Introduction
In the occurrence and development of various chronic
liver diseases, such as chronic viral hepatitis, alcoholic
hepatitis and drug induced hepatitis, liver fibrosis is a notable
indicator of pathological stage (1). The mechanism of liver fibrosis
involves the imbalance of synthesis and degradation of the
extracellular matrix (ECM), and the central feature is the
activation and proliferation of hepatic stellate cells (HSCs)
(1). The activation of HSCs is
mediated by various cytokines, such as TGF-β1, matrix
metalloproteinases and connective tissue growth factor (2). T helper (Th)1/Th2 immune imbalance has
been indicated to contribute to fibroblast activation,
proliferation and transformation to HSCs, leading to increased ECM
production (3,4). Our previous studies have confirmed
that small interfering (si)RNA can successfully interfere with the
expression levels of TGF-β1, TIMP-1 and TIMP-2 in rat liver tissue.
The results indicated that in the TGF-β1, TIMP-1 and TIMP-2 siRNA
treatment groups, TGF-β1, TIMP-1 and TIMP-2 proteins expression
levels were significantly decreased compared with those in the
negative control groups (5,6). Therefore, the expression levels of Th1
(IFN-γ) and Th2 (IL-4 and IL-13) cytokines were investigated to
explore ways to improve hepatic fibrosis in rats with siRNA
treatment under similar experimental conditions.
Materials and methods
Materials and study design
The hepatic tissue was obtained from our previous
studies. Healthy male Sprague-Dawley rats (6-weeks-old) weighing
160-210 g were provided by the Laboratory Animal Center of
Chongqing Medical University. A total of 60 rats were randomly
divided into six groups (10 rats per group): Normal, model,
negative control siRNA, TGF-β1 siRNA, TIMP-1 siRNA and TIMP-2 siRNA
groups. The rat fibrosis model was established using
CCl4 combined with a high fat and high cholesterol diet.
Except for the normal group, all the rats were subcutaneously
injected with 40% CCl4 (ratio, CCl4: Liquid
paraffin; 2:3). The first dose was 3 ml/kg, followed by a dose of 2
ml/kg injected twice a week for 12 weeks. In the second week, the
treatment groups and the negative control group received a 0.25
mg/kg dose of the corresponding viral plasmid which was constructed
from previous experiments (7). The
injection volume was 5 ml/kg, the normal and model groups received
the same volume of a 0.9% sodium chloride solution. All groups
received these injections via the tail vein twice a week for 12
weeks. The normal group was administered a normal diet, while the
other groups were fed with a diet high in fat and cholesterol. All
