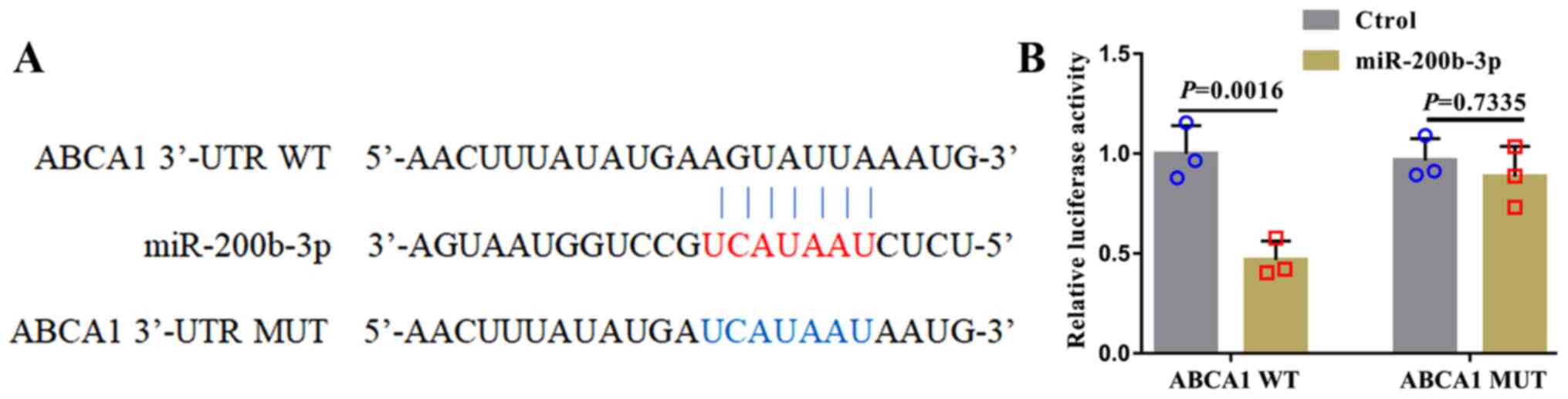Introduction
Atherosclerosis (As) is a chronic cardiovascular
disease that seriously endangers human health. Numerous studies
have confirmed that abnormal levels of blood lipids and cholesterol
are closely related to the occurrence of As (1). Elevated plasma lipids can promote
cholesterol to enter and deposit in the arterial wall, leading to
thickening of the vessel intima, narrowing of the vessels and
atherosclerotic lesions (2). The
main clinical manifestations of As are mononuclear infiltration,
foam cell formation, lipid accumulation in the vascular wall,
plaque formation and inflammation (3,4). Foam
cell formation is characterized by accumulation of lipids and
cholesterol efflux obstruction in macrophages (4). Reverse cholesterol transport (RCT), an
important physiological mechanism for maintaining intracellular
cholesterol balance (5), is also a
crucial process in the occurrence and development of As.
ATP-binding cassette transporter A1 (ABCA1), an
integrated membrane protein, promotes the efflux of free
cholesterol and phospholipids from cells (6). A previous study demonstrated that
ABCA1 mutations can prevent intracellular cholesterol and
phospholipids from being transported out of the cell, causing
accumulation of macrophage-derived foam cells in various tissues,
or even resulting in atherosclerotic lesions (7). It was reported that metastasis
associated lung adenocarcinoma transcript 1 inhibition reduces the
outflow of cholesterol and increases cholesterol accumulation in
macrophages, accelerating therefore the process of As by
downregulating the expression of ABCA1(8). A previous study demonstrated that
urolithin A can increase ABCA1 expression and RCT in macrophages,
thus alleviating the progression of As (9). Hesperidin can attenuate
varenicline-induced oxidized low-density lipoprotein (ox-LDL)
uptake in RAW264.7 cells by upregulating the expression of
ABCA1(10). The accumulation of
lipids and cholesterol is the first step in As formation (1,2).
Although ABCA1 plays a crucial role in regulating cholesterol
efflux (11), the underlying
mechanism remains poorly understood.
Micro-RNAs (miRNAs) are non-coding RNAs of 20-25
nucleotides in length that are involved in gene transcription and
mRNA stability. miRNAs are expressed throughout the body, and
abnormal expression of miRNAs is correlated with numerous diseases,
such as cardiovascular disease (12,13).
For example, the expression of miR-200b-3p is lower in human
cytomegalovirus (HCMV)-infected gastrointestinal tract and
bronchi/lung tissue than in normal tissue, which indicates that low
levels of miR-200b-3p are associated with inflammation due to HCMV
infection in humans (14).
miR-200b-3p inhibits prostate cancer cell proliferation and
promotes cell apoptosis by targeting PPKAR2B (15). miR-200b-3p improves oxaliplatin
resistance and induces migration, growth inhibition and apoptosis
in oxaliplatin-resistant colorectal cancer cells by suppressing
expression of TUBB3(16). Liu et
al (17) demonstrated that
miR-200b-3p is highly expressed in lung adenocarcinoma tissue and
promotes the proliferation and metastasis of lung adenocarcinoma
cells by targeting ABCA1. Furthermore, ABCA1 expression is
associated with lipid accumulation and RCT in macrophage-derived
foam cells and regulates the development of As (7). It is however unclear whether
miR-200b-3p could exacerbate As by promoting lipid accumulation and
inhibiting cholesterol efflux via ABCA1 in macrophage-derived foam
cells.
In the present study, the aim was to investigate
whether miR-200b-3p exacerbates As and the specific mechanism of
miR-200b-3b-induced lipid accumulation and cholesterol efflux
inhibition in macrophage-derived foam cells. The present study
aimed provide a novel basis for targeting miR-200b-3p as a
potential therapeutic strategy for patients with As.
Materials and methods
Clinical samples
Blood samples from 30 patients with As and 30
healthy volunteers were collected at Quanzhou First Hospital
between December 2020 and January 2021. A total of 2 ml blood from
each individual was collected in an EDTA-containing anticoagulant
tube, then centrifuged at 1,000 x g at 4˚C for 10 min. The
supernatant (plasma) was collected to detect the expression of
miR-200b-3p and ABCA1 or stored at -20˚C for further experiments.
