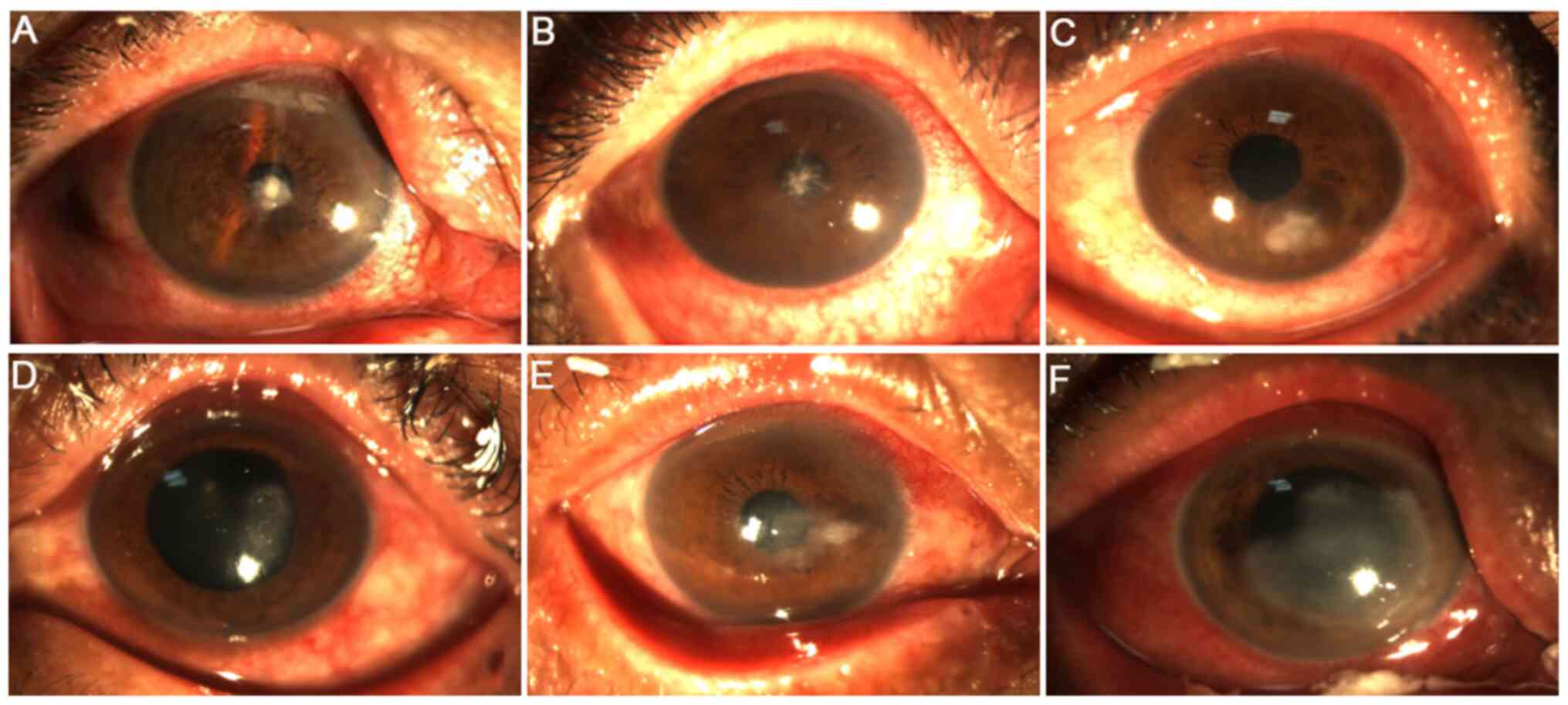
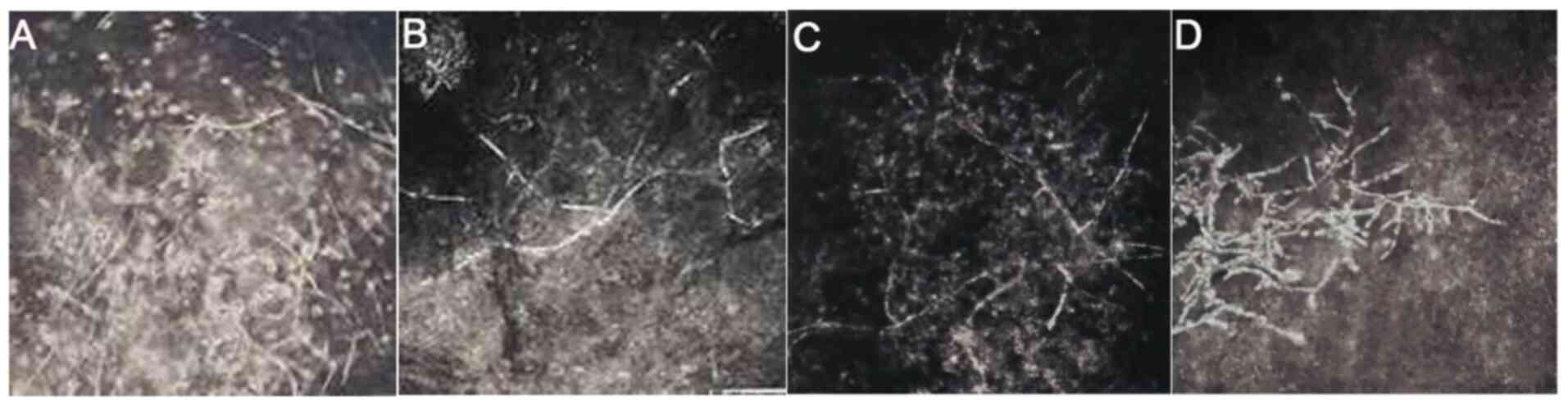
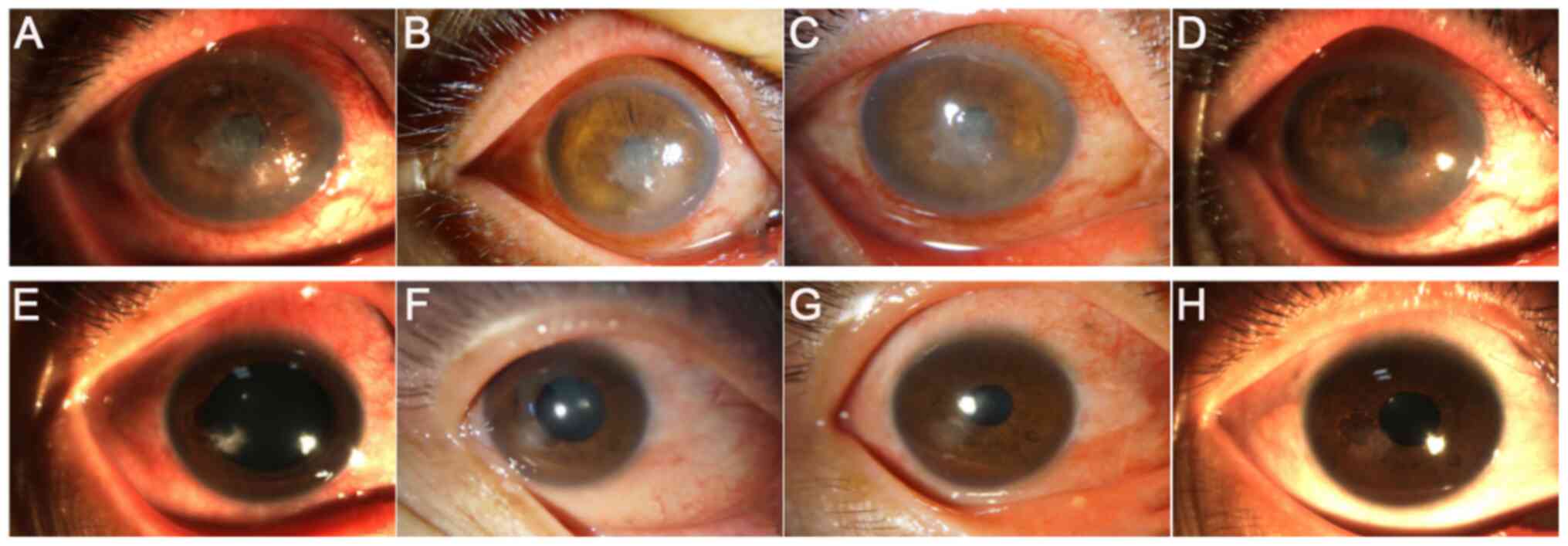
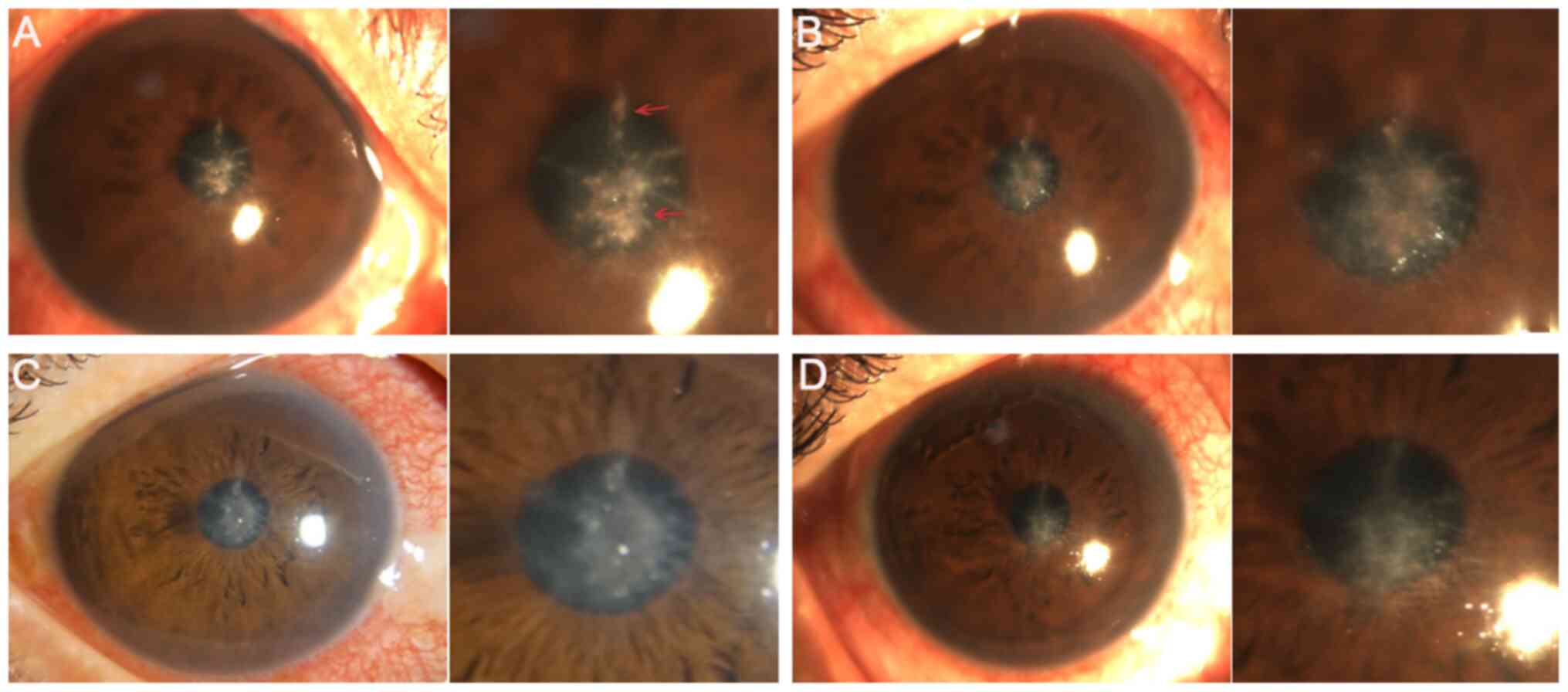
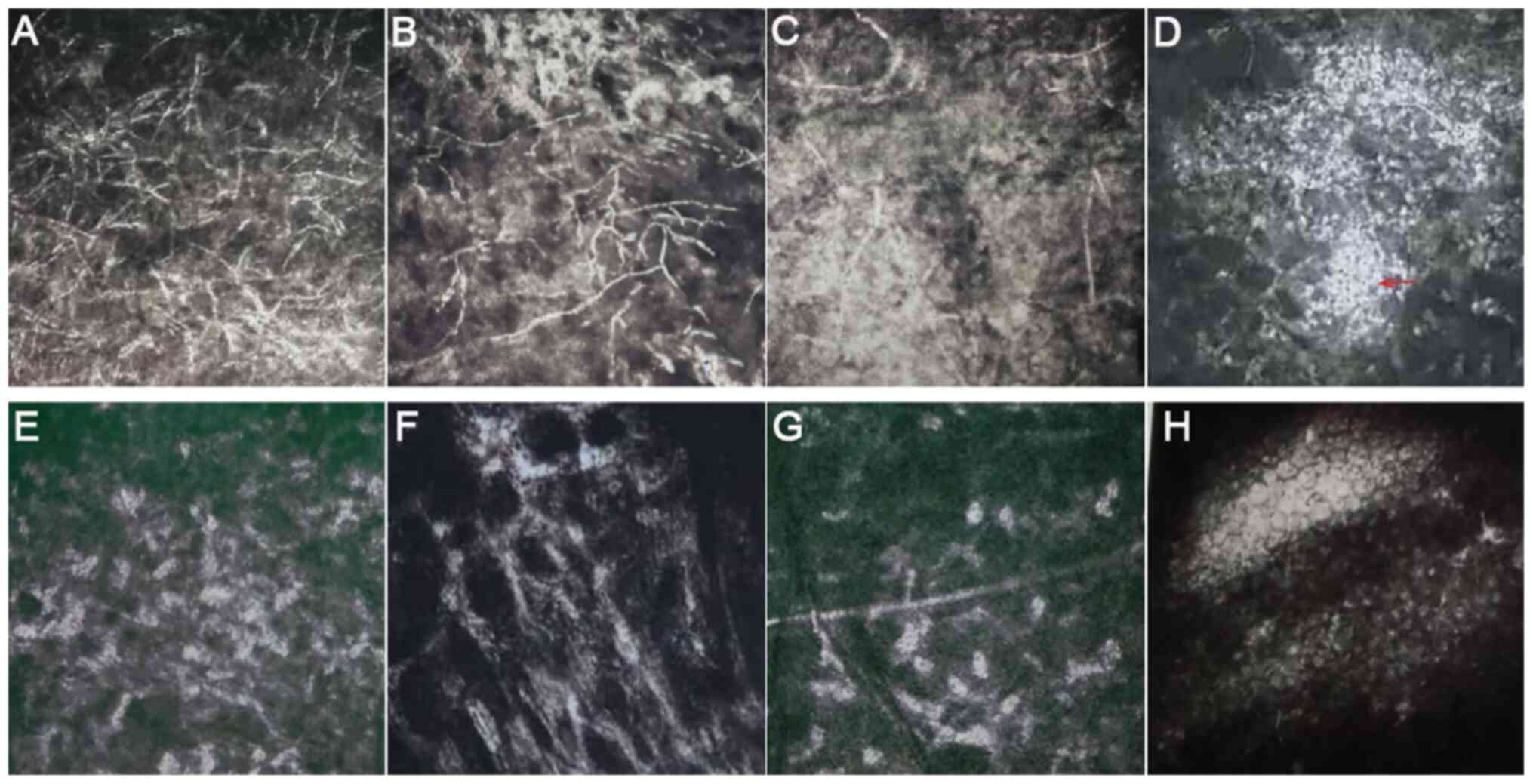
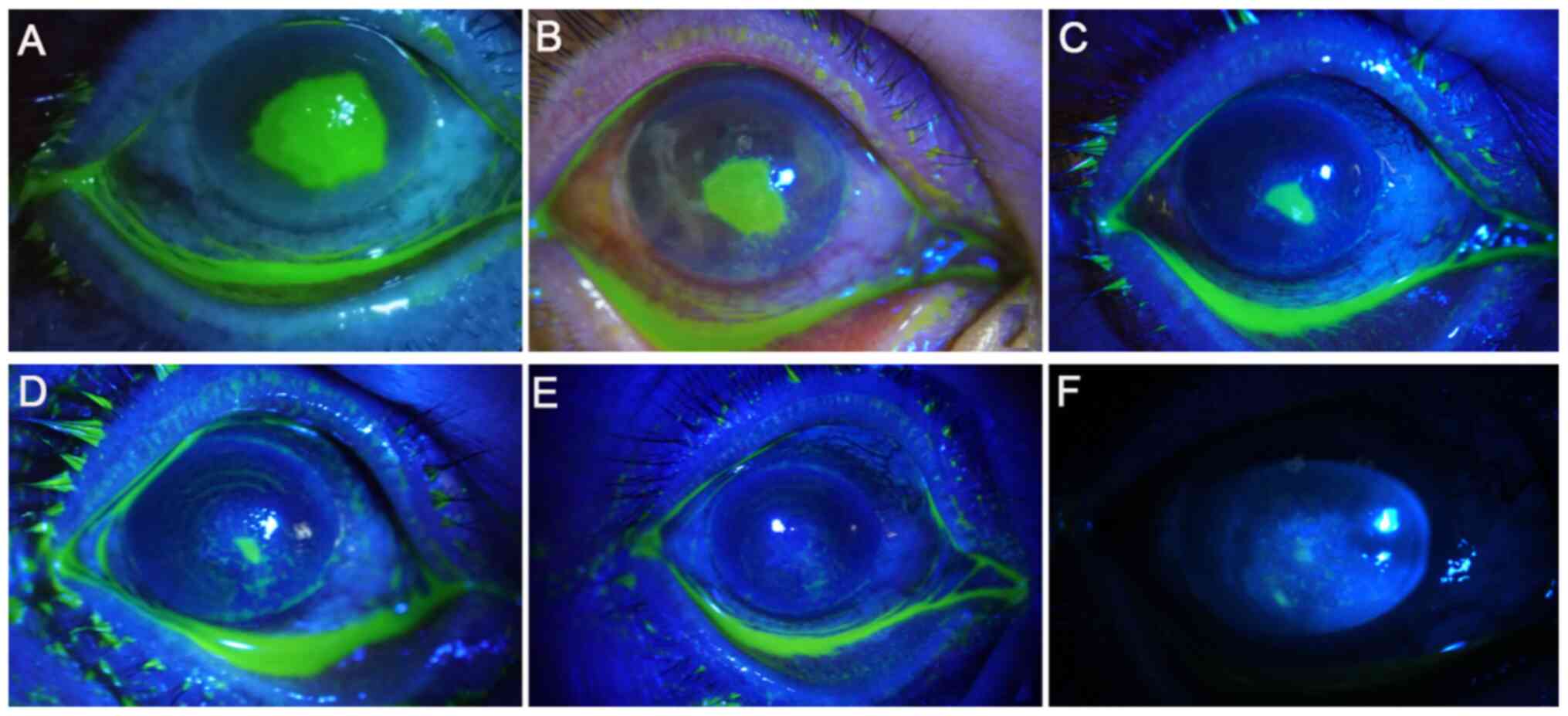
