Introduction
Duodenogastric reflux (DGR, bile reflux) consists of
a retrograde passage of alkaline duodenal and pancreatic content
into the stomach, which can lead to mucous barrier disruption and
direct chemical damage to the surface gastric epithelium. DGR is
common after gastric surgery, cholecystectomy and pyloroplasty, but
it can also occur due to antroduodenal motility disorder (primary
DGR) (1,2). Primary bile reflux does not have a
standardized diagnosis. If an upper digestive endoscopy (UDE)
reveals a large amount of bile in the stomach, mucosal fragility,
erythema/erosions/ulcers in a patient with epigastric pain,
nausea/vomiting, the diagnosis is usually DGR (3).
Gastric cancer (GC), particularly intestinal-type,
arises via the carcinogenic gastric cascade initiated by
Helicobacter (H.) pylori and involves several histological
changes: Active inflammation, non-atrophic chronic gastritis,
multifocal atrophic gastritis (AG), intestinal metaplasia (IM),
dysplasia, and cancer (4-6).
A previous study demonstrated an increased risk of IM in patients
with high levels of bile acid reflux, hence DGR might contribute to
the development of precancerous lesions and GC (7).
The results of studies investigating the
relationship between H. pylori and bile reflux are
contradictory. Some studies report that H. pylori
colonization decreases as a result of high levels of bile acids
(8), while others revealed a
positive relationship between H. pylori and DGR, where DGR
was found to increase the presence of bacteria (9,10).
The present study questioned the importance of
concomitant active H. pylori infection on endoscopic and
histologic gastric changes in patients with biliary aggression. The
objective was to investigate their association with possible
demographic and clinical predictors (digestive symptoms, drug
consumption, social habits, comorbidities) in order to identify
potential strategies to limit progression toward stomach cancer, in
a population with a high prevalence of long-standing H.
pylori infection.
Patients and methods
Subjects and data collection
A total of 2,014 patients with dyspeptic complaints
or anemia, hospitalized at the Medical Clinic No. 2 in Târgu Mureș
Emergency County Hospital, Romania, underwent an upper digestive
endoscopy (UDE) between January 2016 and December 2019. The present
study involved those patients diagnosed with DGR following UDE
(n=560) divided into two groups: Study group (Bile reflux + H.
pylori), 195 patients with H. pylori infection confirmed
upon biopsy specimens utilizing usual histochemical stains or
immunohistochemical methods; and Control group (bile reflux), 365
patients negative for H. pylori infection on all biopsy
sites or immunohistochemistry when performed.
Bile reflux diagnosis was based on detection of DGR
and a large bile pool in the stomach during the endoscopic
examination, in patients with a fasting period of >10 h.
Five random biopsy specimens were taken and examined
for each patient: Two from the antrum, two from the corpus (the
greater and the lesser curvature), and one from incisura angularis.
The tissue samples were fixed in formalin, embedded in paraffin,
and stained with routine hematoxylin and eosin, PAS-Alcian blue and
modified Giemsa, followed by microscopic evaluation. H.
pylori infection was considered negative if the bacteria were
absent from all biopsy sites and positive if identified in at least
one biopsy sample.
