Introduction
Granulosa cell (GC) apoptosis and/or premature
removal of surrounding GCs deprive oocytes of growth factors,
nutrients and survival factors resulting in apoptosis in
diplotene-arrested oocytes cultured in vitro (1), as demonstrated by Tiwari et al
(2). Therefore, GC apoptosis in
ovulated cumulus-oocyte complexes can be used as predictor of
oocyte quality (3). Establishing
appropriate in vitro GC models is important in the study of
ovarian function and assists in elucidation of the mitogenic,
luteotropic and apoptotic mechanisms responsible for selection and
support of the dominant ovarian follicle (4). Furthermore, successful cell isolation
and purification methods are essential for accurate interpretation
of experimental data. A common source of human GCs is from
follicular fluid from patients undergoing in vitro
fertilization (IVF) procedures (5).
Therefore, further high-volume, high-quality studies aimed at
separating GCs from follicular fluid that are high quality and
sufficient in number is essential (6). Several human GC isolation techniques
have been described in the literature (7-12).
The density gradient procedure is a rapid, simple and relatively
inexpensive technique. In addition, it allows the recovery of a
high percentage of luteinized GCs (11). Sun et al (12) confirmed the efficacy of two-step 50%
Percoll gradient centrifugation to separate and extract GCs.
However, to the best of our knowledge, there are no existing
studies that compare the efficacy of a method of isolating GCs in
patients with varying ovarian reserve function. The purification
method for human GCs described in the current study utilizes a
Percoll gradient to remove contaminating red blood cells, followed
by the selection of GC aggregates. This technique was tailored to
produce a pure population of GCs from patients with varying ovarian
reserve function, in order to obtain high-quality RNA for gene
expression studies. The process of the purification and
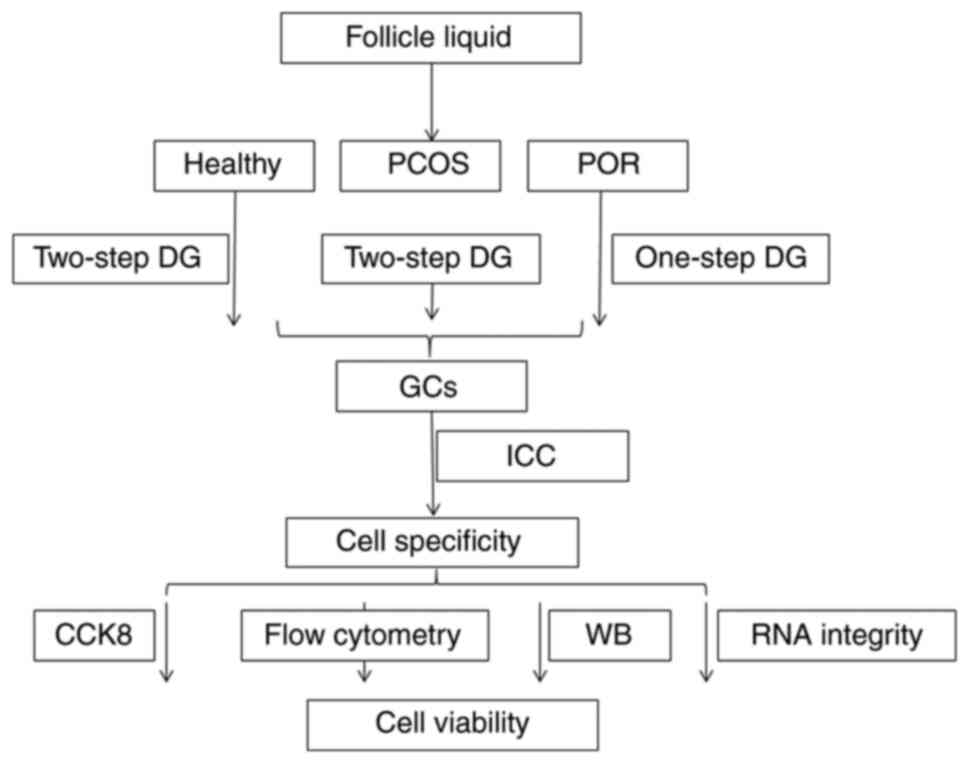
characterization of GCs has been summarized in Fig. 1. Thus, the aim of the current study
was to provide an efficient method of preparing GCs from patients
with varying ovarian reserve functions in order to evaluate their
molecular function.
Materials and methods
Patients and sample preparation
Between December 2015 and June 2016, infertile women
who were eligible for IVF/intracytoplasmic sperm injection (ICSI)
treatment at the Reproductive Medicine Centre of Tianjin Central
Hospital of Obstetrics and Gynecology were examined for eligibility
and those that consented to participate in the present study were
recruited. The study was approved by the hospital's Institutional
Review Board (approval no. TJCOB2016002) and was performed in
accordance with the Declaration of Helsinki. No additional
interventions were included in the routine clinical and laboratory
standards for IVF/ICSI preparation and treatment. Informed written
consent was obtained from all participants. The exclusion criteria
were as follows: Diabetes type 1 or 2, impaired thyroid, renal or
hepatic function, congenital adrenal hyperplasia, endometriosis and
hypothalamic amenorrhea.
Table I summarizes
data from a cohort of 45 patients who underwent oocyte retrieval. A
consensus was reached on the minimal criteria needed to define poor
ovarian response (POR) and polycystic ovary syndrome (PCOS). POR
was defined in accordance with the Bologna criteria (13). At least two of the following three
features had to be present to be categorized as POR: i) Advanced
maternal age (≥40 years) or any other risk factor for POR; ii)
previous POR (≤3 oocytes with a conventional stimulation protocol);
iii) an abnormal ovarian reserve test [for example, antral follicle
count, 5-7 follicles or anti-Müllerian hormone (AMH), 0.5-1.1
ng/ml].
 | Table IPatient characteristics in the three
groups. |
Table I
Patient characteristics in the three
groups.
| | Subject |
|---|
| Characteristic | Patients with POR
(n=5) | Healthy individuals
(n=18) | Patients with PCOS
(n=22) |
|---|
| Age, years | 37±3.16 | 29±3.22 | 27±3.26 |
| No. of oocytes
retrieved | 5.4±1.14 | 16.6±2.81 | 25.7±5.26 |
| Basal FSH | 10.7±2.45 | 6.1±1.10 | 4.5±1.19 |
| Basal FSH/LH | 2.7±1.15 | 1.4±0.99 | 0.6±0.74 |
Only subjects with a diagnosis of PCOS according to
the Rotterdam criteria (14) were
included and one of the features had to be polycystic ovaries on
ultrasound examination. At least two of the following three
features had to be present to be categorized as PCOS: i) Oligo- or
anovulation; ii) clinical and/or biochemical signs of
hyperandrogenism; iii) polycystic ovaries and exclusion of other
etiologies (congenital adrenal hyperplasia, androgen-secreting
tumors, Cushing's syndrome).
