Introduction
Alzheimer's disease (AD) is an age-associated
neurodegenerative disease, where the main symptoms include
progressive decline in cognitive function, memory, spatial
discrimination and language ability, potentially accompanied by
other neurological symptoms (1).
Although AD has been characterized by its pathological features,
including extracellular amyloid β (Aβ) deposition, intracellular
neurofibrillary tangles (NFTs) and neuronal loss (2), there may be other influencing factors
involved in the progression of AD. In AD brains, it has been
reported that lipopolysaccharide (LPS) levels and E. coli
K99 were increased and co-localized with Aβ1-40/42 in
amyloid plaques and around vessels (3). A clinical study also reported that LPS
levels in the brains of patients with AD were two to three times
higher compared with those found in healthy elderly individuals
(4). Therefore, LPS may be involved
in neuronal damage in the progression of AD.
LPS is a toxic component of cell walls of
Gram-negative bacteria, which are widely present in the
gastrointestinal tracts of humans and animals. Increasing evidence
shows that LPS plays important roles in the pathogeneses of
neurodegenerative disorders, such as AD (5). As a pathogen-associated molecular
patterns, LPS can be recognized by the toll-like receptor 4
(TLR4)/MD2 complex on the surface of cells. Microglial TLR4 is a
key regulator of inflammation that may play an essential role in
the complex pathophysiological process of AD (6). LPS-induced hyperactive TLR4 has been
seen to contribute to microglial over-activation, followed by
increased neuronal apoptosis in the cortex of APP/PS1 mice
(6). Additionally, You et al
(7) reported that LPS-induced
hepcidin expression in astrocytes induced the apoptosis of neurons
through iron accumulation in rats. However, it is still not
completely understood whether LPS exposure induces neuronal damage
directly. A recent study showed that LPS could induce inflammatory
injury in a neuronal cell line, HT22, by downregulating
microRNA-132(8). Thus, it is
speculated that LPS exposure may induce neuronal damage directly,
but the precise mechanisms have not been fully elucidated.
Oxidative stress and neuroinflammation have been
reported to play an important role in the progression of AD.
Excessive generation of reactive oxygen species (ROS) induces a
disturbance in the oxidant/anti-oxidant balance and results in
oxidative stress damage in cells (9). ROS-induced oxidative stress has been
considered to be the main cause of various neurological diseases,
such as AD and Parkinson's disease (PD) (10). Nicotinamide adenine dinucleotide
phosphate oxidase (NADPH oxidase, NOX) is a major contributor to
ROS generation in cells (11). NOX2
is extensively expressed in brain, especially in neurons, and is
closely associated with the pathogeneses of several
neurodegenerative diseases, such as AD and PD (12,13).
Inflammasomes are macromolecular complexes that play an important
role in regulating inflammation. Growing evidence shows that the
NOD-like receptor protein 1 (NLRP1) inflammasome is involved in the
pathogenesis of various neurological diseases, such as AD (14). The NLRP1 inflammasome can regulate
the secretion and maturation of pro-inflammatory cytokines, such as
pro-interleukin(IL)-1β into the bioactive form of IL-1β, leading to
an inflammatory response (15). Ma
et al (16) reported that
NOX2 plays an important role in regulating NLRP3 inflammasome
activation in the brain after traumatic brain injury. A previous
study also indicated that NOX2-mediated ROS generation was involved
in NLRP1 inflammasome activation, resulting in age-associated
neuronal damage (17).
Additionally, the latest study reported that LPS could increase the
sensitivity of H9C2 cells to high glucose (HG) and
hypoxia/reoxygenation and aggravate HG- and
hypoxia/reoxygenation-induced H9C2 cell injury by increasing ROS
accumulation and inducing NLRP3 inflammasome activation (18). Therefore, it was hypothesized that
LPS exposure may induce NOX2-NLRP1 inflammasome activation,
resulting in neuronal damage.
Ginseng is a traditional Chinese medicine that has
been used for over 2,000 years as a nourishing drug for improving
health conditions and delaying senescence. Ginsenoside Rg1 (Rg1), a
monomer of a tetracyclic triterpenoid derivative, is mainly
extracted and purified from the root of ginseng (19). It has been reported that Rg1 has
anti-oxidant, anti-inflammatory, anti-apoptotic and autophagic
effects (20). A recent study
showed that Rg1 could attenuate chronic, unpredictable, mild
stress-induced, depressive-like effects in rats via the regulation
of the nuclear factor (NF)-κB/NLRP3 pathway (21). It was also seen that Rg1 could
protect primary astrocyte cultures against oxygen-glucose
deprivation/reoxygenation (OGD/R)-induced injury, by decreasing ROS
generation (22). A recent study
also showed that Rg1 could alleviate age-associated neuronal damage
by decreasing NOX2-mediated ROS generation and inhibiting NLRP1
inflammasome activation in neurons (23). Additionally, recent research
reported that Rg1 had protective effects against LPS-induced
cognitive deficits in rats (24).
Nevertheless, the precise mechanism of Rg1 on LPS-induced neuronal
damage has not been fully elucidated.
Tempol is a ROS scavenger that can promote the
metabolism of ROS. Recently, studies reported that pretreatment
with tempol significantly increased cell viability and anti-oxidant
activity, and decreased ROS production and cyclooxygenase-2
expression (25). Apocynin, a NOX
inhibitor, can inhibit NOX activation by interfering with the
intracellular translocation of two cytosolic components, p47phox
and p67phox (26). Both tempol and
apocynin have been reported to inhibit inflammatory responses in
age-associated kidney damage (27).
In the present study, it was hypothesized that Rg1 could ameliorate
LPS-induced neuronal damage by inhibiting NOX2-NLRP1 inflammasome
activation. In the present study, the effect of LPS exposure on
neuronal damage in HT22 cells, depending on the LPS exposure times
and concentration gradients, was examined. The effect of Rg1,
tempol and apocynin on NOX2-NLPR1 inflammasome activation and
neuronal damage was also examined in HT22 cells.
Materials and methods
Cell culture and treatments
The HT22 cell line is a sub-line derived from parent
HT4 cells that were originally immortalized from mouse hippocampal
neuron primary cultures (28). HT22
cells were cultured in Dulbecco's Modified Eagle Medium (DMEM;
Hyclone; Cytiva), supplemented with 10% fetal bovine serum (FBS;
Zhejiang Tianhang Biotechnology Co., Ltd.) and 1%
penicillin-streptomycin (Beyotime Institute of Biotechnology), in a
5% CO2 incubator (Thermo Fisher Scientific, Inc.) at
37˚C. The cells were cultured for 24 h before being treated with
LPS (Sigma-Aldrich; Merck KGaA), Rg1 (content of Rg1 >98%;
Chengdu Desite Biotechnology Co.), apocynin (Merck KGaA) or tempol
(Merck KGaA). For exploration of the effect of LPS at different
concentrations, HT22 cells were randomly divided into 5 groups and
treated for 24 h, as follows: No-LPS control, 5 mg/l LPS, 10 mg/l
LPS, 20 mg/l LPS, and 40 mg/l LPS. For exploration of the effect of
different lengths of LPS exposure, HT22 cells were randomly divided
into 6 groups: No-LPS 12, 24 and 48 h control groups and LPS (10
mg/l)-treated 12, 24 and 48 h groups. For investigation of the
effect of Rg1, HT22 cells were randomly divided into 6 groups and
treated for 24 h, as follows: No-LPS control, 10 mg/l LPS only, 10
mg/l LPS + Rg1 (1, 5 or 10 µM of Rg1), 10 mg/l LPS + 50 µM tempol,
10 mg/l LPS + 50 µM apocynin. All experiments were repeated three
times.
Measurement of lactate dehydrogenase
(LDH)
To observe the effect of Rg1 on LPS-induced neuronal
damage in HT22 cells, the activity of LDH released to the medium
was detected using an LDH kit (Nanjing KeyGen Biotech Co., Ltd.),
according to the manufacturer's protocol. The cells were seeded
into 96-well plates (5x103 cells per well). When the
cells reached ~60-70% confluence, they were treated with LPS or Rg1
(1, 5 or 10 µM), tempol (50 µM) or apocynin (50 µM). Briefly, the
supernatant was collected and reacted with nicotinamide adenine
dinucleotide (NAD) and lactate solution at 37˚C for 5 min. The
absorbance was detected at 490 nm with a Multiskan™ FC
microplate reader (Thermo Fisher Scientific, Inc.) to calculate the
activity of LDH.
Measurement of ROS production
ROS production was detected by a
H2DCFDA-Cellular ROS Assay kit (Nanjing KeyGen Biotech
Co., Ltd.), according to the manufacturer's protocol. The cells
were seeded into 24-well plates (1x105 cells per well).
When the cells reached ~60-70% confluence, they were treated with
LPS or Rg1 (1, 5 or 10 µM), tempol (50 µM) or apocynin (50 µM). For
ROS detection, the H2DCFDA stock solution was diluted
with serum-free DMEM medium at a ratio of 1:1,000, to prepare a
10-µM working solution of H2DCFDA. Then, the
H2DCFDA working solution was added to the medium and
incubated at 37˚C for 30 min. After incubation, the adherent cells
were washed with PBS 3 times (5 min per wash) and the results were
examined at 488/525 nm excitation/emission using fluorescence
microscopy (Olympus IX71; Olympus Corporation). The mean
fluorescence intensities from three wells and five random fields
(magnification, x200) per well were obtained using Image Pro Plus
6.0 (Media Cybernetics, Inc.) automatic image analysis software to
indicate the changes in ROS production.
