Introduction
Prostate cancer is characterized by a high morbidity
and mortality and is the second most common malignant cancer in men
(1). Salinomycin, a monocarboxylic
ionophore isolated from Streptomyces albus, is widely used
as an animal food additive and has recently been demonstrated to
possess anti-cancer effects (2).
Salinomycin exhibits antibacterial activity, especially against
Gram-positive bacteria and various antibiotic-resistant species of
Streptomyces (3). Gupta
et al (4) reported that
salinomycin selectively kills breast cancer stem cells (CSCs) in
tumor xenograft-inoculated mice. Salinomycin activity toward CSCs
is 100-fold higher than the conventional chemotherapeutic drug
paclitaxel. However, the efficacy and underlying mechanism of
salinomycin in the treatment of prostate cancer remain to be
elucidated.
Increased reactive oxygen species (ROS) production
has been detected in various cancers (5). Malondialdehyde (MDA) and
8-hydroxy-2'-deoxyguanosine (8-OH-dG) are the end-product of
ROS-induced lipid peroxidation and DNA oxidation, respectively,
which are commonly used as oxidative stress biomarkers (6,7).
Recent studies have suggested that MDA and 8-OH-dG levels in the
urine are significantly higher in patients with prostate cancer
compared with healthy volunteers (8,9).
Recently, the anti-nasopharyngeal carcinoma effect of salinomycin
has been widely hypothesized to promote the apoptosis of cancer
cells and decrease cancer radioresistance and relapse using a clone
formation assay (10). A previous
study demonstrated that salinomycin can induce colorectal cancer
cell apoptosis by disrupting the osmotic balance (11). Furthermore, treatment of gastric
cancer, lung cancer and prostate cancer cells with salinomycin
in vitro induces autophagy (12), stimulates oxidative stress (13) and has some anti-angiogenic and
anti-tumorigenic activities (14),
as well as inhibits cancer cell proliferation (15). Furthermore, salinomycin-induced
endoplasmic reticulum (ER) stress could improve apoptosis and serve
therefore an anti-cancer role in prostate cancer cells (16). However, the exact target and
underlying mechanism of salinomycin anti-tumor effect remain
unclear.
Nuclear factor erythroid 2-related factor 2 (Nrf2)
is a crucial regulator of the cellular antioxidant defense system
(17,18). Nrf2 pathway is involved in the
regulation of several antioxidative enzymes, including heme
oxygenase-1 (HO-1), NAD(P)H quinone oxidoreductase 1 (NQO1) and
glutamate-L-cysteine ligase catalytic subunit (GCLc) (19,20).
It has been reported that Nrf2 is overexpressed in squamous cell
carcinoma and that it is associated with a poor prognosis for
patients, since it stimulates cancer cell survival and
proliferation (21). Inhibition of
tumor cell proliferation using Nrf2 inhibitors has therefore become
a potential anti-cancer therapy strategy. Although a previous study
has already shown that salinomycin can inhibit Nrf2 expression and
promote the generation of ROS in nasopharyngeal carcinoma (10), the role of salinomycin on the Nrf2
pathway and redox metabolism of prostate cancer cells and its
potential molecular mechanism are poorly understood.
The present study aimed to elucidate the role of
salinomycin in the treatment of prostate cancer by suppressing Nrf2
antioxidant signaling, promoting oxidative and ER stress and
triggering apoptosis in cancer cells. The findings from the present
study may help understanding the anti-cancer mechanism of
salinomycin.
Materials and methods
Cell culture
The human prostate cancer cell lines PC-3 and DU145
were purchased from The Cell Bank of Type Culture Collection of The
Chinese Academy of Sciences. Cells were cultured in RPMI-1640
medium (cat. no. R0883; Sigma-Aldrich; Merck KGaA) supplemented
with 10% FBS (Welgene, Inc.), 2 mM of L-glutamine, 100 U/ml of
penicillin and 100 µg/ml streptomycin (Welgene, Inc.) and placed at
37˚C in a humidified incubator containing 5% CO2.
Cell viability assay
Cells were seeded into 96-well plates at the density
of 5,000 cells/well. After 24 h incubation, cells were treated with
2 and 5 µM salinomycin, 10 µM tBHQ (Nrf2 activator) or 10 µM ML385
(Nrf2 inhibitor) for 72 h (22-24).
Salinomycin (Sigma-Aldrich; Merck KGaA) was dissolved in DMSO to
create the stock solution. Cell viability was assessed using the
Cell Titer Glo Assay (cat. no. G7570; Promega Corporation)
according to the manufacturer's protocol.
Cell apoptosis analysis
Apoptosis was assessed using a Annexin V-FITC
apoptosis detection kit (BD Biosciences). Briefly, PC-3 and DU145
cells were cultured in 6-well plates at a density of
5x104/well. After treatment with 2 and 5 µM salinomycin
for 72 h, cells were harvested, mixed in 100 µl binding buffer and
stained with 10 µl Annexin-V/propidium iodide (PI) at room
temperature for 15 min. The stained cells were analyzed by flow
cytometry (FACSCalibur; Becton, Dickinson and Company) and the
apoptotic rate was calculated using Cell Quest Pro software
(version 5.1) on Mac®OS 9 (Becton, Dickinson and
Company).
Detection of ROS
The intracellular ROS level was evaluated using the
ROS assay kit (cat. no. MAK145; Sigma-Aldrich; Merck KGaA)
according to the manufacturer's instructions. Briefly, the PC-3 and
DU145 cells were plated in a 96-well plate (5x104
cells/well). Following treatment with 2 and 5 µM salinomycin for 72
h, cells were washed with Hanks balanced salt solution (HBSS) and
incubated with 500 µM of the luminol derivative L-012 in HBSS at
37˚C for 15 min. ROS-induced chemiluminescence was determined every
10 min for a total of 60 min using a Microplate Luminometer (Tropix
TR717; Applied Biosystems; Thermo Fisher Scientific, Inc.).
Detection of MDA
MDA was quantified as thiobarbituric acid reactive
substances (TBARS) as previously described by Xiong et al
(25). Briefly, 5x106
cells were sonicated in 200 µl ice-cold PBS and 100 µl cell lysate
was placed into a 1.5 ml micro-centrifuge tube. After adding 200 µl
ice-cold 10% trichloroacetic acid (TCA) to the cell lysate, the
samples were incubated for 5 min on ice. After centrifugation at
10,000 x g for 15 min at 4˚C, the supernatant was collected and
mixed with 1 ml 10% TCA and 1 ml 0.67% thiobarbituric acid. The
mixture was subsequently heated in a boiling water bath for 30 min.
