Introduction
Trichinellosis is a foodborne parasitic disease with
worldwide distribution (1) that can
cause acute and chronic illness (2). This zoonosis is a public health
problem by affecting human patients and an economic issue in
porcine animal production and food safety (3).
Trichinella infection in humans can be
divided into two phases: An intestinal (enteral) phase and a
muscular (systemic) phase (3). The
symptoms are highly correlated with the stage of the infection
(4) and the severity of clinical
symptoms are directly correlated with the number of larvae ingested
(3) and with the number of larvae
produced by adult worms in the small intestine (5). Therefore, symptoms may range from mild
transient diarrhea, nausea, upper abdominal pain, vomiting (can
appear approximately 2 days post infection) followed by myalgia,
periorbital and/or facial edema, conjunctivitis, fever, headache,
skin rash (3,4) to complications such as myocarditis and
encephalitis (6). Despite therapy,
lethality in severe cases has been reported at a rate of up to 5%
(3).
To ensure food safety for consumers, the European
Commission (EC) legislation requires mandatory meat inspection for
farmed and wild animal species slaughtered for consumption (e.g.,
domestic pigs, horses and wild boar) (3).
Romania, the largest southeast European country, has
reported the highest number of human trichinellosis cases in the
European Union since 2007 (7,8). The
aim of this study was to evaluate the current epidemiological,
laboratory, clinical and therapeutic aspects of human
trichinellosis in Western Romania, addressing the question of
whether this zoonosis is still an important health problem in this
well-known Romanian endemic region.
Patients and methods
This retrospective study was conducted in 2 phases
and included 83 consecutive patients diagnosed and hospitalized
with trichinellosis in Western Romania between January 1st, 2012
and December 31st, 2016 in three counties located in Western
Romania (Timis County, Arad County and Hunedoara County) with a
total population of 1,532,734. In the first phase, we investigated
the clinical database in a major infectious disease hospital from
Timis County, between January 1st, 2012 and June 30th, 2016.
Twenty-three patients were diagnosed and hospitalized with
trichinellosis (9). In the second
phase we extended the studied period (between January 1st, 2012 and
December 31st, 2016) and the research area with two more counties
in Western Romania (Arad County and Hunedoara County). Another 60
patients were identified. Medical records of the patients diagnosed
with trichinellosis were reviewed. From the medical records we
collected data regarding age, sex, environment, clinical
symptomatology, length of hospital stay, laboratory investigation
results (leukocyte and eosinophil count, erythrocyte sedimentation
rate, serologic testing for Trichinella) and specific
therapy. According to the medical records, the diagnosis was
established based on history, clinical symptoms,
Trichinella-specific antibody response (ELISA), detection of
Trichinella larvae (in patient muscle biopsy and/or in the
food item) and epidemiological links (exposure to a common source
or contaminated meat) (10).
Individuals were grouped in two age categories, older and younger
than 15 years of age (Table I).
 | Table IDistribution of trichinellosis cases
in Western Romania by age categories, sex and area of residence
(n=83). |
Table I
Distribution of trichinellosis cases
in Western Romania by age categories, sex and area of residence
(n=83).
| | Sex | Area of
residence |
|---|
| Age categories | No. of cases (%) | Male | Female | Urban | Rural |
|---|
| <15 years | 10(12) | 3 | 7 | 4 | 6 |
| >15 years | 73(88) | 45 | 28 | 23 | 50 |
| Total | 83(100) | 48 | 35 | 27 | 56 |
Data were compiled in a Microsoft Excel database,
version 2011 (Microsoft Corp.). Statistical analysis was performed
using Epi Info Version 7.2 (CDC). Bartlett's Test was used to
determine whether the variables were distributed normally.
Descriptive statistics (mean, standard deviation, percentage,
median and interquartile range) were calculated as appropriate.
Student's t-test and Fisher exact test were used to evaluate
differences between the studied groups. Mann-Whitney U test was
used for non-parametric distribution. A probability level of
P<0.05 was considered to indicate statistical significance.
This study was approved by each of the infectious
disease hospital's ethics committee: ‘Victor Babes’ Infectious
Diseases Hospital Timisoara, Timis County; Infectious Disease
Hospital Arad, Arad County; Municipal Hospital ‘Doctor Alexandru
Simionescu’ Hunedoara, Hunedoara County.
