbase.sysu.edu.cn/) was searched for the
prediction of relationship between miR-184 and TMSB10. The database
revealed potential binding sites of miR-184 in the 3'-UTR of
TMSB10.
Dual-luciferase reporter assay
Transfection experiments were carried out using
Lipofectamine 2000 according to the manufacturer's protocol.
Luciferase plasmids (pGL3-basic; Promega Corporation) encompassing
wild-type or mutant TMSB10 3'-UTR were co-transfected into BCPAP
cells with miR-184 mimic or mimic-NC. At 6 h following
transfection, the transfection medium was replaced with complete
RPMI culture medium. Following incubation for 48 h, the luciferase
activity was determined using Dual-Glo® Luciferase Assay
System (Promega Corporation). Finally, the luminescence intensity
was measured using a microplate reader [Tecan (Shanghai) Trading
Co., Ltd.]. Renilla luciferase was used to be as internal
reference.
Statistical analysis
One-way ANOVA was utilized to determine the
significance among multiple groups using GraphPad Prism software
(version 7.0; GraphPad Software, Inc.), followed by Tukey's post
hoc test. The experimental data are presented as the mean ± SD.
Each experiment was repeated at least three times. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-184 overexpression attenuates
BCPAP cell proliferation
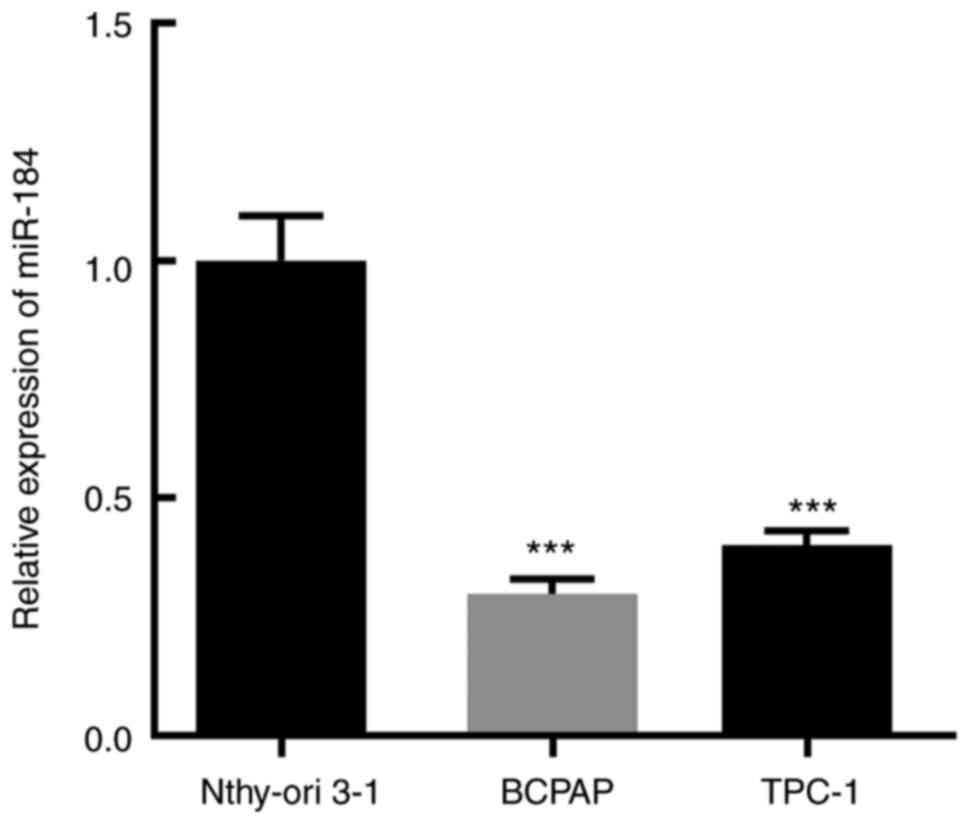
The expression level of miR-184 in the thyroid
cancer cell lines BCPAP and TPC-1 and in the normal thyroid
epithelial cell line Nthy-ori 3-1 was determined via RT-qPCR, and
the results demonstrated that miR-184 expression was most
significantly decreased in BCPAP cells (Fig. 1). It has been reported that the
expression level of miR-184 was markedly downregulated in patients
with thyroid cancer compared with healthy controls and was
positively associated with overall survival (20,21).