TBARS were determined by reading the absorbance at 532 nm.
Detection of 8-OH-dG
Cell nuclear DNA was isolated using the sodium
iodide method. Briefly, total DNA was isolated from cells with
DNAzol reagent (Thermo Fisher Scientific, Inc.). UV
spectrophotometry was used for the quantification of the isolated
DNA (26). 8-OH-dG was measured
using OxiSelect™ Oxidative DNA Damage ELISA kit (cat. no. STA-320;
Cell Biolabs, Inc.) according to the manufacturer's protocol
(26).
Activity of antioxidant enzymes
The activity of the antioxidant enzymes catalase
(CAT), glutathione peroxidase (GSH-Px) and superoxide dismutase
(SOD) was determined by spectrophotometrical methods (27). GSH-Px activity was detected using
the DTNB method (28) (cat. no.
A005-1-2), CAT activity was measured using the ammonium molybdate
method (29) (cat. no. A007-1-1)
and SOD activity was measured using the xanthine-oxidase method
(30) (cat. no. A001-3-2; all from
Nanjing Jiancheng Bioengineering Institute).
Western blot analysis
Cells were lysed in RIPA buffer (cat. no. R0010)
with PMSF (cat. no. P0100; both from Beijing Solarbio Science &
Technology Co., Ltd.) for 30 min on ice. Protein concentration was
determined using a Bradford Protein Assay kit (cat. no. PC0010;
Beijing Solarbio Science & Technology Co., Ltd.). A total of 30
µg protein/lane were separated by 10% SDS-PAGE and transferred onto
PVDF membranes. Membranes were blocked with 5% skimmed milk powder
at 4˚C overnight and incubated with primary antibodies (1:1,000;
Cell Signaling Technology, Inc.) against binding immunoglobulin
protein (Bip; cat. no. 3117), eukaryotic initiation factor 2α
(eIF2α; cat. no. 5324), phosphorylated (p)-eIF2α (cat. no. 5199),
protein kinase RNA-like endoplasmic reticulum kinase (PERK; cat.
no. 5683), p-PERK (cat. no. 3179), activating transcription factor
4 (ATF4; cat. no. 11815), C/EBP homologous protein (CHOP; cat. no.
2895), Nrf2 (cat. no. 12721), GCLc (cat. no. 19689), HO-1 (cat. no.
86806), NQO1 (cat. no. 3187) and GAPDH (cat. no. 5174) overnight at
4˚C. Membranes were then incubated with HRP-conjugated goat
anti-mouse IgG (H+L) secondary antibody (1:3,000; cat. no. SE131)
or HRP-conjugated goat anti-rabbit IgG (H+L) secondary antibody
(1:3,000; cat. no. SE134; both from Beijing Solarbio Science &
Technology Co., Ltd.) at room temperature for 1 h, followed by
washing three times with TBS-Tween (0.1% Tween-20) for 10 min each.
Enhanced chemiluminescence reagent (Thermo Fisher Scientific, Inc.)
was used to detect the signal on the membrane. The data were
analyzed using Tanon-410 automatic gel imaging system (Shanghai
Tianneng Corporation, China) and normalized to expression of the
internal control (GAPDH).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The expression level of CHOP was determined using
RT-qPCR. mRNA was isolated from PC-3 and DU145 cells using TRI
reagent following the manufacturer's instructions (cat. no. T9424;
Sigma-Aldrich; Merck KGaA). cDNA synthesis was performed using the
GoScript Reverse Transcription System (Promega Corporation)
following the manufacturer's instructions. The reaction mixture
contained 6.25 µl 2X GoTaq qPCR Master Mix (Promega Corporation), 1
µl cDNA, 0.25 µl upstream PCR primers and 0.25 µl downstream PCR
primers. Nuclease-free water was added to a final volume of 12.5
µl. Each reaction was run in triplicate. The qPCR reaction
conditions were subjected to an initial predenaturation step at
95˚C for 3 min, followed by 39 cycles of 95˚C for 20 sec and 60˚C
for 30 sec. The relative expression levels were normalized to
endogenous control β-actin and were expressed as 2-ΔΔCq
(31). The sequences of the primers
used were as follows: CHOP, forward 5'-CTTCCATGTAGCGGA GTCCT-3';
reverse 5'-GTGAGAGCCAGTCTCCCTTT-3'; and β-actin, forward
5'-CACCACACCTTCTACAATGAG-3' and reverse
5'-TACGACCAGAGGCATACAG-3'.
Statistical analysis
Statistical analyses were performed using SPSS
software (version 22.0; IBM Corp.). Data were presented as the
means ± standard deviation of three independent experiments.