Cell culture and reagents Healthy
individuals and PCOS patients
Follicular granulosa-luteal cells were obtained
after oocyte retrieval from 38 patients undergoing IVF treatment at
the Reproductive Medicine Centre of Tianjin Central Hospital of
Obstetrics and Gynecology. All patients participating in the
present study underwent controlled ovarian stimulation according to
routine long or short gonadotropin-releasing hormone agonist
protocols. The follicular aspirates from each patient were pooled
in conical bottomed 15-ml polypropylene centrifuge tubes. These
were then centrifuged at 800 x g for 10 min at room temperature
after which the supernatant was discarded. After adding a small
quantity of saline to resuspend the cells, the cell suspension was
transferred to an equal volume of 50% (v/v) Percoll (Sigma-Aldrich;
Merck KGaA) by centrifugation at 500 x g for 20 min at room
temperature. After resuspending the cells using saline, they were
separated with an equal volume of 0.25% trypsin digestion
solution(Sigma-Aldrich; Merck KGaA) for 10 min at room temperature.
Then, after the addition of the same volume of Gibco DMEM/F-12
media (Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum
(ES-Cult™ FBS; Stemcell Technologies, Inc.) to
neutralize trypsin, a centrifugation was performed at 800 x g for 5
min at room temperature and density gradient centrifugation was
repeated. Then, to perform a second density gradient
centrifugation, the supernatant was discarded, and the cells were
mixed well with normal saline and then centrifuged at 500 x g for
20 min at room temperature in an equal volume of 50% Percoll. GCs
were carefully aspirated with pipettes, mixed well with saline and
centrifuged at 800 x g for 5 min at room temperature, after which
the supernatant was discarded. The collected human GCs were
cultured in DMEM/F12 containing 10% FBS and 100 U/ml
penicillin/streptomycin. Samples were taken for cell counting,
viability testing via Trypan blue exclusion, and
immunocytochemistry and western blot analysis. The remainder was
cultured in an incubator under a constant temperature of 37˚C and
5% CO2. Nine samples were processed for RNA extraction
to analyze the integrity.
POR patients
The follicular aspirates from seven POR patients
were pooled. These were then centrifuged at 800 x g for 10 min at
room temperature after which the supernatant was discarded. After
resuspending cells using saline, they were separated using an equal
volume of 0.25% trypsin for digestion at room temperature for 10
min. Subsequent processing was consistent with the abovementioned
protocol. One sample was processed for RNA extraction to analyze
the integrity.
Immunocytochemistry
GCs were seeded at 70% confluence onto small glass
coverslips placed into 24-well plates. After 24 h of culture the
coverslips were removed, washed with PBS three times, fixed with
100% methanol (-20˚C) for 15 min at room temperature, washed with
PBST three times, and blocked for 1 h with 5% (v/v) goat serum
(Gibco; Thermo Fisher Scientific, Inc.) in PBS. Next, the cells
were incubated overnight at 4˚C with the following primary
antibodies: Polyclonal goat anti-human IgG follicle stimulating
hormone receptor (FSHR; 1:100; cat. no. sc-7798) and polyclonal
rabbit anti-human IgG to Müllerian inhibiting substance type II
receptor (MISIIR; 1:200; cat. no. sc-67287) from Santa Cruz
Biotechnology, Inc., and then incubated with secondary antibody
rabbit anti-goat IgG and goat anti-rabbit IgG (1:200; cat. no.
sc2768 and sc2004) at room temperature for 1 h and rinsed with PBS.
Chromogen 3,3'-diaminobenzidine tetrachloride (SERVA
Electrophoresis GmbH) was used as a substrate. The cell nucleus was
stained with Harris hematoxylin solution (cat. no. HY-N0116;
MedChemExpress) for 15 min at room temperature. The expression
levels of FSHR and MISIIR were scored according to the extent and
intensity of staining. The extent of staining was scored by the
percentage of the positively stained area in each region of
interest using the following scale: 0 for <5%; 1 for 5-25%; 2
for 25-50%; 3 for 50-75%; and 4 for ≥75% (15). The staining intensity was scored as
0, 1, 2 and 3 for the representation of negative (no staining),
mild (weak), intermediate (distinct) and intense (strong) staining,
respectively (15). The staining
intensity and stained area percentage were multiplied to establish
a weighted score. Scoring was determined by three independent
evaluators without any knowledge of the pathological and clinical
characteristics of the patients.
Determination of the percentage of GCs
by flow cytometry
To confirm their phenotype, the GCs were
characterized for the expression of specific surface antigens
defining human GCs. Cells incubated for 30 min at 4˚C with 1:200
diluted human FSHR-phycoerythrin (PE) from R&D Systems, Inc.,
(cat. no. FAB65591P), CD9-PE (cat. no. 312105; BioLegend, Inc.) and
CD24-PE (cat. no. 12-0247-42; eBioscience; Thermo Fisher
Scientific, Inc.). After staining, cells were mixed well with PBS
and cell fluorescence was evaluated in 10,000 viable cells using a
BD FACSCanto™ II flow cytometer (BD Biosciences).
Cell viability measurement
Cells were seeded in multi-well plates (96-well) in
DMEM/F12 medium containing 10% FBS, at a density of
1x104 cells/well, and allowed to attach to the DMEM/F12
medium containing 10% FBS for 8 h following analysis. Cells were
counted using Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc.) according to the manufacturer's protocol, and
the absorbance was measured at 450 nm. The cell viability was
analyzed using enzyme-linked immunosorbent assay and optical
density values were read at 450 nm. The survival rate was
calculated with the following formula: [(As-Ab)/(Ac-Ab)] x100%
(16), where As is the absorbance
of PCOS and POR groups; Ab is the absorbance of the blank group,
which contains complete medium without cells; and Ac is the healthy
group.
Measurement of GC apoptosis by flow
cytometry
Cell lines were seeded into 12-well plates and
cultured at 37˚C for 72 h. The cells were collected into centrifuge
tubes, washed three times with PBS and stained with propidium
iodide (PI) and FITC-Annexin V (AnV) from the FITC Annexin V
Apoptosis Detection kit I (BD Biosciences), followed by flow
cytometry using the BD FACSCanto™ II. The percentage of
the cell population in the process of apoptosis was calculated
using FlowJo software (v10.4; FlowJo LLC).
Western blot analysis
The total protein from GCs was collected using RIPA
buffer (Sangon Biotech Co., Ltd.). A total of 1 ml RIPA buffer was
used per 100 mg. Cells were collected from a 6-well plate using 100
µl RIPA buffer per well. The lysates were homogenized and then
centrifuged at 16,000 x g for 30 min at 4˚C. The membrane protein
was extracted using the Membrane and Cytoplasm Protein Extraction
Kit (Nanjing KeyGen Biotech Co., Ltd.) according to the
manufacturer's instructions. Proteins from the supernatant were
quantified using a BCA assay (Thermo Fisher Scientific, Inc.), an
equal volume of 1X loading buffer was added and then the protein
was denatured for 10 min at 100˚C. A total of 20 µg protein from
each sample was separated on a 10% SDS-PAGE gel and transferred to
a PVDF membrane (Bio-Rad Laboratories, Inc.). The membranes were
blocked for 1 h at room temperature in 5% non-fat milk in
Tris-buffered saline with 0.1% Tween-20. Subsequently, the
membranes were incubated overnight at 4˚C with 1:1,000 diluted goat
anti-FSHR (cat. no. sc-7798; Santa Cruz, Biotechnology, Inc.),
1:1,000 diluted rabbit anti-caspase-3 (cat. no. sc-65497; Santa
Cruz Biotechnology, Inc.), 1:1,000 diluted rabbit anti-Bcl-2 (cat.
no. sc-7382; Santa Cruz Biotechnology, Inc.) and 1:10,000 diluted
mouse anti-β-actin (cat. no. C1213; Sungene Biotech, Ltd.). The
next day, the membranes were incubated with the corresponding
secondary antibodies rabbit anti-goat IgG, goat anti-rabbit IgG and
goat anti-mouse (1:200; cat. no. sc2768, sc2004 and sc2005,
respectively) at 1:1,000 for 1 h at room temperature. The immune
complexes were examined by ECL detection (EMD Millipore). The
western blotting bands were quantified using ImageJ Software
(v1.46; National Institutes of Health) after background
subtraction.