Therefore, the BCPAP cell line was selected for the subsequent
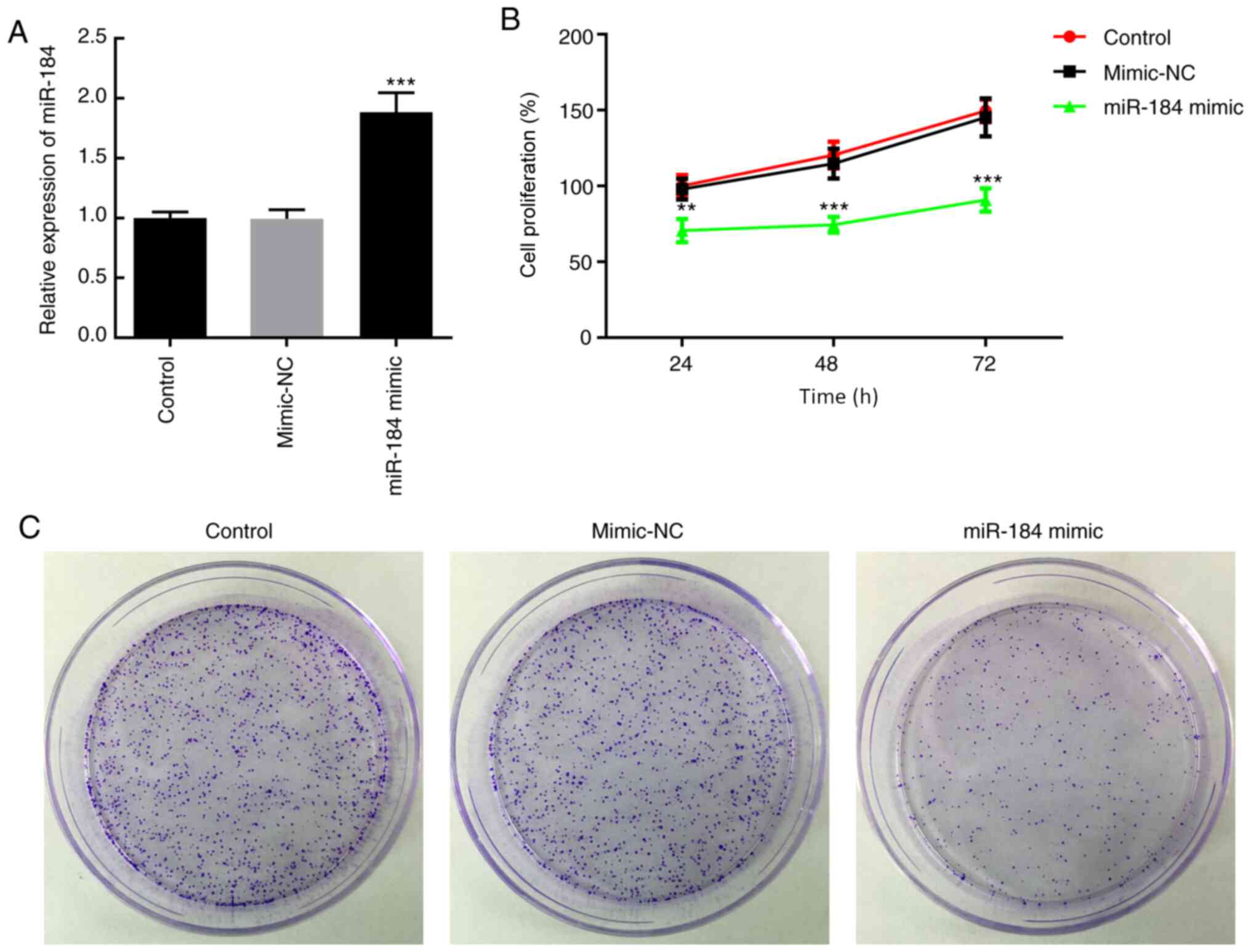
mechanistic experiments. BCPAP cells were transfected with miR-184
mimics or mimic-NC. At 48 h following transfection, miR-184
expression was increased in cells treated with miR-184 mimics
(Fig. 2A). To evaluate cell
proliferation, MTT assays were carried out at 24, 48 and 72 h after
transfection of BCPAP cells with miR-184 mimics. As presented in
Fig. 2B, BCPAP cells overexpressing
miR-184 exhibited reduced proliferative ability compared with cells
in the mimic-NC or control groups (cells receiving no treatment.
