Introduction
Gastric cancer (GC) is one of the major causes of
cancer-related mortality and is a major health burden worldwide
(1). Each year there are ~1 million
new cases of GC worldwide, prompting the World Health Organization
to declare it a public health concern (2). Patients with GC typically exhibit the
‘three high and three low’ characteristic, whereby the incidence,
metastasis and mortality rates are high whereas the early diagnosis
rate, radical resection rate and 5-year survival rate are low
(3). As surgical techniques improve
and progress is made in traditional radiotherapy, chemotherapy and
the implementation of neoadjuvant therapy, the 5-year survival rate
for early GC can reach >95% (2).
However, the low rate of early diagnosis (>70%) means that most
patients will develop advanced-stage disease, resulting in the
optimal surgical window being missed, worsening the overall
prognosis (2). At present, the main
treatment strategy for advanced GC is the combination of
neoadjuvant chemoradiotherapy, molecular-targeted therapy and
immunotherapy (2). However,
development of novel effective therapeutic agents or the discovery
of novel therapeutic targets for GC treatment remains urgently sort
after.
Reversine (Rev) is a 2,6-diamino-substituted purine
analogue that was originally used as a depolarizer to control
cellular dedifferentiation and may ultimately prove to be useful
for in vivo stem cell biology and therapy (4). Rev induces mitotic catastrophe, cell
cycle arrest, polyploidy and cell apoptosis in a number of human
cancer cell types, including in non-small cell lung cancer and
breast cancer (5-7).
A previous study has shown that Rev treatment resulted in
cytotoxicity in human colorectal cancer (CRC) cells and inhibited
cell migration by modulating the JNK signaling pathway (8). Additionally, Rev treatment suppressed
tumor progression by inhibiting cell proliferation, inducing
apoptosis and cell cycle arrest through upregulation of the Fas and
death receptor 5 signaling pathways in CRC cells (9). However, there have been no previous
reports on the effect of Rev on GC cells.
The protein kinase TTK, which is also known as
monopolar spindle 1 or Mps1, has been documented to serve critical
roles in malignant diseases, including hepatocellular carcinoma,
breast cancer, glioblastoma and pancreatic cancer, where has been
reported to promote cell proliferation, invasion and
epithelial-to-mesenchymal transition (10-13).
Frameshift mutations of TTK may alter cell cycle control in the
affected cells and contribute to pathogenesis of GC and CRC with
high microsatellite instability (14). Lower expression levels of TTK was
also reported to be associated with superior prognosis of patients
with glioblastoma and breast cancer (13,15).
In GC, TTK may contribute to tumorigenesis (16), such that TTK expression was found to
be higher in the six GC cell lines AGS, MKN-45, SGC 7901, KATO III,
N-87 and SNU-1 tested compared with that in the normal gastric cell
line GES-1, suggesting that TTK may be a new therapeutic target for
GC (17). However, the role of TTK
and the relationship between TTK and Rev in the regulation of GC
physiology remain ambiguous.
Therefore, the aim of the present study was to
investigate the effect of Rev on GC and its association with TTK in
human GC cells.
Materials and methods
Cell culture and reagents
The two human GC cell lines AGS and NCI-N87, in
addition to the human immortalized gastric epithelial cell line
(GES-1) were obtained from the American Type Culture Collection. GC
cells were cultured in RPMI-1640 medium supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.) and penicillin-streptomycin
(100 U/ml) at 37˚C with 5% CO2. By contrast, GES-1 cells
were grown in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
containing 10% FBS with 100 U/ml P/S at 37˚C with 5%
CO2. Reversine was purchased from Cayman Chemical
Company and was kept as a 10 mM solution in DMSO.
Cell viability assay
Cell viability was detected by Cell Counting Kit-8
(CCK-8) assay (Dojindo Molecular Technologies, Inc.). AGS and
NCI-N87 cells were seeded into a 96-well plate at a density of
1x104 cells/well before they were incubated for 24 h at
37˚C. The cells were then treated with different concentrations of
Rev (0, 0.5, 1, 5, 10 and 20 µM) with DMSO (Sigma-Aldrich; Merck
KGaA) for 24 and 48 h at 37˚C. Subsequently, the cells were treated
with 10 µl CCK-8 solution (Dojindo Molecular Technologies, Inc.)
for 2 h at 37˚C. After treatment, absorbance at 450 nm was measured
in each well using a microplate reader (Varioskan®
Flash; Thermo Fisher Scientific, Inc.). Each experiment was
conducted in triplicate wells and was repeated ≥ three times.
Transfection
AGS and NCI-N87 cells were seeded
(4x105/well) into six-well plates and incubated at 37˚C
overnight. For TTK overexpression, TTK overexpression plasmid
(Oe-TTK) and empty plasmid (Oe-NC) were designed and constructed by
Shanghai GenePharma Co., Ltd. The cells were transfected with
Oe-TTK or Oe-NC using Lipofectamine® 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) for 6 h. The final
concentration of the plasmids was 2 µg/ml. The overexpression of
TTK expression following Oe-TTK transfection was verified by
reverse transcription-quantitative PCR (RT-qPCR), after which the
transfected cells were treated with 20 µM Rev for 24 h at 37˚C.
Colony formation assay
AGS and NCI-N87 cells were seeded into six-well
plates at a low density (1x103) in each well. Cells were
then incubated for 24 h and then treated with 20 µM Rev for 24 h at
37˚C. After 12 days incubation in RPMI-1640 medium with 10% FBS at
37˚C, the plates were washed with PBS and stained with 0.1% crystal
violet at room temperature for 5 min. Colony formation images were
captured using a camera (original magnification x100; Olympus
Corporation). Colonies, defined as clusters of >50 cells, were
counted before colony intensities were calculated using the ImageJ
software Version 1.8.0 (National Institutes of Health). Each
experiment was repeated ≥ three times.
Wound healing assay
AGS and NCI-N87 cells were seeded into a 60-mm dish
and cultured for 24 h at 37˚C. When the cells became confluent, the
monolayer was scratched using a sterile 1 ml pipette tip. The
monolayer was then washed three times with PBS to remove cell
debris and incubated in RPMI-1640 medium containing 2% FBS. After
48-h incubation at 37˚C, images of wound healing were captured
under a light microscope (magnification, x200). The migration rate
was calculated based on the formula: (Wound width at 0 h-wound
width at 24 h)/wound width at 0 h x100%. Each experiment was
repeated ≥ three times.
Invasion assay
Invasion assay was performed using a Transwell
chamber with 8-µm pores (Corning, Inc.). The upper chamber of the
Transwell was first coated with 3 mg/ml Matrigel (BD Biosciences)
and incubated at 37˚C for 1 h. Cells were incubated in RPMI-1640
containing 1% FBS and treated with 20 µM Rev for 24 h at 37˚C. The
cells were trypsinized and suspended at a final concentration of
5x105 cells/ml in RPMI-1640 containing 1% FBS. Cell
suspensions were then loaded into the upper compartment. The lower
chamber was added with 600 µl RPMI-1640 medium containing 10% FBS.
After incubation for 48 h at 37˚C, the cells on the surface of the
upper chamber was wiped off. The invaded cells on the lower chamber
were fixed with 100% methanol for 15 min at room temperature,
stained with 0.5% crystal violet for 10 min at room temperature,
and captured under a light microscope (magnification, x200). Five
randomly chosen fields were counted for each group per chamber.