Apoptosis assay
To confirm neuronal damage, cell apoptosis was
detected using the Annexin V-FITC/PI Apoptosis Detection kit
(Beyotime Institute of Biotechnology), according to the
manufacturer's protocol. The cells were seeded into 24-well plates
(1.0x105 cells per well). When the cells reached ~60-70%
confluence, they were treated with LPS or Rg1 (1, 5 or 10 µM),
tempol (50 µM) or apocynin (50 µM). For Annexin V-FITC/PI staining,
the cells were washed twice with PBS (5 min per wash) then
incubated with binding buffer for 15 min at room temperature. Next,
the cells were incubated with Annexin V-FITC (5 µl/well) and
propidium iodide (PI; 10 µl/well) for 15 min at room temperature.
The results were detected for Annexin V-FITC (excitation/emission,
488/530 nm) and PI (excitation/emission, 488/630 nm) by
fluorescence microscopy (Olympus IX71; Olympus Corporation).
Positively stained areas were analyzed from three wells and five
random fields (magnification, x200) per well by the Image-Pro Plus
6.0 automatic image analysis software. The mean density of Annexin
V-FITC-positive areas was examined to assess early apoptotic cells,
and the mean density of PI-positive areas was examined to assess
late apoptotic cells.
Western blotting
Western blotting was performed to detect the levels
of β-galactosidase (β-Gal), NLRP1, cleaved caspase-1, IL-1β, NF-κB,
phosphorylated (p)-NF-κB, p47phox, p22phox, NOX2, Bax, Bcl-2 and
cleaved caspase-3 proteins. The cells were seeded into 6-well
plates (5.0x105 cells per well). When the cells reached
~60-70%, they were treated with LPS or Rg1 (1, 5 or 10 µM), tempol
(50 µM) or apocynin (50 µM) for 24 h. The total protein was
extracted by a mixture of RIPA lysis buffer (Beyotime Institute of
Biotechnology) with phenylmethanesulfonyl fluoride (Beyotime
Institute of Biotechnology) and phosphatase inhibitors (Beyotime
Institute of Biotechnology) at 100:1:1. The protein concentration
was determined using the BCA Protein Assay kit (Beyotime Institute
of Biotechnology). Equal amounts of protein (30 µg) were separated
by 12% SDS-polyacrylamide gel electrophoresis and transferred onto
polyvinylidene fluoride membranes (Merck KGaA). The membranes were
blocked with 5% skimmed milk for 1 h at room temperature. The
membranes were then washed 3 times (10 min per wash) using
Tris-buffered saline with 0.05% (v/v) Tween-20 (TBST) and incubated
overnight at 4˚C with the primary antibodies for β-Gal (1:1,000;
cat. no. WL03124; Wanleibio Co., Ltd.), NLRP1 (1:1,000; cat. no.
ab3683; Abcam), caspase-1 (1:1,000; cat. no. AF0120; Affinity
Biosciences), IL-1β (1:1,000; cat. no. AF5130; Affinity
Biosciences), NF-κB (1:1,000; cat. no. AF5130; Wanleibio Co.,
Ltd.), p-NF-κB (1:1,000; cat. no. GB11142-1; Wuhan Servicebio
Technology Co., Ltd.), p47phox (1:1,000; cat. no. BS4852; Bioworld
Biotechnology, Inc.), p22phox (1:1,000; cat. no. BS60290; Bioworld
Biotechnology, Inc.), NOX2 (1:1,000; cat. no. ab31092; Abcam), Bax
(1:1,000; AF0120; Affinity Biosciences), Bcl-2 (1:1,000; cat. no.
AF6139; Affinity Biosciences), cleaved caspase-3 (1:1,000; cat. no.
BS7003; Bioworld Technology, Inc.) and β-actin (1:1,000; cat. no.
TA-09; OriGene Technologies, Inc.). The membranes were then
incubated for 1 h at 25˚C with either horseradish
peroxidase-conjugated goat anti-rabbit (1:10,000; cat. no. ZB-2301;
OriGene Technologies, Inc.) or goat anti-mouse (1:10,000; cat. no.
ZB-2305; OriGene Technologies, Inc.) secondary antibodies. The
membranes were incubated with ECL substrate kit (Bio-Rad
Laboratories, Inc.) and the bands were visualized using a Bioshine
Chemi Q4600 Mini Imaging System (Shanghai Bioshine Technology).
ImageJ 1.46R software (National Institutes of Health) was used to
detect the density of immunoreactive bands. The relative density of
each target protein compared to the control was used to represent
the changes in target protein expression levels.
Statistical analysis
Data are presented as the mean ± standard deviation
(SD). The statistical analysis was performed using GraphPad Prism
6.0 software (GraphPad Software, Inc.). Differences among the
experimental groups were evaluated using one-way analysis of
variance (ANOVA) followed by Tukey's multiple comparisons test to
compare the differences between groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
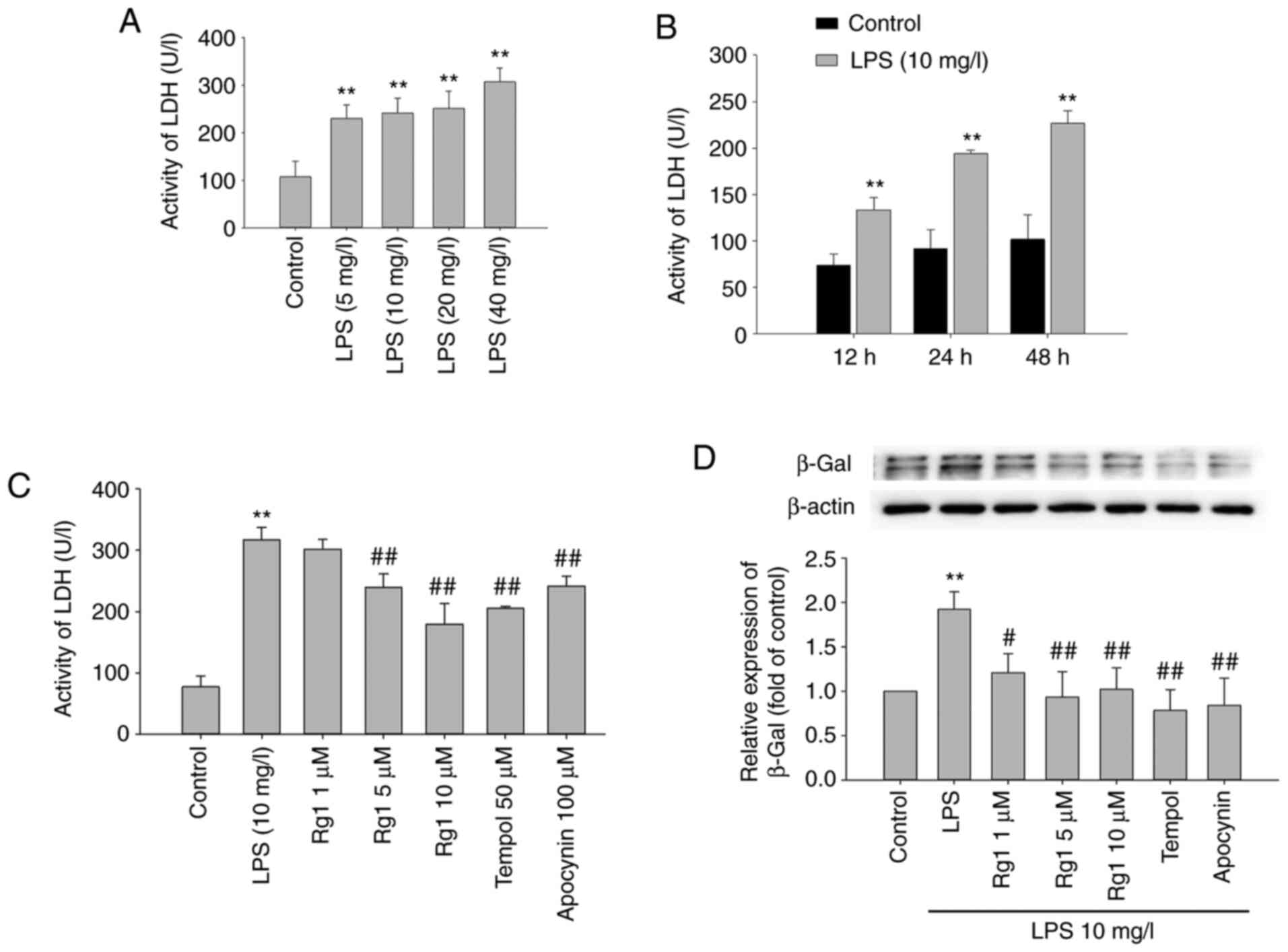
Effects of Rg1 on LPS-induced LDH
release and β-Gal expression in HT22 cells
The effects of LPS exposure on LDH release from HT22
cells were firstly observed. The results showed that LDH release
was significantly increased in LPS-treated (5, 10, 20 or 40 mg/l)
groups compared with the control group (Fig. 1A; P<0.01). LPS (10 mg/l)
treatment for 12, 24 or 48 h also significantly increased the LDH
release compared with the control group (Fig. 1B; P<0.01). The results suggested
that LPS exposure significantly induced neuronal damage in HT22
cells.
The effects of Rg1 treatment on LPS-induced LDH
release in HT22 cells were further observed. The results showed
that LPS (10 mg/l) treatment for 24 h significantly increased the
LDH release in HT22 cells compared with the control group (Fig. 1C; P<0.01). Treatment with Rg1 (5
or 10 µM) significantly decreased the LDH release (Fig. 1C; P<0.01) compared with the
LPS-treated (10 mg/l) group. Meanwhile, treatment with tempol (50
µM) and apocynin (50 µM) also significantly decreased the LDH
release from HT22 cells (Fig. 1C;
P<0.01). The results indicated that Rg1 significantly decreased
LDH release and protected against LPS-induced neuronal damage in
HT22 cells.