Differences were compared using ANOVA followed by Dunnett's or
Tukey's post hoc tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
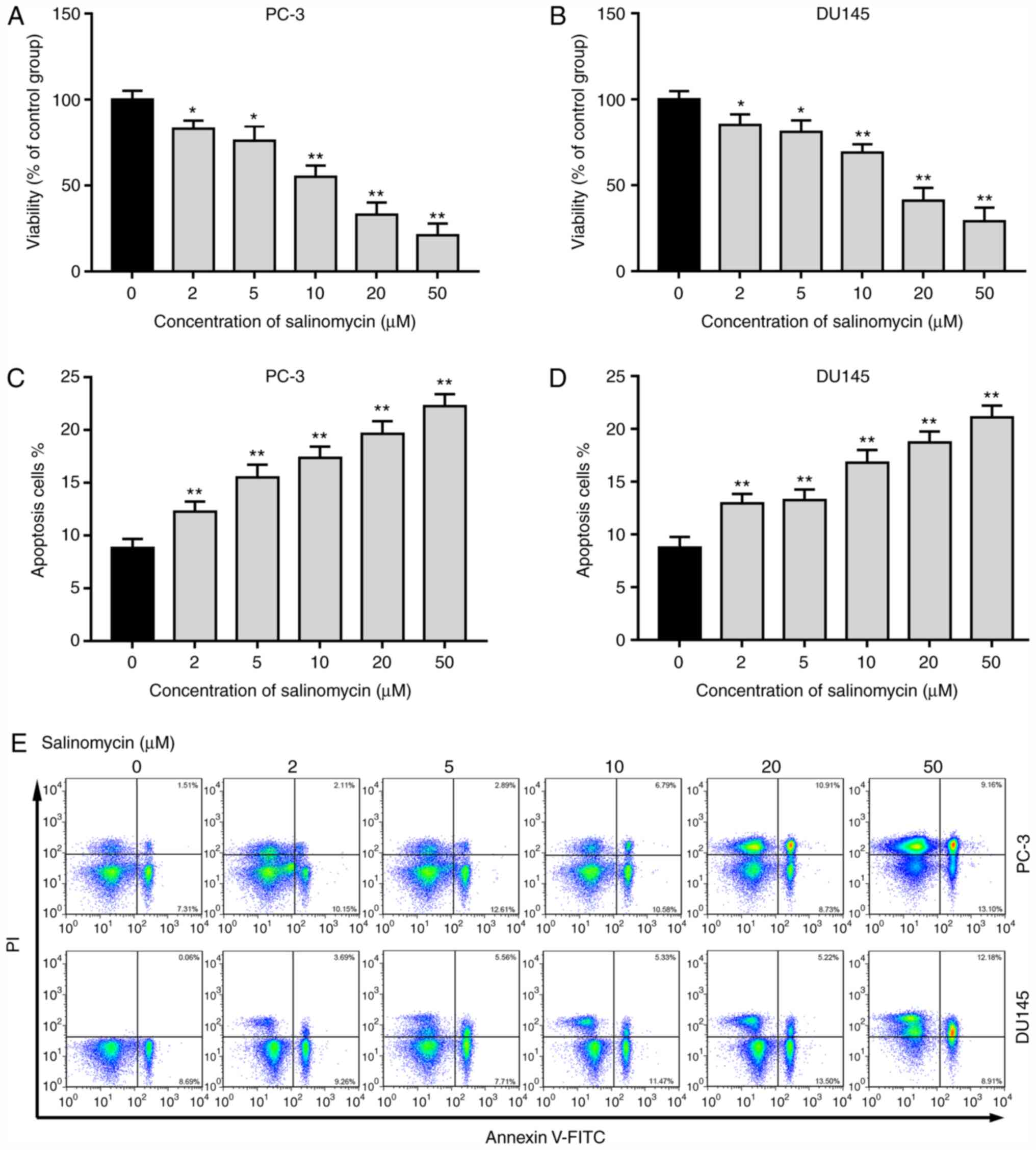
Effects of salinomycin on prostate
cancer cell proliferation and apoptosis
The effects of salinomycin on the proliferation and
apoptosis of PC-3 and DU145 cells were determined for different
concentrations (0, 2, 5, 10, 20 and 50 µM). As seen in Fig. 1A and B, the proliferation of PC-3 and DU145
cells was significantly decreased following treatment with
salinomycin (2-50 µM). In addition, salinomycin treatment
significantly increased the apoptosis of PC-3 and DU145 cells in a
dose-dependent manner (Fig. 1C-E).
These data indicated that salinomycin significantly inhibited the
proliferation and induced the apoptosis of prostate cancer
cells.
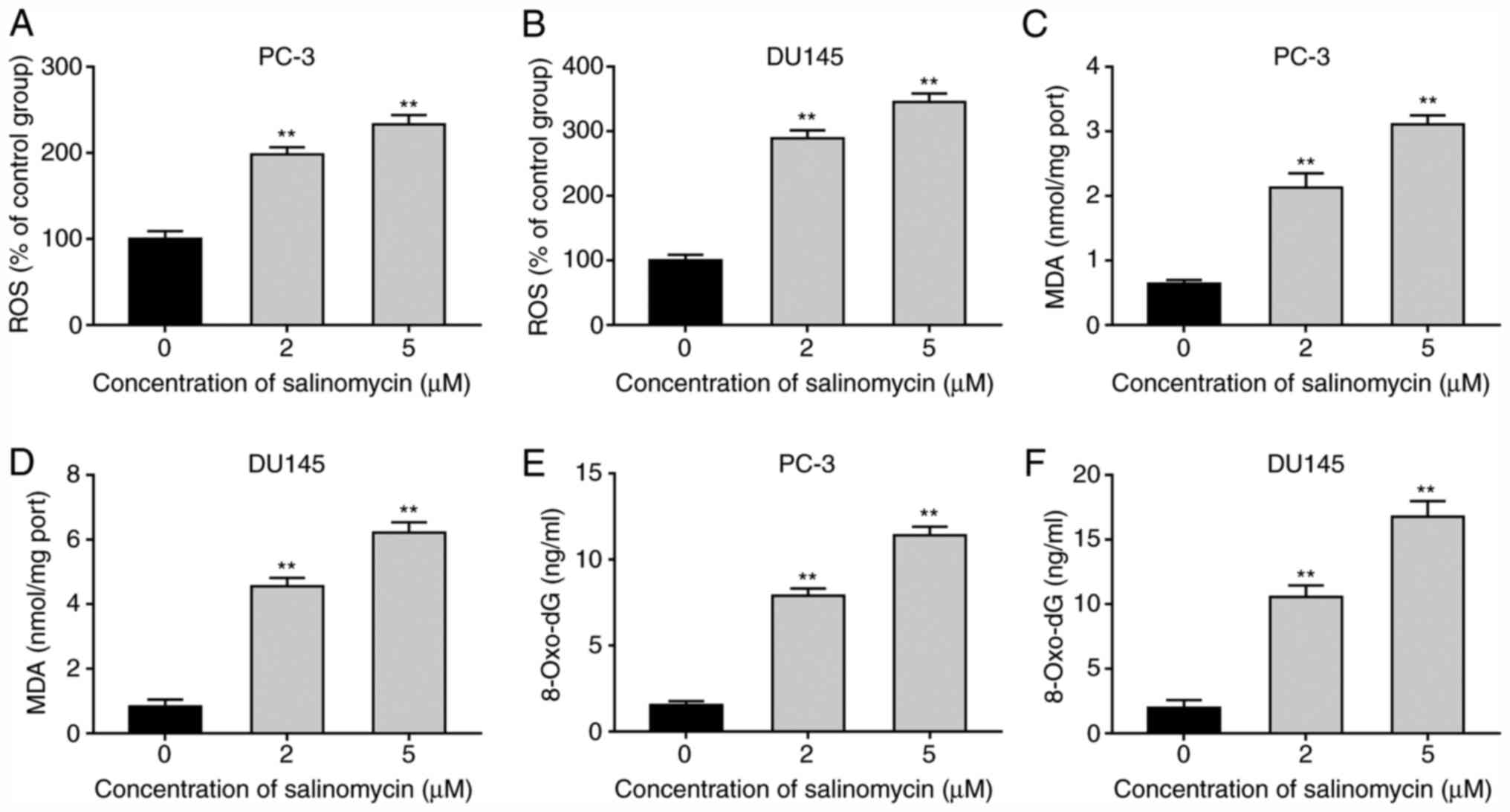
Salinomycin treatment increases
oxidative stress in prostate cancer cells
To determine the impact of salinomycin on prostate
cancer cell oxidative stress, the level of ROS was determined in
PC-3 and DU145 cells following treatment with 2 and 5 µM
salinomycin for 72 h. The results demonstrated that salinomycin
significantly increased the production of ROS in a dose-dependent
manner in PC-3 and DU145 cells (Fig.