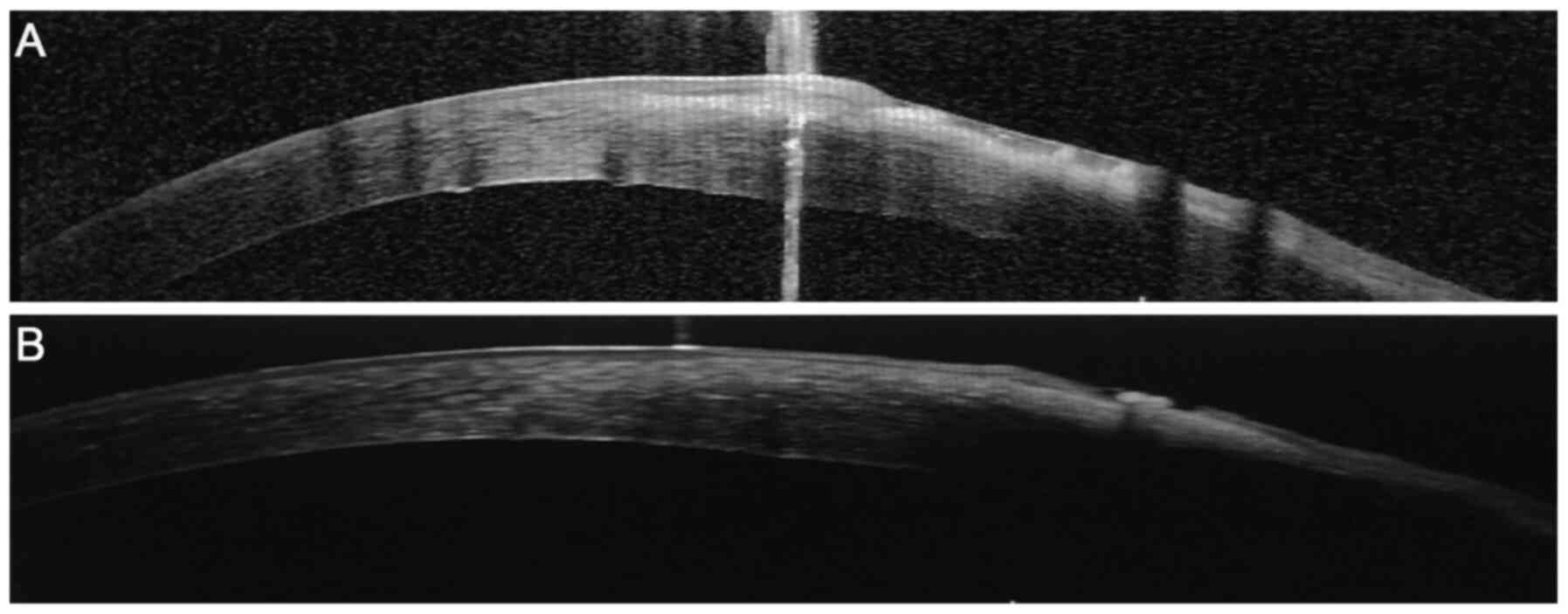
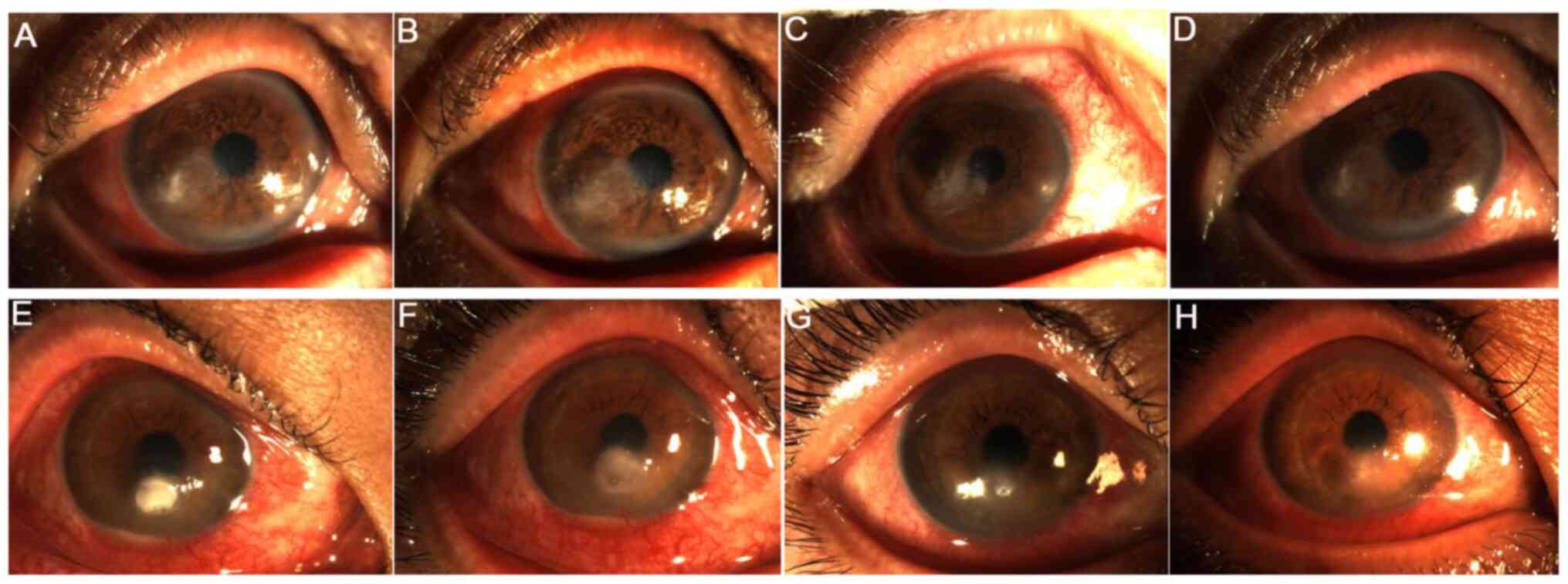
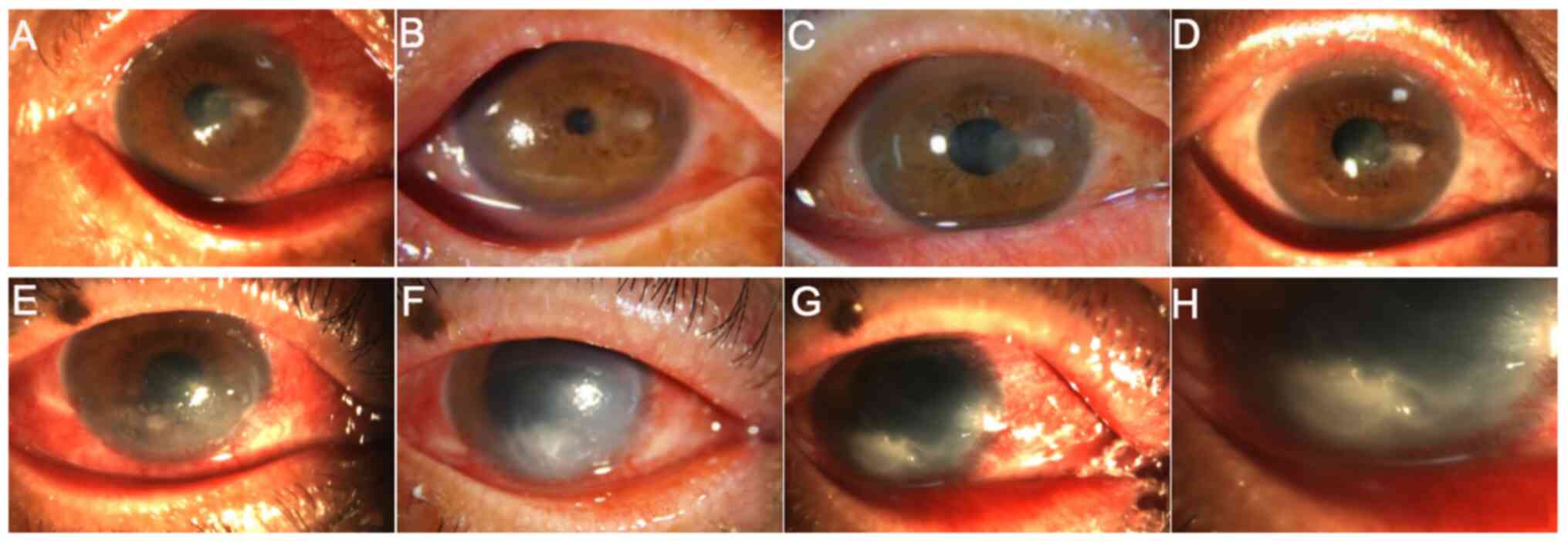
|
1
|
Wang L, Sun S, Jing Y, Han L, Zhang H and
Yue J: Spectrum of fungal keratitis in central China. Clin Exp
Ophthalmol. 37:763–771. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wu J, Zhang WS, Zhao J and Zhou HY: Review
of clinical and basic approaches of fungal keratitis. Int J
Ophthalmol. 9:1676–1683. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fini ME, Cook JR and Mohan R: Proteolytic
mechanisms in corneal ulceration and repair. Arch Dermatol Res. 290
(Suppl 1):S12–S23. 1998.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Xie L, Zhong W, Shi W and Sun S: Spectrum
of fungal keratitis in north China. Ophthalmology. 113:1943–1948.
2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chen Y, Yang W, Gao M, Belin MW, Yu H and
Yu J: Experimental study on cryotherapy for fungal corneal ulcer.
BMC Ophthalmol. 15(29)2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Garg P, Roy A and Roy S: Update on fungal
keratitis. Curr Opin Ophthalmol. 27:333–339. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Spoerl E, Mrochen M, Sliney D, Trokel S
and Seiler T: Safety of UVA-riboflavin cross-linking of the cornea.