The expression levels of miR-200b-3p and ABCA1 in these samples
were evaluated by revers transcription quantitative (RT-q)PCR. The
procedures were approved by the Institutional Ethics Committee of
Quanzhou First Hospital (approval no. 2020-05). Patients and
volunteers signed informed consent and the experiments were
conducted according to the principles of the Declaration of
Helsinki.
Cell culture
RAW264.7 cells were purchased from the American Type
Culture Collection. Cells were cultured in DMEM (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.) and 1% penicillin-streptomycin
(Sigma-Aldrich; Merck KGaA) placed at 37˚C in a humidified
incubator containing 5% CO2.
Foam cells formation
RAW264.7 cells were seeded in 6-well plates at a
density of 5x105 cells/well. When 60% confluence was
reached, cells were exposed to ox-LDL (50 mg/ml; cat. no.
20605ES05; Shanghai Yeasen Biotechnology Co., Ltd.) for 48 h to
induce the formation of foam cells (18,19).
RT-qPCR
Total RNA was isolated from cells and human plasma
samples using TransZol Up Plus RNA kit (cat. no. ER501-01; TransGen
Biotech Co., Ltd.) according to the manufacturers' protocol. The
concentration of total RNA was determined by a NanoDrop ND-2000
spectrophotometer (Thermo Fisher Scientific, Inc.). RNA was reverse
transcribed using an EasyScript First-Strand cDNA Synthesis
SuperMix (AE301-02; TransGen Biotech Co., Ltd.) according to the
manufacturers' instructions. Finally, the expression level of
miR-200b-3p and ABCA1 was evaluated using PerfectStart™ Green qPCR
SuperMix (AQ601-01; TransGen Biotech Co., Ltd.) on the Bio-Rad
C1000 Touch Thermal Cycler CFX96 Real-Time System (Bio-Rad
Laboratories, Inc.). RT-qPCR was performed as follows: Initial
denaturation at 95˚C for 10 min; denaturation at 95˚C for 15 sec
and annealing/extension at 60˚C for 60 sec (40 cycles). The
relative expression levels of miR-200b-3p and ABCA1 were normalized
to the endogenous controls 18S and U6 and were expressed as
2-∆∆Cq (20). The
sequences of the primers for miR-200b-3p and ABCA1 (Sangon Biotech
Co., Ltd.) are presented in Table
I.
 | Table ISequence of the primers used for
reverse transcription quantitative PCR. |
Table I
Sequence of the primers used for
reverse transcription quantitative PCR.
| Primer | Sequence
(5'-3') |
|---|
| miR-200b-3p | |
|
Forward |
GCTGCTGAATTCCATCTAATTTCCAAAAG |
|
Reverse |
TATTATGGATCCGCCCCCAGGGCAATGGG |
| ABCA1 | |
|
Forward |
GGCATCGTGTATGAGAAGG |
|
Reverse |
CTGTAGGGCAGCAGGTTT |
| U6 | |
|
Forward |
GCTTCGGCAGCACATATACT |
|
Reverse |
GCAGGGTCCGAGGTATTC |
| 18S | |
|
Forward |
AGAAACGGCTACCACATCCA |
|
Reverse |
CACCAGACTTGCCCTCCA |
Cell transfection
miR-200b-3p inhibitor control
(5'-CAGUACUUUUGUGUAGUACUAA-3'), miR-200b-3p inhibitor
(5'-UCAUCAUUACCAGGCAGUAUUA-3'), small interfering (si)RNA-control
(si-control, 5'-TAGT TAGACGCGUCACGTAGG-3') and si-ABCA1 (5'-GCAG
TGCCTTTGTAGCCTATG-3') were synthesized by Shanghai GenePharma Co.,
Ltd. The ox-LDL-induced foam cells were seeded in 6-well plates at
5x105 cells/well. When 70-80% confluence was reached,
cells were transfected with the miR-200b-3p inhibitor (2 µg) or
co-transfected with miR-200b-3p inhibitor (1 µg) and
si-ABCA1/si-control (1 µg) using Lipofectamine™ 3000 Transfection
Reagent (cat. no. L3000015; Thermo Fisher Scientific, Inc.)
according to the manufacturers' protocol. The transfected cells
were incubated for 48 h in an incubator with 5% CO2 at
37˚C. After 48 h, the cells were collected for subsequent
experiments.
Western blotting
Cells transfected with si-ABCA1 or miR-200b-3p
inhibitor were collected, washed in cold PBS and lysed on ice for
30 min using RIPA buffer (cat. no. P0013B; Beyotime Institute of
Biotechnology) supplemented with a protease inhibitor cocktail
(cat. no. 04693132001; Roche Diagnostics). The cell lysate was
centrifuged at 12,000 x g at 4˚C for 30 min. The supernatant was
collected and the protein concentration was quantified by a
NanoDrop ND-2000 spectrophotometer (Thermo Fisher Scientific,
Inc.). Proteins (50 µg) were separated by 8% SDS-PAGE and
transferred onto a polyvinylidene fluoride membrane. The membranes
were blocked with 5% skim milk at room temperature for 1 h and then
incubated with a mouse anti-ABCA1 antibody (1:1,000; cat. no.
ab18180; Abcam) and mouse anti-β-actin antibody (1:2,000; cat. no.
HC201-01; TransGen Biotech Co., Ltd.) at room temperature for 2 h.
Subsequently, membranes were incubated with a secondary anti-mouse
antibody (1:5,000; cat. no. 7076P2; Cell Signaling Technology,
Inc.) at room temperature for 1 h. Enhanced chemiluminescence
reagent (cat. no. 170-5061; Bio-Rad Laboratories, Inc.) was used to
detect the signal on the membrane. The data were analyzed via
densitometry using ImageJ software (1.52v, National Institutes of
Health) and normalized to expression of the internal control
β-actin.
