Introduction
Atopic dermatitis (AD) is a chronic inflammatory
skin condition with an increasing prevalence (1). AD affects individuals of all ages,
occurring in 15-20% children and 1-3% adults (2). AD is clinically characterized by skin
dryness and itchy papules (occasionally vesicles in infants) that
become excoriated and lichenified, usually with crusting (3). Accumulating evidence suggests that AD
is elicited by skin barrier dysfunction, which is followed by
immune system activation, which in turn negatively regulates skin
barrier homeostasis, referred to as an ‘outside-inside-outside’
model of AD pathogenesis (4). Thus,
current therapies focus on maintaining skin barrier function and
ameliorating inflammation. For example, skin care and moisturizing
products are recommended as first-line treatment for mild AD
(5). Treatments for
moderate-to-severe AD include dupilumab, cyclosporine, phototherapy
and systemic glucocorticoids, albeit with limited success due to
considerable side effects and inability to affect the recurrence
rate (6). Thus, it is crucial to
identify novel promising therapies, with fewer side effects, for
the effective treatment of AD.
Heme oxygenase-1 (HO-1) catalyzes the first and
rate-limiting step in the oxidative degradation of free heme, and
may affect several biological processes of aneurysmal diseases,
articular diseases and hepatic gluconeogenesis, among others, via
its enzymatic by-products (7).
Previous studies on AD mouse models have demonstrated that certain
agents, including Soshiho-tang, sulforaphane, dihydroaustrasulfone
alcohol (WA-25) and Platycodon grandiflorus root-derived
saponins, can alleviate AD-like skin lesions and skin inflammation
by increasing the expression of HO-1 and nuclear factor erythroid
2-related factor 2 (Nrf2) (8-11).
Another study also concluded that enhancement of HO-1 expression
attenuated the development of skin lesions in mice (12). Based on these findings, HO-1 appears
to hold promise for the treatment of AD; however, whether the
topical use of HO-1 can alleviate AD remains unclear.
Cell-penetrating peptides (CPPs) are short peptides
(<30 amino acids) that are capable of crossing cell membranes
and transport small molecules into cells, such as drugs, peptides,
proteins, nucleic acids, nanoparticles and imaging agents (13). Previous studies have demonstrated
that the CPP-HO-1 fusion protein exerted a protective effect
against renal and intestinal ischemia/reperfusion (I/R) injury
(14,15). In the present study, a CPP was
attached to the HO-1 protein to transportHO-1 into the skin. Given
that the efficacy of the topical use of the CPP-HO-1 fusion protein
in AD treatment remains unclear, the present study aimed to
investigate the efficacy of CPP-HO-1 in a mouse model of AD.
Materials and methods
Chemicals
The H&E staining kit, SDS-PAGE 12% gel
preparation kit and acetone were obtained from Sangon Biotech Co.,
Ltd. 2,4-Dinitrochlorobenzene (DNCB) was purchased from
Sigma-Aldrich (Shanghai) Trading Co., Ltd. Acetone and olive oil
were mixed at a ratio of 4:1. DNCB was dissolved in the
acetone/olive oil mixture at a concentration of 1%. PBS, xylene and
PBST (10% Tween-20) were prepared by the authors' laboratory.
Animals
A total of 18 ICR mice (male, aged 4-5 weeks;
weight, 21±2 g) were purchased from Shanghai Slacker Laboratory
Animal Co., Ltd. [(approval no. SCXK(hu)2017-005; Shanghai, China].
All animals were housed under specific pathogen-free conditions at
a controlled temperature of 20-25˚C and 50-60% humidity with a 12-h
light/dark cycle. The animals were provided access to sterile food
and water ad libitum.
The present study was approved by the Ethics
Committee of Huzhou University (Huzhou, China) and all animal care
and experiments were performed in strict accordance with the Guide
for the Care and Use of Laboratory Animals (published by the
National Institutes of Health and revised in 1996; no. 85-23).