When no organism was detected using histochemical
stains, but a high suspicion of infection existed due to persistent
inflammation, particularly for patients with proton-pump inhibitor
(PPI) therapy, the pathologist performed immunohistochemistry
staining. The immunohistochemical staining was performed with FLEX
polyclonal rabbit anti-Helicobacter pylori antibody, Ready
to Use (Dako Autostainer) and using EnVision FLEX, High pH as a
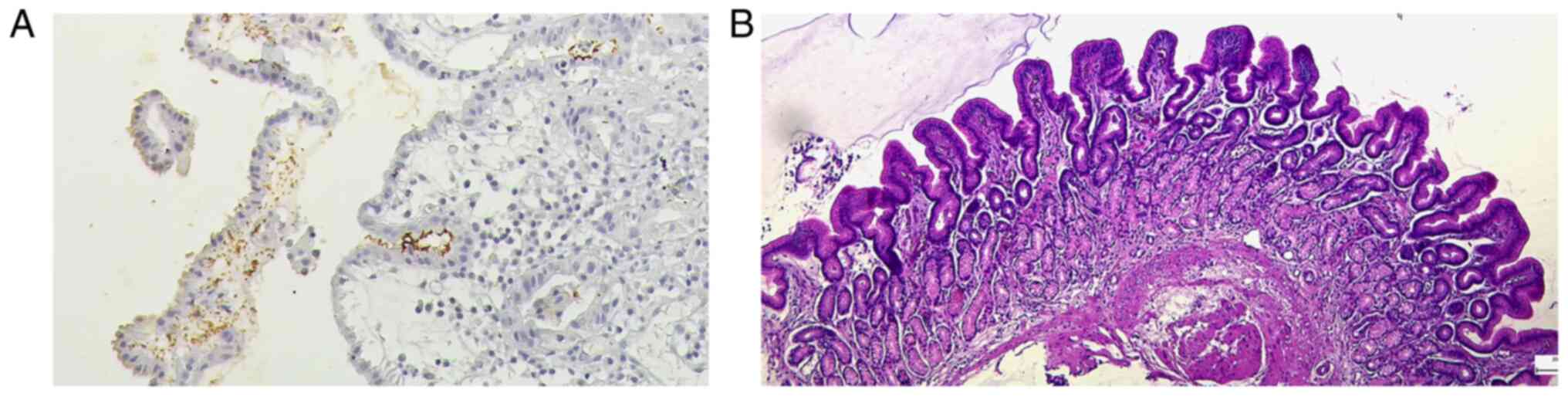
visualization system (Fig. 1).
In the database were noted the clinical examination,
drug exposure, comorbidities, symptoms, and social habits of the
patients. Exclusion criteria were acute bleeding episodes, previous
gastrectomy or H. pylori eradication therapy, dysplasia,
gastric/esophageal neoplasms, and missing data (drug exposure,
social habits, biopsies).
Recorded digestive symptoms included epigastric
pain, heartburn, flatulence, and nausea/vomiting. We considered
chronic proton pump inhibitor therapy (pantoprazole, esomeprazole,
omeprazole) if administered at regular doses for ≥3 months before
investigation.
Anemia and diabetes mellitus were registered when
the diagnosis was made based on international clinical guideline
criteria. For anemia, the cut-off values of hemoglobin levels
according to the WHO definitions were: 12 g/dl (7.5 mmol/l) in
women and <13 g/dl (<8.1 mmol/l) in men (11). We considered alcohol consumption if
patients declared a consumption of ≥10 units (10 ml) of pure
alcohol weekly. Patients registered as smokers were those that
smoked >5 cigarettes/day, including quitters during the past 5
years.
Additional endoscopic and histologic
aspects
During the endoscopic examination of the stomach the
following data were recorded: Mucosal fragility, hyperemia, edema,
petechiae/erosions/ulcers, hiatal hernia, and bile reflux. In order
to describe the endoscopic appearance of reflux esophagitis and
grade its severity, Los Angeles classification system was utilized
(12). In the present study,
esophagitis was recorded as present or absent.
We assessed gastro-duodenal lesions using a modified
Lanza score: Grade 0, no mucosal lesions; Grade 1, one erosion
(mucosal defect <5 mm) or petechiae (hemorrhagic area without
mucosal defect); Grade 2, 2-10 erosions/petechiae; Grade 3, >10
erosions/petechiae; Grade 4, gastric ulcer (defect >5 mm in
diameter). Patients having Lanza scores between 0 and 2 were
registered with no/mild endoscopic lesions, and those with scores
of 3 and 4 were diagnosed with severe endoscopic lesions (13).
Patients with foveolar hyperplasia, vascular
dilation of superficial mucosal capillaries, fibromuscular
replacement of the lamina propria and without inflammation were
diagnosed with reactive gastropathy.