The accumulation of senescence-associated β-Gal is
an important senescence marker (29). To observe the effect of Rg1 on
LPS-induced senescence in HT22 cells, the expression of
senescence-associated β-Gal was detected by western blotting. The
results showed that LPS (10 mg/l) exposure for 24 h significantly
increased β-Gal expression compared with the control group in HT22
cells (Fig. 1D; P<0.01).
Compared with the LPS-treated (10 mg/l) group, the expression of
β-Gal was significantly decreased in Rg1 (1, 5 or 10 µM), tempol
(50 µM) and apocynin (50 µM) groups (Fig. 1D; P<0.01 or P<0.05). The data
suggested that Rg1 treatment delays LPS-induced senescence of HT22
cells.
Effects of Rg1 on ROS generation in
LPS-induced HT22 cells
ROS-mediated oxidative stress plays an important
role in several neurodegenerative diseases. To identify the effects
of LPS on ROS generation in HT22 cells, H2DCFDA
fluorescence staining was performed to examine the levels of ROS.
The results showed that the levels of ROS production were
significantly increased in LPS-treated (10, 20 or 40 mg/l) groups
compared with the control group (Fig.
2A and B; P<0.01).
Meanwhile, the results showed that the ROS production was increased
in the control group and LPS-treated groups when cultured for a
prolonged period. However, compared with the control group, groups
treated with LPS (10 mg/l) for 12, 24 and 48 h showed significant
increases in the ROS levels, especially at 24 h (Fig. 2C and D; P<0.01). Although LPS exposure for 48
h further increased ROS production compared with exposure for 24 h,
the relative ROS production was decreased due to the increase in
ROS production in the control group. These results suggested that
LPS exposure could significantly increase ROS generation in HT22
cells.
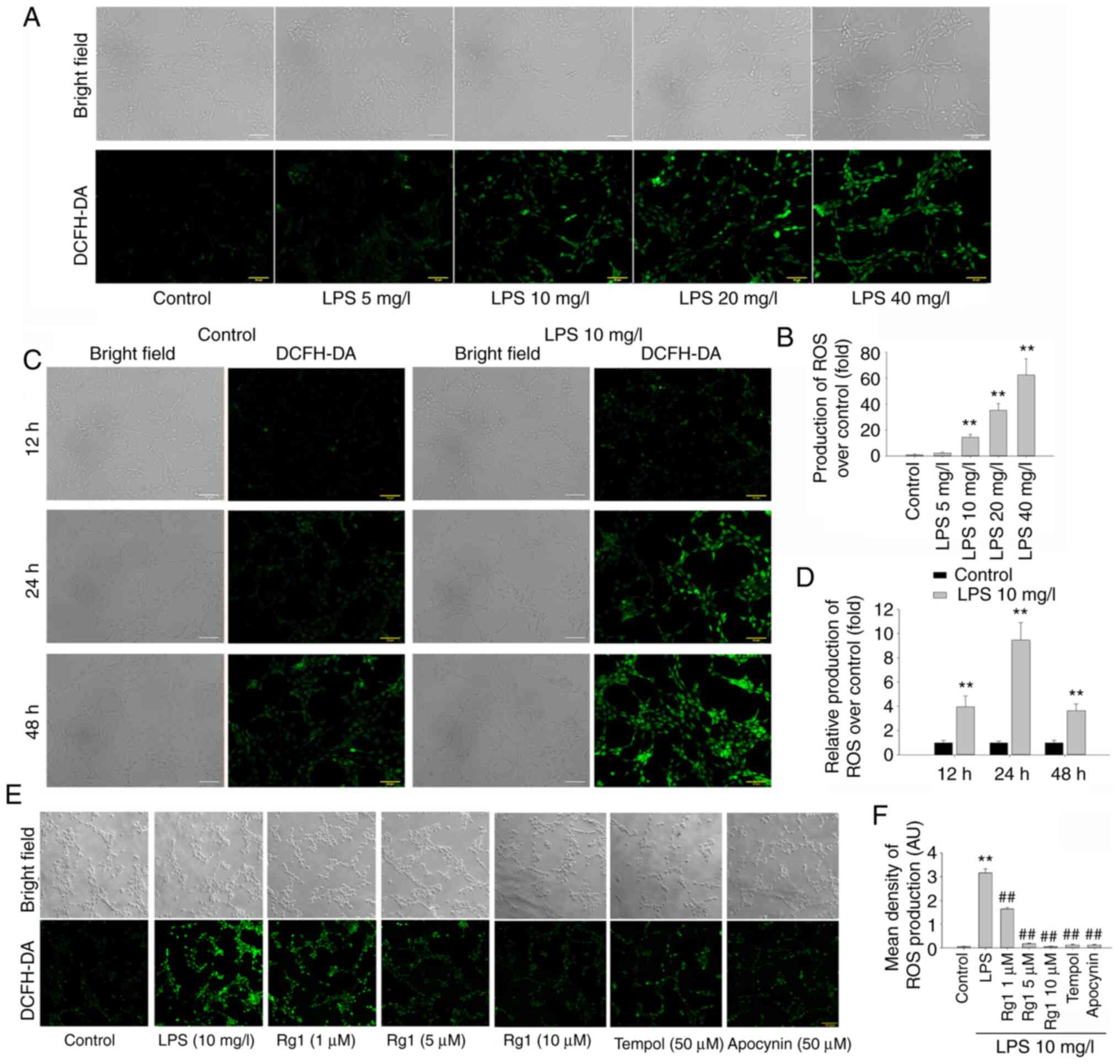 | Figure 2Effects of Rg1 on ROS generation in
LPS-induced HT22 cells. (A) The effects of LPS (5, 10, 20 and 40
mg/l) exposure on ROS generation in HT22 cells. (B) Quantitative
analysis of LPS (5, 10, 20 and 40 mg/l) exposure on ROS production
compared with the control. (C) The effects of LPS (10 mg/l)
exposure for 12, 24 and 48 h on ROS generation in HT22 cells. (D)
Quantitative analysis of LPS (10 mg/l) exposure for 12, 24 and 48 h
on ROS production compared with the control. (E) The effects of Rg1
on ROS production in LPS-induced HT22 cells. (F) Quantitative
analysis of the levels of ROS production. H2DCFDA staining;
magnification, x200, scale bars, 50 µm. Results are expressed as
mean ± SD, n=3. **P<0.01 vs. control group.
##P<0.01 vs. LPS (10 mg/l) group. Rg1, ginsenoside
Rg1; ROS, reactive oxygen species; LPS, lipopolysaccharide. |
The effects of Rg1 treatment on ROS generation was
further observed in LPS-induced HT22 cells. The results showed that
LPS (10 mg/l) treatment for 24 h significantly increased the levels
of ROS production in HT22 cells compared with the control group
(Fig. 2E and F; P<0.01). Compared with the
LPS-treated (10 mg/l) group, treatment with Rg1 (1, 5 or 10 µM)
significantly decreased the levels of ROS (Fig. 2E and F; P<0.01). Meanwhile, treatment with
tempol (50 µM) or apocynin (50 µM) also significantly decreased the
levels of ROS in HT22 cells (Fig.
2E and F; P<0.01). The data
suggested that Rg1 could significantly decrease ROS generation and
could protect against LPS-induced oxidative stress damage in HT22
cells.
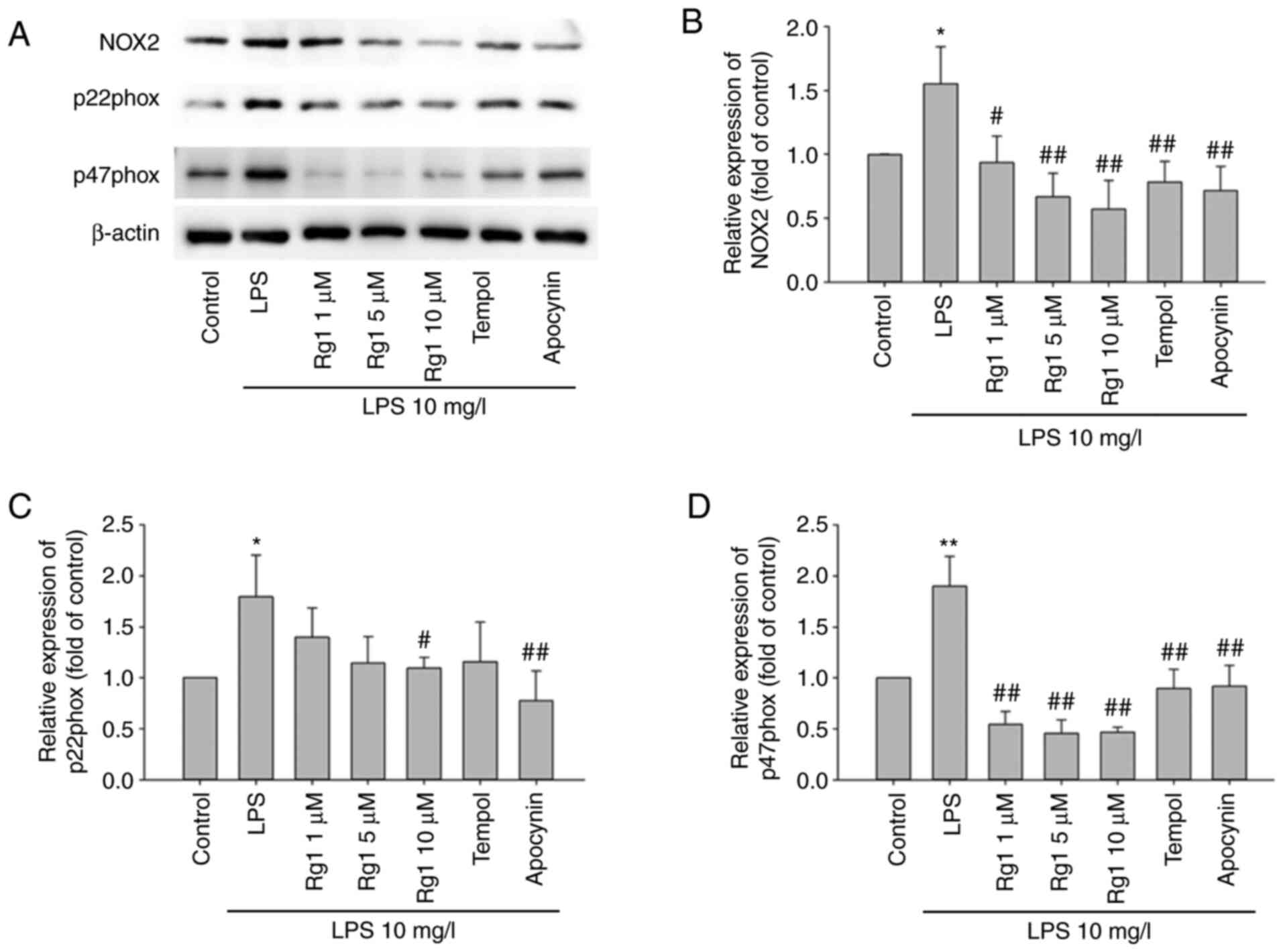
Effects of Rg1 on the expression of
NOX2, p22phox and p47phox in LPS-induced HT22 cells
To confirm whether Rg1 could decrease ROS generation
in LPS-induced HT22 cells by inhibiting NOX2, the expression of
NOX2, p22phox and p47phox levels were detected by western blotting.