Each experiment was repeated ≥ three times.
TdT-mediated dUTP nick-end labeling
(TUNEL) assay
AGS and NCI-N87 cells were seeded
(4x105/well) into six-well plates and incubated at 37˚C.
After treatment with 20 µM Rev in the presence and absence of
Oe-TTK for 24 h at 37˚C, cells were fixed with 4% paraformaldehyde
for 30 min at room temperature and permeabilized with 0.5% Triton
X-100 for 10 min at room temperature. After washing three times
with PBS, the cells were the cells were added with 50 µl TUNEL
(cat. no. 11684817910; Roche diagnostics) at 37˚C for 1 h. After
washing with PBS, the cells were treated with 1 µg/ml DAPI at room
temperature for 5 min. DNA fragmentation was visualized by TUNEL
assay according to the manufacturer's protocols. Finally,
fluorescence images were obtained using a confocal microscope (Carl
Zeiss AG) at magnifications of x200. In total, five randomly chosen
fields were counted for each group per chamber. Each experiment was
repeated ≥ three times.
RT-qPCR
Total RNA extraction in cells was performed using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Reverse transcription was
performed using a RevertAid First Strand cDNA Synthesis Kit (cat.
no. K1622; Thermo Fisher Scientific, Inc.) and the reaction was
incubated at 25˚C for 5 min, 42˚C for 30 min, 85˚C for 5 min and
then kept at 4˚C for 5 min. cDNA was prepared for amplification.
FastStart™ Universal SYBR®-Green Master Mix
(Sigma-Aldrich; Merck KGaA) was used for qPCR following the
manufacturer's protocol. The thermocycling conditions were as
follows: Initial denaturation at 95˚C for 5 min, followed by 35
cycles of denaturation (45 sec at 95˚C), annealing (45 sec at 60˚C)
and extension (8 min at 68˚C), before a final extension at 68˚C for
10 min. GAPDH was used for normalization, where gene expression was
calculated using the 2-ΔΔCq method (18). qPCR amplification was performed
using the following primers: TTK forward,
5'-CGCAGCTTTCTGTAGAAATGGA-3' and reverse,
5'-GAGCATCACTTAGCGGAACAC-3' and GAPDH forward,
5'-GGTGGTCTCCTCTGACTTCAACA-3' and reverse,
5'-GTTGCTGTAGCCAAATTCGTTGT-3'. Each experiment was repeated ≥ three
times.
Western blotting
Cell lysates were prepared in RIPA buffer (Beyotime
Institute of Biotechnology) containing 1% protease and 1%
phosphatase inhibitor cocktail (Sigma Aldrich; Merck KGaA) on ice.
Bicinchoninic acid method was used to measure protein
concentration. Equal amounts of protein lysates (30 µg/lane) were
mixed with loading buffer, separated by 10% SDS-PAGE and
transferred onto PVDF membranes (Invitrogen; Thermo Fisher
Scientific, Inc.). Subsequently, the membranes are blocked with 5%
skimmed milk for 1 h at room temperature and then treated with
primary antibodies (all from Abcam) against TTK (1:1,000, cat. no.
ab11108), matrix metalloproteinase (MMP)-2 (1:1,000, cat. no.
ab92536), MMP-9 (1:1,000, cat. no. ab38898), Bcl-2 (1:2,000, cat.
no. ab182858), Bax (1:2,000, cat. no. ab32503), cleaved caspase-3
(1:500, cat. no. ab32042), cleaved caspase-9 (1:1,500, cat. no.
ab2324), caspase-3 (1:5,000, cat. no. ab32351), caspase-9 (1:1,000,
cat. no. ab32539) and GAPDH (1:1,000, cat. no. ab8245) at 4˚C
overnight. The membranes were then incubated with a HRP-conjugated
secondary antibody (1:2,000, cat. no. 7074, Cell Signaling
Technology Inc.) for 1 h at room temperature. The ECL™ Western
Blotting Analysis System (Cytiva) and the ImageJ software (version
1.46; National Institutes of Health) were used to detect the blots.
Each experiment was repeated at least three times.
Statistical analysis
All experiments were performed ≥ three times. All
data were presented as the mean ± SD. Statistical analyzes were
performed using GraphPad 6 Software (GraphPad Software, Inc.). Two
groups were compared using an unpaired, two-tailed Student's
t-test. Multiple group comparisons were analyzed with one-way ANOVA
followed by the Dunnett's post hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
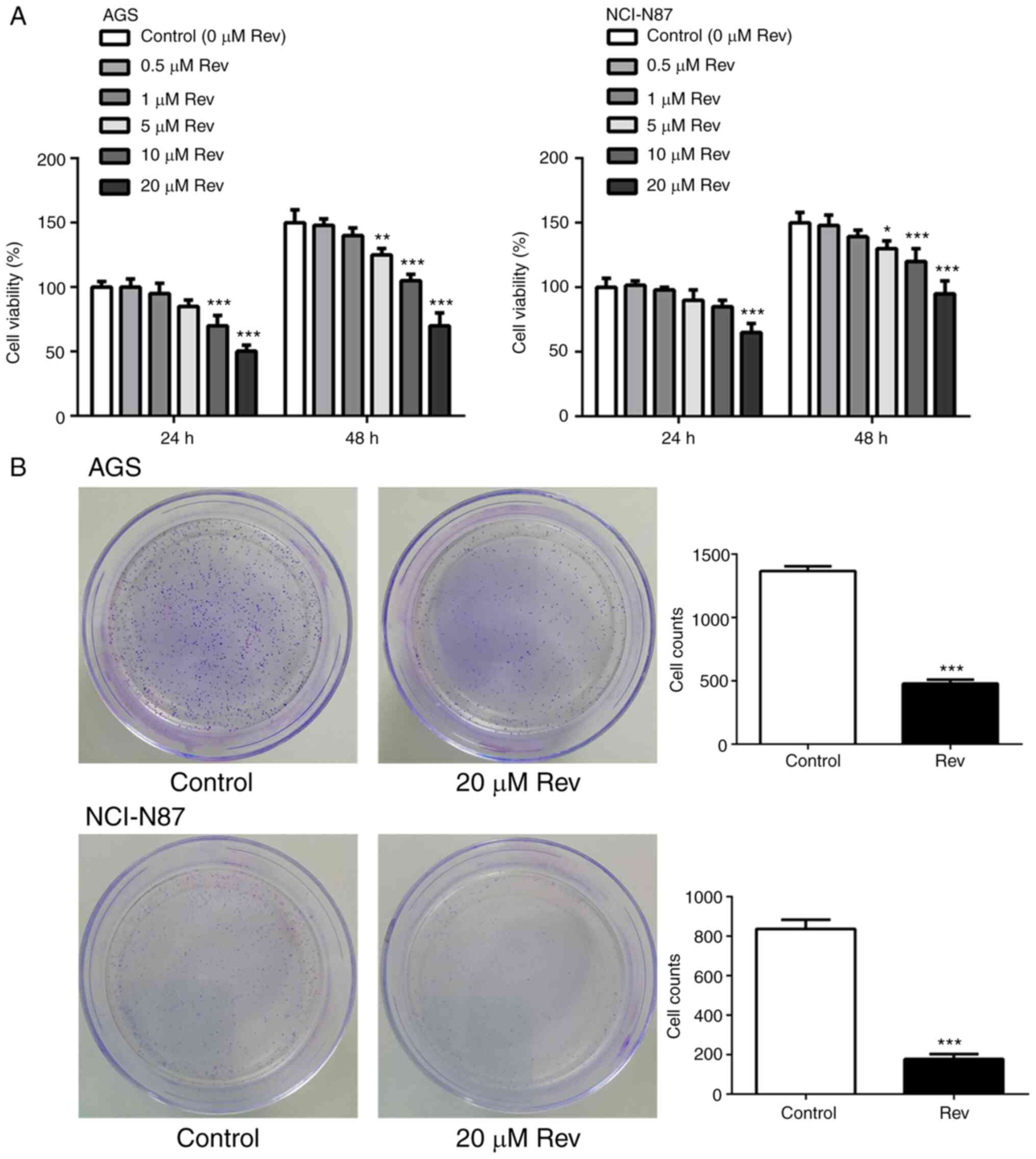
Rev reduces cell viability of human
gastric cancer cells
To analyze the potential cytotoxic effects of Rev,
CCK-8 assay was performed following treatment of GC cell lines AGS
and NCI-N87 cells with different concentrations Rev (0, 0.5, 1, 5,
10 and 20 µM) for 24 and 48 h. Rev treatments markedly inhibited
cell viability of AGS and NCI-N87 cells in a dose-dependent manner
(Fig. 1A), with inhibition becoming
significant compared with that in control from 10 µM upwards,
suggestive of Rev-induced cell death.
