Introduction
Epstein-Barr virus (EBV), also known as human
herpesvirus 4, is a member of the herpesviridae family. EBV
consists of double-stranded DNA (approximately 172 kbp in length)
surrounded by a nucleocapsid and an envelope (1). EBV can be transmitted through saliva,
and most of the adult population may be infected by EBV by the age
of 30 years (2,3). Therefore, it is thought that initial
EBV infection usually occurs in adolescents. EBV has been
recognized as a cause of cancers, such as Burkitt's lymphoma,
Hodgkin's lymphoma, stomach cancer, salivary gland carcinoma and
nasopharyngeal carcinoma (4-7).
The oral cavity is an initial site for persistent
infection of EBV. The specific histological structure of the
tonsillar region may induce sensitivity to EBV infection because
lymphoid tissue is abundant in tonsillar tissue. EBV can infect B
lymphocytes through saliva and can also infect epithelial cells
(8). EBV may also infect both
epithelial cells and blood cells in inflammatory periodontal
pockets. Epithelial cells play a vital role in the amplification of
EBV prior to shedding in saliva (8,9). Thus,
the oral cavity may be involved in virus transmission to other
sites such as the nasopharynx and gastrointestinal tract.
With regard to the association between oral health
status and EBV infection, several previous studies have
demonstrated that EBV is strongly associated with periodontitis
(10-13).
The results indicate that inflammatory periodontal pockets may
serve as a reservoir of viral organisms in the oral cavity and
provide an opportunity for EBV to infect epithelial cells. However,
it remains unknown whether EBV is associated with poor oral health
(i.e., dental plaque accumulation, an increased oral bacterial
count, and a small number of remaining teeth) among middle-aged and
older Japanese people. Smoking and diabetes are strongly related to
oral EBV prevalence (14,15). However, the relationship between
periodontitis and EBV has not been fully elucidated by reducing the
effects of confounding factors (i.e., smoking and a medical history
of diseases such as diabetes). Therefore, the objective of this
study was to clarify the association between oral EBV prevalence
and periodontal health status by considering the effects of
confounding clinical variables in this population group.
Materials and methods
Patients and methods
A total of 150 patients who visited the Department
of Oral Health of Hiroshima University Hospital between October
2018 and December 2019 were enrolled in the present study. We
excluded subjects with oral cancer or potentially malignant oral
disorders (i.e., leukoplakia or lichen planus) (n=1), cancer
patients receiving surgical treatment, chemotherapy or radiotherapy
(n=20), those with auto-immune diseases receiving steroid therapy
(n=3) and those with severe immunodeficiency (n=2). We included
smokers and patients with a medical history of hypertension,
diabetes, hyperlipidemia, stroke, heart disease, or bone and joint
disease. Finally, we analyzed 124 patients (46 males and 78
females; mean age, 69.2 years; age range, 35-90 years) in this
study. The design of this cross-sectional study was approved by the
Ethical Committee of Hiroshima University. All participants signed
an informed consent agreement.
Oral rinse sample processing and DNA
extraction
Oral rinse samples were obtained by asking the
subjects to rinse their mouths with 10 ml of saline for 15 sec.
Samples were collected in sterile 15-ml tubes, and immediately
centrifuged. The supernatant was decanted and the pellets were
stored at -80˚C. DNA was extracted using a PureLink™ Microbiome DNA
Purification kit (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol.
Oral examination
Bacterial numbers on the tongue surface were
measured using a bacterial counter (Panasonic Healthcare Co., Ltd.)
(16). Samples were obtained from
the tongue surface using a cotton swab, according to the
manufacturer's protocol, and then the oral rinse sample was
collected. Probing depth and bleeding on probing (BOP) were then
assessed at six sites (mesiobuccal, mesiolingual, buccal, lingual,
distobuccal, and distolingual) on all remaining teeth. BOP was
recorded as positive when blood flow from the gingival sulcus was
observed. BOP was recorded as negative when no bleeding or a small
bleeding point was observed. Next, plaque control record scores
were recorded according to O'Leary's method using a plaque
disclosing agent to examine dental plaque accumulation (17). The number of remaining teeth and
denture use were also recorded.
Quantitation of human cell
numbers
In accordance with the methods of a previous study
(18), the human endogenous
retrovirus group 3 member 1 (ERV3-1) gene was employed to
quantitate human cells using quantitative polymerase chain reaction
(PCR). DNA levels were quantitated using a CFX Connect real-time
PCR detection system (Bio-Rad Laboratories, Inc.). The reaction
mixture contained SYBR Green PCR Master Mix (Toyobo Life Science),
1.0 µl DNA and 10 µmol of each pair of oligonucleotide primers.
Amplifications were performed with initial melting at 95˚C for 5
min, followed by 40 cycles of 95˚C for 30 sec, 57˚C for 30 sec and
72˚C for 1 min.
EBV DNA detection
Real-time PCR analysis was performed to determine
EBV DNA copy number in the samples using a CFX Connect real-time
PCR detection system. A 180-bp fragment within the EBV genome was
prepared, which was cloned into a pUC57 vector (GenScript Biotech
Corporation,). We generated a standard curve using real-time PCR
analysis, which indicated the CT value vs. the copy number of EBV.