rats had free access to food and water ad libitum. All rats
were kept at 25˚C under 12 h dark/light cycles inside the
air-conditioned room with ~70% humidity. All rats were sacrificed
after 12 weeks. The liver tissues from the middle left lobe of each
liver were stored in 4% formalin and embedded in paraffin; a
portion of the liver tissue samples were stored in liquid nitrogen
in a -80˚C refrigerator for further analysis. All animal
experimental operations were in accordance with Chongqing
Management Approach of Laboratory Animals (Chongqing Government
order no. 195). The animals from the present study received humane
care that was approved by the Institutional Animal Care and Ethics
Committee of the Second Affiliated Hospital of Chongqing Medical
University (Chongqing, China). Additionally, our previous study
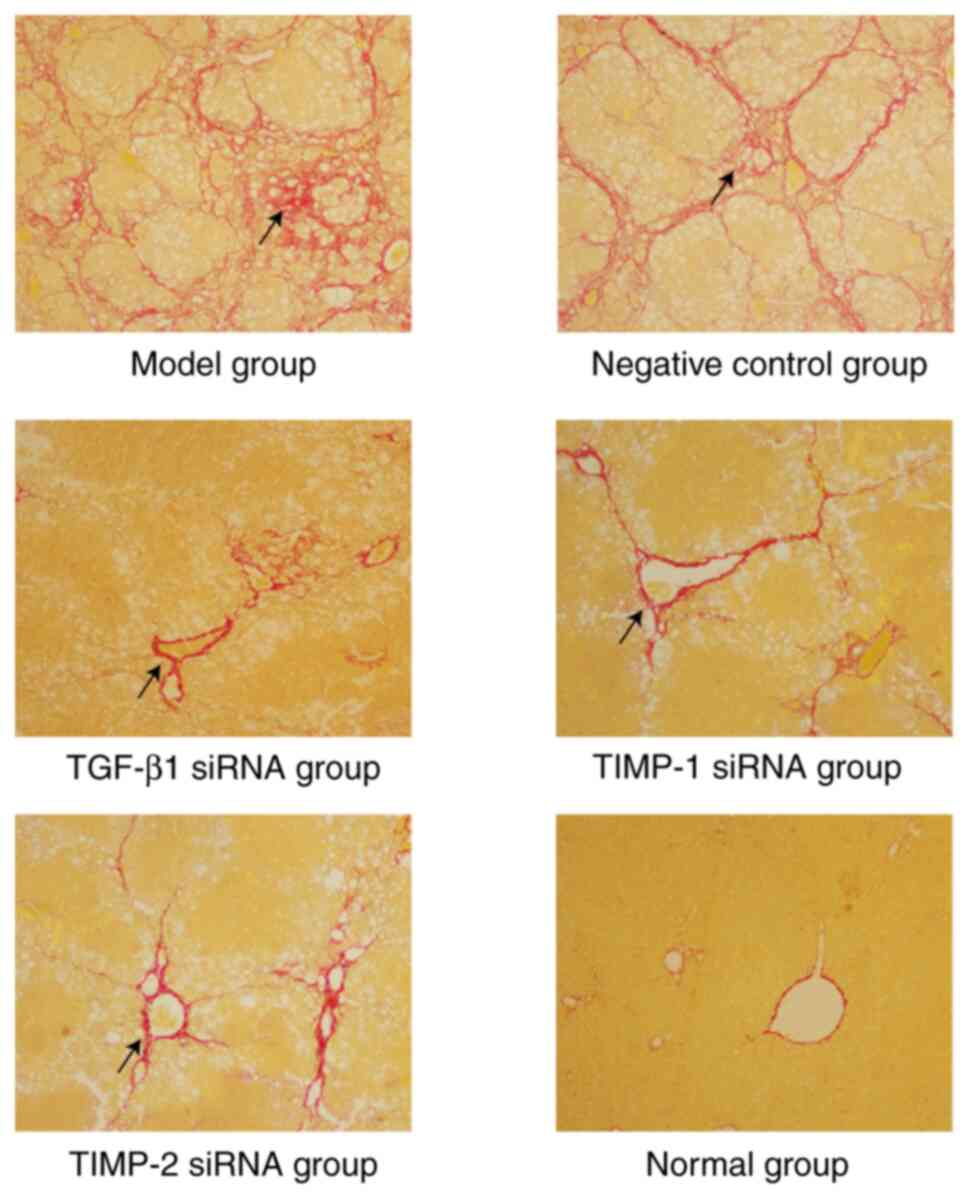
demonstrated the results of a comprehensive analysis of H&E and
Sirius red staining as follows: i) In the normal group, the
structure of the hepatic lobule was normal and fibrosis was grade
0; ii) in the model group and the negative control group, a typical
pseudo-lobular structure was formed and fibrosis was grade V-VI;
iii) compared with the model group and negative control group, the
damage of hepatic lobules in the treatment group was significantly
reduced, and the majority of the pathological grades were II-VI
(Fig. 1) (5).
Immunohistochemical staining
Paraffin-embedded sections were deparaffinized with
xylene, sliced to a thickness of 3-5 µm, and dehydrated in
decreasing concentrations of alcohol. The tissue sections were
incubated with 3% hydrogen peroxide for 5-10 min to block
endogenous peroxidase activity and 5-10% normal goat serum (OriGene
Technologies, Inc.) was used to block non-specific binding sites at
room temperature for 20 min. The sections were incubated at 4˚C
overnight with rabbit polyclonal antibodies against bioactive IFN-γ
(1:100; cat. no. bs-0480R, BIOSS), IL-4 (1:200; cat. no. bs-0581R,
BIOSS) and IL-13 (1:200; cat. no. bs-0560R, BIOSS). Unbound
antibody was washed off with PBS, and the slides were incubated
with the secondary antibody biotin-labeled sheep anti-rabbit IgG
(1:100; cat. no. SA1096; Wuhan Boster Biological Technology, Ltd.)
at 37˚C for 20 min. The antigen-antibody complexes were detected by
the Type I SABC immunohistochemical kit (cat. no. SA1094, Wuhan
Boster Biological Technology, Ltd.) for immunostaining
visualization. All the above procedures were performed in
accordance with the manufacturer's protocol. The staining
intensities of IFN-γ, IL-4 and IL-13 were quantified using
Image-Pro Plus software 6.0 (Media Cybernetics, Inc.).
