Introduction
Lung cancer is one of the predominant causes of
tumor lethality worldwide, with a 5-year survival rate of only
19.3%. Additionally, ~85% of lung cancers are non-small cell lung
cancers (NSCLCs) that are resistant to radiotherapy and
chemotherapy (1-5).
Therefore, the present study explored the molecular mechanisms
involved in the resistance of chemotherapy drugs in NSCLC.
Circular RNAs (circRNAs), a newly appreciated class
of RNA expressed across diverse phyla, have covalently closed-loop
structures without a 5 ‘cap or a 3’ poly A tail (6,7). In
human cancers, the abnormal expression of circRNAs has been
observed (8-12).
A recent study has shown a significant role of the circRNA
circRNA_100876 in chemotherapy resistance, development, and
migration, as well as tumor invasion of NSCLC (13). circRNA circ_0067934 regulates the
miR-1324/FZD5/Wnt/β-catenin axis in hepatocellular carcinoma
promoting tumor growth and metastasis (14). In esophageal squamous cell
carcinoma, circ_0067934 is upregulated and promotes proliferation
(15). In gastric cancer, cisplatin
resistance is enhanced by suppression of miR-198 by PIK3R1 that is
upregulated by circular RNA AKT3(16). Although these circRNAs have
important roles in the development and metastasis of NSCLC, the
chemoresistance regulatory mechanisms of circRNA in NSCLC remain
unclear.
In the present study, the differentially expressed
circRNAs between NSCLC tissues and matched adjacent normal tissues
were investigated by retrieving the microarray data in the
GSE112214 dataset, normalizing the data and analyzing them by using
GEO2R (https://www.ncbi.nlm.nih.gov/geo/geo2r/) after
applying log2 transformation. The results showed that
several circRNAs were significantly increased in NSCLC tissues.
circRNA_103615 is a newly identified circRNA, and was one of the
significantly overexpressed circRNAs in NSCLC in microarray
analysis. However, the function and mechanism of circRNA_103615
have not been reported in cancer. Therefore, the present study
investigated the circRNA_103615 expression levels in NSCLC cell
lines and tissues and explored its function and mechanism in NSCLC
cells.
Materials and methods
Patients and tissue specimens
The samples used in the present study were NSCLC
tissue samples and adjacent non-cancerous tissue samples, which
were obtained from 60 patients who underwent primary surgical
resection of NSCLC at the Fuda Cancer Hospital (Guangzhou, China)
from May 2010 to May 2015. Patients were included in the current
study if they did not receive any therapy other than surgery.
Written informed consent was obtained from all the participants
before enrollment. No preoperative therapy was received by any of
the patients. All procedures involving human samples were approved
and performed in accordance with the ethical standards of the Human
Resources Ethics Committee of the Fuda Cancer Hospital.
Cell culture
The human NSCLC cell lines A549, NCI-H1299, H1650
and H358, and the normal lung cell line Beas-2B were obtained from
the Shanghai Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). All cells were cultured in DMEM (Gibco; Thermo
Fisher Scientific, Inc.) with 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.).
Bioinformatics analysis
Microarray data from the Gene Expression Omnibus
dataset GSE112214 (https://www.ncbi.nlm.nih.gov/geo/geo2r/?acc=GSE112214)
were retrieved to examine the expression of circRNAs in NSCLCs.
GEO2R was used to analyze normalized microarray data after applying
log2 transformation.
Cell transfection
All cells were maintained at 37˚C with 5%
CO2. Cells (2x105 cells/well) were plated in
6-well plates at 24 h and then transfected with control, small
interfering (si) RNA si-circRNA_103615 (100 nM/well in 6-well
plates) or the overexpression plasmid pcDNA3.1-ABCB1 (4.0 µg/well
in 6-well plates; Genepharm, Inc.) at 80% confluence using
Lipofectamine 3000 (Life Technologies; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. The
transfection assay was performed at 37°C for 48 h. After
48 h of transfection, subsequent experiments were performed. The
sequence of si-circRNA_103615 was 5'-CTGCTGACCGCTTCCGGGAGG-3'. The
sequence of si-NC was 5'-GCAAGCTGACCCTGAAGTT-3'. A negative control
oligo (si-NC) or the empty vector pcDNA3.1 were used as
controls.
Cell proliferation assay
A549 cells transfected with either si-circRNA_103615
or si-NC were seeded into 96-well plates at 5,000 cells/well and
examined after 24, 48, 72 and 96 h. Methyl thiazolyl tetrazolium
(MTT; Sigma-Aldrich; Merck KGaA) assay was used to examine cell
proliferation as per the manufacturer's protocol (Sigma-Aldrich;
Merck KGaA). A microplate reader was used to detect the optical
density at 490 nm.
Cell migration and invasion
For cell migration assays, uncoated 24-well
Transwell chambers (8 µm pores; BD Biosciences) were used. For cell
invasion assays, the 24-well Transwell chambers were coated with
Matrigel (BD Biosciences) for cell invasion. A549 cells transfected
with either si-circRNA_103615 or si-NC (1x105 cell/well)
suspended in 200 µl DMEM (with 1% FBS) were added into each
chamber. DMEM medium with 20% FBS was added to the bottom wells.
Subsequently, the cells were allowed to migrate or invade for 48 h
at 37˚C with 5% CO2. Migratory or invasive cells were
stained with DAPI. The stained cells in four random visual fields
were counted under a fluorescence microscope (IX71-F22FL/PH;
Olympus Corporation).
