Introduction
Inorganic arsenic (iAs) is a natural pollutant found
in the air and soil, as well as dissolved in groundwater. The main
source of human exposure to iAs is through contaminated drinking
water and the maximum allowed limit established by the World Health
Organization for iAs in drinking water is 10 µg/l (1). However, an estimated 500 million
individuals worldwide still are exposed to high concentrations of
iAs, ranging from 400 to 25,000 µg/l (2). Chronic exposure to iAs through
contaminated drinking water may lead to non-malignant disorders and
to various types of cancer, including bladder, prostate, lung and
skin (3). For this reason, the
presence of this metalloid in drinking water has been recognized as
a significant global health issue (4).
Recently, the use of natural compounds with
chemopreventive properties has been explored as a way to reduce
iAs-related cellular damage. Curcumin is a phytochemical with
potent antioxidant properties against a variety of environmental
and intracellular hazards, including iAs (5). Curcumin is consumed worldwide, mainly
through using turmeric as a spice. This spice is common in
traditional dishes of India, including curry, and is also used as a
food colorant and preservative (6).
In certain regions such as India, the population normally consumes
around 60-100 mg of curcumin per day without any apparent adverse
health effects (7). As a
chemopreventive compound, curcumin reduces the generation of
oxidative stress and DNA damage associated with iAs exposure in
different cell types, including keratinocytes, lung cells and
lymphocytes (8-10).
Of note, the ability of curcumin to mitigate iAs-related DNA damage
has been demonstrated in circulating lymphocytes obtained from
individuals chronically exposed to iAs-contaminated drinking water
(11,12).
Epstein-Barr virus (EBV) belongs to the
γ-herpesvirus family and is among the most prevalent human viruses,
affecting ~90% of the population worldwide (13). Although EBV infection has been
associated with several human diseases, including infectious
mononucleosis and Burkitt's lymphoma, in most cases, infected
individuals remain asymptomatic (13,14).
It is worth noting that in cellular models derived from gastric and
nasopharyngeal carcinomas that are positive for EBV, curcuminoids
have higher toxicity (15).
Similarly, experimental treatment with curcumin promoted apoptosis
in EBV-related Hodgkin's and Burkitt's lymphoma cells (16,17).
Furthermore, curcumin promotes apoptosis in EBV-immortalized
lymphoblastoid cell lines (LCLs) (18,19).
These results suggest that EBV infection may
influence the chemopreventive properties exerted by curcumin
against iAs-induced toxicity. Thus, in the present study, two
independent EBV-immortalized LCLs were used to evaluate whether the
presence of EBV may modify the protective properties of curcumin
against the cytostatic and cytotoxic effects of iAs. The results
suggested that curcumin pre-treatment sensitized LCLs to
iAs-associated toxicity, induced a slight proportion of G1 phase
arrest and promoted cell death.
Materials and methods
Cell culture and reagents
The lymphoblastoid-derived cell lines CL-45 and
CL-49 had been generated by EBV immortalization of peripheral blood
mononuclear cells obtained from non-related healthy Mexican-mestizo
donors, as described in previous studies by our group (20,21).
The cell lines were cultured in RPMI-1640 medium supplemented with
10% fetal bovine serum, 1% L-alanyl-L-glutamine (GlutaMax 100x), 1%
non-essential amino acids and 1% antibiotic-antimycotic (10,000
U/ml penicillin and 10 mg/ml streptomycin). All cell culture
reagents were purchased from Gibco (Thermo Fisher Scientific,
Inc.). Cells were cultured at 37˚C in a humidified atmosphere with
5% CO2. Sodium arsenite (NaAsO2) as the iAs
and curcumin were purchased from Sigma-Aldrich (Merck KGaA) and
dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich; Merck KGaA)
or nuclease-free water (Sigma-Aldrich; Merck KGaA) to obtain 20 and
50 mM stock solutions, respectively. Curcumin and iAs stocks were
protected from light and aliquots were maintained at -20˚C until
use.
Cell treatments
CL-49 cells were seeded at a density of 200,000
cells/ml in 4 ml of complete growth medium and allowed to grow for
24 h prior to incubation with different concentrations of curcumin
(5, 10 or 15 µM) for 24 h or iAs (5, 10 or 20 µM) for 15 h. In the
case of iAs, the incubation time was selected considering a 9 h
pre-treatment with curcumin followed by the 15 h of iAs treatment,
which corresponds to a total incubation time of 24 h, the
approximate duration time of cell cycle in LCL. For time-response
assays, CL-49 cells were treated with 5 µM curcumin for 12, 18 or
24 h. As a negative control, cell cultures were treated with the
respective vehicles used with curcumin (0.1% DMSO) and iAs (0.1%
H2O) for 24 h in the concentration-response and
time-response assays. For the pre-treatment assays,
8x105 CL-45 and CL-49 cells were grown for 24 h and
treated with 5 µM curcumin for 9 h. Subsequently, the culture
medium with curcumin was removed and the cells were washed with 20
ml of 1X PBS. The cells were then re-cultured in fresh culture
medium and treated with 10 µM iAs for a further 15 h. Similar
washing and re-seeding protocols were used for the following
individual treatments groups: i) In the vehicle control group, cell
cultures were pre-treated with 0.1% DMSO for 9 h, followed by 15 h
with 0.1% nuclease-free water; ii) curcumin treatment alone
included incubation of cell lines with 5 µM curcumin for 9 h
followed by 0.1% H2O for 15 h; and iii) in the iAs-only
group, LCLs were pretreated with 0.1% DMSO for 9 h and then
incubated with 10 µM iAs for 15 h.