Western blotting
Frozen hepatic tissues were mixed well with ice-cold
buffer (50 mM pH 8.0 Tris; 5 mM EDTA; 150 mM NaCl; 0.5% Nonidet
P-40; 100 mM PMSF; 1 mg/ml leupeptin; 1 mg/ml aprotinin; and 1 M
DTT) for 30 min on ice, the samples were centrifuged at 12,000 x g
at 4˚C for 5 min and the supernatant was collected. Protein
concentration was determined using the BCA method. Subsequently, 50
µg of total protein per lane was resolved on 15% SDS-PAGE and
transferred to PVDF membranes (MilliporeSigma), which were blocked
with 5% BSA (Beijing Solarbio Science & Technology Co., Ltd.)
at room temperature for 1 h while shaking. The membranes were
incubated overnight at 4˚C with primary antibodies [IFN-γ (1:100;
cat. no. bs-0480R, BIOSS), IL-4 (1:200; cat. no. bs-0581R, BIOSS),
IL-13 (1:200; cat. no. bs-0560R, BIOSS) and β-actin (1:400; cat.
no. BM387; Wuhan Boster Biological Technology, Ltd.)] while
shaking, washed three times with 0.05% TBS-Tween-20 and then
incubated with HRP-conjugated goat anti-rabbit secondary antibody
(1:2,000; cat. no. BM2006; Wuhan Boster Biological Technology,
Ltd.) for 1 h with shaking at room temperature. Finally,
chemiluminescence (DAB kit; cat. no. SA2025; Wuhan Boster
Biological Technology, Ltd.) was used to detect the expression
levels of IFN-γ, IL-4, IL-13 and β-actin. The intensity of the
protein bands was measured using Bandscan version 5.0 (Bio-Rad
Laboratories, Inc.) and β-actin was used as the internal
control.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated with a high purity total RNA
rapid extraction kit (cat. no. RP1202; BioTeke Corporation), and
then the concentration was determined with electrophoresis and a UV
spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific,
Inc.). Total RNA was converted into cDNA at 37˚C for 15 min
followed by 85˚C for 5 sec using the ExScript™ RT reagent kit (cat.
no. RR037B; Takara Biotechnology Co., Ltd.). RT-qPCR was performed
on an ABI Prism 7300 PCR instrument (Applied Biosystems; Thermo
Fisher Scientific, Inc.) using SYBR® Green as the
detection fluorophore. Each 20 µl reaction mixture contained 2 µl
cDNA, 10 µl 2x SYBR® Premix Ex Taq (Takara Biotechnology
Co., Ltd.), 1.6 µl of 10 µmol/µl forward and reverse primers and
ddH2O to final volume. Optimization was performed for each
gene-specific primer prior to the experiment to confirm that the
primer concentrations and reaction conditions did not produce
artificial amplification signals. Primers were designed using the
IFN-γ, IL-4 and IL-13 sequences provided by GenBank (https://www.ncbi.nlm.nih.gov/genbank/),
and the endogenous GAPDH gene was used as a control. The sequences
of the primers were as follows: IFN-γ forward,
5'-GCGTCCCAAGAAGCAGAATGA-3' and reverse,
5'-TCCGTGTGGACGAATCATCA-3'; IL-4 forward,
5'-ACTCCATGCACCGAGATGTTT-3' and reverse,
5'-CTGGAAGCCCTGCAGATGAG-3'; IL-13 forward,
5'-CTGAGCAACATCACACAAG-3' and reverse, 5'-GGTTACAGAGGCCATTCAAT-3';
and GAPDH forward, 5'-TGATTCTACCCACGGCAAGTT-3' and reverse,
5'-TGATGGGTTTCCCATTGATGA-3'. A standard two-step PCR amplification
procedure was used. The PCR parameters were as follows: 95˚C for 10
sec, followed by 40 cycles of 5 sec at 95˚C and 31 sec at 60˚C.
