Introduction
Cervical carcinoma (CaCx) is one of the most common
cancers and one of the leading causes of cancer death among
females, worldwide (1). Despite the
recent advances in the understanding and treatment of this
distressing disease, the prognosis of patients with CaCx remains
unsatisfactory due to the advanced stage of the disease at
diagnosis (2). Thus, there is an
urgent need for the identification of biological factors in the
tumorigenesis and development of CaCx in order to improve prognosis
of advanced CaCx.
The Ca2+-activated Cl- channel
A2 (CLCA2) is a member of the Ca2+-activated
Cl- channel (CACC) family, characterized by a single
COOH-terminal transmembrane segment followed by a 16 amino acid
cytoplasmic tail, a structure incompatible with function as a
channel (3). CLCA2 serves vital
roles in a variety of biological processes, including cell
proliferation, DNA damage and other stressors (4). In human stratified epithelium, the
expression of CLCA2 has been reported to be strongly associated
with basal cell adhesion molecules, suggesting its role in basal
cell detachment (5). Several
studies have focused on the function of CLCA2 in tumorigenesis and
tumor development. For example, as a p53-inducible growth
inhibitor, CLCA2 induced the expression of p53, cyclin-dependent
kinase inhibitors p21 and p27, and cell cycle arrest in breast
cancer cells (4). In addition,
CLCA2 interacts with the cell junctional protein eva-1 homolog A to
mediate homophilic cell-cell adhesion and epithelial-to-mesenchymal
transition in breast cancer cells (6). Additionally, an in vivo study
reported that CLCA2 is an ultraviolet (UV) B target gene that may
serve a role in epidermal differentiation and UV-dependent skin
malignancies (7). However, the role
of CLCA2 in CaCx has not been well elucidated. The present study
focused on the expression of CLCA2 in CaCx and the role of CLCA2 in
the prognosis of patients with CaCx.
Materials and methods
Patients and tissue specimens
The samples were collected from 144 cervical cancer
patients (mean age, 43.7 years; age range, 25-68 years) who
underwent radical hysterectomy from January 2007 to December 2013
at Guangzhou Women and Children's Medical Center (Guangdong,
China). The tumor stage of patients was classified according to the
2018 International Federation of Gynecology and Obstetrics staging
system (8). The overall survival
(OS) and recurrence-free survival (RFS) was calculated as the time
from radical hysterectomy to the date of death and recurrence,
respectively. The resected specimens were promptly laid out and
fixed in 10% phosphate-buffered formalin at room temperature, and
then processed for pathological assessment. Additionally, eight
pairs of snap-frozen cervical cancer and paired adjacent
non-cancerous tissues (>5 cm from the tumor) were collected for
reverse transcription-quantitative (RT-q)PCR in 2018 at Guangzhou
Women and Children's Medical Center. The tissues were carefully
excised, frozen at -20˚C and evaluated to exclude dysplasia and the
presence of inflammatory cells.
This study was approved by the Research Ethics
Committee of Guangzhou Women and Children's Medical Center
(approval no. GWCMC201224) and written informed consent was
obtained from each patient.
Cell Lines
Cervical cancer cell lines, SiHa, C33A, HeLa, CaSki,
and ME-180 were cultured in DMEM medium (Invitrogen; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.). Primary normal cervical epithelial
cells (NCEC) were obtained from the patient's fresh tissues and
cultured in keratinocyte serum-free medium (Invitrogen; Thermo
Fisher Scientific, Inc.) (9).
Small interfering RNA (siRNA)
transfection
CLCA2 knockdown was performed by transfecting the
synthetic siRNA duplexes in SiHa and CaSki cells (3x105
cells/well) for 24-96 h. The CLCA2 siRNA sequences were designed
and synthesized by RiboBio Co., Ltd. The sequences of the sense
strand of siRNA duplexes were as follows: SiControl,
5'-CATTAATGTCGGACAAC-3'; CLCA2-Ri1, 5'-CGAAGTTCTGTTACCCATA-3';
CLCA2-Ri2, 5'-GGTCGTTGTATAAGTCGAA-3'. Cells were transfected with
siRNA using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
Briefly, a total of 5 µl Lipofectamine® 2000 and 20 µM
siRNA were added into 120 µl 1X RiboFECT™ CP Buffer (Guangzhou
RiboBio Co., Ltd.), and the mixture incubated for 5 min at 37˚C.
Subsequently, 12 µl RiboFECT™ CP Reagent (Guangzhou RiboBio Co.,
Ltd.) was added into the mixture and incubated for 15 min at 37˚C.