Atrophy was registered as the loss of normal glands
in both the antrum/corpus. IM has various aspects on histology. The
pathologist considered complete IM when some parts of the gastric
epithelium had a small intestinal epithelium aspect, with
eosinophilic enterocytes, goblet cells and brush border. Incomplete
IM was defined as colonic epithelium in gastric mucosa, containing
mucin droplets with irregular form and different sizes, as well as
the absence of the brush border. The Updated Sydney System was used
to assess the intensity of mononuclear inflammatory cellular
infiltrates and inflammatory activity (neutrophilic infiltrations)
in the diagnosis of gastritis. The degree of the atrophy and IM was
defined using the OLGA/OLGIM (Operative Link on Gastritis/IM
Assessment) (14). In addition,
patients with antral-type glands in the body of the stomach were
diagnosed with pseudopyloric metaplasia.
Statistical analysis
For statistical analysis, JASP (JASP graphical
program for statistical analysis supported by the University of
Amsterdam) program v.0.12.1 (https://jasp-stats.org/) was applied. Mean ± standard
deviation or absolute (relative) frequencies were used to summarize
the demographic and clinical data. To identify significant
differences in terms of quantitative demographic characteristics,
Mann Whitney U test was applied, while for comparison of
categorical variables the Chi-square or Fisher's exact test were
utilized, both followed by univariate logistic regression analysis.
All significant factors and factors whose unadjusted estimated
significance level was P<0.25 in univariate analysis were
selected as candidates for multivariate logistic regression. The
partial likelihood ratio test was applied to decide the analyzed
variables in the final model. The odds ratio (OR) and 95% CI
quantified the magnitude of the association. A two-sided P-value
<0.05 was considered statistically significant in all tests.
Results
The observed relative frequency of H. pylori
infection among patients with bile reflux was 34.82%. There was no
significant difference in terms of age between the Study and
Control groups: Median (Q1-Q3) 62 years (52-72) for controls vs. 60
years (50.5-69.5) for cases, U=38206, P=0.151, but patients >65
years, although without a statistically significant level, were
more frequent in the Bile reflux group (without H. pylori
infection). The differences between demographic and clinical
characteristics are presented in Table
I. Severe endoscopic lesions (score 3 and 4) and premalignant
gastric lesions (IM and AG) were more common in patients with DGR
reflux and H. pylori infection [45.1% (64) vs. 28.4% (104)
and 37.4% (73) vs. 32.3% (118), respectively], but without a
statistically significant association (Table I).
 | Table IGroup differences regarding the
studied factors in patients with DGR ± H. pylori
infection. |
Table I
Group differences regarding the
studied factors in patients with DGR ± H. pylori
infection.
| | Bile reflux + H.
pylori (study group) (n1=195) | Bile reflux (control
group) (n2=365) | |
|---|
| Variables | No. | % | No. | % | P-valuea |
|---|
| Age >65 years | 65 | 33.3 | 150 | 41 | 0.0800 |
| Female sex | 98 | 50.3 | 199 | 54.6 | 0.3700 |
| Premalignant gastric
lesions | 73 | 37.4 | 118 | 32.3 | 0.2200 |
| Severe endoscopic
lesions | 64 | 45.1 | 104 | 28.4 | 0.2800 |
| Anemia | 32 | 16.4 | 67 | 18.3 | 0.6400 |
| Esophagitis | 43 | 22.0 | 72 | 19.7 | 0.5100 |
| Diabetes | 34 | 17.4 | 62 | 16.9 | 0.9000 |
| Heartburn | 58 | 29.7 | 98 | 26.8 | 0.4800 |
| Epigastric
pain | 103 | 52.8 | 156 | 42.7 | 0.0261 |
|
Nausea/Vomiting | 33 | 16.9 | 55 | 15.0 | 0.6200 |
| Flatulence | 50 | 25.6 | 67 | 18.3 | 0.0496 |
|
Smokingb | 31 | 15.8 | 34 | 9.3 | 0.0263 |
| Alcohol
consumptionc | 40 | 20.5 | 54 | 14.7 | 0.0900 |
| PPI therapy | 73 | 37.4 | 185 | 50.6 | 0.0033 |
In the Bile reflux group, the stomach histology most
frequently demonstrated reactive gastropathy (44.4% patients),
followed by inactive chronic gastritis (40.5%). Also, 15.1% of the
patients were without histologic lesions.