RT-qPCR was performed at least three times per gene, with a
no-template control as a negative control. The relative mRNA levels
of the target genes were calculated using the 2-ΔΔCq
method (8).
Statistical analysis
Each experiment was repeated at least three times,
and the experimental data are presented as the mean ± standard
deviation. Statistical analysis was performed using SPSS (version
13.0; SPSS, Inc.). Differences between groups were analyzed using
one-way ANOVA followed by Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Immunohistochemical characteristics of
each group of rats
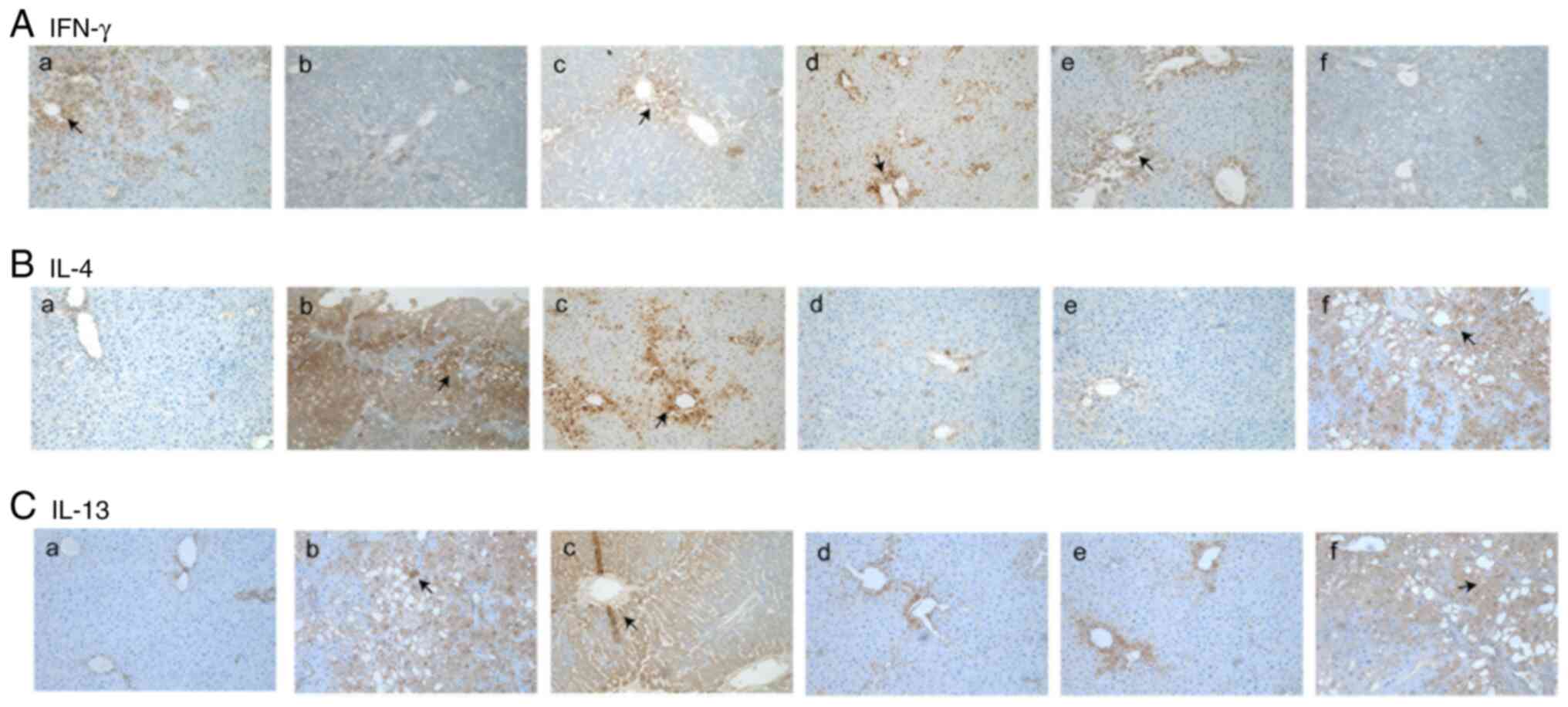
The expression level of IFN-γ in the normal group
was mainly localized in the portal area and the area surrounding
the central vein. Compared with that of the normal group, the
expression level of IFN-γ in the model group was significantly
decreased (Fig. 2A-a and b; Table
I). The expression levels of IFN-γ in the TGF-β1, TIMP-1 and
TIMP-2 siRNA treatment groups were significantly increased compared
with that in the model group (Fig.