The results showed that the expression of NOX2, p22phox and p47phox
were significantly increased in the LPS-treated (10 mg/l) group
compared with the control group (Fig.
3A-D; P<0.05 or P<0.01, respectively). Compared with the
LPS-treated (10 mg/l) group, treatment with Rg1 (1, 5 or 10 µM),
tempol (50 µM) or apocynin (50 µM) significantly decreased the
expression of NOX2 and p47phox (Fig.
3A and C; P<0.05 and
P<0.01, respectively). Meanwhile, treatment with Rg1 (10 µM) or
apocynin (50 µM) significantly decreased the expression of p22phox
in LPS-induced HT22 cells (Fig. 3B;
P<0.05 and P<0.01, respectively). These data suggest that LPS
exposure-induced ROS generation might be associated with the
activation of NOX2, and that Rg1 could significantly decrease NOX2
expression and decrease ROS generation in LPS-induced HT22
cells.
Effects of Rg1 on the expression of
NLRP1, caspase-1, IL-1β, NF-κB and p-NF-κB in LPS-induced HT22
cells
The elevation of ROS production is involved in NLRP1
inflammasome activation, which plays an important role in
neurodegenerative diseases. To confirm whether Rg1 treatment could
protect against LPS-induced neuronal damage by inhibiting NLRP1
inflammasome activation, the expression of NLRP1, cleaved
caspase-1, IL-1β, NF-κB and p-NF-κB was investigated in LPS-induced
HT22 cells. The results showed that LPS (10 mg/l) exposure
significantly increased the expression of NLRP1, cleaved caspase-1,
IL-1β, NF-κB and p-NF-κB, in comparison to the control group
(Fig. 4A-F; P<0.05 and
P<0.01, respectively). When compared with the LPS-treated group,
treatment with Rg1 (5 or 10 µM) and apocynin (50 µM) significantly
decreased the expression of NLRP1 (Fig.
4A and B; P<0.01). Treatment
with Rg1 (5 or 10 µM), tempol (50 µM) or apocynin (50 µM) also
significantly decreased the expression of cleaved caspase-1, IL-1β,
NF-κB and p-NF-κB (Fig. 4A,
C-F; P<0.05 and P<0.01,
respectively). Since the expression levels of NF-κB and p-NF-κB
were increased in the LPS group and decreased in Rg1, tempol or
apocynin groups, the relative expression of p-NF-κB/NF-κB has an
increasing trend only in the LPS group and a decreasing trend in
the Rg1 group. The aforementioned results indicated that activation
of the NLRP1 inflammasome was involved in LPS exposure-induced
neuronal damage. The aforementioned results also suggest that Rg1
might protect against LPS-induced HT22 cells damage by inhibiting
the expression of the NLRP1 inflammasome.
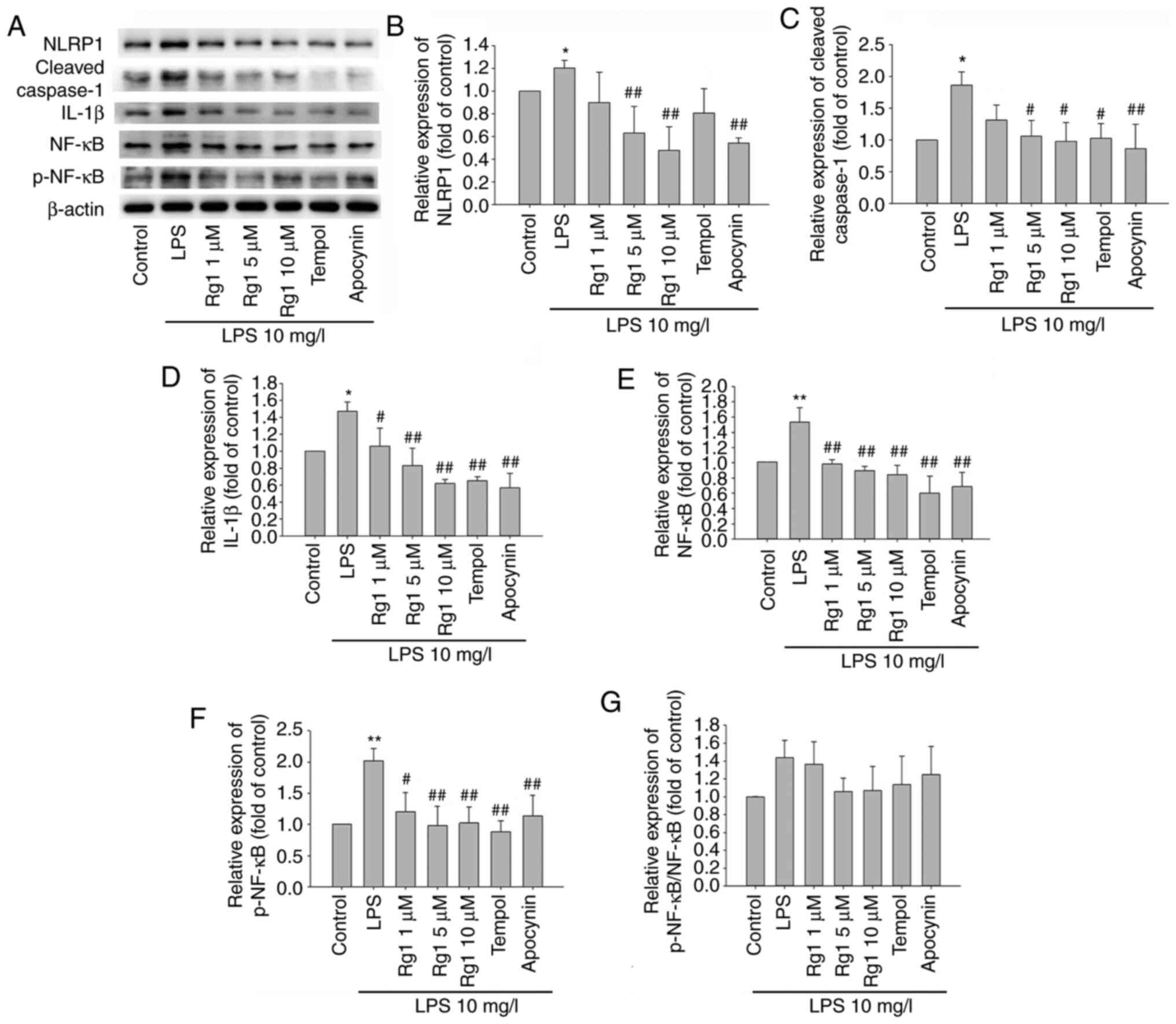 | Figure 4Effects of Rg1 on the expression