(Fig. 2B). Additionally, miR-184
overexpression reduced the colony formation ability of BCPAP cells
compared with the mimic-NC and control groups (Fig. 2C).
miR-184 overexpression suppresses
EMT
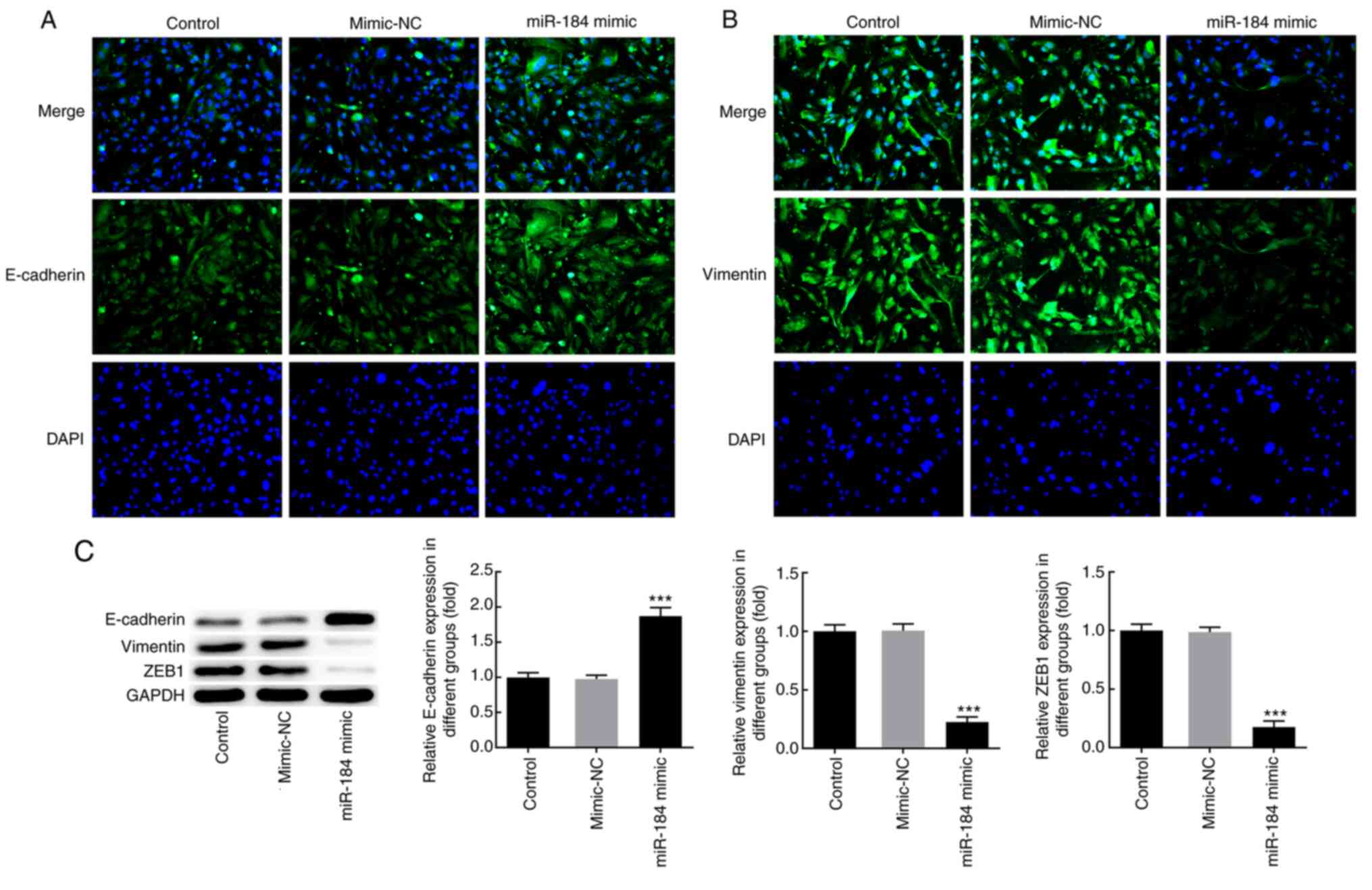
To further characterize the effects of miR-184 at
the molecular level, BCPAP cells were transfected with miR-184
mimics, and the expression of the EMT markers E-cadherin and
vimentin was detected via IF analysis. The results demonstrated
that miR-184 overexpression notably increased the expression levels
of E-cadherin and decreased those of vimentin compared with the
mimic-NC and control groups (Fig.