Cell apoptosis and cell cycle
Cell apoptosis was detect using an Annexin V-FITC
Apoptosis kit (cat. no. 556547; BD Biosciences), as per the
manufacturer's protocol. Cell cycle distribution was detected using
a propidium iodide (PI)/RNase Staining buffer (cat. no. 550825; BD
Biosciences), as per manufacturer's protocol. The percentage of
apoptotic cells was determined by flow cytometry (C6; BD
Biosciences), and the data were analyzed using FlowJo software
(V10.7.1; FlowJo LLC)
Colony formation
Cells were plated in 6-well plates at
1x103 per well and incubated for 14 days. Subsequently,
cells were fixed with 4% paraformaldehyde at 37˚C, and stained with
crystal violet (Sigma-Aldrich; Merck KGaA). The number of colonies
were counted with ImageJ (1.52v; National Institutes of
Health).
Reverse transcription-quantitative PCR
(RT-qPCR)
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract RNA from NSCLC tissues and
cells, as per the manufacturer's instructions. PrimeScript RT
Reagent kit (Takara Biotechnology Co., Ltd.) was used to
synthesized cDNA for analysis of circRNA_103615, multidrug
resistance-associated protein 1 (MRP1), breast cancer resistance
protein (BCRP), glutathione S-transferase π (GST-π), high mobility
group box 1 (HMGB1), and ATP binding cassette subfamily Bmember 1
(ABCB1) expression levels. SYBR Green kit (Qiagen GmbH) was used to
run qPCR on an ABI Prism 7500 Sequence Detector (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The temperature
protocol for the reaction was as follows: 95˚C for 10 min; 40
cycles at 95˚C for 15 sec and 60˚C for 1 min. GAPDH was used as an
internal reference gene. Relative fold changes in expression were
calculated using the formula 2-ΔΔCq. The primers for the
circRNA_103615 and GAPDH are listed in Table I.
 | Table IPrimer sequences used in the present
study. |
Table I
Primer sequences used in the present
study.
| Gene | Primer | Sequence (5'-3') |
|---|
| circRNA_103615 | Forward |
CTGACCCTTGACAGCATACGGATG |
| | Reverse |
GAAGTTACGGTCGGTGTATGGACGAG |
| GAPDH | Forward |
GCACCGTCAAGGCTGAGAAC |
| | Reverse |
ATGGTGGTGAAGACGCCAGT |
| MRP1 | Forward |
GCACTGGCTTCTAACTATTGG |
| | Reverse |
TCTCATTGAAGTGTGAGTACAC |
| BCRP | Forward |
TGGCTTAGACTCAAGCACAGC |
| | Reverse |
TCGTCCCTGCTTAGACATCC |
| GST-π | Forward |
AATGGATCCTCCACCATGCCGCCCTACACCGTGGT |
| | Reverse |
GACCTCGAGCTACTGTTTCCCGTTGCCAT |
| HMGB1 | Forward |
ATATGGCAAAAGCGGACAAG |
| | Reverse |
GCAACATCACCAATGGACAG |
| ABCB1 | Forward |
TGGTTCAGGTGGCTCTGGAT |
| | Reverse |
CTGTAGACAAACGATGAGCTAT |
Half-maximal inhibitory concentration
(IC50) value
A549 cells transfected with either si-circRNA_103615
or si-NC were seeded into 96-well plates at 5,000 cells/well. The
culture medium was subsequently replaced with medium containing
cisplatin (DDP) (32, 16, 8, 4, 2, 1, 0.5, 0.25 and 0.125 mg/ml) for
48 h, and cell viability was evaluated with an MTT assay
(Sigma-Aldrich; Merck KGaA), as per the manufacturer's protocol
(Sigma-Aldrich; Merck KGaA). A microplate reader was used to detect
the optical density at 490 nm.
Western blot analysis
RIPA lysis extraction buffer (Thermo Fisher
Scientific, Inc.) was used for total protein extraction from NSCLC
cells and protein concentration was determined by performing a
bicinchoninic acid assay (Thermo Fisher Scientific, Inc.).
Subsequently, 8-12% SDS-PAGE was used to separate protein samples
(25 µg), and then the samples were transferred on PVDF membranes
(EMD Millipore). The membrane was blocked in PBS containing 0.1%
Tween-20 (Beyotime Institute of Biotechnology) and 5% non-fat dry
milk at room temperature for 2 h. The membranes were first
incubated with the following primary antibodies: Anti-ABCB1
(1:1,000; cat. no. 13342; Cell Signaling Technology, Inc.) or
anti-GAPDH (1:1,000; cat. no. 5174; Cell Signaling Technology,
Inc.) for overnight incubation at 4˚C. The membranes were then
incubated with horseradish peroxidase-anti-rabbit secondary
antibody (1:2,000; cat. no. 14708; Cell Signaling Technology, Inc.)
for 1 h at room temperature. Finally, the protein bands were
detected using ECL solution (Pierce; Thermo Fisher Scientific,
Inc.) and images were captured using a FluorChem imaging system
(ProteinSimple) GADPH was used as a loading control.
Statistical analysis
All experiments were repeated three times, and
quantitative data were presented as the mean ± SD. Student's t-test
was used for statistical analysis (two groups that were not
matched/paired were compared using an unpaired t-test, and two
groups that were matched/paired were compared using a paired
t-test). Comparisons among >2 groups were performed using
analysis of variance followed by Dunnett's or Tukey's test.
Correlation analysis was conducted using Pearson's correlation
coefficient. The association between circRNA_103615 expression
levels and the patient clinicopathological characteristics was
determined by χ2 test. Kaplan-Meier survival analysis
was used to calculate the survival curve, and the log-rank test was
employed to determine statistical significance. P<0.05 was
considered to indicate a statistically significant difference.