levels of NLRP1, cleaved-caspase-1, IL-1β, NF-κB and p-NF-κB in
LPS-induced HT22 cells determined by western blotting. (A) The
bands of NLRP1, cleaved-caspase-1, IL-1β, NF-κB and p-NF-κB in
LPS-induced HT22 cells. Quantitative analysis of (B) NLRP1, (C)
cleaved-caspase-1, (D) IL-1β, (E) NF-κB, (F) p-NF-κB expression
levels relative to the control and (G) p-NF-κB/NF-κB. Results are
expressed as mean ± SD, n=3. *P<0.05;
**P<0.01 vs. control group. #P<0.05;
##P<0.01 vs. LPS (10 mg/l) group. Rg1, ginsenoside
Rg1; LPS, lipopolysaccharide; IL, interleukin, p-, phosphorylated-;
NF-κB, nuclear factor-κB. |
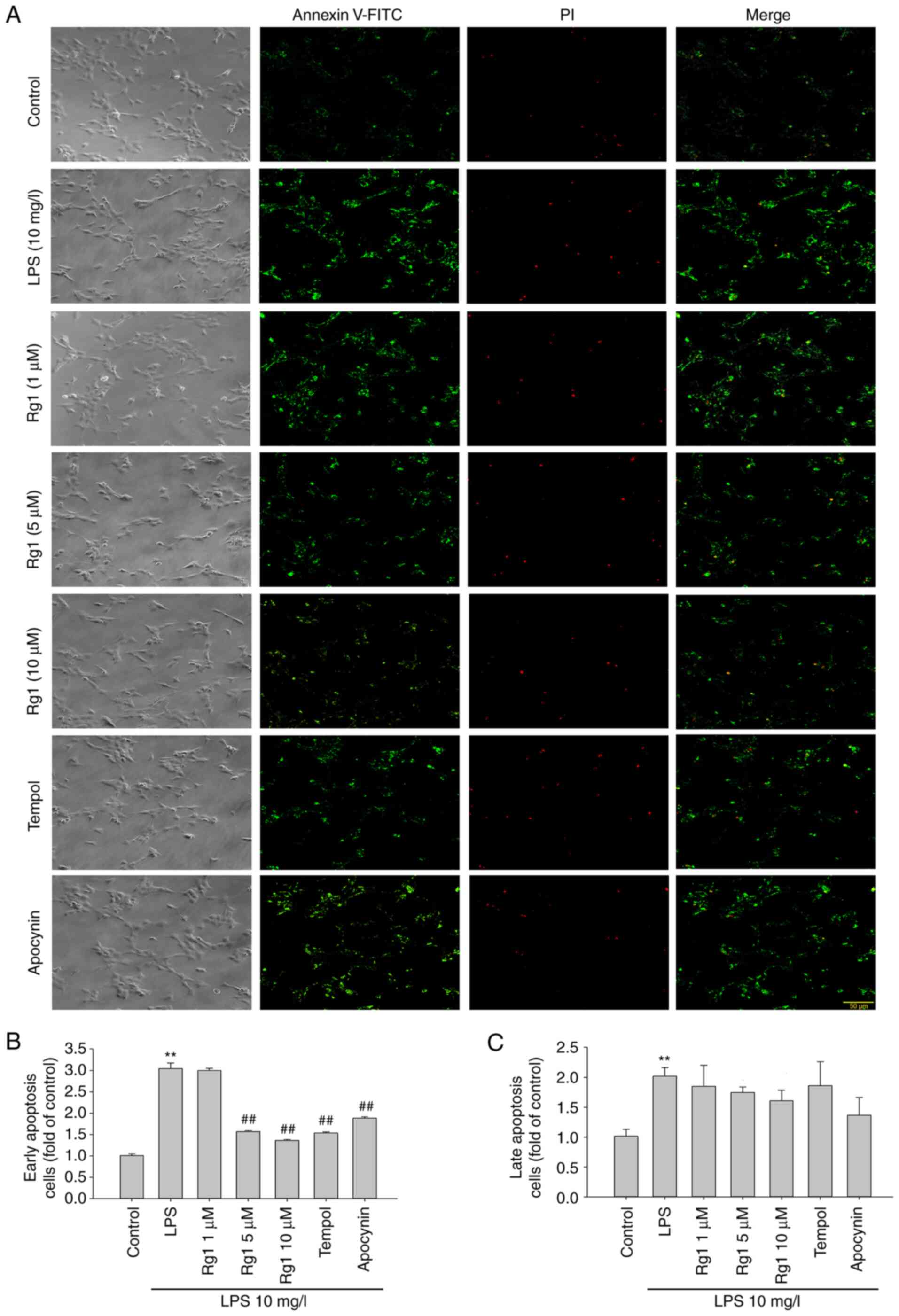
Effects of Rg1 on LPS-induced
apoptosis in HT22 cells
Neuronal apoptosis is involved in the pathogenesis
of neurodegenerative diseases. To confirm the protective effect of
Rg1 on LPS-induced neuronal damage, the effect of Rg1 on
LPS-induced apoptosis was observed by Annexin V FITC/PI staining in
HT22 cells. The results showed that LPS (10 mg/l) exposure for 24 h
significantly increased both the early and late apoptotic cells, in
comparison to the control group (Fig.
5A-C; P<0.01). Compared with the LPS-treated (10 mg/l)
group, treatment with Rg1 (5 or 10 µM), tempol (50 µM) or apocynin
(50 µM) significantly decreased the early apoptotic cells (Fig. 5A and B; P<0.01). However, treatment with Rg1,
tempol or apocynin had no significant effect on the late apoptotic
cells (Fig. 5A and C; P>0.05). The aforementioned data
indicated that Rg1 could effectively alleviate LPS-induced
apoptosis in HT22 cells.
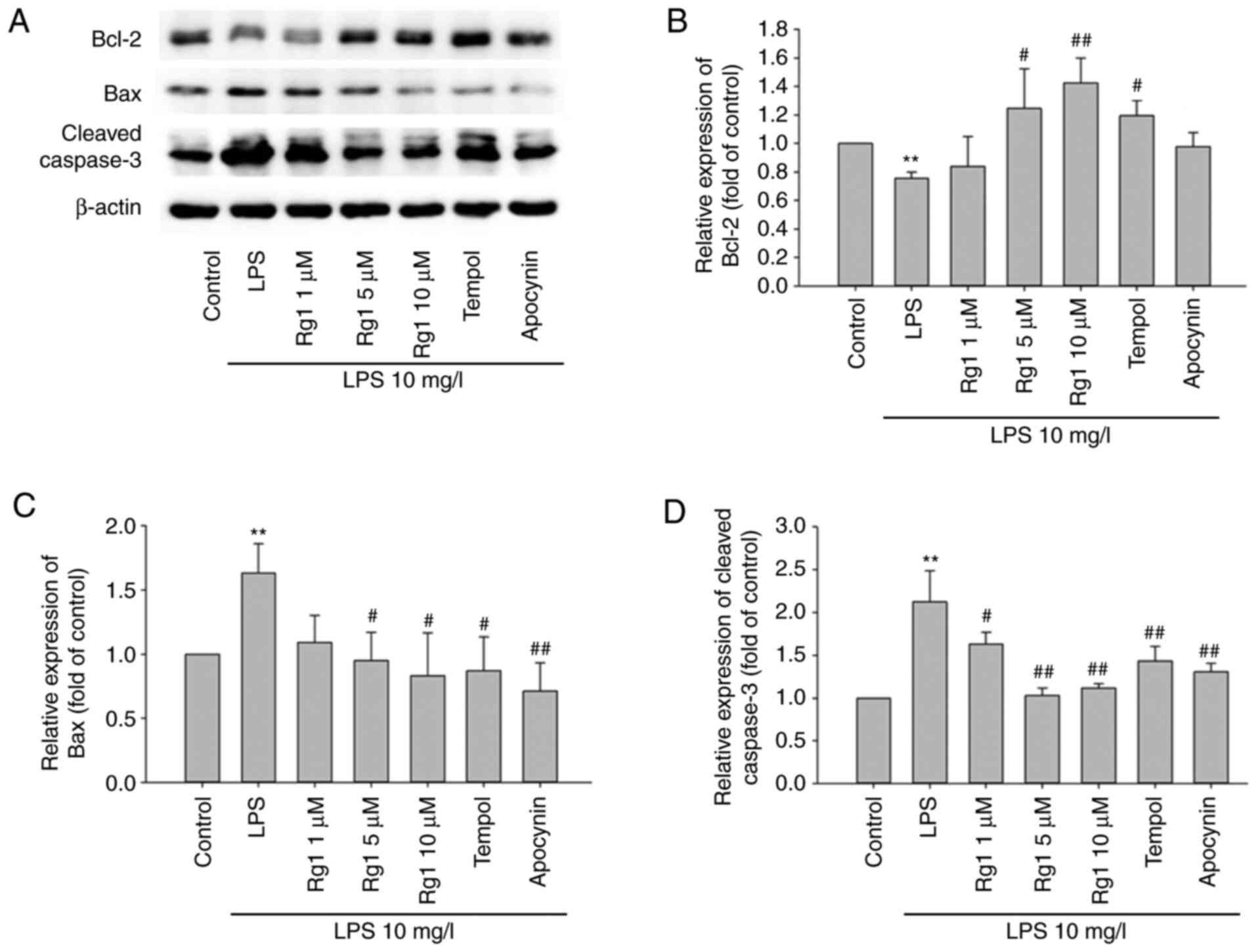
Effects of Rg1 on the expression of
Bax, Bcl-2 and cleaved caspase-3 in LPS-induced HT22 cells
The effect of Rg1 was investigated on the expression
of apoptosis-associated proteins Bax, Bcl-2 and cleaved caspase-3,
in LPS-induced HT22 cells. The results showed that LPS (10 mg/l)
exposure for 24 h significantly increased the expression of Bax and
cleaved caspase-3, and significantly decreased Bcl-2 expression, in
comparison to the control group (Fig.
6A-D; P<0.01). When compared with the LPS-treated (10 mg/l)
group, treatment with Rg1 (5 or 10 µM), tempol (50 µM) or apocynin
(50 µM) significantly decreased the expression levels of Bax and
cleaved caspase-3 expressions (Fig.
6A-D; P<0.05 or P<0.01). Meanwhile, Rg1 (5 or 10 µM) and
tempol treatment significantly increased Bcl-2 expression level
(Fig. 6A and B; P<0.05 and P<0.01, respectively).
The results suggested that sRg1 could alleviate LPS-induced
apoptosis by regulating the expression of Bax, Bcl-2 and cleaved
caspase-3 in HT22 cells.