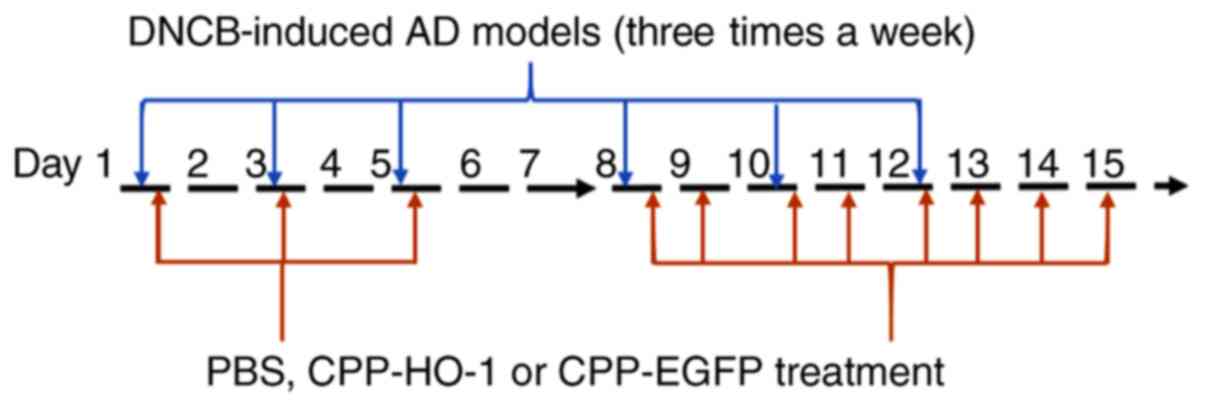
Induction of AD-like lesions and drug
treatment
After 2 weeks of acclimation, the mice were divided
into three groups (n=6/group) as follows: AD, CPP-enhanced green
fluorescent protein (EGFP) (DNCB with 0.5 µg/µl CPP-EGFP) and
CPP-HO-1 (DNCB with 0.5 µg/µl CPP-HO-1) groups.
The method for inducing AD was modified from a
previous study (10). The dorsal
skin hair was clipped and an area ~1x2 cm2 was depilated
with a hair removal cream. A total of 50 µl 1% DNCB was added to
the dorsal skin three times per week for a total of 2 weeks.
Treatment with 50 µl PBS, 50 µl CPP-EGFP (0.5 µg/µl) or 50 µl
CPP-HO-1 (0.5 µg/µl) was applied 3 times for the first week 1 h
following DNCB and 7 times for the second week. In order to
increase cell penetrating efficiency, CPP-HO-1, CPP-EGFP or PBS
solution was kept on the skin for at least 3 h. The animals were
sacrificed on day 15, and dorsal dermal tissues were collected for
further analysis. The experimental schedule is summarized in
Fig. 1.
Preparation of CPP-HO-1
The preparation method of the CPP-HO-1 was as
follows: The CPP-HO-1 and CPP-EGFP genes were synthesized by Sangon
Biotech Co., Ltd. and inserted into the pET28b vector by the
NdeI and EcoRI restriction enzymes and T4 DNA ligase
(Sangon Biotech Co., Ltd.), and subsequently transferred into the
Novagen's Rosetta™ 2 (pLysS) host strains from Hangzhou Biogroup
Technology Co., Ltd. CPP-HO-1 was induced at 0.7 mm IPTG for 18 h
at 37˚C, and was subsequently centrifuged at 13.8 x g for 15 min at
4˚C. The resultant pellet was stored at -80˚C for at least 24 h.
For protein extraction, the E. coli were released with PBS
buffer (pH 7.4) containing 20 mM imidazole and sonicated with an
ultrasonic homogenizer (Ningbo Xinzhi Biological Technology Co.,
Ltd.) at 60% amplitude for 5 min on ice, for 15 cycles of 5 sec on
and 15 sec off. The supernatant was subsequently centrifuged at
14.95 x g for 20 min at 4˚C and bound to a HiTrap His column (1 ml,
GE Healthcare) at a rate of 0.5 ml/min. Elution was performed using
500 mM imidazole. Subsequently, 2.5 ml eluent was added to the
pre-balanced G25 desalination column (GE Healthcare) for
desalination and was finally stored in 10% glycerol (Sangon Biotech
Co., Ltd.) at -80˚C.