Cell death assay
After the treatments, LCLs were harvested and cell
death levels determined using the LIVE/DEAD® Fixable
Dead Cell Stain kit (Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. This assay is based on the use of a
cell-impermeable fluorescent dye that reacts with the amines of
cellular proteins. In viable cells, cell membrane integrity
prevents cell entry of the fluorescent compound, resulting in its
reaction with only the cell-surface amines and the production of a
low-intensity staining. However, in cells with compromised
membranes, i.e., dying cells, the fluorescent reagent reacts with
amines inside the cell and on its surface, producing intense
fluorescent staining. In brief, 8x105 cells were
resuspended in 1 ml of 1X PBS, stained with 1 µl of a 1:20 dilution
of LIVE/DEAD red fluorescent reactive dye and incubated for 30 min
at room temperature in the dark. The cell lines were then washed
and resuspended in 1 ml 1X PBS with 1% bovine serum albumin
(Sigma-Aldrich; Merck KGaA). Samples were analyzed with a BD
FACSAria III flow cytometer system (BD Biosciences, Inc.),
capturing at least 10,000 events for each sample. Data analysis was
performed using Flowing Software version 2.5.1 (Turku Centre for
Biotechnology).
Cell cycle assay
To analyze cell cycle profile changes in the
pre-treatment assays, cell lines were harvested, washed with 1X PBS
and fixed overnight at -20˚C with 1 ml of ice-cold 70% ethanol.
After fixation, the cells were washed with 1 ml cold 1X PBS and
resuspended in 250 µl of 1X PBS. The cellular suspension was then
incubated with 0.5 mg/ml RNAse A (Sigma-Aldrich; Merck KGaA) for 1
h at 37˚C. Finally, cells were stained with 10 µg/ml propidium
iodide (Sigma-Aldrich; Merck KGaA) for 60 min on ice. Samples were
analyzed using the BD FACSAria III flow cytometer system (BD
Biosciences, Inc.), capturing at least 20,000 events for each
sample. Cell cycle histograms were generated and analyzed using
ModFit LT 3.2 software (Verity Software House).
Statistical analysis
Values are expressed as the mean ± standard
deviation. Differences between groups were analyzed by one-way
analysis of variance followed by Tukey's multiple-comparisons test.
Statistical analysis was performed using GraphPad Prism 7 software
(version 5.01; GraphPad Software, Inc.). All data analyses were
performed using results from at least three independent biological
experiments. P<0.05 was considered to indicate statistical
significance.
Results
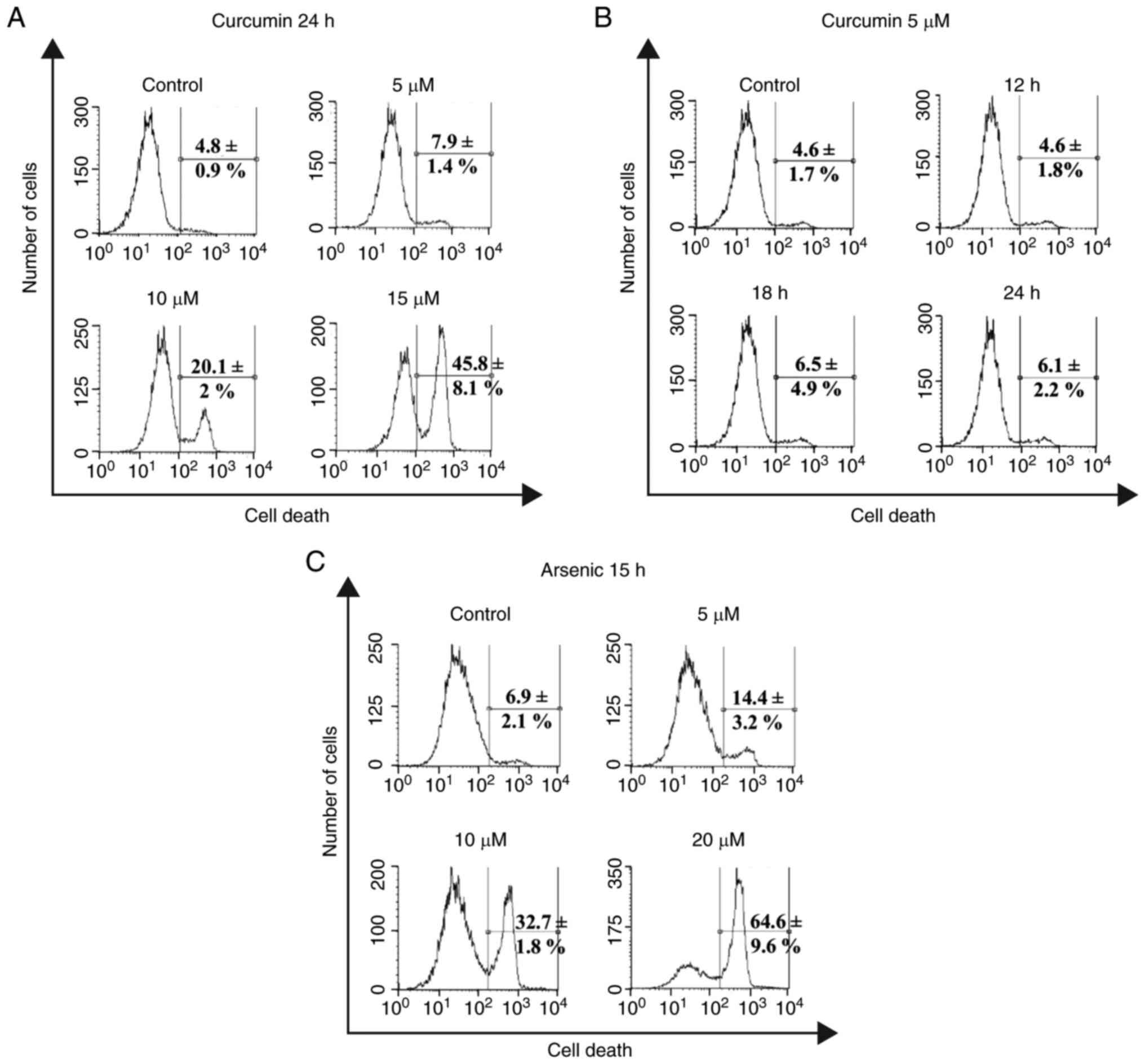
Cytotoxic effects of curcumin and
arsenic in EBV-immortalized LCLs
Curcumin is a phytochemical with chemopreventive