The riboFECT™ CP mixture was diluted with 1,863 µl RPMI-1640
(Gibco; Thermo Fisher Scientific, Inc.) to a total volume of 2 ml.
The cells were cultured for another 48 h, at which point the
subsequent experiments were performed. The non-specific siRNA
vector was used as a control.
RT-qPCR
Total RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The resulting
RNA was subsequently reversed transcribed into cDNA using
PrimeScript™ RT Master Mix (Takara Biotechnology Co.,
Ltd.). qPCR was performed using the SYBR-Green mix kit (Bio-Rad
Laboratories, Inc.). The thermocycling conditions of PCR
amplification consisted of an initial denaturation at 95˚C for 10
min, followed by 40 cycles of denaturation at 95˚C for 10 sec and
annealing at 60˚C for 30 sec. The primers used were as follows:
CLCA2 forward, 5'-ACAGGCTGGTGACAAAGTGGTC-3' and reverse,
5'-ACTGGCAATGCCCACGAAGGTA-3'; β-actin forward,
5'-TCATGAAGTGTGACGTTGACATCCGT-3' and reverse
5'-CCTAGAAGCATTTGCGGTGCACGATG-3'. The 2-ΔΔCq method was
used to calculate relative expression of CLCA2 mRNA (10).
Immunohistochemistry (IHC)
Tissues were fixed in 4% paraformaldehyde solution
at room temperature for 24 h, embedded in paraffin and sliced into
sections (4 µM thick). Sections were then dewaxed and rehydrated in
an ethanol series (100, 95, 75 and 50%, each for 5 min). Endogenous
peroxidase activity was blocked by immersing the sections in 3%
H2O2 in methanol for 10 min at room
temperature. The sections were blocked with 1% BSA for 15 min at
room temperature. and then incubated overnight at 4˚C with rabbit
anti-CLCA2 antibody (1:200; cat. no. HPA047192; Sigma-Aldrich;
Merck KGaA). The following day, the slides were incubated with
biotinylated anti-rabbit secondary antibody (1:1,000; cat. no.
66467-1-Ig; Proteintech Group, Inc.) for 30 min at room
temperature, followed by further incubation with
streptavidin-horseradish peroxidase (HRP) at 37˚C for 30 min.
Slides were observed under a light microscope (magnification,
x40).
The CLCA2 IHC results were assessed by two
pathologists using a blind protocol design. The analysis was
assessed according to both the intensity of staining and the
proportion of positively stained tumor cells. The area of staining
was stratified into five scoring groups: 0, not detected; 1,
<10% positive cells; 2, 11-50% positive cells; 3, 51-80%
positive cells; 4, >80% positive cells. Intensity of stained
cells was graded into four levels: 0, no staining; 1, weak
staining; 2, moderate staining; 3, strong staining. Using this
method, the expression of CLCA2 was evaluated by the staining index
(SI; multiplying values of the above two scores). The SI score of
≥6 was considered as high CLCA2 expression and SI <6 was
considered as low CLCA2 expression.
Western blotting
Western blotting was performed according to standard
methods as previously described (11). Cells and tissues were placed in RIPA
lysis buffer (Beyotime Institute of Biotechnology) at 4˚C for 1 h.
A Pierce BCA Protein assay kit (Thermo Fisher Scientific, Inc.) was
used to quantify protein expression. Protein samples (30 µg/lane)
were separated using 8-15% sodium dodecyl sulfate-polyacrylamide
gels and transferred to PVDF membranes. After the blots were
blocked in 5% milk for 1 h at room temperature, the membranes were
incubated overnight at 4˚C. With primary antibodies against CLCA2
(1:5,000, cat. no. HPA047192; Sigma-Aldrich; Merck KGaA), β-catenin
(1:4,000; cat. no. ab22656; Abcam). The membranes were then
incubated with HRP-conjugated anti-rabbit secondary antibody
(1:4,000; cat. no. ab6112; Abcam) for 2 h at room temperature. A
membrane-enhanced chemiluminescence reagent kit (Amersham; Cytiva)
was used to detect the immunoreactive bands. Nuclear extracts were
prepared using the Nuclear Extraction kit (Active Motif, Inc.),
according to the manufacturer's instructions. Anti-GAPDH (1:5,000;
cat. no. ab9485; Abcam) and anti-P84 (1:10,000; cat. no. ab131268;
Abcam) antibodies were used as loading controls.
MTT assay
Cell viability was measured using MTT assay. After
CLCA2 knockdown, growing cells (2x103/well) were seeded
in 96-well plates. At each time point (day 1, 2, 3, 4 and 5), cells
were stained with 100 µl sterile MTT dye (0.5 mg/ml, Sigma-Aldrich;
Merck KGaA), followed by removal of the culture medium and addition
of 150 µl of dimethyl sulfoxide to dissolve the purple formazan
(Sigma-Aldrich; Merck KGaA). Absorbance was determined at 570 nm
immediately. Experiments were repeated three times, and data were
represented as the mean ± SEM.