Based on these results, the maximum safe drug
concentration of Rev of 20 µM was selected for subsequent
experiments. To verify the Rev-induced reductions of AGS and
NCI-N87 cell proliferation, colony formation assay was performed.
As shown in Fig. 1B, the number of
colonies formed by AGS and NCI-N87 cells stained with crystal
violet was significantly decreased following treatment with Rev (20
µM) for 24 h compared with that in control. These findings suggest
that Rev inhibits cell viability and prolifertion in human gastric
cancer cells.
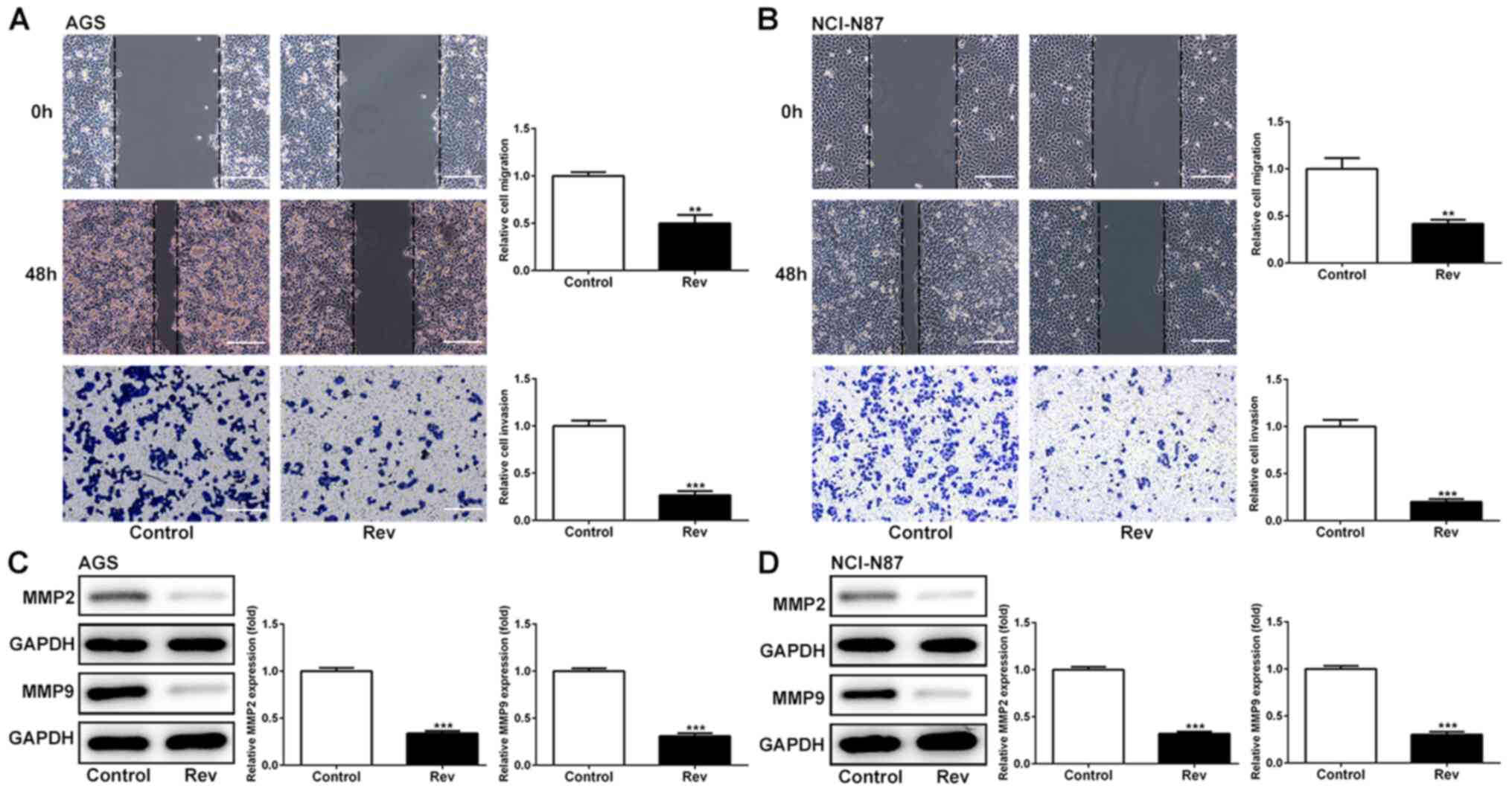
Rev inhibits cell migration and
invasion of human gastric cancer cells
To explore the effects of Rev on cell migration and
invasion in AGS and NCI-N87 cells, wound healing and Transwell
assays were conducted. It was found that reductions in the cell
free area in the wound observed in those in the control group were
significantly attenuated by the presence of Rev in both cell lines
(Fig. 2A and B), suggestive of anti-migratory effects
mediated by Rev. Using Transwell assay, it was observed that cell
invasion was significantly reduced in cells treated with Rev for 48
h when compared with that in control cells (Fig. 2A and B). In addition, effects of Rev treatment
on the expression levels of regulatory proteins of migration were
further examined. As shown in Fig.
2C and D, Rev treatment
resulted in significant decreases in the levels of MMP-2 and MMP-9
in AGS and NCI-N87 cells. Taken together, these results suggested
the anti-migratory and anti-invasive effects of Rev in human
gastric cancer cells.