RNA extraction and amplification
For RNA analysis, GC samples were obtained from:
Patients with PCOS (n=5); healthy patients (n=2); and patients with
POR (n=2). Total RNA was extracted using an
RNAequeous®-Micro kit (Ambion; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. The samples
were analyzed for total RNA concentration using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), and total RNA
quality and level of degradation using an Agilent 2100 Bioanalyzer
(Agilent Technologies Deutschland GmbH) according to the
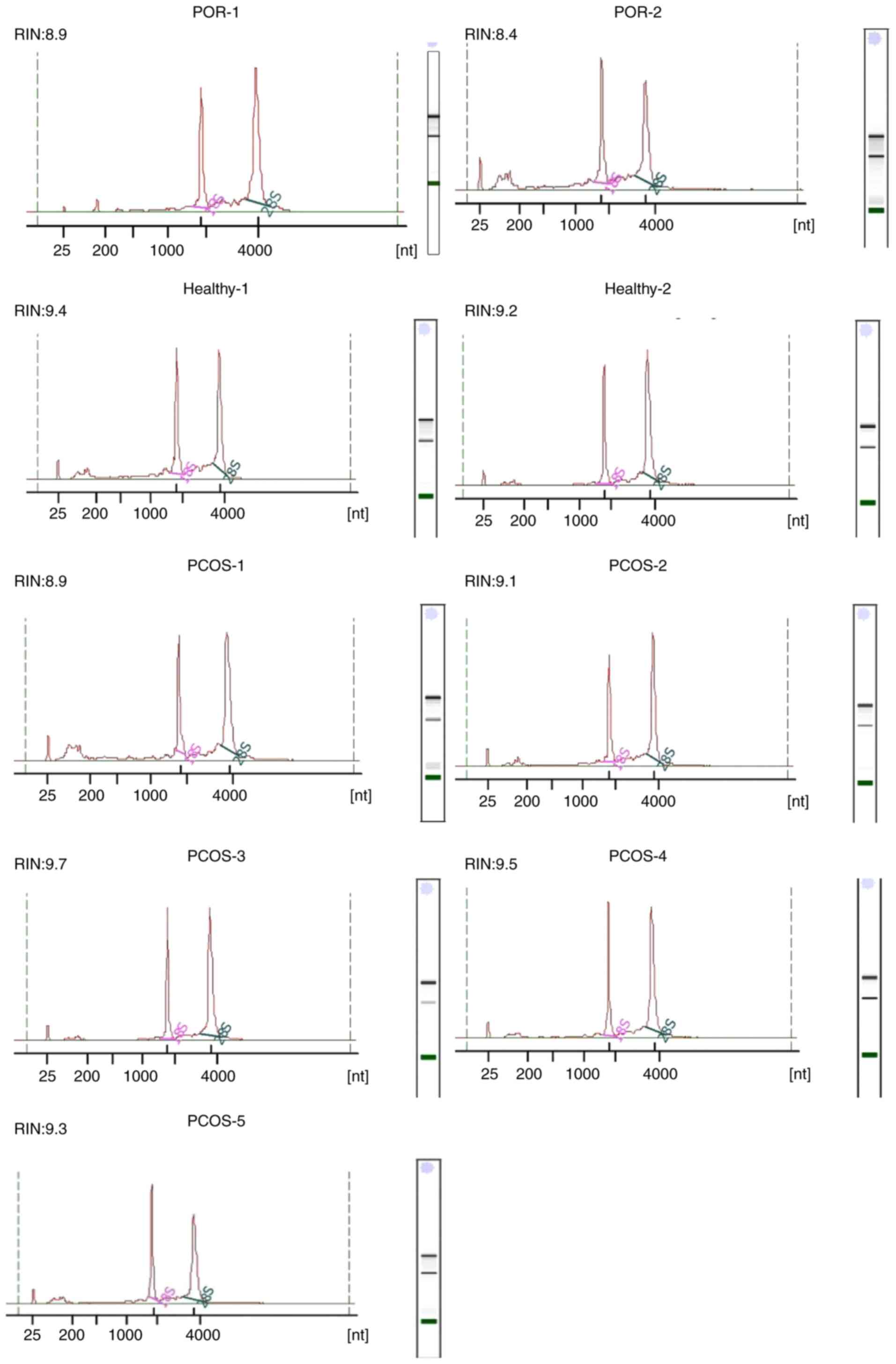
manufacturer's instructions. All of the RNA samples showed two
distinct peaks representing 18S and 28S ribosomal RNA, which
indicated good quality RNA, and presented an RIN of 8-10.
Statistical analysis
The data were analyzed using SPSS 19.0 software (IBM
Corp.). Student's unpaired t-test was performed for mean comparison
between two groups. The comparisons among multiple groups were
analyzed using ANOVA followed by the Least significant difference
post hoc test. Experimental data are presented as means ± standard
deviation and P<0.05 was considered to indicate a statistically
significant difference.