Amplifications were performed with a cycle of 95˚C for 5 min,
followed by 40 cycles of 95˚C for 1 min, 58˚C for 1 min and 72˚C
for 1 min. Next, real-time PCR was performed to detect EBV DNA. DNA
samples containing 1,000-10,000 human cells per 1.0 µl DNA were
used for PCR analysis. The primer sequences for EBV were
5'-CCTGGTCATCCTTTGCCA-3' (sense) and 5'-TGCTTCGTTATAGCCGTAGT-3'
(antisense) (19). Copy numbers
above the detection limit in a standard curve for EBV DNA were
assessed as EBV positive. EBV DNA copy number was presented as the
mean ± standard deviation of three independent experiments. ERV3-1
gene was used as an internal control for real-time PCR
analysis.
Periodontal disease-related bacteria
detection by PCR
According to our previous study, periodontal
disease-related bacteria were detected by PCR with specific DNA
primer sets (20). After the PCR
reaction, the PCR product was electrophoresed on 2% agarose gels
with ethidium bromide staining.
Statistical analysis
The χ2 test or Fisher's exact test were
used to evaluate significant differences between positive rates of
EBV DNA and clinical factors. The Mann-Whitney U test was used to
compare differences in clinical parameters between two groups. The
Kruskal-Wallis test was used to compare differences in clinical
parameters among three groups. If the Kruskal-Wallis test was
significant, the Nemenyi test was performed. A propensity
score-matched analysis was employed to decrease the effects of
confounders. Propensity scores were calculated by logistic
regression analysis of 11 clinical factors (age, sex, remaining
teeth, denture use, smoking, hypertension, diabetes,
hyperlipidemia, stroke, heart disease, and bone and joint disease).
The results of the Hosmer-Lemeshow test was not statistically
significant (P=0.44), suggesting good fitness of the model. A
caliper of 0.25 standard deviation of the propensity score was used
for analysis. Statistical analysis was performed using SPSS version
24.0 (IBM Corp.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Association between EBV DNA positivity
and clinical factors
EBV DNA positivity was examined in a total of 124
oral rinse samples using real-time PCR. EBV DNA was determined as
positive in 16 of 124 participants (12.9%). The number of EBV DNA
copies was evaluated as copy number/100 human cells. The average
number of EBV viral copies was 5.3±4.8 copies/100 cells (range,
0.8-13.8 copies/100 cells). Table I
summarizes the relationship between EBV DNA and clinical
parameters. No significant difference was found between EBV DNA and
clinical factors (i.e., sex, age, remaining teeth, denture use or
medical history). Smokers exhibited a higher EBV DNA positivity
rate (25.0%) than non-smokers (12.5%), but a significant
association was not found.
 | Table IAssociation between oral EBV DNA and
clinical parameters. |
Table I
Association between oral EBV DNA and
clinical parameters.
| | EBV DNA | |
|---|
| Clinical factor
(n) | Negative | Positive | P-value |
|---|
| Age,
yearsa | 69.1±11.6 | 69.4±12.6 | 0.74 |
| Sex, n (%) | | | 0.59 |
|
Male
(46) | 39 (84.8) | 7 (15.2) | |
|
Female
(78) | 69 (88.5) | 9 (11.5) | |
| Remaining
teetha | 23.0±6.7 | 23.9±4.5 | 0.77 |
| Denture user, n
(%) | | | >0.99 |
|
Non-user
(90) | 78 (86.7) | 12 (13.3) | |
|
User
(34) | 30 (88.2) | 4 (11.8) | |
| Smoking, n (%) | | | 0.43 |
|
No
(120) | 105 (87.5) | 15 (12.5) | |
|
Yes (4) | 3 (75.0) | 1 (25.0) | |
| Hypertension, n
(%) | | | 0.20 |
|
No (96) | 86 (89.6) | 10 (10.4) | |
|
Yes
(28) | 22 (78.6) | 6 (21.4) | |
| Diabetes, n
(%) | | | |
|
No
(111) | 96 (86.5) | 15 (13.5) | >0.99 |
|
Yes
(13) | 12 (92.3) | 1 (7.7) | |
| Hyperlipidemia, n
(%) | | | |
|
No
(100) | 88 (88.0) | 12 (12.0) | 0.51 |
|
Yes
(24) | 20 (83.3) | 4 (16.7) | |
| Stroke, n (%) | | | |
|
No
(118) | 104 (88.1) | 14 (11.9) | 0.17 |
|
Yes (6) | 4 (66.7) | 2 (33.3) | |
| Heart disease, n
(%) | | | |
|
No
(117) | 102 (87.2) | 15 (12.8) | 0.68 |
|
Yes (7) | 6 (85.7) | 1 (14.3) | |
| Bone and joint
disease, n (%) | | | |
|
No
(117) | 102 (87.2) | 15 (12.8) | 0.68 |
|
Yes (7) | 6 (85.7) | 1 (14.3) | |
Association between EBV DNA positivity
and dental plaque accumulation and periodontal condition
Next, the relationship between EBV DNA positivity
and dental plaque accumulation and periodontal health condition was
examined. Associations between EBV DNA positivity and dental plaque
accumulation and periodontal health condition are summarized in
Table II. There was no significant
difference between EBV DNA positivity and the plaque control record
score. Ten of the 38 participants with periodontal pockets ≥6 mm
were EBV DNA positive (26.3%). There was a significant association
between EBV DNA positivity and probing depth. Subjects with BOP
exhibited a higher EBV DNA positivity rate (20.8%) than those
without BOP (7.0%). A significant association was found between BOP
and EBV DNA positivity. Additionally, subjects with ≥6 mm
periodontal pockets with BOP recorded a higher EBV DNA positivity
rate (28.0%) than those without ≥6 mm periodontal pockets with BOP
(9.1%). There was a significant association between ≥6 mm
periodontal pockets with BOP and EBV DNA positivity. However, no
significant difference was found between EBV DNA positivity and
bacterial numbers.