Discussion
Although AD has been characterized by its
pathological features, such as Aβ deposition, NFTs and neuron loss,
LPS is also involved in the progression of AD. The effects of Rg1
are associated to its properties, such as its anti-oxidant effects
and ability to inhibit inducible nitric oxide synthase (iNOS)
expression and nitric oxide (NO) overgeneration (30). In addition, Rg1 has been reported to
attenuate LPS-induced inflammatory responses in murine BV-2
microglial cells (31). However,
whether Rg1 can alleviate LPS-induced neuronal damage and its
precise mechanism of action needs to be further investigated. In
the present study, the results demonstrated that LPS exposure could
significantly induce NOX2 and NLRP1 inflammasome activation,
resulting in neuronal damage and apoptosis in HT22 cells. More
importantly, Rg1 treatment could significantly decrease the
expression of NOX2 and NLRP1 inflammasomes, decrease ROS and
cytokine generation, and alleviate neuronal damage and apoptosis in
LPS-induced HT22 cells. Meanwhile, the results showed that
treatment with tempol (a ROS scavenger) and apocynin (a NOX
inhibitor) also significantly decreased the expression of NOX2 and
NLRP1 inflammasomes and alleviated neuronal damage and apoptosis in
LPS-induced HT22 cells. These findings suggest that NOX2 expression
and NLRP1 inflammasome activation is closely associated with
LPS-induced neuronal damage, and that Rg1 treatment can protect
against LPS-induced neuronal damage by inhibiting NOX2-NLRP1
inflammasomes in HT22 cells.
LPS is a bacterial cell wall endotoxin that can
cause systemic inflammation and neuroinflammation through
activation of the systemic or neurological immune system and
generation of pro-inflammatory mediators, such as IL-Iβ, IL-6 and
IL-8 (32,33). LPS is composed of lipid A, a core
oligosaccharide chain, and an O-antigenic polysaccharide side
chain. LPS is commonly used as an inflammatory inducer and an
oxidative stress stimulant in both in vitro and in
vivo experiments to activate cells to produce various
inflammatory components (34).
Oxidative stress can damage the mitochondria and other cellular
components of neurons (35),
ultimately contributing to the pathogenesis of AD. LPS has also
been used to stimulate ROS accumulation and induce neuronal damage
(36). Overproduction of ROS
participates in cell suicide, by initiating programmed cell death
(apoptosis) pathways (37). In the
present study, the results of LDH release indicated that HT22 cells
were significantly damaged by increasing concentrations and times
of LPS exposure. The results of H2DCFDA staining showed
that ROS production was significantly increased with increasing
concentration and time of LPS exposure in HT22 cells. In addition,
Annexin V FITC/PI staining showed that apoptotic cells were
significantly increased in LPS-induced HT22 cells. These data
indicated that LPS exposure could significantly increase ROS
generation and cell apoptosis in HT22 cells, contributing to
neuronal damage and becoming more serious with increasing
concentration and time of LPS exposure.
Ginseng is a traditional Chinese medicine which has
been used for thousands of years to improve the health of the
elderly and delay ageing. Ginsenoside Rg1, a main active component,
has neurotrophic, neuroprotective and anti-ageing effects, and it
has often been used to prevent neuronal damage by inhibiting
oxidative stress and inflammation (38,39).
Additionally, it has been reported that Rg1 has anti-senescence
effects on neural stem cells and delays brain aging by activating
the Wnt/β-catenin signaling pathway (40). Rg1 can also protect PD mice (where
PD is induced through continuous LPS injections) by inhibiting the
nuclear entry of NF-κB and regulating the polarization balance of
microglia (41). In the present
study, the results showed that Rg1, tempol and apocynin treatments
significantly decreased LDH release, ROS generation and IL-1β
expression compared with the LPS group in HT22 cells. Meanwhile,
the results indicated that Rg1, tempol or apocynin treatments
significantly inhibited the expression of β-Gal and alleviated
LPS-induced cell apoptosis in HT22 cells. All these data suggest
that Rg1 treatment could effectively protect against LPS-induced
neuronal damage. However, the mechanism underlying the protective
effects of Rg1 on LPS-induced neuronal damage remains to be fully
elucidated.
NADPH oxidase (NOX) belongs to a group of
electron-transporting transmembrane enzymes that contribute to ROS
generation in many tissues (42)
and it has been reported to be vital in the progression of
neurodegenerative diseases, including AD (43). In turn, the accumulation of ROS also
could upregulate NOX expression. It has been reported that
H2O2 treatment can significantly increase
NOX2 expression in PC12 cells, a cell line similar to neurons
(44). NOX is a complex membrane
protein that is composed of membrane subunits of catalyzed gp91phox
(NOX2) and p22phox, several regulatory catalytic subunits of
p40phox, p47phox, p67phox and the small GTPase Rac. NOX2 is
considered to be the major subtype of NOX in the brain, especially
in neurons (45). A recent study
suggested that inhibition of NOX activity could be a viable
neuroprotective strategy for brain injury and senescence (46). It has been reported that walnut
polyphenol extract protects against malathion- or
chlorpyrifos-mediated immunotoxicity and inhibits oxidative damage
by modulating the TLRx-NOX-ROS signaling pathway (47). In the present study, the results
indicated that the expression of NOX2, p22phox and p47phox were
significantly increased in LPS-induced HT22 cells. It was also seen
that Rg1, tempol or apocynin treatment significantly decreased the
expression of NOX2, p22phox and p47phox in LPS-induced HT22 cells.
It was speculated that Rg1, tempol or apocynin treatment might
downregulate NOX2 expression by decreasing ROS accumulation. The
detailed mechanism of Rg1 on the regulation of NOX2 requires
further study.
Increasing evidence suggests that inflammatory
responses are an important feature and the leading cause of
age-associated neuronal damage (48). NLRP1 inflammasomes play a pivotal
role in promoting inflammation throughout the body, and
particularly in neurons (49,50).
The NLRP1 inflammasome is composed of NLRP1, apoptosis-associated
speck-like protein (ASC) and procaspase-1. ASC can interact with
NLRP1 and procaspase-1, and activate procaspase-1 to caspase-1,
which cleaves the pro-IL-1β to generate the mature form of IL-1β
(51). This effect can also be
achieved by activating the NF-κB signaling pathway, which promotes
the transcription of inflammation-associated genes, such as IL-1β
and IL-6(52). It has been reported
that the inhibition of NLRP3 inflammasome activity could
significantly decrease tissue inflammatory damage and inhibit
apoptosis in LPS-induced acute kidney injury (53). A recent study reported that Rg1
could attenuate LPS-induced inflammation and apoptosis both in
neonatal rat cardiomyocytes and septic mice and restore impaired
cardiac function by blocking the TLR4/NF-κB/NLRP3 pathway (54). It has been reported that excessive
ROS generation can activate the NLRP1 inflammasome and induce
macrophage apoptosis in vitro and in vivo; although
these effects can be significantly inhibited by pretreatment with
ROS inhibitors (55). The latest
study also showed that the inhibition of NOX significantly
suppressed the production of pro-inflammatory cytokines, both in
vitro and in vivo (56).
However, it is still unknown whether Rg1 can protect against
neuronal damage by inhibiting the expression of NOX2 and NLRP1
inflammasomes in LPS-induced HT22 cells. In the present study, it
was found that LPS treatment significantly increased the expression
of NLRP1, IL-1β, cleaved caspase-1, NF-κB and p-NF-κB in HT22
cells. The results also showed that Rg1, tempol and apocynin
treatments significantly decreased the expression of NLRP1, IL-1β,
cleaved caspase-1, NF-κB and p-NF-κB in LPS-induced HT22 cells.
Tempol, similar to catalase, is often used as a ROS scavenger in
some models of neurodegeneration (57,58).
Apocynin can inhibit NOX2 (IC50, ~10 µM) and NOX4
(IC50, ~200 µM) to prevent ROS generation (59). The present results showed that Rg1
had a similar function to tempol and apocynin, suggesting that Rg1
might inhibit NLRP1 inflammasome activation by inhibiting NOX2
activity, decreasing ROS production in LPS-induced HT22 cells.
Apoptosis is a process of programmed cell suicide,
which can be induced by senescence (60). It has been reported that both
oxidative stress and inflammatory responses can induce cell
apoptosis, which is involved in neuronal damage in
neurodegenerative diseases, such as AD. Liu et al (61) reported that the inhibition of
hippocampal neuron apoptosis could protect against neuron damage in
ischemic stroke. Phosphatidylserine (PS) is located on the inner
side of the cell membrane and turns to the outer surface of the
cell membrane in early apoptotic cells. Annexin V can bind to
phosphatidylserine exposed on the outside of the membrane of early
apoptotic cells. Propidium iodide (PI), a nucleic acid dye, cannot
penetrate the membrane of normal cells or early apoptotic cells,
but can stain the nucleus of late apoptotic cells (62). The present study results indicated
that Rg1 and apocynin treatment could inhibit apoptosis, shown by a
decrease in both early and late apoptotic cells. However, tempol
(50 µM) treatment only showed a decrease in the early apoptotic
cells in LPS-induced HT22 cells. The doses of tempol used (50-200
µM) are commonly used in vitro to protect against cell
apoptosis (63). In the present
study, a smaller dose of tempol (50 µM) was used, which may be the
reason why only early apoptotic cells were inhibited. The B-cell
lymphoma-2 (Bcl-2) family is known for their regulatory effects on
apoptosis, and contains the anti-apoptotic gene, Bcl-2, and the
pro-apoptotic gene, Bcl-2 associated X protein (Bax). An increase
in Bax can promote apoptosis, while an increase in Bcl-2 can
inhibit apoptosis. Caspase-3, the most critical protease for
promoting the apoptosis cascade, plays the final pivotal role in
apoptotic pathways (64). The
present results showed that LPS exposure significantly increased
the expression of Bax and cleaved caspase-3 and decreased the
expression of Bcl-2 in HT22 cells. However, treatment with Rg1,
tempol or apocynin significantly decreased the expression levels of
Bax and cleaved caspase-3 and increased the expression level of
Bcl-2 in LPS-induced HT22 cells. The results suggested that Rg1
could alleviate LPS-induced neuronal apoptosis via the regulation
of the expression of Bax, Bcl-2 and cleaved caspase-3 in HT22
cells.
