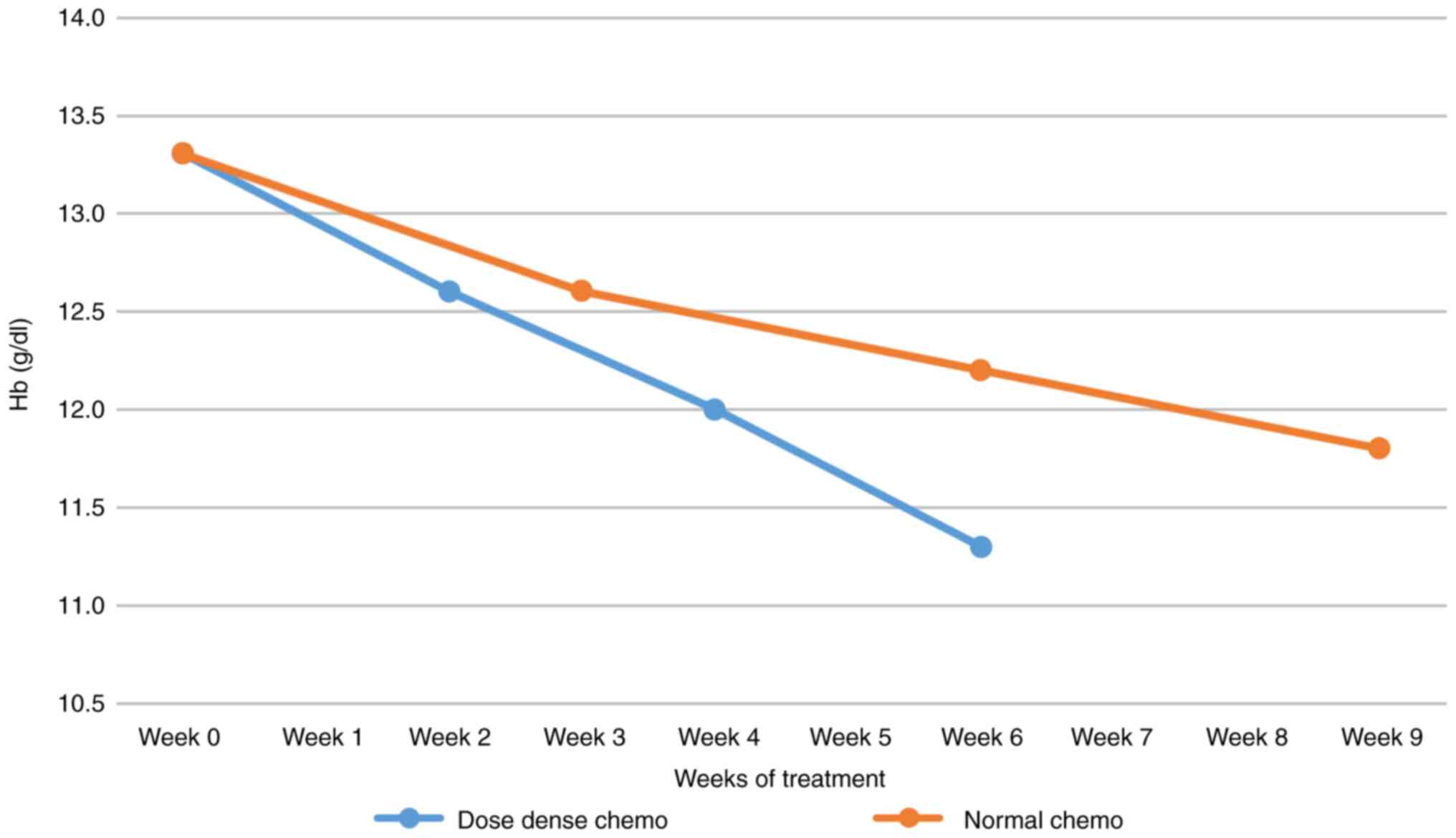
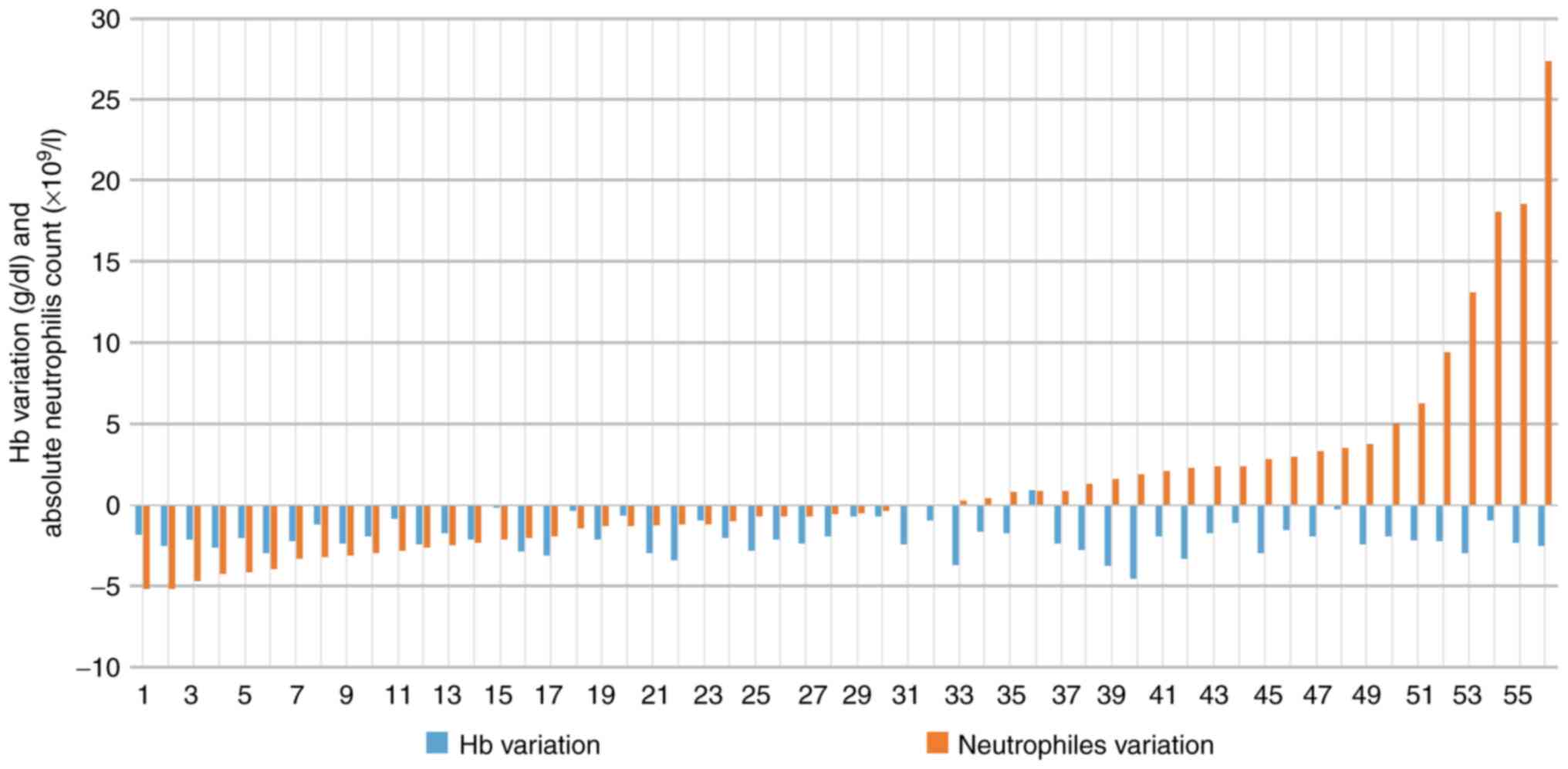
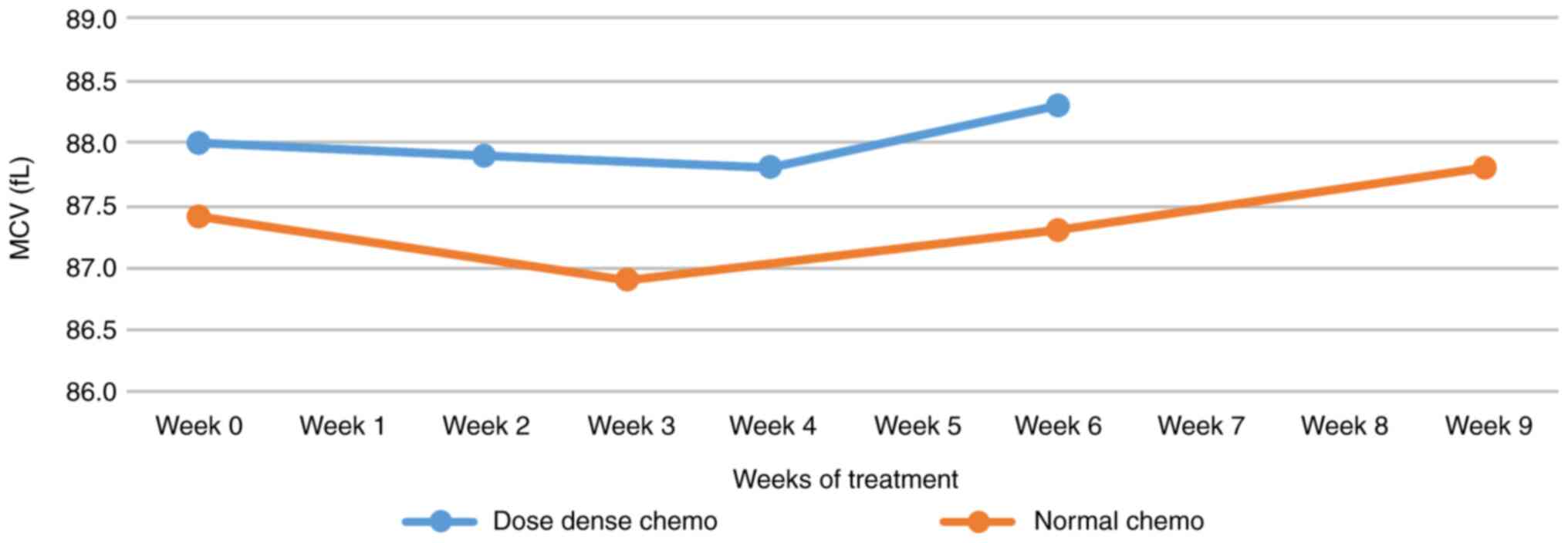
