Introduction
Ischemia/reperfusion (I/R) injury remains the major
cause of liver injury during major liver resection and
transplantation (1,2). Oxidative stress is supposed to be the
major initiating mechanism (3,4). In
recent decades, increasing studiesintended to illuminate the
molecular and pathological mechanisms in liver I/R injury have been
reported; however, only a partial understanding of these processes
is currently available (1,2). I/R injury is a critical condition
caused by interrupted blood supply, and potentially, it is caused
by hypoxia/reoxygenation (H/R) (5-7).
The in vitro H/R-induced injury model is the best model
available with which to mimic and study the in vivo I/R
injury (5-7).
Excessive production of reactive oxygen species (ROS) along with a
dramatic decrease in antioxidant defenses is observed during H/R
(3,8). Oxidative damage and inflammatory
responses are responsible for I/R injury and H/R injury (3,8). Thus,
these attributes could serve as therapeutic targets for the
prevention and treatment of I/R injury.
Hesperidin (HDN) is a bioflavonoid with
antibacterial, anti-inflammatory, and antioxidant effects (9-12).
It was reported that HDN has hydrogen radical- and hydrogen
peroxide-removal activities and serves an antioxidant role in
biological systems (9,10). We and others have revealed that HDN
has a protective effect against I/R injury (13-17).
It is also reported that HDN protects against H/R injury in
vitro in rat cardiomyocytes and a human first-trimester
trophoblast cell line (18,19). However, whether HDN protects
hepatocytes (HCs) against H/R-induced injury remains unknown.
The present study hypothesized that HDN may
ameliorate H/R-induced injury in HCs in vitro. HCs were
isolated and cultured under H/R conditions with or without HDN
exposure. The present study revealed that HDN ameliorated
H/R-induced injury in HCs in vitro. Furthermore, the results
demonstrated that HDN attenuated HC oxidative stress and
inflammatory responses while enhancing autophagy during H/R. Thus,
exposure to HDN may have a protective effect on HCs during
H/R-induced injury.
Materials and methods
Hepatocyte isolation and culture
A total of 3 male C57BL/6J WT mice aged 8-12 weeks
and weighing 22-30 g were purchased from and housed in Guangxi
Medical University Laboratory Animal Center (Guangxi, China). The
mice were bred in a specific pathogen-free animal facility under
controlled conditions at 19-23˚C and 40-60% humidity with a 12-h
dark/light cycle and had free access to food and water. HCs were
isolated from mice as described previously (17,20).
Briefly, the mice wereeuthanized with 5% isoflurane for 5 min in a
plexiglass chamber, followed by bilateral thoracotomy for a
secondary confirmation of death. Thenthe mice were perfused using
an in situ collagenase (type VI; WorthingtonBiochemical
Corporation) technique in vivo. HCs were separated from
nonparenchymal cells and purified to >99% purity with a
viability >95%, as confirmed by trypan blue exclusion. HCs were
cultured as described previously (17,21).
HCs (1.5x105 cells/ml) were plated on gelatin-coated
culture plates with collagen I (BD Pharmingen; BD Biosciences) in
cell culture medium. The cell culture media contained Williams
medium E (Invitrogen; Thermo Fisher Scientific, Inc.) with 10% calf
serum (Thermo Fisher Scientific, Inc.), 15 mM HEPES (Thermo Fisher
Scientific, Inc.), 1 µM insulin (Eli Lilly and Company), 2 mM
L-glutamine (Thermo Fisher Scientific, Inc.), penicillin (100 U/ml)
and streptomycin (100 U/ml; Invitrogen; Thermo Fisher Scientific,
Inc.). HCs were cultured overnight at 37˚C, and the culture medium
was replaced with fresh medium before the experimental treatment.
For H/R treatment, HCs were incubated under hypoxic conditions (1%
oxygen for 10 h) following reoxygenation (normoxic conditions for 8
h). HCs were divided into the following groups: Control PBS group
(HCs were subjected to normoxia with PBS, 18 h), control HDN group
(HCs were subjected to normoxia with 50 µg/ml HDN, 18 h), H/R PBS
group (HCs were subjected to H/R with PBS, H/R for 10 and 8 h as
aforementioned) and H/R HDN group (HCs were subjected to H/R with
50 µg/ml HDN, H/R for 10 and 8 h as aforementioned).
Reagents
For western blotting, the autophagy antibody sample
kit (cat. no. 4445; Cell Signaling Technology, Inc.; 1:1,000) was
used, which targets the proteins microtubule-associated protein 1
light chain 3α (MAP1LC3A, also known as LC3), Beclin-1, and
sequestosome 1 (SQSTM1, also known as P62). GAPDH (cat. no. ab8245;
Abcam; 1:2,500) was used as the internal control. The goat
anti-mouse secondary antibody (cat. no. 31430) and goat anti-rabbit
secondary antibody (cat. no. 31460) (both at 1:20,000 dilution)
were from Thermo Fisher Scientific, Inc. HDN (HPLC >98%; cat.
no. XW05202631) was obtained from Sinopharm Chemical Reagent Co.,
Ltd. The malondialdehyde assay kit (MDA; cat. no. A003-1),
superoxide dismutase assay kit (SOD; cat. no. A001-1), glutathione
assay kit (GSH; cat. no. A006-1), interleukin-6 assay kit (IL-6;
cat. no. H007) and tumor necrosis factor-α assay kit (TNF-α; cat.
no. H052) were purchased from Nanjing Jiancheng Bioengineering
Institute.
Supernatant sample assays
Supernatant alanine aminotransferase (ALT) levels
were measured using an ALT assay kit according to the
manufacturer's instructions (cat. no. C009-2-1; Nanjing Jiancheng
Institute of Biotechnology) and analyzed byspectrophotometry. The
levels of ALT were expressed as units per liter of supernatant
(U/l). Lactate dehydrogenase (LDH) released into the medium
solution from dead cells was measured by a LDH assay kit (cat. no.
A020-2-2; Nanjing Jiancheng Institute of Biotechnology) according
to the manufacturer's instructions and analyzed by
spectrophotometry. The levels of LDH were expressed as units per
liter of supernatant (U/l).
Cellular viability assay
The images of the cell morphology were captured
using a light microscope (XDS-1A; Shanghai Precision Instrument
Co., Ltd.). Cell viability was measured using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay (Sigma-Aldrich; Merck KGaA), according to the manufacturer's
instructions. The MTT assays were quantified by reading the
absorbance on a plate reader. The wavelength to measure the
absorbance (Abs) of each sample was 492 nm. MTT reduction measured
at 492 nm was converted to percentage cell viability according to
the following formula: % cell viability=[(Abs 492 nm of treated
group-blank)/(Abs 492 nm of control-blank)] x100.
Intracellular ROS assessment
Intracellular ROS levels were assessed using a
ROSkit (cat. no. CA1410; Beijing Solarbio Science﹠Technology Co.,
Ltd.). Dichlorodihydrofluorescein diacetate (DCFH-DA) was diluted
(1:1,000) with serum-free medium to 10 µmol/l. After the HCs were
treated, the cells were washed and incubated with DCFH-DA for 20
min at 37˚C. Then the cells were washed three times before
assessment. Fluorescence was detected and photographed with an
inverted fluorescent microscope (magnification, x200; IX71; Olympus
Corporation).