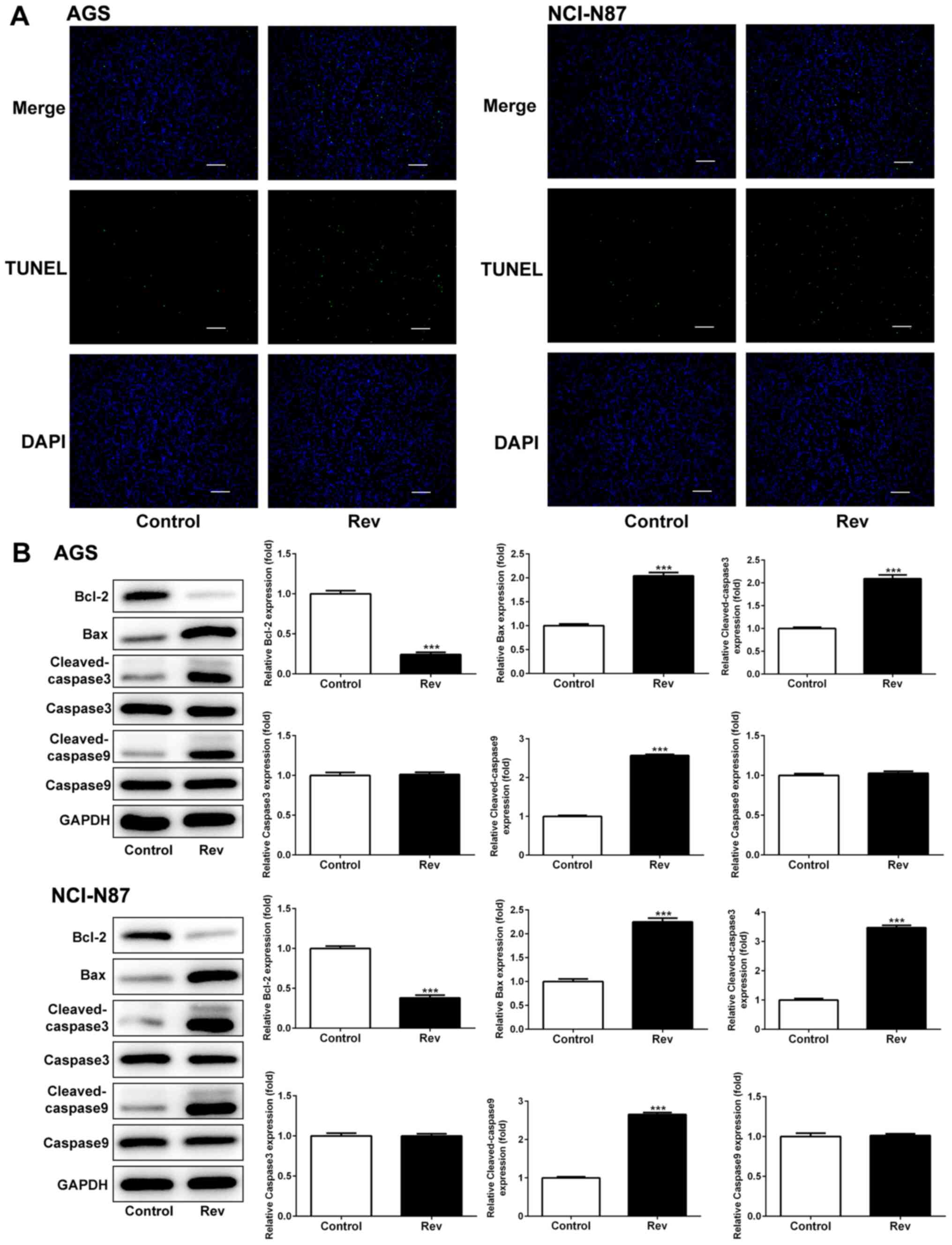
Rev treatment induces apoptosis in
human gastric cancer cells
To determine if Rev can induce apoptosis in human GC
cells, AGS and NCI-N87 cells were treated with Rev for 24 h and
subsequently used for TUNEL analysis to assess cell apoptosis. The
number of GC cells with condensed nuclei (Green) was markedly
increased following Rev treatment compared with that in control
cells (Fig. 3A). To examine the
apoptotic properties of Rev, the expressions of Bax, Bcl-2 and
caspase-3/9 were further investigated. Rev treatment resulted in
significant increases in the levels of pro-apoptotic proteins Bax,
cleaved-caspase-3/9 in AGS and NCI-N87 cells whilst significantly
reducing the levels of the anti-apoptotic protein Bcl-2 in response
to Rev treatment (Fig. 3B).
However, uncleaved caspase-3/9 were not altered in response to Rev
treatment. Therefore, these results suggest that 24 h treatment of
gastric cancer cells with Rev can induce cell apoptosis.
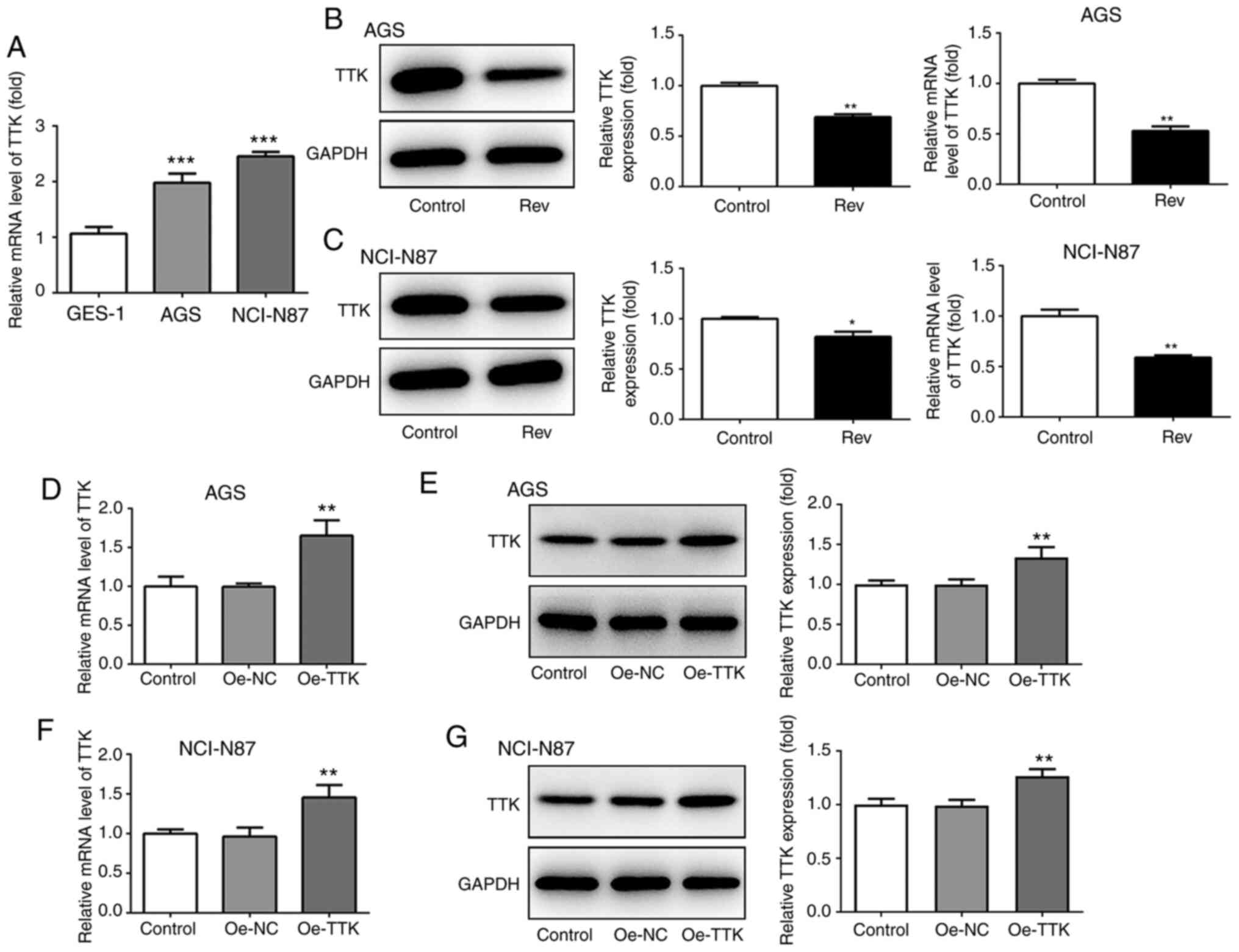
Expression of TTK in human gastric
cancer cells
To confirm if Rev can target the TTK, TTK expression
was detected in GC cells in the presence of Rev using western
blotting and RT-qPCR. It was found that TTK expression was
significantly higher in both AGS and NCI-N87 cells compared with
that GES-1 cells (Fig. 4A). Rev
treatment significantly reduced both protein and mRNA TTK
expression in both GC cell lines tested (Fig. 4B and C). To explore the effects of TTK further,
overexpression plasmids of TTK were used overexpress TTK in AGS and
NCI-N87 cells. As shown in Fig.