Results
circRNA_103615 expression is increased
in NSCLC clinical specimens and cell lines
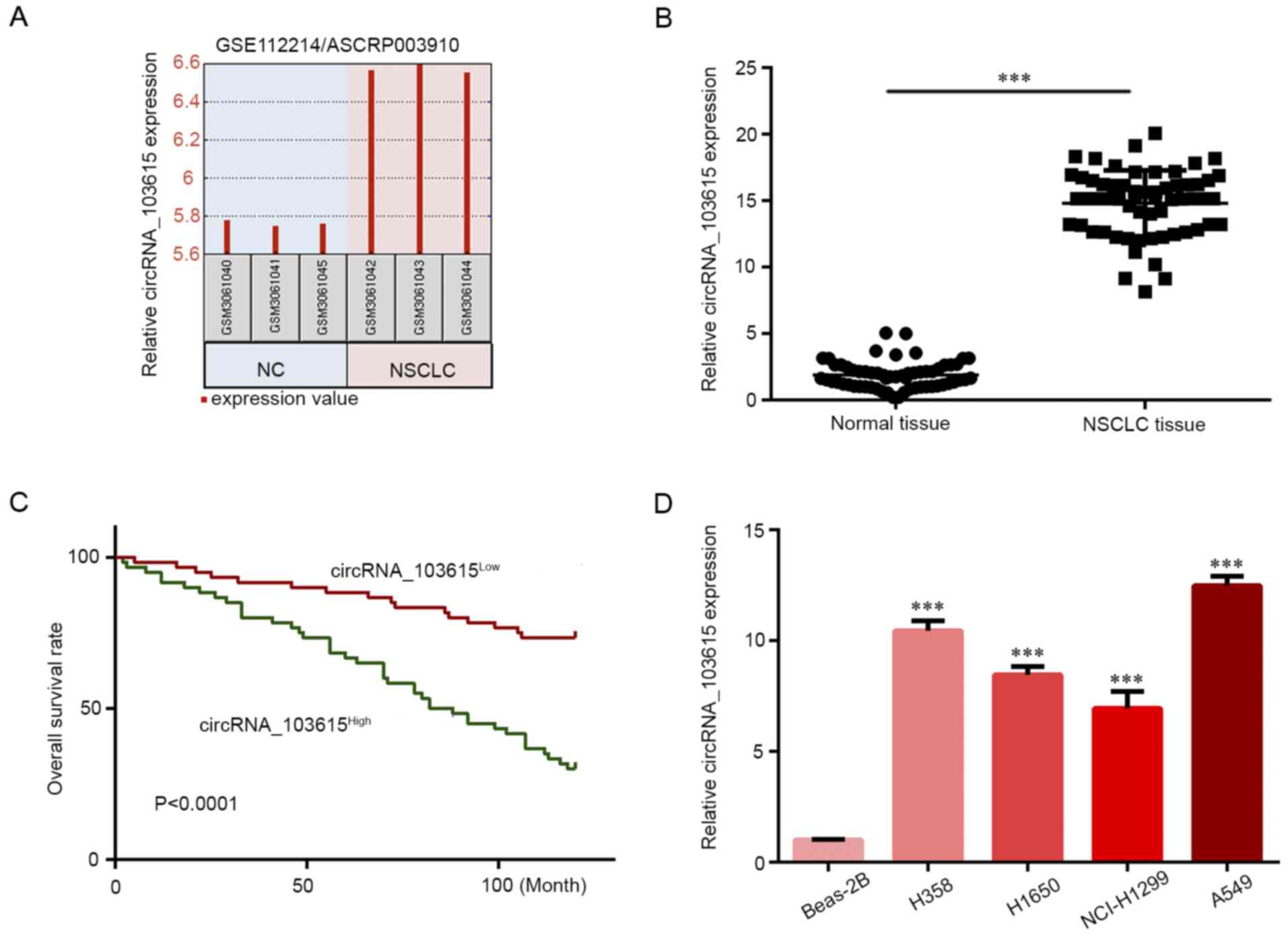
Microarray data in the Gene Expression Omnibus
dataset GSE112214 were retrieved to examine the expression of
circRNAs in NSCLC, and GEO2R was used to analyze normalized
microarray data after applying log2 transformation. The
data showed that circRNA_103615 expression was increased in NSCLC
tissues compared with normal tissues (Fig. 1A). Therefore, circRNA_103615 was
selected for further investigation in the present study. RT-qPCR
was used to detect the circRNA_103615 expression levels in 60 NSCLC
tissue samples and matched normal tissues. The results demonstrated
that circRNA_103615 expression levels were significantly increased
in NSCLC tissues compared with normal tissues (Fig. 1B). Next, the patient cohort was
separated into high circRNA 103615 (n=30) and low circRNA_103615
groups (n=30) according to the median circRNA_103615 expression
(15.1585), and Kaplan-Meier survival analysis revealed that low
circRNA_103615 expression levels in NSCLC patients were associated
with a higher survival rate (Fig.
1C). Furthermore, the circRNA_103615 expression levels of NSCLC
cell lines A549, NCI-H1299, H1650 and H358 and a normal lung cell
line Beas-2B were examined by RT-qPCR assay. The results revealed
that the circRNA_103615 expression levels were significantly
increased in NSCLC cell lines compared with the normal Beas-2B
cells (Fig. 1D). The present
findings demonstrated that circRNA_103615 was significantly
upregulated in NSCLC clinical samples and cell lines, suggesting
that its dysregulation may promote NSCLC progression.