2A-b-e). In addition, there was no notable difference in the
expression levels of IFN-γ between the negative control group and
the model group (Fig. 2A-b and
f; Table I).
 | Table IExpression of IFN-γ, IL-4 and IL-13
detected via immunohistochemistry. |
Table I
Expression of IFN-γ, IL-4 and IL-13
detected via immunohistochemistry.
| Cytokine | Normal group | Model group | TGF-β1 siRNA
treatment group | TIMP-1 siRNA
treatment group | TIMP-2 siRNA
treatment group | Negative control
group |
|---|
| IFN-γ | 13.035±1.517 |
2.931±0.903a |
8.178±0.562b |
9.289±1.026b |
9.534±0.627b |
2.752±0.045a |
| IL-4 | 5.482±1.313 |
16.737±2.011a |
14.787±1.068c |
7.486±0.448b |
7.513±1.152b |
15.260±1.205a |
| IL-13 | 4.457±0.895 |
15.531±2.066a |
15.036±0.447c |
8.401±0.679b |
8.292±0.847b |
15.586±0.885a |
The normal group presented low expression levels of
IL-4 and IL-13 (brown particles in the cytoplasm; Fig. 2B-a and C-a). These cytokines were mainly
concentrated in the area surrounding the central vein and in the
portal area. The expression levels of IL-4 and IL-13 in the model
group were significantly increased compared with those of the
normal group, as indicated by immunohistochemical staining
(Fig. 2B-b and C-b; Table
I). It was observed that the expression levels of IL-4 and
IL-13 in the TGF-β1 siRNA treatment group were similar to those in
the model group (Fig. 2B-c and
C-c); however, compared with the
model and negative control group, the expression levels of IL-4 and
IL-13 in the TIMP-1 and TIMP-2 siRNA treatment groups were
significantly decreased (Fig. 2B
and C-d and e; Table
I).
Expression levels of IFN-γ, IL-4 and
IL-13 as determined by western blotting
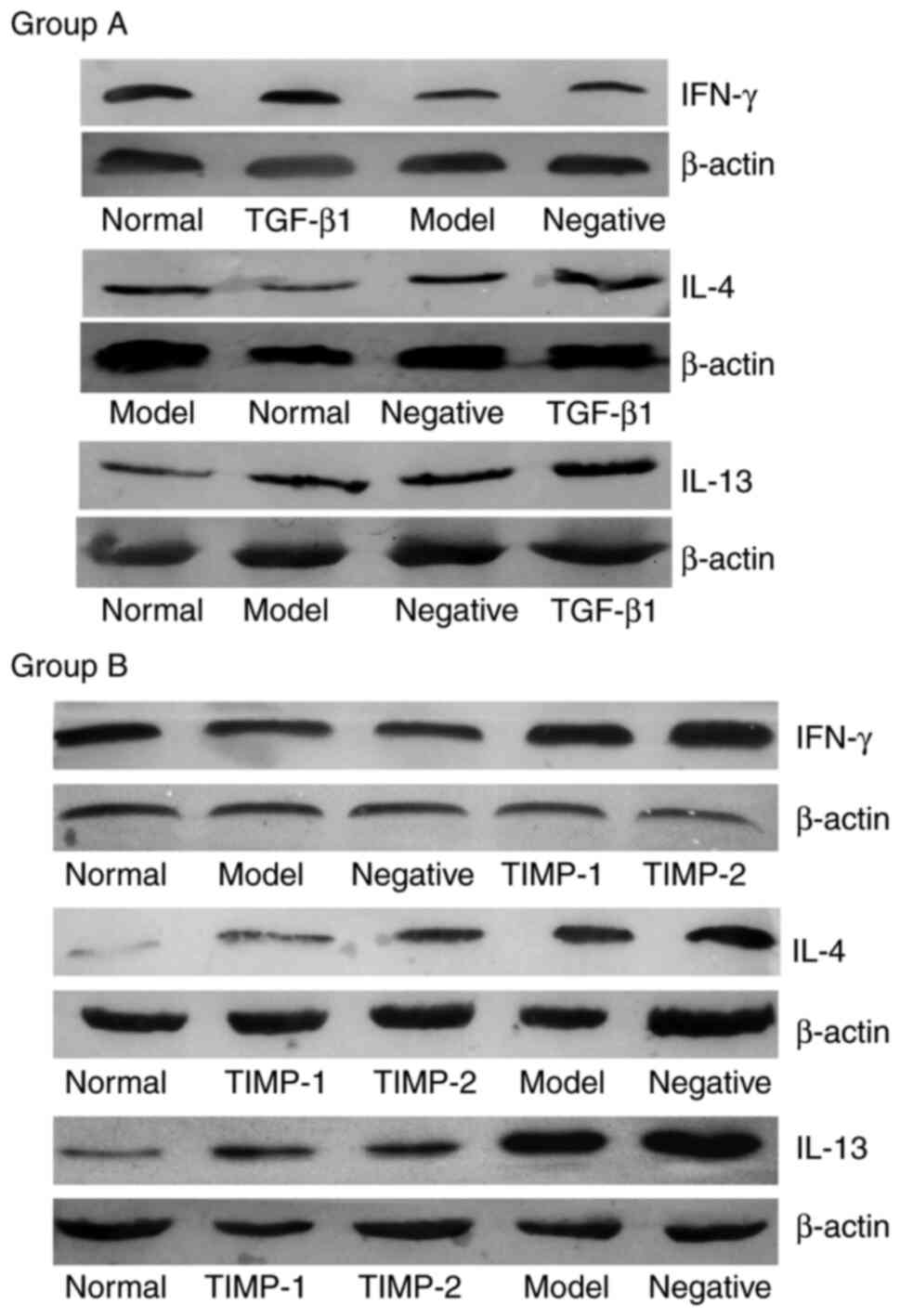
