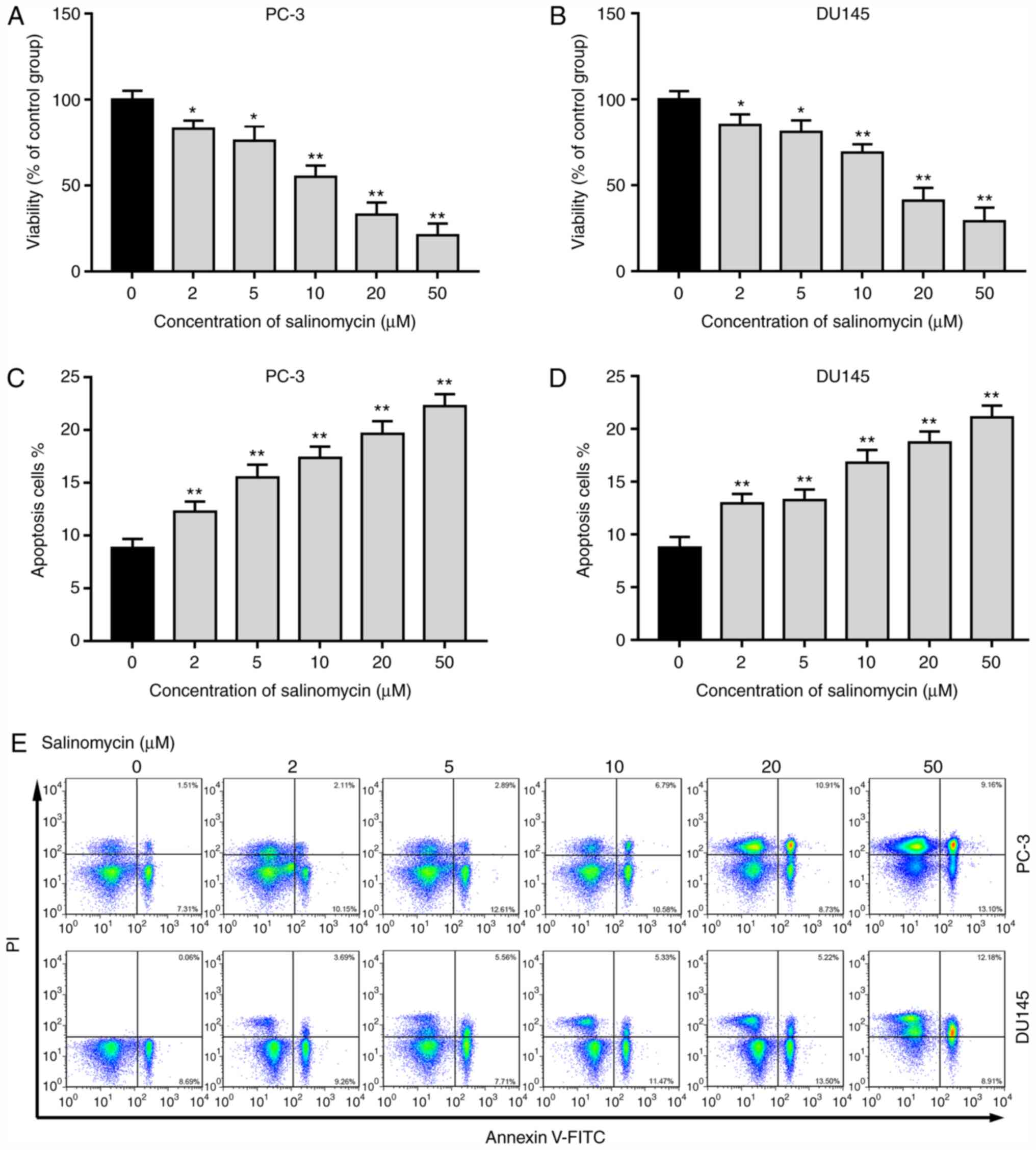
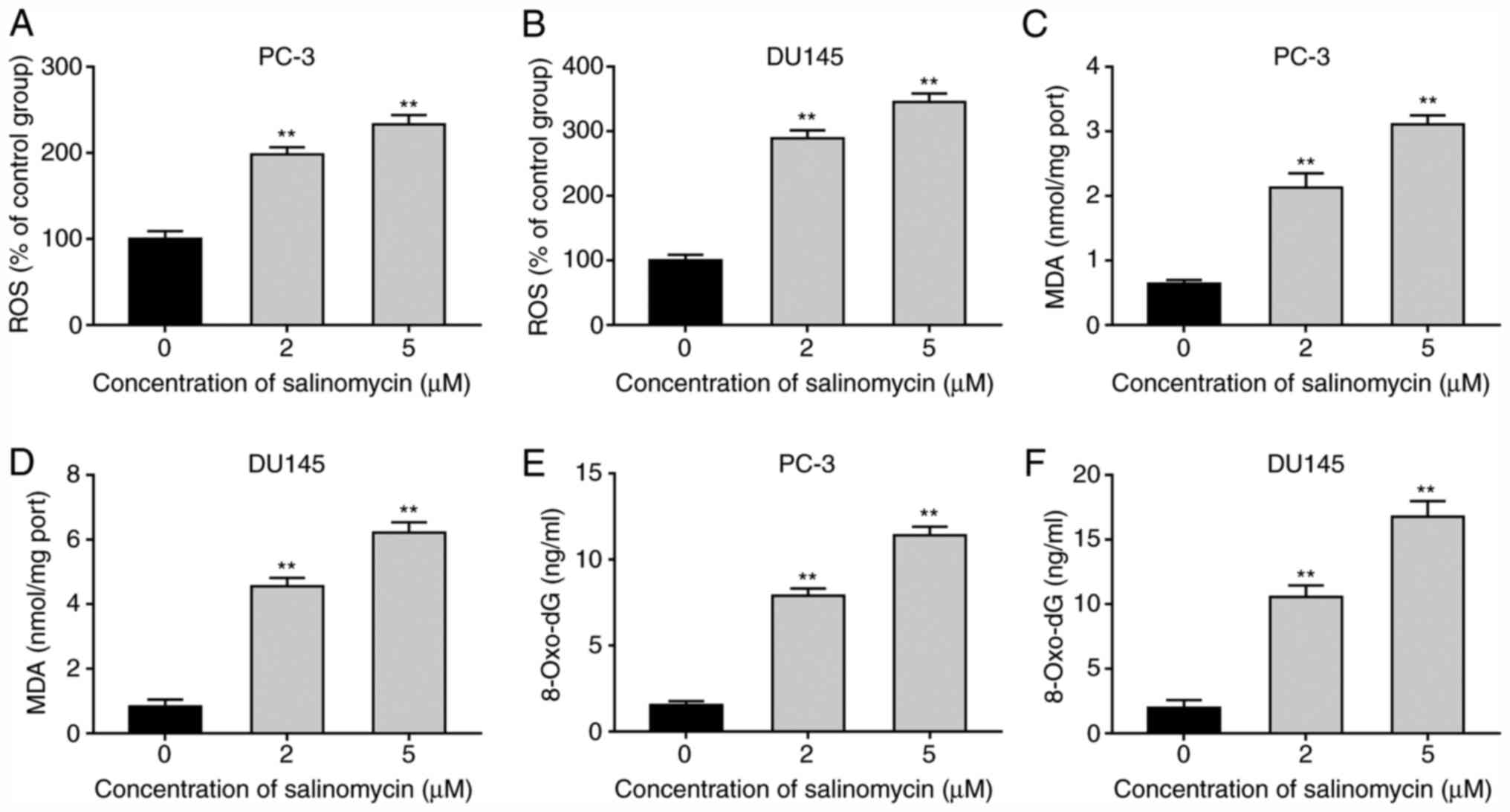
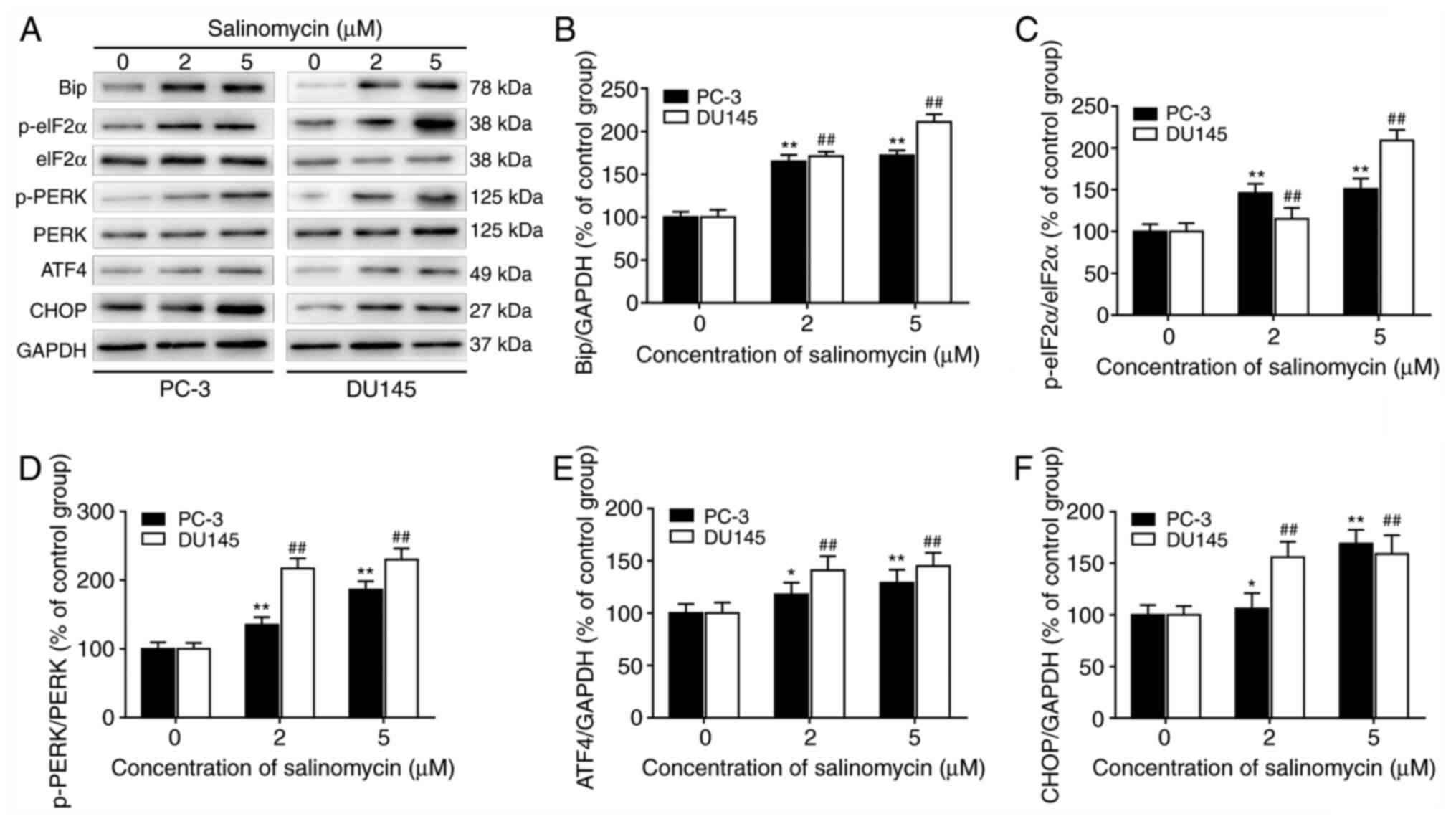
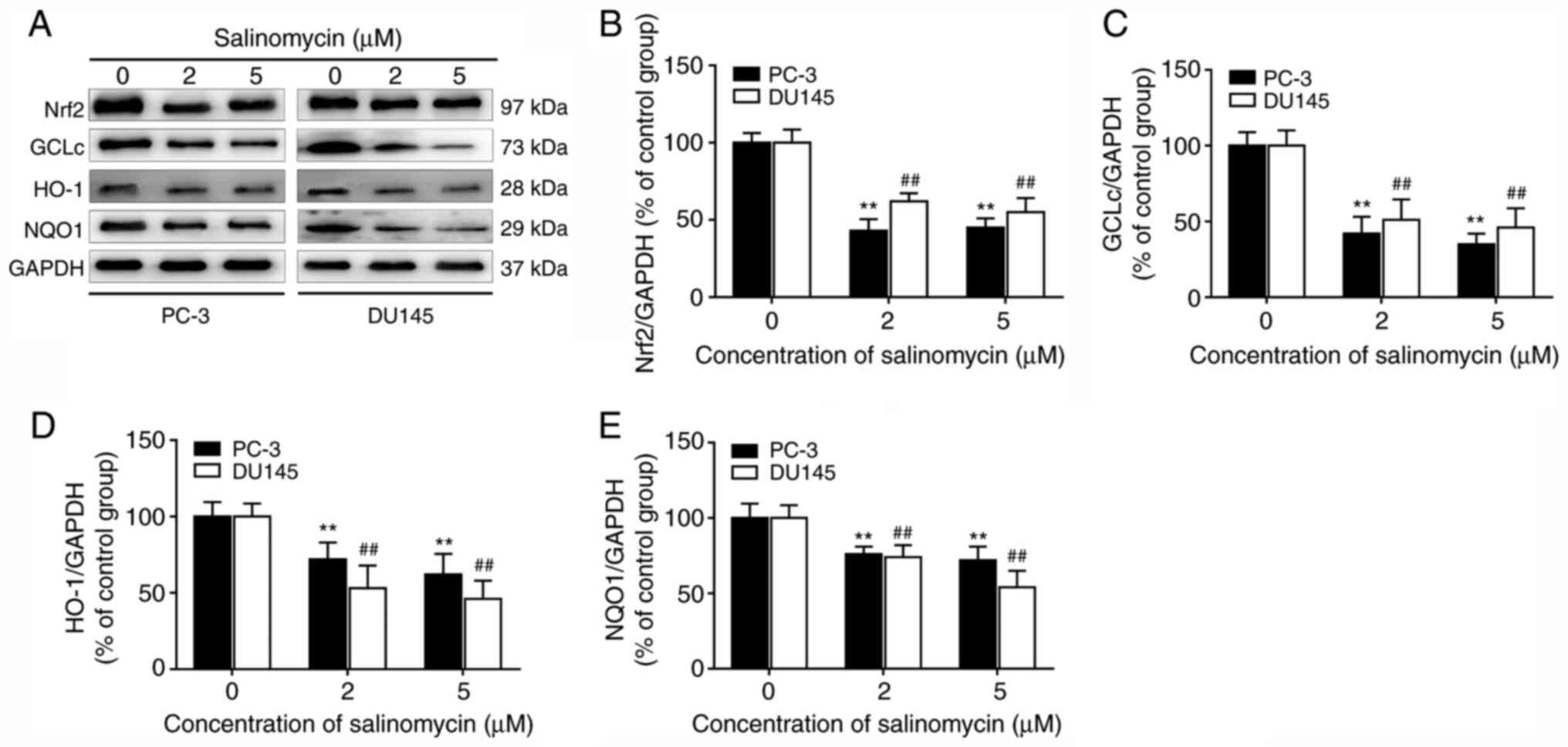
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Naujokat C, Fuchs D and Opelz G:
Salinomycin in cancer: A new mission for an old agent. Mol Med Rep.
3:555–559. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Huczyński A, Janczak J, Antoszczak M,
Wietrzyk J, Maj E and Brzezinski B: Antiproliferative activity of
salinomycin and its derivatives. Bioorg Med Chem Lett.
22:7146–7150. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gupta PB, Onder TT, Jiang G, Tao K,
Kuperwasser C, Weinberg RA and Lander ES: Identification of
selective inhibitors of cancer stem cells by high-throughput
screening. Cell. 138:645–659. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Moloney JN and Cotter TG: ROS signalling
in the biology of cancer. Semin Cell Dev Biol. 80:50–64.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mizoue T, Tokunaga S, Kasai H, Kawai K,
Sato M and Kubo T: Body mass index and oxidative DNA damage: A
longitudinal study. Cancer Sci. 98:1254–1258. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Xiao MH, Xia JY, Wang ZL, Hu WX, Fan YL,
Jia DY, Li J, Jing PW, Wang L and Wang YP: Ginsenoside Rg1
attenuates liver injury induced by D-galactose in mice. Exp Ther
Med. 16:4100–4106. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chang WH, Lee CC, Yen YH and Chen HL:
Oxidative damage in patients with benign prostatic hyperplasia and
prostate cancer co-exposed to phthalates and to trace elements.
