Introduction
Fungal keratitis (FK) is a devastating corneal
disorder, with ~40% prevalence of infectious keratitis prevalence
in China, which is mainly associated with agricultural trauma
(1,2). Once infected, corneal antigenicity
changes and the immune system is activated, resulting in increased
inflammatory cell infiltration, which release a large number of
inflammatory factors (3). FK
remains to be the major cause of fungal corneal ulcers in northern
China (4). At present, antifungal
medications and surgery are the main treatment approaches for
fungal corneal ulcer (5). However,
several problems remain, including the emergence of drug-resistant
strains of pathogens, long treatment cycles, recurrence, high
treatment costs and complications such as hepatorenal function
impairment and rejection (6).
Therefore, development of novel and effective treatment strategies
for fungal corneal ulcer is urgently required.
Corneal cross-linking (CXL) is a photochemical
reaction mediated by ultraviolet A (UV-A) radiation and the
photosensitizer riboflavin (7,8).
Previous studies have demonstrated that CXL is effective against
bacterial and fungal corneal ulcers (9,10). The
traditional CXL procedure involves irradiation of the eye at 3
mW/cm2 for 30 min using a total energy dose of 5.4
J/cm2 (7). However,
certain limitations remain for the clinical application of CXL,
including long irradiation time, limited dose of irradiation and
severe corneal irritation (11,12).
Currently, although new CXL technologies have been developed to
shorten the operation time and improve the comfort level of
patients postoperatively, their effects, including localized lesion
and infection control, remain unsatisfactory compared with those
following traditional CXL (13-15).
Therefore, irradiation time and total irradiation dose was improved
according to the principle of the biological efficacy associated
with total energy dose (16). A
similar study indicated that increased irradiation at 7-45
mW/cm2 shortened irradiation time, whilst a total energy
dose of 7.2 J/cm2 was also reasonable and safe (17). Furthermore, Özdemir et al
(18) demonstrated that CXL
combined with voriconazole exhibited beneficial effects in a rabbit
model of fungal keratitis. However, the effect of CXL compared with
that of intrastromal voriconazole injection for treating fungal
corneal ulcers remains unclear.
The present study aimed to evaluate the efficiency
of a modified CXL procedure, which involves irradiation at 30
mW/cm2 for 4 min using a total energy dose of 7.2
J/cm2, compared with that of intrastromal voriconazole
injection. The findings of the present study could reveal a novel
therapeutic option for treating fungal corneal ulcer.
Materials and methods
Patients
In the present study, 31 patients (sex, 19 males and
12 females; age, 55.3±9.0 years) with fungal corneal ulcer who were
treated at the General Hospital of Northern Theater Command
(Shenyang, China) between October 2017 and October 2019 were
enrolled.
The inclusion criteria were as follows: i) Patients
diagnosed with fungal corneal ulcer by corneal scraping, fungal
culture or in vivo confocal microscopy (IVCM; Figs. 1 and 2); ii) the ulcer depth did not exceed the
2/3 of the corneal thickness, as measured using slit lamp or
anterior segment optical coherence tomography (AS-OCT); iii)
patients did not heal despite conservative therapy for ≥7 days; iv)
no indication for emergency surgery; v) patients cooperated with
eye examination and aged from 18 to 90 years; and vi) followed up
for ≥6 months. Exclusion criteria were as follows: i) Patients with
systemic diseases that could potentially influence vision,
including diabetes, hypertension and cardiovascular diseases; ii)
lesions with full-thickness or even perforated, observation of
obvious endothelial plaques and anterior chamber empyema; and iii)
with fundus lesions, including fundus hemorrhage, macular
degeneration and optic nerve atrophy, which affected postoperative
vision in the past.
According to the surgical approach, patients who
were treated with CXL were defined as the CXL group (n=10), whilst
patients with corneal debridement combined with intrastromal
voriconazole injection were defined as the stromal injection group
(n=21). The advantages and disadvantages of two treatment methods
were addressed for each patient, following which a surgical plan
was developed according to the patients' choice. In addition, some
patients did not accept CXL due to economic conditions. Therefore,
the differences in sample size between the two groups appear to be
large. The present study was approved by the Ethics Committee of
the General Hospital of Northern Theater Command (approval no.
201736). All eligible patients provided written informed consent
prior to treatment and all operations were completed by Dr MG.
Microbiological examination
Specimens were collected from the surface of corneal
ulcer, and then cultured on Sabouraud agar medium at 28˚C for 1-7
days (cat. no. YC-SDA-90; Shenyang Yancheng Biological Products
Co., Ltd.). Species or genus was identified by colony morphology
referring to Manual of Clinical Microbiology, 11th Edition
(19). For filamentous fungus that
cannot be identified by morphology, 18S rRNA or ITS sequencing was
conducted by BGI Genomics.
Surgical procedures
In the CXL group, patients were treated two or three
times with pilocarpine nitrate for 10 min prior surgery.
Subsequently, local anesthetic was administered before the
conjunctival sac and surface of lesions were washed with 0.9% NaCl.
Following removal of the epithelium and necrotic tissue, a corneal
ring was placed on the surface of the cornea and 0.1% of riboflavin
(Avedro Inc.) was instilled every 5 min for 30 min. Until
riboflavin entered the anterior chamber, the cornea was irradiated
for 4 min using a KXL I UV-A source (Avedro Inc.) at a wavelength
of 370 nm, beam diameter of 9 mm, irradiance of 30
mW/cm2 with a total energy dose of 7.2 J/cm2.
Following irradiation, ofloxacin ointment (Shenyang Xingqi
Pharmaceutical Co., Ltd.) was applied to the eyes and sterile
auxiliary materials were used to cover them.
For patients in the stromal injection group, local
infiltration anesthesia was performed around the eyeball using 0.5%
proparacaine hydrochloride (Alcaine, Alcon Laboratories, Inc.).
Subsequently, the conjunctival sac and surface of the lesions were
washed for two to three times with 0.9% NaCl before the lesion
necrotic tissue was removed without exceeding 1/2 of the corneal
thickness. A 1-ml syringe attached to a 30 G needle was then used
to penetrate the corneal stroma at a relatively horizontal angle
from the transparent cornea area of the lesion edge. Voriconazole
(0.5 mg/ml; Sichuan Meidakang Pharmaceutical Co., Ltd.) was slowly
injected to form an edema before the extent of corneal edema was
used to evaluate the coverage of the drug. The injection dose of
voriconazole depended on the size of the lesion. In general, the
infiltration area of voriconazole was larger than the lesion
coverage by 0.5 mm. The injection was repeated after 3-5 days if
lesions were not localized or were deepened. Following surgery,
ofloxacin ointment was applied to the eyes, which were finally
covered with sterile auxiliary materials.
Postoperative management
Antibiotic therapy was administrated after the
surgical procedure. Briefly, the eyes were treated postoperatively
with 5% natamycin eye drops (four to six times/day; one drop each
time), 0.5% levofloxacin eye drops (twice/day; one drop each time)
and 0.3% sodium hyaluronate eye drops (four times/day; one drop
each time), 3% ofloxacin eye ointment (once/day; one drop) and oral
fluconazole capsules (0.2 g; first dosage, 0.4 g). The
aforementioned therapies were administrated for ≥4 weeks. If the
lesions were not improved or worsen, lamellar corneal transplant
surgery would be performed. The follow-up of all cases lasted for
≥6 months.
Postoperative evaluation indices
Treatment efficacy was evaluated according to the
following efficacy evaluation criteria (20): i) ‘Cured’ was defined as healed
ulcer and no fungal hyphae; ii) ‘effective’ treatment was defined
as partially healed ulcers, alleviated inflammatory responses,
relieved lesions and no fungal hyphae or reduction in hyphae, or
treatment being effective at first, followed by reoccurrence of
infection and wound not healing; and iii) ‘ineffective’ treatment
was defined by increased numbers of fungal hyphae, aggravated area
and depth of ulcer. Infection control and total efficacy rates were
calculated according to the following equations: i) Infection
control rate (%) = cured cases/total cases x100; ii) total
effective rate (%) = (cured cases + effective cases)/total cases
x100.
The definition of localized lesions was that the
area and depth of ulcer did not increase or decrease and the
inflammation was reduced, as previously described (21). IVCM was used to evaluate the
structure of corneal lesions, fungal hyphae, infection depth, cell
morphology and arrangement. In addition, 1 week after surgery,
sodium fluorescein eye detection test paper (Tianjin Jingming New
Technology Development Co., Ltd.) was applied to stain the cornea
as previously described (22). No
staining of the cornea would indicate that the ulcer was cured.