Reverse transcription-quantitative PCR
(RT-qPCR)
RT-qPCR was performed as previously described
(17). Briefly, total RNA was
extracted with the RNeasy mini kit (QiagenGmbH), according to the
manufacturer's instructions. Then, complementary DNA (cDNA) was
generated from 1 µg of total RNA with 2 µM oligo-dT primersand the
Omniscript™ reverse transcriptase (both Qiagen GmbH). The iTaq
SYBR-Green PCR Master Mix (Applied Biosystems; Thermo Fisher
Scientific, Inc.) with specific primers for β-actin, IL-6, and
TNF-α (Qiagen GmbH) was used to perform qPCR. All samples were
assayed in duplicate and normalized to the β-actin mRNA abundance.
Gene expression levels were quantified using the 2-ΔΔCq
method (22). The primers used for
qPCR were the same as previously described (17).
Western blotting analysis
The western blotting protocol was the same as that
previously described (17,23). Briefly, HCs were collected in lysis
buffer (Cell Signaling Technology, Inc.), sonicated, and
centrifuged (16,000 x g for 15 min, 4˚C), after which the
supernatant was collected. Protein concentrations were measured
using a bicinchoninic acid protein assay kit (Thermo Fisher
Scientific, Inc.). Samples were then run on SDS-PAGE and
transferred to a polyvinylidene difluoride membrane. Then the
membrane was incubated with primary and secondary antibodies,
before being developed by an enhanced chemiluminescence kit (Thermo
Fisher Scientific, Inc.). The signal was acquired with a ChemiDoc
MP Imaging System (Bio-RadLaboratories, Inc.).
Autophagic flux
Autophagic flux was assessed as described previously
(23). HCs were first cultured
under hypoxic conditions, and then received reoxygenation with
exposure to bafilomycin A1 (50 nM) treatment for 1 h (final hour of
8 h reoxygenation procedure). Western blotting was performed to
analyse LC3 I: II conversion. In order to measure the accumulation
of LC3 in HCs, the GFP-LC3 adenovirus was transfected into HCs
before hypoxia, and then the HCs received the H/R procedure
involving bafilomycin A1 exposure. HCs transfected with GFP-LC3
were photographed with a Zeiss LSM510 laser-scanning confocal
microscope (Carl Zeiss AG). GFP-LC3 puncta were counted with at
least 5 green dots/cell from at least 30 cells/treatment.
Results
Hesperidin protects HCs against H/R
injury
To determine the appropriate dose of HDN for HC
treatment, HCs were isolated and exposed to different doses of HDN
under normoxic conditions. As shown in Fig. 1A, there were significant side
effects on cell viability with HDN doses >50 µg/ml. Therefore,
the dose 50 µg/ml of HDN was used for subsequent experiments.
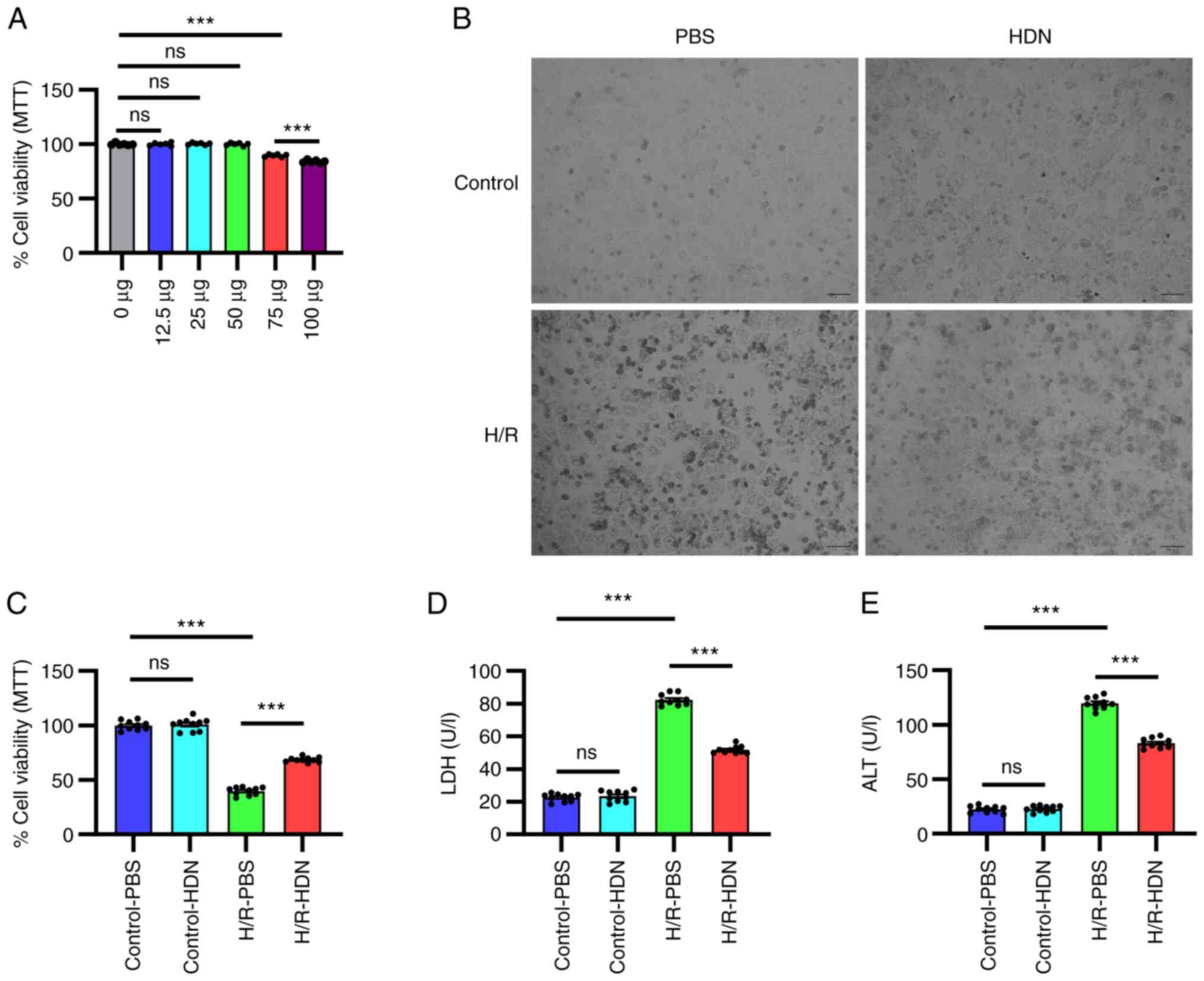 | Figure 1HDN protects HCs against H/R injury.
(A) Cell viability was determined by MTT assay for HCs exposed to
different doses of HDN under normoxic conditions. (B) Morphology of
HCs in control PBS group, control HDN group, H/R PBS group and H/R
HDN group under a light microscope (scale bar, 50 µm). (C) Cell
viability was determined by MTT assayfor HCs in control PBS group,
control HDN group, H/R PBS group and H/R HDN group. (D) Levels of
supernatant LDH and (E) ALT in control PBS group, control HDN
group, H/R PBS group and H/R HDN group. Results areshown as the
mean ± SEM of three independent experiments.