Oil Red O stain
Oil Red O (0.5%; cat. no. O1391-250ML;
Sigma-Aldrich; Merck KGaA) was diluted with distilled water at a
ratio of 3:2 and filtered with filter paper to generate the working
solution. The cells were fixed with 4% paraformaldehyde at room
temperature for 30 min and washed three times with PBS. The cells
were stained with Oil Red O working solution at 37˚C for 30 min and
then rinsed with 65% isopropanol. Finally, the cells were observed
under a 10x inverted microscope (magnification, x10).
Enzyme-linked immunosorbent assay
(ELISA)
Total cholesterol (TC) and free cholesterol (FC)
levels were evaluated using ELISA kits (cat. nos. E1015-105 and
E1016-105, respectively; Applygen Technologies, Inc.) according to
the manufacturers' protocol. Briefly, cells were washed in cold PBS
and harvested. The cells were mixed with the appropriate lysis
buffer and allowed to stand for 10 min for lysis. The protein
concentration was measured in some lysates with an Easy II Protein
Quantitative kit (BCA; TransGen Biotech Co., Ltd.). The rest of the
lysates was centrifuged at 2,000 x g for 5 min at room temperature
and supernatants were collected. TC and FC levels were measured
with a microplate reader (Molecular Devices, LLC). The cholesterol
ester (CE) level was calculated using the following equation:
CE=TC-FC. The data were normalized to the total cellular protein
concentration.
Luciferase reporter assay
miR-200b-3p mimic, wild-type (WT) and mutant (MUT)
3'-UTRs of ABCA1 were synthesized by Sangon Biotech Co, Ltd. The WT
and MUT ABCA1 3'-UTR were inserted into the luciferase reporter
vector (pGL3; Promega Corporation) to construct pGL3-ABCA1-WT or
pGL3-ABCA1-MUT plasmids, respectively. The foam cells were
co-transfected with plasmid DNA and miR-200b-3p mimic with
Lipofectamine 3000 Transfection Reagent (cat. no. L300015, Thermo
Fisher Scientific, Inc.) following the manufacturers' protocol.
After transfection for 48 h, the cells were collected for analysis
of luciferase activity. Luciferase activity was measured by a
microplate reader (Tecan Infinite 200, Tecan Group, Ltd.) with Dual
Luciferase Reporter Gene Assay kit (cat. no. RG027; Beyotime
Institute of Biotechnology) in according to the manufacturers'
protocol. The luciferase activity of cells was analyzed using
Renilla luciferase activity as an internal reference.
Statistical analysis
All data from three independent experiments were
presented as the means ± standard deviation and analyzed using
GraphPad Prism 7.0 software (GraphPad Software, Inc.). Comparison
between two groups was performed using Student's t-test. Spearman
correlation analysis was used to analyze the correlation between
the expression of miR-200b-3p and ABCA1 in patients with As.
P<0.05 was considered to indicate a statistically significant
difference.
Results
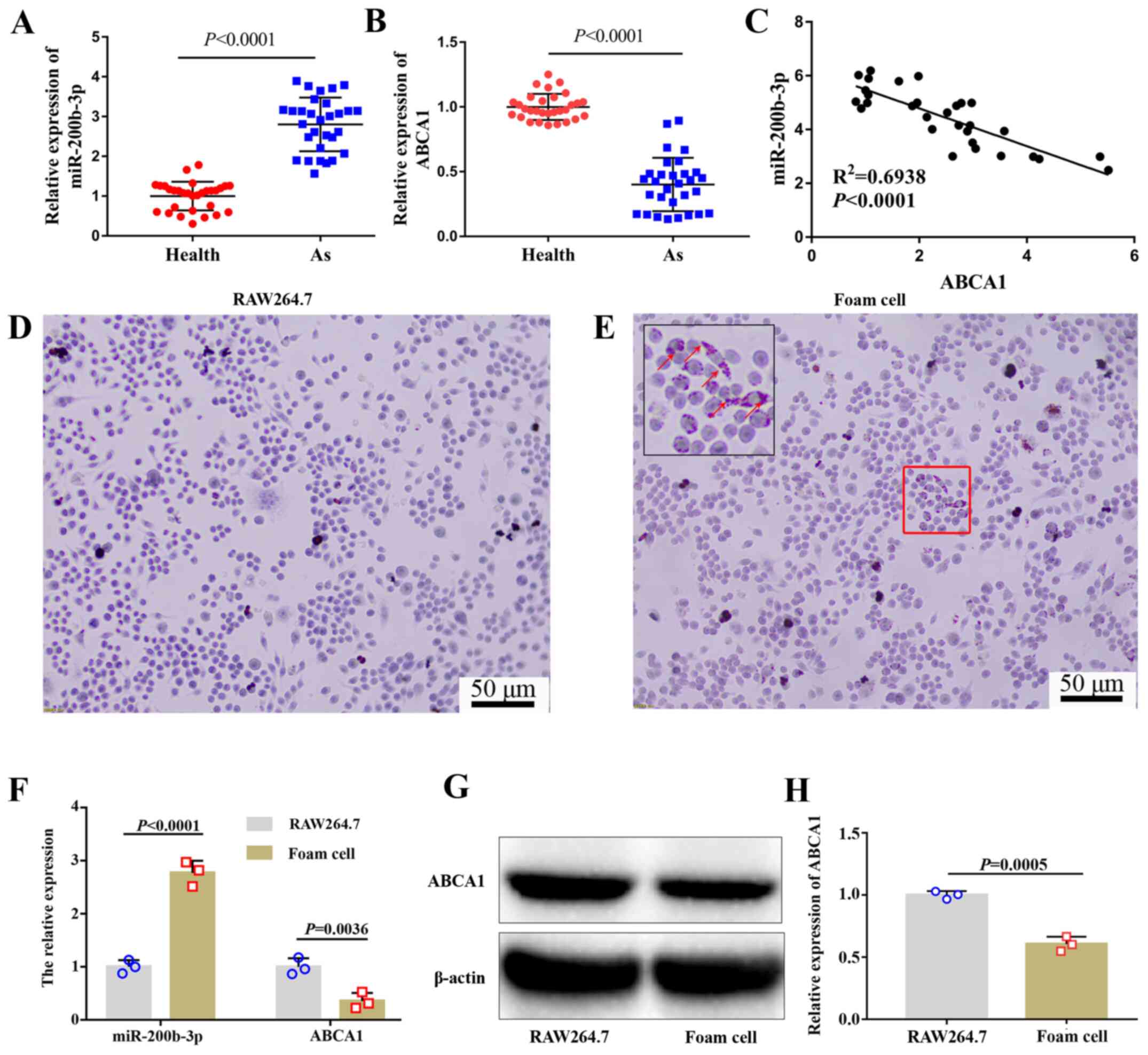
miR-200b-3p expression is upregulated
and ABCA1 expression is downregulated in patients with As and foam
cells
The expression of miR-200b-3p and ABCA1 in patients
with or without As was evaluated by RT-qPCR. The results
demonstrated that miR-200b-3p expression in patients with As was
significantly higher compared with healthy volunteers (Fig. 1A), whereas the expression of ABCA1
in patients with As was significantly lower compared with healthy
people (Fig. 1B). Furthermore,
miR-200b-3p expression was negatively correlated with ABCA1
expression (Fig. 1C).