Evaluation of AD severity
AD was observed in all mice and the score was
recorded on the last day of the experiment, according to the
criteria described in Table I. The
severity of AD was determined based on four symptoms: i) Erythema;
ii) erosion; iii) scar; and iv) edema. The score of each clinical
symptom ranged from 0 to 3 (none, 0; mild, 1; moderate, 2; and
severe, 3). The total AD score (maximum score, 12) was the sum of
individual scores.
 | Table IEvaluation criteria of atopic
dermatitis (8). |
Table I
Evaluation criteria of atopic
dermatitis (8).
| Items | Response
intensity | Score |
|---|
| Erythema | None | 0 |
| | Mild | 1 |
| | Moderate | 2 |
| | Severe | 3 |
| Erosion | None | 0 |
| | Mild | 1 |
| | Moderate | 2 |
| | Severe | 3 |
| Scarring | None | 0 |
| | Mild | 1 |
| | Moderate | 2 |
| | Severe | 3 |
| Edema | None | 0 |
| | Mild | 1 |
| | Moderate | 2 |
| | Severe | 3 |
Measurement of scratching
behavior
All mice were acclimated in acrylic cages for 15
min. Following acclimation, the mice were videotaped for 30 min and
the number of scratches was counted by the same observer.
Histological analysis
The mice were anesthetized with an i.p. injection of
a mixed solution containing xylazine (20 mg/kg; Bayer) and ketamine