Cell migration and invasion assay
Cell migration and invasion abilities were analyzed
by Transwell chamber assays. A total of 200 µl cell medium
(1x105 cells/100 µl in RPMI 1640 medium) were added to
the upper chamber of Transwell equipment, and medium containing 10%
FBS (HyClone; GE Healthcare Life Sciences) was added to the lower
chamber. For invasion assays, Matrigel-coated Transwell cell
culture chambers (1:5; BD Biosciences) were introduced. After 24 h,
SiHa and CaSki cells on the upper side of the filter were removed
with a cotton swab. Migrated and invaded cells on the lower
membrane surface were fixed in 4% paraformaldehyde for 30 min at
room temperature, stained with 0.1% crystal violet for 15 min at
25˚C and visualized using a light microscope (Olympus Corp.;
magnification, x20).
Gene set enrichment analysis
(GSEA)
GSEA was performed using the GSEA platform (version
2.0.9; http://www.broadinstitute.org/gsea/) to analyze the
correlation between CLCA2 expression and Wnt pathway.
Statistical analysis
All statistical analyses were performed with SPSS
18.0 software (SPSS, Inc.). Comparison of CLCA2 expression between
different groups was assessed with paired Student's t-tests or
one-way analysis of variance (ANOVA) followed by a
Student-Newman-Keuls post-hoc test. Continuous variables were
expressed as mean ± SD. A standard χ2 test was performed
to assess the association between CLCA2 expression and the
clinicopathological characteristics. OS and RFS curves were
obtained by the Kaplan-Meier method and were compared with the
log-rank test. Multivariate analysis was performed using the Cox
regression model. P<0.05 was considered to indicate a
statistically significant difference.
Results
CLCA2 is downregulated in cervical
cancer
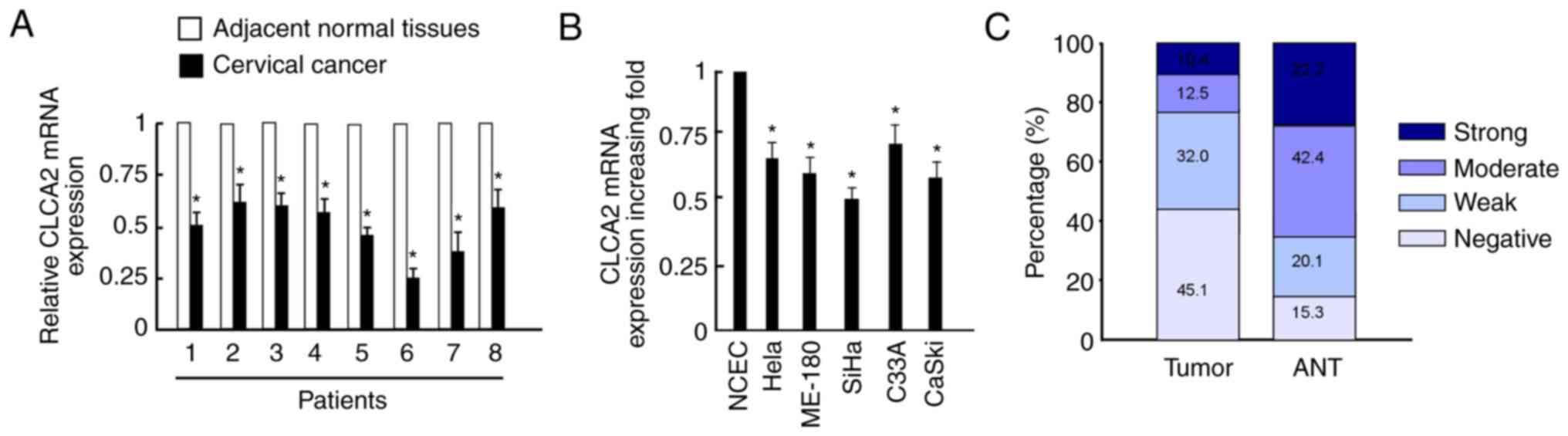
RT-qPCR revealed that CLCA2 mRNA expression levels
were significantly downregulated in cervical cancer tissues
compared with those in adjacent normal tissues (Fig. 1A). Additionally, CLCA2 mRNA
expression levels were significantly decreased in cervical cancer
cell lines compared with those in NCEC (Fig. 1B). CaSki and SiHa cells were
selected for further analysis as their expression levels were
decreased more obviously in cervical cancer lines compared with
NCEC. Additionally, CLCA2 staining intensity was scored as strong
or moderate intensity in 22.9% of tumor tissues, whereas 64.6% of
corresponding adjacent normal cervical tissues were scored as
strong or moderate intensity (Fig.