 | Table IIAssociation between oral EBV DNA and
oral health. |
Table II
Association between oral EBV DNA and
oral health.
| | EBV DNA | |
|---|
| Factor (n) | Negative | Positive | P-value |
|---|
| Plaque control
record scores (%)a | 36.2±18.4 | 40.3±19.4 | 0.34 |
| Probing depth [mm,
n (%)] | | | 0.01 |
|
<4(52) | 49 (96.4) | 3 (3.6) | |
|
≥4 and
<6(34) | 31 (91.2) | 3 (8.8) | |
|
≥6(38) | 28 (73.7) | 10 (26.3) | |
| BOP, n (%) | | | 0.03 |
|
No (71) | 66 (93.0) | 5 (7.0) | |
|
Yes
(53) | 42 (79.2) | 11 (20.8) | |
| ≥6 mm periodontal
pocket with BOP, n (%) | | | 0.02 |
|
No (99) | 90 (90.9) | 9 (9.1) | |
|
Yes
(25) | 18 (72.0) | 7 (28.0) | |
| Oral bacteria
numbera
(1.0x106 CFU/ml) | 8.1±6.1 | 7.8±9.0 | 0.30 |
Association between EBV DNA positivity
and periodontal disease-related bacteria
In this study, we investigated the so-called red
complex bacteria which are the most vital pathogens in chronic
periodontal disease (21). PCR was
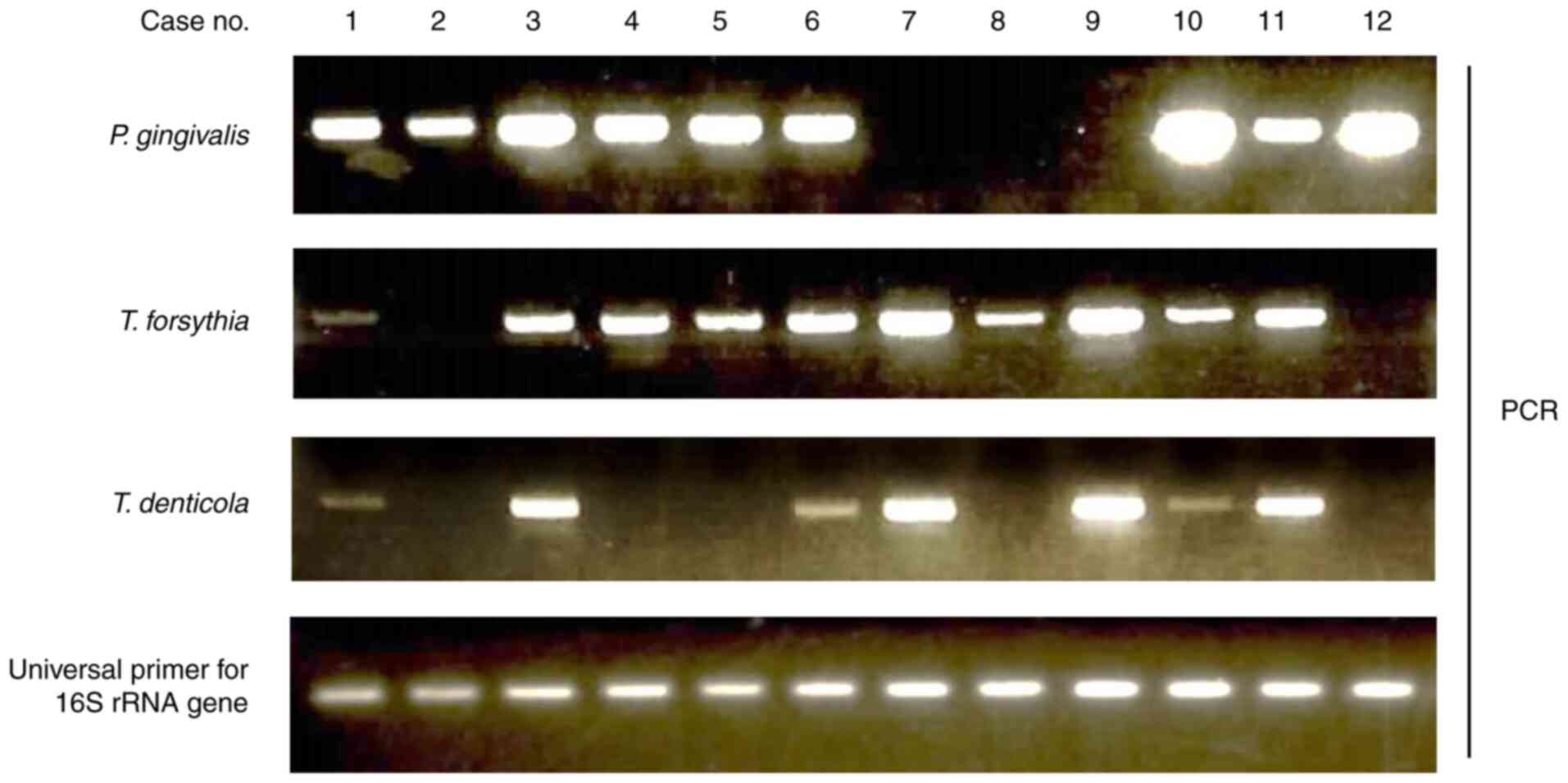
performed to detect P. gingivalis, T. forsythia, and
T. denticola DNA (Fig. 1).
Associations between EBV DNA positivity and red complex bacteria
are summarized in Table III.
T. denticola positive participants exhibited a higher EBV
DNA positivity rate than negative participants (17.6% vs. 9.6%).
However, there was no significant association between EBV DNA
positivity and periodontal disease-related bacteria.
 | Table IIIAssociation between oral EBV DNA and
periodontal disease-related bacteria. |
Table III
Association between oral EBV DNA and
periodontal disease-related bacteria.
| | EBV DNA | |
|---|
| Periodontal
bacteria (n) | Negative, n
(%) | Positive, n
(%) | P-value |
|---|
| P.
gingivalis | | | 0.79 |
|
Negative
(56) | 48 (85.7) | 8 (14.3) | |
|
Positive
(68) | 60 (88.2) | 8 (11.8) | |
| T.
forsythia | | | 0.56 |
|
Negative
(32) | 27 (84.4) | 5 (15.6) | |
|
Positive
(92) | 81 (88.0) | 11 (12.0) | |