***P<0.001 with comparisons shown by lines. HDN,
hesperidin; HC, hepatocyte; H/R, hypoxia/reoxygenation; LDH,
lactate dehydrogenase; ALT, alanine aminotransferase; ns, not
significant. |
To determine the role of HDN in H/R-induced HC
injury, HCs were isolated and cultured under H/R conditions with or
without HDN treatment. HC injury was evaluated by microscopic
visualization of cell morphology and measured by an MTT assay and
by detecting supernatant LDH and ALT levels. HCs appeared to be
swelled into spherical shapes, and exhibited membrane rupture,
nuclei pyknotic and nuclear condensation after H/R-induced injury
under a light microscope (Fig. 1B).
HDN treatment appeared to partially maintain HC cell morphology and
numbers (Fig. 1B). The results of
the MTT assay revealed that HC damage was significantly induced
under H/R (Fig. 1C). As shown in
Fig. 1B and C, HDN alone had no effect on HC morphology
and cell viability. However, HDN treatment significantly
ameliorated the cell viability under H/R conditions (Fig. 1C). Similarly, supernatant LDH levels
and ALT levels increased significantly during H/R, and these levels
were significantly reversed by HDN treatment (Fig. 1D and E). Taken together, these data indicated
that HDN protected HCs against H/R-induced injury.
Hesperidin attenuates HC oxidative
stress induced by H/R
The most characteristic mechanism of H/R-induced
injury is oxidative stress (3,8). HDN
has been reported to attenuate oxidative stress in many situations,
such as in I/R injury and toxin-induced damage (17,24).
To explore whether HDN attenuates oxidative stress during H/R, the
levels of MDA, SOD, GSH and ROS were detected using commercial
kits. Notably, compared with that of the control group, the MDA
content increased significantly during H/R; however, this change
was significantly reversed by exposure to HDN (Fig. 2A). By contrast, compared with that
of the control group the antioxidant activities of SOD and GSH
decreased significantly during H/R, and these effects were reversed
by exposure to HDN (Fig. 2B and
C). Similar findings were obtained
using a fluorescent dye assay to detect the levels of ROS; H/R
significantly induced ROS levels in HCs, while this was effectively
reversed by HDN treatment (Fig.
2C). Taken together, these data indicated that HDN attenuated
HC oxidative stress induced by H/R.
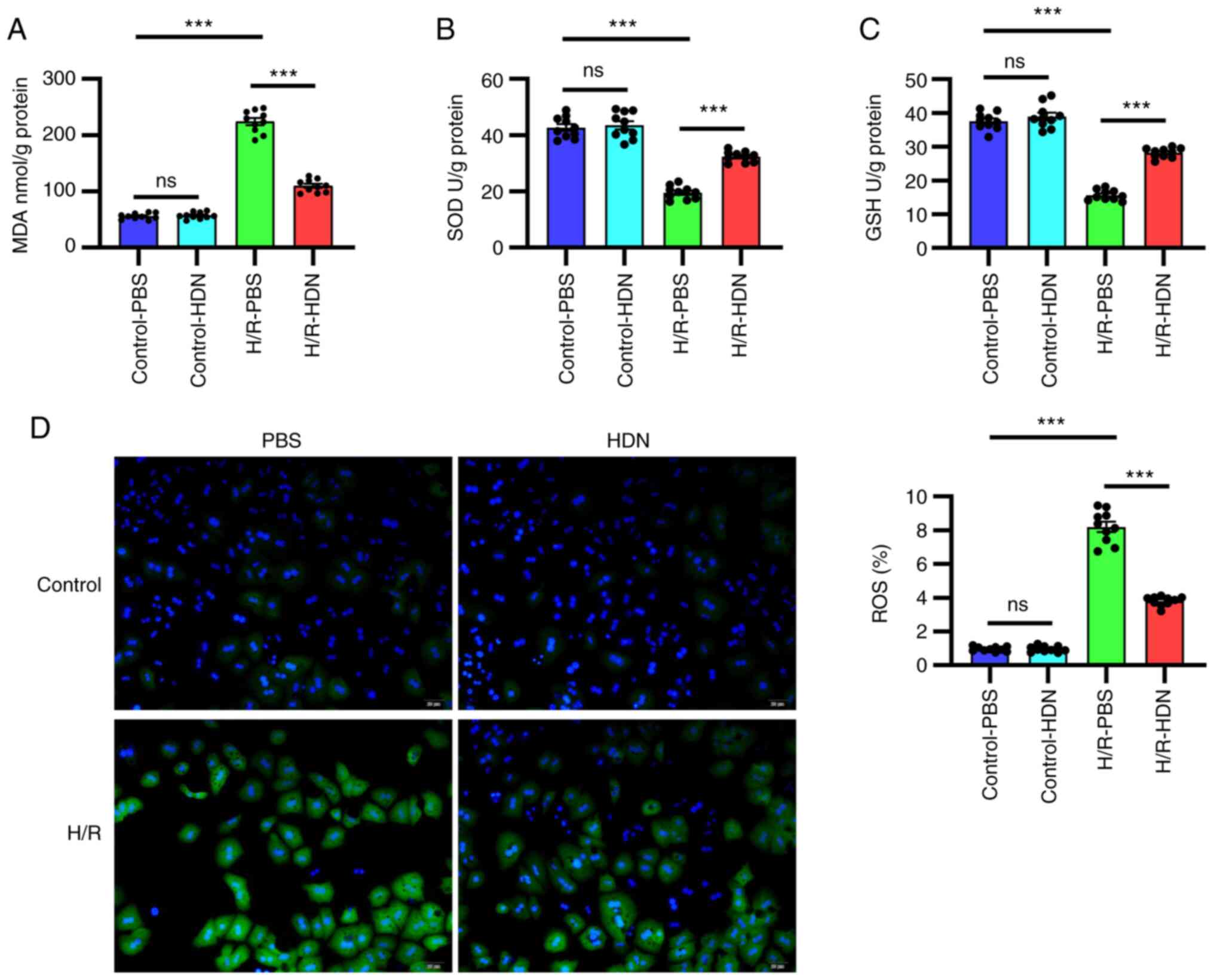 | Figure 2HDN attenuates hepatocyte oxidative
stress induced by H/R. (A) MDA, (B) SOD, (C) GSH and (D) ROSlevels
in control PBS group, control HDN group, H/R PBS group and H/R HDN
group. ROS (green) was detected by fluorescence microscopy
(magnification, x40). Results are shown as the mean ± SEM of three
independent experiments. ***P<0.001 with comparisons
shown by lines. HDN, hesperidin; H/R, hypoxia/reoxygenation; MDA,