4D-G, the plasmids of Oe-TTK exhibited good transfection
efficiency in both cell lines, which significantly upregulated mRNA
and protein levels of TTK compared with those in cells transfected
with the Oe-NC plasmid.
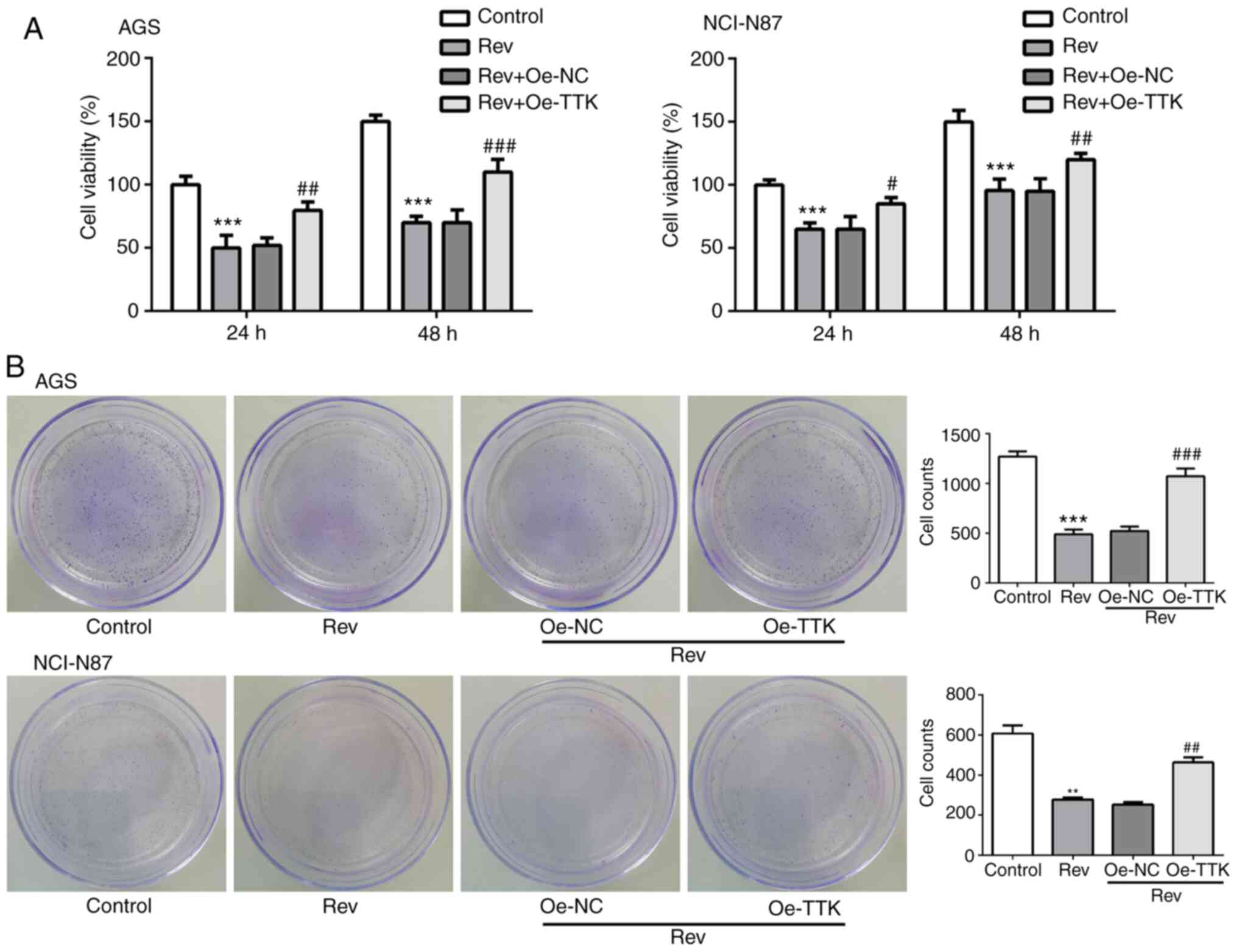
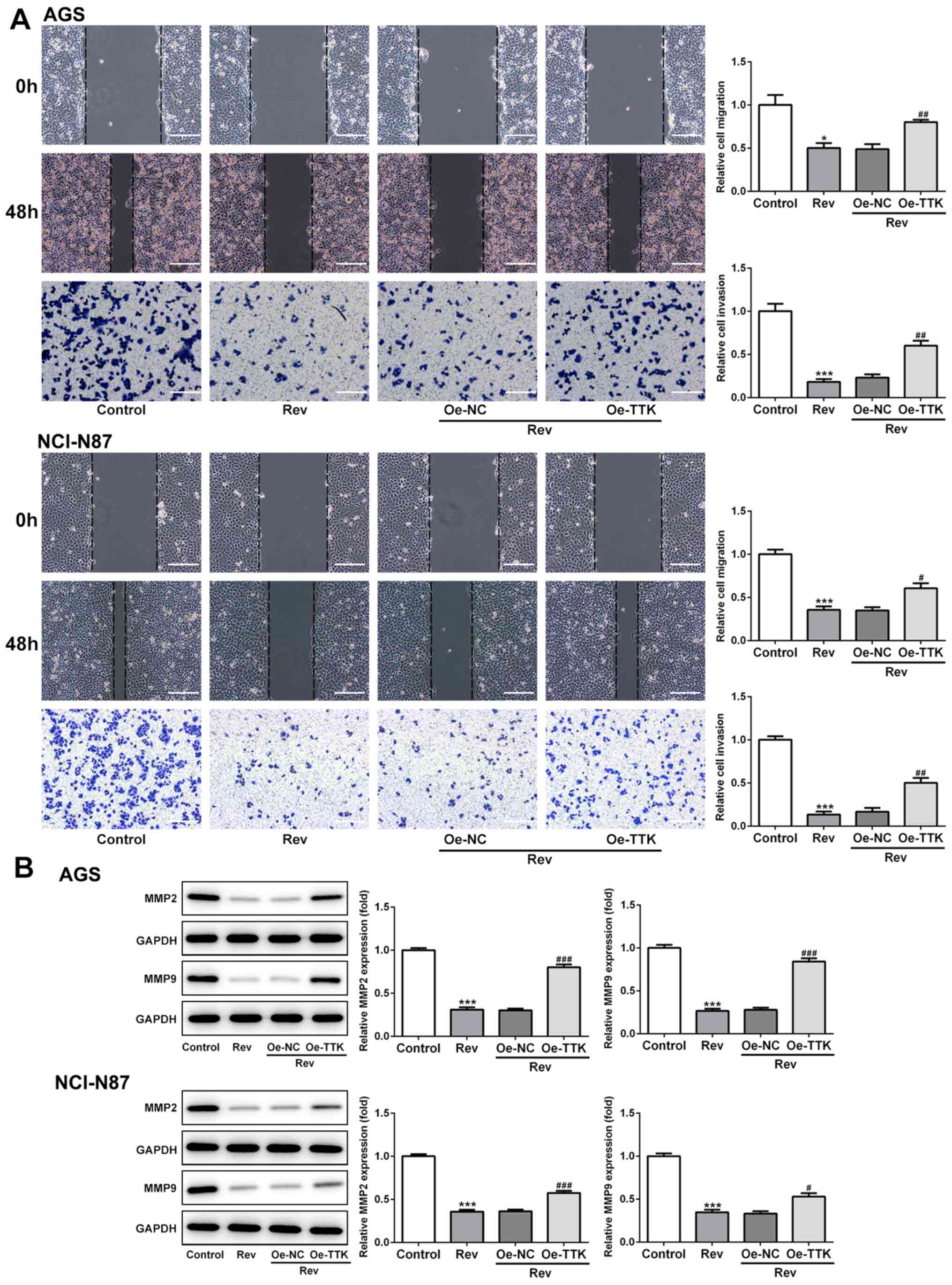
Overexpression of TTK attenuates
Rev-induced inhibition of cell viability, migration and invasion in
human gastric cancer cells
The effect of TTK overexpression on cell viability,
migration and invasion following Rev treatment was next examined.