Ulcer healing rate was calculated according to the following
equation: Ulcer healing rate (%) = cured cases/total cases x100.
Furthermore, the best corrected visual acuity (BCVA) was first
determined, which was then converted to logarithm of minimal angle
of resolution (logMAR) to evaluate the outcome of the surgery.
AS-OCT was used to evaluate the postoperative corneal parameters
included corneal thickness, stromal infiltration and corneal
epithelium healing.
Complications
In the present study, the incidence of
complications, including corneal epithelial haze, loss of corneal
transparency, corneal melting, viral keratitis or iritis and other
types of eye tissue damage were recorded at postoperative 1, 2, 4,
12 and 24 weeks.
Statistical analysis
All data were analyzed using the SPSS 22.0 software
(IBM Corp.). BCVA, expressed as the logMAR, was analyzed using the
Mann-Whitney U test. The ages between the groups were compared with
a Student's t-test, whilst the enumeration data, including sex,
pathogenesis, lesion coverage, infection control, total effective
and ulcer healing rates were analyzed by Fisher's exact test.
Mixed-model ANOVA was used to analyze the effects of
between-groups, within-group and interaction of group and time on
BCVA. If the time or interaction effects were significant,
Bonferroni-adjusted post hoc analysis was done to assess the
comparison. P<0.05 was considered to indicate a statistically
significant difference.
Results
General characteristics
The baseline characteristics of the patients in the
CXL and stromal injection groups are shown in Table I. There were no significant
differences in age, sex, pathogenesis, lesion coverage and BCVA
between the two groups.
 | Table IGeneral characteristics of patients
in the two treatment groups. |
Table I
General characteristics of patients
in the two treatment groups.
| Parameter | CXL (n=10) | Stromal injection
(n=21) | Z/t/χ2
value | P-value |
|---|
| Age, years | 56.4±6.5 | 54.7±10.1 | -0.48 | 0.63 |
| Sex (male/female),
n (%) | 7/3
(70.0/30.0) | 12/9
(57.1/42.9) | 0.47 | 0.70 |
| Pathogenesis, n
(%) | | | 1.75 | 0.83 |
|
Trauma
history | 8 (80.0) | 16 (76.2) | | |
|
History of
medication and surgery | 2 (20.0) | 2 (9.5) | | |
| Other | 0 (0.0) | 3 (14.3) | | |
| Lesion coverage, n
(%) | | | 0.41 | >0.99 |
|
≤5 mm | 9 (90.0) | 17 (81.0) | | |
|
>5
mm | 1 (10.0) | 4 (19.0) | | |
| BCVA (logMAR) | 1.28±1.08 | 1.62±1.33 | -0.47 | 0.66 |
Efficacy evaluation after surgery
The infection control rates in the CXL and stromal
injection groups were 90.0 (9/10 eyes) and 47.6% (10/21 eyes),
respectively (P=0.04; Table II),
whilst the total effective rate was 90.0% (9/10 eyes) for the CXL
and 80.9% (17/21 eyes) for the stromal injection group (Table III). However, 7 eyes (33.3%) in
the stromal injection group were temporarily relieved 1 week after
surgery, infection recurred and the wound was not healed between
weeks 2-4, which belonged to effective group (Table III). To treat these fungal corneal
ulcers further, corneal transplantation was performed. At 4 weeks
after surgery, lesion was localized and the epithelium was healed
in the CXL group, where the corneal transparency was increased at
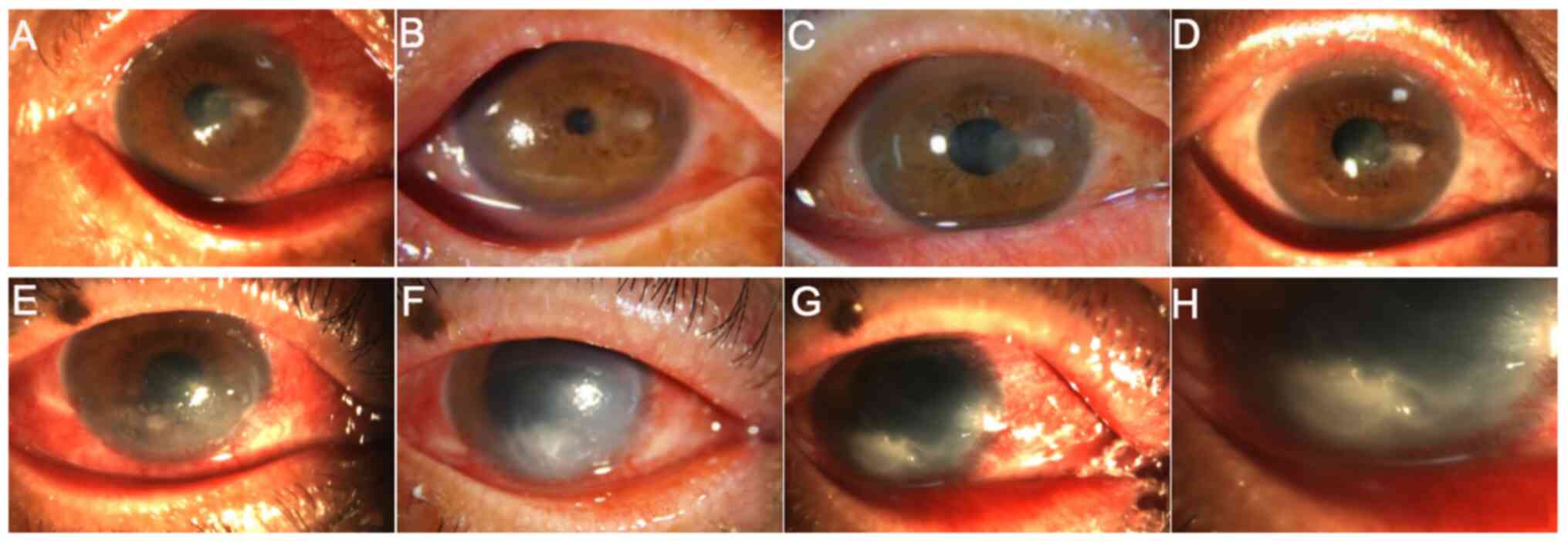
12 weeks after surgery (Fig. 3).
Similarly, in the stromal injection group, the border of the
corneal ulcer was localized, which was cleared 1 week after the
operation (Fig. 3).
 | Table IIComparison of infection control
between the two groups. |
Table II
Comparison of infection control
between the two groups.
| Infection
control | CXL (n=10) | Stromal injection
(n=21) | P-value |
|---|
| Controlled
infection, n (%) | 9 (90.0) | 10 (47.6%) | |
| Uncontrolled
infection, n (%) | 1 (10.0) | 11 (52.4%) | 0.04 |
 | Table IIIComparison of treatment efficacy
between the two groups. |
Table III
Comparison of treatment efficacy
between the two groups.
| Treatment
outcome | CXL (n=10) | Stromal injection
(n=21) | P-value |
|---|
| Cured, n (%) | 9 (90.0) | 10 (47.6) | |
| Effective, n
(%) | 0 (0.0) | 7 (33.3) | |
| Ineffective, n
(%) | 1 (10.0) | 4 (19.1) | >0.99 |
Localized lesion
The localized lesions in both groups at 1, 2, 4, 12
and 24 weeks after surgery were shown in Table IV. Localized lesions were observed
in nine eyes (90.0%) in the CXL group and nine eyes (42.9%) in the
stromal injection group 4, 12 and 24 weeks after surgery (P=0.02).