Silencing of circRNA_103615 attenuates
tumor progression in NSCLC
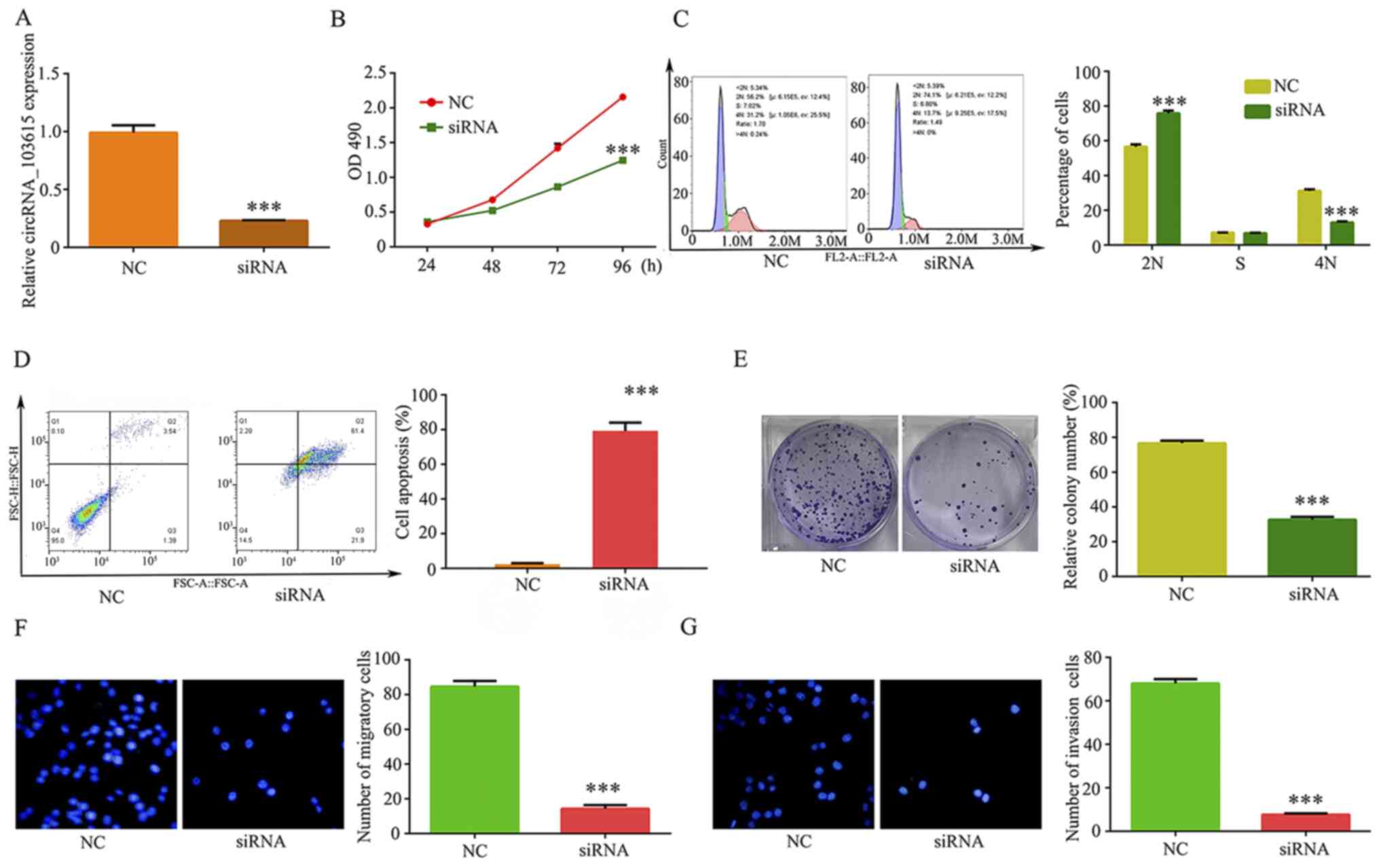
To investigate the function of circRNA_103615 in
NSCLC, an siRNA that specifically targeted the circRNA_103615
junction sites was constructed (si-circRNA_103615) and transfected
into the A549 cells. RT-qPCR was used to examine the transfection
and silencing efficiency of si-circRNA_103615. The results
confirmed that circRNA_103615 expression levels were significantly
decreased in A549 cells transfected with the si-circRNA_103615
compared with the control (Fig.
2A). The effect of circRNA_103615 silencing in cell
proliferation was analyzed by MTT assay. The results showed that
the numbers of viable A549 cells were suppressed at 48-96 h
following si-circRNA_103615 transfection (Fig. 2B), suggesting that knockdown of
circRNA_103615 attenuated NSCLC cell proliferation in vitro.
In addition, G1 arrest was induced by si-circRNA_103615
transfection (Fig. 2C), and cell
apoptosis was promoted (Fig. 2D)
compared with the NC-transfected A549 cells. Finally, the clone
formation ability of A549 cell was significantly decreased
following silencing of circRNA_103615 (Fig. 2E).
The association analysis of circRNA_103615
expression with the clinicopathological features of the patients
enrolled in the present study revealed that increased expression of
circRNA_103615 was significantly associated with increased tumor
size, advanced TNM stage and the presence of metastases (Table II). Because cell migration and
invasion are processes associated with tumor progression and
metastasis, the present study explored the effect of circRNA_103615
on NSCLC cell migration and invasion. Transwell assays were
utilized to examine migration and invasion of A549 cells
transfected with either si-circRNA_103615 or si-NC in vitro.