(150 mg/kg; Bayer). All mice were decapitated and the dorsal dermal
skin tissues were collected and fixed in optimal cutting
temperature (OCT) compound (Sakura Finetek USA, Inc.) for 24 h at
-80˚C. Tissue samples were cut into 50-µm sections. H&E
staining was performed at 37˚C to determine epidermal thickness and
inflammatory cell infiltration in each group. The tissue sections
were observed under an optical light microscope (magnification,
x1,000), and the degrees of keratinization, dermaledema and
lymphocyte infiltration were analyzed.
Statistical analysis
Statistical analysis was performed using SPSS 20.0
(IBM Corp.). All the experiments were performed in triplicate (n=6
mice per group) and data are presented as median and interquartile
range. Kruskal-Wallis test followed by Dunn's post hoc test was
used to compare differences among multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
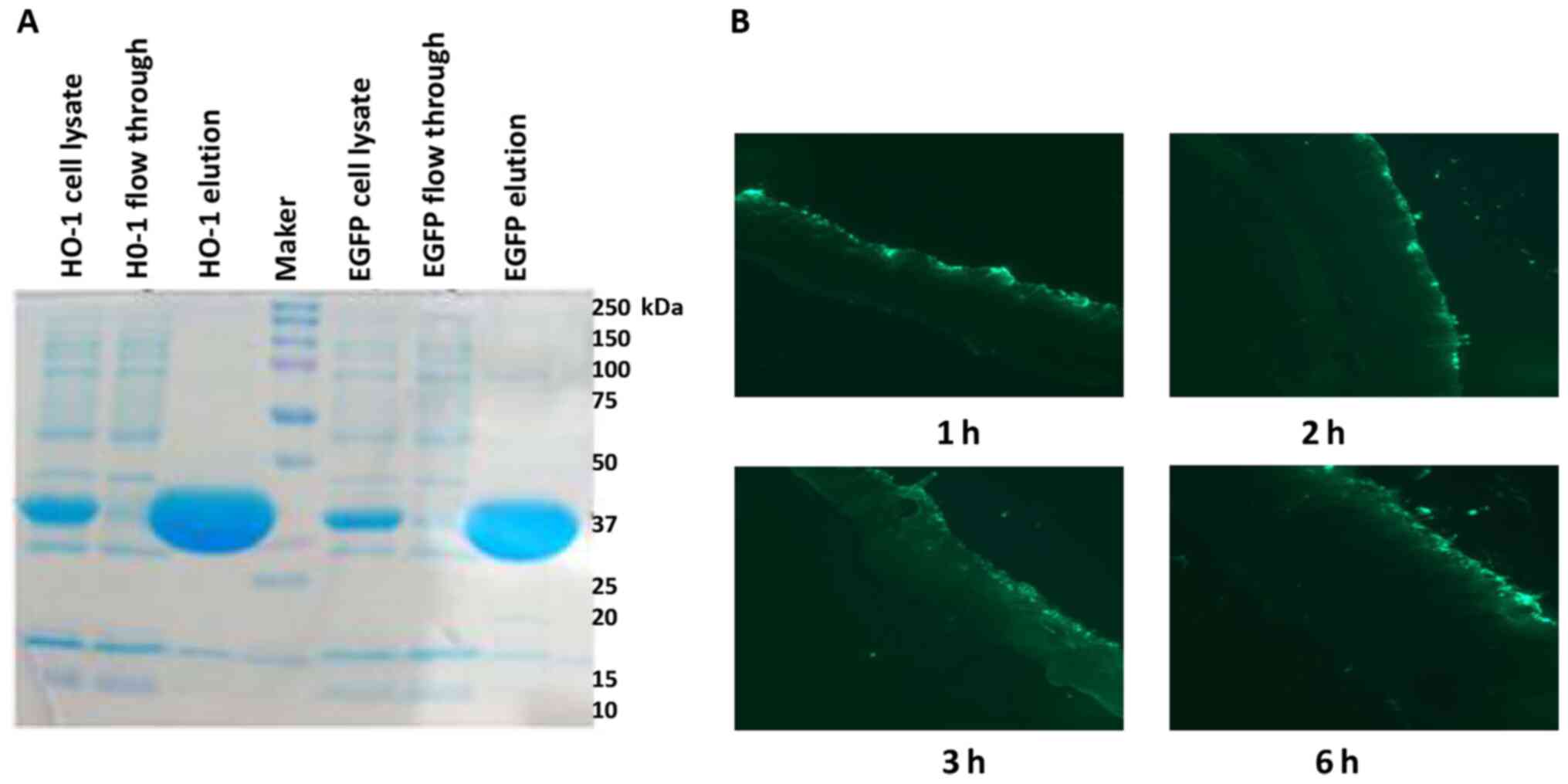
CPP-HO-1 purification
The SDS-PAGE (coloration) results demonstrated high
purity of CPP-HO-1 and CPP-EGFP (Fig.
2A). CPP-EGFP was applied to the dorsal skin of the mice for 1,
2, 3 and 6 h, and examination under a fluorescence microscope
revealed that CPP-EGFP infiltrated through the mouse skin barrier