These findings suggest that treatment with CXL was superior
compared with intrastromal voriconazole in the aspect of localized
lesions. Furthermore, the eyes were examined for focal lesions
before and after surgery in the CXL group. The examination revealed
that an ulcer area of 2x2 mm was present in the center of the
cornea preoperatively. In addition, pseudopods and satellite foci
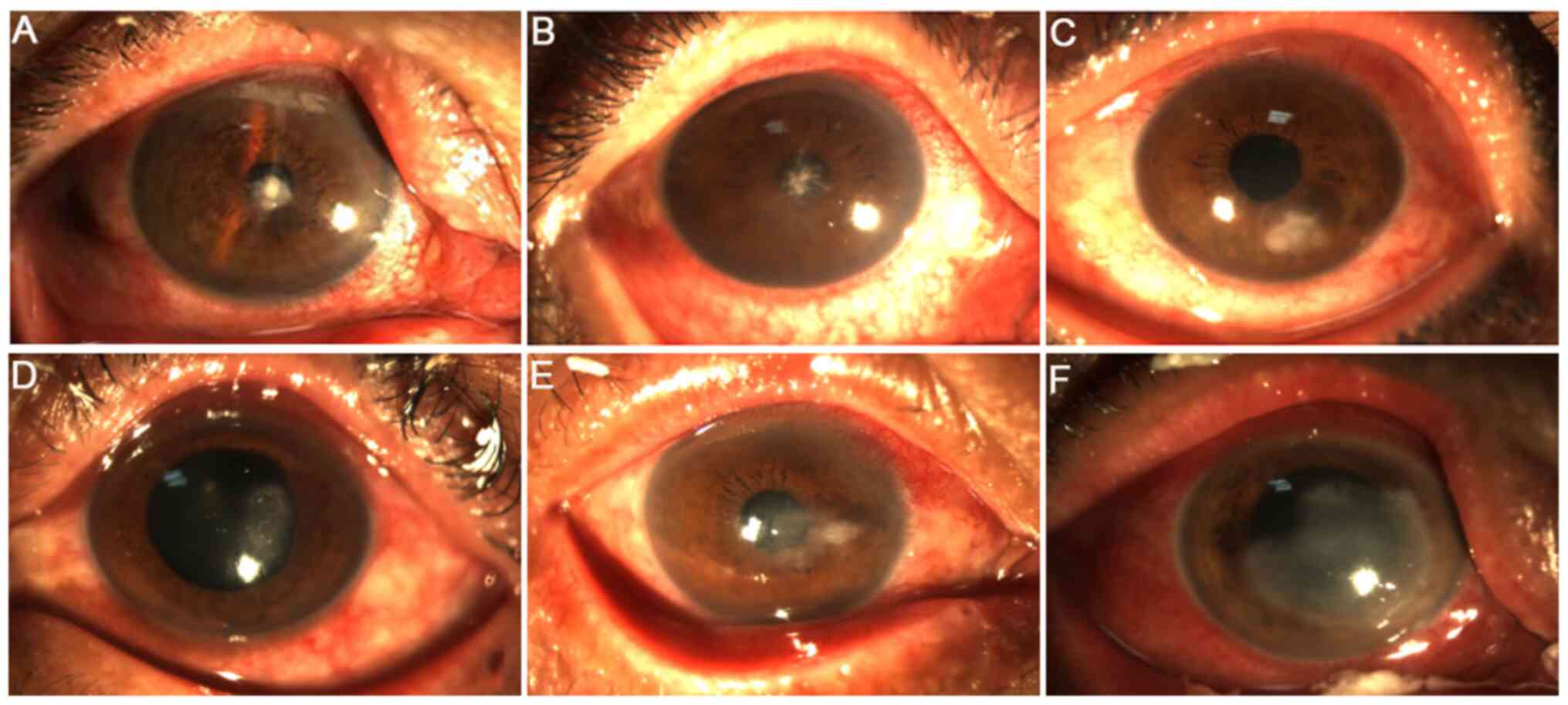
were also present (Fig. 4A). At 3,
5 and 14 days after surgery, the ulcer surface became more diffused
with poorly defined borders, where the pseudopods and satellite
foci disappeared (Fig. 4B-D).
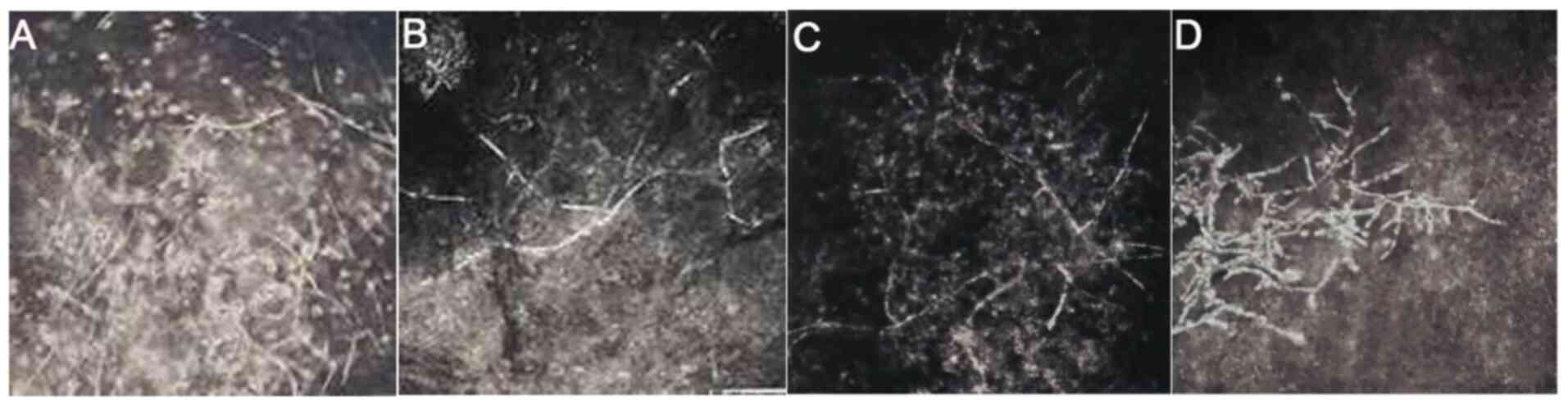
Furthermore, a large number of fungal mycelia, defects in the
epithelial tissue, irregular cell morphology and inflammatory cell
infiltration was observed in the shallow stromal layer before
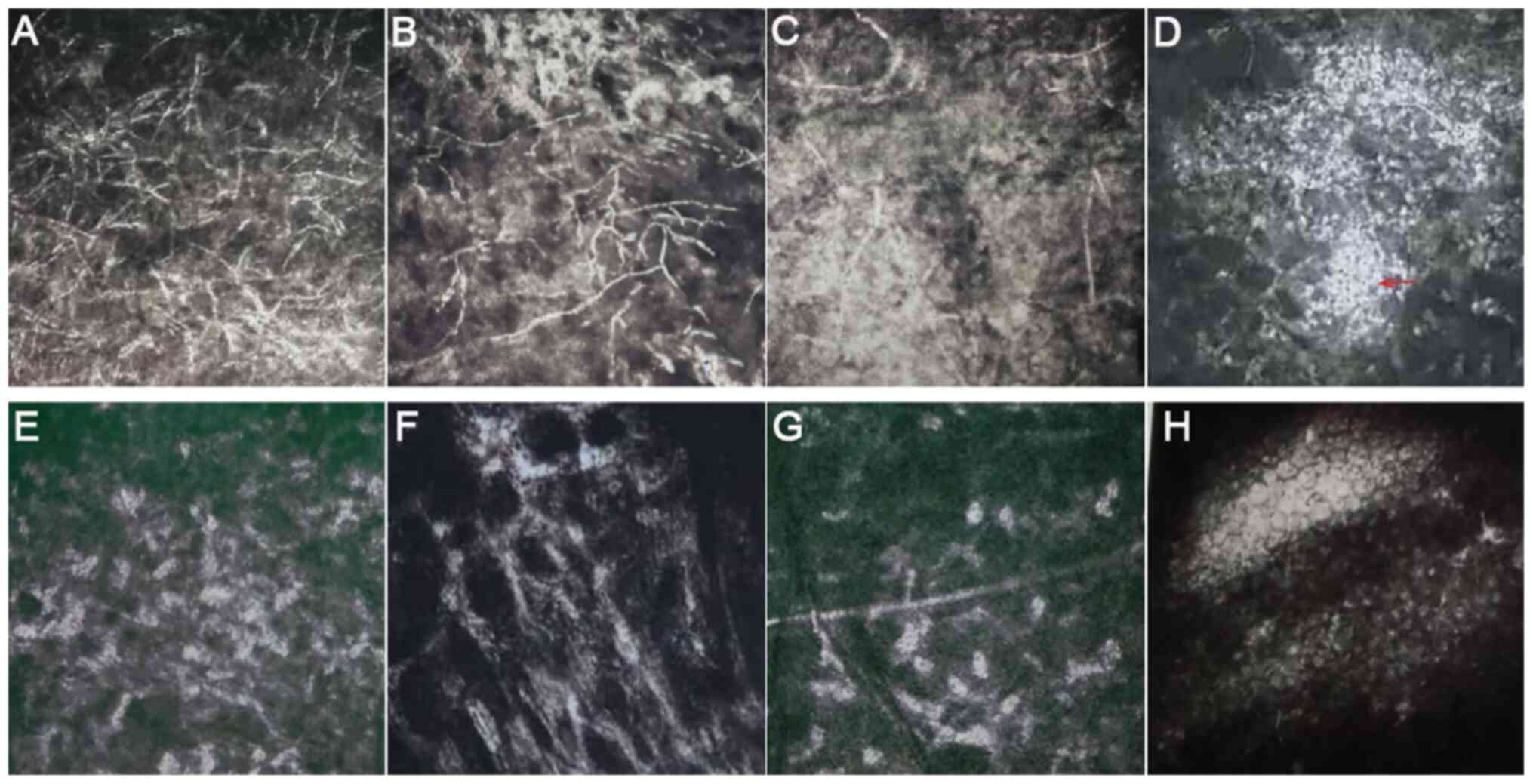
operation using IVCM (Fig. 5A-D).