| T.
denticola | | | 0.28 |
|
Negative
(73) | 66 (90.4) | 7 (9.6) | |
|
Positive
(51) | 42 (82.4) | 9 (17.6) | |
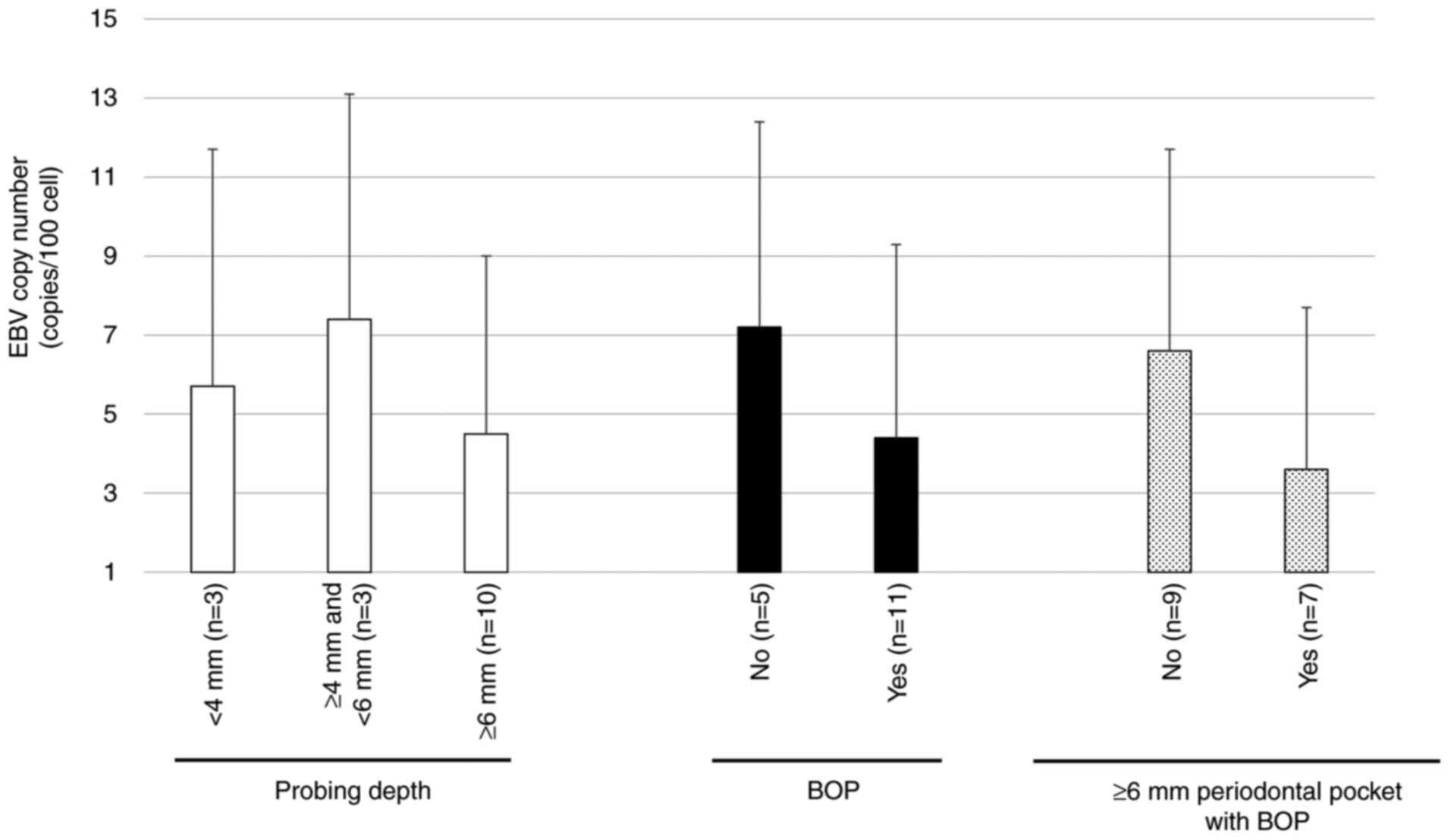
Association between EBV DNA copy
number and periodontal condition
To examine the relationship between virus DNA
amplification and periodontal tissue condition (i.e., periodontal
pocket depth or BOP), we investigated the EBV DNA copy number in 16
EBV positive cases. Associations between EBV DNA copy number/100
human cells and the periodontal condition of EBV positive cases are
presented in Fig. 2. There was no
significant association between EBV DNA copy number and periodontal
pocket depth or BOP. Additionally, there was no significant
increase in the EBV DNA copy number in people with ≥6 mm
periodontal pockets and BOP as compared to those without ≥6 mm
periodontal pockets and BOP.
Association between EBV DNA positivity
and periodontal health status in propensity score-matched
cases
Propensity score-matching was performed between
participants without ≥4 mm periodontal pockets and BOP
(participants with good periodontal health) and those with ≥4 mm
periodontal pockets, BOP or both (participants with poor
periodontal health) using propensity scores generated from 11
clinical factors (age, sex, remaining teeth, denture use, smoking,
hypertension, diabetes, hyperlipidemia, stroke, heart disease, and
bone and joint disease). A total of 70 propensity score-matched
cases (35 participants in matched pairs) were evaluated by
univariate analysis. We confirmed that none of the 11 clinical
variables were significantly associated with EBV DNA positivity
(Table IV). Next, we investigated
the association between EBV DNA positivity and periodontal health
status. Participants with poor periodontal health exhibited a
higher EBV DNA positivity rate (25.7%) than those with good
periodontal health (0.0%). There was a significant association