properties in normal cells but with cytotoxic effects in tumoral
and immortalized cells. Thus, the cytotoxic effects of curcumin
were evaluated in the EBV-immortalized lymphoblast cell line CL-49,
including the measurement of the fluorescent intensity of the
LIVE-DEAD viability dye through flow cytometry in
concentration-response assays. A concentration-dependent increase
was observed in the percentage of cell death after 24 h of
incubation with 10 µM (20.1±2%) and 15 µM (45.8±8.1%) curcumin
(Fig. 1A). By contrast, treatment
with 5 µM curcumin exhibited low toxicity compared with the vehicle
control (7.9±1.4 vs. 4.8±0.9%; Fig.
1A). Accordingly, in time-response assays using 5 µM curcumin,
in comparison with the vehicle control (4.6±1.7%), no significant
increase in the percentage of cell death was obtained after
incubation for 12 h (4.6±1.8%), 18 h (6.5±4.9%) or 24 h (6.1±2.2%)
(Fig. 1B). These results suggested
that 5 µM curcumin had no toxic effects on the CL-49 cell line.
The cytotoxic effect of iAs exposure for 15 h was
then evaluated in CL-49 cells using concentration-response curves.
This incubation time was selected based on the consideration of
using a curcumin pre-treatment time of 9 h, which together with iAs
incubation time for 15 h covers the LCL cell cycle of ~24 h. As
expected, iAs treatment increased the levels of cell death in a
concentration-dependent manner, starting at a concentration of as
low as 5 µM (14.4±3.2%) and up to 20 µM (64.6±9.6%), which was the
highest concentration used (Fig.
1C). The intermediate concentration of 10 µM iAs was used for
further curcumin pre-treatment assays because this concentration
may not be associated with massive necrotic cell death events, as
may occur with a highly toxic concentration of 20 µM. In addition,
the 10-µM concentration of iAs is equivalent to a high level of
human exposure through iAs-contaminated drinking water (750
µg/l).
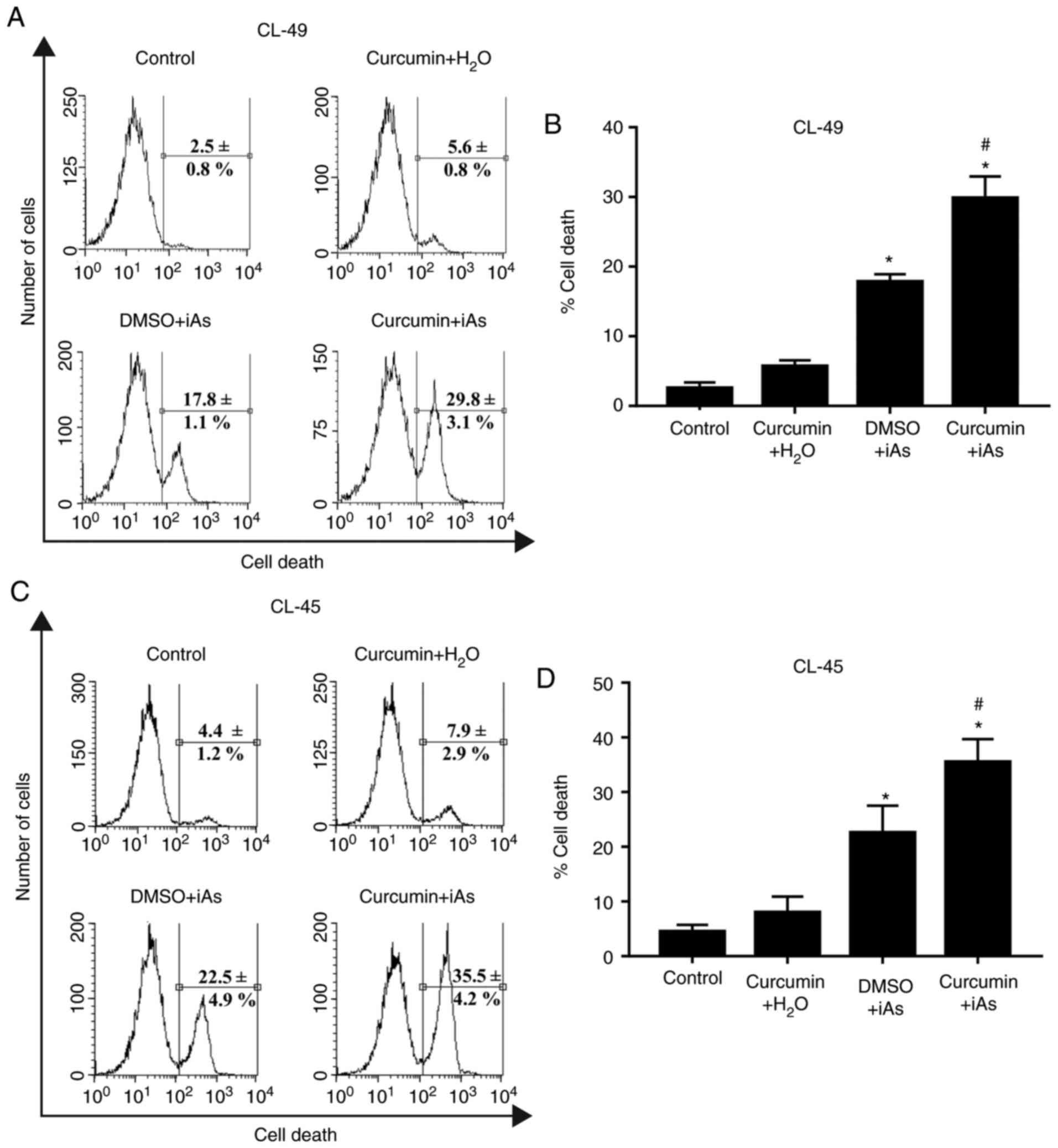
Curcumin enhances arsenic-induced
toxicity in EBV-immortalized lymphocytes
To explore the effect of curcumin on iAs-induced
toxicity on CL-49 cells, levels of cell death were analyzed after
treatment with 10 µM iAs for 15 h with or without pre-treatment
with 5 µM curcumin for 9 h. The percentage of cell death increased
from ~18% in the iAs-treated cultures in the absence of curcumin
pre-treatment to almost 30% in CL-49 cells incubated with curcumin
prior to iAs treatment (Fig. 2A and
B). As expected, pre-treatment with
5 µM curcumin followed by incubation with iAs vehicle
(H2O) resulted in a non-significant increase in cellular
toxicity in comparison with the vehicle control (5.6±0.8 vs.
2.5±0.8%; Fig. 2A and B). To rule out any cell line-specific