Environ Int. 121:1179–1184. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Arif M, Rashid A, Majeed A, Qaiser F and
Razak S: Evaluation of correlation between expression of P53 and
Malondialdehyde levels in prostate cancer patients. J Pak Med
Assoc. 68:1373–1377. 2018.PubMed/NCBI
|
|
10
|
Zhang G, Wang W, Yao C, Ren J, Zhang S and
Han M: Salinomycin overcomes radioresistance in nasopharyngeal
carcinoma cells by inhibiting Nrf2 level and promoting ROS
generation. Biomed Pharmacother. 91:147–154. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhou J, Li P, Xue X, He S, Kuang Y, Zhao
H, Chen S, Zhi Q and Guo X: Salinomycin induces apoptosis in
cisplatin-resistant colorectal cancer cells by accumulation of
reactive oxygen species. Toxicol Lett. 222:139–145. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li T, Su L, Zhong N, Hao X, Zhong D,
Singhal S and Liu X: Salinomycin induces cell death with autophagy
through activation of endoplasmic reticulum stress in human cancer
cells. Autophagy. 9:1057–1068. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ketola K, Hilvo M, Hyötyläinen T, Vuoristo
A, Ruskeepää AL, Orešič M, Kallioniemi O and Iljin K: Salinomycin
inhibits prostate cancer growth and migration via induction of
oxidative stress. Br J Cancer. 106:99–106. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li T, Liu X, Shen Q, Yang W, Huo Z, Liu Q,
Jiao H and Chen J: Salinomycin exerts anti-angiogenic and
anti-tumorigenic activities by inhibiting vascular endothelial
growth factor receptor 2-mediated angiogenesis. Oncotarget.
7:26580–26592. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wu D, Zhang Y, Huang J, Fan Z, Shi F and
Wang S: Salinomycin inhibits proliferation and induces apoptosis of
human nasopharyngeal carcinoma cell in vitro and suppresses tumor
growth in vivo. Biochem Biophys Res Commun. 443:712–717.
2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang Y, Li F, Liu L, Jiang H, Hu H, Du X,
Ge X, Cao J and Wang Y: Salinomycin triggers endoplasmic reticulum
stress through ATP2A3 upregulation in PC-3 cells. BMC Cancer.
19(381)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Menegon S, Columbano A and Giordano S: The
dual roles of Nrf2 in cancer. Trends Mol Med. 22:578–593.
2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lau A, Villeneuve NF, Sun Z, Wong PK and
Zhang DD: Dual roles of Nrf2 in cancer. Pharmacol Res. 58:262–270.
2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kubben N, Zhang W, Wang L, Voss TC, Yang
J, Qu J, Liu GH and Misteli T: Repression of the antioxidant Nrf2
pathway in premature aging. Cell. 165:1361–1374. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhou L, Zhang H, Davies KJA and Forman HJ:
Aging-related decline in the induction of Nrf2-regulated
antioxidant genes in human bronchial epithelial cells. Redox Biol.
14:35–40. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shibata T, Ohta T, Tong KI, Kokubu A,
Odogawa R, Tsuta K, Asamura H, Yamamoto M and Hirohashi S: Cancer
related mutations in Nrf2 impair its recognition by Keap1-Cul3 E3
ligase and promote malignancy. Proc Natl Acad Sci USA.
105:13568–13573. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kim KY, Park KI, Kim SH, Yu SN, Park SG,
Kim YW, Seo YK, Ma JY and Ahn SC: Inhibition of autophagy promotes
salinomycin-induced apoptosis via reactive oxygen species-mediated
PI3K/AKT/mTOR and ERK/p38 MAPK-dependent signaling in human
prostate cancer cells. Int J Mol Sci. 18(1088)2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Xiong Y, Wang Y, Zhang J, Zhao N, Zhang H,
Zhang A, Zhao D, Yu Z, Yin Y, Song L, et al: hPMSCs protects
against D-galactose-induced oxidative damage of CD4+ T
cells through activating Akt-mediated Nrf2 antioxidant signaling.
Stem Cell Res Ther. 11(468)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang Y, Xiong Y, Zhang A, Zhao N, Zhang J,
Zhao D, Yu Z, Xu N, Yin Y, Luan X, et al: Oligosaccharide
attenuates aging-related liver dysfunction by activating Nrf2
antioxidant signaling. Food Sci Nutr. 8:3872–3881. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xiong Y, Xiong Y, Zhou S, Yu Z, Zhao D,
Wang Z, Li Y, Yan J, Cai Y and Zhang W: Inhibition of glutathione
synthesis induced by exhaustive running exercise via the decreased
influx rate of L-cysteine in rat erythrocytes. Cell Physiol
Biochem. 40:1410–1421. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Stasiolek M, Adamczewski Z, Sliwka PW,
Puła B, Karwowski B, Merecz-Sadowska A, Dedecjus M and Lewiński A:
The molecular effect of diagnostic absorbed doses from
131I on papillary thyroid cancer cells in vitro.
Molecules. 22(993)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Xiong Y, Xiong Y, Zhou S, Sun Y, Zhao Y,
Ren X, Zhang Y and Zhang N: Vitamin C and E supplements enhance the
antioxidant capacity of erythrocytes obtained from aged rats.