At 4 weeks following surgery, the mycelium-like structures in the
shallow stromal layer disappeared and 'honeycomb'-like structures
were visible (Fig. 5E). However,
the number of honeycomb structures in the deep stromal layer was
decreased compared with that of 4 weeks after surgery (Fig. 5G). Additionally, the number of
inflammatory cells was reduced, where some scar tissues were formed
(Fig. 5F) and endothelial cells
were regular with normal morphology and arrangement (Fig. 5H).
 | Table IVComparison of localized lesion at the
indicated time-points post-operation. |
Table IV
Comparison of localized lesion at the
indicated time-points post-operation.
| Time | CXL (n=10) | Stromal injection
(n=21) | P-value |
|---|
| 1 week, n (%) | 9 (90.0) | 16 (76.2) | 0.63 |
| 2 weeks, n (%) | 9 (90.0) | 13 (61.9) | 0.21 |
| 4 weeks, n (%) | 9 (90.0) | 9 (42.9) | 0.02 |
| 12 weeks, n
(%) | 9 (90.0) | 9 (42.9) | 0.02 |
| 24 weeks, n
(%) | 9 (90.0) | 9 (42.9) | 0.02 |
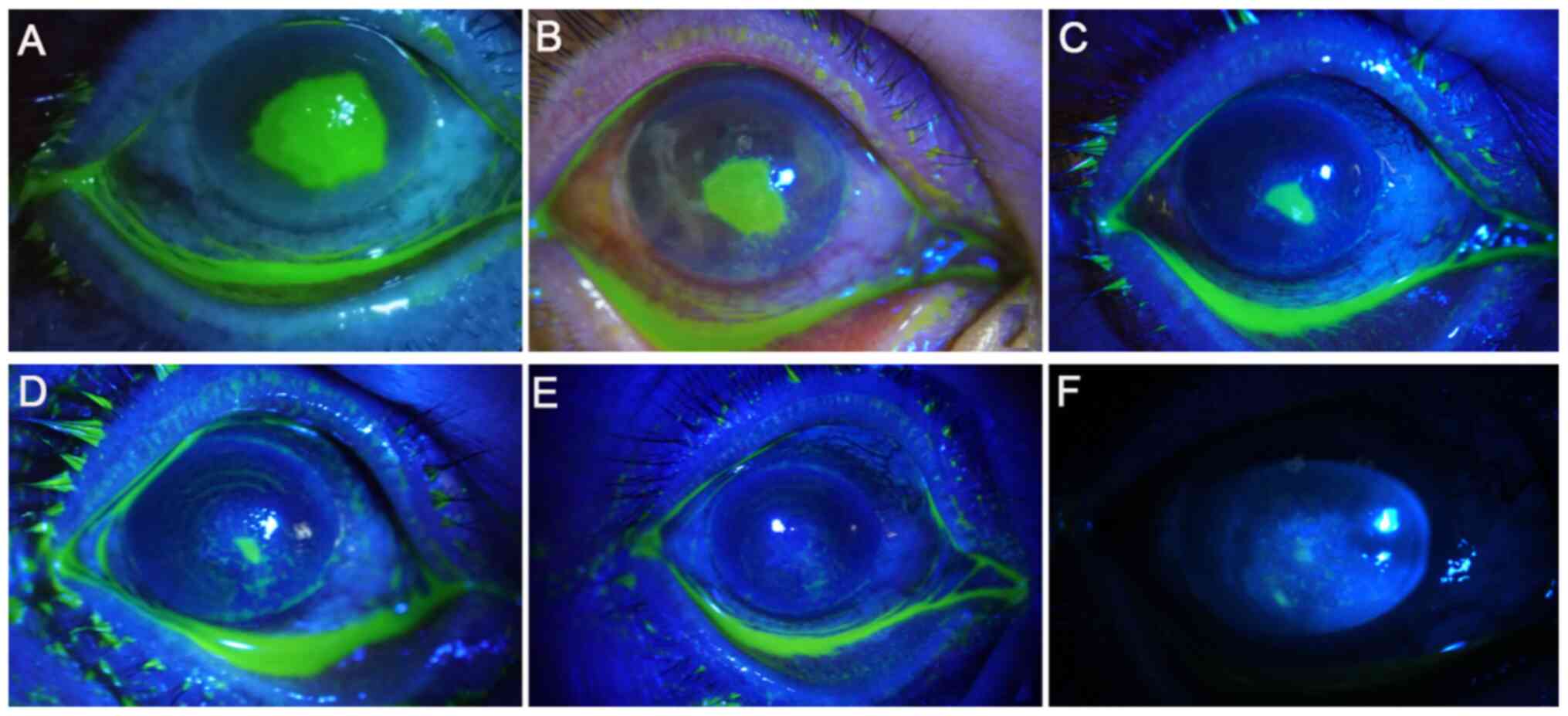
Ulcer healing after surgery
The ulcer healing rate 1 week after surgery in the
CXL and stromal injection groups was 60.0 (6/10 eyes) and 23.8%
(5/21 eyes), respectively. However, no statistically significant
difference between the two groups were observed (P=0.11; Table V). The degree of ulcer healing
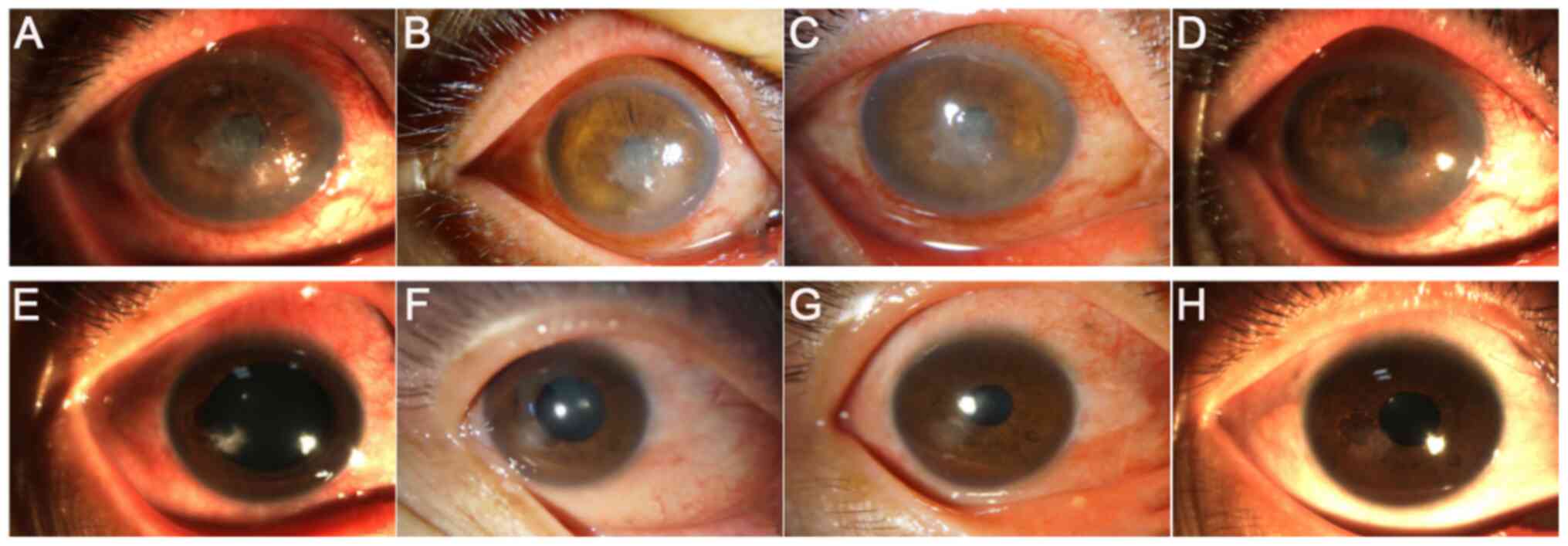
following treatment with UV A-riboflavin CXL is shown in Fig. 6. Prior to surgery, the cornea was
extensively stained with sodium fluorescein, whilst at 7 days
post-surgery, no staining was observed, suggesting that the corneal
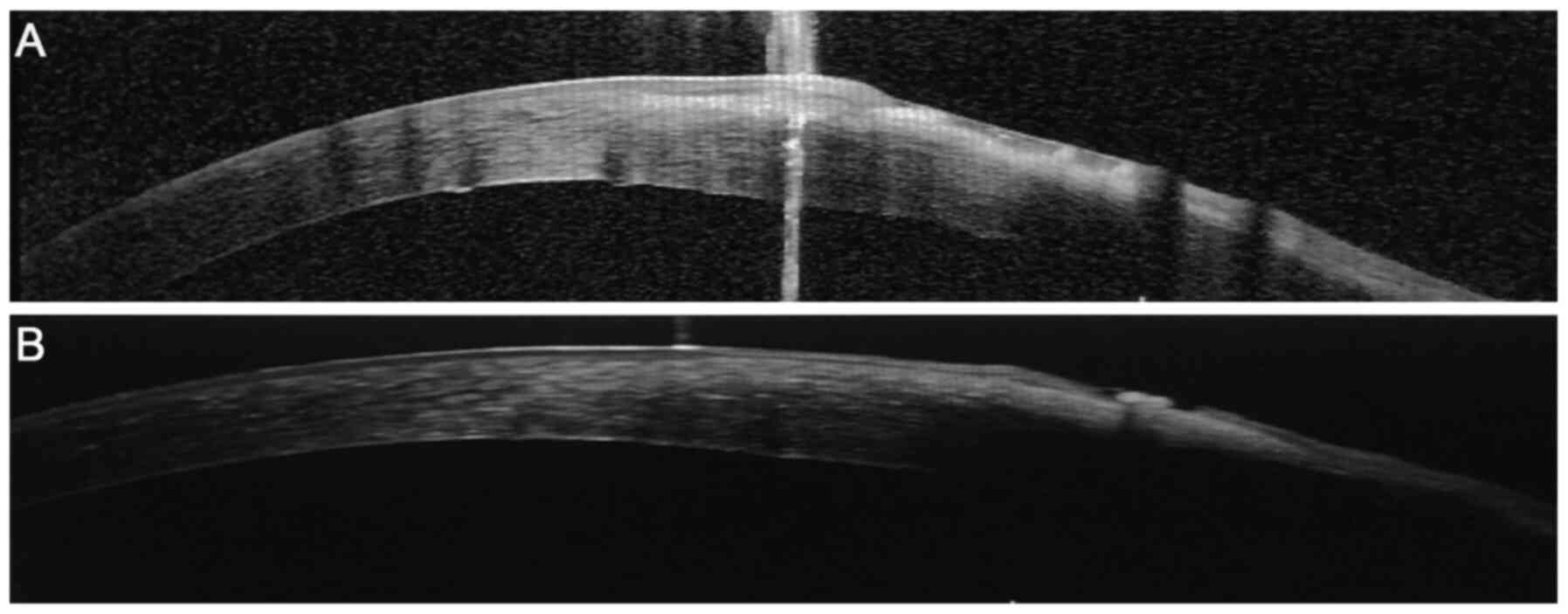
ulcer was cured. Furthermore, the images of AS-OCT revealed that
patients in the CXL group were completely healed 3 months after
surgery (Fig. 7). Most patients
with fungal keratitis in China are farmers (1). Due to economic conditions, no AS-OCT
images were available for the stromal injection group. The
association between corneal lesion diameter and healing process is
shown in Fig. 8. The corneal
epithelium was healed on weeks 4 and 1 after surgery in patients,
and the lesion diameter was ≥5 and <5 mm, respectively. This
indicated that the healing time of lesions with a diameter <5 mm
was decreased compared with that of lesions with a diameter ≥5
mm.
 | Table VComparison of ulcer healing rates 1
week after surgery. |
Table V
Comparison of ulcer healing rates 1
week after surgery.
| Status | CXL (n=10) | Stromal injection
(n=21) | P-value |
|---|
| Healing, n (%) | 6 (60.0) | 5 (23.8) | |
| Not healing, n
(%) | 4 (40.0) | 16 (76.2) | |
| Total, n (%) | 10 (100.0) | 21 (100.0) | 0.11 |
Visual prognosis
As shown in Table