Inhibitions of cell viability and colony formation induced by Rev
treatment were significantly reversed by Oe-TTK transfection in AGS
and NCI-N87 cells (Fig. 5A and
B). In addition, overexpression of
TTK significantly reversed the inhibitory effects of Rev on the
migratory and invasive capacities of GC cells (Fig. 6A). Expression levels of MMP2 and
MMP9 were also significantly elevated in Rev-treated GC cells
transfected with Oe-TTK compared with those in cells treated with
Rev alone (Fig. 6B). These results
suggest that the Rev-induced inhibition of GC cell migration and
invasion is mediated by suppressing TTK expression.
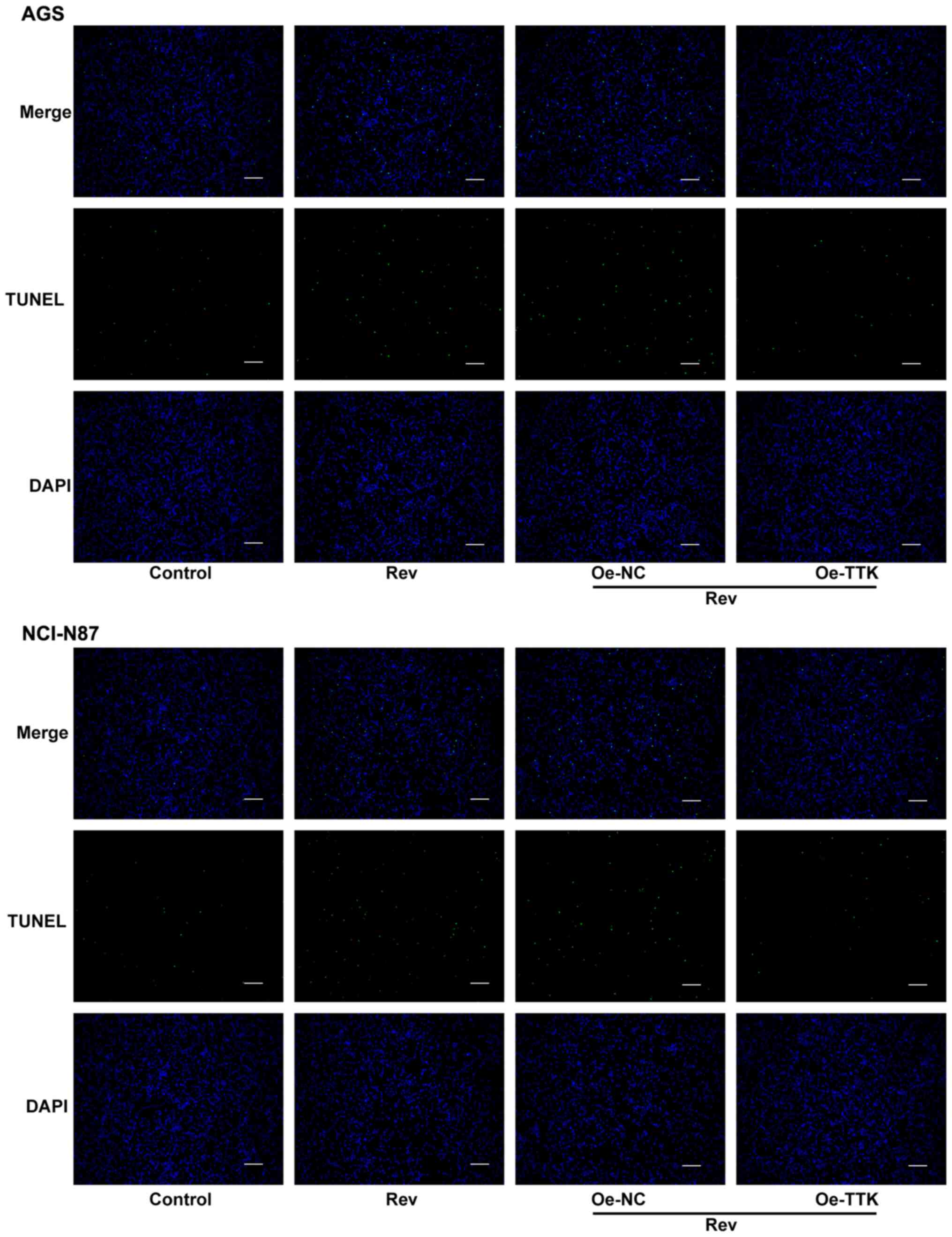
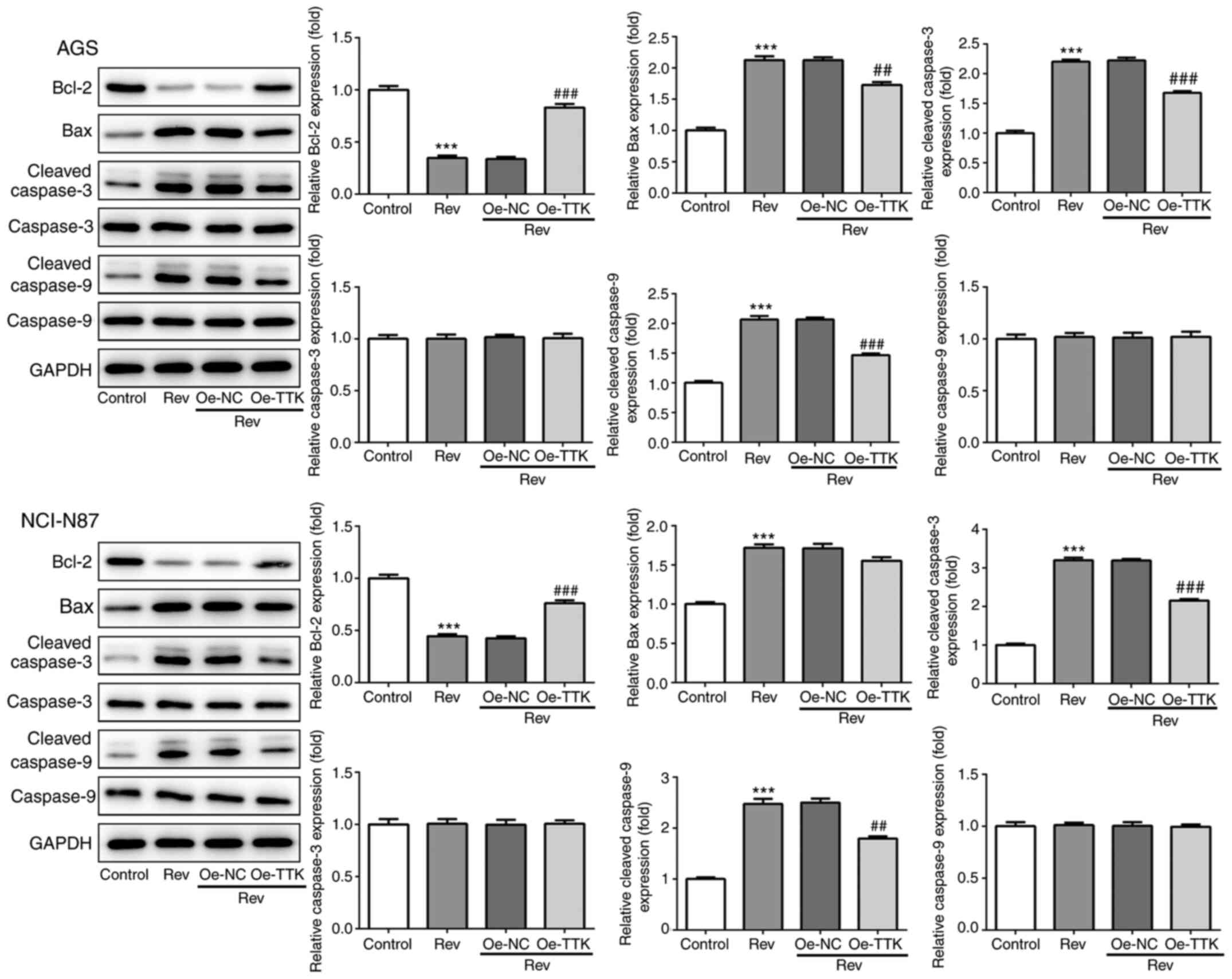
Overexpression of TTK attenuates
reversine-induced induction of apoptosis in human gastric cancer
cells
To determine if Rev can induce apoptosis in GC cells
by targeting TTK, TUNEL and western blotting were performed to
assess cell apoptosis and expression of apoptosis-related proteins.
Increases in the number of apoptotic cells, which were stained
green by TUNEL, following Rev treatment was markedly attenuated in
AGS and NCI-N87 cells by Oe-TTK transfection (Fig. 7). Furthermore, TTK overexpression
significantly abrogated the Rev-induced elevations in
cleaved-caspase-3 and -9 activation and Bcl-2 inhibition. However,
TTK overexpression did not significantly affect uncleaved caspase-3
and -9 protein expression (Fig. 8).
Therefore, these observations suggest that Rev treatment promotes
apoptosis by directly inhibiting TTK in human gastric cancer
cells.
Discussion
Rev is a synthetic purine analogue that has been
previously reported to induce the de-differentiation of the murine
myoblast cell line into multipotent progenitor cells, which can
subsequently re-differentiate into other different cell types
(4). Rev increased the plasticity
of bone marrow-derived mesenchymal stem cells for the generation of
cardiomyocytes in vitro (19). In addition, Rev induced myoblast
redifferentiation into cell types of neural and mesodermal lineages
(20). Rev also exerted an
inhibitive effect on human renal carcinoma cells via induction of
cell apoptosis and polyploidy (21). A number of studies have suggested
that Rev confers tumor-suppressive effects against several cancer
cells types, including colorectal and breast cancer (5,7,9).
However, studies evaluating the effects of Rev on human GCs has not
been previously reported. Therefore, the impact of Rev on tumor
cell behavior and its association with TTK expression in human
gastric cancer cells were investigated in the present study, which
found Rev to be a potential anti-GC agent.
Dysregulation of cell growth and apoptosis are
important for cancer development and progression (22). In the present study, Rev notably
reduced the cell viability of the two GC cell lines tested in a
concentration-dependent manner, consistent with previous findings
that Rev exerted anticancer effects by suppressing CRC cell growth
(9). Furthermore, Rev treatment
significantly reduced colony formation. TUNEL staining was
performed and nuclear condensation was also evaluated in the
present study. To verify he effects of Rev on the apoptosis of GC