2A and B). Furthermore, a
significantly increase in MDA levels in PC-3 and DU145 cells
following salinomycin treatment was observed (Fig. 2C and D). In addition, the levels of 8-OH-dG,
which is a marker of oxidative stress-induced DNA damage, were
determined. As presented in Fig. 2E
and F, 8-OH-dG levels were
significantly increased in PC-3 and DU145 cells after salinomycin
treatment. These data indicated that salinomycin treatment promoted
the production of ROS and caused lipid peroxidation of
polyunsaturated fatty acid and DNA damage.
Salinomycin increased ER stress in
prostate cancer cells
To determine whether salinomycin could induce ER
stress in PC-3 and DU145 cells, the expression of ER stress-related
proteins was determined by western blotting (Fig. 3A). The results demonstrated that
salinomycin treatment caused the significant upregulation of the ER
stress biomarkers Bip (Fig. 3B),
ATF4 (Fig. 3E) and CHOP (Fig. 3F) in both PC-3 and DU145 cells.
Furthermore, the expression of p-eIF2α (Fig. 3C) and p-PERK (Fig. 3D) was significantly increased in
both PC-3 and DU145 cells following treatment with salinomycin.
These findings indicated that salinomycin could increase the
expression of ER stress regulatory proteins in prostate cancer
cells.
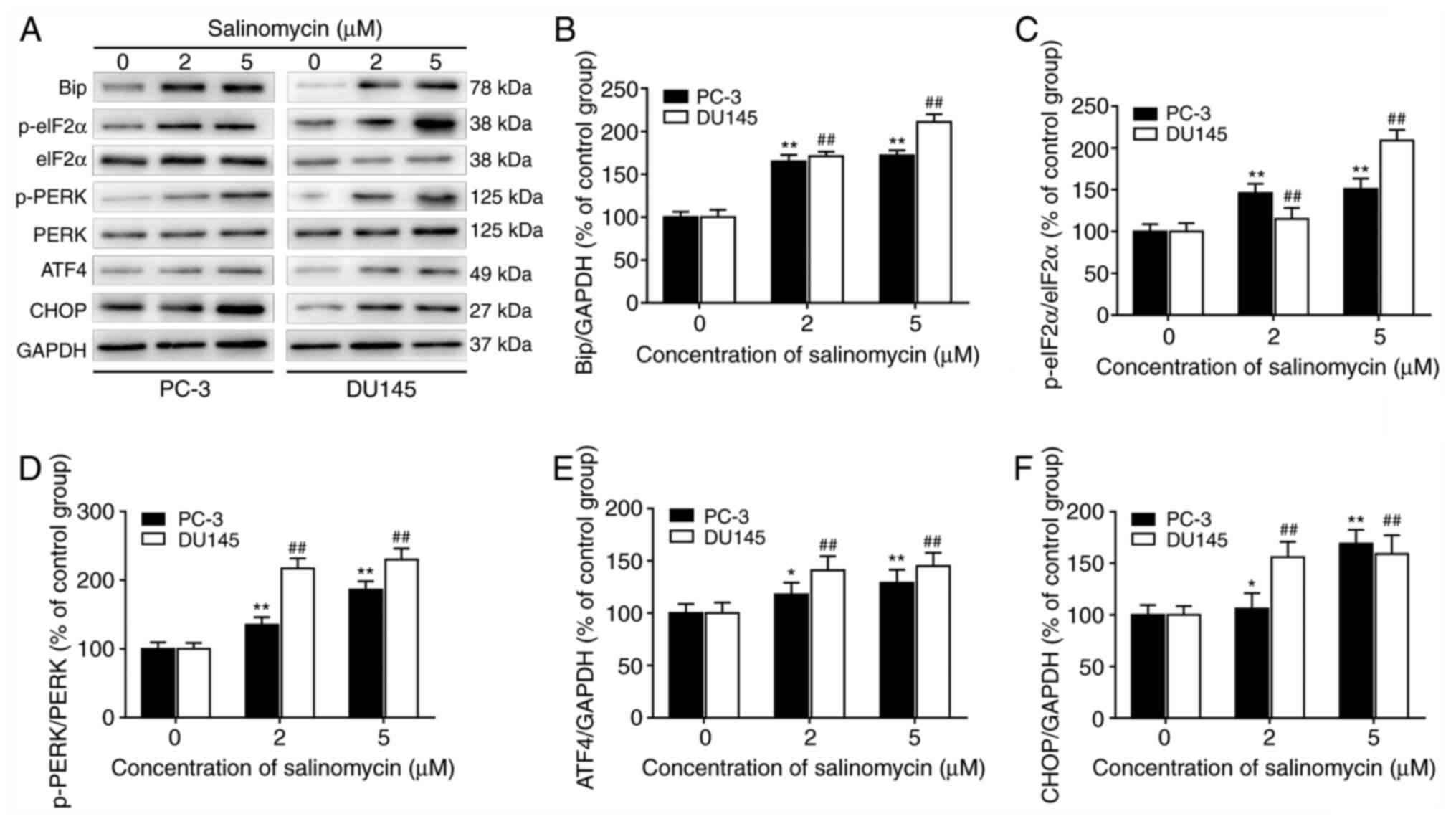 | Figure 3Salinomycin promotes prostate cancer
cell ER stress. Prostate cancer cells PC-3 and DU145 were treated
with 2 and 5 µM salinomycin for 72 h. (A) Effect of salinomycin on
ER stress biomarker was assessed by western. Bip, p-eIF2α, eIF2α,
p-PERK, PERK, ATF4, CHOP and GAPDH protein electrophoresis images.
(B-F) Relative quantification of protein expression from bands in
(A). Data were presented as the means ± standard deviation.