In summary, the present study demonstrated that LPS
exposure could significantly induce neuronal damage and apoptosis
in HT22 cells. Rg1 treatment could significantly alleviate neuronal
damage and apoptosis, and the mechanisms may be associated with the
inhibition of NOX2 and NLRP1 inflammasome expression, since these
were significantly increased in LPS-induced HT22 cells. However,
the present study only observed the protective effect of Rg1 on
LPS-induced neuronal damage in vitro, and the protective
effect and mechanism in vivo warrant further
investigation.
Acknowledgements
The authors would like to thank Mr. Dake Huang and
Mr. Bao Li from the Synthetic Laboratory of Basic Medicine College,
Anhui Medical University for their technical assistance.
Funding
Funding: This study was supported by the National Natural
Science Foundation of China (grant nos. 81671384 and 81371329) and
Natural science research projects of universities in Anhui province
(grant no. KJ2019A0226).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ, SD and YC performed the experiments, analyzed
the data, and were the major contributors in writing the
manuscript. JZ, ZF and ZS collated the data. YH and XD contributed
to the interpretation of the results. ZF and ZS confirm the
authenticity of all the raw data. WL designed the study, critically
revised the manuscript for intellectually important content and
supervised the study. All authors read and approved the final
submitted manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Freeman LC and Ting JP: The pathogenic
role of the inflammasome in neurodegenerative diseases. J
Neurochem. 136 (Suppl 1):S29–S38. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Boutajangout A, Lindberg H, Awwad A, Paul
A, Baitalmal R, Almokyad I, Höidén-Guthenberg I, Gunneriusson E,
Frejd FY, Härd T, et al: Affibody-mediated sequestration of amyloid
β demonstrates preventive efficacy in a transgenic Alzheimer's
disease mouse model. Front Aging Neurosci. 11(64)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhan X, Stamova B, Jin LW, DeCarli C,
Phinney B and Sharp FR: Gram-negative bacterial molecules associate
with Alzheimer disease pathology. Neurology. 87:2324–2332.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhao Y, Jaber V and Lukiw WJ: Secretory
products of the human GI tract microbiome and their potential
impact on Alzheimer's disease (AD): Detection of lipopolysaccharide
(LPS) in AD hippocampus. Front Cell Infect Microbiol.
7(318)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ju IG, Choi JG, Kim N, Kwak C, Lee JK and
Oh MS: Peucedani Japonici Radix ameliorates
lipopolysaccharide-induced neuroinflammation by regulating
microglial responses. Neurosci Lett. 686:161–167. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhou J, Yu W, Zhang M, Tian X, Li Y and Lü
Y: Imbalance of microglial TLR4/TREM2 in LPS-treated APP/PS1
transgenic mice: A potential link between Alzheimer's disease and
systemic inflammation. Neurochem Res. 44:1138–1151. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
You LH, Yan CZ, Zheng BJ, Ci YZ, Chang SY,
Yu P, Gao GF, Li HY, Dong TY and Chang YZ: Astrocyte hepcidin is a
key factor in LPS-induced neuronal apoptosis. Cell Death Dis.
8(e2676)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ji YF, Wang D, Liu YR, Ma XR, Lu H and
Zhang BA: MicroRNA-132 attenuates LPS-induced inflammatory injury
by targeting TRAF6 in neuronal cell line HT-22. J Cell Biochem.
119:5528–5537. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Christensen LP and Christensen KB: The
role of direct and indirect polyphenolic antioxidants in protection
against oxidative stress. In: Polyphenols in Human Health and
Disease. Watson RR, Preedy VR and Zibadi S (eds). Academic Press,
San Diego, CA, pp289-309, 2014.
|
|
10
|
Bhat AH, Dar KB, Anees S, Zargar MA,
Masood A, Sofi MA and Ganie SA: Oxidative stress, mitochondrial
dysfunction and neurodegenerative diseases; a mechanistic insight.
Biomed Pharmacother. 74:101–110. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dasoveanu DC, Park HJ, Ly CL, Shipman WD,
Chyou S, Kumar V, Tarlinton D, Ludewig B, Mehrara BJ and Lu TT:
Lymph node stromal CCL2 limits antibody responses. Sci Immunol.
5(eaaw0693)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
d'Avila JC, Siqueira LD, Mazeraud A,
Azevedo EP, Foguel D, Castro-Faria-Neto HC, Sharshar T, Chrétien F
and Bozza FA: Age-related cognitive impairment is associated with
long-term neuroinflammation and oxidative stress in a mouse model
of episodic systemic inflammation. J Neuroinflammation.
15(28)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fan LM, Cahill-Smith S, Geng L, Du J,
Brooks G and Li JM: Aging-associated metabolic disorder induces
Nox2 activation and oxidative damage of endothelial function. Free
Radic Biol Med. 108:940–951. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zotova E, Bharambe V, Cheaveau M, Morgan
W, Holmes C, Harris S, Neal JW, Love S, Nicoll JA and Boche D:
Inflammatory components in human Alzheimer's disease and after
active amyloid-β42 immunization. Brain. 136:2677–2696.
2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Abulafia DP, de Rivero Vaccari JP, Lozano
JD, Lotocki G, Keane RW and Dietrich WD: Inhibition of the
inflammasome complex reduces the inflammatory response after
thromboembolic stroke in mice. J Cereb Blood Flow Metab.
29:534–544. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ma MW, Wang J, Dhandapani KM and Brann DW:
NADPH oxidase 2 regulates NLRP3 inflammasome activation in the
brain after traumatic brain injury. Oxid Med Cell Longev.
2017(6057609)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xu T, Sun L, Shen X, Chen Y, Yin Y, Zhang
J, Huang D and Li W and Li W: NADPH oxidase 2-mediated NLRP1
inflammasome activation involves in neuronal senescence in
hippocampal neurons in vitro. Int Immunopharmacol. 69:60–70.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Qiu Z, He Y, Ming H, Lei S, Leng Y and Xia
ZY: Lipopo-lysaccharide (LPS) aggravates high glucose- and
hypoxia/reoxygenation-induced injury through activating
ROS-dependent NLRP3 inflammasome-mediated pyroptosis in H9C2
cardiomyocytes. J Diabetes Res. 2019(8151836)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xiao Q, Zhang S, Ren H, Du R, Li J, Zhao
J, Gao Y, Zhu Y and Huang W: Ginsenoside Rg1 alleviates
ANIT-induced intrahepatic cholestasis in rats via activating
farnesoid X receptor and regulating transporters and metabolic
enzymes. Chem Biol Interact. 324(109062)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhu G, Wang Y, Li J and Wang J: Chronic
treatment with ginsenoside Rg1 promotes memory and hippocampal
long-term potentiation in middle-aged mice. Neuroscience.
292:81–89. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang YQ, Wang XB, Xue RR, Gao XX and Li
W: Ginsenoside Rg1 attenuates chronic unpredictable mild
stress-induced depressive-like effect via regulating NF-κB/NLRP3
pathway in rats. Neuroreport. 30:893–900. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Giuliani C: The flavonoid quercetin
induces AP-1 activation in FRTL-5 thyroid cells. Antioxidants.
8(112)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chen Y, Ding S, Zhang H, Sun Z, Shen X,
Sun L, Yin Y, Qun S and Li W: Protective effects of ginsenoside Rg1
on neuronal senescence due to inhibition of NOX2 and NLRP1
inflammasome activation in SAMP8 mice. J Funct Foods.
65(103713)2020.
|
|
24
|
Jin Y, Peng J, Wang X, Zhang D and Wang T:
Ameliorative effect of ginsenoside Rg1 on
lipopolysaccharide-induced cognitive impairment: Role of
cholinergic system. Neurochem Res. 42:1299–1307. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Crupi R, Impellizzeri D, Gugliandolo E,
Cordaro M, Siracusa R, Britti D, Cuzzocrea S and Di Paola R: Effect
of tempol, a membrane-permeable free radical scavenger, on in vitro
model of eye inflammation on rabbit corneal cells. J Ocul Pharmacol
Ther. 35:571–577. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li M, Liu Z, Zhuan L, Wang T, Guo S, Wang
S, Liu J and Ye Z: Effects of apocynin on oxidative stress and
expression of apoptosis-related genes in testes of diabetic rats.
Mol Med Rep. 7:47–52. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Shen X, Dong X, Han Y, Li Y, Ding S, Zhang
H, Sun Z, Yin Y and Li W and Li W: Ginsenoside Rg1 ameliorates
glomerular fibrosis during kidney aging by inhibiting NOX4 and
NLRP3 inflammasome activation in SAMP8 mice. Int Immunopharmacol.
82(106339)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu J, Li L and Suo WZ: HT22 hippocampal
neuronal cell line possesses functional cholinergic properties.
Life Sci. 84:267–271. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
El-Far AH, Darwish NHE and Mousa SA:
Senescent colon and breast cancer cells induced by doxorubicin
exhibit enhanced sensitivity to curcumin, caffeine, and
thymoquinone. Integr Cancer Ther.