effect on the drug-sensitizing capacity of curcumin, the same
experimental conditions were applied in a second EBV-positive LCL
(CL-45 cells) derived from an independent healthy donor. A previous
study by our group suggested that treatment of CL-45 cells with 5
µM curcumin for 24 h did not significantly increase cell death
(19). Similar to the effect on
CL-49 cells, the CL-45 cell line treated with 10 µM iAs after
incubation with curcumin vehicle (DMSO) exhibited lower levels of
cell death than those observed in cell cultures treated with 5 µM
curcumin prior to iAs incubation (22.5±4.9 vs. 35.5±4.2%; Fig. 2C and D). These results suggested that curcumin
sensitizes EBV-immortalized LCLs to iAs-related toxicity.
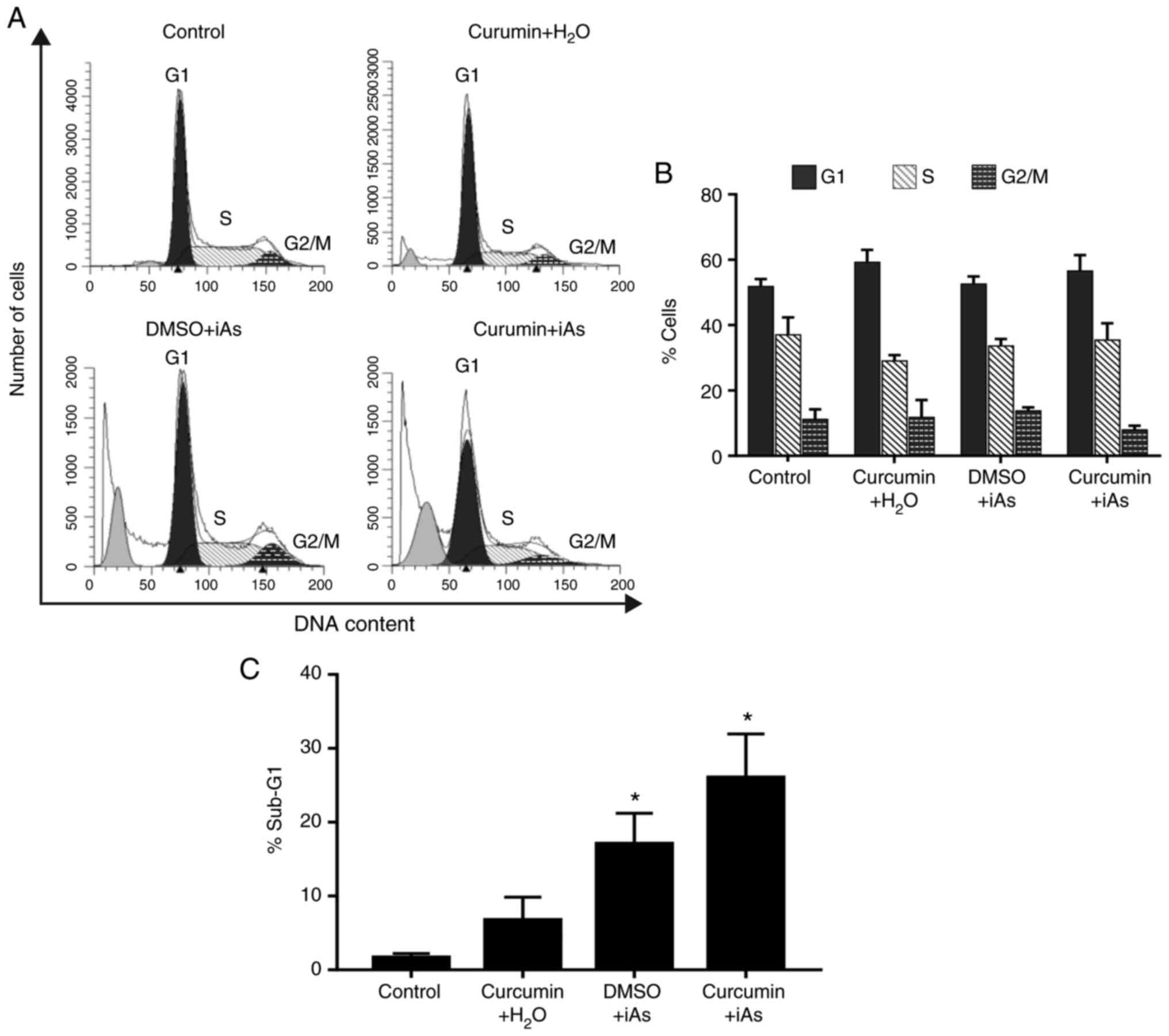
Cytostatic effect of curcumin and
arsenic on LCLs
Next, alterations in cell cycle progression induced
by curcumin sensitization to iAs toxicity were examined. As the
cytotoxic effect of curcumin on CL-49 and CL-45 was similar and
both cell lines were obtained from healthy individuals using the
same strain of EBV, it was decided to evaluate the cytostatic
effects of curcumin only in CL-49 cells. The results suggested that
treatment with 5 µM curcumin and iAs vehicle (H2O),
compared with the vehicle control, induced a significant
accumulation of cells in G1 phase (59.2±3.8 vs. 51.8±2.3%), without
any evident changes in the sub-G1 population (Fig. 3). In the group treated with iAs
alone, no apparent effect on cell cycle progression was observed,
while a significant increase in sub-G1 cells was detected in
comparison with the vehicle group (17.1±4.2 vs. 1.6±0.6%; Fig. 3).
Of note, CL-49 cells treated with curcumin followed
by iAs treatment exhibited a not significant increase in the number
of cells in the G1 and S phases in comparison with curcumin vehicle
and iAs treatment (G1: 56.5±4.9 vs. 52.5±2.3%; S: 35.4±5.1 vs.
33.7±2.1%; Fig. 3A and B). Consistent with the previous results
for cell death, the sub-G1 phase levels in the curcumin + iAs group
increased significantly with respect to cell cultures treated only
with iAs (26.0±5.9 vs. 17.1±4.2%; Fig.
3C). These results indicated that curcumin may enhance
iAs-related toxicity, possibly through cytostatic effects.
Discussion
Hydroarsenicism is a major health issue worldwide,
as ~100 million individuals are chronically exposed to this
pollutant (22). The natural
compound curcumin has emerged as an important alternative strategy
for diminishing the toxic effects caused by arsenic exposure
(8,9,11,12,23).
However, previous studies documented the cytotoxic effects of
curcumin in EBV immortalized lymphoblastoid cells lines and in cell