Rejuvenation Res. 20:85–92. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Rotruck JT, Pope AL, Ganther HE, Swanson
AB, Hafeman DG and Hoekstra WG: Selenium: Biochemical role as a
component of glutathione peroxidase. Science. 179:588–590.
1973.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Góth L: A simple method for determination
of serum catalase activity and revision of reference range. Clin
Chim Acta. 196:143–151. 1991.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sun Y, Oberley LW and Li Y: A simple
method for clinical assay of superoxide dismutase. Clin Chem.
34:497–500. 1988.PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Dewangan J, Srivastava S and Rath SK:
Salinomycin: A new paradigm in cancer therapy. Tumour Biol.
39(1010428317695035)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hail N Jr and Lotan R: Cancer
chemoprevention and mitochondria: Targeting apoptosis in
transformed cells via the disruption of mitochondrial
bioenergetics/redox state. Mol Nutr Food Res. 53:49–67.
2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yadav RK, Chae SW, Kim HR and Chae HJ:
Endoplasmic reticulum stress and cancer. J Cancer Prev. 19:75–88.
2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lin Y, Jiang M, Chen W, Zhao T and Wei Y:
Cancer and ER stress: Mutual crosstalk between autophagy, oxidative
stress and inflammatory response. Biomed Pharmacother.
118(109249)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li W, Yang J, Luo L, Jiang M, Qin B, Yin
H, Zhu C, Yuan X, Zhang J, Luo Z, et al: Targeting photodynamic and
photothermal therapy to the endoplasmic reticulum enhances
immunogenic cancer cell death. Nat Commun. 10(3349)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Jeong S, Kim DY, Kang SH, Yun HK, Kim JL,
Kim BR, Park SH, Na YJ, Jo MJ, Jeong YA, et al: Docosahexaenoic
acid enhances oxaliplatin-induced autophagic cell death via the ER
stress/Sesn2 pathway in colorectal cancer. Cancers (Basel).
11(982)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Peñaranda Fajardo NM, Meijer C and Kruyt
FA: The endoplasmic reticulum stress/unfolded protein response in
gliomagenesis, tumor progression and as a therapeutic target in
glioblastoma. Biochem Pharmacol. 118:1–8. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Shen Y, Yang J, Zhao J, Xiao C, Xu C and
Xiang Y: The switch from ER stress-induced apoptosis to autophagy
via ROS-mediated JNK/p62 signals: A survival mechanism in
methotrexate-resistant choriocarcinoma cells. Exp Cell Res.
334:207–218. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Xian M, Cao H, Cao J, Shao X, Zhu D, Zhang
N, Huang P, Li W, Yang B, Ying M, et al: Bortezomib sensitizes
human osteosarcoma cells to adriamycin-induced apoptosis through
ROS-dependent activation of p-eIF2α/ATF4/CHOP axis. Int J Cancer.
141:1029–1041. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wardyn JD, Ponsford AH and Sanderson CM:
Dissecting molecular cross-talk between Nrf2 and NF-κB response
pathways. Biochem Soc Trans. 43:621–626. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wang Y, Xiong Y, Zhang A, Zhao N, Zhang J,
Zhao D, Yu Z, Xu N, Yin Y, Luan X, et al: Oligosaccharide
attenuates aging-related liver dysfunction by activating Nrf2
antioxidant signaling. Food Sci Nutr. 8:3872–3881. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Towers CG, Fitzwalter BE, Regan D,
Goodspeed A, Morgan MJ, Liu CW, Gustafson DL and Thorburn A: Cancer
cells upregulate Nrf2 signaling to adapt to autophagy inhibition.
Dev Cell. 50:690–703.e6. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Rodrigues C, Milkovic L, Bujak IT,
Tomljanovic M, Soveral G and Cipak Gasparovic A: Lipid profile and
aquaporin expression under oxidative stress in breast cancer cells
of different malignancies. Oxid Med Cell Longev.
2019(2061830)2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhang XQ, Yao C, Bian WH, Chen X, Xue JX,
Zhu ZY, Ying Y, Xu YL and Wang C: Effects of Astragaloside IV on
treatment of breast cancer cells execute possibly through
regulation of Nrf2 via PI3K/AKT/mTOR signaling pathway. Food Sci
Nutr. 7:3403–3413. 2019.PubMed/NCBI View Article : Google Scholar
|