Based on previous research (8,11,18),
RAW264.7 cells were selected to explore the specific molecular
mechanism of miR-200b-3p in As. RAW246.7 cells were treated with
ox-LDL for 48 h. To investigate whether foam cells were formed
following this treatment, lipid content was evaluated by Oil Red O
staining. The results demonstrated that the number of lipid
particles was markedly increased in cells treated with ox-LDL
(Fig. 1D and E), which confirmed the formation of
RAW264.7-derived foam cells induced by ox-LDL treatment. The
expression levels of miR-200b-3p and ABCA1 were measured by
RT-qPCR. As shown in Fig. 1F,
miR-200b-3p was upregulated whereas ABCA1 was downregulated in
RAW264.7-derived foam cells compared with RAW264.7 cells. In
addition, the protein expression of ABCA1 was significantly
decreased in foam cells compared with RAW264.7 cells (Fig. 1G and H).
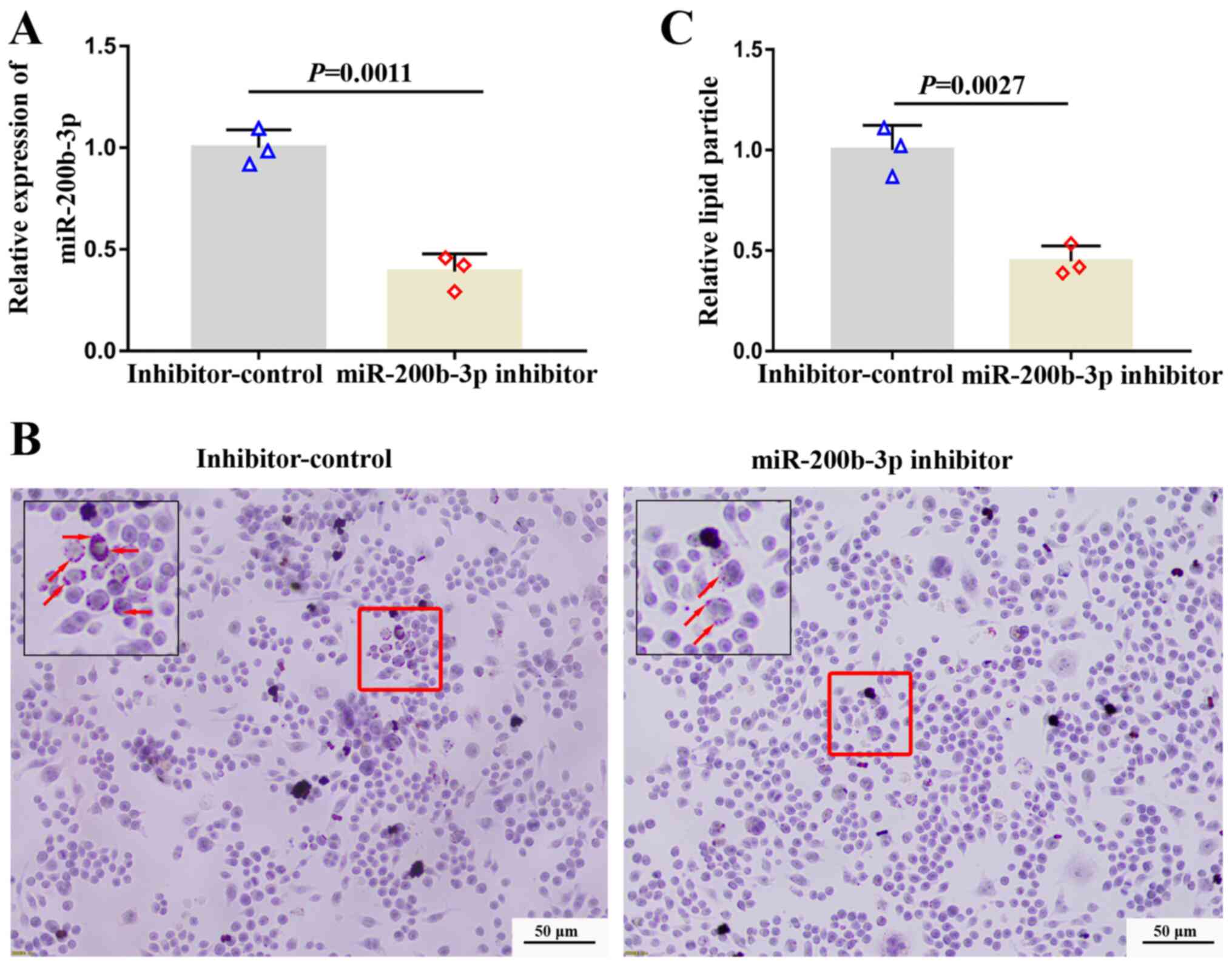
Inhibition of miR-200b-3p decreases
lipid accumulation and enhances cholesterol efflux
To investigate the effect of miR-200b-3p on lipid
accumulation and cholesterol efflux, foam cells were transfected
with a miR-200b-3p inhibitor and the expression of miR-200b-3p was
measured by RT-qPCR. The expression of miR-200b-3p was distinctly
decreased in foam cells transfected with miR-200b-3p inhibitor
compared with cells transfected with inhibitor-control (Fig. 2A), suggesting the successful
transfection. Subsequently, lipid content was evaluated by Oil Red
O staining. The results demonstrated that the number of orange
particles in the cytoplasm was decreased in cells transfected with
the miR-200b-3p inhibitor compared with cells transfected with
inhibitor control (Fig. 2B and
C), suggesting that miR-200b-3p
inhibition could decrease lipid accumulation.