The distribution of premalignant gastric lesions was
similar between the Study and Control groups. Patients presented
more frequent both IM and AG, but there were 41% cases and 39%
controls of IM alone. AG, without IM, was found only in a small
percentage of patients with premalignant gastric lesions (Table II).
 | Table IIPremalignant gastric lesion
distribution. |
Table II
Premalignant gastric lesion
distribution.
| | Bile reflux + H.
pylori (Study group) (n1=73) | Bile reflux
(Control group) (n2=118) | |
|---|
| Premalignant
gastric lesions (n=191) | No. | % | No. | % |
P-valuea | OR | 95% CI |
|---|
| IM | 30 | 41.1 | 46 | 39 | 0.80 | 1.07 | 0.65 to 1.75 |
| AG | 2 | 2.7 | 8 | 6.8 | 0.32 | 0.38 | 0.07 to 1.87 |
| IM + AG | 41 | 56.2 | 64 | 54.2 | 0.88 | 1.08 | 0.60 to 1.94 |
In the univariate logistic regression model, smoking
was a significant factor associated with DGR and H. pylori
infection, while pre-treatment with PPI had a negative effect.
Regarding the symptomatology, epigastric pain and flatulence were
significant factors associated with H. pylori infection and
bile reflux (Table III).
 | Table IIIResults from the univariate binary
logistic regression. |
Table III
Results from the univariate binary
logistic regression.
| Variable | Statistics Z |
P-valuea | Crude OR | 95% CI |
|---|
| Age >65
years | -1.79 | 0.07 | 0.71 | 0.49 to 1.03 |
| Female sex | -0.96 | 0.33 | 0.84 | 0.59 to 1.19 |
| Severe endoscopic
lesions | 1.06 | 0.28 | 1.22 | 0.84 to 1.78 |
| Anemia | -0.57 | 0.56 | 0.87 | 0.54 to 1.38 |
| Esophagitis | 0.64 | 0.51 | 1.15 | 0.75 to 1.76 |
| Diabetes | 0.13 | 0.89 | 1.03 | 0.65 to 1.63 |
| Heartburn | 0.72 | 0.46 | 1.15 | 0.78 to 1.69 |
| Epigastric
pain | 2.27 | 0.023 | 1.50 | 1.05 to 2.12 |
|
Nausea/Vomiting | 0.57 | 0.56 | 1.14 | 0.71 to 1.84 |
| Flatulence | 2.09 | 0.036 | 1.56 | 1.02 to 2.37 |
|
Smokingb | 2.29 | 0.022 | 1.84 | 1.09 to 3.10 |
| Alcohol
consumptionc | 1.17 | 0.08 | 1.48 | 0.94 to 2.33 |
| PPI therapy | -2.98 | 0.003 | 0.58 | 0.40 to 0.83 |
According to the multivariate logistic analysis
results, in patients with DGR, smoking was significantly associated
with H. pylori infection, while PPI therapy remained
negatively associated with the immunohistochemically confirmed
infection. The odds of H. pylori infection increased by 1.88
(95% CI 1.10 to 3.21) in smokers with bile reflux, effect adjusted
for other covariates. Epigastric pain was related to H.
pylori-positive patients, while flatulence had only a tendency
towards statistical significance (P=0.09) (Table IV).
 | Table IVFinal multivariable logistic
regression model. |
Table IV
Final multivariable logistic
regression model.
| Variables | ba | SE |
P-valueb | Adjusted OR | 95% CI |
|---|
| Smoking | 0.63 | 0.27 | 0.020 | 1.88 | 1.10 to 3.21 |
| PPI therapy | -0.67 | 0.19 |
<0.001 | 0.50 | 0.35 to 0.73 |
| Flatulence | 0.36 | 0.21 | 0.090 | 1.44 | 0.93 to 2.21 |
| Epigastric
pain | 0.52 | 0.18 | 0.005 | 1.69 | 1.17 to 2.45 |
| Intercept | -0.73 | 0.14 |
<0.001 | 0.47 | 0.35 to 0.64 |
The final multivariable logistic regression model
was statistically significant, χ2(555)=26.52,
P<0.001. The model correctly classified 66% of patients and the
Brier score of 0.21 indicates a good accuracy of the model.