The results revealed that cell migration (Fig. 2F) and invasion (Fig. 2G) of A549 cells significantly
decreased following silencing of circRNA_103615 expression. These
findings suggested that the downregulation of circRNA_103615 may
attenuate tumor progression in NSCLC.
 | Table IIAssociation between clinical features
and circRNA_103615 expression in 60 patients with non-small cell
lung carcinoma. |
Table II
Association between clinical features
and circRNA_103615 expression in 60 patients with non-small cell
lung carcinoma.
| | circRNA_103615
expression | |
|---|
| Clinical
feature | No. of cases | Low | High | P-value |
|---|
| Age (years) | | | | 0.452 |
|
<55 | 34 | 18 | 16 | |
|
≥55 | 26 | 12 | 14 | |
| Sex | | | | 0.845 |
|
Male | 30 | 16 | 14 | |
|
Female | 30 | 14 | 16 | |
| TNM stage | | | | <0.01 |
|
I-II | 24 | 20 | 4 | |
|
III-IV | 36 | 10 | 26 | |
| Tumor size | | | | <0.01 |
|
<5
cm | 27 | 19 | 8 | |
|
≥5 cm | 33 | 11 | 22 | |
| Metastasis | | | | <0.01 |
|
No | 27 | 22 | 5 | |
|
Yes | 33 | 8 | 25 | |
Silencing of circRNA_103615 reverses
the cisplatin resistance of NSCLC cells in vitro
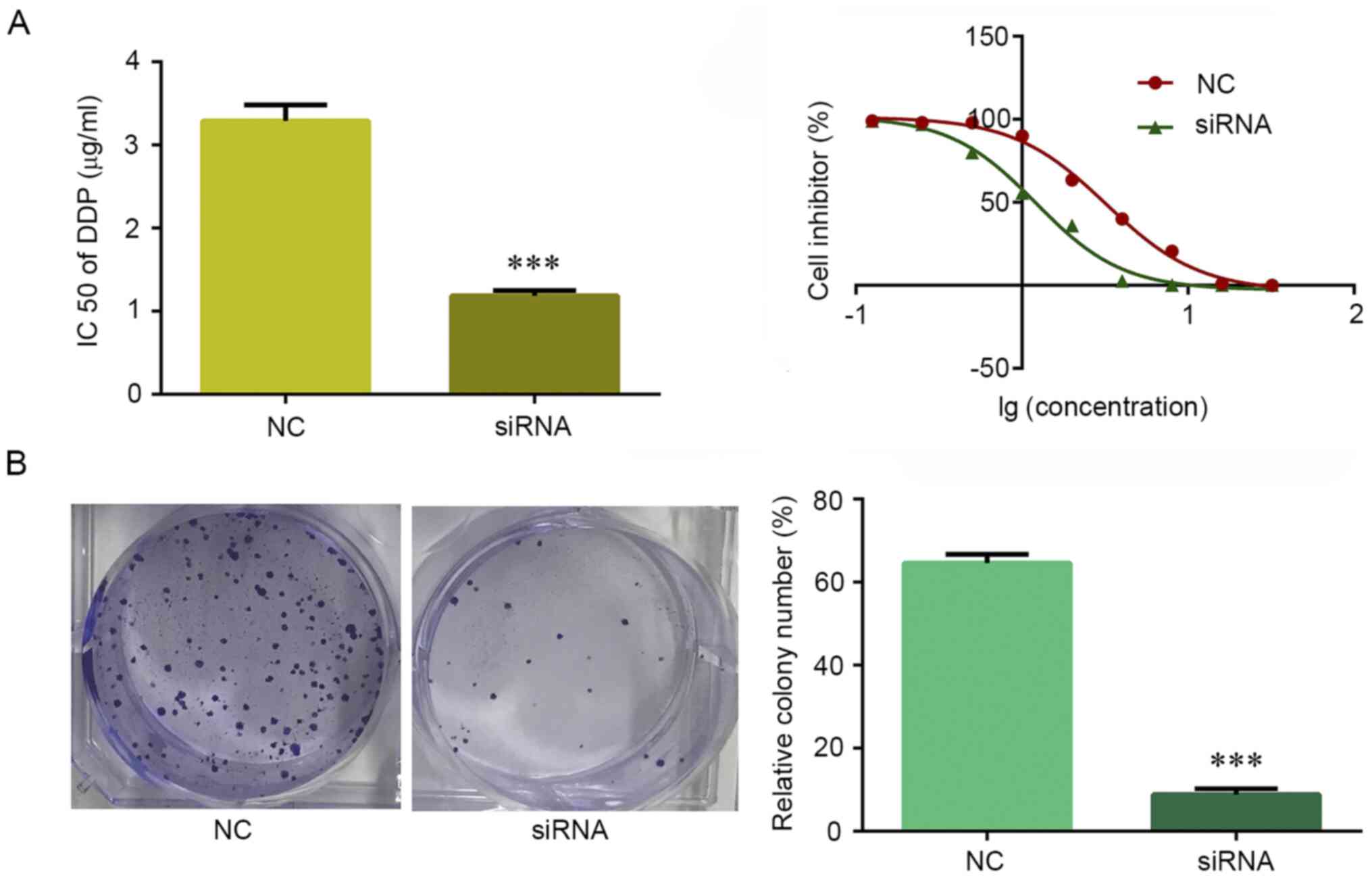
The most commonly used drug for the treatment of
NSCLC is cisplatin. MTT assay was used to explore the potential
role of circRNA_103615 in cisplatin resistance in NSCLC. The
results demonstrated that, after 48 h of treatment, the
IC50 values of cisplatin in A549 cells transfected with
si-circRNA_103615 (1.18 µg/ml) were decreased compared with those
in control A549 cells (3.29 µg/ml) (Fig. 3A). In addition, when A549 cells were
treated with 2 µg/ml cisplatin for a week in colony formation
assays, the cell growth rate was significantly inhibited in A549
cells following si-circRNA_103615 transfection compared with
control A549 cells (Fig. 3B).
Therefore, downregulation of circRNA_103615 reversed the cisplatin
resistance of NSCLC cells in vitro.
circRNA_103615 enhances cisplatin
resistance by promoting ABCB1 expression in NSCLC cells
To explore the molecular mechanism underlying the
role of circRNA_103615 in cisplatin resistance in NSCLC, RT-qPCR
was used to examine the expression of a panel of classical
multidrug resistance-related genes. The results reveled that the
mRNA expression levels of ABCB1 were significantly decreased in
A549 cells transfected with si-circRNA_103615 compared with
NC-transfected A549 cells (Fig.