3A and B). In addition, western
blot analysis revealed that miR-184-overexpressing cells displayed
significantly increased E-cadherin and decreased vimentin and zinc
finger E-box-binding homeobox 1 (ZEB1) expression levels compared
with those in the control and mimic-NC groups (Fig. 3C).
miR-184 directly targets TMSB10
3'-UTR
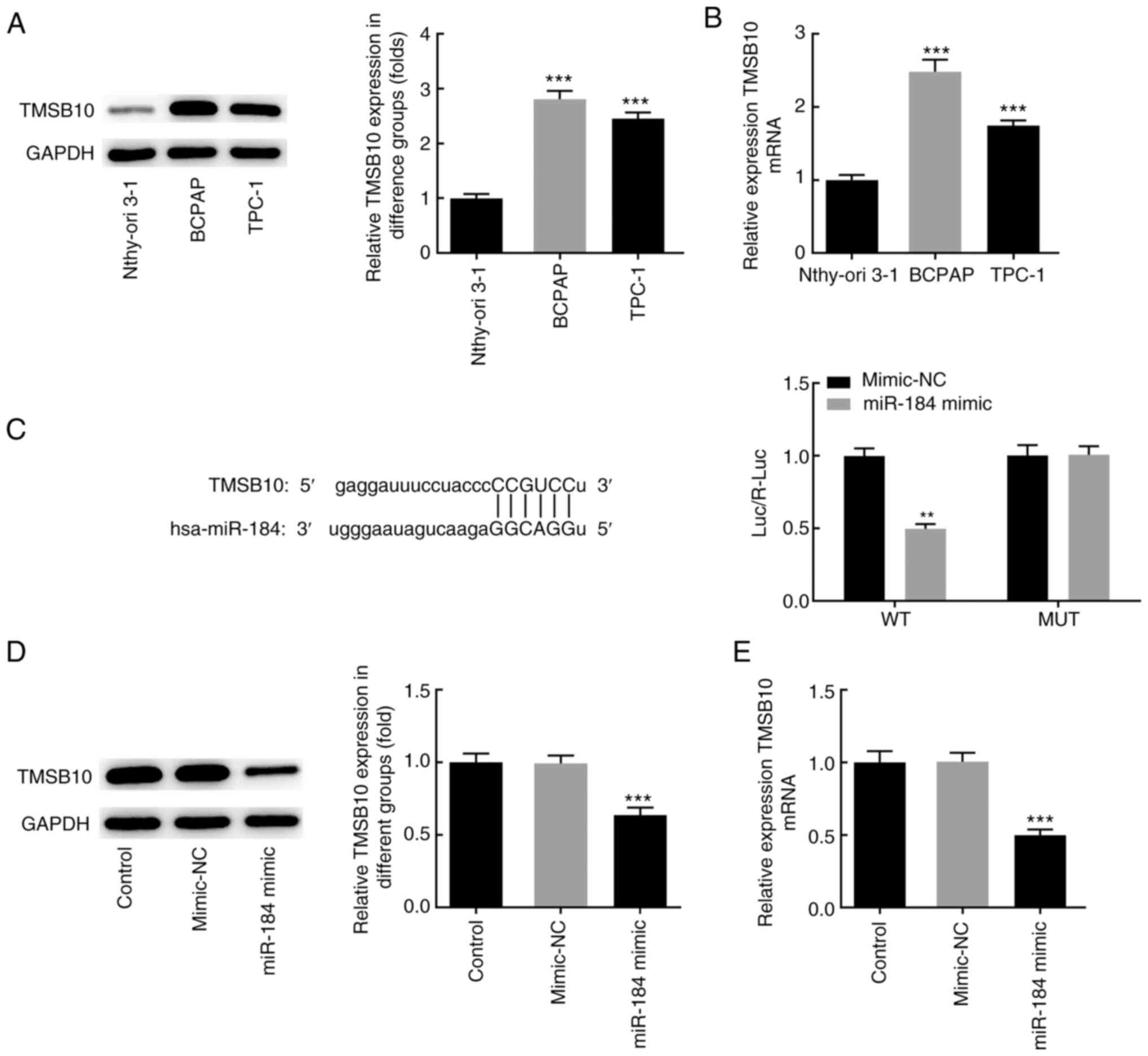
The aforementioned findings suggested that miR-184
was downregulated in BCPAP cells. Subsequently, the mRNA and
protein levels of TMSB10 were detected in different thyroid cancer
cell lines. The results demonstrated that the expression level of
TMSB10 was increased in BCPAP cells compared with Nthy-ori 3-1
normal thyroid epithelial cell line, suggesting a possible
association between miR-184 and TMSB10 (Fig. 4A and B). Subsequently, it was examined whether
the expression of TMSB10 could be regulated by miR-184. Mutations
of five bases were generated in the potential binding site of
miR-184 in the TMSB10 3'-UTR. Co-transfection of BCPAP cells with
the wild-type TMSB10 3'-UTR luciferase plasmids and miR-184 mimics
resulted in decreased luciferase activity compared with the
mimic-NC group (Fig. 4C). No
significant difference was observed in luciferase activity between
the cotransfection group of mutant-type TMSB10 3'-UTR luciferase
plasmids and miR-184 mimics and the mimic-NC group. In addition,
miR-184 mimics notably decreased the mRNA and protein expression
levels of TMSB10 compared with the mimic-NC or control groups
(Fig. 4D and E). Taken together, the aforementioned
findings suggested that the regulation of TMSB10 expression could
be partially mediated by miR-184.