between EBV DNA positivity and periodontal health status (P=0.001)
(Table V).
 | Table IVCharacteristics of participants with
good periodontal health and those with poor periodontal health
comprising 70 propensity score matched participants. |
Table IV
Characteristics of participants with
good periodontal health and those with poor periodontal health
comprising 70 propensity score matched participants.
| Clinical factor
(n) | Participants with
good periodontal health (n=35) | Participants with
poor periodontal health (n=35) | P-value |
|---|
| Age,
yearsa | 68.7±10.3 | 68.4±9.4 | 0.77 |
| Sex, n (%) | | | >0.99 |
|
Male
(24) | 12 (50.0) | 12 (50.0) | |
|
Female
(46) | 23 (50.0) | 23 (50.0) | |
| Remaining
teetha | 22.5±7.3 | 22.2±7.0 | 0.31 |
| Denture user, n
(%) | | | >0.99 |
|
Non-user
(50) | 25 (50.0) | 25 (50.0) | |
|
User
(20) | 10 (50.0) | 10 (50.0) | |
| Smoking, n (%) | | | >0.99 |
|
No (67) | 33 (49.3) | 34 (50.7) | |
|
Yes (3) | 2 (66.7) | 1 (33.3) | |
| Hypertension, n
(%) | | | >0.99 |
|
No (57) | 28 (49.1) | 29 (50.9) | |
|
Yes
(13) | 7 (53.8) | 6 (46.2) | |
| Diabetes, n
(%) | | | >0.99 |
|
No (62) | 31 (50.0) | 31 (50.0) | |
|
Yes (8) | 4 (50.0) | 4 (50.0) | |
| Hyperlipidemia, n
(%) | | | >0.99 |
|
No (55) | 28 (50.9) | 27 (49.1) | |
|
Yes
(15) | 7 (46.7) | 8 (53.3) | |
| Stroke, n (%) | | | >0.99 |
|
No (66) | 33 (50.0) | 33 (50.0) | |
|
Yes (4) | 2 (50.0) | 2 (50.0) | |
| Heart disease, n
(%) | | | >0.99 |
|
No (68) | 34 (50.0) | 34 (50.0) | |
|
Yes (2) | 1 (50.0) | 1 (50.0) | |
| Bone and joint
disease, n (%) | | | >0.99 |
|
No (67) | 34 (50.7) | 33 (49.3) | |
|
Yes (3) | 1 (33.3) | 2 (66.7) | |
 | Table VAssociation between oral EBV DNA and
periodontal health status in 70 propensity score-matched cases. |
Table V
Association between oral EBV DNA and
periodontal health status in 70 propensity score-matched cases.