VI, BCVA was not significantly different between the CXL and
stromal injection group (F=1.58; P=0.22), and significant time
effect was found (F=21.99; P<0.0001). There was also significant
interaction between the time and surgery method (F=5.46; P=0.03).
Additionally, the results showed that BCVA was significantly
decreased after surgery in the CXL group compared with that before
surgery but not in the stromal injection group. BCVA was not
significantly different between the CXL and stromal injection group
at 6 months postoperatively (P=0.08; Table VI). Furthermore, the percentage of
patients LogMAR <0.05 in the CXL group was 80%, but in the
stromal injection group was 42.85%, as demonstrated by the BCVA
interval distribution at 6 months after surgery. However, there was
no obvious difference on visual acuity at postoperative 6 months
between the two groups (P=0.22; Table
VII).
 | Table VIComparison of BCVA at 6 months
postoperativelya-c. |
Table VI
Comparison of BCVA at 6 months
postoperativelya-c.
| Time | CXL (n=10) | Stromal injection
(n=21) |
|---|
| Pre-operative
BCVA | 1.28±1.08 | 1.62±1.33 |
| Post-operative
BCVA |
0.56±0.90d |
1.40±1.35e |
 | Table VIIInterval distribution of BCVA at
post-operation in the two groups. |
Table VII
Interval distribution of BCVA at
post-operation in the two groups.
| BCVA category | CXL (n=10) | Stromal injection
(n=21) | P-value |
|---|
| LogMAR ≤0.2 | 5 (50.00) | 7 (33.33) | |
| 0.2< logMAR
≤0.5 | 3 (30.00) | 2 (9.52) | |
| 0.5< logMAR
≤1.0 | 0 (0.00) | 2 (9.53) | |
| LogMAR >1.0 | 2 (20.00) | 10 (47.62) | 0.22 |
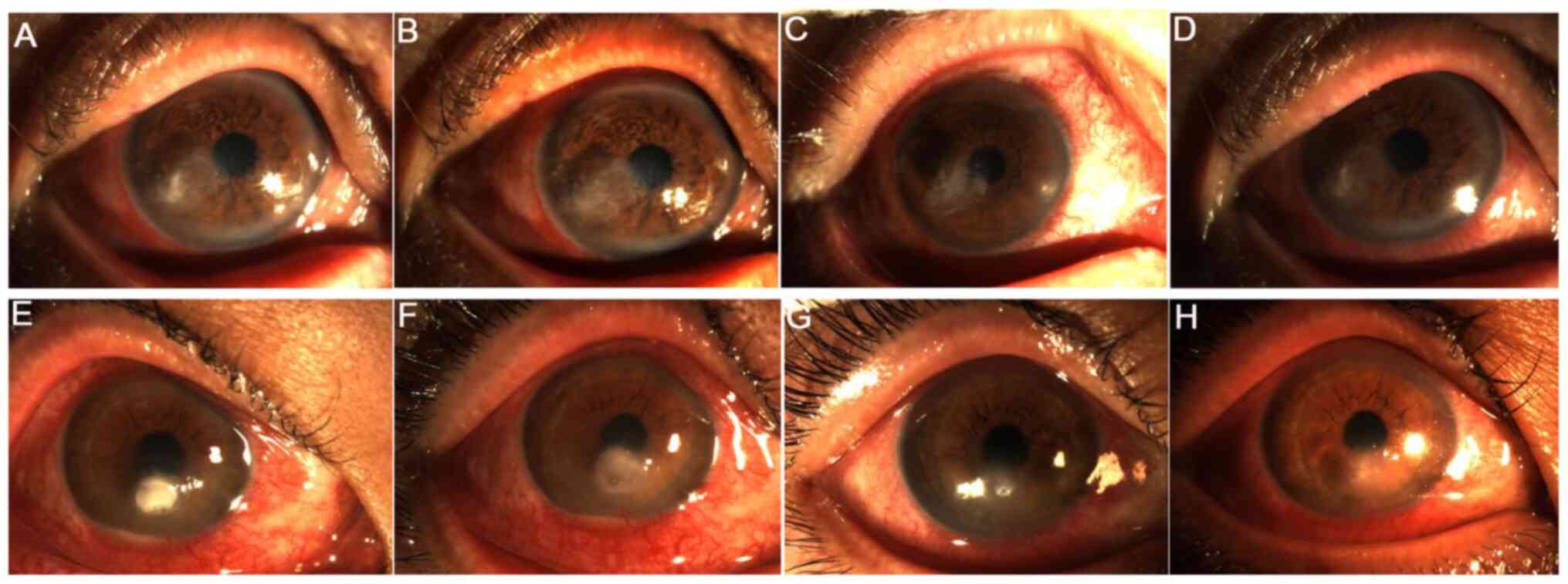
Postoperative complications and
management
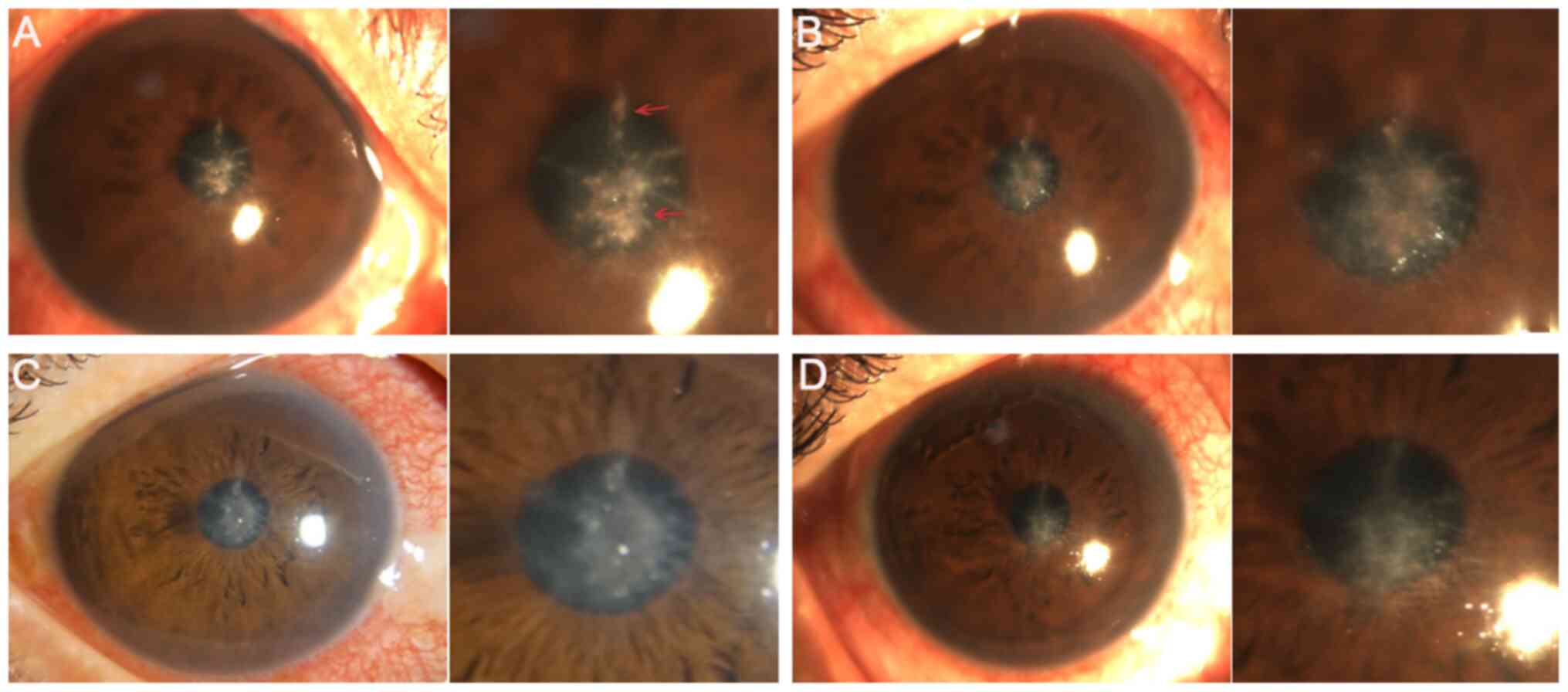
In the CXL group, loss of corneal transparency
occurred in one case 4 weeks after surgery (Fig. 9A), whilst one case of corneal
thinning was observed in the stromal injection group 3 days
postoperatively (Fig. 9B). During
follow-up, the patient in the stromal injection group finally
underwent corneal transplantation due to poor infection control.
However, the infection in the CXL group was controlled when the
visual acuity recovered at postoperative 24 weeks. Nevertheless, no
other complications were recorded.
Microbiological examination
Prior to surgery, fungal culture results showed that
7 (70.0%) and 16 cases (76.2%) were positive for fungal infection
in the CXL and stromal injection groups, respectively.
Specifically, using strain identification tests, 11 cases of
Fusarium, 6 cases of Alternaria alternata, 3 cases of
Aspergillus, 1 case of Candida albicans, 1 case of
Absidia orchidis and 1 case of Penicillium were
identified. However, there was no significant difference in species