Results
Eighty-three patients were diagnosed and
hospitalized with trichinellosis in Western Romania. Patients were
aged between 2 and 78 years (mean age, 35.9); 57.8% (48/83) were
males and 32.5% (27/83) were inhabitants of urban areas. The
majority of patients were aged over 15 years [88% (73/83)]
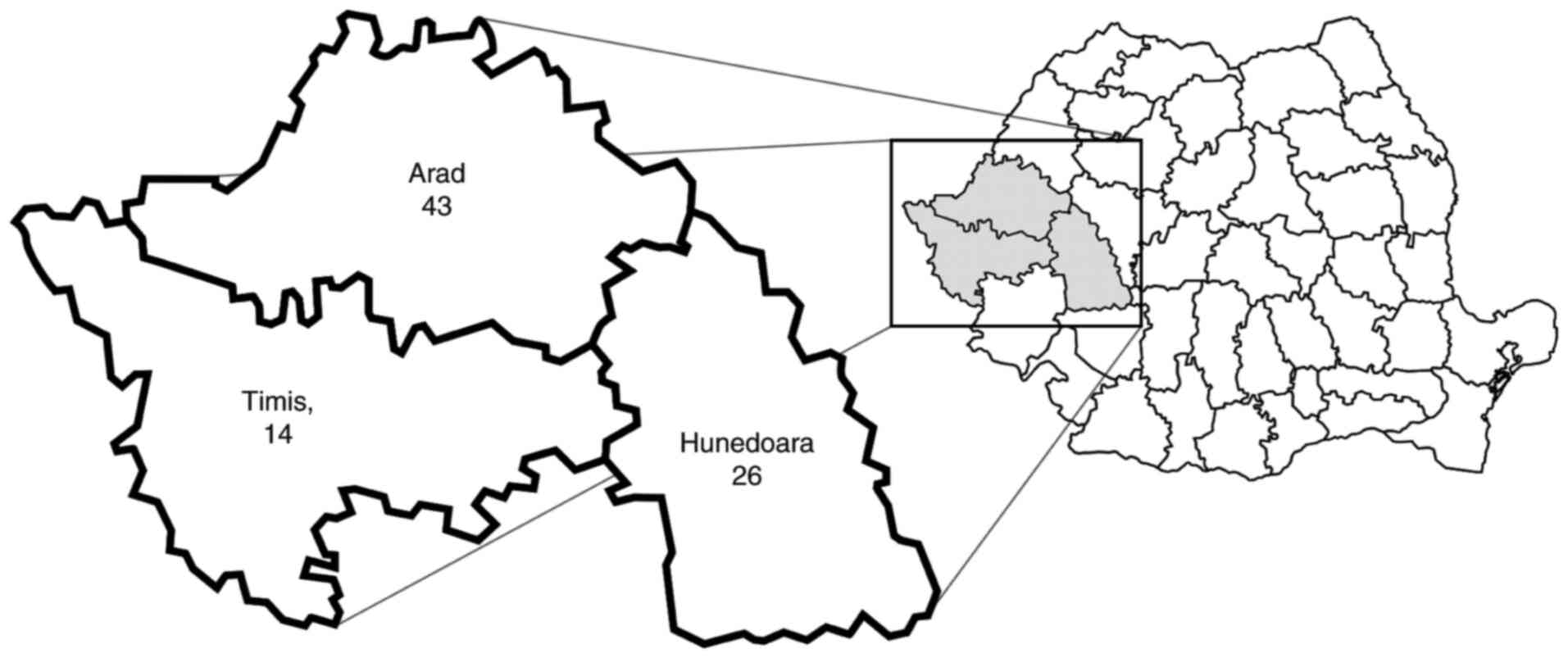
(Table I). The patients were
residents of Arad (n=43; 51.8%), Timis (n=14; 16.9%) and Hunedoara
(n=26; 31.3%) counties (Fig. 1).
The incidence (cases per 100,000 inhabitants) for the studied
period was 9.98 in Arad, 2.04 in Timis and 6.21 in Hunedoara.