Discussion
The diagnosis and clinical consequences of DGR are
challenging, particularly for patients without known risk factors
(gastric surgery) (15). High
amounts of bile acids can cause direct gastric mucosal injury by
its components, triggering inflammation (16). Because bile reflux and H.
pylori often coexist and both are involved in the pathogenesis
of gastritis, research has examined the relationship between these
two entities. All patients included in this study had endoscopic
criteria for biliary reflux and potential chemical aggression of
the gastric mucosa. Only less than half of patients without active
H. pylori infection (Control group) presented histologic
features of gastropathy (44.4%), while 15% had no histologic
changes. The absence of histologic gastropathy supports less
aggressive or recurrent exposure to bile acid or no biopsy from
those areas with chemical aggression. In patients with active H.
pylori infection, the chemical aggression is ‘covered’ by
histologic inflammatory changes. Reexamination after eradication
therapy may appreciate the role of each factor in the changes in
gastric mucosa.
A recent article suggests a molecular mechanism
related to the FXR/NF-κB signaling pathway, CDX2, and MUC2
implicated in IM occurrence and progression in patients with bile
reflux (17). In addition,
researchers have questioned the role of cytokines and their gene
variants in premalignant gastric lesion development in patients
with H. pylori infection (18). The risk of GC, mucosal aggression,
symptoms and interaction with H. pylori infection are
important issues in managing patients with DGR in daily practice as
H. pylori favors the release of gastrin in the antrum,
decreases peristaltic movements, and determines bile reflux
(16). Although a higher prevalence
of IM has been reported in patients with both DGR and H.
pylori (10), in the studied
population, H. pylori infection over bile reflux appeared
not to exert significant additional influence on IM and AG
development.
Severe endoscopic lesions were not significantly
associated with concomitant H. pylori infection in the
studied groups, sustaining previous findings in patients with
gastric surgery in whom H. pylori did not play a substantial
role in ulcer recurrence (19).
In the present study, epigastric pain was the most
common complaint in both groups and was significantly associated
with H. pylori infection, an effect that remained
significant also in multivariate analysis. Another study
investigating the symptoms in patients with H. pylori
infection and bile reflux, reported nausea as the main complaint in
patients with DGR, while in patients with H. pylori
gastritis, epigastric pain was the most frequent symptom, followed
by heartburn (20). Nevertheless,
H. pylori infection was detected in only 40% of dyspeptic
patients in the Romanian population, the role of other underlying
conditions playing an important role (21).
The role of bile reflux in reflux esophagitis
pathogenesis assumes chemical injury, but a recent study suggests
that acidic bile salts induce esophagitis by a cytokine-mediated
inflammation injury, driven by hypoxia-inducible factor 2α (HIF-2α)
(22). Regarding the relationship
between H. pylori and esophagitis, a meta-analysis showed
that eradication of H. pylori increased the risk of
esophagitis development, compared with patients without
eradication. However, in the present study, the number of cases
with esophagitis was similar between the two groups (23).
In the studied population, patients with DGR and
negative for H. pylori infection were found to be consuming
more frequent proton-pump inhibitors (PPIs). The effect of PPIs on
decreasing the bacterial load in biopsy samples is well-known
(24); therefore, to counteract the
possible false-negative results when the suspicion of infection
persisted due to inflammatory changes on histology, pathologists
performed immunohistochemistry staining. High number of PPI
prescriptions may be explained by the rich symptomatology
attributed to both gastric acid secretion and DGR.
In the studied population, the multivariate logistic
analysis revealed smoking is associated with H. pylori
infection in patients with DGR. This result is in line with studies
demonstrating that smoking affects gastro-duodenal motility and
favors bile reflux into the stomach. Furthermore, smoking
imbalances the antioxidant equilibrium and increases the risk of
H. pylori infection (25).
The strength of the present study is the systematic
histologic assessment of both antrum and corpus changes in patients
with biliary reflux on endoscopy, tied with a thorough description
of the most relevant clinical and endoscopic findings. Patients
with a non-invasive diagnosis of H. pylori infection often
complain about the persistence of symptoms after eradication
therapy. Identifying associations between bile reflux and
clinicopathological parameters may offer possible strategies to
manage the symptoms and follow-up of this group of patients. The
study has all the limitations of a retrospective observational
study. The lack of bile reflux measurement and pH monitoring, and
patients enrolled from the same geographical area are aspects to be
improved.