malondialdehyde; SOD, superoxide dismutase; GSH, glutathione; ROS,
reactive oxygen species; ns, not significant. |
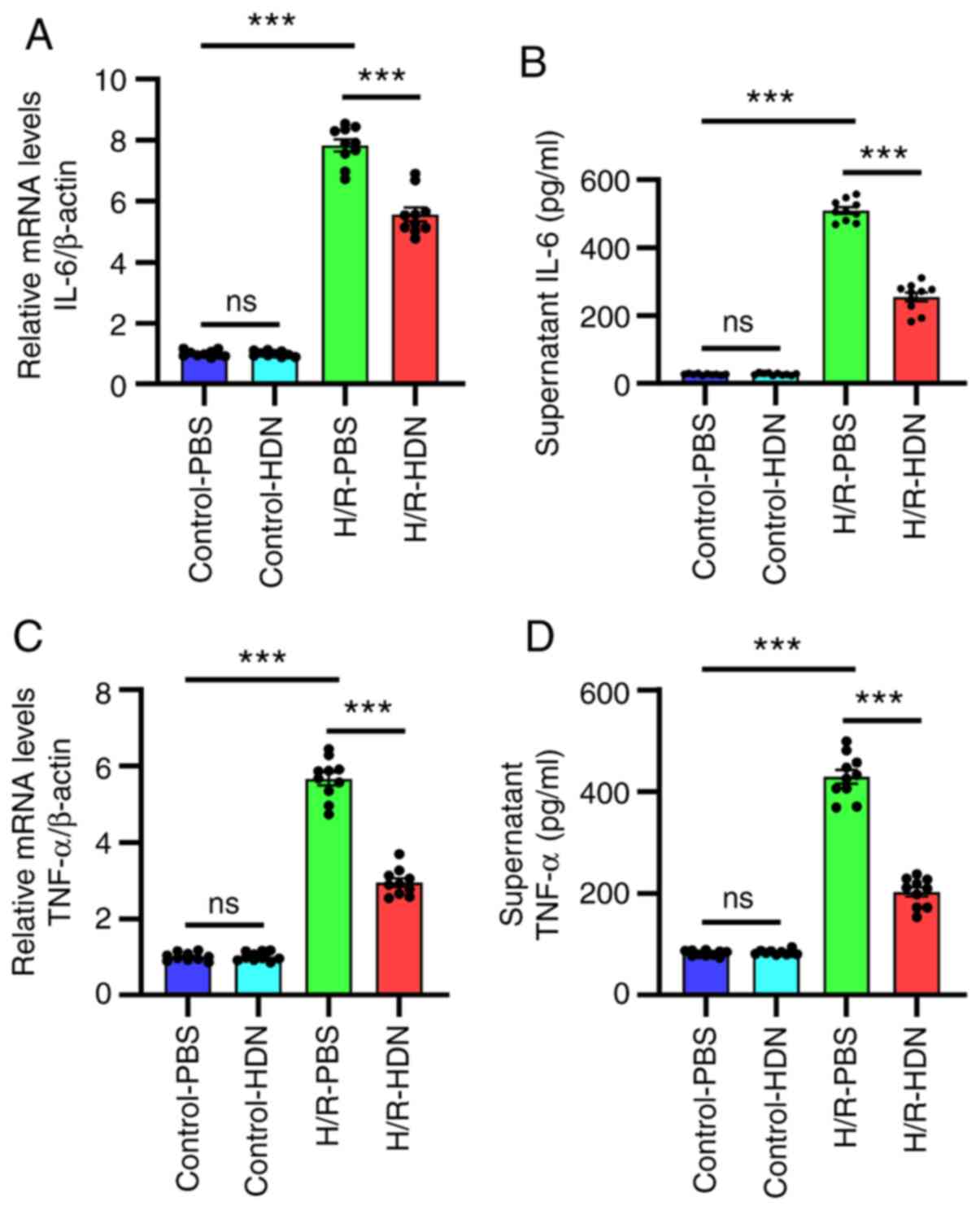
Hesperidin ameliorates HC inflammatory
responses during H/R
The mechanisms of H/R-induced injury include both
direct cellular damage resulting from oxidative stress and injury
resulting from non-sterile inflammatory responses (3,8). It
has been reported that numerous cytokines are involved in
H/R-induced injury (25-27).
To ascertain the relationship between HDN and inflammation during
H/R-induced injury, the levels of IL-6 and TNF-α were assessed by
RT-qPCR and ELISA. The results demonstrated that, compared with
those of the control group, the mRNA expression levels of IL-6 and
TNF-α in HCs and the concentrations of IL-6 and TNF-α in the
supernatants increased significantly following H/R, and HDN
treatment significantly ameliorated these effects (Fig. 3). Thus, these data indicated that
HDN ameliorated HC inflammatory responses during H/R.
Hesperidin induces autophagy to
protect HCs against H/R injury
Cell death is the end result of H/R-induced injury
and can propagate injury through the activation of inflammatory
pathways (25-27).
It has been reported that autophagy protects against liver I/R
injury in vivo and protects HCs against H/R injury in
vitro (23). We and others have
demonstrated that HDN protects against I/R injury (13-17).
To determine whether HDN may regulate protective autophagy during
H/R injury, the present study assessed the levels of specific
autophagic markers in HCs with or without HDN exposure during H/R
injury. Western blotting results demonstrated that LC3-Ⅱ and
Beclin-1 protein expression levels increased, while P62 levels
decreased during H/R upon exposure to HDN (Fig. 4A). It is important to know whether
autophagosomes were formed from new autophagosome formation or from
the blockade of autophagosome degradation during autophagy. To
investigate these possibilities, HCs were cultured with or without
HDN treatment under exposure to bafilomycin A1, which inhibits
autophagolysosomal fusion and degradation, and then the HCs were
subjected to normoxia or H/R. Western blotting results demonstrated
that LC3-Ⅱ levels increased in HCs with or without HDN treatment
under the normoxia condition (Fig.
4B). However, LC3-Ⅱ levels only increased in HCs exposed to HDN
under the H/R conditions (Fig. 4C).
In addition, GFP-LC3 was transfected into HCs and the accumulation
of GFP-LC3 puncta was quantified by confocal microscopy. Consistent
with the western blotting results, the numbers of GFP-LC3 puncta in
HCs with exposure to HDN were greater compared with those observed
in HCs without HDN exposure under the H/R conditions after
bafilomycin A1 treatment (Fig. 4D).
Taken together, these data indicated that HDN induced autophagy to
protect HCs against H/R injury.