4A). No significant differences were observed in the expression
levels of the classical multidrug resistance-related genes MRP1,
BCRP, GST-π and HMGB1 (Fig. 4A).
Furthermore, western blot analysis was used to detect the protein
expression levels of ABCB1. The results demonstrated that ABCB1
protein expression levels were also markedly decreased in A549
cells following si-circRNA_103615 transfection compared with
control A549 cells (Fig. 4B). In
addition, ABCB1 mRNA expression levels were significantly increased
in NSCLC tissues compared with normal tissues (Fig. 4C), and ABCB1 expression was
positively correlated with circRNA_103615 expression in NSCLC
tissues (Fig. 4D).
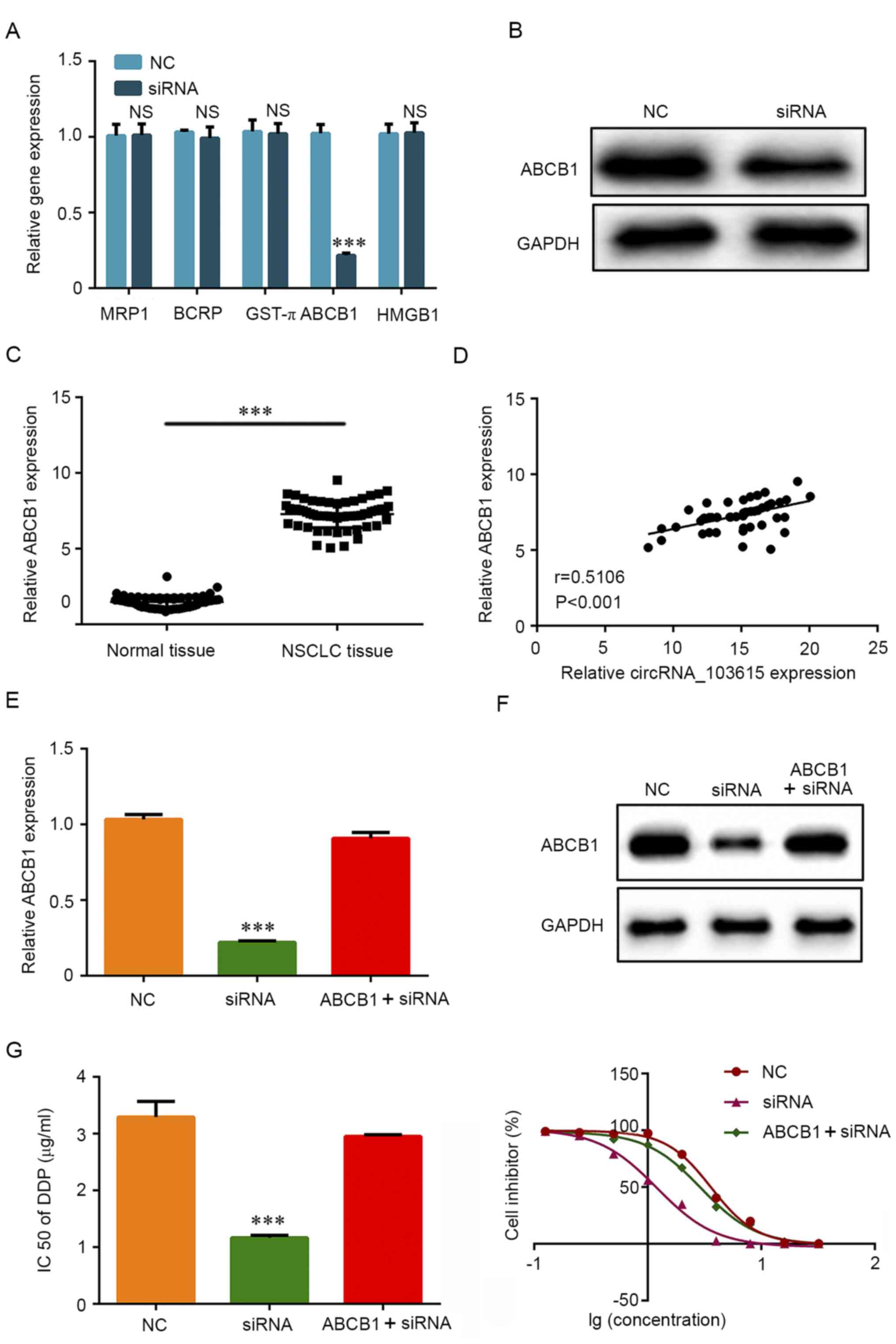 | Figure 4circRNA_103615 enhances cisplatin
resistance by promoting ABCB1 expression in NSCLC cells. (A) The
mRNA expression levels of classical multidrug resistance-related
genes were detected by RT-qPCR in A549 cells transfected with
si-circRNA_103615 or si-NC. (B) The protein expression levels of
ABCB1 were detected by western blotting in A549 cells transfected
with si-circRNA_103615 or si-NC. (C) ABCB1 expression levels were
examined by RT-qPCR in 60 NSCLC tissues and their adjacent normal
tissues collected in the present study. ***P<0.001.
(D) The correlation between circRNA_103615 and ABCB1 expression in
the 60 NSCLC tissues was evaluated using Pearson's correlation
analysis (n=60, r=0.5106, P<0.001). (E) A549 cells were
transfected with si-NC or si-circRNA_103615, or co-transfected with
si-circRNA_103615 and an ABCB1-overexpression plasmid. Successful
ABCB1 overexpression was confirmed by RT-PCR and (F) western blot
analysis. ***P<0.001 vs. NC. (G) The IC50
value of cisplatin was detected by MTT assay.