in a time-dependent manner (Fig.
2B).
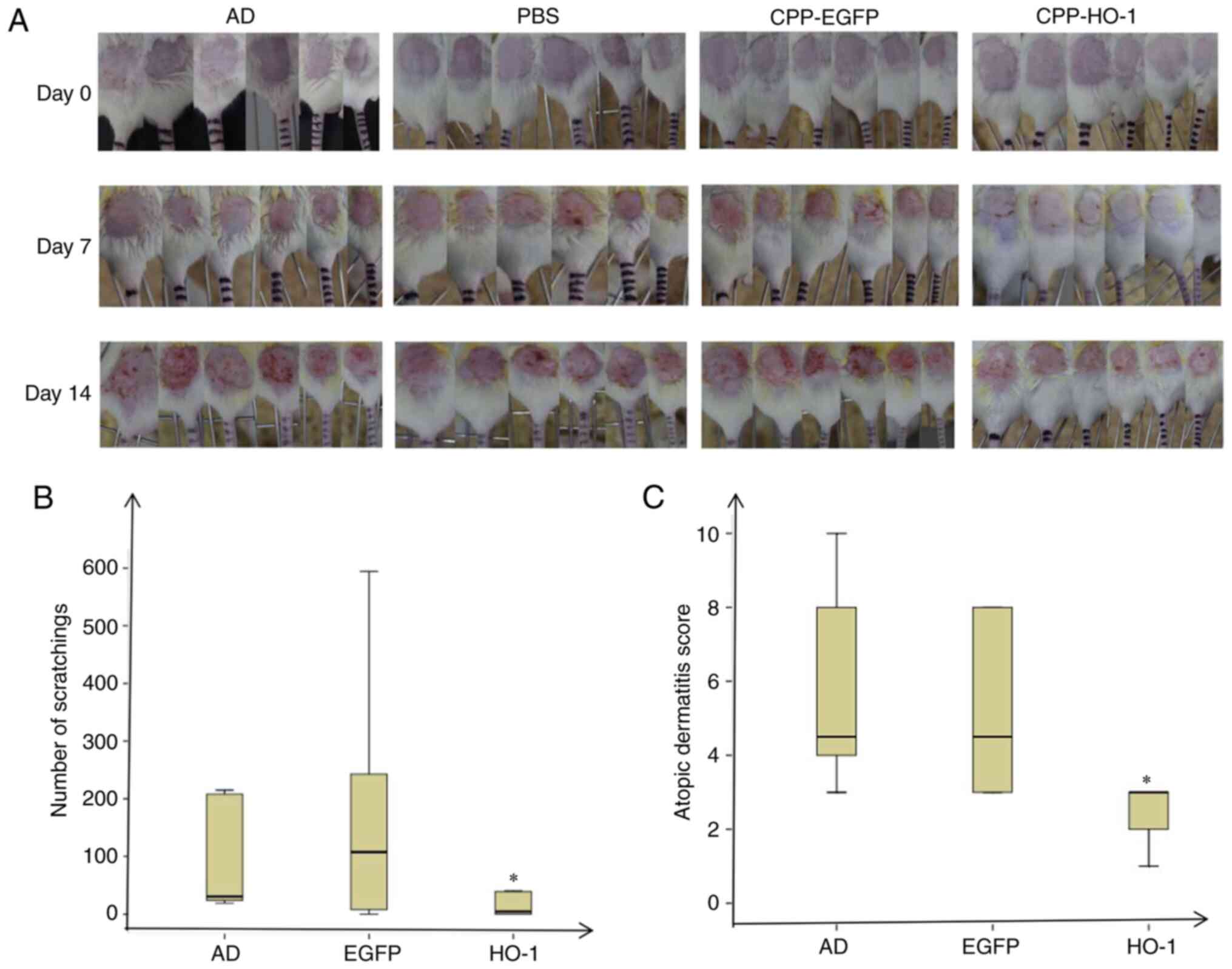
Dermatitis severity and scratching
behavior
The AD model was induced by DNCB, and 50 µl CPP-EGFP
(0.5 µg/µl) or 50 µl CPP-HO-1 (0.5 µg/µl) was added to study the
therapeutic effect (Fig. 1). The AD
group exhibited severe dermatitis with erythema, scarring, edema
and erosion on day 14 (Fig. 3). The
skin condition was significantly improved in CPP-HO-1-treated mice
compared with those in the AD group (Fig. 3A). All mice were acclimated in
acrylic cages for 15 min and subsequently videotaped for 30 min on
day 15, and the number of scratches was counted by the same
observer. The results demonstrated that the number of scratching
events was lower in the CPP-HO-1 group compared with the AD group
(Fig. 3B; P<0.05), while there
was no significant difference between the AD and CPP-EGFP groups
(P>0.05). The score was recorded on the last day of the
experiment, according to the criteria described in Table I (8). The results demonstrated that CPP-HO-1
decreased the dermatitis score of DNCB-induced skin lesions on day
15 compared with the AD group (Fig.
3C; P<0.05), whereas the AD score was not significantly
improved in the CPP-EGFP group (P>0.05).