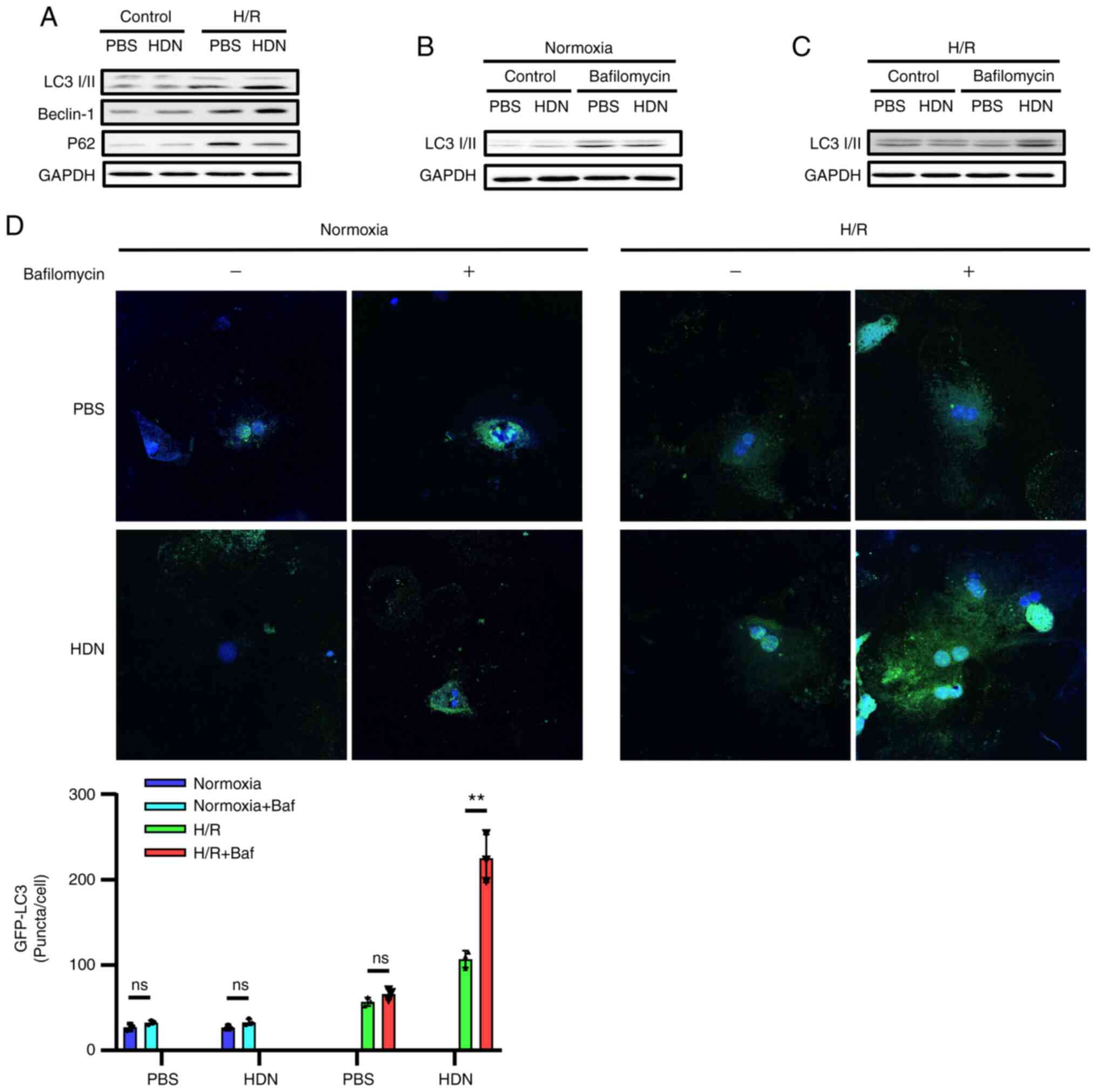 | Figure 4HDN induces autophagy to protect HCs
against H/R injury. (A) Representative western blots showing the
protein expression levels of LC3, Beclin-1 and P62 in control PBS
group, control HDN group, H/R PBS group and H/R HDN group. (B)
Representative western blots showing the levels of LC3 in HCs
subjected to normoxia with or without bafilomycin A1 (50 nM). (C)
Representative western blots showing the levels of LC3 in HCs
subjected to H/R with or without bafilomycin A1 (50 nM). (D)
Confocal microscopy images of HCs overexpressing GFP-LC3 (green)
and subjected to normoxia or H/R, with/without bafilomycin A1 (50
nM) treatment (magnification, x40). Nuclei were counterstained with
Hoechst 33342 (blue). The numbers of GFP-LC3 puncta were counted
per cell and represented in the bar graph. Results are shown as the
mean ± SEM of three independent experiments. **P<0.01
with comparisons shown by lines. HDN, hesperidin; HC, hepatocyte;
H/R, hypoxia/reoxygenation; LC3, microtubule-associated protein 1
light chain 3α; P62, sequestosome 1; ns, not significant. |
Discussion
HDN is a flavonoid extracted from plants belonging
to the families Lamiaceae and Betulaceae with a variety of
bioactivities (9). It has been
revealed that HDN has a variety of beneficial qualities, such as
attenuating apoptosis, ameliorating hypotension, preventing hepatic
steatosis, impairing dengue virus replication, and suppressing
cancer proliferation (9-12).
Additionally, it ameliorates I/R injury by attenuating oxidative
stress and inflammation responses (17). However, whether HDN protects HCs
against H/R-induced injury in vitro was largely unknown. The
present study provided strong evidence that HDN protected HCs
against H/R-induced injury by attenuating oxidative stress and
ameliorating inflammatory responses while promoting autophagy.
Thus, the current results identified HDN as a potential protective
factor for HCs. These findings indicated that HDN may serve as a
natural compound for ameliorating HC injury during H/R.
I/R injury remains a critical challenge because it
results in cell death and organ failure (28-30).
Increasing evidence has revealed that the basic pathophysiology of
I/R is initiated from anaerobic metabolism and increasing ROS
production (31,32). The dramatically increased content of
ROS is due to excessive production of ROS and lower levels of
antioxidant factors (33,34). Thus, how to balance the oxidative
stress is a crucial point in dealing with I/R injury. Increasing
studies have revealed that HDN possesses an antioxidant effect
(9). The present studydemonstrated
that the MDA content and ROS levels increased during H/R and were
significantly reversed following exposure to HDN. However, the
antioxidant indicators SOD and GSH decreased during H/R and were
reversed following exposure to HDN. Taken together, these results
suggested that HDN attenuated HC oxidative stress induced by H/R,
which is consistent with previous studies (19,35,36).
Ebegboni et al (19) found
that flavonoids were associated with ROS modulation, reducing the
generation of superoxide/hydrogen peroxide during H/R-induced
oxidative stress in a human first-trimester trophoblast cell line.
Chen et al (35) observed
that hesperitin (an active metabolite of HDN) significantly
attenuated oxidative stress-induced apoptosis by reducing ROS
levels in cisplatin-treated HK-2 cells in vitro. In a sodium
arsenite (SA)-induced nephrotoxicity and hepatotoxicity model, Turk
et al (36) observed that
HDN co-treatment had an antioxidant effect on SA-induced toxicity
and aided in protecting the tissue architecture by decreasing MDA
and 8-hydroxy-2'-deoxyguanosine levels and increasing the GSH level
and SOD, catalase (CAT), and glutathione peroxidase (GPx)
activities. However, the mechanism by which HDN regulates oxidative
stress remains unknown.
Anti-inflammatory effects are one of the most
important effect types of HDN. Excessive inflammatory responses are
supposed to be the major mechanism of I/R injury in the later phase
(8). Regulating inflammatory
responses provides a novel therapeutic and preventive target for
I/R injury. The present study found that the mRNA expression levels
of IL-6 and TNF-α in HCs and the concentrations of IL-6 and TNF-α
in the supernatants increased significantly after H/R, and HDN
significantly ameliorated these effects. These results suggested
that HDN may ameliorate inflammation in H/R. We and others have
confirmed that HDN attenuates inflammatory responses in I/R injury
(9,17). In a chronic unpredictable mild
stress (CUMS)-induced rat model, Xie et al (37) found that HDN treatment significantly
relieved depressive-like behaviors in CUMS rats by decreasing the
expression levels of IL-6 and TNF-α in the prefrontal cortex and
microglia. In addition, they found that the relative mechanisms
were based on the NLR family pyrin domain containing 3 inflammatory
signaling pathway (37). Heo et
al (38) revealed that HDN
treatment in rats with spinal cord injury reduced neuropathological
changes (includinghemorrhage, inflammatory cell infiltration, and
tissue loss) and levels of proinflammatory cytokines, such as
TNF-α. Collectively, these data imply that HDN ameliorates
inflammatory responses to protect against tissue injury.
