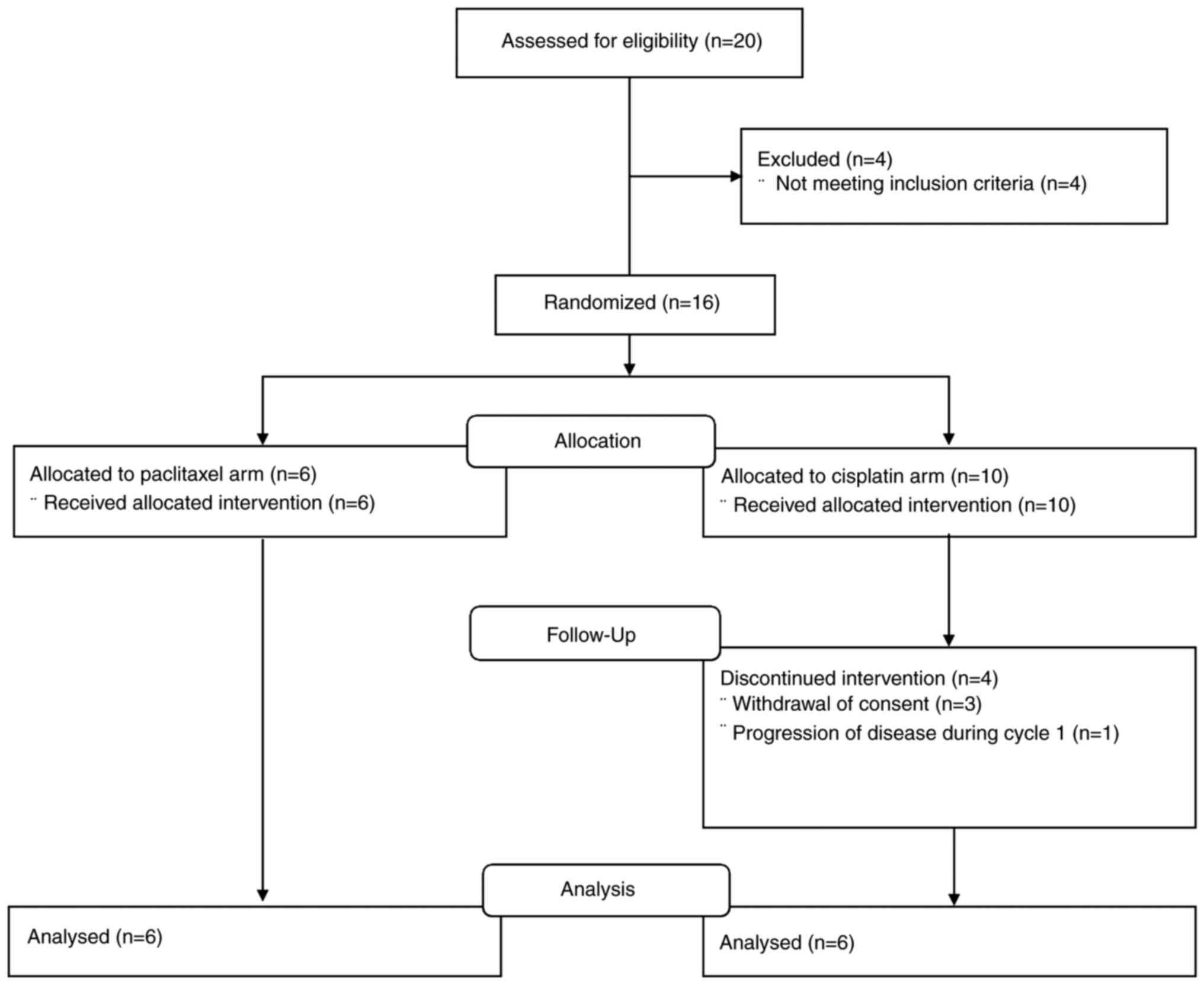
|
1
|
Hanker LC, Loibl S, Burchardi N, Pfisterer
J, Meier W, Pujade-Lauraine E, Ray-Coquard I, Sehouli J, Harter P
and du Bois A: AGO and GINECO study group. The impact of second to
sixth line therapy on survival of relapsed ovarian cancer after
primary taxane/platinum-based therapy. Ann Oncol. 23:2605–2612.