distribution between the two groups (P>0.99; Table VIII).
 | Table VIIIComparison of species distribution
between the two treatment groups. |
Table VIII
Comparison of species distribution
between the two treatment groups.
| Species | CXL (n=10) | Stromal group
(n=21) | P-value |
|---|
| Fusarium, n
(%) | 4 (40.0) | 7 (33.3) | |
| Alternaria
alternata, n (%) | 2 (20.0) | 4 (19.1) | |
| Aspergillus,
n (%) | 1 (10.0) | 2 (9.5) | |
| Candida
albicans, n (%) | 0 (0.0) | 1 (4.8) | |
| Other, n (%) | 0 (0.0) | 2 (9.5) | |
| Total, n (%) | 7 (70.0) | 16 (76.2) | >0.99 |
Discussion
CXL is fast becoming a method of choice for treating
ocular surface diseases, including keratoplasty, fungal and
bacterial corneal ulcers (10,13,23).
CXL controls infection by enhancing the biomechanical strength of
the cornea and increasing collagen resistance to enzymatic
hydrolysis (24,25). Although the effectiveness of CXL
application on fungal corneal ulcers have been previously reported
(26), its efficacy on infection
control and ulcer healing requires further investigation in a
larger population. In the present study, the CXL procedure was
improved, following 4 min irradiation at 30 mW/cm2 and a
total dose of 7.2 J/cm2, its efficacy was evaluated. The
findings suggested that this type of modified CXL conferred
superior outcome compared with that by voriconazole in terms of the
infection control rate and localized lesions. Furthermore, the
cured patients in the CXL group overall exhibited reduced risks for
further medication and surgery.
FK exists in two forms, the first of which is
filamentous fungal keratitis that is most commonly caused by
Fusarium and Aspergillus; the second form is yeast
keratitis, which results from Candida albicans infection
(27). In the present study,
microbiological examination demonstrated that Fusarium,
Alternaria alternata and Aspergillus, belonging to
filamentary fungi, accounted for the majority of fungal
distribution. A previous study has revealed that Fusarium
and Aspergillus are the most commonly isolated pathogens for
FK and account for >80% cases in Northern China (4). These findings were consistent with the
results of the present study, indicating that filamentous FK was a
major cause of severe corneal ulcers in China. At present, an
increasing number of studies have been focused on the development
of effective treatments for fungal corneal ulcer. Hariprasad et
al (28) demonstrated that
voriconazole could effectively use to treat fungal eye infections.
In addition, a number of studies have previously shown that
intrastromal voriconazole injection is effective against
filamentous fungi infections (29-31).