Results
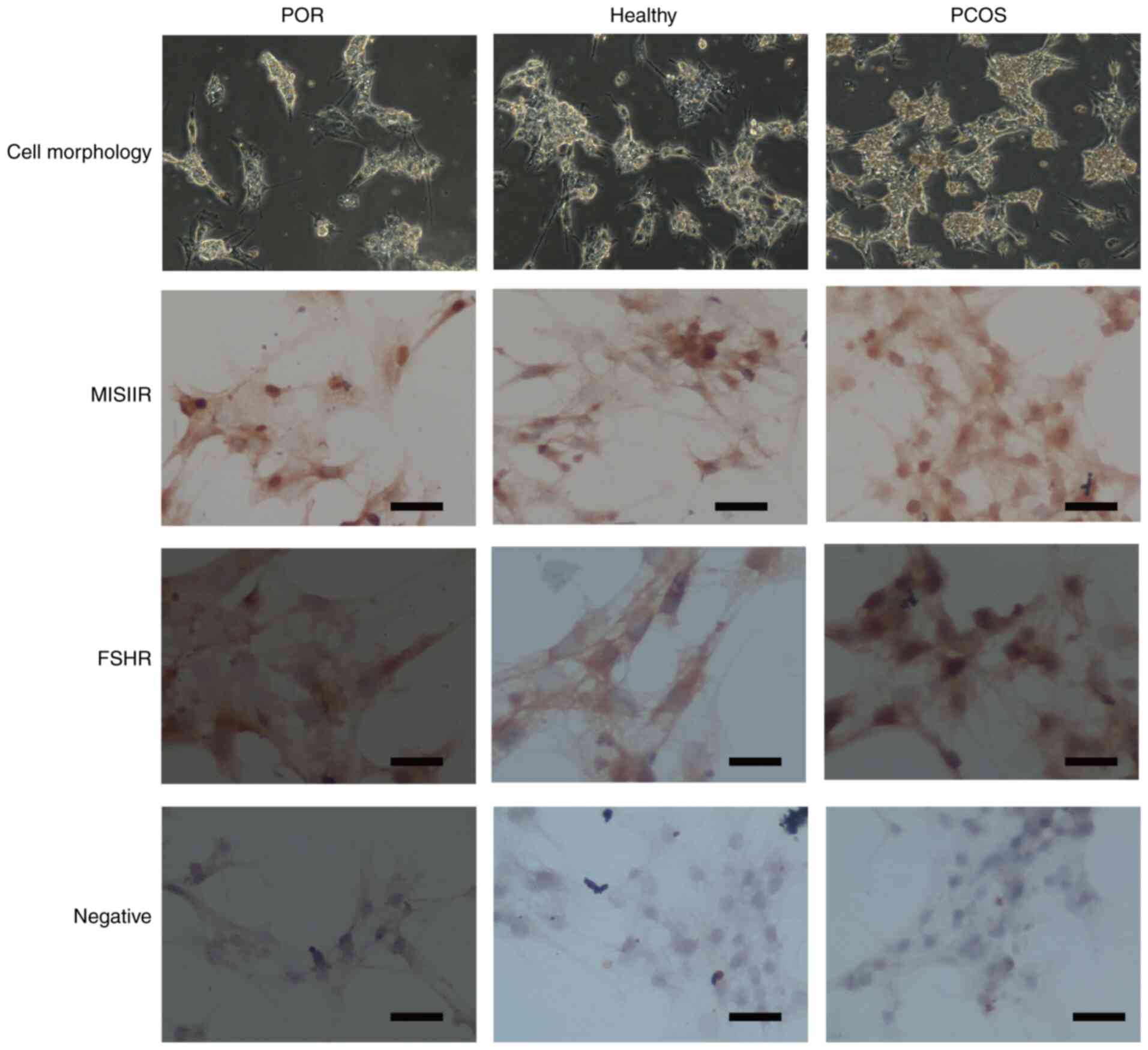
Human GCs express MISIIR and FSHR
Following 24 h of primary culture, single adherent
cells were observed in a distinct area of the culture dish. These
cells were rhombic or irregular in shape, inconsistent in size and
shape, and had clear boundaries. Additionally, connections between
the cells were observed under a microscope (Fig. 2). As all cells plated on the cover
slips exhibited MISIIR and FSHR (Fig.
2), immunocytochemistry demonstrated that the majority, if not
all, of human GCs express these receptors. The mean purity of GC
samples obtained using this method was >95%.
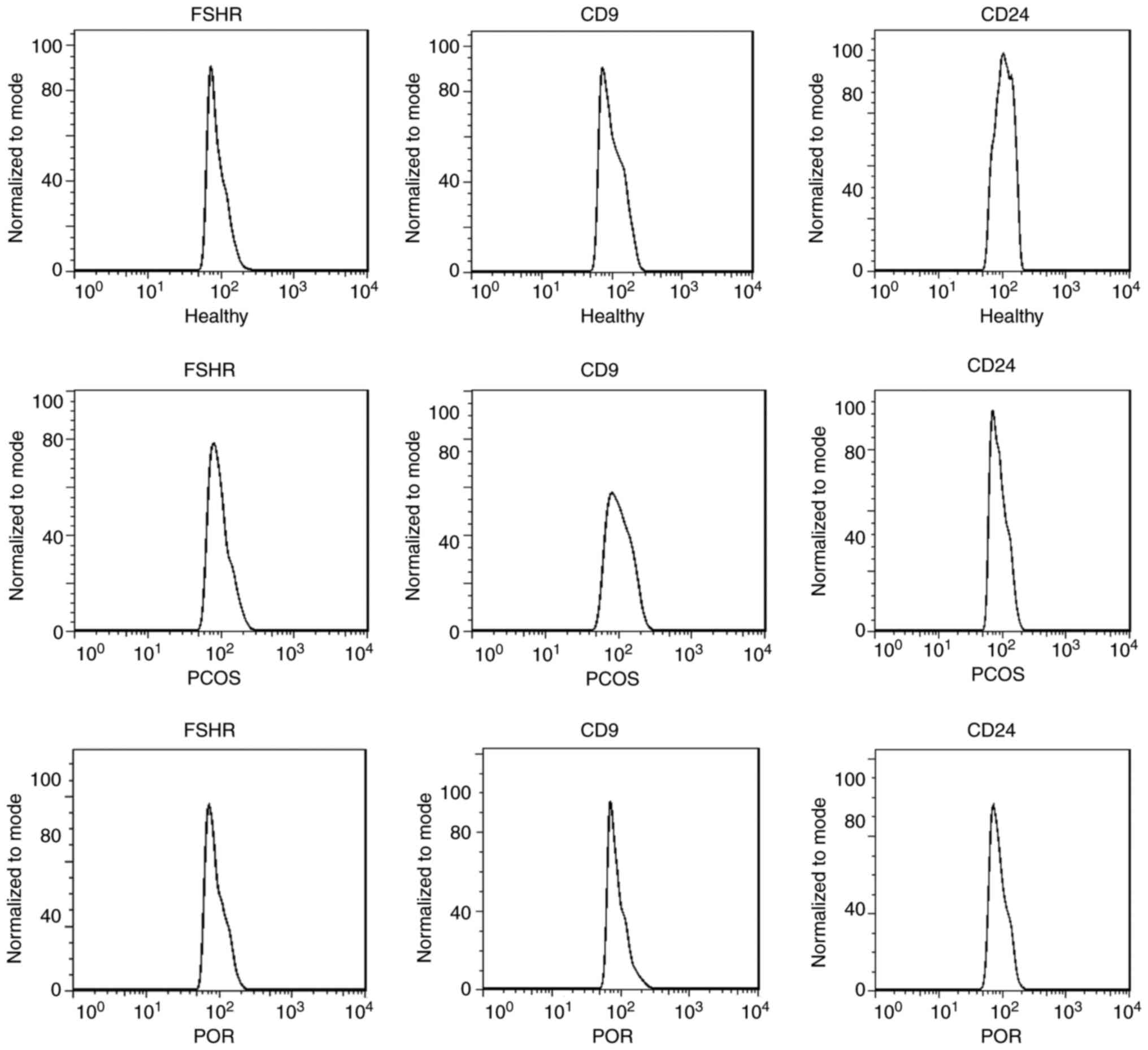
Human GCs express surface
antigens
In the three groups >95% of GCs were positive for
FSHR (Fig. 3). The CD9 and CD24
molecules are cell surface glycoproteins, which are also expressed
on the GCs surrounding the oocytes. Flow cytometry demonstrated
that CD9 is present on the cell surface of, on arithmetic average,
70% of GCs among the three groups and CD24 is present on the cell
surface of 90% of GCs among the three groups. The expression of CD9
on GCs in patients with PCOS was significantly lower than that in
the other groups (Fig. 3).