2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Li Z, Sun Q, Huang X, Zhang J, Hao J, Li Y
and Zhang S: The efficacy of radiofrequency hyperthermia combined
with chemotherapy in the treatment of advanced ovarian cancer. Open
Med (Wars). 13:83–89. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Vasanthan A, Mitsumori M, Park JH, Zhi-Fan
Z, Yu-Bin Z, Oliynychenko P, Tatsuzaki H, Tanaka Y and Hiraoka M:
Regional hyperthermia combined with radiotherapy for uterine
cervical cancers: A multi-institutional prospective randomized
trial of the international atomic energy agency. Int J Radiat Oncol
Biol Phys. 61:145–153. 2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yang W, Han GH, Shin HY, Lee EJ, Cho H,
Chay DB and Kim JH: Combined treatment with modulated
electro-hyperthermia and an autophagy inhibitor effectively inhibit
ovarian and cervical cancer growth. Int J Hyperthermia. 36:9–20.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lee SY, Lee NR, Cho DH and Kim JS:
Treatment outcome analysis of chemotherapy combined with modulated
electro-hyperthermia compared with chemotherapy alone for recurrent
cervical cancer, following irradiation. Oncol Lett. 14:73–78.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Minnaar CA, Kotzen JA, Ayeni OA, Naidoo T,
Tunmer M, Sharma V, Vangu MDT and Baeyens A: The effect of
modulated electro-hyperthermia on local disease control in
HIV-positive and -negative cervical cancer women in South Africa:
Early results from a phase III randomised controlled trial. PLoS
One. 14(e0217894)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yoo HJ, Lim MC, Seo SS, Kang S, Joo J and
Park SY: Phase I/II clinical trial of modulated
electro-hyperthermia treatment in patients with relapsed,
refractory or progressive heavily treated ovarian cancer. Jpn J
Clin Oncol. 49:832–838. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hegyi G, Szigeti GP and Szász A:
Hyperthermia versus oncothermia: Cellular effects in complementary
cancer therapy. Evid Based Complement Alternat Med.
2013(672873)2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yang KL, Huang CC, Chi MS, Chiang HC, Wang
YS, Hsia CC, Andocs G, Wang HE and Chi KH: In vitro comparison of
conventional hyperthermia and modulated electro-hyperthermia.
Oncotarget. 7:84082–84092. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Szasz AM, Minnaar CA, Szentmártoni G,
Szigeti GP and Dank M: Review of the clinical evidences of
modulated electro-hyperthermia (mEHT) method: An update for the
practicing oncologist. Front Oncol. 9(1012)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Schulz KF, Altman DG and Moher D: CONSORT
Group. CONSORT 2010 statement: Updated guidelines for reporting
parallel group randomized trials. Ann Intern Med. 152:726–732.
2010.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Haveman J, Bergs JWJ, Franken NAP, van
Bree C and Stalpers LJA: Effect of hyperthermia on uptake and
cytotoxicity of cisplatin in cultured murine mammary carcinoma
cells. Oncol Rep. 14:561–567. 2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Raaphorst GP and Yang DP: The evaluation
of thermal cisplatin sensitization in normal and XP human cells
using mild hyperthermia at 40 and 41 degrees C. Anticancer Res.
25:2649–2653. 2005.PubMed/NCBI
|
|
14
|
Gynecologic Oncology Group. Markman M,
Blessing J, Rubin SC, Connor J, Hanjani P and Waggoner S: Phase II
trial of weekly paclitaxel (80 mg/m2) in platinum and
paclitaxel-resistant ovarian and primary peritoneal cancers: A
gynecologic oncology group study. Gynecol Oncol. 101:436–440.
2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982.PubMed/NCBI
|
|
16
|
U.S.Department of Health and Human
Services: Common Terminology Criteriafor Adverse Events (CTCAE).
Version 4.0. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
Accessed June 14, 2010.
|
|
17
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jiang Z, Yan W and Ming J and Y: Docetaxel
weekly regimen in conjunction with RF hyperthermia for pretreated
locally advanced non-small cell lung cancer. A preliminary study.
7(189)2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Leal BZ, Meltz ML, Mohan N, Kuhn J,
Prihoda TJ and Herman TS: Interaction of hyperthermia with taxol in
human MCF-7 breast adenocarcinoma cells. Int J Hyperthermia.
15:225–236. 1999.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rietbroek RC, Katschinski DM, Reijers MH,
Robins HI, Geerdink A, Tutsch K, d'Oleire F and Haveman J: Lack of
thermal enhancement for taxanes in vitro. Int J Hyperthermia.
13:525–533. 1997.PubMed/NCBI View Article : Google Scholar
|
|
21
|
van der Burg MEL, de Wit R, Van Putten WL,
Logmans A, Kruit WH, Stoter G and Verweij J: Weekly cisplatin and
daily oral etoposide is highly effective in platinum pretreated
ovarian cancer. Br J Cancer. 86:19–25. 2002.PubMed/NCBI View Article : Google Scholar
|