1C).
Correlation of CLCA2 expression and
clinicopathological features
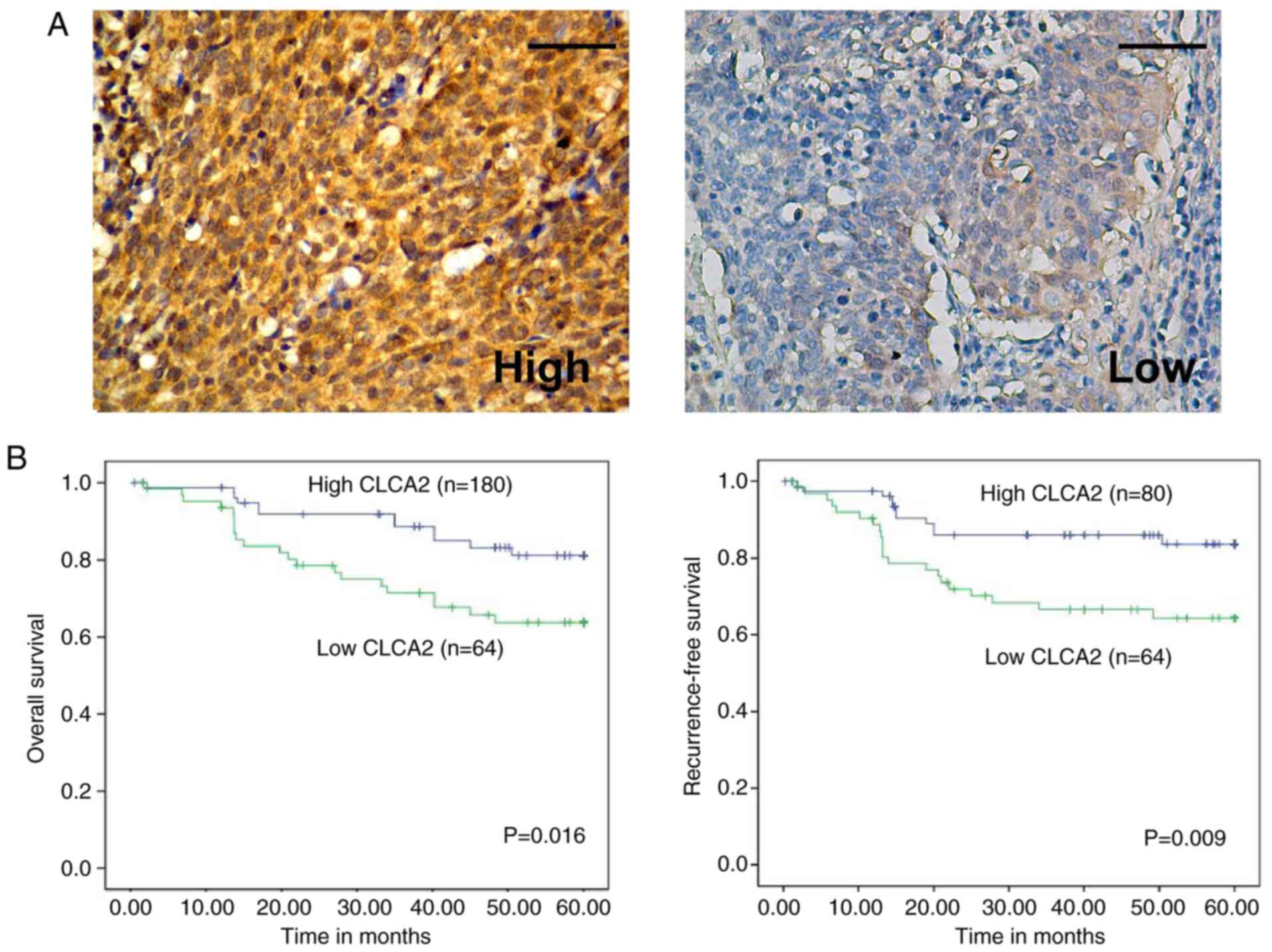
The expression of CLCA2 in 144 cervical cancer
specimens was examined by IHC. In Table
I, patient age was divided at 45 years on the basis of several
previous studies (12). The
representative immunostaining of high and low CLCA2 expression in
cervical cancer tissues was demonstrated in Fig. 2A. Statistical analysis further
revealed a positive correlation between CLCA2 expression and tumor
size (P=0.009), tumor stage (P=0.028) and HPV status (P=0.041;
Table I). In addition, no
significant differences were observed between high and low
expression levels of CLCA2 in terms of patient's age, tumor
differentiation, histological type or lymph node involvement
(Table I).