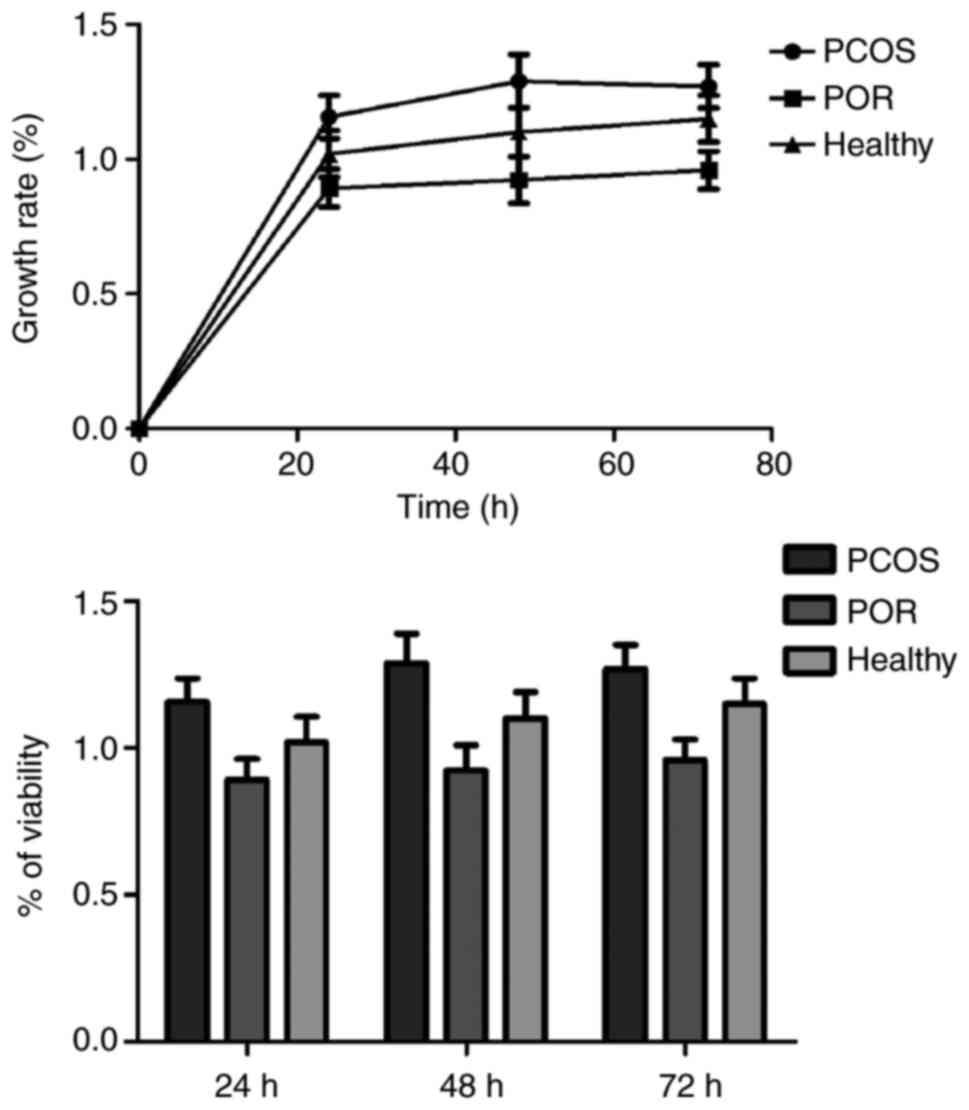
Proliferation rate of PCOS and healthy
GCs are increased compared with the POR group
Cell viability was assessed by CCK-8 assay. An
increase of ~50-70% in cell viability was observed in the cells
over time, with no significant differences identified among the
three groups. The histogram in Fig.
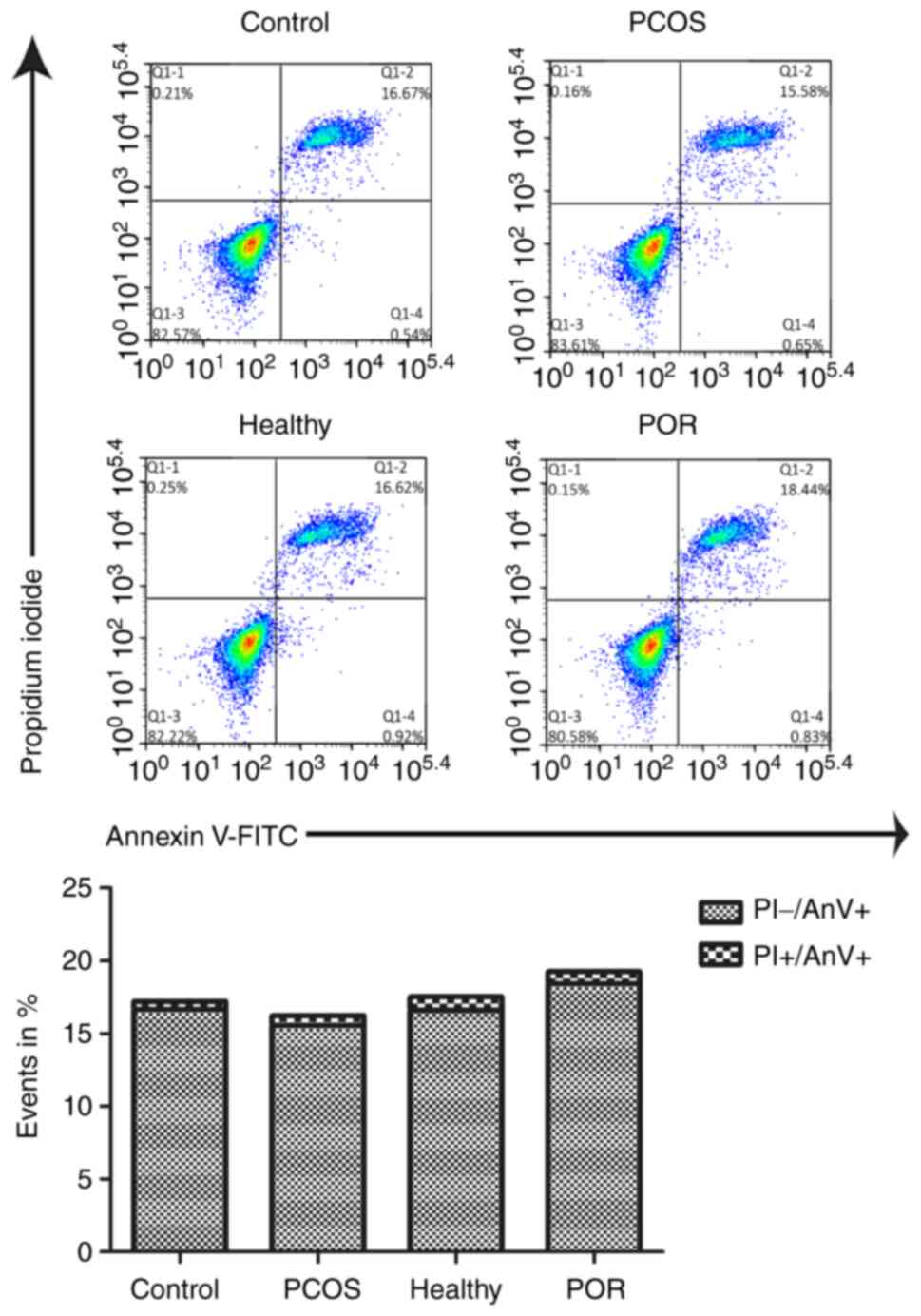
4 further illustrates the experimental results. Flow cytometry
was then performed to evaluate GC apoptosis and the arithmetic
average percentages of cells in two quadrants are presented. The
early apoptotic cells (PI-/AnV+) and late
apoptotic cells (PI+/AnV+) are presented as
percentages of events and no statistically significant differences
in the levels of apoptosis were identified among the different
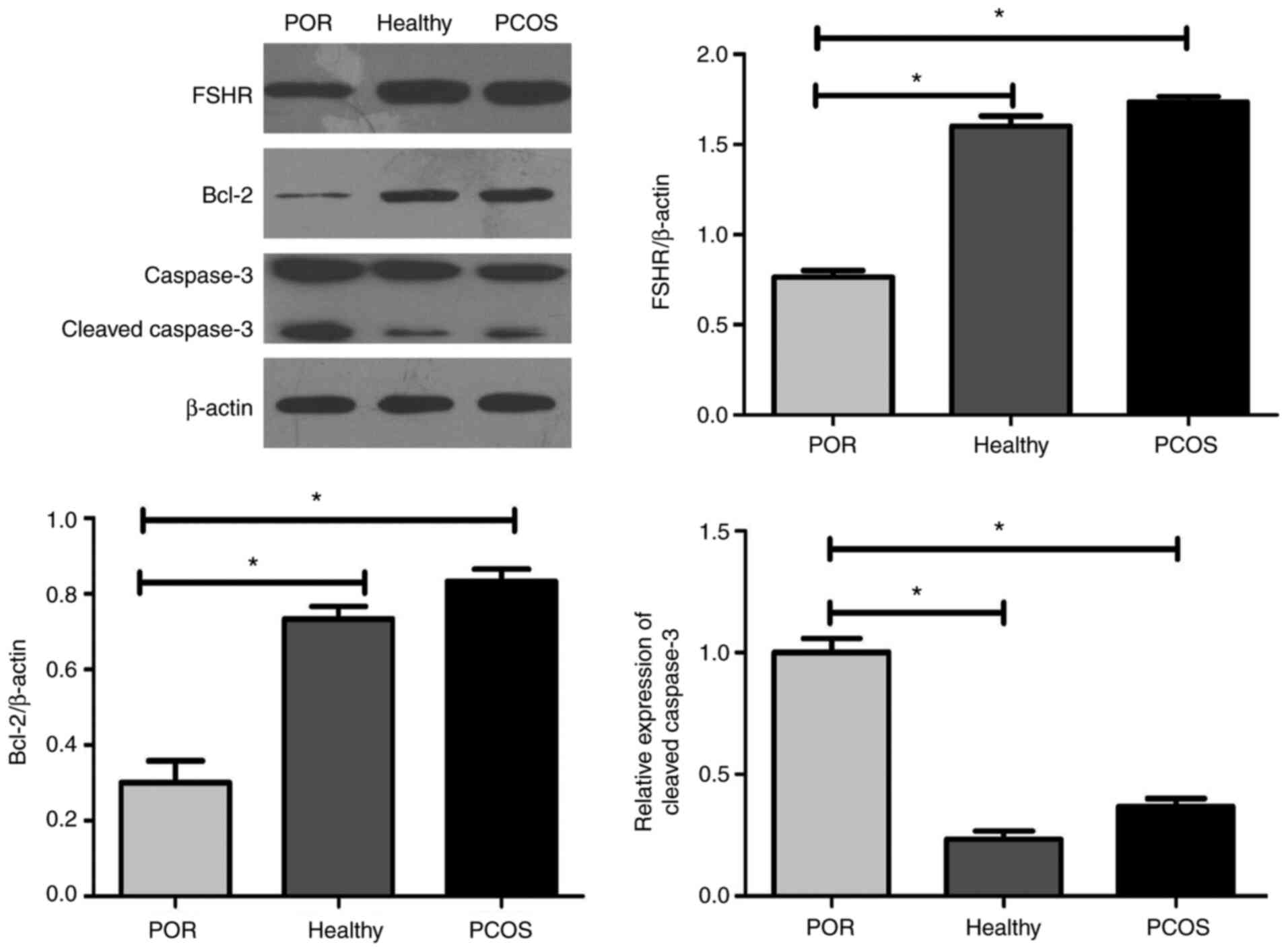
groups (Fig. 5). Higher levels of
FSHR were observed in the GCs of healthy individuals and patients
with PCOS when compared with those of patients with POR (P<0.01;
Fig. 6). The results showed that
GCs from healthy individuals and patients with PCOS exhibited
increased Bcl-2 expression levels and inhibited early cleavage of
caspase-3 when compared with patients with POR.
Analysis of RNA integrity
An RIN was then determined for each sample using an
established algorithm following sample assessment in an automated
electrophoresis system (Agilent 2100 Bioanalyzer) (17). No statistically significant RNA
degradation was observed in the nine samples of the GCs, as
demonstrated by the representative electropherograms and
corresponding RIN values (Fig. 7).
The mean RIN for all patient samples was 9.1. Moreover, the