Furthermore, the levels of cholesterol in the foam
cells and in foam cell culture media were analyzed by ELISA. The
TC, FC and EC levels in the foam cells transfected with the
miR-200b-3p inhibitor were significantly decreased compared with
those in foam cells transfected with the inhibitor-control
(P<0.05; Table II); however,
the EC/TC ratio was not significantly changed in foam cells
transfected with the miR-200b-3p inhibitor (Table II). Furthermore, FC level in the
culture media of foam cells transfected with the miR-200b-3p
inhibitor was significantly increased compared with that in the
culture media of foam cells transfected with inhibitor control
(P<0.05; Table III). To
analyze the effect of miR-200b-3p on cholesterol efflux in foam
cells derived from macrophages, the cholesterol efflux rate of the
foam cells was calculated according to the following equation:
Cholesterol efflux rate % = culture media FC/(FC in culture media +
FC in foam cells) x100%. As seen in Table IV, the cholesterol outflow of the
foam cells transfected with the miR-200b-3p inhibitor was
significantly higher than that of the inhibitor-control cells
(P<0,05), which indicated that miR-200b-3p inhibition may
enhance cholesterol efflux in RAW264.7-derived foam cells.
 | Table IIEffect of miR-200b-3p on
intracellular cholesterol content in foam cells. |
Table II
Effect of miR-200b-3p on
intracellular cholesterol content in foam cells.
| | Inhibitor
control | miR-200b-3p
inhibitor | P-value |
|---|
| TC (nmol/mg total
protein) | 362.74±11.04 | 285.28±8.11 | 0.0006 |
| FC (nmol/mg total
protein) | 151.74±5.85 | 109.28±8.42 | 0.0020 |
| CE (nmol/mg total
protein) | 211.00±5.37 | 176.04±11.30 | 0.0084 |
| CE/TC (%) | 58.17±0.41 | 61.69±3.04 | 0.1181 |
 | Table IIIFree cholesterol level in the culture
media of foam cells. |
Table III
Free cholesterol level in the culture
media of foam cells.
| | Inhibitor
control | miR-200b-3p
inhibitor | P-value |
|---|
| FC (nmol/mg total
protein) | 53.73±4.05 | 82.42±3.29 | 0.0007 |
 | Table IVEffect of miR-200b-3p on cholesterol
efflux in foam cells. |
Table IV
Effect of miR-200b-3p on cholesterol
efflux in foam cells.
| | Inhibitor
control | miR-200b-3p
inhibitor | P-value |
|---|
| Cholesterol efflux
(%) | 26.14±1.16 | 43.04±0.91 | <0.0001 |
Taken together, these findings suggested that
inhibition of miR-200b-3p may decrease lipid accumulation and
enhance cholesterol efflux.
miR-200b-3p downregulates the
expression of ABCA1 in foam cells
In human lung adenocarcinoma cell lines, miR-200b-3p
targets ABCA1 and negatively regulates the expression of
ABCA1(17). To investigate whether
miR-200b-3p could target ABCA1 in RAW264.7-derived foam cells, WT
and MUT ABCA1 3'-UTRs were designed (Fig. 3A) and co-transfected into foam
cells. Then, luciferase activity was evaluated. As presented in
Fig. 3B, luciferase activity in
cells transfected with miR-200b-3p mimic and the WT ABCA1 3'-UTR
was decreased compared with that in cells transfected with
miR-200b-3p mimic and MUT ABCA1 3'-UTR.
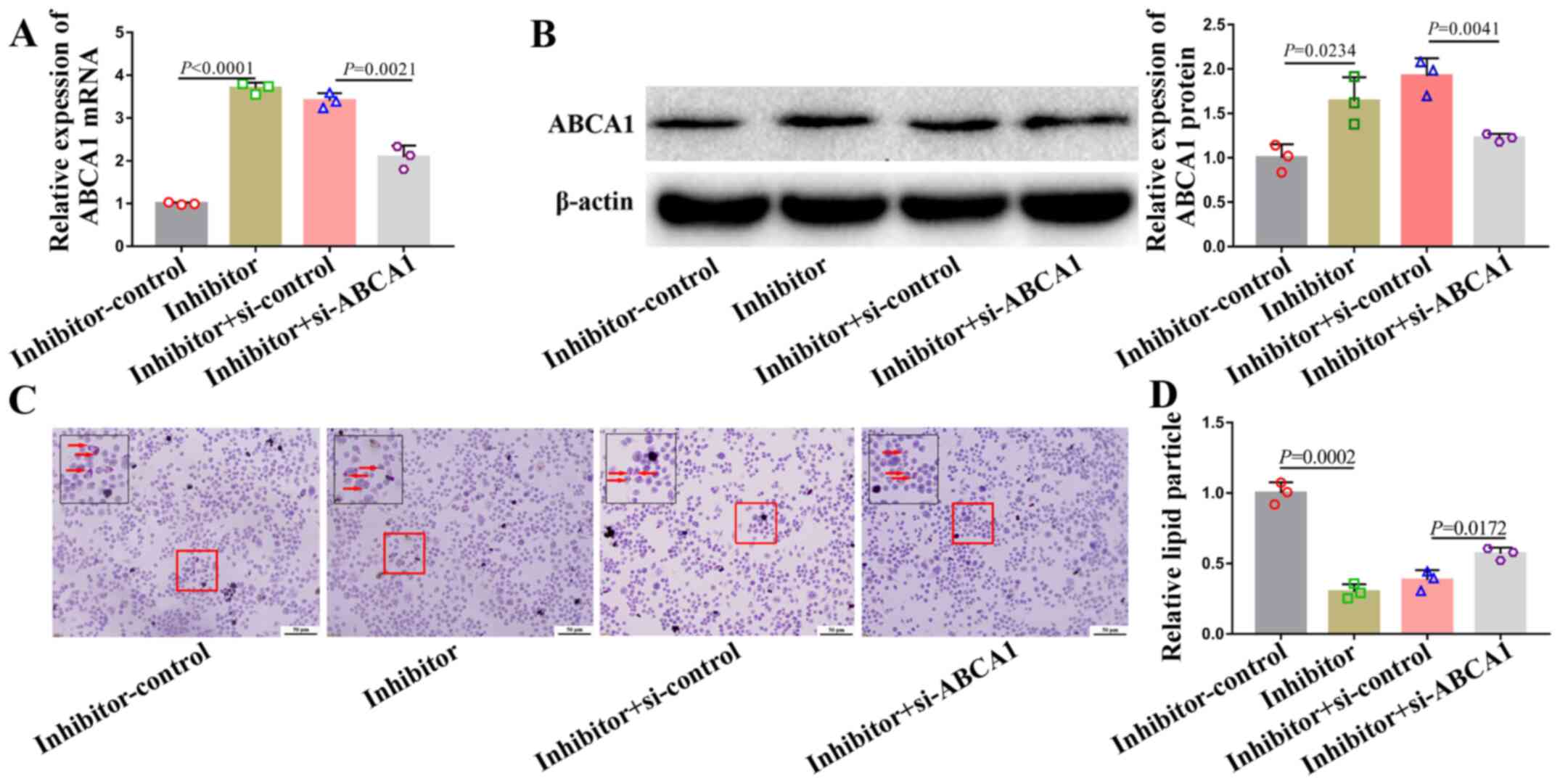
miR-200b-3p inhibitor alleviates lipid
accumulation and accelerates cholesterol efflux by upregulating
ABCA1 expression
A rescue experiment was performed to determine the
relationship between miR-200b-3p and ABCA1. To do so, foam cells
were co-transfected with a miR-200b-3p inhibitor and si-ABCA1. The
mRNA and protein expression of ABCA1 in cells transfected with
si-ABCA1 and miR-200b-3p inhibitor was partly downregulated
(Fig. 4A and B). Furthermore, si-ABCA1 partly abrogated
the alleviation of lipid accumulation (Fig. 4C and D) and acceleration of cholesterol efflux
(Table V) induced by miR-200b-3p
inhibitor.