 | Table IThe association between clinical
parameters with Ca2+-activated Cl- channel
A2. |
Table I
The association between clinical
parameters with Ca2+-activated Cl- channel
A2.
| | CLCA2 | |
|---|
| Features | Total | High | Low | P-value |
|---|
| Age, years | | | | |
|
≤45 y | 90 | 46 | 44 | 0.166 |
|
>45
y | 54 | 34 | 20 | |
| Tumor stage | | | | |
|
IB | 95 | 59 | 36 | 0.028a |
|
IIA | 49 | 21 | 28 | |
| Tumor size, cm | | | | |
|
≤4 | 87 | 56 | 31 | 0.009a |
|
>4 | 57 | 24 | 33 | |
| Differentiation | | | | |
|
1/2 | 108 | 43 | 65 | 0.174 |
|
3 | 36 | 19 | 17 | |
| Histological
type | | | | |
|
Squamous
cell cancer | 89 | 49 | 40 | 0.878 |
|
Adenocarcinoma | 55 | 31 | 24 | |
| Human papillomavirus
16/18 | | | | |
|
+ | 115 | 59 | 56 | 0.041a |
|
- | 29 | 21 | 8 | |
| LN metastasis | | | | |
|
No | 110 | 64 | 46 | 0.254 |
|
Yes | 34 | 16 | 18 | |
| Total | 144 | 80 | 64 | |
CLCA2 expression predicts favorable
prognosis in cervical cancer
Statistical analysis also revealed a correlation
between CLCA2 expression and patients' clinical prognosis.
Kaplan-Meier analysis indicated that higher expression of CLCA2 was
correlated with more favorable OS (P=0.016) and RFS (P=0.009;
Fig. 2B). Multivariate Cox
regression analysis revealed that CLCA2 expression was an
independent predictor for OS (P=0.017) and RFS (P=0.025) in
patients with CaCx (Table II).
 | Table IIMultivariate Cox proportional hazards
regression models for estimating overall survival and
recurrence-free survival. |
Table II
Multivariate Cox proportional hazards
regression models for estimating overall survival and
recurrence-free survival.
| | Overall
survival | Recurrence-free
survival |
|---|
| Prognostic
variables | Hazard ratio (95%
CI) | P-values | Hazard ratio (95%
CI) | P-values |
|---|
| Age, years | | | | |
|
≤45 vs.
>45 y | - | | - | |
| Tumor stage | | | | |
|
IB vs.
IIA | 2.769
(0.401-7.158) | 0.022a | - | |
| Tumor size, cm | | | | |
|
≤4 vs.
>4 | 2.293
(0.097-8.340) | 0.034a | - | |
| Histological
type | | | | |
|
SCC vs.
AC | - | | - | |
| LN metastasis | | | | |
|
+ vs. - | - | | 33.278
(1.071-10.432) | 0.026a |
| HPV 16/18 | | | | |
|
+ vs. - | 2.178
(0.242-5.219) | 0.015a | - | |
| CLCA2
expression | | | | |
|
Low vs.