Autophagy is required in diverse paradigms of
lifespan extension, leading to the prevailing notion that autophagy
can maintain cell homeostasis and ensure cell survival under
stressful conditions (39,40). We and others have revealed that
autophagy has a pivotal role in maintaining cell survival following
liver I/R injury (23,41). The present study found that the
protein levels of autophagy makers LC3-Ⅱ and Beclin-1 increased,
while P62 levels decreased during H/R under exposure to HDN. Under
exposure to bafilomycin A1, which inhibits autophagolysosomal
fusion and degradation, LC3-Ⅱ levels increased in HCs exposed to
HDN under the H/R conditions. Furthermore, the results were
confirmed by microscopic evaluation of GFP-LC3 puncta in HCs
through confocal microscopy. Taken together, these results
suggested that HDN induced autophagy to protect HCs against H/R
injury. In a previous study, Saiprasad et al (42) found that HDN supplementation
initiated apoptosis via targeted inhibition of constitutively
activated Aurora-A-mediated PI3K/Akt/glycogen synthase kinase-3β
and mTOR pathways coupled with autophagic stimulation against
azoxymethane-induced colon carcinogenesis. Zhu et al
(43) revealed that autophagic
activation may be involved in the mechanism of hesperidin's
therapeutic effects on cognitive impairment. However, Li et
al (15) found that excessive
autophagy exacerbates myocardial I/R injury and that HDN could
reduce myocardial I/R injury by suppressing excessive autophagy.
These opposing observations deserve further study and discussion.
In a previous study, Turk et al (36) found that HDN could decrease
oxidative stress, inhibit inflammation and reduce apoptosis to
protect against SA-induced liver toxicity. The present study
revealed that HDN attenuated oxidative stress, inhibited the
production of pro-inflammatory cytokines and induced autophagy to
protect hepatocytes against H/R-induced injury. Induction of
autophagy may be the underlying mechanism by which HDN aids in
hepatoprotection.
As aforementioned, the effects of HDN on autophagy
appear to be controversial. In the present study, cultured HCs were
injured by H/R stimulation, presumably by oxidative stress. HDN
exposure rescued the damaging effects of H/R stimulation.
Additionally, exposure toHDNincreased autophagy in H/R-treated HCs,
as shown in Fig. 4. It would be
interesting to know how much death in HCs after H/R treatment was
due to autophagy and how much was due to oxidative injury, however
this is difficult to determine. It appears that HDN exposure could
reduce oxidative stress levels by approximately half, according to
Fig. 2. It has been reported that
oxidative stress and inflammatory activity are two early events in
the cascade of I/R injury and H/R injury (41,44).
These two factors also directly trigger the development of
autophagy (41,44). Taken together, it can be assumed
that appropriate autophagy activity contributes to HC recovery by
reducing oxidative stress and inflammatory activity, while
autophagy dysfuntion aggravates HC injury (Fig. 5). The detailed mechanisms by which
HDN regulates oxidative stress, inflammatory activity and autophagy
balance deserve further study in the future.
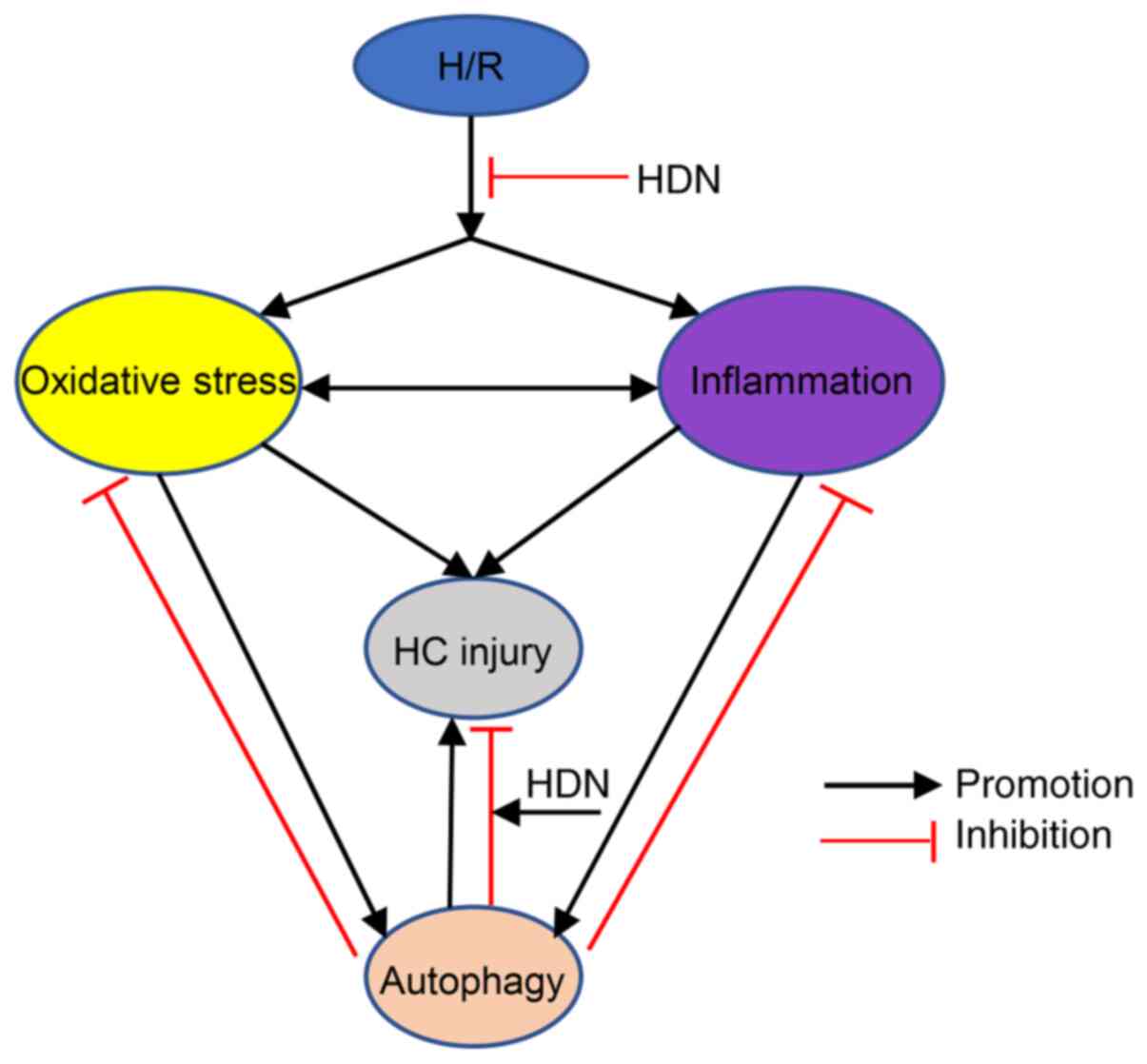 | Figure 5Schematic demonstrating the interplay
among oxidative stress, inflammatory activity, and autophagy
following H/R injury. H/R leads to oxidative stress, inflammatory
activity, and autophagy, while HDN exposure inhibits these effects.