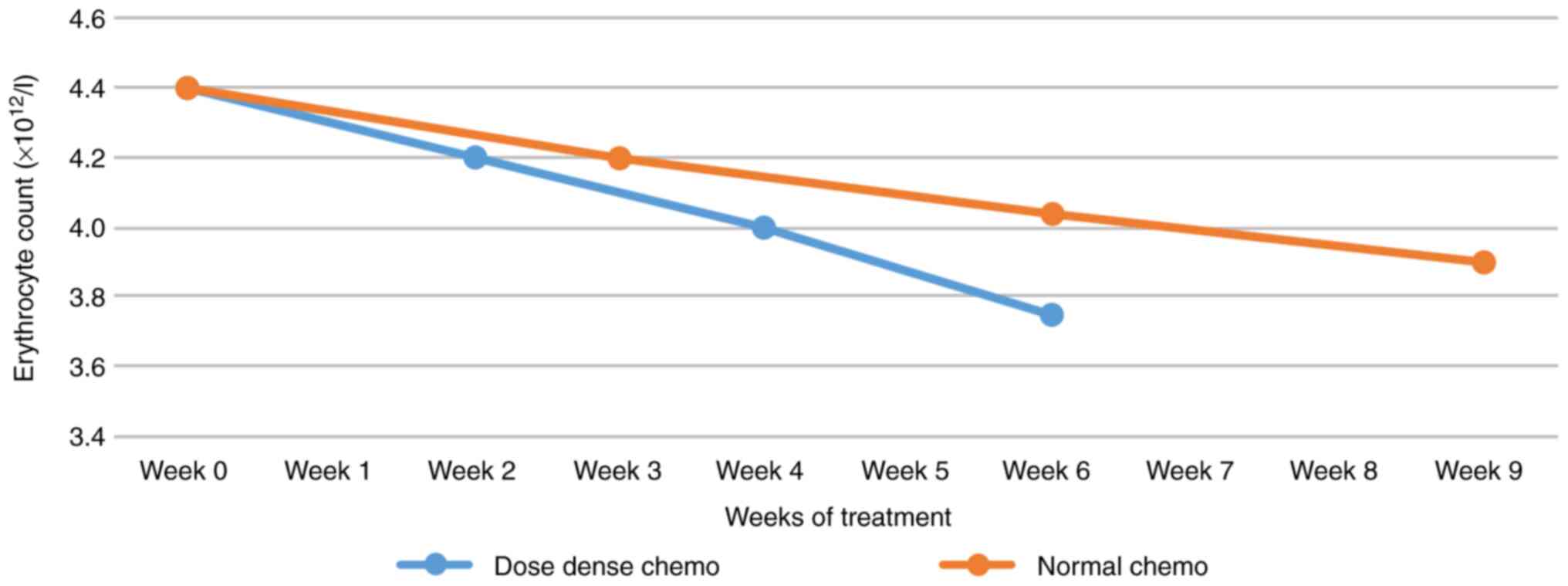
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Romania: Cluj Regional Cancer Registry,
Timisoara Regional Cancer Registry. http://ghdx.healthdata.org/organizations/timisoara-regional-cancer-registry-romania.