miR-184 regulates the effects of
TMSB10
RT-qPCR results demonstrated that transfection of
BCPAP cells with pcDNA-TMSB10 markedly increased the TMSB10 mRNA
expression level compared with empty vector or control cells
(Fig. 5A). To further evaluate the
effects of TMSB10 and the regulatory mechanism of miR-184, the cell
proliferation rate and the expression of EMT-associated markers was
determined in BCPAP cells overexpressing miR-184 and TMSB10. Of
note, TMSB10 overexpression reversed the effects of miR-184
overexpression on cell proliferation, colony formation and
expression of EMT markers (Fig.
5B-F). These findings collectively indicated that alterations
in the expression of miR-184 may result in increased TMSB10 levels
in thyroid cancer, thereby promoting thyroid cancer cell
proliferation and EMT.
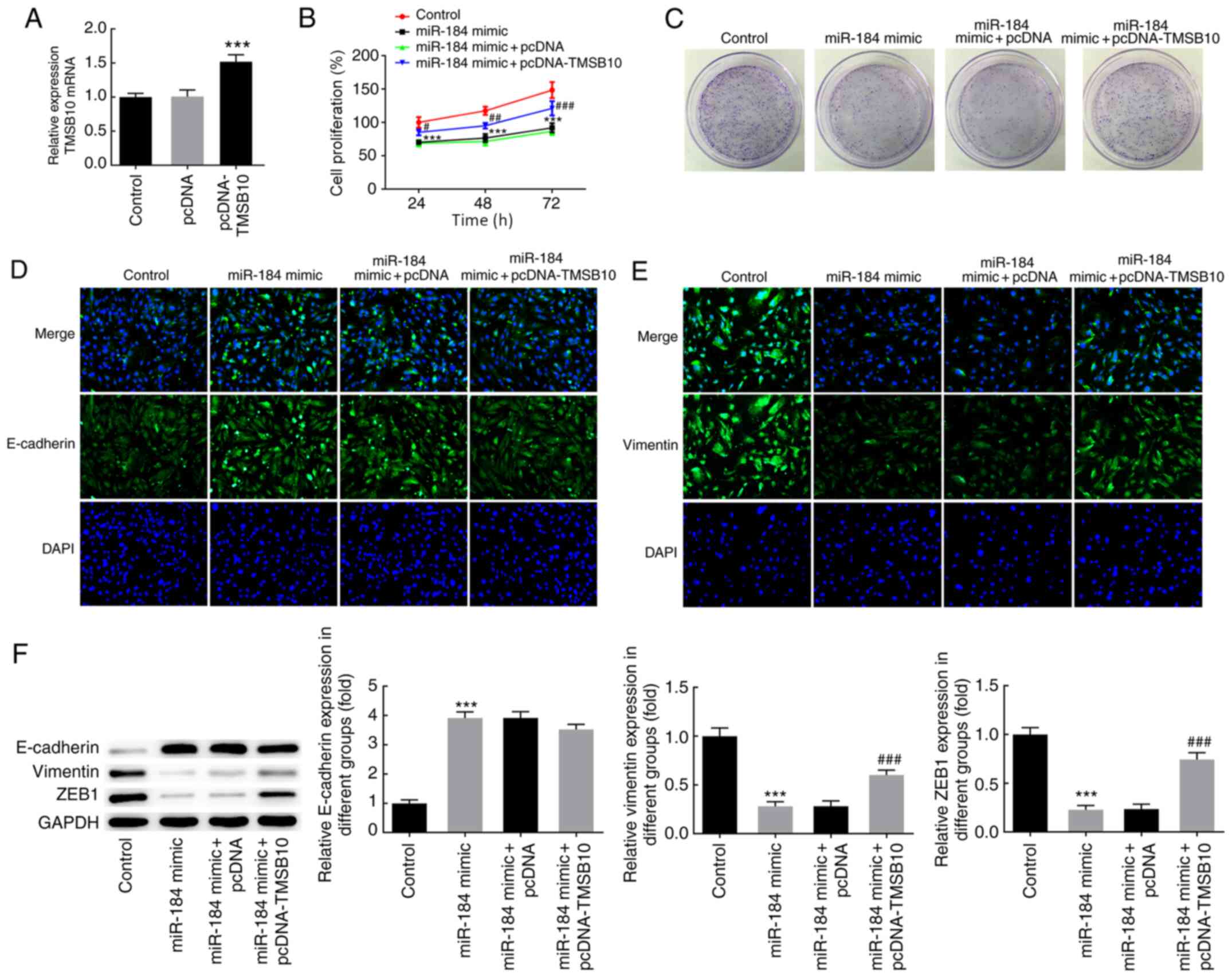 | Figure 5miR-184 regulated the expression of
TMSB10. (A) TMSB10 mRNA expression following cell transfection with
pcDNA-TMSB10. ***P<0.001 vs. pcDNA. (B) Cell
proliferation was evaluated using an MTT assay.
#P<0.05, ##P<0.01,
###P<0.001 vs. miR-184 mimic + pcDNA at 24, 48 and 72
h, respectively. ***P<0.001 vs. control at 24, 48 and
72 h, respectively. (C) Colony formation assay following cell
transfection with miR-184 mimics and pcDNA-TMSB10.
Immunofluorescence staining demonstrating the protein expression of
(D) E-cadherin and (E) vimentin. (F) Protein expression of
E-cadherin, vimentin and ZEB1 as determined by western blot
analysis, ***P<0.001 vs. control;
###P<0.001 vs. miR-184 mimic + pcDNA. The
experimental data are presented as the mean ± SD. Magnification,
x200. TMSB10, thymosin β10; ZEB1, zinc finger E-box-binding
homeobox 1; miR, microRNA; NC, negative control. |
Discussion
The present study demonstrated that miR-184
overexpression could markedly reduce TMSB10 expression in BCPAP