high | 2.838
(0.651-6.519) | 0.017a | 3.265
(1.282-7.984) | 0.025a |
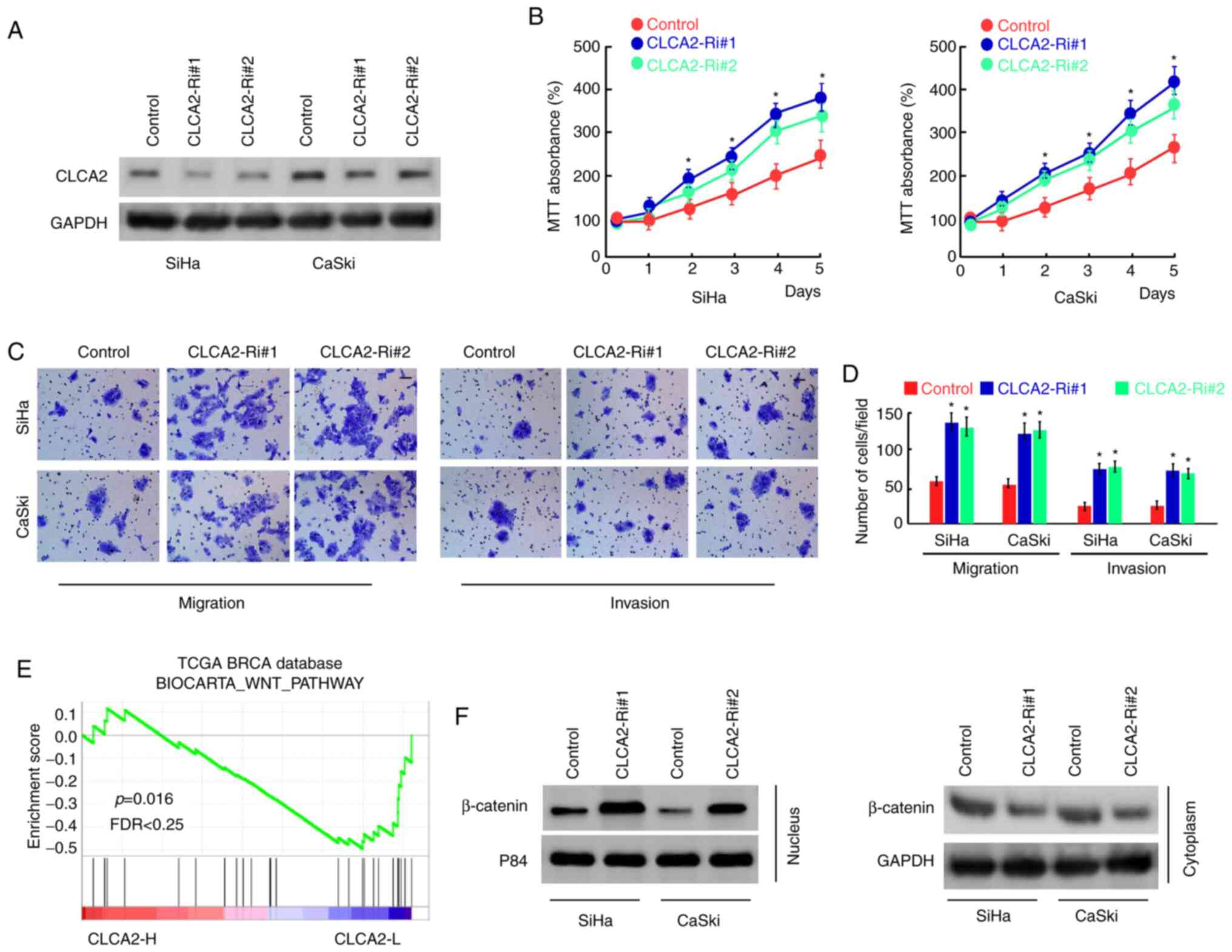
CLCA2 inhibition promotes CaCx cell
proliferation, migration, and invasion
To further examine the role of CLCA2 in CaCx cells,
CLCA2 expression was inhibited using siRNAs in SiHa and CaSki cells
(Fig. 3A). The results of MTT
assays demonstrated that the silencing of CLCA2 expression
significantly increased the proliferation of SiHa and CaSki cells
compared with that of the control group (Fig. 3B). In addition, Transwell assays
demonstrated that knockdown of CLCA2 effectively promoted CaCx cell
migration and invasion (Fig. 3C and
D). These results indicated that
CLCA2 inhibits the proliferation, migration and invasion of CaCx
cells.
CLCA2 suppresses Wnt/β-catenin
signaling in cervical cancer cells
In order to further explore the mechanism of CLCA2
regulating cell functions, the publicly available gene expression
array data for CaCx using GSEA was analyzed. The results
demonstrated that CLCA2 expression was negatively correlated with
the activation of the Wnt/β-catenin pathway (Fig. 3E). In addition, the effect of CLCA2
on the subcellular localization of β-catenin was assessed. The
results demonstrated that knockdown of CLCA2 increased the
translocation of β-catenin into the nucleus (Fig. 3F), indicating that CLCA2 suppressed
the activation of Wnt/β-catenin signaling in cervical cancer
cells.
Discussion
This study demonstrated that CLCA2 functions as a
tumor suppressor to inhibit CaCx progression for the very
first-time, to the best of our knowledge. This study also
demonstrated that the expression of CLCA2 is negatively correlated
with tumor stage, tumor size, HPV status and the prognosis of
patients. In the in vitro assays, decreased expression of
CLCA2 promoted CaCx cell proliferation, migration and invasion.
These findings suggested that CLCA2 may function as a
tumor-suppressive protein in CaCx and may be regarded as a
potential prognostic marker of CaCx.