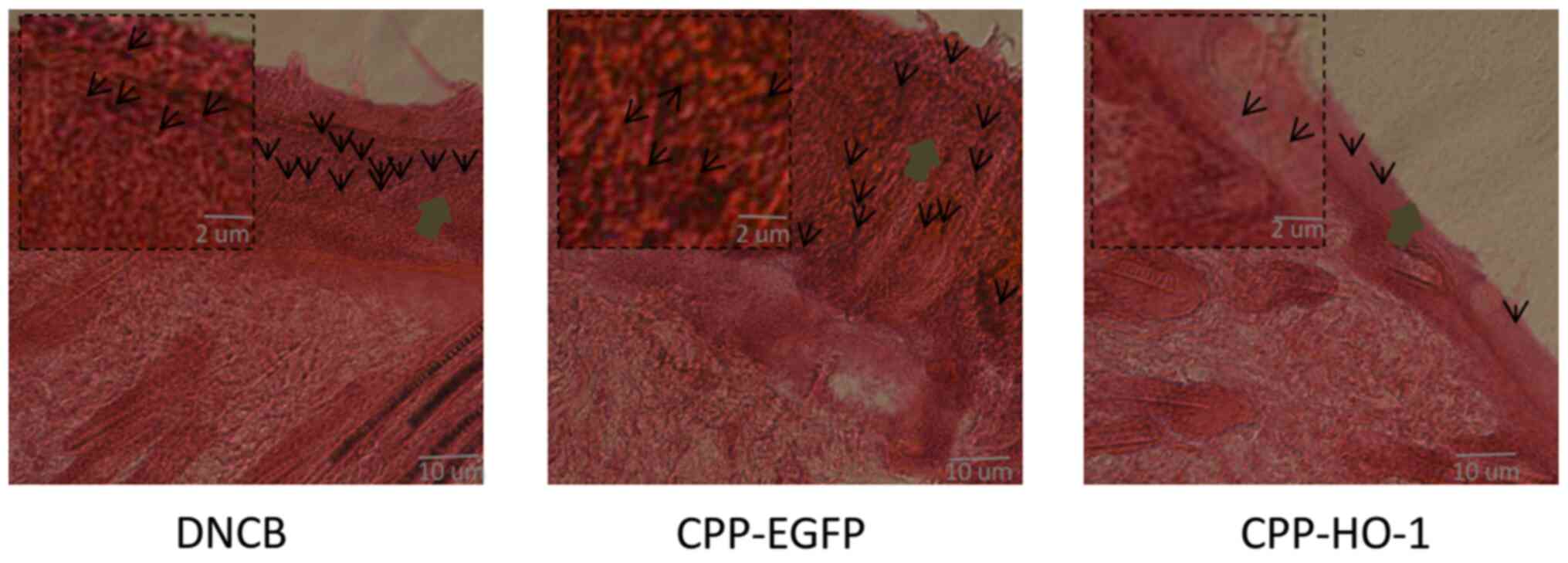
H&E staining
Examination of H&E stained sections of the skin
lesions revealed decreased epidermal thickness and inflammatory
cells in the CPP-HO-1 group. However, the epidermal thickness and
inflammatory cell infiltration exhibited no significant differences
between the CPP-EGFP and AD groups (Fig. 4). Taken together, these results
indicated that topical application of CPP-HO-1 can improve the
histological signs of AD.
Discussion
It was previously indicated that induction of HO-1
plays a protective role in several inflammation-related diseases
(7), including AD, and HO-1 has
been reported to exhibit therapeutic efficiency in AD (8-11).
Chen and Zhong (16) revealed that
HO-1 combined with microRNAs may affect certain skin diseases, such
as ischemia, hypoxia, rheumatoid arthritis and AD, by regulating
the functions of T cells, dendritic cells and mast cells, and the
release of chemokines and cytokines. Another study reported that
the HO-1 inducer, cobaltic protoporphyrin, inhibited
T-cell-dependent skin inflammation by suppressing
antigen-presenting cells (17). In
2016, Kim et al (18)
reported that 2,3-dimethoxy-2'-hydroxychalcone, a derivative of
2'-hydroxychalcone in the flavonoid family, can alleviate skin
inflammation by inhibiting TNF-α-induced intercellular adhesion
molecule-1 expression and adhesion of monocytes to keratinocytes,
by suppressing NF-κB activation and inducing HO-1 expression in
keratinocytes. Hung et al (10) demonstrated that WA-25 can protect
against AD by increasing the expression levels of HO-1 and Nrf2.
Gene therapy of AD via targeting the HO-1 gene has been reported in
several studies; however, AD therapy using the HO-1 protein is
limited by its inability to enter cells. In previous studies, CPPs
have been conjugated with HO-1 to form the CPP-HO-1 fusion protein,
which can transfer the HO-1 protein into cells and decrease the
extent of I/R injury (14,15); however, to the best of our
knowledge, this method has not been reported in AD to date.
In the present study, the CPP was conjugated to HO-1
or EGFP (control protein with similar molecular weight) (19) to form the fusion proteins CPP-HO-1
and CPP-EGFP, and CPP-HO-1 was studied in the therapy of AD in
mice. The CPP-EGFP could effetely enter the skin time-dependently,
as shown in Fig. 2A, similar to
CPP-EGFP penetration reported in heart tissues (20,21).