Cornea. 26:385–389. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang Y, Conrad AH and Conrad GW: Effects
of ultraviolet-A and riboflavin on the interaction of collagen and
proteoglycans during corneal cross-linking. J Biol Chem.
286:13011–13022. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wei S, Zhang C, Zhang S, Xu Y and Mu G:
Treatment results of corneal collagen cross-linking combined with
riboflavin and 440 Nm blue light for bacterial corneal ulcer in
rabbits. Curr Eye Res. 42:1401–1406. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tal K, Gal-Or O, Pillar S, Zahavi A, Rock
O and Bahar I: Efficacy of primary collagen cross-linking with
photoactivated chromophore (PACK-CXL) for the treatment of
staphylococcus aureus-induced corneal ulcers. Cornea. 34:1281–1286.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Raiskup F and Spoerl E: Corneal
crosslinking with riboflavin and ultraviolet A. I. Principles. Ocul
Surf. 11:65–74. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ghanem VC, Ghanem RC and de Oliveira R:
Postoperative pain after corneal collagen cross-linking. Cornea.
32:20–24. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Taneri S, Oehler S, Lytle G and Dick HB:
Evaluation of epithelial integrity with various transepithelial
corneal cross-linking protocols for treatment of keratoconus. J
Ophthalmol. 2014(614380)2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lombardo M, Giannini D, Lombardo G and
Serrao S: Randomized controlled trial comparing transepithelial
corneal cross-linking using iontophoresis with the dresden protocol
in progressive keratoconus. Ophthalmology. 124:804–812.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kim M, Takaoka A, Hoang QV, Trokel SL and
Paik DC: Pharmacologic alternatives to riboflavin photochemical
corneal cross-linking: A comparison study of cell toxicity
thresholds. Invest Ophthalmol Vis Sci. 55:3247–3257.
2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gatzioufas Z, Richoz O, Brugnoli E and
Hafezi F: Safety profile of high-fluence corneal collagen
cross-linking for progressive keratoconus: Preliminary results from
a prospective cohort study. J Refract Surg. 29:846–848.
2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chan TC, Chow VW, Jhanji V and Wong VW:
Different topographic response between mild to moderate and
advanced keratoconus after accelerated collagen cross-linking.
Cornea. 34:922–927. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Özdemir HB, Kalkancı A, Bilgihan K, Göçün
PU, Öğüt B, Karakurt F and Erdoğan M: Comparison of corneal
collagen cross-linking (PACK-CXL) and voriconazole treatments in
experimental fungal keratitis. Acta Ophthalmol. 97:e91–e96.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jorgensen JH, Pfaller MA, Carroll KC,
Funke G, Landry ML, Richter SR and Warnock DW (eds): Manual of
Clinical Microbiology. 11th edition. American Society for
Microbiology, p2892, 2015.
|
|
20
|
Mutoh T, Ishikawa I, Matsumoto Y and
Chikuda M: A retrospective study of nine cases of Acanthamoeba
keratitis. Clin Ophthalmol. 4:1189–1192. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Karti O, Zengin MO, Cinar E, Tutuncu M,
Karahan E, Celik A and Kucukerdonmez C: Effect of 1- and
6-hour-delayed corneal collagen cross-linking on corneal healing in
a rabbit alkali-burn model: Clinical and histological observations.
Cornea. 35:1644–1649. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sharma S: Diagnosis of infectious diseases
of the eye. Eye (Lond). 26:177–184. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bamdad S, Malekhosseini H and Khosravi A:
Ultraviolet A/riboflavin collagen cross-linking for treatment of
moderate bacterial corneal ulcers. Cornea. 34:402–406.
2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kohlhaas M, Spoerl E, Schilde T, Unger G,
Wittig C and Pillunat LE: Biomechanical evidence of the
distribution of cross-links in corneas treated with riboflavin and
ultraviolet A light. J Cataract Refract Surg. 32:279–283.
2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Spoerl E, Wollensak G and Seiler T:
Increased resistance of crosslinked cornea against enzymatic
digestion. Curr Eye Res. 29:35–40. 2004.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kasparova EA, Sobkova OI and Yang B:
Corneal collagen cross-linking in the treatment of infectious
keratitis and corneal ulcers. Vestn Oftalmol. 133:113–119.
2017.PubMed/NCBI View Article : Google Scholar : (In Russian).
|
|
27
|
Lakhundi S, Siddiqui R and Khan NA:
Pathogenesis of microbial keratitis. Microb Pathog. 104:97–109.
2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hariprasad SM, Mieler WF, Lin TK, Sponsel
WE and Graybill JR: Voriconazole in the treatment of fungal eye
infections: A review of current literature. Br J Ophthalmol.
92:871–878. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Siatiri H, Daneshgar F, Siatiri N and
Khodabande A: The effects of intrastromal voriconazole injection
and topical voriconazole in the treatment of recalcitrant Fusarium
keratitis. Cornea. 30:872–875. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Prakash G, Sharma N, Goel M, Titiyal JS
and Vajpayee RB: Evaluation of intrastromal injection of
voriconazole as a therapeutic adjunctive for the management of deep
recalcitrant fungal keratitis. Am J Ophthalmol. 146:56–59.