*P<0.05 and **P<0.01 vs. PC-3 cells
control. ##P<0.01 vs. DU145 cells control. ER,
endoplasmic reticulum; Bip, binding immunoglobulin protein; eIF2α,
eukaryotic initiation factor 2α; p, phosphorylated; PERK, protein
kinase RNA-like endoplasmic reticulum kinase; ATF4, activating
transcription factor 4; CHOP, C/EBP homologous protein. |
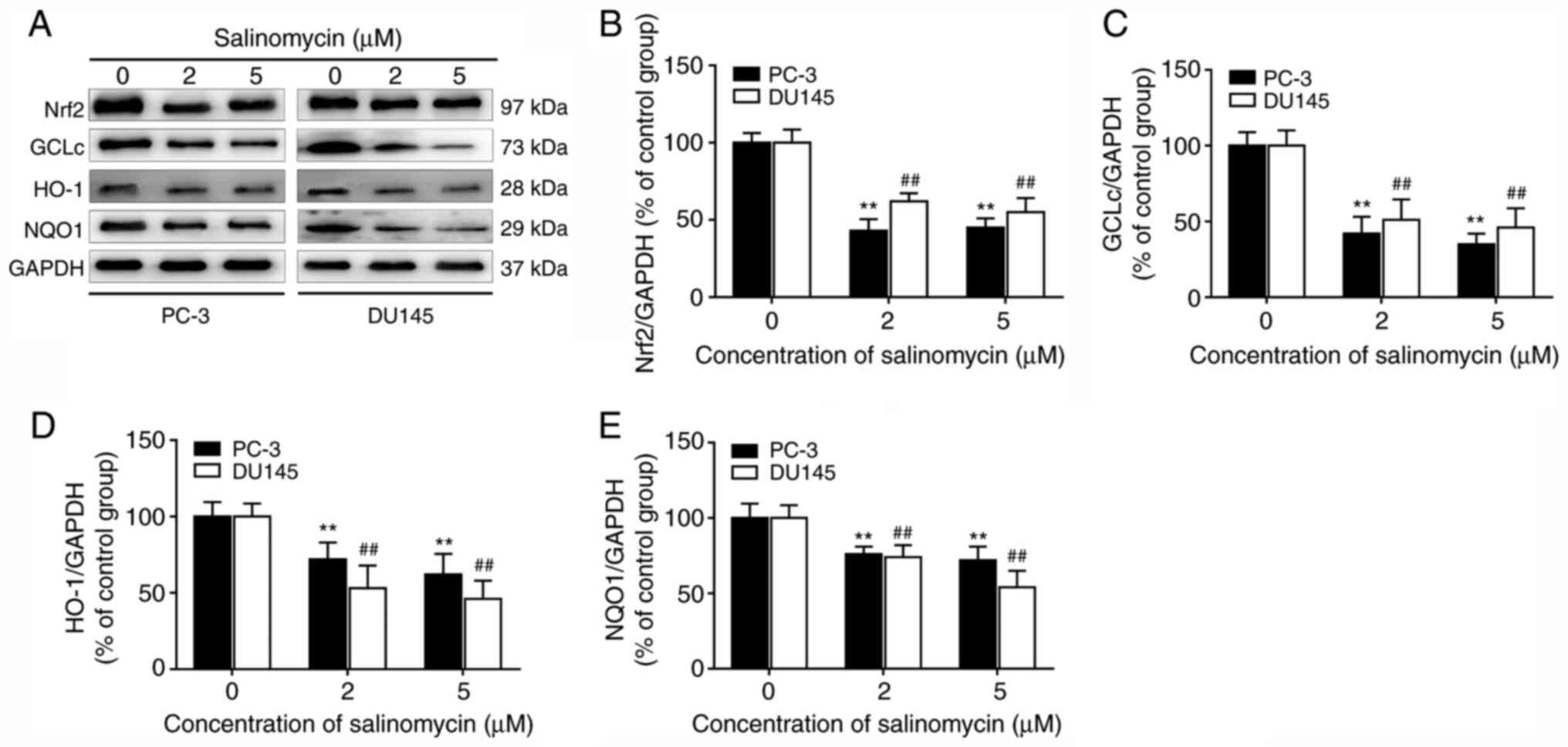
Salinomycin inhibits the activation of
Nrf2 signaling in prostate cancer cells
Whether salinomycin could regulate the activation of
Nrf2 pathway, which is the essential signaling transduction pathway
involved in anti-oxidative response, was further investigated. As
shown in Fig. 4A and B, salinomycin treatment significantly
decreased Nrf2 expression in both PC-3 and DU145 cells. In
addition, the expression of Nrf2 downstream target proteins GCLc,
HO-1 and NQO1, was significantly decreased in salinomycin treated
groups compared with control group (Fig. 4A and C-E).
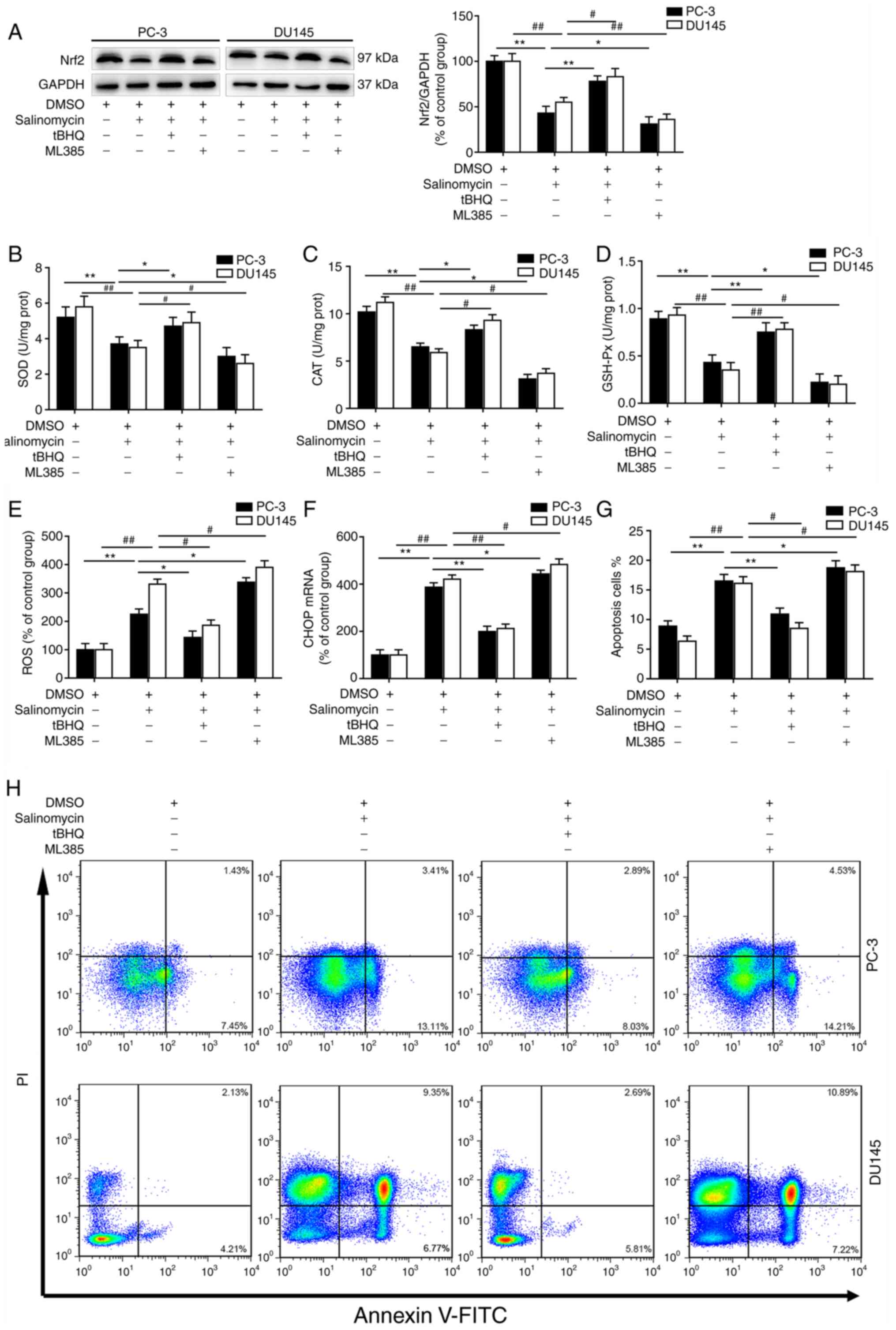
Furthermore, cell treatment with salinomycin and the
Nrf2 activator tert-butylhydroquinone (tBHQ) significantly
increased the expression of Nrf2 in both PC-3 and DU145 cells
compared with treatment with salinomycin alone (Fig. 5A). Conversely, cell treatment with
salinomycin and the Nrf2 inhibitor ML385 significantly decreased
the expression of Nrf2 in both PC-3 and DU145 cells compared with
treatment with salinomycin alone (Fig.
5A). These data indicated that salinomycin treatment could
inhibit the activity of Nrf2 signaling in prostate cancer
cells.
Salinomycin decreases the activity of
antioxidant enzymes and increases the apoptosis of prostate cancer
cells via inhibiting Nrf2 pathway
To determine the relationship between
salinomycin-induced Nrf2 signaling inhibition and the change in
antioxidant enzyme activity in prostate cancer cells, the
activities of SOD, CAT and GSH-Px were determined following
treatment of PC-3 and DU145 cells with 5 µM salinomycin, 10 µM
tBHQ, 10 µM ML385 or DMSO vehicle for 72 h. As presented in
Fig. 5B-D, the activities of SOD,
CAT and GSH-Px were significantly decreased after salinomycin
treatment. Furthermore, tBHQ treatment signifcantly increased the
activities of SOD, CAT and GSH-Px in the two prostate cancer cell
lines compared with treatment with salinomycin alone. Conversely,
ML385 treatment significantly decreased the activities of SOD, CAT
and GSH-Px in both PC-3 and DU145 cells compared with treatment
with salinomycin alone.
The effects of Nrf2 inhibitor and activator on
salinomycin-induced oxidative and ER stress in prostate cancer
cells were further investigated. As presented in Fig. 5E and F, ROS levels and CHOP expression were
significantly decreased folowing tBHQ treatment compared with
treatment with salinomycin alone. Furthermore, tBHQ treatment
signifcantly decreased the apoptosis in both PC-3 and DU145 cells
compared with treatment with salinomycin alone (Fig. 5G). Conversely, ML385 treatment