***P<0.001 vs. NC. Data are presented as the mean ±
SD (n=3). circRNA, circular RNA; ABCB1, ATP binding cassette
subfamily Bmember 1; NSCLC, non-small cell lung carcinoma; RT-qPCR,
reverse transcription-quantitative PCR; si, small interfering; NC,
negative control; IC50, half-maximal inhibitory
concentration. |
To further examine whether the effects of
circRNA_103615 were mediated by ABCB1, an ABCB1-overexpressing
plasmid was transfected in A549 cell lines to restore ABCB1
expression. Firstly, the efficiency of the ABCB1-overexpressing
plasmid pcDNA3.1-ABCB1 was confirmed by RT-qPCR (Fig. S1). The results showed that pcDNA3.1
could be used as a transfection control. RT-qPCR results (Fig. 4E) and western blotting results
(Fig. 4F) confirmed that the
expression of ABCB1 was successfully increased in cells
co-transfected with si-circRNA_103615 and pcDNA3.1-ABCB1 compared
with cells transfected with si-circRNA_103615 alone. Of note, the
IC50 values of cisplatin in the ABCB1-overexpressing,
circRNA_103615-silenced A549 cells (2.95 µg/ml) were significantly
increased compared with the cells transfected with
si-circRNA_103615 alone (1.17 µg/ml) (Fig. 4G). The present findings indicated
that circRNA_103615 promoted ABCB1 expression and subsequently
enhanced cisplatin resistance in NSCLC cells.
Discussion
Highly efficient sequencing and bioinformatic
analysis technologies have aided in the identification and
functional study of circRNAs. Several circRNAs have been found to
be associated with progression, migration and invasion of NSCLC.
For example, the upregulation of circRNA hsa_circ_0007534 predicts
an unfavorable prognosis for NSCLC and exerts oncogenic properties
in vitro and in vivo (17). Overexpression of circRNA_100876 has
been shown to have prognostic value in NSCLC (13). Microarray analysis has shown that
circRNA hsa_circ_0007385 functions as an oncogene in NSCLC
tumorigenesis (18). Notably,
circRNA acts as a prognostic biomarker and inhibits tumor
progression through the mechanism of sponge miRNA (19,20).
For example, a novel circular RNA, hsa_circ_0043278, regulates
miR-520, acts as a potential biomarker and promotes NSCLC
proliferation and migration (21).
A microarray profile of circRNAs has identified hsa_circ_0014130 as
a new circRNA biomarker in NSCLC (22). CircFADS2 regulates the proliferation
and invasion of lung cancer cells via the sponging of
miR-498(23). circRNA circ_0001649
acts as a prognostic biomarker and inhibits NSCLC progression via
sponging miR-331-3p and miR-338-5p (24).
In the present study and for the first time,
circRNA_103615 was investigated in tissues from patients with
NSCLC, and circRNA_103615 expression levels were demonstrated to be
significantly upregulated in NSCLC tissues and cell lines. In
addition, functional assays revealed that silencing of
circRNA_103615 attenuated the proliferation, migration, and
invasion of NSCLC cells and promoted cell apoptosis. Further
studies will be required in the future to explore the signaling
pathways by which circRNA_103615 affects cell proliferation,
migration and invasion.
Research in the chemoresistance mechanisms in NSCLC
is rare. Thus, investigating the molecular mechanisms involved in
the chemoresistance of NSCLC may provide an understanding of
improved therapies for NSCLC. In the present study, silencing of
circRNA_103615 significantly decreased the cisplatin
IC50 values of A549 cells and reduced the expression
levels of the classical multidrug resistance gene ABCB1, suggesting
that circRNA_103615 promoted ABCB1 expression in NSCLC cells, thus,
enhancing cisplatin resistance. It is worth noting that the
expression of other classical multidrug resistance-related genes,
including MRP1, BCRP, GST-π and HMGB1, was not affected. Therefore,
ABCB1 appears to be the predominant protein mediating the role of
circRNA_103615 in chemoresistance. Further studies will be needed
in the future to explore the signaling pathways affected by
circRNA_103615 and ABCB1 in chemoresistance.
In conclusion, the present study demonstrated that
circRNA_103615 promoted proliferation, migration, invasion and
cisplatin resistance by enhancing ABCB1 expression in NSCLC cells
in vitro. Therefore, circRNA_103615 may serve as a potential
biomarker in NSCLC, and circRNA_103615 suppression may serve as a
potential therapeutic strategy for NSCLC.
Supplementary Material
ABCB1 overexpression in NSCLC cells.
ABCB1 mRNA expression levels were detected in co-transfection with
si-NC and pcDNA3-1 by RT-qPCR in A549 cells. *P<0.05.
ABCB1, ATP binding cassette subfamily B member 1; NSCLC, non-small
cell lung carcinoma; RT-qPCR, reverse transcription-quantitative
PCR; NC, negative control.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WH, HoL and ZL conceived the current study. HoL and
WH conceived and designed the experiments. HoL and ZL performed the
experiments. HuL and LZ analyzed statistical data. WH interpreted
the data and revised the manuscript. HoL and WH confirmed the
authenticity of all the raw data All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
All procedures involving human samples were approved
by the Human Resources Ethics Committee of the Fuda Cancer
Hospital. Written informed consent was obtained from all the
participants before enrollment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chang A: Chemotherapy, chemoresistance and
the changing treatment landscape for NSCLC. Lung Cancer. 71:3–10.
2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jeremic B, Milicic B, Dagovic A,
Aleksandrovic J and Nikolic N: Pretreatment clinical prognostic
factors in patients with stage IV non-small cell lung cancer
(NSCLC) treated with chemotherapy. J Cancer Res Clin Oncol.