cells, indicating that decreased miR-184 expression levels may
contribute to upregulated TMSB10 expression in BCPAP cells. It has
been previously demonstrated that TMSB10 was upregulated in
squamous cell carcinoma and its upregulated levels were not
considered to be due to its hypomethylation status; however, this
mechanism was not considered as the main mechanism underlying
TMSB10 upregulation (22). In the
present study, TMSB10 mediated the effects of miR-184 on thyroid
cancer cell proliferation and EMT. TMSB10, which is an
actin-sequestering protein belonging to the β-thymosin family, has
been associated with LNM in PTC (15). Additionally, it has been reported
that TMSB10 promotes the malignant behavior of breast cancer cells,
and is therefore considered to be a potential therapeutic target in
breast cancer (18).
In breast cancer cells, TMSB10 has been observed to
promote cell proliferation and invasion via the AKT/FOXO signaling
pathway (22). In addition, several
studies have reported that TMSB10 expression is closely associated
with disease progression and unfavorable prognosis in different
types of cancer, such as hepatocellular carcinoma and bladder
cancer (23,24). EMT is an important process involved
in normal embryonic development, and is a key factor in tumor
invasion and metastasis (25),
which is characterized by loss of cell adhesion, downregulation of
E-cadherin expression and enhanced cell mobility (26). Immunostaining results on the
expression of vimentin and E-cadherin indicated that the expression
levels of both proteins were closely associated with the size and
progression of thyroid tumors (27). ZEB1 is a key molecule in EMT,
directly binding to the E-box element via its zinc finger domain
and suppressing the transcription of the CDH1 gene, which encodes
E-cadherin protein; ZEB1 has been indicated to promote EMT,
invasion and metastasis of thyroid cancer cells (28,29).