The most frequent symptoms included: myalgia in 66
(79.5%), fever in 55 (66.3%), eyelid edema in 40 (48.2%) and
asthenia in 35 (42.2%). Other clinical findings included headache,
diarrhea, abdominal pain and cutaneous rash (Table II). Clinical signs were reported 1
to 30 days (average incubation period was 7.6 days) after eating
contaminated meat (raw pork products from domestic pigs raised and
slaughtered in their own backyard and/or wild boar from
noncommercial sources). Sixty-eight (81.9%) patients presented
symptoms in the first 10 days.
 | Table IIReported clinical symptoms in patients
hospitalized with trichinellosis included in the study (n=83). |
Table II
Reported clinical symptoms in patients
hospitalized with trichinellosis included in the study (n=83).
| | Symptoms n (%) |
|---|
| Year | No. of cases | Myalgia | Fever | Eyelid edema | Asthenia | Headache | Diarrhea | Abdominal pain | Cutaneous rash |
|---|
| 2012 | 26 | 23 (88.4) | 19 (73.1) | 6 (23.1) | 6 (23.8) | 2 (7.7) | 8 (30.7) | 2 (7.7) | 5 (19.2) |
| 2013 | 2 | 2(100) | 1 (50.0) | 2(100) | 1 (50.0) | 2(100) | 0 (0) | 0 (0) | 0 (0) |
| 2014 | 41 | 32 (78.0) | 23 (56.1) | 28 (68.3) | 24 (58.5) | 16 (39.0) | 11 (26.8) | 2 (4.9) | 1 (2.44) |
| 2015 | 4 | 2 (50.0) | 4(100) | 1 (25.0) | 1 (25.0) | 3 (75.0) | 2 (50.0) | 0 (0) | 0 (0) |
| 2016 | 10 | 7 (70.0) | 8 (80.0) | 3 (30.0) | 3 (30.0) | 2 (20.0) | 1 (10.0) | 1 (10.0) | 1 (10.0) |
| Total | 83 | 66 (79.5) | 55 (66.3) | 40 (48.2) | 35 (42.2) | 25 (30.1) | 22 (26.5) | 5 (6.0) | 7 (8.43) |
Hospitalization period ranged between 1 and 17 days
(average, 7.5 days). In 22 (26.5%) patients, this period ranged
between 10 and 17 days and required significant health care
resources.
Of the 83 patients, 43 (51.8%) had high levels of
white blood cells (more than 10,000/mm3). The mean value
of the leukocyte count was higher in patients aged over 15 years
than in patients aged below 15 years (P=0.070). Seventy-six (91.6%)
patients had eosinophilia more than 5%. The mean value of the
eosinophil count was significantly higher in patients aged over 15
years compared to patients aged below 15 years (P=0.003). The
median erythrocyte sedimentation rate (ESR) value was significantly
higher in patients aged over 15 years compared to patients aged
below 15 years (P=0.020; Table
III). Sixty-four patients (77.1%) had ESR higher than 10
mm/h.
 | Table IIILaboratory data in patients diagnosed
with trichinellosis. |
Table III
Laboratory data in patients diagnosed
with trichinellosis.
| | Age group
(years) | |
|---|
| | | | <15 | >15 | |
|---|
| Variable | Normal values | Range of values
(N=83) | Range of values | P-value |
|---|
| Leukocyte count
(cells/mm3) | 4,500-9,999 | 4,100-45,560
(12,526.8±6,941.8)a | 5,700-18,480
(9,896±4,265.9)a | 4,100-45,560
(12,887.3±7,177.4)a | 0.070 |
| Eosinophil count
(%) | 0-4.9 | 0.2-69.9
(21±15.9)a | 0.3-30.8
(10±9.8)a | 0.2-69.9
(22.5±15.9)a | 0.003 |
| Erythrocyte
sedimentation rate (mm/h) | 0-10 | 2-80 15
(10-23)b | 5-26 9
(5-14)b | 2-80 15
(10-23)b | 0.020 |
Sixty-nine patients (83.1%) received treatment with
albendazole, 14 (16.9%) with mebendazole and 47 (56.6%) patients
received associated corticotherapy. Seven patients continued
antiparasitic treatment after discharge between 2 and 13 days.