In the present study, in order to increase cell penetrating
efficiency, the CPP-HO-1, CPP-EGFP or PBS solution were kept on the
skin for at least 3 h. The AD model was induced using DNCB, the
immune phenotype of AD, such as upregulated iNOS protein
expression, was not included in the present study, as it was
described in a previous study (10). The DNCB-treated skin area exhibited
itchy, red, swollen and cracked skin, which indicated that the AD
model had been successfully established. In order to determine the
optimal time and concentration of CPP-HO-1 treatment, the benefits
in mice were compared between different concentrations of CPP-HO-1
(0.1 and 0.5 µg/µl) in a pre-study (data not shown), and the result
revealed that 0.5 µg/µl was the most effective. However, the hair
in the depilated area grew rapidly, and the frequent use of hair
remover would also further damage the skin; therefore, in order to
reduce the use of hair remover, the CPP-HO-1, CPP-EGFP or PBS
treatment was applied 1 h after DNCB induction. Furthermore, the
treatment times were also increased in the second week to increase
the therapeutic efficacy, according to a previously published study
(10). Compared with the PBS and
CPP-EGFP groups, CPP-HO-1 effectively alleviated scratching,
lowered skin score, and decreased skin swelling and inflammatory
cell infiltration, as shown in Fig.
3. Similar conclusions were also reported by Kirino et
al (12) and Hung et al
(10) via enhancing HO-1
expression. H&E staining of the skin lesions also revealed that
CPP-HO1 decreased epidermal thickness and inflammatory cell
infiltration compared with the PBS and CPP-EGFP groups (Fig. 4). Taken together, these results
suggest that CPP-HO-1 may have a therapeutic effect on AD.
However, there were certain limitations to the
present study. A normal control group, which was not set in our
research, is required to better demonstrate the effect of HO-1 on
AD. The present study used 50-µm OCT sections for HE staining,
although 5-µm sections may exhibit a higher resolution and thus
will be used in future studies. To strengthen the evidence on the
benefits of HO-1 for AD, immunohistochemistry and ELISA must also
be performed to investigate additional parameters of AD in mice,
such as the serum levels of IL-4, IL-13 and inducible nitric oxide
synthase. Furthermore, the detailed mechanism underlying the
therapeutic effect of HO-1 on AD must be further elucidated.
In conclusion, the results of the present study
suggested that CPP-HO-1 may have a therapeutic effect on AD, and
thus may hold promise as a therapeutic strategy.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the National Key
Research and Development Program of China (grant no.
SQ2020YFF0401041) and the Zhejiang Provincial Natural Science
Foundation of China (grant nos. LGF18C050001 and 2021C03036).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL designed the study and edited manuscript; FT
drafted the initial manuscript. WJ, TQ and SQ isolated the
proteins; FT, JS, XM and MR performed the animal experiments. WJ
and TQ confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Huzhou University (Huzhou, China) and all animal care
and experiments were performed in strict accordance with the Guide
for the Care and Use of Laboratory Animals (published by the
National Institutes of Health and revised in 1996; no. 85-23).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kapur S, Watson W and Carr S: Atopic
dermatitis. Allergy Asthma Clin Immunol. 14 (Suppl
2)(52)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kowalska-Olędzka E, Czarnecka M and Baran
A: Epidemiology of atopic dermatitis in Europe. J Drug Assess.
8:126–128. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Silverberg JI: Atopic Dermatitis in
Adults. Med Clin North Am. 104:157–176. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Elias PM and Steinhoff M:
‘Outside-to-inside’ (and now back to ‘outside’) pathogenic
mechanisms in atopic dermatitis. J Invest Dermatol. 128:1067–1070.
2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Puar N, Chovatiya R and Paller AS: New
treatments in atopic dermatitis. Ann Allergy Asthma Immunol.
126:21–31. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Johnson BB, Franco AI, Beck LA and
Prezzano JC: Treatment-resistant atopic dermatitis: Challenges and
solutions. Clin Cosmet Investig Dermatol. 12:181–192.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pae HO, Lee YC and Chung HT: Heme
oxygenase-1 and carbon monoxide: Emerging therapeutic targets in
inflammation and allergy. Recent Pat Inflamm Allergy Drug Discov.