baseline of the electrophoretic pattern was stable, therefore RNA
integrity was good, the results were reliable and follow-up studies
are now required.
Discussion
The present study describes a method for purifying
human GCs from patients with different ovarian function derived
from follicular aspirates. The present study was performed to
enable a range of future molecular studies for the analysis of gene
expression in order to provide high-quality RNA. As such, a means
of purifying GCs was required that maintained RNA integrity and was
suitable for all cell types. These aims have been successfully
achieved by using the Percoll gradient method described in the
current study. In the majority of studies, a Percoll method is used
for GC extraction, which separates single GCs and bundles of GCs
(6,18-20).
Aghadavod et al (6) compared
Ficoll, Percoll and Red Blood Cell Lysing buffer (RBL) methods for
the extraction of GCs and demonstrated that the Percoll method is
less likely to cause cellular damage and is more suitable for GC
extraction. However, the authors did not evaluate different types
of patients.
The developmental capacity of an oocyte is largely
dependent on its interaction with GCs through transzonal
projections, gap junctions and the secretion of paracrine factors
(21). Therefore, the study of GCs
is a good starting point when examining oocyte maturation. The
specificity of cells cultured in vitro should first be
identified; some data indicate that GCs specifically express FSHR
as soon as they reach the pre-antral follicle stage (22) and secrete AMH as soon as they reach
the primary follicle stage (23,24).