significantly elevated ROS level, CHOP expression and apoptosis in
both prostate cancer cell lines compared with treatment with
salinomycin alone (Fig. 5E-H).
Discussion
Prostate cancer is a commonly diagnosed cancer in
men. The present study demonstrated a potential important role of
salinomycin in mediating the apoptosis of prostate cancer cells by
increasing oxidative and ER stress through the inhibition of Nrf2
antioxidant signaling.
Salinomycin is a monocarboxylic ionophore isolated
from Streptomyces albus (2).
Salinomycin ability to kill cancer stem cells and therapy-resistant
cancer cells may allow this compound to be considered as a
potential novel chemotherapeutic drug (32). The present study demonstrated that
salinomycin significantly inhibited the proliferation and induced
the apoptosis of PC-3 and DU145 cells in a dose-dependent manner.
It was previously reported that cancer chemopreventive agents
cancer induce apoptosis partly via ROS generation and disruption of
the redox homeostasis (33). The
results from the present study demonstrated that salinomycin could
promote ROS accumulation, lipid peroxidation of polyunsaturated
fatty acids and DNA damage. These findings were consistent with
results from a previous study demonstrating that salinomycin
inhibits prostate cancer cell proliferation and migration by
reducing the expression of key prostate cancer oncogenes, inducing
oxidative stress, decreasing the antioxidative capacity and
diminishing cancer stem cell fraction (13).
The ER is an essential intracellular organelle that
is crucial for multiple cellular functions, including protein
folding and maturation and maintenance of cellular homeostasis. ER
stress is activated by a variety of factors and triggers the
unfolded protein response (UPR), which restores homeostasis or
activates cell death (34).
Previous studies have reported that UPR signaling pathways are
closely related to autophagy, apoptosis, inflammatory response and
oxidative stress in cancer cells (35-37).
The PERK/ATF4/CHOP pathway plays an important role in modulating
anti- and pro-apoptotic proteins, such as B-cell lymphoma-2 (Bcl-2)
family members that control mitochondrial apoptosis (38). Similarly, the present study
demonstrated that the expression of UPR proteins, including Bip,
ATF4, p-PERK, p-eIF2α and CHOP, was significantly increased in PC-3
and DU145 cells treated with salinomycin. Previous studies have
reported that ROS activate PERK signal transduction, which induces
apoptosis via ER stress in choriocarcinoma cells (39). Furthermore, ROS may play a role in
cell death via the p-eIF2α/ATF4/CHOP axis (40). Taken together, these results
indicate that salinomycin may initiate prostate cancer cell
apoptosis by promoting ROS production and ER stress.
Nrf2 is a key regulator of the antioxidant system
(41,42). Recent studies have demonstrated that
regulation of the Nrf2 signaling pathway is involved in the
pathogenesis and treatment of various cancers (43-45).