Both oxidative stress and inflammation induce autophagy and HC
injury. Appropriate autophagy activity contributes to HC recovery
by reducing oxidative stress and inflammatory activity, while
autophagy dysfunction aggravates HC injury. HDN exposure promotes
the effects of autophagy, thus reducing HCinjury. H/R,
hypoxia/reoxygenation; HDN, hesperidin; HC, hepatocyte. |
In summary, the present study demonstrated that HDN
attenuated HC oxidative stress and inflammatory responses while
enhancing autophagy during H/R. HDN may have a protective effect on
HCs during H/R-induced injury. These findings broaden our
understanding of the functions of HDN in acute sterile inflammation
and raise the possibility that HDN may be used to protect organs
against I/R injury.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by The Young Teachers' Basic
Ability Improvement in Guangxi University Project (grant no.
2019KY0108), the National Natural Science Foundation of China
(grant no. 81960358),Talents Sub-Highland of Emergency and Medical
Rescue of Guangxi Province in China (grant no. GXJZ201405), Health
Commission of Guangxi (grant no. Z2016289), the Guangxi Natural
Science Foundation (grant nos. 2018JJB140279 and
2020GXNSFAA159127), Guangxi Key Laboratory for the Prevention and
Control of Viral Hepatitis (grant no. GXCDCKL201902) and the
Medical Excellence Award funded by the Creative Research
Development Grant from the First Affiliated Hospital of Guangxi
Medical University (grant no. 2017-11-7).
Availability of data and materials
The datasets used and /or analyzed during the
current study are available from the corresponding authors on
reasonable request.
Authors' contributions
SL and JZ designed and performed the majority of
experiments, isolated and cultured hepatocytes, collected and
analyzed the experimental data, and drafted the manuscript. LP, QQ
and DL performed the supernatant analysis, MTT assay and ELISA. PW,
WP and YW performed autophagic flux and conducted the
immunofluorescence staining. YX performed the RT-qPCR and western
blot analysis. LS and XY supervised the project, provided technical
advice, conducted analyses of the raw data, and reviewed and edited
the manuscript. SL and XY confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Protocols involving animals were approved by The
Animal Care and Use Committee of The First Affiliated Hospital of
Guangxi Medical University (Nanning, China), and the experiments
were performed in adherence to the National Institutes of Health
guidelines for the use of laboratory animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nastos C, Kalimeris K, Papoutsidakis N,
Tasoulis MK, Lykoudis PM, Theodoraki K, Nastou D, Smyrniotis V and
Arkadopoulos N: Global consequences of liver ischemia/reperfusion
injury. Oxid Med Cell Longev. 2014(906965)2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Schemmer P, Lemasters JJ and Clavien PA:
Ischemia/Reperfusion injury in liver surgery and transplantation.
HPB Surg. 2012(453295)2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sinning C, Westermann D and Clemmensen P:
Oxidative stress in ischemia and reperfusion: Current concepts,
novel ideas and future perspectives. Biomark Med. 11:11031–11040.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Halladin NL: Oxidative and inflammatory
biomarkers of ischemia and reperfusion injuries. Dan Med J.
62(B5054)2015.PubMed/NCBI
|
|
5
|
Tan S, Yokoyama Y, Wang Z, Zhou F, Nielsen
V, Murdoch AD, Adams C and Parks DA: Hypoxia-Reoxygenation is as
damaging as ischemia-reperfusion in the rat liver. Crit Care Med.
26:1089–1095. 1998.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Portal L, Martin V, Assaly R, de Tassigny
A, Michineau S, Berdeaux A, Ghaleh B and Pons S: A model of
hypoxia-reoxygenation on isolated adult mouse cardiomyocytes:
Characterization, comparison with ischemia-reperfusion, and
application to the cardioprotective effect of regular treadmill
exercise. J Cardiovasc Pharmacol Ther. 18:367–375. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zou X, Liu Q, Guo S, Zhu J, Han J, Xia Z,
Du Y, Wei L and Shang J: A novel zebrafish larvae
hypoxia/reoxygenation model for assessing myocardial
ischemia/reperfusion injury. Zebrafish. 16:434–442. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jimenez-Castro MB, Cornide-Petronio ME,
Gracia-Sancho J and Peralta C: Inflammasome-Mediated inflammation
in liver ischemia-reperfusion injury. Cells. 8(1131)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Parhiz H, Roohbakhsh A, Soltani F, Rezaee
R and Iranshahi M: Antioxidant and anti-inflammatory properties of
the citrus flavonoids hesperidin and hesperetin: An updated review
of their molecular mechanisms and experimental models. Phytother
Res. 29:323–331. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Elhelaly AE, AlBasher G, Alfarraj S,
Almeer R, Bahbah EI, Fouda MMA, Bungau SG, Aleya L and Abdel-Daim
MM: Protective effects of hesperidin and diosmin against
acrylamide-induced liver, kidney, and brain oxidative damage in
rats. Environ Sci Pollut Res Int. 26:35151–35162. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pandey P, Sayyed U, Tiwari RK, Siddiqui
MH, Pathak N and Bajpai P: Hesperidin induces ROS-mediated
apoptosis along with cell cycle arrest at G2/M phase in human gall
bladder carcinoma. Nutr Cancer. 71:676–687. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mo'men YS, Hussein RM and Kandeil MA:
Involvement of PI3K/Akt pathway in the protective effect of
hesperidin against a chemically induced liver cancer in rats. J
Biochem Mol Toxicol. 33(e22305)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Park HK, Kang SW and Park MS: Hesperidin
ameliorates hepatic ischemia-reperfusion injury in sprague-dawley
rats. Transplant Proc. 51:2828–2832. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Park WS, Park MS, Kang SW, Jin SA, Jeon Y,
Hwang J and Kim SK: Hesperidin shows protective effects on renal
function in ischemia-induced acute kidney injury (Sprague-Dawley
Rats). Transplant Proc. 51:2838–2841. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li X, Hu X, Wang J, Xu W, Yi C, Ma R and
Jiang H: Inhibition of autophagy via activation of PI3K/Akt/mTOR
pathway contributes to the protection of hesperidin against
myocardial ischemia/reperfusion injury. Int J Mol Med.