In conclusion, in the studied population, H.
pylori infection over DGR did not exert significant additional
influence on the severity of endoscopic lesions, neither in
premalignant gastric lesion development. Epigastric pain is more
frequent in patients with bile reflux and H. pylori
infection than in uninfected patients. Smoking was associated with
H. pylori infection in patients with DGR, while H.
pylori infection did not influence esophagitis occurrence. In
patients with DGR diagnosis, PPI therapy had a negative association
with immunohistochemically assessed H. pylori infection
active gastritis.
Acknowledgements
Not applicable.
Funding
Funding: This research study was supported by an Internal
Research Grant from the University of Medicine, Pharmacy, Sciences,
and Technology of Târgu Mureș (no. 615/12/17.01.2019).
Availability of data and materials
The datasets analyzed during the current study are
available from the corresponding author on request.
Authors' contributions
Conceptualization of the study design was achieved
by AN and AS. Methodology was the responsibility of AN. Formal
analysis was conducted by AN and AS. Study investigation was
conducted by AN and SM. Resources were obtained by AN and data
curation was performed by AN and AS. Writing and original draft
preparation was done by AS, AN and SM. Writing, review and editing
was done by AN and AS. Supervision was conducted by AN, and project
administration and funding acquisition were the responsibility of
AN. All authors have read and agreed to the published version of
the manuscript.
Ethics approval and consent to
participate
The research was approved by the Ethics Committee of
the University of Medicine and Pharmacy of Târgu Mureș, Romania
(282, 19.07.2019). The patient informed consent was obtained for
regular endoscopic investigation.
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Tatsugami M, Ito M, Tanaka S, Yoshihara M,
Matsui H, Haruma K and Chayama K: Bile acid promotes intestinal
metaplasia and gastric carcinogenesis. Cancer Epidemiol Biomarkers
Prev. 21:2101–2107. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Agin M and Kayar Y: The effect of primary
duodenogastric bile reflux on the presence and density of
Helicobacter pylori and on gastritis in childhood. Medicina
(Kaunas). 55(775)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chang WK, Lin CK, Chuan DC and Chao YC:
Duodenogastric reflux: Proposed new endoscopic classification in
sypmtomatic patients. J Med Sci. 36:1–5. 2016.
|
|
4
|
Amieva M and Peek RM Jr: Pathobiology of
Helicobacter pylori-induced gastric cancer.
Gastroenterology. 150:64–78. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
González CA, Sanz-Anquela JM, Gisbert JP
and Correa P: Utility of subtyping intestinal metaplasia as marker
of gastric cancer risk. A review of the evidence. Int J Cancer.
133:1023–1032. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jencks DS, Adam JD, Borum ML, Koh JM,
Stephen S and Doman DB: Overview of current concepts in gastric
intestinal metaplasia and gastric cancer. Gastroenterol Hepatol (N
Y). 14:92–101. 2018.PubMed/NCBI
|
|
7
|
Matsuhisa T, Arakawa T, Watanabe T,
Tokutomi T, Sakurai K, Okamura S, Chono S, Kamada T, Sugiyama A,
Fujimura Y, et al: Relation between bile acid reflux into the
stomach and the risk of atrophic gastritis and intestinal
metaplasia: A multicenter study of 2283 cases. Dig Endosc.
25:519–525. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Asfeldt AM, Steigen SE, Løchen ML, Straume
B, Johnsen R, Bernersen B, Florholmen J and Paulssen EJ: The
natural course of Helicobacter pylori infection on
endoscopic findings in a population during 17 years of follow-up:
The Sørreisa gastrointestinal disorder study. Eur J Epidemiol.
24:649–658. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li D, Zhang J, Yao WZ, Zhang DL, Feng CC,
He Q, Lv HH, Cao YP, Wang J, Qi Y, et al: The relationship between
gastric cancer, its precancerous lesions and bile reflux: A
retrospective study. J Dig Dis. 21:222–229. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ladas SD, Katsogridakis J, Malamou H,
Giannopoulou H, Kesse-Elia M and Raptis SA: Helicobacter
pylori may induce bile reflux: Link between H. pylori
and bile induced injury to gastric epithelium. Gut. 38:15–18.