2:159–165. 2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wu W, Peng G, Yang F, Zhang Y, Mu Z and
Han X: Sulforaphane has a therapeutic effect in an atopic
dermatitis murine model and activates the Nrf2/HO-1 axis. Mol Med
Rep. 20:1761–1771. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Choi JH, Jin SW, Han EH, Park BH, Kim HG,
Khanal T, Hwang YP, Do MT, Lee HS, Chung YC, et al: Platycodon
grandiflorum root-derived saponins attenuate atopic
dermatitis-like skin lesions via suppression of NF-κB and STAT1 and
activation of Nrf2/ARE-mediated heme oxygenase-1. Phytomedicine.
21:1053–1061. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hung HC, Feng CW, Lin YY, Chen CH, Tsui
KH, Chen WF, Pan CY, Sheu JH, Sung CS and Wen ZH: Nucleophosmin
modulates the alleviation of atopic dermatitis caused by the
marine-derived compound dihydroaustrasulfone alcohol. Exp Mol Med.
50(e446)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lee JH, Jo EH, Lee B, Noh HM, Park S, Lee
YM, Kim DK and Park MC: Soshiho-Tang, a Traditional Herbal
Medicine, Alleviates Atopic Dermatitis Symptoms via Regulation of
Inflammatory Mediators. Front Pharmacol. 10(742)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kirino M, Kirino Y, Takeno M, Nagashima Y,
Takahashi K, Kobayashi M, Murakami S, Hirasawa T, Ueda A, Aihara M,
et al: Heme oxygenase 1 attenuates the development of atopic
dermatitis-like lesions in mice: Implications for human disease. J
Allergy Clin Immunol. 122:290–297, 297.e1-297.e8. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Morris MC, Deshayes S, Heitz F and Divita
G: Cell-penetrating peptides: From molecular mechanisms to
therapeutics. Biol Cell. 100:201–217. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
He XH, Tang JJ, Wang YL, Zhang ZZ and Yan
XT: Transduced heme oxygenase-1 fusion protein reduces renal
ischemia/reperfusion injury through its antioxidant and
antiapoptotic roles in rats. Transplant Proc. 47:1627–1632.
2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
He XH, Li QW, Wang YL, Zhang ZZ, Ke JJ,
Yan XT and Chen K: Transduced PEP-1-heme oxygenase-1 fusion protein
reduces remote organ injury induced by intestinal
ischemia/reperfusion. Med Sci Monit. 21:1057–1065. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen L and Zhong JL: MicroRNA and heme
oxygenase-1 in allergic disease. Int Immunopharmacol.
80(106132)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Listopad J, Asadullah K, Sievers C, Ritter
T, Meisel C, Sabat R and Döcke WD: Heme oxygenase-1 inhibits T
cell-dependent skin inflammation and differentiation and function
of antigen-presenting cells. Exp Dermatol. 16:661–670.
2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kim H, Youn GS, An SY, Kwon HY, Choi SY
and Park J: 2,3-Dimethoxy-2'-hydroxychalcone ameliorates
TNF-α-induced ICAM-1 expression and subsequent monocyte
adhesiveness via NF-kappaB inhibition and HO-1 induction in HaCaT
cells. BMB Rep. 49:57–62. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Brunet AA, Fuller-Carter PI, Miller AL,
Voigt V, Vasiliou S, Rashwan R, Hunt DM and Carvalho LS: Validating
fluorescent Chrnb4.EGFP mouse models for the study of cone
photoreceptor degeneration. Transl Vis Sci Technol.
9(28)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ma J, Lau CK, Obed A, Dada A, Doenecke A,
Fan ST, Schlitt HJ and Tsui TY: A cell penetrating heme oxygenase
protein protects heart graft against ischemia/reperfusion injury.
Gene Ther. 16:320–328. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li H, Zheng X, Koren V, Vashist YK and
Tsui TY: Highly efficient delivery of siRNA to a heart transplant
model by a novel cell penetrating peptide-dsRNA binding domain. Int
J Pharm. 469:206–213. 2014.PubMed/NCBI View Article : Google Scholar
|