The CLCA proteins, originally named Cl-
channels, have various members expressed throughout epithelia,
endothelia, and other cell types, and mediate a constitutively
expressed endogenous Cl- current (13). The CLCAs are transmembrane proteins
and distinguished by the juxtaposition of metalloprotease and VWA
domains and the capacity to self-cleave (14). Overexpression of CLCA proteins
produce a novel Cl- current that can be activated by
Ca2+ and blocked by typical Cl- channel
inhibitors, which results in their designation as Cl-
channels (15). As a type I
integral transmembrane protein, CLCA2 is induced by p53 in response
to cell detachment, DNA damage and other stressors (4). CLCA2 was originally identified as a
tumor suppressor in breast cancer, and its ectopic expression
retarded tumor formation (16). A
subsequent study revealed that CLCA2 was downregulated in cervical
and ovarian cancer cells, suggesting its potential
tumor-suppressing role in these malignancies (17). The results of the present study
demonstrated that CLCA2 expression was decreased in CaCx compared
with that in adjacent normal cervix tissues. This study also
demonstrated significant association between CLCA2 and tumor size,
tumor stage and HPV status, indicating the specific involvement of
CLCA2 in CaCx development.
CLCA2 has been reported to negatively regulate
cancer cell migration and invasion (18), however, the underlying mechanism has
not been defined yet. Qiang et al (19) demonstrated that CLCA2 inhibits
growth and metastasis of nasopharyngeal carcinoma cells via
inhibition of focal adhesion kinase (FAK)/extracellular
signal-regulated kinases signaling. In corroboration with their
study, Sasaki et al (18)
revealed that CLCA2 function as a target of P53 to inhibit cancer
cell migration and invasion through suppression of the FAK
signaling pathway. The results of the present study demonstrated
that attenuated expression of CLCA2 increased β-catenin nuclear
translocation in CaCx cells, suggesting that the tumor-suppressing
role of CLCA2 in CaCx is related to the inactivation of
Wnt/β-catenin signaling. To the best of our knowledge, this study
is the first study to suggest that CLCA2 may contribute to the
development of CaCx. As CLCA2 is a CACC regulator, the underlying
mechanisms of CLCA2 mediated cancer cell migration and invasion
need further investigation.
The clinical significance of CLCA2 has been shown in
previous studies. Jiang et al (20) investigated the expression of acute
myeloid leukemia 1 protein (AML1) fusion genes and demonstrated
that AML1-CLCA2 can be potential molecular markers for diagnostics
and prognostic evaluation of acute myeloid leukemia. Man et
al (21) suggested that the
combination of the four markers cytokine 7, CLCA2, hyaluronan
mediated motility receptor or telomerase reverse transcriptase has
great value in lung adenocarcinoma prognosis evaluation. However,
the role of CLCA2 in predicting the survival of cancer patients has
never been reported. This study demonstrated that patients with
lower levels of CLCA2 had a significantly poorer OS and RFS
compared with patients with higher levels of CLCA2, suggesting that
CLCA2 may be used as a prognostic marker for predicting clinical
outcome of patients with CaCx.
In conclusion, this is the first study that explored
the role of CLCA2 in CaCx and demonstrated that CLCA2 may act as a
tumor suppressor in CaCx, to the best of our knowledge.
Downregulation of CLCA2 in CaCx, which is strongly correlated with
tumor progression and clinical prognosis, merits further
development as a prognostic marker.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Innovation Fund
of Guangzhou Women and Children's Medical Center (grant no.
201513521).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
PZ participated in data collection and drafted the
manuscript. YLin performed the statistical analysis. YLiu
participated in the design of the study. All authors analyzed and
interpreted the patient data. PZ and YLiu confirm the authenticity
of all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Research Ethics
Committee of Guangzhou Women and Children's Medical Center
(approval no. GWCMC201224). Written informed consent was obtained
from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Peiretti M, Zapardiel I, Zanagnolo V,
Landoni F, Morrow CP and Maggioni A: Management of recurrent
cervical cancer: A review of the literature. Surg Oncol.
21:e59–e66. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cerrotta A, Gardan G, Cavina R,
Raspagliesi F, Stefanon B, Garassino I, Musumeci R, Tana S and De
Palo G: Concurrent radiotherapy and weekly paclitaxel for locally
advanced or recurrent squamous cell carcinoma of the uterine
cervix. A pilot study with intensification of dose. Eur J Gynaecol
Oncol. 23:115–119. 2002.PubMed/NCBI
|
|
3
|
Elble RC, Walia V, Cheng HC, Connon CJ,
Mundhenk L, Gruber AD and Pauli BU: The putative chloride channel
hCLCA2 has a single C-terminal transmembrane segment. J Biol Chem.