lines derived from tumors associated with chronic EBV infection
(15-19).
Considering the high prevalence of EBV infection in human
populations, the use of this phytochemical as a chemo-preventive
compound may be associated with different side effects on human
health.
Thus, in the present study, EBV-immortalized
lymphocytes were used as an experimental model to evaluate the
effect of latent EBV infection on the chemopreventive properties of
curcumin against iAs. First, a significant increase in CL-49 cell
death was observed after individual treatment with curcumin or iAs,
at concentrations ranging from 10 to 15 µM in the case of curcumin
and from 5 to 20 µM for iAs. Previous studies have described the
induction of cell death in EBV-immortalized LCLs treated with
curcumin in the range of 15 to 20 µM (18,19).
Furthermore, iAs-related induction of cell death by autophagy in
LCLs and by apoptosis in EBV-positive Burkitt's lymphoma cells has
been described at similar concentrations (24,25).
When the protective effect of non-toxic
concentrations of curcumin against iAs was evaluated in
EBV-immortalized lymphocytes, higher levels of cell death were
detected in cell cultures treated with curcumin prior to incubation
with iAs than after treatment with iAs alone. This result is in
clear contradiction with the chemopreventive effect exerted by
curcumin against iAs-induced genotoxic damage and toxicity reported
in different cellular and animal models (10,21,26-28).
In addition, pre-treatment with curcumin enhanced iAs-related
alterations in cell cycle progression, provoking a not significant
arrest in G1 and S phases and a reduction of cells in G2/M
phase.
In the flow cytometry assays, the presence of a
population of sub-G1 cells was observed after iAs treatment, with
or without curcumin pre-treatment (Fig.
3A), which is indicative of apoptosis. iAs promotes genotoxic
damage in LCLs and activates apoptosis with caspase processing in
EBV-positive Burkitt's lymphoma cells (25,29,30).
However, it was reported that autophagy is the predominant type of
cell death induced by iAs in human lymphocytes (24). Thus, further studies are necessary
to conclusively determine the type of cell death induced by
curcumin and iAs treatment in LCLs.
Previous studies also have described a delayed
progression from S to M phase in circulating lymphocytes extracted
from individuals chronically exposed to iAs through drinking water
(31,32). Similarly, iAs concentrations ranging
from 5 to 10 µM induce S-phase accumulation in cell lines derived
from bladder and breast carcinomas and in myeloid leukemia cell
lines after treatments for 12 to 24 h (33-36).