cells, expression of the anti-apoptotic protein Bcl-2, the
pro-apoptotic protein Bax and the central component of the
apoptotic cascade caspase-3/9, were measured. Rev treatment induced
apoptosis by activating Bax and caspase-3/9 whilst suppressing the
expression of Bcl-2. These results suggest that Rev exerts
cytotoxicity on GC cells and has potential applications for
anticancer treatment.
Metastasis is a major cause of chemotherapeutic
failure and cancer mortality, such that gastric cancer is one of
the most invasive and metastatic type of cancer (2). Recently, Rev was demonstrated to
suppress the migration and invasion of human CRC (9). In the present study, GC cells were
exposed to Rev for 24 h, where it was found that Rev significantly
suppressed the migration and invasion of GC cells using wound
healing and Transwell assays. The potential anti-metastatic effects
of Rev was in line with previous findings that Rev functions as a
potent anti-migratory agent in invasive cancer cells (8,23).
MMPs serve important roles in many physiological and pathological
processes, including angiogenesis, tumor invasion and metastasis
(24). Lyu et al (25) found that Marimastat, an MMP
inhibitor, could inhibit MMP2 and MMP 9, thereby inhibiting tumor
growth, invasion and metastasis in breast cancer cells. In the
present study, Rev reduced the expression of MMP2 and MMP9 in GC
cells. This suggest that Rev can potentially inhibit GC cancer cell
metastasis, consistent with a previous finding (8).
A previous study performed in vitro showed
that TTK knockdown inhibited the proliferation, invasion and
migration of prostate cancer cells whilst promoting cell apoptosis
(26). In addition, in vivo
experiments showed that TTK gene knockout inhibited tumorigenesis
in mice injected with prostate cancer cells (26). Hudler et al (16) confirmed that polymorphisms in the
gene of the mitotic kinase TTK could have an effect on the risk of
gastric tumorigenesis and adenocarcinoma development. In the
present study, it was found that TTK was overexpressed in GC cells
compared with that in the normal human gastric epithelial cell
line, which was in turn reduced by Rev treatment. Therefore, it
could be hypothesized that TTK can serve an important role in GC as
a downstream target of Rev. Therefore, overexpression of TTK was
subsequently used to assess the association between Rev and TTK
expression in GC cells. The present study demonstrated that TTK
overexpression reversed the Rev-induced reductions in cell
viability, colony formation, migration, invasion and Rev-induced
apoptosis in GC cells, consistent with a previous study, which also
reported that TTK can inhibit the proliferation of prostate cancer
(26). However, further
experimentation is required to explore the specific underlying
mechanism. Therefore, TTK appears to be an attractive target for
the development of novel therapeutics against gastric cancer. In
the present study, the effects and potential mechanism of Rev on GC
was only tested on cell lines without further verification by in
vivo experiments. The in vivo role of Rev in GC would
need to be explored in further studies.