| | EBV DNA | |
|---|
| Periodontal health
status | Negative, n
(%) | Positive, n
(%) | P-value |
|---|
| Participants with
good periodontal health (n=35) | 35 (100.0) | 0 (0.0) | 0.001 |
| Participants with
poor periodontal health (n=35) | 26 (74.3) | 9 (25.7) | |
Discussion
Periodontitis is a chronic inflammatory disease, and
periodontitis-related bacteria play a vital role in the initiation
and progression of periodontitis. Herpes viruses as well as
periodontitis-related bacteria have been reported to be involved in
aggressive periodontal disease (22). The herpes virus may be involved in
the change of cell structure in periodontal tissues and has
cytopathic effects on inflammatory cells such as leukocytes,
lymphocytes and macrophages (22).
High levels of cytomegalovirus and P. gingivalis were
detected in school children with juvenile periodontitis in Jamaica
(23). HSV-1 and EBV were detected
more frequently in Brazilian people with chronic periodontitis or
aggressive periodontal disease than in people without periodontitis
(11). EBV DNA was more frequently
detected in individual periodontal pockets with ≥5 mm probing depth
than in healthy sites with ≤3 mm probing depth in patients with
periodontal disease in the US population (12). A meta-analysis based on case control
studies revealed that there was a significant relationship between
EBV or human cytomegalovirus infection and chronic periodontitis
(13,24). In this study, we found there was a
significant association between oral EBV and periodontal pocket
depth and BOP. BOP is considered to be a significant indicator of
periodontal tissue inflammation (25). Additionally, subjects with ≥6 mm
periodontal pockets with BOP exhibited a higher EBV DNA positivity
rate than those without ≥6 mm periodontal pockets with BOP.
Furthermore, a propensity score-matched analysis revealed that
people with poor periodontal health exhibited a significantly
higher EBV DNA positivity rate than those with good periodontal
health, suggesting that there was a close association between
periodontitis and EBV prevalence.
There was no significant association between EBV DNA
positivity and periodontal disease-related bacteria such as P.
gingivalis, T. forsythia and T. denticola. It
remains unclear whether periodontal disease-related bacteria are
associated with EBV prevalence. In contrast, human cytomegalovirus
promotes the virulence of periodontal disease-related bacteria such
as Actinobacillus actinomycetemcomitans (26). EBV prevalence may be associated with
enhanced pathogenicity of periodontal disease-related bacteria.
Additionally, there was no significant relationship between EBV DNA
copy number and periodontal condition. In contrast, the EBV DNA
copy number was significantly higher in subjects with periodontitis
than in those with gingivitis and normal subjects, indicating that
EBV gene amplification may be related to periodontal inflammation
(27). Further study is required to
clarify the relationship between EBV DNA amplification and the
severity of periodontal inflammation.
The results of this study suggest that local
periodontal inflammation and an inhibited local immune system may
provide the opportunity for EBV to infect epithelial cells in the
oral cavity (i.e., recent EBV infection). In addition, persistent
EBV-infected blood cells may have been reactivated in accordance
with periodontal inflammation (i.e., reactivation of EBV-infected
lymphocytes). However, it remains unknown which of these factors is
a major cause of the prevalence of oral EBV in this study.
As for the association between EBV DNA and clinical
factors, there was no significant difference between EBV DNA and
sex, age, remaining teeth, denture use, or medical history.
Diabetic patients were more susceptible to oral EBV than
non-diabetic patients (15). Thus,
diabetes is thought to be an important risk factor in the
prevalence of oral EBV DNA. However, no significant relationship
was found between oral EBV prevalence and diabetes in this study.
Good diabetes control in diabetic patients may have contributed to
their lower oral EBV positivity rate.
Research has shown that current smoking increases
the oral EBV load in people in China (14). This result indicates that smoking
may enhance EBV activity and induce persistent EBV infection.
Smoking is thought to be a significant risk factor in the
prevalence of oral EBV DNA due to the smoking-inhibited immune
response. In this study, no association was found between smoking
and EBV positivity, possibly because the number of smokers in this
study was small. Further study will be required to clarify the
association between smoking and EBV by including many more smokers
in the study cohort. Acharya et al reported that there was a
significant relationship between betel nut chewing and oral EBV
prevalence (28). It is
hypothesized that betel nut chewing may have an impact on EBV
infection in the oral cavity. Betel nut chewing may inhibit immune
responses due to the action of alkaloids such as arecoline
(29). Betel nut chewing also
results in constant injury to the oral mucosa, providing an
opportunity for EBV to infect oral epithelial cells. Smoking and
betel nut chewing may contribute to persistent EBV infection and
reactivation of EBV in the oral cavity.