 | Table Vsi-ABCA1 partly abrogates miR-200b-3p
inhibitor-induced accelerated cholesterol efflux in foam cells. |
Table V
si-ABCA1 partly abrogates miR-200b-3p
inhibitor-induced accelerated cholesterol efflux in foam cells.
| Groups | Cholesterol efflux
rate % | P-value |
|---|
| miR-200b-3p
inhibitor control | 27.13±2.67 | 0.0019 |
| miR-200b-3p
inhibitor | 47.71±4.12 | |
| miR-200b-3p
inhibitor + si-control | 45.63±3.58 | 0.0310 |
| miR-200b-3p
inhibitor + si-ABCA1 | 35.31±4.15 | |
Taken together, these data suggested that
miR-200b-3p inhibitor may alleviate lipid accumulation and
accelerate cholesterol efflux by positively regulating ABCA1 in
RAW264.7-derived foam cells.
Discussion
As is the main cause of cardiovascular and
cerebrovascular diseases (21,22).
The pathogenesis of As involves multiple genetic and environmental
factors. For example, abnormal of SOS3 methylation and histone
results in As by disrupting the function of vascular endothelium
(23). Smoking accelerates As
formation by impairing endothelial function and increasing
inflammation/oxidative stress (24). Lipid metabolism disorders are the
main pathological bases of As and are mainly characterized by
macrophage infiltration, foam cell formation, lipid accumulation in
the blood vessel wall and plaque formation (3,4,25).
Cholesterol outflow disorder is one of the main causes of
macrophage lipid accumulation and foam cell formation and it leads
to the development of As (25,26).
Studying cholesterol efflux disorders is therefore beneficial when
investigating the underlying mechanism of As and determining
strategies for the prevention and treatment of As.
In the early development of As, macrophages
aggregate under local inflammatory stimulation, excessive lipids
accumulate intracellularly and cholesterol outflow decreases in
macrophages, resulting in the formation of macrophage-derived foam
cells (27). Macrophage-derived
foam cells are a sign of plaque formation and early development of
As. ABCA1, an integrin on the cytomembrane, plays an important role
in high density lipoprotein (HDL) and very low-density lipoprotein
production, inflammation, insulin-glucose imbalance and obesity
(28,29). Furthermore, ABCA1 combined with the
apolipoprotein apoA-I promotes the outflow of FC from macrophage
cells (30). A previous study on
the structure of ABCA1 protein demonstrated that the C-terminus of
ABCA1 regulates cholesterol flippase activity and cholesterol
efflux (31). Previous studies have
reported that ABCA1 mutations can cause Tangier disease (32) and familial HDL deficiency (7). Although abnormalities in ABCA1 can
cause a variety of diseases, the role of ABCA1 in the regulation of
lipid accumulation and cholesterol efflux is a current focus of
research. Previous studies have demonstrated that abnormal
expression of ABCA1 disturbs lipid accumulation and cholesterol
efflux in foam cells, resulting in As (33,34).
ABCA1 is therefore considered as a biomarker for predicting As and
a potential target gene for the treatment of As (35,36).
Previous studies have suggested that the targeting
of ABCA1 by many miRNAs can exacerbate/attenuate the development of
As, such as miR-30e/92a (37),
miR-143/145(38) and miR-148a
(39). Therefore, miRNAs can
regulate the progression of As by affecting the expression of
ABCA1.
In the present study, RAW264.7 macrophages were
treated with ox-LDL to induce the formation of RAW264.7-derived
foam cells. The expression of ABCA1 was significantly decreased in
the foam cells, whereas the expression of miR-200b-3p was
significantly increased. Results from Oil red O staining
demonstrated that lipid accumulation in foam cells was
significantly increased compared with that in RAW264.7 cells.