In the present study, miR-184 overexpression in
BCPAP cells upregulated E-cadherin expression levels, and
downregulated vimentin and ZEB1 expression levels, suggesting that
miR-184 may suppress EMT. Notably, TMSB10 overexpression partly
restored the effects of miR-184 overexpression on BCPAP cells. An
increasing number of studies have focused on the regulation of EMT
in thyroid cancer, shedding light on its pathogenesis, and various
signaling pathways have been associated with the regulation of EMT
(30-32).
The present study highlighted the potential regulatory effect of
the miR-184/TMSB10 axis on EMT in thyroid cancer. The regulatory
role of the miR-184/TMSB10 axis in cell proliferation and EMT
requires further validation in vivo, which is a limitation
of the present study and a further research direction. To the best
of our knowledge, the present study is the first to report the
regulatory role of TMSB10 in human thyroid cancer and provide
preliminary findings on its underlying mechanism of action, thereby
indicating a potential novel target for inhibiting the
proliferation of thyroid cancer cells.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CY, YL and KF made substantial contributions to the
conception and design of the study, performed the experiments,
interpreted the data and drafted and revised the manuscript for
important intellectual content. CY and KF confirm the authenticity
of the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Davies L and Welch HG: Current thyroid
cancer trends in the United States. JAMA Otolaryngol Head Neck
Surg. 140:317–322. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Roman BR, Morris LG and Davies L: The
thyroid cancer epidemic, 2017 perspective. Curr Opin Endocrinol
Diabetes Obes. 24:332–336. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cabanillas ME, McFadden DG and Durante C:
Thyroid cancer. Lancet. 388:2783–2795. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Luo L, Xia L, Zha B, Zuo C, Deng D, Chen
M, Hu L, He Y, Dai F, Wu J, et al: miR-335-5p targeting ICAM-1
inhibits invasion and metastasis of thyroid cancer cells. Biomed
Pharmacother. 106:983–990. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Li R, Teng X, Zhu H, Han T and Liu Q:
MiR-4500 regulates PLXNC1 and inhibits papillary thyroid cancer
progression. Horm Cancer. 10:150–160. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liu F, Lou K, Zhao X, Zhang J, Chen W,
Qian Y, Zhao Y, Zhu Y and Zhang Y: miR-214 regulates papillary
thyroid carcinoma cell proliferation and metastasis by targeting
PSMD10. Int J Mol Med. 42:3027–3036. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Minna E, Romeo P, Dugo M, De Cecco L,
Todoerti K, Pilotti S, Perrone F, Seregni E, Agnelli L, Neri A, et
al: miR-451a is underexpressed and targets AKT/mTOR pathway in
papillary thyroid carcinoma. Oncotarget. 7:12731–12747.
2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Qiu Z, Li H, Wang J and Sun C: miR-146a
and miR-146b in the diagnosis and prognosis of papillary thyroid
carcinoma. Oncol Rep. 38:2735–2740. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Condello V, Torregrossa L, Sartori C,
Denaro M, Poma AM, Piaggi P, Valerio L, Materazzi G, Elisei R,
Vitti P and Basolo F: mRNA and miRNA expression profiling of
follicular variant of papillary thyroid carcinoma with and without
distant metastases. Mol Cell Endocrinol. 479:93–102.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhu HM, Jiang XS, Li HZ, Qian LX, Du MY,
Lu ZW, Wu J, Tian XK, Fei Q, He X and Yin L: miR-184 inhibits tumor
invasion, migration and metastasis in nasopharyngeal carcinoma by
targeting Notch2. Cell Physiol Biochem. 49:1564–1576.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang JX, Gao J, Ding SL, Wang K, Jiao JQ,
Wang Y, Sun T, Zhou LY, Long B, Zhang XJ, et al: Oxidative
modification of miR-184 enables it to target Bcl-xL and Bcl-w. Mol
Cell. 59:50–61. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zheng X, Carstens JL, Kim J, Scheible M,
Kaye J, Sugimoto H, Wu CC, LeBleu VS and Kalluri R:
Epithelial-to-mesenchymal transition is dispensable for metastasis
but induces chemoresistance in pancreatic cancer. Nature.
527:525–530. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Da C, Wu K, Yue C, Bai P, Wang R, Wang G,
Zhao M, Lv Y and Hou P: N-cadherin promotes thyroid tumorigenesis
through modulating major signaling pathways. Oncotarget.
8:8131–8142. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lv N, Shan Z, Gao Y, Guan H, Fan C, Wang H
and Teng W: Twist1 regulates the epithelial-mesenchymal transition
via the NF-κB pathway in papillary thyroid carcinoma. Endocrine.