Of the 83 patients included in the study, one male
patient maintained his motor deficit on the left side after
discharge and two patients with neurological complications (stroke,
right hemiplegia) died. The other patients had favorable
outcome.
Over a 5-year period, the year with the highest
reported number of trichinellosis cases was 2014 (41/83, 49.4%)
(Table II). November was the month
with the highest reported rate, with 32 (38.6%) cases, followed by
January with 26 (31.3%), December with 9 (10.8%), April with 6
(7.2%), May with 3 (3.6%), February and March with 2 (2.4%), June,
August and September with one case (1.2%).
Seventy-five patients from 10 different family
outbreaks met the epidemiological criteria of exposure to a common
source of infection. Five of these patients were from three family
outbreaks and met the epidemiological criteria of exposure to
confirmed contaminated meat products (traced back to
trichinelloscopy-positive meat). Eight patients were sporadic
cases. Eleven patients from 5 different family outbreaks and one
sporadic case were tested for Trichinella-specific antibody
response (ELISA) and 8 had positive results. Patients who had
negative results met the epidemiological criteria for diagnosis.
Two sporadic cases were confirmed by muscle biopsy (Table IV).
 | Table IVDistribution of trichinellosis cases
in Western Romania by family outbreak, county, epidemiological
link, laboratory criteria and infection source (n=83). |
Table IV
Distribution of trichinellosis cases
in Western Romania by family outbreak, county, epidemiological
link, laboratory criteria and infection source (n=83).
| | Laboratory
criteria | Epidemiological
link | |
|---|
| Studied period | County | Family outbreak
(cases) | Common source | Contaminated meat
products (trichineloscopy) | Trichinella-specific
antibody (ELISA)+ |
Trichinella-specific antibody
(ELISA)- | Muscle biopsy | Food source |
|---|
| 2012 | Timis | 4 | Yes | - | - | 2 | - | Wild boar |
| | | 3 | Yes | - | 1 | - | - | Pork |
| | Hunedoara | 6 | Yes | - | 1 | 1 | - | Pork |
| | | 4 | Yes | - | - | - | - | Pork |
| | | 8 | Yes | 2 yes | - | - | - | Pork |
| | | 1 (sporadic) | - | - | - | - | - | Pork |
| 2013 | Hunedoara | 2 (sporadic) | - | - | - | - | - | Pork |
| 2014 | Arad | 34 | Yes | 1 yes | 3 | - | - | Pork |
| | | 1 (sporadic) | - | - | - | - | - | Unknown |
| | Hunedoara | 5 | Yes | 2 yes | - | - | - | Pork |
| | Timis | 1 (sporadic) | - | - | - | - | Yes | Pork |
| 2015 | Timis | 2 | Yes | - | 2 | - | - | Pork |
| | Arad | 1 (sporadic) | - | - | | - | Yes | Unknown |
| | | 1 (sporadic) | - | - | 1 | - | - | Unknown |
| 2016 | Timis | 3 | Yes | - | - | - | - | Pork |
| | | 1 (sporadic) | - | - | - | - | - | Pork |
| | Arad | 6 | Yes | - | - | - | - | Pork |
Overall 73 patients were classified as probable
cases and 10 were classified as confirmed cases.
Pork meat products from privately raised animals
were consumed in 76 (91.6%) patients and wild boar meat products
from noncommercial sources in 4 (4.8%); these 4 patients were all
hospitalized in 2012 and were residents of Timis county. The
proportion of pork meat products as food source cases was
significantly higher in patients with exposure to a common source
(epidemiological links) [odds ratio (OR)=10.6; 95% confidence
interval (CI), 1.85-61.3; P=0.01].
Discussion
Modern pork production systems and slaughter
inspection programs have reduced or eliminated pork as a source for
trichinellosis in many European Union (EU) countries (1). In the EU, most pigs are subject to
official meat inspection at slaughter in accordance with Regulation
(EC) No 2015/1375; only pigs slaughtered for own consumption are
not covered by this regulation (11).
The number of cases and the EU notification rate
have been steadily decreasing since 2012, and in 2016 the lowest
rate (0.02) was reported since the beginning of the EU-level
surveillance. The decrease was mainly due to a markedly reduced
number of trichinellosis cases reported by Bulgaria and Romania,
which had experienced most Trichinella outbreaks in previous
years (11). Educating consumers
(eating only well-cooked meat), improvements in pig-rearing
practices, and increased control measures at slaughterhouses may
explain this decrease (12).