Furthermore, in RAW264.7-derived foam cells transfected with
miR-200b-3p inhibitor, lipid accumulation was alleviated,
cholesterol efflux was enhanced, which suggested that miR-200b-3p
may promote lipid accumulation and inhibit cholesterol efflux.
Subsequently, miR-200b-3p may be considered as a biomarker for the
prediction of As; however, the underlying mechanisms of miR-200b-3p
remain to be further explored. Bioinformatics analysis and
luciferase reporter experiments verified that miR-200b-3p could
target ABCA1 in RAW264.7 macrophages, which was consistent with Liu
et al (17). Rescue
experiments revealed that miR-200b-3p exacerbated lipid
accumulation, suppressed cholesterol efflux in macrophages and
accelerated the formation of foam cells, which ultimately led to
the formation of As.
This study presented some limitations. Ox-LDL was
used for foam cell formation, which was recognized by the majority
of scientific researchers. However, ox-LDL can elevate the
oxidative stress in macrophages and potentially affect the
expression of numerous genes not related to cholesterol efflux. Due
to this limitation, other triggers, such as acetylated and
aggregated LDL and intermediate-density lipoprotein, will be
further assessed in cholesterol and lipid load into macrophages in
the future. The ABCA1 mediated cholesterol efflux is most relevant
for As, not only in macrophages, but also in liver, intestine and
other organs. The ApoE-/- mice model with As will be used to
further verify that miR-200b-3p could target ABCA1 to promote the
formation and progression of As in macrophages, liver, intestine
and other organs. In addition, regulation of cholesterol efflux by
miR-200b-3p and ABCA1 in hepatocytes and intestinal cells will be
investigated in the future.
In summary, the present study demonstrated that
inhibition of miR-200b-3p alleviated lipid accumulation and
promoted cholesterol efflux by targeting ABCA1 in
macrophage-derived foam cells. miR-200b-3p could therefore be
considered as a potential target gene for treating As.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZJG and YBY designed the study and helped to revise
the manuscript. YTW, JBL, HQL, GXZ, CMH and ML performed the
experiments and analyzed the data. YTW wrote the manuscript. YTW,
ZJG and YBY confirm the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study adhered to the principles in the
Declaration of Helsinki. All participants provided written informed
consent. This study was approved by the Institutional Ethics
Committee of Quanzhou First Hospital (approval no. 2020-05).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ference BA, Kastelein JJP and Catapano AL:
Lipids and lipoproteins in 2020. JAMA. 324:595–596. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Aguilar-Ballester M, Herrero-Cervera A,
Vinué Á, Martínez-Hervás S and González-Navarro H: Impact of
cholesterol metabolism in immune cell function and atherosclerosis.
Nutrients. 12(12)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li D, Liu Y, Zhang X, Lv H, Pang W, Sun X,
Gan LM, Hammock BD, Ai D and Zhu Y: Inhibition of soluble epoxide
hydrolase alleviated atherosclerosis by reducing monocyte
infiltration in Ldlr(-/-) mice. J Mol Cell Cardiol. 98:128–137.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Webb NR and Moore KJ: Macrophage-derived
foam cells in atherosclerosis: Lessons from murine models and
implications for therapy. Curr Drug Targets. 8:1249–1263.
2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jin P, Bian Y, Wang K, Cong G, Yan R, Sha
Y, Ma X, Zhou J, Yuan Z and Jia S: Homocysteine accelerates
atherosclerosis via inhibiting LXRα-mediated ABCA1/ABCG1-dependent
cholesterol efflux from macrophages. Life Sci. 214:41–50.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Demina EP, Miroshnikova VV and Schwarzman
AL: Role of the ABC transporters A1 and G1, key reverse cholesterol
transport proteins, in atherosclerosis. Mol Biol (Mosk).
50:223–230. 2016.PubMed/NCBI View Article : Google Scholar : (In Russian).
|
|
7
|
Maranghi M, Truglio G, Gallo A, Grieco E,
Verrienti A, Montali A, Gallo P, Alesini F, Arca M and Lucarelli M:
A novel splicing mutation in the ABCA1 gene, causing Tangier
disease and familial HDL deficiency in a large family. Biochem
Biophys Res Commun. 508:487–493. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu L, Tan L, Yao J and Yang L: Long
non-coding RNA MALAT1 regulates cholesterol accumulation in
ox-LDL-induced macrophages via the microRNA-17-5p/ABCA1 axis. Mol
Med Rep. 21:1761–1770. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Han QA, Su D, Shi C, Liu P, Wang Y, Zhu B
and Xia X: Urolithin A attenuated ox-LDL-induced cholesterol
accumulation in macrophages partly through regulating miR-33a and
ERK/AMPK/SREBP1 signaling pathways. Food Funct. 11:3432–3440.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Koga M, Kanaoka Y, Inada K, Omine S,
Kataoka Y and Yamauchi A: Hesperidin blocks varenicline-aggravated
atherosclerotic plaque formation in apolipoprotein E knockout mice
by downregulating net uptake of oxidized low-density lipoprotein in
macrophages. J Pharmacol Sci. 143:106–111. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ren K, Li H, Zhou HF, Liang Y, Tong M,
Chen L, Zheng XL and Zhao GJ: Mangiferin promotes macrophage
cholesterol efflux and protects against atherosclerosis by
augmenting the expression of ABCA1 and ABCG1. Aging (Albany NY).
11:10992–11009. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang F, Zhang R, Zhang X, Wu Y, Li X,
Zhang S, Hou W, Ding Y, Tian J, Sun L, et al: Comprehensive
analysis of circRNA expression pattern and circRNA-miRNA-mRNA
network in the pathogenesis of atherosclerosis in rabbits. Aging
(Albany NY). 10:2266–2283. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Laffont B and Rayner KJ: MicroRNAs in the
pathobiology and therapy of atherosclerosis. Can J Cardiol.
33:313–324. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lee KH, Lim BJ, Ferreira VH, Min SY, Hong
YM, Jo JH and Han SH: Expression of human miR-200b-3p and -200c-3p
in cytomegalovirus-infected tissues. Biosci Rep.