In the present study, salinomycin effectively downregulated the
expression of Nrf2, HO-1, NQO1 and GCLc in prostate cancer cell
lines. Cell treatment with the Nrf2 activator tBHQ significantly
reversed the therapeutic effects of salinomycin by promoting the
expression of the Nrf2 pathway and antioxidant enzyme activities.
In addition, cell treatment with the Nrf2 inhibitor ML385 promoted
the therapeutic effects of salinomycin. These results indicated
that salinomycin may promote some anti-cancer effects by inhibiting
the Nrf2 pathway in prostate cancer cells. These results were in
agreement with previous results demonstrating that salinomycin
overcomes radioresistance in nasopharyngeal carcinoma cells by
inhibiting Nrf2 levels and promoting ROS production (10).
To test our hypothesis, we explored the influence of
salinomycin on antioxidant enzyme activity in prostate cancer
cells. The results demonstrated that salinomycin treatment
significantly downregulated the activities of CAT, GSH-Px and SOD.
Furthermore, Nrf2 activator tBHQ treatment significantly increased
the activities of antioxidant enzymes and inhibited the apoptosis
promoting effect of salinomycin on prostate cancer cells.
Conversely, the Nrf2 inhibitor ML385 promoted apoptosis of prostate
cancer cells and enhanced the anti-cancer effect of salinomycin by
reducing the antioxidant capacity and increasing oxidative stress
and ER stress. These results suggested that salinomycin treatment
may reduce the activity of antioxidant enzymes by inhibiting the
Nrf2 pathway in prostate cancer cells that become more susceptible
to oxidative damage. These results were in line with the
upregulated ROS levels, CHOP expression and apoptosis in prostate
cancer cells.
In conclusion, to the best of our knowledge, the
present study demonstrated for the first time that salinomycin,
which is a potent antitumor agent, exhibited some anti-cancer
activities on human prostate cancer cells by inducing ROS and ER
stress-dependent apoptosis via suppressing Nrf2 antioxidant
signaling. These findings elucidated the anti-cancer effects and
underlying mechanism of salinomycin and may help the development of
novel therapeutic strategies against prostate cancer.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PM and JY conceived and designed the experiments and
wrote the manuscript. SL and YY performed the experiments and were
responsible for data analysis and interpretation. PM and JY
confirmed the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Naujokat C, Fuchs D and Opelz G:
Salinomycin in cancer: A new mission for an old agent. Mol Med Rep.
3:555–559. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Huczyński A, Janczak J, Antoszczak M,
Wietrzyk J, Maj E and Brzezinski B: Antiproliferative activity of
salinomycin and its derivatives. Bioorg Med Chem Lett.
22:7146–7150. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gupta PB, Onder TT, Jiang G, Tao K,
Kuperwasser C, Weinberg RA and Lander ES: Identification of
selective inhibitors of cancer stem cells by high-throughput
screening. Cell. 138:645–659. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Moloney JN and Cotter TG: ROS signalling
in the biology of cancer. Semin Cell Dev Biol. 80:50–64.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mizoue T, Tokunaga S, Kasai H, Kawai K,
Sato M and Kubo T: Body mass index and oxidative DNA damage: A
longitudinal study. Cancer Sci. 98:1254–1258. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Xiao MH, Xia JY, Wang ZL, Hu WX, Fan YL,
Jia DY, Li J, Jing PW, Wang L and Wang YP: Ginsenoside Rg1
attenuates liver injury induced by D-galactose in mice. Exp Ther
Med. 16:4100–4106. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chang WH, Lee CC, Yen YH and Chen HL:
Oxidative damage in patients with benign prostatic hyperplasia and
prostate cancer co-exposed to phthalates and to trace elements.
Environ Int. 121:1179–1184. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Arif M, Rashid A, Majeed A, Qaiser F and
Razak S: Evaluation of correlation between expression of P53 and
Malondialdehyde levels in prostate cancer patients. J Pak Med
Assoc. 68:1373–1377. 2018.PubMed/NCBI
|
|
10
|
Zhang G, Wang W, Yao C, Ren J, Zhang S and
Han M: Salinomycin overcomes radioresistance in nasopharyngeal
carcinoma cells by inhibiting Nrf2 level and promoting ROS
generation. Biomed Pharmacother. 91:147–154. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhou J, Li P, Xue X, He S, Kuang Y, Zhao
H, Chen S, Zhi Q and Guo X: Salinomycin induces apoptosis in
cisplatin-resistant colorectal cancer cells by accumulation of
reactive oxygen species. Toxicol Lett. 222:139–145. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li T, Su L, Zhong N, Hao X, Zhong D,
Singhal S and Liu X: Salinomycin induces cell death with autophagy
through activation of endoplasmic reticulum stress in human cancer
cells. Autophagy. 9:1057–1068. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ketola K, Hilvo M, Hyötyläinen T, Vuoristo
A, Ruskeepää AL, Orešič M, Kallioniemi O and Iljin K: Salinomycin
inhibits prostate cancer growth and migration via induction of
oxidative stress. Br J Cancer. 106:99–106. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li T, Liu X, Shen Q, Yang W, Huo Z, Liu Q,
Jiao H and Chen J: Salinomycin exerts anti-angiogenic and
anti-tumorigenic activities by inhibiting vascular endothelial
growth factor receptor 2-mediated angiogenesis. Oncotarget.
7:26580–26592. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wu D, Zhang Y, Huang J, Fan Z, Shi F and
Wang S: Salinomycin inhibits proliferation and induces apoptosis of
human nasopharyngeal carcinoma cell in vitro and suppresses tumor
growth in vivo. Biochem Biophys Res Commun. 443:712–717.
2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang Y, Li F, Liu L, Jiang H, Hu H, Du X,
Ge X, Cao J and Wang Y: Salinomycin triggers endoplasmic reticulum
stress through ATP2A3 upregulation in PC-3 cells. BMC Cancer.
19(381)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Menegon S, Columbano A and Giordano S: The
dual roles of Nrf2 in cancer. Trends Mol Med. 22:578–593.
2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lau A, Villeneuve NF, Sun Z, Wong PK and
Zhang DD: Dual roles of Nrf2 in cancer. Pharmacol Res. 58:262–270.
2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kubben N, Zhang W, Wang L, Voss TC, Yang
J, Qu J, Liu GH and Misteli T: Repression of the antioxidant Nrf2
pathway in premature aging. Cell. 165:1361–1374. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhou L, Zhang H, Davies KJA and Forman HJ:
Aging-related decline in the induction of Nrf2-regulated
antioxidant genes in human bronchial epithelial cells. Redox Biol.