19(1534735419901160)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Xu TZ, Shen XY, Sun LL, Chen YL, Zhang BQ,
Huang DK and Li WZ: Ginsenoside Rg1 protects against
H2O2-induced neuronal damage due to
inhibition of the NLRP1 inflammasome signalling pathway in
hippocampal neurons in vitro. Int J Mol Med. 43:717–726.
2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zong Y, Ai QL, Zhong LM, Dai JN, Yang P,
He Y, Sun J, Ling EA and Lu D: Ginsenoside Rg1 attenuates
lipopolysaccharide-induced inflammatory responses via the
phospholipase C-γ1 signaling pathway in murine BV-2 microglial
cells. Curr Med Chem. 19:770–779. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zanoni I, Bodio C, Broggi A, Ostuni R,
Caccia M, Collini M, Venkatesh A, Spreafico R, Capuano G and
Granucci F: Similarities and differences of innate immune responses
elicited by smooth and rough LPS. Immunol Lett. 142:41–47.
2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Catorce MN and Gevorkian G: LPS-induced
murine neuroinflammation model: Main features and suitability for
pre-clinical assessment of nutraceuticals. Curr Neuropharmacol.
14:155–164. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lopes PC: LPS and neuroinflammation: A
matter of timing. Inflammopharmacology. 24:291–293. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Xu C, Hou B, He P, Ma P, Yang X, Yang X,
Zhang L, Qiang G, Li W and Du G: Neuroprotective effect of
salvianolic acid A against diabetic peripheral neuropathy through
modulation of Nrf2. Oxid Med Cell Longev.
2020(6431459)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Velagapudi R, El-Bakoush A and Olajide OA:
Activation of Nrf2 pathway contributes to neuroprotection by the
dietary flavonoid tiliroside. Mol Neurobiol. 55:8103–8123.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wan JZ, Wang R, Zhou ZY, Deng LL, Zhang
CC, Liu CQ, Zhao HX, Yuan CF, He YM, Dun YY, et al: Saponins of
Panax japonicus attenuate neuronal apoptosis through oxidative
stress-related pathways and autophagy in natural aging rats. Curr
Pharm Biotechnol. 21:2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wu J, Pan Z, Wang Z, Zhu W, Shen Y, Cui R,
Lin J, Yu H, Wang Q, Qian J, et al: Ginsenoside Rg1 protection
against β-amyloid peptide-induced neuronal apoptosis via estrogen
receptor α and glucocorticoid receptor-dependent anti-protein
nitration pathway. Neuropharmacology. 63:349–361. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ma J, Liu J, Wang Q, Yu H, Chen Y and
Xiang L: The beneficial effect of ginsenoside Rg1 on Schwann cells
subjected to hydrogen peroxide induced oxidative injury. Int J Biol
Sci. 9:624–636. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Xiang Y, Wang SH, Wang L, Wang ZL, Yao H,
Chen LB and Wang YP: Effects of ginsenoside Rg1 regulating
Wnt/β-catenin signaling on neural stem cells to delay brain
senescence. Stem Cells Int. 2019(5010184)2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Liu JQ, Zhao M, Zhang Z, Cui LY, Zhou X,
Zhang W, Chu SF, Zhang DY and Chen NH: Rg1 improves LPS-induced
Parkinsonian symptoms in mice via inhibition of NF-κB signaling and
modulation of M1/M2 polarization. Acta Pharmacol Sin. 41:523–534.
2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Xu J and Wei X, Gao F, Zhong X, Guo R, Ji
Y, Zhou X, Chen J, Yao P, Liu X and Wei X: Nicotinamide adenine
dinucleotide phosphate oxidase 2-derived reactive oxygen species
contribute to long-term potentiation of C-fiber-evoked field
potentials in spinal dorsal horn and persistent mirror-image pain
following high-frequency stimulus of the sciatic nerve. Pain.
161:758–772. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wang Y, Lv W, Li Y, Liu D, He X and Liu T:
Ampelopsin improves cognitive impairment in Alzheimer's disease and
effects of inflammatory cytokines and oxidative stress in the
hippocampus. Curr Alzheimer Res. 17:44–51. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Chen XH, Zhou X, Yang XY, Zhou ZB, Lu DH,
Tang Y, Ling ZM, Zhou LH and Feng X: Propofol protects against
H2O2-induced oxidative injury in differentiated PC12 cells via
inhibition of Ca(2+)-dependent NADPH oxidase. Cell Mol Neurobiol.
36:541–551. 2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sun L, Chen Y, Shen X, Xu T, Yin Y, Zhang
H, Ding S, Zhao Y, Zhang Y, Guan Y and Li W: Inhibition of
NOX2-NLRP1 signaling pathway protects against chronic
glucocorticoids exposure-induced hippocampal neuronal damage. Int
Immunopharmacol. 74(105721)2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Tenkorang MAA, Duong P and Cunningham RL:
NADPH oxidase mediates membrane androgen receptor-induced
neurodegeneration. Endocrinology. 160:947–963. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zhao Y, Fan C, Zhang A, Zhang Y, Wang F,
Weng Q and Xu M: Walnut polyphenol extract protects against
Malathion- and chlorpyrifos-induced immunotoxicity by modulating
TLRx-NOX-ROS. Nutrients. 12(616)2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Liu L and Chan C: The role of inflammasome
in Alzheimer's disease. Ageing Res Rev. 15:6–15. 2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Yin Y, Yan Y, Jiang X, Mai J, Chen NC,
Wang H and Yang XF: Inflammasomes are differentially expressed in
cardiovascular and other tissues. Int J Immunopathol Pharmacol.
22:311–322. 2009.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Tan CC, Zhang JG, Tan MS, Chen H, Meng DW,
Jiang T, Meng XF, Li Y, Sun Z, Li MM, et al: NLRP1 inflammasome is
activated in patients with medial temporal lobe epilepsy and
contributes to neuronal pyroptosis in amygdala kindling-induced rat
model. J Neuroinflammation. 12(18)2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
de Rivero Vaccari JP, Dietrich WD and
Keane RW: Therapeutics targeting the inflammasome after central
nervous system injury. Transl Res. 167:35–45. 2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Martins JD, Liberal J, Silva A, Ferreira
I, Neves BM and Cruz MT: Autophagy and inflammasome interplay. DNA
Cell Biol. 34:274–281. 2015.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Gao Q and Zhu H: The overexpression of
sirtuin1 (SIRT1) alleviated lipopolysaccharide (LPS)-induced acute
kidney injury (AKI) via inhibiting the activation of
nucleotide-binding oligomerization domain-like receptors (NLR)
family pyrin domain containing 3 (NLRP3) inflammasome. Med Sci
Monit. 25:2718–2726. 2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Luo M, Yan D, Sun Q, Tao J, Xu L, Sun H
and Zhao H: Ginsenoside Rg1 attenuates cardiomyocyte apoptosis and
inflammation via the TLR4/NF-κB/NLRP3 pathway. J Cell Biochem.
121:2994–3004. 2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Gan P, Gao Z, Zhao X and Qi G: Surfactin
inducing mitochondria-dependent ROS to activate MAPKs, NF-κB and
inflammasomes in macrophages for adjuvant activity. Sci Rep.
6(39303)2016.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Peng X, Yang Y, Tang L, Wan J, Dai J, Li
L, Huang J, Shen Y, Lin L, Gong X and Zhang L: Therapeutic benefits
of apocynin in mice with lipopolysaccharide/D-galactosamine-induced
acute liver injury via suppression of the late stage pro-apoptotic
AMPK/JNK pathway. Biomed Pharmacother. 125(110020)2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Youn CK, Kim J, Jo ER, Oh J, Do NY and Cho
SI: Protective effect of tempol against cisplatin-induced
ototoxicity. Int J Mol Sci. 17(1931)2016.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Wilcox CS: Effects of tempol and
redox-cycling nitroxides in models of oxidative stress. Pharmacol
Ther. 126:119–145. 2010.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Serrander L, Cartier L, Bedard K, Banfi B,
Lardy B, Plastre O, Sienkiewicz A, Fórró L, Schlegel W and Krause
KH: NOX4 activity is determined by mRNA levels and reveals a unique
pattern of ROS generation. Biochem J. 406:105–114. 2007.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Barman J, Kumar R, Saha G, Tiwari K and
Dubey VK: Apoptosis: Mediator molecules, interplay with other cell
death processes and therapeutic potentials. Curr Pharm Biotechnol.
16:644–663. 2018.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Liu QS, Deng R, Li S, Li X, Li K,
Kebaituli G, Li X and Liu R: Ellagic acid protects against neuron
damage in ischemic stroke through regulating the ratio of Bcl-2/Bax
expression. Appl Physiol Nutr Metab. 42:855–860. 2017.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Zhou H, Wang Q, Yuan D, Wang J, Huang Y,
Wu H, Jian J, Yang D, Huang N, Haisch C, Jiang Z and Chen S: Early
apoptosis real-time detection by label-free SERS based on
externalized phosphatidylserine. Analyst. 141:4293–4298.
2016.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Qi C, Liu X, Xiong T and Wang D: Tempol
prevents isoprenaline-induced takotsubo syndrome via the reactive
oxygen species/mitochondrial/anti-apoptosis/p38 MAPK pathway. Eur J
Pharmacol. 886(173439)2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Ju Y, Su Y, Chen Q, Ma K, Ji T, Wang Z and
Li W and Li W: Protective effects of Astragaloside IV on
endoplasmic reticulum stress-induced renal tubular epithelial cells
apoptosis in type 2 diabetic nephropathy rats. Biomed Pharmacother.
109:84–92. 2019.PubMed/NCBI View Article : Google Scholar
|