Our findings were lower than the number of
trichinellosis hospitalized cases previously reported in Western
Romania: during 2007-2009, trichinellosis was diagnosed in 91
individuals from Arad (n=49) and Timis (n=42) counties (13); during 1996-2005, 492 patients with
trichinellosis were reported in Hunedoara county alone (14). Therefore, our results suggest a
decreasing trend of trichinellosis in Western Romania. However, the
number of cases from Western Romania exceeded the number of
confirmed cases at a national level in countries such as Belgium,
Germany, Latvia, Lithuania, Poland and Spain according to the ECDC
database (7).
Prevention and control of trichinellosis is a matter
of education and hygiene in the environment and at home (2). Of the 83 patients with trichinellosis,
10 (12%) were aged below 15 years. This suggests a lack of
education in parents (15). Western
Romania was part of the Austrian-Hungarian empire until 1918 and
populations living in this area may have maintained various customs
and food habits even today (raw meat products consumption), which
are known to be risk factors for trichinellosis (16).
Generally, traditional pork products made with the
infected meat are often given as gifts to relatives or friends
residing in urban regions, because there is a preference for
home-made products over commercially processed meat products and
often cause small and clustered outbreaks in
Trichinella-endemic regions. Frequently, people from urban
areas buy their own pigs, which are raised and slaughtered by their
friends or relatives in the countryside (13). Between 1998 and 2011, in Western
Romania, the annual average number of infected pigs on farms was
48.6 and in private households 29.1(17).
No species identification of Trichinella was
carried out in this study. We focused on the epidemiological
aspects of trichinellosis in Western Romania based on the available
information collected from the patient medical records.
Trichinellosis can be a serious human disease,
particularly in elderly individuals, in whom severe complications
such as myocarditis or encephalitis can lead to death (18).
In Romania, trichinellosis may not be diagnosed and
reported unless infection is sufficiently severe to require
hospitalization or the cases are part of a larger outbreak that
requires attention from Public Health Authorities (19). It is highly likely that the true
prevalence of human trichinellosis in Romania is higher than the
reported cases, due to multiple causes. Asymptomatic and
subclinical infections may be undiagnosed. Consequently, they are
not usually officially recorded as having trichinellosis (19).
Public health strategies should be maintained and
permanently improved. Good management practices that eliminate the
risk of exposure to Trichinella infection should be
administratively organized, and more efficient and rigorous
educational programs for both swine breeders and consumers
(especially through the mass media) are imperative in order to
prevent and eradicate trichinellosis in endemic areas.
Implementation of strict hygienic measures aimed to control
infection transmission are strongly recommended. When an acute
infection is diagnosed, screening of family members and/or
individuals who consumed the same infected meat products should be
highly considered, in order to apply the preventive strategies
and/or therapeutic actions.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data analyzed during this study are included in
this published article.
Authors' contributions
MAL was responsible for the study design,
implementation of the research, data collection, data analysis, and
writing. RP was responsible for the study design, implementation of
the research, data collection, data analysis, and writing. VEL was
responsible for the data collection and implementation of the
research. EDP conducted the data analysis, and epidemiological
study. TRO was responsible for the study design, devised the
project, verified the analytical methods, and helped supervise the
project. All authors discussed the results, provided critical
feedback and contributed to the final draft of the manuscript. All
authors read and approved the final manuscript for publication.
Ethics approval and consent to
participate
This study was approved by each of the infectious
disease hospital's ethics committee: ‘Victor Babes’ Infectious
Diseases Hospital Timisoara, Timis County; Infectious Disease
Hospital Arad, Arad County; Municipal Hospital ‘Doctor Alexandru
Simionescu’ Hunedoara, Hunedoara County.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rostami A, Gamble HR, Dupouy-Camet J,
Khazan H and Bruschi F: Meat sources of infection for outbreaks of
human trichinellosis. Food Microbiol. 64:65–71. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Foreyt WJ: Trichinosis. U.S. Geological
Survey Circular 1388 Reston, Va, p60, 2013.
|
|
3
|
Gottstein B, Pozio E and Nöckler K:
Epidemiology, diagnosis, treatment, and control of trichinellosis.