14:35–40. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shibata T, Ohta T, Tong KI, Kokubu A,
Odogawa R, Tsuta K, Asamura H, Yamamoto M and Hirohashi S: Cancer
related mutations in Nrf2 impair its recognition by Keap1-Cul3 E3
ligase and promote malignancy. Proc Natl Acad Sci USA.
105:13568–13573. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kim KY, Park KI, Kim SH, Yu SN, Park SG,
Kim YW, Seo YK, Ma JY and Ahn SC: Inhibition of autophagy promotes
salinomycin-induced apoptosis via reactive oxygen species-mediated
PI3K/AKT/mTOR and ERK/p38 MAPK-dependent signaling in human
prostate cancer cells. Int J Mol Sci. 18(1088)2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Xiong Y, Wang Y, Zhang J, Zhao N, Zhang H,
Zhang A, Zhao D, Yu Z, Yin Y, Song L, et al: hPMSCs protects
against D-galactose-induced oxidative damage of CD4+ T
cells through activating Akt-mediated Nrf2 antioxidant signaling.
Stem Cell Res Ther. 11(468)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang Y, Xiong Y, Zhang A, Zhao N, Zhang J,
Zhao D, Yu Z, Xu N, Yin Y, Luan X, et al: Oligosaccharide
attenuates aging-related liver dysfunction by activating Nrf2
antioxidant signaling. Food Sci Nutr. 8:3872–3881. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xiong Y, Xiong Y, Zhou S, Yu Z, Zhao D,
Wang Z, Li Y, Yan J, Cai Y and Zhang W: Inhibition of glutathione
synthesis induced by exhaustive running exercise via the decreased
influx rate of L-cysteine in rat erythrocytes. Cell Physiol
Biochem. 40:1410–1421. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Stasiolek M, Adamczewski Z, Sliwka PW,
Puła B, Karwowski B, Merecz-Sadowska A, Dedecjus M and Lewiński A:
The molecular effect of diagnostic absorbed doses from
131I on papillary thyroid cancer cells in vitro.
Molecules. 22(993)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Xiong Y, Xiong Y, Zhou S, Sun Y, Zhao Y,
Ren X, Zhang Y and Zhang N: Vitamin C and E supplements enhance the
antioxidant capacity of erythrocytes obtained from aged rats.
Rejuvenation Res. 20:85–92. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Rotruck JT, Pope AL, Ganther HE, Swanson
AB, Hafeman DG and Hoekstra WG: Selenium: Biochemical role as a
component of glutathione peroxidase. Science. 179:588–590.
1973.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Góth L: A simple method for determination
of serum catalase activity and revision of reference range. Clin
Chim Acta. 196:143–151. 1991.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sun Y, Oberley LW and Li Y: A simple
method for clinical assay of superoxide dismutase. Clin Chem.
34:497–500. 1988.PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Dewangan J, Srivastava S and Rath SK:
Salinomycin: A new paradigm in cancer therapy. Tumour Biol.
39(1010428317695035)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hail N Jr and Lotan R: Cancer
chemoprevention and mitochondria: Targeting apoptosis in
transformed cells via the disruption of mitochondrial
bioenergetics/redox state. Mol Nutr Food Res. 53:49–67.
2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yadav RK, Chae SW, Kim HR and Chae HJ:
Endoplasmic reticulum stress and cancer. J Cancer Prev. 19:75–88.
2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lin Y, Jiang M, Chen W, Zhao T and Wei Y:
Cancer and ER stress: Mutual crosstalk between autophagy, oxidative
stress and inflammatory response. Biomed Pharmacother.
118(109249)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li W, Yang J, Luo L, Jiang M, Qin B, Yin
H, Zhu C, Yuan X, Zhang J, Luo Z, et al: Targeting photodynamic and
photothermal therapy to the endoplasmic reticulum enhances
immunogenic cancer cell death. Nat Commun. 10(3349)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Jeong S, Kim DY, Kang SH, Yun HK, Kim JL,
Kim BR, Park SH, Na YJ, Jo MJ, Jeong YA, et al: Docosahexaenoic
acid enhances oxaliplatin-induced autophagic cell death via the ER
stress/Sesn2 pathway in colorectal cancer. Cancers (Basel).
11(982)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Peñaranda Fajardo NM, Meijer C and Kruyt
FA: The endoplasmic reticulum stress/unfolded protein response in
gliomagenesis, tumor progression and as a therapeutic target in
glioblastoma. Biochem Pharmacol. 118:1–8. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Shen Y, Yang J, Zhao J, Xiao C, Xu C and
Xiang Y: The switch from ER stress-induced apoptosis to autophagy
via ROS-mediated JNK/p62 signals: A survival mechanism in
methotrexate-resistant choriocarcinoma cells. Exp Cell Res.
334:207–218. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Xian M, Cao H, Cao J, Shao X, Zhu D, Zhang
N, Huang P, Li W, Yang B, Ying M, et al: Bortezomib sensitizes
human osteosarcoma cells to adriamycin-induced apoptosis through
ROS-dependent activation of p-eIF2α/ATF4/CHOP axis. Int J Cancer.
141:1029–1041. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wardyn JD, Ponsford AH and Sanderson CM:
Dissecting molecular cross-talk between Nrf2 and NF-κB response
pathways. Biochem Soc Trans. 43:621–626. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wang Y, Xiong Y, Zhang A, Zhao N, Zhang J,
Zhao D, Yu Z, Xu N, Yin Y, Luan X, et al: Oligosaccharide
attenuates aging-related liver dysfunction by activating Nrf2
antioxidant signaling. Food Sci Nutr. 8:3872–3881. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Towers CG, Fitzwalter BE, Regan D,
Goodspeed A, Morgan MJ, Liu CW, Gustafson DL and Thorburn A: Cancer
cells upregulate Nrf2 signaling to adapt to autophagy inhibition.
Dev Cell. 50:690–703.e6. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Rodrigues C, Milkovic L, Bujak IT,
Tomljanovic M, Soveral G and Cipak Gasparovic A: Lipid profile and
aquaporin expression under oxidative stress in breast cancer cells
of different malignancies. Oxid Med Cell Longev.
2019(2061830)2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhang XQ, Yao C, Bian WH, Chen X, Xue JX,
Zhu ZY, Ying Y, Xu YL and Wang C: Effects of Astragaloside IV on
treatment of breast cancer cells execute possibly through
regulation of Nrf2 via PI3K/AKT/mTOR signaling pathway. Food Sci
Nutr. 7:3403–3413. 2019.PubMed/NCBI View Article : Google Scholar
|