38(BSR20180961)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xia L, Han Q, Chi C, Zhu Y, Pan J, Dong B,
Huang Y, Xia W, Xue W and Sha J: Transcriptional regulation of
PRKAR2B by miR-200b-3p/200c-3p and XBP1 in human prostate cancer.
Biomed Pharmacother. 124(109863)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wu YZ, Lin HY, Zhang Y and Chen WF:
miR-200b-3p mitigates oxaliplatin resistance via targeting TUBB3 in
colorectal cancer. J Gene Med. 22(e3178)2020.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Liu K, Zhang W, Tan J, Ma J and Zhao J:
MiR-200b-3p functions as an oncogene by targeting ABCA1 in lung
adenocarcinoma. Technol Cancer Res Treat.
18(1533033819892590)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cao H, Jia Q, Yan L, Chen C, Xing S and
Shen D: Quercetin suppresses the progression of atherosclerosis by
regulating MST1-mediated autophagy in ox-LDL-induced RAW264.7
macrophage foam cells. Int J Mol Sci. 20(6093)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yin M, Lu J and Guo Z, Zhang Y, Liu J, Wu
T, Guo K, Luo T and Guo Z: Reduced SULT2B1b expression alleviates
ox-LDL-induced inflammation by upregulating miR-148-3P via
inhibiting the IKKβ/NF-κB pathway in macrophages. Aging (Albany
NY). 13:3428–3442. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rahman MS and Woollard K: Atherosclerosis.
Adv Exp Med Biol. 1003:121–144. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kalaria RN: The pathology and
pathophysiology of vascular dementia. Neuropharmacology.
134:226–239. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Grimaldi V, Vietri MT, Schiano C, Picascia
A, De Pascale MR, Fiorito C, Casamassimi A and Napoli C: Epigenetic
reprogramming in atherosclerosis. Curr Atheroscler Rep.
17(476)2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lechner K, von Schacky C, McKenzie AL,
Worm N, Nixdorff U, Lechner B, Kränkel N, Halle M, Krauss RM and
Scherr J: Lifestyle factors and high-risk atherosclerosis: Pathways
and mechanisms beyond traditional risk factors. Eur J Prev Cardiol.
27:394–406. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chistiakov DA, Bobryshev YV and Orekhov
AN: Macrophage-mediated cholesterol handling in atherosclerosis. J
Cell Mol Med. 20:17–28. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Boshuizen MC, Hoeksema MA, Neele AE, van
der Velden S, Hamers AA, Van den Bossche J, Lutgens E and de
Winther MP: Interferon-β promotes macrophage foam cell formation by
altering both cholesterol influx and efflux mechanisms. Cytokine.
77:220–226. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yuan Y, Li P and Ye J: Lipid homeostasis
and the formation of macrophage-derived foam cells in
atherosclerosis. Protein Cell. 3:173–181. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Babashamsi MM, Koukhaloo SZ, Halalkhor S,
Salimi A and Babashamsi M: ABCA1 and metabolic syndrome; a review
of the ABCA1 role in HDL-VLDL production, insulin-glucose
homeostasis, inflammation and obesity. Diabetes Metab Syndr.
13:1529–1534. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Jung D, Cao S, Liu M and Park S: A
Meta-analysis of the associations between the ATP-binding cassette
transporter ABCA1 R219K (rs2230806) polymorphism and the risk of
type 2 diabetes in Asians. Horm Metab Res. 50:308–316.
2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liu M, Mei X, Herscovitz H and Atkinson D:
N-terminal mutation of apoA-I and interaction with ABCA1 reveal
mechanisms of nascent HDL biogenesis. J Lipid Res. 60:44–57.
2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Okamoto Y, Tomioka M, Ogasawara F, Nagaiwa
K, Kimura Y, Kioka N and Ueda K: C-terminal of ABCA1 separately
regulates cholesterol floppase activity and cholesterol efflux
activity. Biosci Biotechnol Biochem. 84:764–773. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Puntoni M, Sbrana F, Bigazzi F and
Sampietro T: Tangier disease: Epidemiology, pathophysiology, and
management. Am J Cardiovasc Drugs. 12:303–311. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ouimet M, Barrett TJ and Fisher EA: HDL
and reverse cholesterol transport. Circ Res. 124:1505–1518.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Price NL, Rotllan N, Zhang X,
Canfrán-Duque A, Nottoli T, Suarez Y and Fernández-Hernando C:
Specific disruption of Abca1 targeting largely mimics the effects
of miR-33 knockout on macrophage cholesterol efflux and
atherosclerotic plaque development. Circ Res. 124:874–880.
2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wu YR, Shi XY, Ma CY, Zhang Y, Xu RX and
Li JJ: Liraglutide improves lipid metabolism by enhancing
cholesterol efflux associated with ABCA1 and ERK1/2 pathway.
Cardiovasc Diabetol. 18(146)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tan YL, Ou HX, Zhang M, Gong D, Zhao ZW,
Chen LY, Xia XD, Mo ZC and Tang CK: Tanshinone IIA promotes
macrophage cholesterol efflux and attenuates atherosclerosis of
apoE-/- mice by Omentin-1/ABCA1 pathway. Curr Pharm
Biotechnol. 20:422–432. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wang Z, Zhang J, Zhang S, Yan S, Wang Z,
Wang C and Zhang X: MiR-30e and miR-92a are related to
atherosclerosis by targeting ABCA1. Mol Med Rep. 19:3298–3304.
2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yue Y, Zhang Z, Zhang L, Chen S, Guo Y and
Hong Y: miR-143 and miR-145 promote hypoxia-induced proliferation
and migration of pulmonary arterial smooth muscle cells through
regulating ABCA1 expression. Cardiovasc Pathol. 37:15–25.
2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Goedeke L, Rotllan N, Canfrán-Duque A,
Aranda JF, Ramírez CM, Araldi E, Lin CS, Anderson NN, Wagschal A,
de Cabo R, et al: MicroRNA-148a regulates LDL receptor and ABCA1
expression to control circulating lipoprotein levels. Nat Med.
21:1280–1289. 2015.PubMed/NCBI View Article : Google Scholar
|