51:469–477. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chandrashekar DS, Bashel B, Balasubramanya
SAH, Creighton CJ, Rodriguez IP, Chakravarthi BVSK and Varambally
S: UALCAN: A portal for facilitating tumor subgroup gene expression
and survival analyses. Neoplasia. 19:649–658. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang XJ, Su YR, Liu D, Xu DB, Zeng MS and
Chen WK: Thymosin beta 10 correlates with lymph node metastases of
papillary thyroid carcinoma. J Surg Res. 192:487–493.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xiao R, Shen S, Yu Y, Pan Q, Kuang R and
Huang H: TMSB10 promotes migration and invasion of cancer cells and
is a novel prognostic marker for renal cell carcinoma. Int J Clin
Exp Pathol. 12:305–312. 2019.PubMed/NCBI
|
|
18
|
Zhang X, Ren D, Guo L, Wang L, Wu S, Lin
C, Ye L, Zhu J, Li J, Song L, et al: Thymosin beta 10 is a key
regulator of tumorigenesis and metastasis and a novel serum marker
in breast cancer. Breast Cancer Res. 19(15)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tang J, Kong D, Cui Q, Wang K, Zhang D,
Yuan Q, Liao X, Gong Y and Wu G: Bioinformatic analysis and
identification of potential prognostic microRNAs and mRNAs in
thyroid cancer. PeerJ. 6(e4674)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xu Y, Chen J, Yang Z and Xu L:
Identification of RNA expression profiles in thyroid cancer to
construct a competing endogenous RNA (ceRNA) Network of mRNAs, Long
noncoding RNAs (lncRNAs), and microRNAs (miRNAs). Med Sci Monit.
25:1140–1154. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lee SM, Na YK, Hong HS, Jang EJ, Yoon GS,
Park JY and Kim DS: Hypomethylation of the thymosin β(10) gene is
not associated with its overexpression in non-small cell lung
cancer. Mol Cells. 32:343–348. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Song C, Su Z and Guo J: Thymosin β10 is
overexpressed and associated with unfavorable prognosis in
hepatocellular carcinoma. Biosci Rep.
39(BSR20182355)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang B, Wang Z, Zhang T and Yang G:
Overexpression of thymosin β10 correlates with disease progression
and poor prognosis in bladder cancer. Exp Ther Med. 18:3759–3766.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Paolillo M and Schinelli S: Extracellular
matrix alterations in metastatic processes. Int J Mol Sci.
20(4947)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pradella D, Naro C, Sette C and Ghigna C:
EMT and stemness: Flexible processes tuned by alternative splicing
in development and cancer progression. Mol Cancer.
16(8)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Calangiu CM, Simionescu CE, Stepan AE,
Cernea D, Zăvoi RE and Mărgăritescu C: The expression of CK19,
vimentin and E-cadherin in differentiated thyroid carcinomas. Rom J
Morphol Embryol. 55:919–925. 2014.PubMed/NCBI
|
|
28
|
Morillo-Bernal J, Fernandez LP and
Santisteban P: FOXE1 regulates migration and invasion in thyroid
cancer cells and targets ZEB1. Endocr Relat Cancer. 27:137–151.
2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Xia W and Jie W:
ZEB1-AS1/miR-133a-3p/LPAR3/EGFR axis promotes the progression of
thyroid cancer by regulating PI3K/AKT/mTOR pathway. Cancer Cell
Int. 20(94)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li T, Zhao N, Lu J, Zhu Q, Liu X, Hao F
and Jiao X: Epigallocatechin gallate (EGCG) suppresses
epithelial-Mesenchymal transition (EMT) and invasion in anaplastic
thyroid carcinoma cells through blocking of TGF-β1/Smad signaling
pathways. Bioengineered. 10:282–291. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Werner TA, Forster CM, Dizdar L, Verde PE,
Raba K, Schott M, Knoefel WT and Krieg A: CXCR4/CXCR7/CXCL12 axis
promotes an invasive phenotype in medullary thyroid carcinoma. Br J
Cancer. 117:1837–1845. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yang Z, Yu W, Huang R, Ye M and Min Z:
SIRT6/HIF-1α axis promotes papillary thyroid cancer progression by
inducing epithelial-mesenchymal transition. Cancer Cell Int.
19(17)2019.PubMed/NCBI View Article : Google Scholar
|