281:29448–29454. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Walia V, Ding M, Kumar S, Nie D, Premkumar
LS and Elble RC: hCLCA2 is a p53-inducible inhibitor of breast
cancer cell proliferation. Cancer Res. 69:6624–6632.
2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Connon CJ, Kawasaki S, Yamasaki K,
Quantock AJ and Kinoshita S: The quantification of hCLCA2 and
colocalisation with integrin beta4 in stratified human epithelia.
Acta Histochem. 106:421–425. 2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ramena G, Yin Y, Yu Y, Walia V and Elble
RC: CLCA2 interactor EVA1 is required for mammary epithelial cell
differentiation. PLoS One. 11(e0147489)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bart G, Hämäläinen L, Rauhala L, Salonen
P, Kokkonen M, Dunlop TW, Pehkonen P, Kumlin T, Tammi MI,
Pasonen-Seppänen S and Tammi RH: rClca2 is associated with
epidermal differentiation and is strongly downregulated by
ultraviolet radiation. Br J Dermatol. 171:376–387. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Grigsby PW, Massad LS, Mutch DG, Powell
MA, Thaker PH, McCourt C, Hagemann A, Fuh K, Kuroki L, Schwarz JK,
et al: FIGO 2018 staging criteria for cervical cancer: Impact on
stage migration and survival. Gynecol Oncol. 157:639–643.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang J, Ou J, Guo Y, Dai T, Li X, Liu J,
Xia M, Liu L and He M: TBLR1 is a novel prognostic marker and
promotes epithelial-mesenchymal transition in cervical cancer. Br J
Cancer. 111:112–124. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang B, Zhang L and Zhao L, Zhou R, Ding
Y, Li G and Zhao L: LASP2 suppresses colorectal cancer progression
through JNK/p38 MAPK pathway meditated epithelial-mesenchymal
transition. Cell Commun Signal. 15(21)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Park S, Kim J, Eom K, Oh S, Kim S, Kim G,
Ahn S, Park KH, Chung D and Lee H: microRNA-944 overexpression is a
biomarker for poor prognosis of advanced cervical cancer. BMC
Cancer. 19(419)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pauli BU, Abdel-Ghany M, Cheng HC, Gruber
AD, Archibald HA and Elble RC: Molecular characteristics and
functional diversity of CLCA family members. Clin Exp Pharmacol
Physiol. 27:901–905. 2000.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pawlowski K, Lepisto M, Meinander N,
Sivars U, Varga M and Wieslander E: Novel conserved hydrolase
domain in the CLCA family of alleged calcium-activated chloride
channels. Proteins. 63:424–439. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fuller CM, Ji HL, Tousson A, Elble RC,
Pauli BU and Benos DJ: Ca(2+)-activated Cl(-) channels: A newly
emerging anion transport family. Pflugers Archiv. 443 (Suppl
1):S107–S110. 2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li X, Cowell JK and Sossey-Alaoui K: CLCA2
tumour suppressor gene in 1p31 is epigenetically regulated in
breast cancer. Oncogene. 23:1474–1480. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhao G, Chen J, Deng Y, Gao F, Zhu J, Feng
Z, Lv X and Zhao Z: Identification of NDRG1-regulated genes
associated with invasive potential in cervical and ovarian cancer
cells. Biochem Biophys Res Commun. 408:154–159. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sasaki Y, Koyama R, Maruyama R, Hirano T,
Tamura M, Sugisaka J, Suzuki H, Idogawa M, Shinomura Y and Tokino
T: CLCA2, a target of the p53 family, negatively regulates cancer
cell migration and invasion. Cancer Biol Ther. 13:1512–1521.
2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Qiang YY, Li CZ, Sun R, Zheng LS, Peng LX,
Yang JP, Meng DF, Lang YH, Mei Y, Xie P, et al: Along with its
favorable prognostic role, CLCA2 inhibits growth and metastasis of
nasopharyngeal carcinoma cells via inhibition of FAK/ERK signaling.
J Exp Clin Cancer Res. 37(34)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jiang MM, Gao L, Jing Y, Ding Y, Xu YY,
Zhou MH, Ma C, Wang N, Wang W, Han XP, et al: Rapid detection of
AML1 associated fusion genes in patients with adult acute myeloid
leukemia and its clinical significance. Zhongguo Shi Yan Xue Ye Xue
Za Zhi. 21:821–829. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Man Y, Cao J, Jin S, Xu G, Pan B, Shang L,
Che D, Yu Q and Yu Y: Newly identified biomarkers for detecting
circulating tumor cells in lung adenocarcinoma. Tohoku J Exp Med.
234:29–40. 2014.PubMed/NCBI View Article : Google Scholar
|