Of note, iAs-treated cells exhibit high caspase activity (35).
In the case of curcumin, its anti-proliferative
effects in EBV-immortalized LCLs have been reported to be
predominantly exerted through causing G1-phase arrest. However, in
cell lines derived from monocytic leukemia and gallbladder, breast
and colorectal cancer cells, the phytochemical was observed to
induce arrest in S phase (37-41).
In addition, curcumin causes DNA damage in human lymphocytes
(42,43) and activates apoptosis with
phosphatidylserine exposure, cytochrome C release, poly(ADP-ribose)
polymerase (PARP) cleavage and DNA damage in LCLs and EBV-positive
B-lymphoma cell lines (17,18,44,45).
Thus, in the current model, curcumin pretreatment increased the
genotoxic properties of iAs promoting an initial arrest in the cell
cycle, followed by induction of apoptosis.
The chemosensitizing effects of curcumin in
EBV-positive cells may be explained by its capacity to modulate the
EBV life cycle. Curcumin reduces levels of Epstein-Barr nuclear
antigen 1, a critical protein for viral latency maintenance,
promoting cell cycle arrest and apoptosis of EBV-nasopharyngeal
carcinoma cells (46). Similarly, a
previous study indicated that curcuminoids promote EBV lytic cycle
reactivation, increasing the cell death of gastric and
nasopharyngeal cell lines when combined with other lytic activators
such as gemcitabine and valproic acid (15).
Of note, the concentration of curcumin used in the
present study was significantly lower than that associated with
adverse effects on human health. For instance, a study evaluating
the effect of curcumin on DNA damage repair potential used an
intervention of 500 mg (1.36 mM) twice a day for 3 months in 66
healthy volunteers without any apparent adverse effects on their
health (12). Another study
demonstrated the safety of ingesting increasing concentrations of a
commercial curcumin formulation (C3 Complex™) of up to 12,000 mg
(32.6 mM) in healthy individuals, revealing minimal toxicity that
did not appear to be dose-related. The adverse health effects of
the highest doses were headache and diarrhea, both classified as
grade I according to National Cancer Institute, Common Toxicity
Criteria version 2.0(47).
The present study, as far as may be discerned, was
the first to assess the chemosensitizing capacity of curcumin to
iAs toxicity in LCLs. However, the present study had several
limitations. For instance, the type of cell death (e.g. necrosis or
apoptosis) observed after the different treatments (i.e. curcumin,
iAs and curcumin plus iAs) was not characterized by using
differential assays such as Annexin V/PI staining, caspase
processing, PARP cleavage or DNA fragmentation. In addition,
evaluation of the alteration of RNA or protein levels of cell cycle
regulators was also lacking. This indicates the necessity of
characterizing the specific type of cell death and the cellular
mechanisms activated by curcumin pre-treatment before LCLs are
exposed to iAs. In the same sense, the cell cycle alterations
associated with the sensitizing effects of curcumin should be
evaluated. Further studies are warranted to test the sensitizing
effect of this phytochemical to iAs in primary lymphocytes and
other EBV-infected cells.
In conclusion, the results of the present study
indicated that curcumin pre-treatment sensitized EBV-positive LCLs
to iAs toxicity. Thus, further studies analyzing the use of
curcumin as a strategy for reducing iAs toxicity in individuals
with chronic EBV infection are required.
Acknowledgements
The authors would like to acknowledge Mrs. Linda
Nelly Patiño Uriostegui (National Institute of Genomic Medicine,
Flow cytometry Lab; Mexico) and Mr. José Luis Cruz-Colin (National
Institute of Genomic Medicine, Clinic Research; Mexico) for their
valuable technical assistance.
Funding
Funding: This work was supported by a grant from Secretary of
Public Education-National Council for Science and Technology
(SEP-CONACYT; grant no. 243587) and from the National Institute of
Genomic Medicine (INMEGEN; grant no. CON31/2015). MMC received a
fellowship from the National System of Researchers in Mexico.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
Conceived and designed the experiments: EJC, AHZ and
MMC. Performed the experiments: MMC and GCR. Wrote the manuscript:
EJC, AHZ and MMC. Coordinated and facilitated the project: EJC. EJC
and AHZ confirmed the authenticity of the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chung J, Yu S and Hong Y: Environmental
source of arsenic exposure. J Prev Med Public Health. 47:253–257.
2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shaji E, Santosh M, Sarath KV, Prakash
Pranav, Deepchand V and Divya BD: Arsenic contamination of
groundwater: A global synopsis with focus on the Indian peninsula.
Geosci Front. 12(101079)2021.
|
|
3
|
Sinha D and Prasad P: Health effects
inflicted by chronic low-level arsenic contamination in
groundwater: A global public health challenge. J Appl Toxicol.
40:87–131. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Carlin DJ, Naujokas MF, Bradham KD, Cowden
J, Heacock M, Henry HF, Lee JS, Thomas DJ, Thompson C, Tokar EJ, et
al: Arsenic and environmental health: State of the science and
future research opportunities. Environ Health Perspect.
124:890–899. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Amadi CN, Offor SJ, Frazzoli C and
Orisakwe OE: Natural antidotes and management of metal toxicity.