Previous studies identified that CD9 is associated with the
integrin α6β1 involved in GC adhesion and regulation of GC
luteinization (25-27).
CD9 expression on GCs increases through ovulation, but at ovulation
CD9 expression is reduced on GCs of mature follicles when compared
with immature follicles (25-27).
CD24 is an important mediator of ovulation and CD24(+) GCs are
essential for triggering ovulation. Treatment with human chorionic
gonadotropin significantly increases the expression of CD24 in GCs
(28,29). Furthermore, CD9 and CD24 expression
on GCs has been linked with female reproductive function (25-29).
Clavero et al (26)
identified that CD9 may be involved in the regulation of follicular
luteinization, CD9 and CD24 producing an inhibition of the
luteinization of GC and preventing premature follicular
luteinization. Therefore, in the present study, the expression
levels of FSHR and MISIIR served as the standards for the
identification of GCs. The results of immunocytochemistry
demonstrated that almost all cells plated on the cover slips showed
presence of MISIIR and FSHR. Flow cytometry showed that the
positive rate of FSHR in cultured GCs reached >95% and that CD9
is present on the cell surface of, on arithmetic average, 70% of
GCs among the three groups and CD24 is present on the cell surface
of 90% of GCs among the three groups, which reinforces the
credibility of the obtained experimental data. These results
suggested that the expression levels of FSHR in humans with
different ovarian reserve function gradually decrease with
increasing age and degeneration. Thus, the downregulation of CD9
antigen expression in PCOS ovaries would be consistent with
arrested folliculogenesis in patients with PCOS (30). The advantage of primary cell culture
is to maximize the preservation of cell characteristics, so that
in vitro results are more consistent with the environment
in vivo.
However, research by Aghadavod et al
(6) demonstrated that when GCs were
extracted using the RBL method, there were large clumps of
aggregated GCs and the number of dead leukocyte and erythrocyte
carcasses was negligible. The use of RBL solution can result in
more thoroughly dissolved follicular fluid mixed with red blood
cells, but a larger quantity of cell debris was apparent in the GCs
in our preliminary experiment. In the method used in the current
study, the small quantity of red blood cell residues did not
significantly affect the adherence rate of GCs cultured at 24 h. In
the present study, the red blood cells were removed using the
direct Percoll density gradient separation method and two-step
centrifugation and, thus, ensured cell purity. Follicular fluid
often contains white mucus, which supports and protects oocytes
after the removal of cumulus-GC clusters (5). Following centrifugation, mucus is
located in the upper layer of erythrocyte sedimentation and under
microscopic examination, a large number of cumulus GCs are observed
(5). In the study by Sun et
al (12), the gradient
centrifugation method combined with trypsin digestion was used to
separate human GCs, which also removes other mixed cells and tissue
fragments and helps to obtain higher purity ovarian GCs. Liu et
al (31) confirmed that the
cell clusters were significantly reduced after trypsin digestion
and the cells were easier to adhere to. The cells then grew well
and were evenly distributed (31).
The present study used trypsin to digest the extracellular matrix
components within the mucus. It can be effectively removed from
adhering to GCs and therefore significantly improves GC purity.
Due to different ovarian reserve functions, a single
method may not be applicable to all types of patients. To the best
of our knowledge, there are no studies that compare the efficacy of
a method of isolating GCs in patients with different ovarian
reserve function. In the current study, cells from healthy
individuals and patients with PCOS were isolated using two-step
Percoll density gradient centrifugation. Cells from patients with
POR were isolated using a one-step method owing to the lower number
of cells. The limitation of the current study was that the number
of GCs in the follicular fluid of patients with POR was very small
due to the two-step centrifugation method. Therefore, the results
of GC extraction in patients with POR using the two- and one-step
Percoll protocols were not compared. The aim of the present study
was to evaluate the efficacy of one-step and two-step DG for the
extraction of GCs in patients with different ovarian reserve
functions.
Immunocytochemistry and the CCK-8\flow cytometry
results demonstrated that the purity and activity of the cells in
the POR group were similar to those in the healthy and PCOS groups,
and that the observed differences were not statistically
significant. RNA integrity in the POR group patients, although
slightly lower than the other two groups of patients, was high
enough that one step DG could be conducted in follow-up
experiments. This extraction method for GCs shows that it is
effective in patients with differing ovarian reserve functions.
Decreased ovarian reserve, such as during POR, may
be due to ovarian cell apoptosis (32). One of the primary mechanisms of
ovarian apoptosis involves mitochondrial apoptotic signaling
cascades, which are mediated by mitochondrial Bcl-2 family members
(33). In the current study, the
expression of Bcl-2 and caspase-3 were compared in three different
patient groups. Active caspase-3 and Bcl-2 expression levels were
significantly downregulated in the GCs of patients with PCOS when
compared with those of individuals in the POR and healthy groups.
This result is consistent with a previous study by Chuffa et
al (34).
Thus, the present study demonstrated that the newly
modified procedure used in the current study was highly efficient
for GC isolation in patients with differing ovarian reserve
functions. Use of this optimized protocol will enable the study of
GC complex functions and molecular functions, and extends our
knowledge regarding the mechanisms involved in oocyte
apoptosis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was performed with financial support
from the Tianjin Health and Family Planning Commission (grant no.
2018007) and the National Natural Science Foundation of China
(grant no. 81803927).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH conceived and designed the research, and
interpreted the results of the experiments. GG contributed to the
design of the study and the interpretation of experimental results.
GG, SL, NX, YZ and HL performed experiments, analyzed data,
prepared figures and drafted the manuscript. GG and YZ also edited
and revised manuscript. YH and HL confirm the authenticity of all
the raw data. All authors have read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
Institutional Review Board of Tianjin Central Hospital of
Obstetrics and Gynecology (approval no. TJCOB2016002) and were
performed in accordance with the Declaration of Helsinki. Informed
consent was obtained from all individual participants included in
the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tiwari M, Prasad S, Tripathi A, Pandey AN,
Ali I, Singh AK, Shrivastav TG and Chaube SK: Apoptosis in
mammalian oocytes: A review. Apoptosis. 20:1019–1025.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tiwari M, Tripathi A and Chaube SK:
Presence of encircling granulosa cells protects against oxidative
stress-induced apoptosis in rat eggs cultured in vitro. Apoptosis.
22:98–107. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Levay PF, Huyser C, Fourie FL and Rossouw
DJ: The detection of blood contamination in human follicular fluid.
J Assist Reprod Genet. 14:212–217. 1997.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Havelock JC, Rainey WE and Carr BR:
Ovarian granulosa cell lines. Mol Cell Endocrinol. 228:67–78.
2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chilvers RA, Bodenburg YH, Denner LA and
Urban RJ: Development of a novel protocol for isolation and
purification of human granulosa cells. J Assist Reprod Genet.
29:547–556. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Aghadavod E, Zarghami N, Farzadi L, Zare
M, Barzegari A, Movassaghpour AA and Nouri M: Isolation of
granulosa cells from follicular fluid; applications in biomedical
and molecular biology experiments. Adv Biomed Res.