Clin Microbiol Rev. 22:127–145. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Capó V and Despommier DD: Clinical aspects
of infection with Trichinella spp. Clin Microbiol Rev. 9:47–54.
1996.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Faber M, Schink S, Mayer-Scholl A, Ziesch
C, Schönfelder R, Wichmann-Schauer H, Stark K and Nöckler K:
Outbreak of trichinellosis due to wild boar meat and evaluation of
the effectiveness of post exposure prophylaxis, Germany, 2013. Clin
Infect Dis. 60:e98–e104. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dupouy-Camet J, Kociecka W, Bruschi F,
Bolas-Fernandez F and Pozio E: Opinion on the diagnosis and
treatment of human trichinellosis. Expert Opin Pharmacother.
3:1117–1130. 2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
European Centre for Disease Prevention and
Control (ECDC): Surveillance Atlas of Infectious Diseases. ECDC,
Solna, 2018. http://atlas.ecdc.europa.eu/public/index.aspx?Instance=GeneralAtlas.
Accessed May 5, 2018.
|
|
8
|
Pavel R, Popovici ED and Olariu TR:
Epidemiology of human trichinellosis in the European Union,
2007-2015. J Med Evol. 23(No 4)2017.
|
|
9
|
Lupu MA, Lazureanu EV and Olariu TR:
Trichinellosis in western Romania a 4 year retrospective study. Int
J Infect Dis. 53(83)2016.
|
|
10
|
Dupouy-Camet J and Murrell KD (eds):
FAO/WHO/OIE Guidelines for the surveillance, management, prevention
and control of Trichinellosis. Food and Agriculture Organization of
the United Nations, World Health Organization, World Organisation
for Animal Health, 2007. http://www.fao.org/3/a0227e/a0227e.pdf.
|
|
11
|
European Food Safety Authority and
European Centre for Disease Prevention and Control. The European
Union summary report on trends and sources of zoonoses, zoonotic
agents and food-borne outbreaks in 2016. EFSA J.
15(5077)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pozio E, Ludovisi A, Pezzotti P, Bruschi F
and Gómez-Morales MÁ: Retrospective analysis of hospital discharge
records for cases of trichinellosis does not allow evaluation of
disease burden in Italy. Parasite. 26(42)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Neghina R, Neghina AM and Marincu I:
Trichinellosis in hospitalized patients from a Romanian endemic
area, 2007-2009. Clin Microbiol Infect. 18:86–90. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Neghina R, Neghina AM, Marincu I, Moldovan
R and Iacobiciu I: Trichinellosis and poverty in a Romanian
industrial area: An epidemiological study and brief review of
literature. Foodborne Pathog Dis. 7:757–761. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tatulescu DF, Muntean MI, Cismaru C,
Astilean AE, Sabou MC and Crisan AM: An update on human
trichinellosis in Romania-a 15 years retrospective study on the
epidemiology and clinical signs. Sci Parasitol. 11:51–54. 2010.
|
|
16
|
Blaga R, Durand B, Antoniu S, Gherman C,
Cretu CM, Cozma V and Boireau P: A dramatic increase in the
incidence of human trichinellosis in Romania over the past 25
years: Impact of political changes and regional food habits. Am J
Trop Med Hyg. 76:983–986. 2007.PubMed/NCBI
|
|
17
|
Borza C, Neghina AM, Dumitrascu V, Tirnea
L, Calma CL and Neghina R: Epizootiology of trichinellosis in pigs
and wild boars in Western Romania, 1998-2011. Vector Borne Zoonotic
Dis. 12:712–713. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ruetsch C, Delaunay P, Armengaud A,
Peloux-Petiot F, Dupouy-Camet J, Vallée I, Polack B, Boireau P and
Marty P: Inadequate labeling of pork sausages prepared in Corsica
causing a trichinellosis outbreak in France. Parasite.
23(27)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Murrell KD and Pozio E: Worldwide
occurrence and impact of human trichinellosis, 1986-2009. Emerg
Infect Dis. 170:2194–2202. 2011.PubMed/NCBI View Article : Google Scholar
|