Environ Sci Pollut Res Int. 26:18032–18052. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hewlings SJ and Kalman DS: Curcumin: A
review of its effects on human health. Foods. 6(92)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shah BH, Nawaz Z, Pertani SA, Roomi A,
Mahmood H, Saeed SA and Gilani AH: Inhibitory effect of curcumin, a
food spice from turmeric, on platelet-activating factor- and
arachidonic acid-mediated platelet aggregation through inhibition
of thromboxane formation and Ca2+ signaling. Biochem Pharmacol.
58:1167–1172. 1999.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhao R, Yang B, Wang L, Xue P, Deng B,
Zhang G, Jiang S, Zhang M, Liu M, Pi J and Guan D: Curcumin
protects human keratinocytes against inorganic arsenite-induced
acute cytotoxicity through an NRF2-dependent mechanism. Oxid Med
Cell Longev. 2013(412576)2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hosseinzadehdehkordi M, Adelinik A and
Tashakor A: Dual effect of curcumin targets reactive oxygen
species, adenosine triphosphate contents and intermediate steps of
mitochondria-mediated apoptosis in lung cancer cell lines. Eur J
Pharmacol. 769:203–210. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tiwari H and Rao MV: Curcumin
supplementation protects from genotoxic effects of arsenic and
fluoride. Food Chem Toxicol. 48:1234–1238. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Biswas J, Sinha D, Mukherjee S, Roy S,
Siddiqi M and Roy M: Curcumin protects DNA damage in a chronically
arsenic-exposed population of West Bengal. Hum Exp Toxicol.
29:513–524. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Roy M, Sinha D, Mukherjee S and Biswas J:
Curcumin prevents DNA damage and enhances the repair potential in a
chronically arsenic-exposed human population in West Bengal, India.
Eur J Cancer Prev. 20:123–131. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ali AS, Al-Shraim M, Al-Hakami AM and
Jones IM: Epstein-Barr virus: Clinical and epidemiological revisits
and genetic basis of oncogenesis. Open Virol J. 9:7–28.
2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Geng L and Wang X: Epstein-Barr
virus-associated lymphoproliferative disorders: Experimental and
clinical developments. Int J Clin Exp Med. 8:14656–14671.
2015.PubMed/NCBI
|
|
15
|
Ramayanti O, Brinkkemper M, Verkuijlen
SAWM, Ritmaleni L, Go ML and Middeldorp JM: Curcuminoids as EBV
lytic activators for adjuvant treatment in EBV-positive carcinomas.
Cancers (Basel). 10(89)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mackenzie GG, Queisser N, Wolfson ML,
Fraga CG, Adamo AM and Oteiza PI: Curcumin induces cell-arrest and
apoptosis in association with the inhibition of constitutively
active NF-kappaB and STAT3 pathways in Hodgkin's lymphoma cells.
Int J Cancer. 123:56–65. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li ZX, Ouyang KQ, Jiang X, Wang D and Hu
Y: Curcumin induces apoptosis and inhibits growth of human
Burkitt's lymphoma in xenograft mouse model. Mol Cells. 27:283–289.
2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ranjan D, Johnston TD, Reddy KS, Wu G,
Bondada S and Chen C: Enhanced apoptosis mediates inhibition of
EBV-transformed lymphoblastoid cell line proliferation by curcumin.
J Surg Res. 87:1–5. 1999.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Méndez-García LA, Martínez-Castillo M,
Villegas-Sepúlveda N, Orozco L and Córdova EJ: Curcumin induces
p53-independent inactivation of Nrf2 during oxidative
stress-induced apoptosis. Hum Exp Toxicol. 38:951–961.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Córdova EJ, Martínez-Hernández A,
Uribe-Figueroa L, Centeno F, Morales-Marín M, Koneru H, Coleman MA
and Orozco L: The NRF2-KEAP1 pathway is an early responsive gene
network in arsenic exposed lymphoblastoid cells. PLoS One.
9(e88069)2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Morales-Marin ME, Cordova EJ, Centeno F,
Martínez-Hernández A, Méndez-García A, Molina B, Frías S and Orozco
L: NFE2L2 gene variants and arsenic susceptibility: A
lymphoblastoid model. J Toxicol Environ Health A. 78:628–634.
2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Shankar S, Shanker U and Shikha :
Arsenic contamination of groundwater: A review of sources,
prevalence, health risks, and strategies for mitigation.
ScientificWorldJournal. 2014(304524)2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mukherjee S, Roy M, Dey S and Bhattacharya
RK: A mechanistic approach for modulation of arsenic toxicity in
human lymphocytes by curcumin, an active constituent of medicinal
herb curcuma longa linn. J Clin Biochem Nutr. 41:32–42.
2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bolt AM, Byrd RM and Klimecki WT:
Autophagy is the predominant process induced by arsenite in human
lymphoblastoid cell lines. Toxicol Appl Pharmacol. 244:366–373.
2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zebboudj A, Maroui MA, Dutrieux J,
Touil-Boukoffa C, Bourouba M, Chelbi-Alix MK and Nisole S: Sodium
arsenite induces apoptosis and Epstein-Barr virus reactivation in
lymphoblastoid cells. Biochimie. 107:247–256. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Khan S, Vala JA, Nabi SU, Gupta G, Kumar
D, Telang AG and Malik JK: Protective effect of curcumin against
arsenic-induced apoptosis in murine splenocytes in vitro. J
Immunotoxicol. 9:148–159. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
El-Demerdash FM, Yousef MI and Radwan FME:
Ameliorating effect of curcumin on sodium arsenite-induced
oxidative damage and lipid peroxidation in different rat organs.