However, other studies have reached the opposite conclusion,
suggesting that voriconazole is not sufficient for treating
filamentous fungi. Narayana et al (32) indicated that voriconazole was not
effective in moderating severe filamentous fungal ulcers. Another
similar study in Japan demonstrated in three cases of keratitis
caused by Fusarium and Aspergillus that were not
completely cured after treatment with voriconazole. Briefly, in
corneal infections, fungi are normally observed in the deep stroma,
but since voriconazole could not reach Descemet's membrane,
infection recurrence occurred in one case. Another patient was
treated with topical steroids in the early postoperative stage
(33). Regarding topical steroids,
Lin et al (34) found a case
of a patient who was treated with topical steroids in the early
stage and who relapsed after voriconazole treatment, suggesting
that treatment ineffectiveness is associated with the use of
topical steroids. Furthermore, Cheng et al (35) suggested that failure of voriconazole
in the treatment for filamentous fungal keratitis may be associated
with the rapid reproduction and mutations of Fusarium
induced by the administration of glucocorticoids during the early
postoperative period. A similar study revealed that treatment with
polyene combined with azole not only reduces the effectiveness of
azole against filamentous fungal keratitis, but also enhances drug
toxicity (36). In addition, a
previous study suggested that not reaching the minimum inhibitory
concentration (MIC) for a specific strain is another cause for
voriconazole inefficiency against filamentous fungi (37). Specifically, the genus
Fusarium consists of >100 species, where ~15 of them may
cause corneal infections in humans (38) and exhibit different MIC (29,39),
indicating that different Fusarium species is one of the
reasons leading to the different conclusions In the present study,
77 eyes (33.3%) were temporarily relieved 1 week after surgery, but
the infection recurred and the wound was not healed after 2-4 weeks
in the stromal injection group. Additionally, two of the four
patients who failed to respond to treatment had a history of
surgery and medication. The aforementioned findings suggested that
voriconazole could be effective for treating filamentous fungal
corneal ulcer. However, its administration should be carefully
considered for patients with history of surgery and medication and
moderate to severe infection.
UV A-riboflavin CXL has been widely used to treat a
variety of fungal infections, including Fusarium,
Aspergillus and Candida albicans (40). A previous study demonstrated that
treatment with 0.1 and 0.25% riboflavin combined with UV could
effectively treat Alternaria alternata infection (41). Another study reported that ulcer
healing was achieved in all patients following treatment with
traditional CXL, where ~80% presented significantly improved visual
acuity (42). In the present study,
the modified CXL procedure with irradiation for 4 min at 30
mW/cm2 and a total dose of 7.2 J/cm2 was used
to treat fungal infections. This modified CXL procedure was found
to be effective in controlling infection and improving visual
acuity. According to the principle that the biological efficacy is
associated with total energy dose, Chan et al (17) demonstrated that treatment with
increased irradiation of 7-45 mW/cm2, shortened UV
exposure time (from 30 to 5 min) and a total dose of 7.2
J/cm2 was reasonable and safe. A similar study
previously revealed that higher fluences of UV-light substantially
increased the anti-bacterial efficacy of CXL combined with
photoactivated chromophore (43).
Therefore, it was hypothesized that the CXL procedure with
irradiation for 4 min at 30 mW/cm2 and a total dose of
7.2 J/cm2 for treating patients with fungal corneal
ulcer was safe and effective.
Fungal infection is characterized by a large number
of hyphae or spores in the focal lesions (44). In the present study, fungal mycelia,
defects in the epithelial tissue, irregular cell morphology and
inflammatory cell infiltration were observed prior to surgery.
However, following CXL therapy, the number of fungal mycelia and
inflammatory cell infiltration were decreased and endothelial cells
acquired their typical morphology. These findings were consistent
with previous studies. Wei et al (9) reported that the symptoms were rapidly
relieved in the majority of rabbits with bacterial corneal ulcer,
where the area of inflammatory cell infiltration was decreased
after treatment with CXL combined with riboflavin and 440 nm blue
light. Additionally, Bamdad et al (23) demonstrated that the defects in
endothelial cells were smaller postoperationally compared with
those before surgery. Another study also previously showed that
re-epithelization occurred in 56% of patients within 1 week in
terms of healing (45), which was
consistent with results from the present study, where the rate of
ulcer healing 1 week after surgery in the CXL group was 60.0%. The
aforementioned findings suggested that treatment with modified CXL
accelerated corneal repair and promoted ulcer healing to a certain
extent.
Previous studies have suggested that the diameter of
corneal lesion is closely associated with the healing process. For
example, a prospective study found that the healing time was
increased with increasing ulcer and infiltration area (46). Furthermore, Li et al
(31) demonstrated that the
majority of patients with healed cornea at 1 week postoperatively
exhibited corneal lesion diameter <5.0 mm; however the healing
time was >1 week in patients with corneal lesion diameter
>5.0 mm. In the present study, the healing time of the
epithelium in the subgroup with lesion coverage <5.0 mm was
shorter compared with that in the >5.0 mm subgroup, indicating
that corneal lesion diameter was closely associated with the
healing process. Furthermore, You et al (47) indicated that lesions with maximum
diameters of <5 mm could reduce the difficulty of surgery and
instability of the cornea. These results suggested that the
treatment efficacy of this modified CXL surgery in lesions with
coverage ≤5.0 mm could be superior to that in the ≥5.0 mm
subgroup.
In the present study, corneal transparency was
decreased in one case in the CXL group. Generally, apoptosis occurs
to a depth of <300 µm of the corneal stroma 24 h after surgery,
resulting in the loss of corneal transparency (48,49).
In the present study, corneal thinning was observed in one case in
the stromal injection group. A study also previously showed that
cell apoptosis was observed following the surgical removal of the
corneal ulcer lesion, leading to the decreased number of corneal
cells, which in turn resulted in corneal thinning (50). However, no other complications were
observed in the present study. The aforementioned data suggested
that the modified CXL procedure with irradiation for 4 min at 30
mW/cm2 and a total energy dose of 7.2 J/cm2
was safe and effective for treating fungal corneal ulcers.
Nevertheless, this procedure requires to further optimization to
relief pain and related complications.
Limitations of the present study included the small
sample size. Therefore, a large randomized controlled study should
be performed in the future to confirm the aforementioned findings
and to explore the risk factors affecting the treatment efficacy
and healing rate, including the fungal species and lesion
coverage.
In conclusion, modified CXL treatment could result
in favorable effects in patients with fungal corneal ulcer
regarding the control of infection, localized lesions and
accelerated epithelialization. In addition to the reduced risk for
complications, including loss of corneal transparency, modified CXL
treatment could also minimize the need for surgery.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YC, MG and LS conceived the study. XM and YC
collected materials and samples. YC, XM and LS contributed to data
analysis and interpretation of the results. MG and YC provided
administrative support. YC and LS confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of General Hospital of Northern Theater Command (approval
no. 201736). Written informed consent was obtained from each
patient. All procedures were performed in accordance with the
Declaration of Helsinki developed by the World Medical
Association.
Patient consent for publication
All patients provided written informed consent for
the publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang L, Sun S, Jing Y, Han L, Zhang H and
Yue J: Spectrum of fungal keratitis in central China. Clin Exp
Ophthalmol. 37:763–771. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wu J, Zhang WS, Zhao J and Zhou HY: Review
of clinical and basic approaches of fungal keratitis. Int J
Ophthalmol. 9:1676–1683. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fini ME, Cook JR and Mohan R: Proteolytic
mechanisms in corneal ulceration and repair. Arch Dermatol Res. 290
(Suppl 1):S12–S23. 1998.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Xie L, Zhong W, Shi W and Sun S: Spectrum
of fungal keratitis in north China. Ophthalmology. 113:1943–1948.
2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chen Y, Yang W, Gao M, Belin MW, Yu H and
Yu J: Experimental study on cryotherapy for fungal corneal ulcer.
BMC Ophthalmol. 15(29)2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Garg P, Roy A and Roy S: Update on fungal
keratitis. Curr Opin Ophthalmol. 27:333–339. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Spoerl E, Mrochen M, Sliney D, Trokel S
and Seiler T: Safety of UVA-riboflavin cross-linking of the cornea.
Cornea. 26:385–389. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang Y, Conrad AH and Conrad GW: Effects
of ultraviolet-A and riboflavin on the interaction of collagen and
proteoglycans during corneal cross-linking. J Biol Chem.
286:13011–13022. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wei S, Zhang C, Zhang S, Xu Y and Mu G:
Treatment results of corneal collagen cross-linking combined with
riboflavin and 440 Nm blue light for bacterial corneal ulcer in
rabbits. Curr Eye Res. 42:1401–1406. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tal K, Gal-Or O, Pillar S, Zahavi A, Rock
O and Bahar I: Efficacy of primary collagen cross-linking with
photoactivated chromophore (PACK-CXL) for the treatment of
staphylococcus aureus-induced corneal ulcers. Cornea. 34:1281–1286.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Raiskup F and Spoerl E: Corneal
crosslinking with riboflavin and ultraviolet A. I. Principles. Ocul
Surf. 11:65–74. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ghanem VC, Ghanem RC and de Oliveira R:
Postoperative pain after corneal collagen cross-linking. Cornea.