129:114–122. 2003.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yano T, Okamoto T, Fukuyama S and Maehara
Y: Therapeutic strategy for postoperative recurrence in patients
with non-small cell lung cancer. World J Clin Oncol. 5:1048–1054.
2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kondo S, Iwata S, Yamada T, Inoue Y,
Ichihara H, Kichikawa Y, Katayose T, Souta-Kuribara A, Yamazaki H,
Hosono O, et al: Impact of the integrin signaling adaptor protein
NEDD9 on prognosis and metastatic behavior of human lung cancer.
Clin Cancer Res. 18:6326–6338. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jeck WR, Sorrentino JA, Wang K, Slevin MK,
Burd CE, Liu J, Marzluff WF and Sharpless NE: Circular RNAs are
abundant, conserved, and associated with ALU repeats. RNA.
19:141–157. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang HD, Jiang LH, Sun DW, Hou JC and Ji
ZL: circRNA: A novel type of biomarker for cancer. Breast Cancer.
25:1–7. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yao Z, Luo J, Hu K, Lin J, Huang H, Wang
Q, Zhang P, Xiong Z, He C, Huang Z, et al: ZKSCAN1 gene and its
related circular RNA (circZKSCAN1) both inhibit hepatocellular
carcinoma cell growth, migration, and invasion but through
different signaling pathways. Mol Oncol. 11:422–437.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tian M, Chen R, Li T and Xiao B: Reduced
expression of circRNA hsa_circ_0003159 in gastric cancer and its
clinical significance. J Clin Lab Anal. 32(e22281)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Du WW, Fang L, Yang W, Wu N, Awan FM, Yang
Z and Yang BB: Induction of tumor apoptosis through a circular RNA
enhancing Foxo3 activity. Cell Death Differ. 24:357–370.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yang C, Yuan W, Yang X, Li P, Wang J, Han
J, Tao J, Li P, Yang H, Lv Q and Zhang W: Circular RNA circ-ITCH
inhibits bladder cancer progression by sponging miR-17/miR-224 and
regulating p21, PTEN expression. Mol Cancer. 17(19)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gong Y, Mao J, Wu D, Wang X, Li L, Zhu L
and Song R: Circ-ZEB1.33 promotes the proliferation of human HCC by
sponging miR-200a-3p and upregulating CDK6. Cancer Cell Int.
18(116)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yao JT, Zhao SH, Liu QP, Lv MQ, Zhou DX,
Liao ZJ and Nan KJ: Over-expression of circRNA_100876 in non-small
cell lung cancer and its prognostic value. Pathol Res Pract.
213:453–456. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhu Q, Lu G, Luo Z, Gui F, Wu J, Zhang D
and Ni Y: circRNA circ_0067934 promotes tumor growth and metastasis
in hepatocellular carcinoma through regulation of
miR-1324/FZD5/Wnt/β-catenin axis. Biochem Biophys Res Commun.
497:626–632. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xia W, Qiu M, Chen R, Wang S, Leng X, Wang
J, Xu Y, Hu J, Dong G, Xu PL and Yin R: Circular RNA
has_circ_0067934 is upregulated in esophageal squamous cell
carcinoma and promoted proliferation. Sci Rep.
6(35576)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Huang X, Li Z, Zhang Q, Wang W, Li B, Wang
L, Xu Z, Zeng A, Zhang X, Zhang X, et al: Circular RNA AKT3
upregulates PIK3R1 to enhance cisplatin resistance in gastric
cancer via miR-198 suppression. Mol Cancer. 18(71)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Qi Y, Zhang B, Wang J and Yao M:
Upregulation of circular RNA hsa_circ_0007534 predicts unfavorable
prognosis for NSCLC and exerts oncogenic properties in vitro and in
vivo. Gene. 676:79–85. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jiang MM, Mai ZT, Wan SZ, Chi YM, Zhang X,
Sun BH and Di QG: Microarray profiles reveal that circular RNA
hsa_circ_0007385 functions as an oncogene in non-small cell lung
cancer tumorigenesis. J Cancer Res Clin Oncol. 144:667–674.
2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jin X, Feng CY, Xiang Z, Chen YP and Li
YM: circRNA expression pattern and circRNA-miRNA-mRNA network in
the pathogenesis of nonalcoholic steatohepatitis. Oncotarget.
7:66455–66467. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ashwal-Fluss R, Meyer M, Pamudurti NR,
Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N and
Kadener S: circRNA biogenesis competes with pre-mRNA splicing. Mol
Cell. 56:55–66. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cui J, Li W, Liu G, Chen X, Gao X, Lu H
and Lin D: A novel circular RNA, hsa_circ_0043278, acts as a
potential biomarker and promotes non-small cell lung cancer cell
proliferation and migration by regulating miR-520f. Artif Cells
Nanomed Biotechnol. 47:810–821. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang S, Zeng X, Ding T, Guo L, Li Y, Ou S
and Yuan H: Microarray profile of circular RNAs identifies
hsa_circ_0014130 as a new circular RNA biomarker in non-small cell
lung cancer. Sci Rep. 8(2878)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhao F, Han Y, Liu Z, Zhao Z, Li Z and Jia
K: circFADS2 regulates lung cancer cells proliferation and invasion
via acting as a sponge of miR-498. Biosci Rep.
38(BSR20180570)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liu T, Song Z and Gai Y: Circular RNA
circ_0001649 acts as a prognostic biomarker and inhibits NSCLC
progression via sponging miR-331-3p and miR-338-5p. Biochem Biophys
Res Commun. 503:1503–1509. 2018.PubMed/NCBI View Article : Google Scholar
|