4(250)2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Asem EK, Feng S, Stingley-Salazar SR,
Turek JJ, Peter AT and Robinson JP: Basal lamina of avian ovarian
follicle: Influence on morphology of granulosa cells in-vitro. Comp
Biochem Physiol C Toxicol Pharmacol. 125:189–201. 2000.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lobb DK and Younglai EV: A simplified
method for preparing IVF granulosa cells for culture. J Assist
Reprod Genet. 23:93–95. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Grondahl ML, Borup R, Lee YB, Myrhoj V,
Meinertz H and Sorensen S: Differences in gene expression of
granulosa cells from women undergoing controlled ovarian
hyperstimulation with either recombinant follicle-stimulating
hormone or highly purified human menopausal gonadotropin. Fertil
Steril. 91:1820–1830. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ferrero H, Delgado-Rosas F, Garcia-Pascual
CM, Monterde M, Zimmermann RC, Simón C, Pellicer A and Gómez R:
Efficiency and purity provided by the existing methods for the
isolation of luteinized granulosa cells: A comparative study. Hum
Reprod. 27:1781–1789. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Raad G, Bazzi M, Tanios J, Mourad Y,
Azouri J, Azouri J and Fakih C: Optimization of the cell aggregates
method for isolation and purification of human granulosa cells from
follicular fluid. Int J Fertil Steril. 13:339–345. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sun Y, Lin Y, Li H, Liu J, Sheng X and
Zhang W: 2,5-Hexanedione induces human ovarian granulosa cell
apoptosis through BCL-2, BAX, and CASPASE-3 signaling pathways.
Arch Toxicol. 86:205–215. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ferraretti AP, La Marca A, Fauser BC,
Tarlatzis B, Nargund G and Gianaroli L: ESHRE consensus on the
definition of ‘poor response’ to ovarian stimulation for in vitro
fertilization: The Bologna criteria. Hum Reprod. 26:1616–1624.
2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rotterdam ESHRE/ASRM-Sponsored PCOS
Consensus Workshop Group. Revised 2003 consensus on diagnostic
criteria and long-term health risks related to polycystic ovary
syndrome. Fertil Steril. 81:19–25. 2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wei W, Sun HH, Li N, Li HY, Li X, Li Q and
Shen XH: WNT5A modulates cell cycle progression and contributes to
the chemoresistance in pancreatic cancer cells. Hepatobiliary
Pancreat Dis Int. 13:529–538. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Giovannetti E, Mey V, Danesi R, Mosca I
and Del Tacca M: Synergistic cytotoxicity and pharmacogenetics of
gemcitabine and pemetrexed combination in pancreatic cancer cell
lines. Clin Cancer Res. 10:2936–2943. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Schroeder A, Mueller O, Stocker S,
Salowsky R, Leiber M, Gassmann M, Lightfoot S, Menzel W, Granzow M
and Ragg T: The RIN: An RNA integrity number for assigning
integrity values to RNA measurements. BMC Mol Biol.
7(3)2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chin EC, Harris TE and Abayasekara DR:
Changes in cAMP-dependent protein kinase (PKA) and progesterone
secretion in luteinizing human granulosa cells. J Endocrinol.
183:39–50. 2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Shahnazi V, Zaree M, Nouri M,
Mehrzad-Sadaghiani M, Fayezi S and Darabi M, Khani S and Darabi M:
Influence of ω-3 fatty acid eicosapentaenoic acid on IGF-1 and
COX-2 gene expression in granulosa cells of PCOS women. Iran J
Reprod Med. 13:71–78. 2015.PubMed/NCBI
|
|
20
|
Motta PM, Nottola SA, Familiari G, Makabe
S, Stallone T and Macchiarelli G: Morphodynamics of the
follicular-luteal complex during early ovarian development and
reproductive life. Int Rev Cytol. 223:177–288. 2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gilchrist RB, Lane M and Thompson JG:
Oocyte-secreted factors: Regulators of cumulus cell function and
oocyte quality. Hum Reprod Update. 14:159–177. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gougeon A: Regulation of ovarian
follicular development in primates: Facts and hypotheses. Endocr
Rev. 17:121–155. 1996.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Durlinger AL, Visser JA and Themmen AP:
Regulation of ovarian function: The role of anti-Müllerian hormone.
Reproduction. 124:601–609. 2002.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Weenen C, Laven JS, Von Bergh AR,
Cranfield M, Groome NP, Visser JA, Kramer P, Fauser BC and Themmen
AP: Anti-Müllerian hormone expression pattern in the human ovary:
Potential implications for initial and cyclic follicle recruitment.
Mol Hum Reprod. 10:77–83. 2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Takao Y, Fujiwara H, Yamada S, Hirano T,
Maeda M, Fujii S and Ueda M: CD9 is expressed on the cell surface
of human granulosa cells and associated with integrin alpha6beta1.
Mol Hum Reprod. 5:303–310. 1999.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Clavero A, Castilla JA, Martinez L,
Mendoza N, Fontes J and Maldonado V: Expression of integrin
fraction and adhesion molecules on human granulosa cells and its
relation with oocyte maturity and follicular steroidogenesis. J
Assist Reprod Genet. 21:187–195. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jaslow CR, Patterson KS, Cholera S,
Jennings LK, Ke RW and Kutteh WH: CD9 Expression by human granulosa
cells and platelets as a predictor of fertilization success during
IVF. Obstet Gynecol Int. 2010(192461)2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wissing ML, Kristensen SG, Andersen CY,
Mikkelsen AL, Høst T, Borup R and Grøndahl ML: Identification of
new ovulation-related genes in humans by comparing the
transcriptome of granulosa cells before and after ovulation
triggering in the same controlled ovarian stimulation cycle. Hum
Reprod. 29:997–1010. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Dong JP, Dai ZH, Jiang ZX, He Y, Wang L,
Liao QY, Sun NX, Wang YN, Sun SH, Lin W, et al: CD24: A marker of
granulosa cell subpopulation and a mediator of ovulation. Cell
Death Dis. 10(791)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Oksjoki S, Söderström M, Inki P, Vuorio E
and Anttila L: Molecular profiling of polycystic ovaries for
markers of cell invasion and matrix turnover. Fertil Steril.
83:937–944. 2005.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liu Y, Chen X, Xue X, Shen C, Shi C, Dong
J, Zhang H, Liang R, Li S and Xu J: Effects of Smad3 on the
proliferation and steroidogenesis in human ovarian luteinized
granulosa cells. IUBMB Life. 66:424–437. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
32
|
Seifer DB, Gardiner AC, Ferreira KA and
Peluso JJ: Apoptosis as a function of ovarian reserve in women
undergoing in vitro fertilization. Fertil Steril. 66:593–598.
1996.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hussein MR, Haemel AK and Wood GS:
Apoptosis and melanoma: Molecular mechanisms. J Pathol.
199:275–288. 2003.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chuffa LG, Lupi Júnior LA and da Maia Lima
AF: Sex steroid receptors and apoptosis-related proteins are
differentially expressed in polycystic ovaries of adult dogs.
Tissue Cell. 48:10–17. 2016.PubMed/NCBI View Article : Google Scholar
|