32:20–24. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Taneri S, Oehler S, Lytle G and Dick HB:
Evaluation of epithelial integrity with various transepithelial
corneal cross-linking protocols for treatment of keratoconus. J
Ophthalmol. 2014(614380)2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lombardo M, Giannini D, Lombardo G and
Serrao S: Randomized controlled trial comparing transepithelial
corneal cross-linking using iontophoresis with the dresden protocol
in progressive keratoconus. Ophthalmology. 124:804–812.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kim M, Takaoka A, Hoang QV, Trokel SL and
Paik DC: Pharmacologic alternatives to riboflavin photochemical
corneal cross-linking: A comparison study of cell toxicity
thresholds. Invest Ophthalmol Vis Sci. 55:3247–3257.
2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gatzioufas Z, Richoz O, Brugnoli E and
Hafezi F: Safety profile of high-fluence corneal collagen
cross-linking for progressive keratoconus: Preliminary results from
a prospective cohort study. J Refract Surg. 29:846–848.
2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chan TC, Chow VW, Jhanji V and Wong VW:
Different topographic response between mild to moderate and
advanced keratoconus after accelerated collagen cross-linking.
Cornea. 34:922–927. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Özdemir HB, Kalkancı A, Bilgihan K, Göçün
PU, Öğüt B, Karakurt F and Erdoğan M: Comparison of corneal
collagen cross-linking (PACK-CXL) and voriconazole treatments in
experimental fungal keratitis. Acta Ophthalmol. 97:e91–e96.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jorgensen JH, Pfaller MA, Carroll KC,
Funke G, Landry ML, Richter SR and Warnock DW (eds): Manual of
Clinical Microbiology. 11th edition. American Society for
Microbiology, p2892, 2015.
|
|
20
|
Mutoh T, Ishikawa I, Matsumoto Y and
Chikuda M: A retrospective study of nine cases of Acanthamoeba
keratitis. Clin Ophthalmol. 4:1189–1192. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Karti O, Zengin MO, Cinar E, Tutuncu M,
Karahan E, Celik A and Kucukerdonmez C: Effect of 1- and
6-hour-delayed corneal collagen cross-linking on corneal healing in
a rabbit alkali-burn model: Clinical and histological observations.
Cornea. 35:1644–1649. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sharma S: Diagnosis of infectious diseases
of the eye. Eye (Lond). 26:177–184. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bamdad S, Malekhosseini H and Khosravi A:
Ultraviolet A/riboflavin collagen cross-linking for treatment of
moderate bacterial corneal ulcers. Cornea. 34:402–406.
2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kohlhaas M, Spoerl E, Schilde T, Unger G,
Wittig C and Pillunat LE: Biomechanical evidence of the
distribution of cross-links in corneas treated with riboflavin and
ultraviolet A light. J Cataract Refract Surg. 32:279–283.
2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Spoerl E, Wollensak G and Seiler T:
Increased resistance of crosslinked cornea against enzymatic
digestion. Curr Eye Res. 29:35–40. 2004.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kasparova EA, Sobkova OI and Yang B:
Corneal collagen cross-linking in the treatment of infectious
keratitis and corneal ulcers. Vestn Oftalmol. 133:113–119.
2017.PubMed/NCBI View Article : Google Scholar : (In Russian).
|
|
27
|
Lakhundi S, Siddiqui R and Khan NA:
Pathogenesis of microbial keratitis. Microb Pathog. 104:97–109.
2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hariprasad SM, Mieler WF, Lin TK, Sponsel
WE and Graybill JR: Voriconazole in the treatment of fungal eye
infections: A review of current literature. Br J Ophthalmol.
92:871–878. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Siatiri H, Daneshgar F, Siatiri N and
Khodabande A: The effects of intrastromal voriconazole injection
and topical voriconazole in the treatment of recalcitrant Fusarium
keratitis. Cornea. 30:872–875. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Prakash G, Sharma N, Goel M, Titiyal JS
and Vajpayee RB: Evaluation of intrastromal injection of
voriconazole as a therapeutic adjunctive for the management of deep
recalcitrant fungal keratitis. Am J Ophthalmol. 146:56–59.
2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li SX, Biang J, Li X, Zhang LT and Shi WY:
Keratectomy combined with intrastromal injection of voriconazole in
treating fungal keratitis. Zhonghua Yan Ke Za Zhi. 53:682–688.
2017.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
32
|
Narayana S, Krishnan T, Ramakrishnan S,
Samantaray PP, Austin A, Pickel J, Porco T, Lietman T and
Rose-Nussbaumer J: Mycotic antimicrobial localized injection: A
randomized clinical trial evaluating intrastromal injection of
voriconazole. Ophthalmology. 126:1084–1089. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Niki M, Eguchi H, Hayashi Y, Miyamoto T,
Hotta F and Mitamura Y: Ineffectiveness of intrastromal
voriconazole for filamentous fungal keratitis. Clin Ophthalmol.
8:1075–1079. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lin HC, Chu PH, Kuo YH and Shen SC:
Clinical experience in managing Fusarium solani keratitis. Int J
Clin Pract. 59:549–554. 2005.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Cheng J, Zhai HL, Wang JY and Xie LX:
Clinical features and treatments of retrocorneal fungal infection.
Zhonghua Yan Ke Za Zhi. 53:758–765. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Schacter LP, Owellen RJ, Rathbun HK and
Buchanan B: Letter: Antagonism between miconazole and amphotericin
B. Lancet. 2(318)1976.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Shen YC, Wang MY, Wang CY, Tsai TC, Tsai
HY, Lee HN and Wei LC: Pharmacokinetics of intracameral
voriconazole injection. Antimicrob Agents Chemother. 53:2156–2157.
2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Alastruey-Izquierdo A, Cuenca-Estrella M,
Monzón A, Mellado E and Rodríguez-Tudela JL: Antifungal
susceptibility profile of clinical Fusarium spp. isolates
identified by molecular methods. J Antimicrob Chemother.
61:805–809. 2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Pearson MM, Rogers PD, Cleary JD and
Chapman SW: Voriconazole: A new triazole antifungal agent. Ann
Pharmacother. 37:420–432. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
40
|
Kashiwabuchi RT, Carvalho FR, Khan YA,
Hirai F, Campos MS and McDonnell PJ: Assessment of fungal viability
after long-wave ultraviolet light irradiation combined with
riboflavin administration. Graefes Arch Clin Exp Ophthalmol.
251:521–527. 2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Bilgihan K, Kalkanci A, Ozdemir HB, Yazar
R, Karakurt F, Yuksel E, Otag F, Karabicak N and Arikan-Akdagli S:
Evaluation of antifungal efficacy of 0.1% and 0.25% riboflavin with
UVA: A comparative in vitro study. Curr Eye Res. 41:1050–1056.
2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Li Z, Jhanji V, Tao X, Yu H, Chen W and Mu
G: Riboflavin/ultravoilet light-mediated crosslinking for fungal
keratitis. Br J Ophthalmol. 97:669–671. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kling S, Hufschmid FS, Torres-Netto EA,
Randleman JB, Willcox M, Zbinden R and Hafezi F: High fluence
increases the antibacterial efficacy of PACK cross-linking. Cornea.
39:1020–1026. 2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wu CS, Wu SS and Chen PC: A prospective
study of fungal infection of gastric ulcers: Clinical significance
and correlation with medical treatment. Gastrointest Endosc.
42:56–58. 1995.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Idrus EA, Utti EM and Mattila JS:
Photoactivated chromophore corneal cross-linking (PACK-CXL) for
treatment of severe keratitis. Acta Ophthalmol. 97:721–726.
2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Said DG, Elalfy MS, Gatzioufas Z,
El-Zakzouk ES, Hassan MA, Saif MY, Zaki AA, Dua HS and Hafezi F:
Collagen cross-linking with photoactivated riboflavin (PACK-CXL)
for the treatment of advanced infectious keratitis with corneal
melting. Ophthalmology. 121:1377–1382. 2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
You X, Li J, Li S and Shi W: Effects of
lamellar keratectomy and intrastromal injection of 0.2% fluconazole
on fungal keratitis. J Ophthalmol. 2015(656027)2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Wollensak G, Spoerl E, Wilsch M and Seiler
T: Keratocyte apoptosis after corneal collagen cross-linking using
riboflavin/UVA treatment. Cornea. 23:43–49. 2004.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Linna TU, Vesaluoma MH, Petroll WM,
Tarkkanen AH and Tervo TM: Confocal microscopy of a patient with
irregular astigmatism after LASIK reoperations and relaxation
incisions. Cornea. 19:163–169. 2000.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Sivak JM and Fini ME: MMPs in the eye:
Emerging roles for matrix metalloproteinases in ocular physiology.
Prog Retin Eye Res. 21:1–14. 2002.PubMed/NCBI View Article : Google Scholar
|