Overall, the present study provided evidence that
Rev treatment suppressed GC physiology by inhibiting of cell
viability, migration and invasion, whilst inducing apoptosis, by
downregulating TTK expression in human GC cells. Results from the
present study suggest that Rev may be used as a novel anticancer
agent for human GC, such that TTK may serve as an attractive target
for cancer therapy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
PX and ZJ designed the experiments. PX, JL and DJ
performed the experiments and analyzed the data. JL and DJ
authenticated the raw data. All the authors read and approved the
final version of this manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Correa P: Gastric cancer: Overview.
Gastroenterol Clin North Am. 42:211–217. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Song Z, Wu Y, Yang J, Yang D and Fang X:
Progress in the treatment of advanced gastric cancer. Tumour Biol:
Jul 3, 2017 (Epub ahead of print). doi:
10.1177/1010428317714626.
|
|
3
|
Wu H, Wang W, Tong S and Wu C:
Nucleostemin regulates proliferation and migration of gastric
cancer and correlates with its malignancy. Int J Clin Exp Med.
8:17634–17643. 2015.PubMed/NCBI
|
|
4
|
Chen S, Zhang Q, Wu X, Schultz PG and Ding
S: Dedifferentiation of lineage-committed cells by a small
molecule. J Am Chem Soc. 126:410–411. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hsieh TC, Traganos F, Darzynkiewicz Z and
Wu JM: The 2,6-disubstituted purine reversine induces growth arrest
and polyploidy in human cancer cells. Int J Oncol. 31:1293–1300.
2007.PubMed/NCBI
|
|
6
|
Lu YC, Lee YR, Liao JD, Lin CY, Chen YY,
Chen PT and Tseng YS: Reversine induced multinucleated cells, cell
apoptosis and autophagy in human non-small cell lung cancer cells.
PLoS One. 11(e0158587)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kuo CH, Lu YC, Tseng YS, Shi CS, Chen SH,
Chen PT, Wu FL, Chang YP and Lee YR: Reversine induces cell cycle
arrest, polyploidy, and apoptosis in human breast cancer cells.
Breast Cancer. 21:358–369. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jemaa M, Abassi Y, Kifagi C, Fezai M,
Daams R, Lang F and Massoumi R: Reversine inhibits colon carcinoma
cell migration by targeting JNK1. Sci Rep. 8(11821)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Park YL, Ha SY, Park SY, Choi JH, Jung MW,
Myung DS, Kim HS and Joo YE: Reversine induces cell cycle arrest
and apoptosis via upregulation of the Fas and DR5 signaling
pathways in human colorectal cancer cells. Int J Oncol.
54:1875–1883. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liu X, Liao W, Yuan Q, Ou Y and Huang J:
TTK activates Akt and promotes proliferation and migration of
hepatocellular carcinoma cells. Oncotarget. 6:34309–34320.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kaistha BP, Honstein T, Muller V, Bielak
S, Sauer M, Kreider R, Fassan M, Scarpa A, Schmees C, Volkmer H, et
al: Key role of dual specificity kinase TTK in proliferation and
survival of pancreatic cancer cells. Br J Cancer. 111:1780–1787.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
King JL, Zhang B, Li Y, Li KP, Ni JJ,
Saavedra HI and Dong JT: TTK promotes mesenchymal signaling via
multiple mechanisms in triple negative breast cancer. Oncogenesis.
7(69)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang J, Xie Y, Bai X, Wang N, Yu H, Deng
Z, Lian M, Yu S, Liu H, Xie W and Wang M: Targeting dual
specificity protein kinase TTK attenuates tumorigenesis of
glioblastoma. Oncotarget. 9:3081–3088. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ahn CH, Kim YR, Kim SS, Yoo NJ and Lee SH:
Mutational analysis of TTK gene in gastric and colorectal cancers
with microsatellite instability. Cancer Res Treat. 41:224–228.
2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Finetti P, Cervera N, Charafe-Jauffret E,
Chabannon C, Charpin C, Chaffanet M, Jacquemier J, Viens P,
Birnbaum D and Bertucci F: Sixteen-kinase gene expression
identifies luminal breast cancers with poor prognosis. Cancer Res.
68:767–776. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hudler P, Britovsek NK, Grazio SF and
Komel R: Association between polymorphisms in segregation genes
BUB1B and TTK and gastric cancer risk. Radiol Oncol. 50:297–307.
2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang YW, Zhu ZG, Liu BY, Gu QL, Li JF, Qu
Y, Chen XH and Lin YZ: Expression and clinical significance of
cancer-related gene MPS-1 in gastric cancer. Zhonghua Wei Chang Wai
Ke Za Zhi. 8:503–506. 2005.PubMed/NCBI(In Chinese).
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pikir BS, Susilowati H, Hendrianto E and
Abdulrantam F: Reversin increase the plasticity of bone
marrow-derived mesenchymal stem cell for generation of
cardiomyocyte in vitro. Acta Med Indones. 44:23–27. 2012.PubMed/NCBI
|
|
20
|
Lee EK, Bae GU, You JS, Lee JC, Jeon YJ,
Park JW, Park JH, Ahn SH, Kim YK, Choi WS, et al: Reversine
increases the plasticity of lineage-committed cells toward
neuroectodermal lineage. J Biol Chem. 284:2891–2901.
2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cheng L, Wang H, Guo K, Wang Z, Zhang Z,
Shen C, Chen L and Lin J: Reversine, a substituted purine, exerts
an inhibitive effect on human renal carcinoma cells via induction
of cell apoptosis and polyploidy. Onco Targets Ther. 11:1025–1035.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Llambi F and Green DR: Apoptosis and
oncogenesis: Give and take in the BCL-2 family. Curr Opin Genet
Dev. 21:12–20. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jemaà M, Galluzzi L, Kepp O, Boileve A,
Lissa D, Senovilla L, Harper F, Pierron G, Berardinelli F, Antoccia
A, et al: Preferential killing of p53-deficient cancer cells by
reversine. Cell Cycle. 11:2149–2158. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Huang H, Wu K, Ma J, Du Y, Cao C and Nie
Y: Dopamine D2 receptor suppresses gastric cancer cell invasion and
migration via inhibition of EGFR/AKT/MMP-13 pathway. Int
Immunopharmacol. 39:113–120. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lyu Y, Xiao Q, Yin L, Yang L and He W:
Potent delivery of an MMP inhibitor to the tumor microenvironment
with thermosensitive liposomes for the suppression of metastasis
and angiogenesis. Signal Transduct Target Ther.
4(26)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chen S, Wang J, Wang L, Peng H, Xiao L, Li
C, Lin D and Yang K: Silencing TTK expression inhibits the
proliferation and progression of prostate cancer. Exp Cell Res.
385(111669)2019.PubMed/NCBI View Article : Google Scholar
|