Food Chem Toxicol. 47:249–254. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gao S, Duan X, Wang X, Dong D, Liu D, Li
X, Sun G and Li B: Curcumin attenuates arsenic-induced hepatic
injuries and oxidative stress in experimental mice through
activation of Nrf2 pathway, promotion of arsenic methylation and
urinary excretion. Food Chem Toxicol. 59:739–747. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rasmussen RE and Menzel DB: Variation in
arsenic-induced sister chromatid exchange in human lymphocytes and
lymphoblastoid cell lines. Mutat Res. 386:299–306. 1997.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Guillamet E, Creus A, Ponti J, Sabbioni E,
Fortaner S and Marcos R: In vitro DNA damage by arsenic compounds
in a human lymphoblastoid cell line (TK6) assessed by the alkaline
Comet assay. Mutagenesis. 19:129–135. 2004.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ostrosky-Wegman P, Gonsebatt ME, Montero
R, Vega L, Barba H, Espinosa J, Palao A, Cortinas C, García-Vargas
G and del Razo LM: Lymphocyte proliferation kinetics and genotoxic
findings in a pilot study on individuals chronically exposed to
arsenic in Mexico. Mutat Res. 250:477–482. 1991.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gonsebatt ME, Vega L, Montero R,
Garcia-Vargas G, Del Razo LM, Albores A, Cebrian ME and
Ostrosky-Wegman P: Lymphocyte replicating ability in individuals
exposed to arsenic via drinking water. Mutat Res. 313:293–299.
1994.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hernández-Zavala A, Córdova E, Del Razo
LM, Cebrián ME and Garrido E: Effects of arsenite on cell cycle
progression in a human bladder cancer cell line. Toxicology.
207:49–57. 2005.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Pozo-Molina G, Ponciano-Gómez A,
Rivera-González GC, Hernández-Zavala A and Garrido E:
Arsenic-induced S phase cell cycle lengthening is associated with
ROS generation, p53 signaling and CDC25A expression. Chem Biol
Interact. 238:170–179. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
McCollum G, Keng PC, States JC and McCabe
MJ Jr: Arsenite delays progression through each cell cycle phase
and induces apoptosis following G2/M arrest in U937 myeloid
leukemia cells. J Pharmacol Exp Ther. 313:877–887. 2005.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lehmann GM and McCabe MJ: Arsenite slows S
phase progression via inhibition of cdc25A dual specificity
phosphatase gene transcription. Toxicol Sci. 99:70–78.
2007.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhu GH, Dai HP, Shen Q, Ji O, Zhang Q and
Zhai YL: Curcumin induces apoptosis and suppresses invasion through
MAPK and MMP signaling in human monocytic leukemia SHI-1 cells.
Pharm Biol. 54:1303–1311. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Liu TY, Tan ZJ, Jiang L, Gu JF, Wu XS, Cao
Y, Li ML, Wu KJ and Liu YB: Curcumin induces apoptosis in
gallbladder carcinoma cell line GBC-SD cells. Cancer Cell Int.
13(64)2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Saleh EM, El-awady RA, Eissa NA and
Abdel-Rahman WM: Antagonism between curcumin and the topoisomerase
II inhibitor etoposide: A study of DNA damage, cell cycle
regulation and death pathways. Cancer Biol Ther. 13:1058–1071.
2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lu JJ, Cai YJ and Ding J: Curcumin induces
DNA damage and caffeine-insensitive cell cycle arrest in colorectal
carcinoma HCT116 cells. Mol Cell Biochem. 354:247–252.
2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Xiang L, He B, Liu Q, Hu D, Liao W, Li R,
Peng X, Wang Q and Zhao G: Antitumor effects of curcumin on the
proliferation, migration and apoptosis of human colorectal
carcinoma HCT-116 cells. Oncol Rep. 44:1997–2008. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Błasiak J, Trzeciak A, Małecka-Panas E,
Drzewoski J, Iwanienko T, Szumiel I and Wojewódzka M: DNA damage
and repair in human lymphocytes and gastric mucosa cells exposed to
chromium and curcumin. Teratog Carcinog Mutagen. 19:19–31.
1999.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Sebastià N, Soriano JM, Barquinero JF,
Villaescusa JI, Almonacid M, Cervera J, Such E, Silla MA and
Montoro A: In vitro cytogenetic and genotoxic effects of curcumin
on human peripheral blood lymphocytes. Food Chem Toxicol.
50:3229–3233. 2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Chen C, Johnston TD, Jeon H, Gedaly R,
McHugh PP, Burke TG and Ranjan D: An in vitro study of liposomal
curcumin: Stability, toxicity and biological activity in human
lymphocytes and Epstein-Barr virus-transformed human B-cells. Int J
Pharm. 366:133–139. 2009.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Han SS, Chung ST, Robertson DA, Ranjan D
and Bondada S: Curcumin causes the growth arrest and apoptosis of B
cell lymphoma by downregulation of egr-1, C-myc, Bcl-XL, NF-kappaB,
and p53. Clin Immunol. 93:152–161. 1999.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Liu L, Yang J, Ji W and Wang C: Curcumin
inhibits proliferation of epstein–barr virus-associated human
nasopharyngeal carcinoma cells by inhibiting EBV nuclear antigen 1
expression. BioMed Res Int. 2019(8592921)2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Lao CD, Ruffin MT IV, Normolle D, Heath
DD, Murray SI, Bailey JM, Boggs ME, Crowell J, Rock CL and Brenner
DE: Dose escalation of a curcuminoid formulation. BMC Complement
Altern Med. 6(10)2006.PubMed/NCBI View Article : Google Scholar
|