Latent EBV genomes express EBV-encoded nuclear
antigens 1, 2, 3A, 3B, and 3C, latent membrane proteins and small
noncoding RNAs (30). These
proteins are importantly related to B-cell immortalization and
development of B-cell lymphoma (30). BamHI A rightward transcripts (BARTs)
and EBV-encoded microRNAs derived from the BARTs are involved in
malignant transformation of epithelial cells (31). These results indicate that EBV
infection is implicated in the development of epithelial malignant
tumors. EBV has been detected not only in normal oral epithelium
but also in oral lichen planus and oral squamous cell carcinoma
(28,32,33),
supporting the idea that EBV plays a vital role in oral cavity
cancer development. Furthermore, persistent EBV infection due to
smoking may increase the risk of EBV-related epithelial malignant
tumors. However, it remains unknown how EBV induces the development
of cancer in the oral cavity. The oral cavity mainly acts as a
reservoir to provide EBV for other anatomical sites such as the
stomach and nasopharynx. Accordingly, it is essential to prevent
primary oral EBV infection to prevent secondary EBV infections in
the nasopharynx and gastrointestinal tract.
In this study, the plaque control record scores were
higher in EBV-positive individuals than in EBV-negative
individuals, but there was no significant association between
dental plaque accumulation and the EBV positivity rate. EBV was
detected in the subgingival dental plaque in deep periodontal
pockets (12). Dental plaque
accumulation may be related to EBV prevalence in the oral cavity.
Thus, regular oral health care (i.e., subgingival plaque removal
using a toothbrush and subgingival scaling) is necessary not only
to prevent periodontal disease, but also to prevent EBV infection
in periodontal tissue.
In conclusion, oral EBV infection is thought to be
associated with periodontitis in middle-aged and older Japanese
people. However, it remains unknown whether EBV can localize in
periodontal tissues. The association between oral EBV and the
severity of periodontitis (i.e., the degree of alveolar bone loss)
was not elucidated in this study. Accordingly, further study is
required to uncover the presence of EBV in inflammatory periodontal
tissues and to determine the relationship between EBV and the
severity of periodontitis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was financially supported by
university grants from Hiroshima University.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CYS performed experiments and analyzed and
interpreted the data. HS designed the study, performed the
experiments, analyzed and interpreted the data, and wrote the
manuscript. HM performed the experiments and analyzed the data. KO
and TT discussed and analyzed the data and aided in writing the
paper. MS designed the current study and discussed data analysis.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethical Committee of Hiroshima University
approved the present study (approval no. E-1115). All participants
provided their written informed consent prior to participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Baer R, Bankier AT, Biggin MD, Deininger
PL, Farrell PJ, Gibson TJ, Hatfull G, Hudson GS, Satchwell SC,
Séguin C, et al: DNA sequence and expression of the B95-8
Epstein-Barr virus genome. Nature. 310:207–211. 1984.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Houldcroft CJ and Kellam P: Host genetics
of Epstein-Barr virus infection, latency and disease. Rev Med
Virol. 25:71–84. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Cohen JI: Epstein-Barr virus infection. N
Engl J Med. 343:481–492. 2000.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Epstein MA: Reflections on Epstein-Barr
virus: Some recently resolved old uncertainties. J Infect.
43:111–115. 2001.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hjalgrim H, Askling J, Rostgaard K,
Hamilton-Dutoit S, Frisch M, Zhang JS, Madsen M, Rosdahl N,
Konradsen HB, Storm HH and Melbye M: Characteristics of Hodgkin's
lymphoma after infectious mononucleosis. N Engl J Med.
349:1324–1332. 2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cho WC: Nasopharyngeal carcinoma:
Molecular biomarker discovery and progress. Mol Cancer.
6(1)2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shibata D, Tokunaga M, Uemura Y, Sato E,
Tanaka S and Weiss LM: Association of Epstein-Barr virus with
undifferentiated gastric carcinomas with intense lymphoid
infiltration. Lymphoepithelioma-like carcinoma. Am J Pathol.
139:469–474. 1991.PubMed/NCBI
|
|
8
|
Jiang R, Scott RS and Hutt-Fletcher LM:
Epstein-Barr virus shed in saliva is high in B-cell-tropic
glycoprotein gp42. J Virol. 80:7281–7283. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hadinoto V, Shapiro M, Sun CC and
Thorley-Lawson DA: The dynamics of EBV shedding implicate a central
role for epithelial cells in amplifying viral output. PLoS Pathog.
5(e1000496)2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kamma JJ, Contreras A and Slots J: Herpes
viruses and periodontopathic bacteria in early-onset periodontitis.
J Clin Periodontol. 28:879–885. 2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Imbronito AV, Okuda OS, Maria de Freitas
N, Moreira Lotufo RF and Nunes FD: Detection of herpesviruses and
periodontal pathogens in subgingival plaque of patients with
chronic periodontitis, generalized aggressive periodontitis, or
gingivitis. J Periodontol. 79:2313–2321. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dawson DR, Wang C, Danaher RJ, Lin Y,
Kryscio RJ, Jacob RJ and Miller CS: Real-time polymerase chain
reaction to determine the prevalence and copy number of
Epstein-Barr virus and cytomegalovirus DNA in subgingival plaque at
individual healthy and periodontal disease sites. J Periodontol.