Accessed: 29 Sept., 2020.
|
|
3
|
DeSantis CE, Ma J, Gaudet MM, Newman LA,
Miller KD, Sauer AG, Jemal A and Siegel RL: Breast cancer
statistics, 2019. CA Cancer J Clin. 69:438–451. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ward EM, Sherman RL, Henley SJ, Jemal A,
Siegel DA, Feuer EJ, Firth AU, Kohler BA, Scott S, Ma J, et al:
Annual Report to the nation on the status of cancer, featuring
cancer in men and women age 20-49 years. J Natl Cancer Inst.
111:1279–1297. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Merlo DF, Ceppi M, Filiberti R, Bocchini
V, Znahor A, Gamulin M, Primic-Žakelj M, Bruzzi P, Bouchardy C and
Fucci A: Breast cancer incidence trends in European women aged
20-39 years at diagnosis. Breast Cancer Res Treat. 134:363–370.
2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bodmer A, Feller A, Bordoni A, Bouchardy
C, Dehler S, Ess S, Levi F, Konzelmann I, Rapiti E, Steiner A, et
al: Breast cancer in younger women in Switzerland 1996-2009: A
longitudinal population-based study. Breast. 24:112–117.
2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Guo F, Kuo YF, Shih YCT, Giordano SH and
Berenson AB: Trends in breast cancer mortality by stage at
diagnosis among young women in the United States. Cancer.
124:3500–3509. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Brenner DR, Ruan Y, Shaw E, O'Sullivan D,
Poirier AE, Heer E, Villeneuve PJ, Walter SD, Friedenreich CM,
Smith L and De P: Age-Standardized cancer-incidence trends in
Canada, 1971-2015. Can Med Assoc J. 191:E1262–E1273.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Leclère B, Molinié F, Trétarre B, Stracci
F, Daubisse-Marliac L and Colonna M: GRELL Working Group. Trends in
the incidence of breast cancer among women under 40 in seven
European countries: A GRELL cooperative study. Cancer Epidemiol.
37:544–549. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Baeyens-Fernández JA, Molina-Portillo E,
Pollán M, Rodriguez-Barranco M, Del Moral R, Arribas-Mir L,
Sánchez-Cantalejo Ramírez E and Sánchez MJ: Trends in incidence,
mortality and survival in women with breast cancer from 1985 to
2012 in Granada, Spain: A population-based study. BMC Cancer.
18(781)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ruggeri M, Pagan E, Bagnardi V, Bianco N,
Gallerani E, Buser K, Giordano M, Gianni L, Rabaglio M, Freschi A,
et al: Fertility concerns, preservation strategies and quality of
life in young women with breast cancer: Baseline results from an
ongoing prospective cohort study in selected European centers.
Breast. 47:85–92. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ganz PA, Greendale GA, Petersen L, Kahn B
and Bower JE: Breast cancer in younger women: Reproductive and late
health effects of treatment. J Clin Oncol. 21:4184–4193.
2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG). Increasing the dose intensity of
chemotherapy by more frequent administration or sequential
scheduling. A patient-level meta-analysis of 37 298 women with
early breast cancer in 26 randomised trials. Lancet. 393:1440–1452.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Papakonstantinou A, Matikas A, Bengtsson
NO, Malmström P, Hedayati E, Steger G, Untch M, Hübbert L,
Johansson H, Hellström M, et al: Efficacy and safety of tailored
and dose-dense adjuvant chemotherapy and trastuzumab for resected
HER2-positive breast cancer: Results from the phase 3 PANTHER
trial. Cancer. 15:1175–1182. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhou W, Chen S, Xu F and Zeng X: Survival
benefit of pure dose-dense chemotherapy in breast cancer: A
meta-analysis of randomized controlled trials. World J Surg Oncol.
16(144)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cocciolone V, Cannita K, Tessitore A,
Mastroiaco V, Rinaldi L, Paradisi S, Irelli A, Baldi PL, Sidoni T,
Ricevuto E, et al: Neoadjuvant chemotherapy in breast cancer: A
dose-dense schedule in real life and putative role of PIK3CA
mutations. Oncotarget. 9:27380–27396. 2018.PubMed/NCBI View Article : Google Scholar
|