1996.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kasi M and Bowling T: Anaemia in
gastroenterology. Medicine. 43:153–156. 2015.
|
|
12
|
Lundell LR, Dent J, Bennet JR, Blum AL,
Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler
SJ, et al: Endoscopic assessment of oesophagitis: Clinical and
functional correlates and further validation of the Los Angeles
classification. Gut. 45:172–180. 1999.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lanza FL: Endoscopic studies of gastric
and duodenal injury after the use of ibuprofen, aspirin, and other
nonsteroidal anti-inflammatory agents. Am J Med. 77:19–24.
1984.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Capelle LG, de Vries AC, Haringsma J, Ter
Borg F, de Vries RA, Bruno MJ, van Dekken H, Meijer J, van Grieken
NC and Kuipers EJ: The staging of gastritis with the OLGA system by
using intestinal metaplasia as an accurate alternative for atrophic
gastritis. Gastrointest Endosc. 71:1150–1158. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
McCabe ME IV and Dilly CK: New causes for
the old problem of bile reflux gastritis. Clin Gastroenterol
Hepatol. 16:1389–1392. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Huang H, Tian J, Xu X, Liang Q, Huang X,
Lu J and Yao Y: A study on the roles of Helicobacter pylori
in bile reflux gastritis and gastric cancer. J BUON. 23:659–664.
2018.PubMed/NCBI
|
|
17
|
Yu JH, Zheng JB, Qi J, Yang K, Wu YH, Wang
K, Wang CB and Sun XJ: Bile acids promote gastric intestinal
metaplasia by upregulating CDX2 and MUC2 expression via the
FXR/NF-κB signalling pathway. Int J Oncol. 54:879–892.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Negovan A, Iancu M, Fülöp E and Bănescu C:
Helicobacter pylori and cytokine gene variants as predictors
of premalignant gastric lesions. World J Gastroenterol.
25:4105–4124. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pantea M, Negovan A, Banescu C, Bataga S,
Neagoe R, Mocan S and Iancu M: Factors associated with recurrent
ulcers in patients with gastric surgery after more than 15 years: A
cross-sectional single-center study. Gastroenterol Res Pract.
2018(8319481)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Barakat EAME, Abbas NF and El-Kholi NY:
Primary bile reflux gastritis versus Helicobacter pylori
gastritis: A comparative study. Egypt J Intern Med. 30:23–27.
2018.
|
|
21
|
Corojan AL, Dumitrașcu DL, Ciobanca P and
Leucuta DC: Prevalence of Helicobacter pylori infection
among dyspeptic patients in Northwestern Romania: A decreasing
epidemiological trend in the last 30 years. Exp Ther Med.
20:3488–3492. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Souza RF, Bayeh L, Spechler SJ, Tambar UK
and Bruick RK: A new paradigm for GERD pathogenesis. Not acid
injury, but cytokine-mediated inflammation driven by HIF-2α: A
potential role for targeting HIF-2α to prevent and treat reflux
esophagitis. Curr Opin Pharmacol. 37:93–99. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhao Y, Li Y, Hu J, Wang X, Ren M, Lu G,
Lu X, Zhang D and He S: The effect of Helicobacter pylori
eradication in patients with gastroesophageal reflux disease: A
meta-analysis of randomized controlled studies. Dig Dis.
38:261–268. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bazin T, Nchare Mfondi A, Julie C, Émile
JF, Raymond J and Lamarque D: Contribution of genetic amplification
by PCR for the diagnosis of Helicobacter pylori infection in
patients receiving proton pump inhibitors. United European
Gastroenterol J. 6:1267–1273. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Butt J, Varga MG, Wang T, Tsugane S,
Shimazu T, Zheng W, Abnet CC, Yoo KY, Park SK, Kim J, et al:
Smoking, Helicobacter pylori serology and gastric cancer
risk in prospective studies from China, Japan, and Korea. Cancer
Prev Res (Phila). 12:667–674. 2019.PubMed/NCBI View Article : Google Scholar
|