80:1133–1140. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhu C, Li F, Wong MC, Feng XP, Lu HX and
Xu W: Association between Herpesviruses and chronic periodontitis:
A meta-analysis based on case-control studies. PLoS One.
10(e0144319)2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
He YQ, Liao XY, Xue WQ, Xu YF, Xu FH, Li
FF, Li XZ, Zhang JB, Wang TM, Wang F, et al: Association between
environmental factors and oral Epstein-Barr virus DNA loads: A
multicenter cross-sectional study in China. J Infect Dis.
219:400–409. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dworzański J, Drop B, Kliszczewska E,
Strycharz-Dudziak M and Polz-Dacewicz M: Prevalence of Epstein-Barr
virus, human papillomavirus, cytomegalovirus and herpes simplex
virus type 1 in patients with diabetes mellitus type 2 in
south-eastern Poland. PLoS One. 14(e0222607)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hamada R, Suehiro J, Nakano M, Kikutani T
and Konishi K: Development of rapid oral bacteria detection
apparatus based on dielectrophoretic impedance measurement method.
IET Nanobiotechnol. 5:25–31. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
O'Leary TJ, Drake RB and Naylor JE: The
plaque control record. J Periodontol. 43(38)1972.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Shigeishi H, Sugiyama M, Ohta K, Rahman MZ
and Takechi M: Higher prevalence and gene amplification of HPV16 in
oropharynx as compared to oral cavity. J Appl Oral Sci. 24:397–403.
2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kato A, Imai K, Sato H and Ogata Y:
Prevalence of Epstein-Barr virus DNA and Porphyromonas
gingivalis in Japanese peri-implantitis patients. BMC Oral
Health. 17(148)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Su CY, Shigeishi H, Nishimura R, Ohta K
and Sugiyama M: Detection of oral bacteria on the tongue dorsum
using PCR amplification of 16S ribosomal RNA and its association
with systemic disease in middle-aged and elderly patients. Biomed
Rep. 10:70–76. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Socransky SS, Haffajee AD, Cugini MA,
Smith C and Kent RL Jr: Microbial complexes in subgingival plaque.
J Clin Periodontol. 25:134–144. 1998.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Slots J and Contreras A: Herpesviruses: A
unifying causative factor in periodontitis? Oral Microbiol Immunol.
15:277–280. 2000.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Michalowicz BS, Ronderos M, Camara-Silva
R, Contreras A and Slots J: Human herpesviruses and
Porphyromonas gingivalis are associated with juvenile
periodontitis. J Periodontol. 71:981–988. 2000.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gao Z, Lv J and Wang M: Epstein-Barr virus
is associated with periodontal diseases: A meta-analysis based on
21 case-control studies. Medicine (Baltimore).
96(e5980)2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
de Souza PH, de Toledo BE, Rapp GE, Zuza
EP, Neto CB and Mendes AJ: Reliability of bleeding and non-bleeding
on probing to gingival histological features. J Int Acad
Periodontol. 5:71–76. 2003.PubMed/NCBI
|
|
26
|
Teughels W, Sliepen I, Quirynen M, Haake
SK, Van Eldere J, Fives-Taylor P and Van Ranst M: Human
cytomegalovirus enhances A. actinomycetemcomitans adherence to
cells. J Dent Res. 86:175–180. 2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Srivastava AK, Shukla S, Srivastava P,
Dhole TN, Nayak MT, Nayak A and Mathur A: Real time detection and
quantification of Epstein Barr virus in different grades of oral
gingivitis and periodontitis patients. J Exp Ther Oncol. 13:9–14.
2019.PubMed/NCBI
|
|
28
|
Acharya S, Ekalaksananan T, Vatanasapt P,
Loyha K, Phusingha P, Promthet S, Kongyingyoes B and Pientong C:
Association of Epstein-Barr virus infection with oral squamous cell
carcinoma in a case-control study. J Oral Pathol Med. 44:252–257.
2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Singh PN, Natto Z, Yel D, Job J and
Knutsen S: Betel quid use in relation to infectious disease
outcomes in Cambodia. Int J Infect Dis. 16:e262–e267.
2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Saha A and Robertson ES: Epstein-Barr
virus-associated B-cell lymphomas: Pathogenesis and clinical
outcomes. Clin Cancer Res. 7:3056–3063. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tsao SW, Tsang CM, To KF and Lo KW: The
role of Epstein-Barr virus in epithelial malignancies. J Pathol.
235:323–333. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mao EJ and Smith CJ: Detection of
Epstein-Barr virus (EBV) DNA by the polymerase chain reaction (PCR)
in oral smears from healthy individuals and patients with squamous
cell carcinoma. J Oral Pathol Med. 22:12–17. 1993.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sand LP, Jalouli J, Larsson PA and Hirsch
JM: Prevalence of Epstein-Barr virus in oral squamous cell
carcinoma, oral lichen planus, and normal oral mucosa. Oral Surg
Oral Med Oral Pathol Oral Radiol Endod. 93:586–592. 2002.PubMed/NCBI View Article : Google Scholar
|