2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li SX, Biang J, Li X, Zhang LT and Shi WY:
Keratectomy combined with intrastromal injection of voriconazole in
treating fungal keratitis. Zhonghua Yan Ke Za Zhi. 53:682–688.
2017.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
32
|
Narayana S, Krishnan T, Ramakrishnan S,
Samantaray PP, Austin A, Pickel J, Porco T, Lietman T and
Rose-Nussbaumer J: Mycotic antimicrobial localized injection: A
randomized clinical trial evaluating intrastromal injection of
voriconazole. Ophthalmology. 126:1084–1089. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Niki M, Eguchi H, Hayashi Y, Miyamoto T,
Hotta F and Mitamura Y: Ineffectiveness of intrastromal
voriconazole for filamentous fungal keratitis. Clin Ophthalmol.
8:1075–1079. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lin HC, Chu PH, Kuo YH and Shen SC:
Clinical experience in managing Fusarium solani keratitis. Int J
Clin Pract. 59:549–554. 2005.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Cheng J, Zhai HL, Wang JY and Xie LX:
Clinical features and treatments of retrocorneal fungal infection.
Zhonghua Yan Ke Za Zhi. 53:758–765. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Schacter LP, Owellen RJ, Rathbun HK and
Buchanan B: Letter: Antagonism between miconazole and amphotericin
B. Lancet. 2(318)1976.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Shen YC, Wang MY, Wang CY, Tsai TC, Tsai
HY, Lee HN and Wei LC: Pharmacokinetics of intracameral
voriconazole injection. Antimicrob Agents Chemother. 53:2156–2157.
2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Alastruey-Izquierdo A, Cuenca-Estrella M,
Monzón A, Mellado E and Rodríguez-Tudela JL: Antifungal
susceptibility profile of clinical Fusarium spp. isolates
identified by molecular methods. J Antimicrob Chemother.
61:805–809. 2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Pearson MM, Rogers PD, Cleary JD and
Chapman SW: Voriconazole: A new triazole antifungal agent. Ann
Pharmacother. 37:420–432. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
40
|
Kashiwabuchi RT, Carvalho FR, Khan YA,
Hirai F, Campos MS and McDonnell PJ: Assessment of fungal viability
after long-wave ultraviolet light irradiation combined with
riboflavin administration. Graefes Arch Clin Exp Ophthalmol.
251:521–527. 2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Bilgihan K, Kalkanci A, Ozdemir HB, Yazar
R, Karakurt F, Yuksel E, Otag F, Karabicak N and Arikan-Akdagli S:
Evaluation of antifungal efficacy of 0.1% and 0.25% riboflavin with
UVA: A comparative in vitro study. Curr Eye Res. 41:1050–1056.
2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Li Z, Jhanji V, Tao X, Yu H, Chen W and Mu
G: Riboflavin/ultravoilet light-mediated crosslinking for fungal
keratitis. Br J Ophthalmol. 97:669–671. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kling S, Hufschmid FS, Torres-Netto EA,
Randleman JB, Willcox M, Zbinden R and Hafezi F: High fluence
increases the antibacterial efficacy of PACK cross-linking. Cornea.
39:1020–1026. 2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wu CS, Wu SS and Chen PC: A prospective
study of fungal infection of gastric ulcers: Clinical significance
and correlation with medical treatment. Gastrointest Endosc.
42:56–58. 1995.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Idrus EA, Utti EM and Mattila JS:
Photoactivated chromophore corneal cross-linking (PACK-CXL) for
treatment of severe keratitis. Acta Ophthalmol. 97:721–726.
2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Said DG, Elalfy MS, Gatzioufas Z,
El-Zakzouk ES, Hassan MA, Saif MY, Zaki AA, Dua HS and Hafezi F:
Collagen cross-linking with photoactivated riboflavin (PACK-CXL)
for the treatment of advanced infectious keratitis with corneal
melting. Ophthalmology. 121:1377–1382. 2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
You X, Li J, Li S and Shi W: Effects of
lamellar keratectomy and intrastromal injection of 0.2% fluconazole
on fungal keratitis. J Ophthalmol. 2015(656027)2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Wollensak G, Spoerl E, Wilsch M and Seiler
T: Keratocyte apoptosis after corneal collagen cross-linking using
riboflavin/UVA treatment. Cornea. 23:43–49. 2004.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Linna TU, Vesaluoma MH, Petroll WM,
Tarkkanen AH and Tervo TM: Confocal microscopy of a patient with
irregular astigmatism after LASIK reoperations and relaxation
incisions. Cornea. 19:163–169. 2000.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Sivak JM and Fini ME: MMPs in the eye:
Emerging roles for matrix metalloproteinases in ocular physiology.
Prog Retin Eye Res. 21:1–14. 2002.PubMed/NCBI View Article : Google Scholar
|