42:1917–1924. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Meng X, Wei M, Wang D, Qu X, Zhang K,
Zhang N and Li X: The protective effect of hesperidin against renal
ischemia-reperfusion injury involves the TLR-4/NF-κB/iNOS pathway
in rats. Physiol Int. 107:82–91. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li S, Qin Q, Luo D, Pan W, Wei Y, Xu Y,
Zhu J and Shang L: Hesperidin ameliorates liver
ischemia/reperfusion injury via activation of the akt pathway. Mol
Med Rep. 22:4519–4530. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
He S, Wang X, Zhong Y, Tang L, Zhang Y,
Ling Y, Tan Z, Yang P and Chen A: Hesperetin post-treatment
prevents rat cardiomyocytes from hypoxia/reoxygenation injury in
vitro via activating PI3K/Akt signaling pathway. Biomed
Pharmacother. 91:1106–1112. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ebegboni VJ, Dickenson JM and
Sivasubramaniam SD: Antioxidative effects of flavonoids and their
metabolites against hypoxia/reoxygenation-induced oxidative stress
in a human first trimester trophoblast cell line. Food Chem.
272:117–125. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lei Z, Deng M, Yi Z, Sun Q, Shapiro RA, Xu
H, Li T, Loughran PA, Griepentrog JE, Huang H, et al: cGAS-mediated
autophagy protects the liver from ischemia-reperfusion injury
independently of STING. Am J Physiol Gastrointest Liver Physiol.
314:G655–G667. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sun Q, Loughran P, Shapiro R, Shrivastava
IH, Antoine DJ, Li T, Yan Z, Fan J, Billiar TR and Scott MJ:
Redox-Dependent regulation of hepatocyte absent in melanoma 2
inflammasome activation in sterile liver injury in mice.
Hepatology. 65:253–268. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li S, Yi Z, Deng M, Scott MJ, Yang C, Li
W, Lei Z, Santerre NM, Loughran P and Billiar TR: TSLP protects
against liver I/R injury via activation of the PI3K/Akt pathway.
JCI Insight. 4(e129013)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Iranshahi M, Rezaee R, Parhiz H,
Roohbakhsh A and Soltani F: Protective effects of flavonoids
against microbes and toxins: The cases of hesperidin and
hesperetin. Life Sci. 137:125–132. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cernanec J, Guilak F, Weinberg JB,
Pisetsky DS and Fermor B: Influence of hypoxia and reoxygenation on
cytokine-induced production of proinflammatory mediators in
articular cartilage. Arthritis Rheum. 46:968–975. 2002.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cheng Y, Xiong J, Chen Q, Xia J, Zhang Y,
Yang X, Tao K, Zhang S and He S: Hypoxia/reoxygenation-induced
HMGB1 translocation and release promotes islet proinflammatory
cytokine production and early islet graft failure through TLRs
signaling. Biochim Biophys Acta Mol Basis Dis. 1863:354–364.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chenevier-Gobeaux C, Simonneau C,
Lemarechal H, Bonnefont-Rousselot D, Poiraudeau S, Rannou F,
Ekindjian OG, Anract P and Borderie D: Effect of
hypoxia/reoxygenation on the cytokine-induced production of nitric
oxide and superoxide anion in cultured osteoarthritic synoviocytes.
Osteoarthritis Cartilage. 21:874–881. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Fernandez AR, Sánchez-Tarjuelo R, Cravedi
P, Ochando J and López-Hoyos M: Review: Ischemia reperfusion
injury-A translational perspective in organ transplantation. Int J
Mol Sci. 21(8549)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Arriel RA, Rodrigues JF, Souza HLR,
Meireles A, Leitao LFM, Crisafulli A and Marocolo M:
Ischemia-Reperfusion intervention: From enhancements in exercise
performance to accelerated performance recovery-a systematic review
and meta-analysis. Int J Environ Res Public Health.
17(8161)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Nieuwenhuijs-Moeke GJ, Pischke SE, Berger
SP, Sanders JSF, Pol RA, Struys M, Ploeg RJ and Leuvenink HGD:
Ischemia and reperfusion injury in kidney transplantation: Relevant
mechanisms in injury and repair. J Clin Med. 9(253)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yi Z, Deng M, Scott MJ, Fu G, Loughran PA,
Lei Z, Li S, Sun P, Yang C, Li W, et al: Immune-Responsive gene
1/itaconate activates nuclear factor erythroid 2-related factor 2
in hepatocytes to protect against liver ischemia-reperfusion
injury. Hepatology. 72:1394–1411. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Guan LY, Fu PY, Li PD, Li ZN, Liu HY, Xin
MG and Li W: Mechanisms of hepatic ischemia-reperfusion injury and
protective effects of nitric oxide. World J Gastrointest Surg.
6:122–128. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Nita M and Grzybowski A: The role of the
reactive oxygen species and oxidative stress in the pathomechanism
of the age-related ocular diseases and other pathologies of the
anterior and posterior eye segments in adults. Oxid Med Cell
Longev. 2016(3164734)2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
He L, He T, Farrar S, Ji L, Liu T and Ma
X: Antioxidants maintain cellular redox homeostasis by elimination
of reactive oxygen species. Cell Physiol Biochem. 44:532–553.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chen X, Wei W, Li Y, Huang J and Ci X:
Hesperetin relieves cisplatin-induced acute kidney injury by
mitigating oxidative stress, inflammation and apoptosis. Chem Biol
Interact. 308:269–278. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Turk E, Kandemir FM, Yildirim S, Caglayan
C, Kucukler S and Kuzu M: Protective effect of hesperidin on sodium
arsenite-induced nephrotoxicity and hepatotoxicity in rats. Biol
Trace Elem Res. 189:95–108. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Xie L, Gu Z, Liu H, Jia B, Wang Y, Cao M,
Song R, Zhang Z and Bian Y: The anti-depressive effects of
hesperidin and the relative mechanisms based on the NLRP3
inflammatory signaling pathway. Front Pharmacol.
11(1251)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Heo SD, Kim J, Choi Y, Ekanayake P, Ahn M
and Shin T: Hesperidin improves motor disability in rat spinal cord
injury through anti-inflammatory and antioxidant mechanism via
Nrf-2/HO-1 pathway. Neurosci Lett. 715(134619)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhou B, Kreuzer J, Kumsta C, Wu L, Kamer
KJ, Cedillo L, Zhang Y, Li S, Kacergis MC, Webster CM, et al:
Mitochondrial permeability uncouples elevated autophagy and
lifespan extension. Cell. 177:299–314. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wang K: Autophagy and apoptosis in liver
injury. Cell Cycle. 14:1631–1642. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Li J, Lin W and Zhuang L: CD5L-Induced
activation of autophagy is associated with hepatoprotection in
ischemic reperfusion injury via the CD36/ATG7 axis. Exp Ther Med.
19:2588–2596. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Saiprasad G, Chitra P, Manikandan R and
Sudhandiran G: Hesperidin induces apoptosis and triggers autophagic
markers through inhibition of Aurora-A mediated
phosphoinositide-3-kinase/Akt/mammalian target of rapamycin and
glycogen synthase kinase-3 beta signalling cascades in experimental
colon carcinogenesis. Eur J Cancer. 50:2489–2507. 2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhu B, Yang C, Wu J and Hua F: Autophagic
activation may be involved in the mechanism of hesperidin's
therapeutic effects on cognitive impairment. J Neurol Sci.
351:202–203. 2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
He J, Liu J, Huang Y, Tang X, Xiao H and
Hu Z: Oxidative stress, inflammation, and autophagy: Potential
targets of mesenchymal stem cells-based therapies in ischemic
stroke. Front Neurosci. 15(641157)2021.PubMed/NCBI View Article : Google Scholar
|