Western blotting was used to examine the protein
expression levels in each group. Analysis of the gray values of the
bands revealed that the β-actin bands were uniform, but the
densities of the target gene bands were different (Fig. 3). Compared with those of the normal
group, the expression level of IFN-γ in the model group was
decreased, but the levels of IL-4 and IL-13 were increased. This
effect was the same in the TGF-β1 siRNA treatment group (group A)
and the TIMP-1 and TIMP-2 siRNA treatment groups (group B). In the
TGF-β1, TIMP-1 and TIMP-2 siRNA treatment groups, the expression
level of IFN-γ was significantly increased compared with that of
the model group (Table II). The
expression levels of IL-4 and IL-13 in the TGF-β1 siRNA treatment
group were similar to those in the model group, while the
expression levels of IL-4 and IL-13 in the TIMP-1 and TIMP-2 siRNA
treatment groups were significantly decreased compared with those
of the model group. There was no difference in the expression
levels of IFN-γ, IL-4 and IL-13 between the negative control group
and the model group in either group A or B (Fig. 3; Table
II).
 | Table IIExpression levels of IFN-γ, IL-4 and
IL-13 detected via western blotting. |
Table II
Expression levels of IFN-γ, IL-4 and
IL-13 detected via western blotting.
| Cytokine | Normal group | Model group | TGF-β1
siRNA treatment group | TIMP-1 siRNA
treatment group | TIMP-2 siRNA
treatment group | Negative control
group |
|---|
| IFN-γ | 0.835±0.189 |
0.562±0.133a |
0.746±0.091b |
0.785±0.024b |
0.853±0.027b |
0.410±0.016a |
| IL-4 | 0.354±0.018 |
0.871±0.003a |
0.798±0.020c |
0.390±0.018b |
0.473±0.120b |
0.798±0.090a |
| IL-13 | 0.461±0.128 |
0.923±0.156a |
0.819±0.343c |
0.392±0.039b |
0.426±0.007b |
0.819±0.091a |
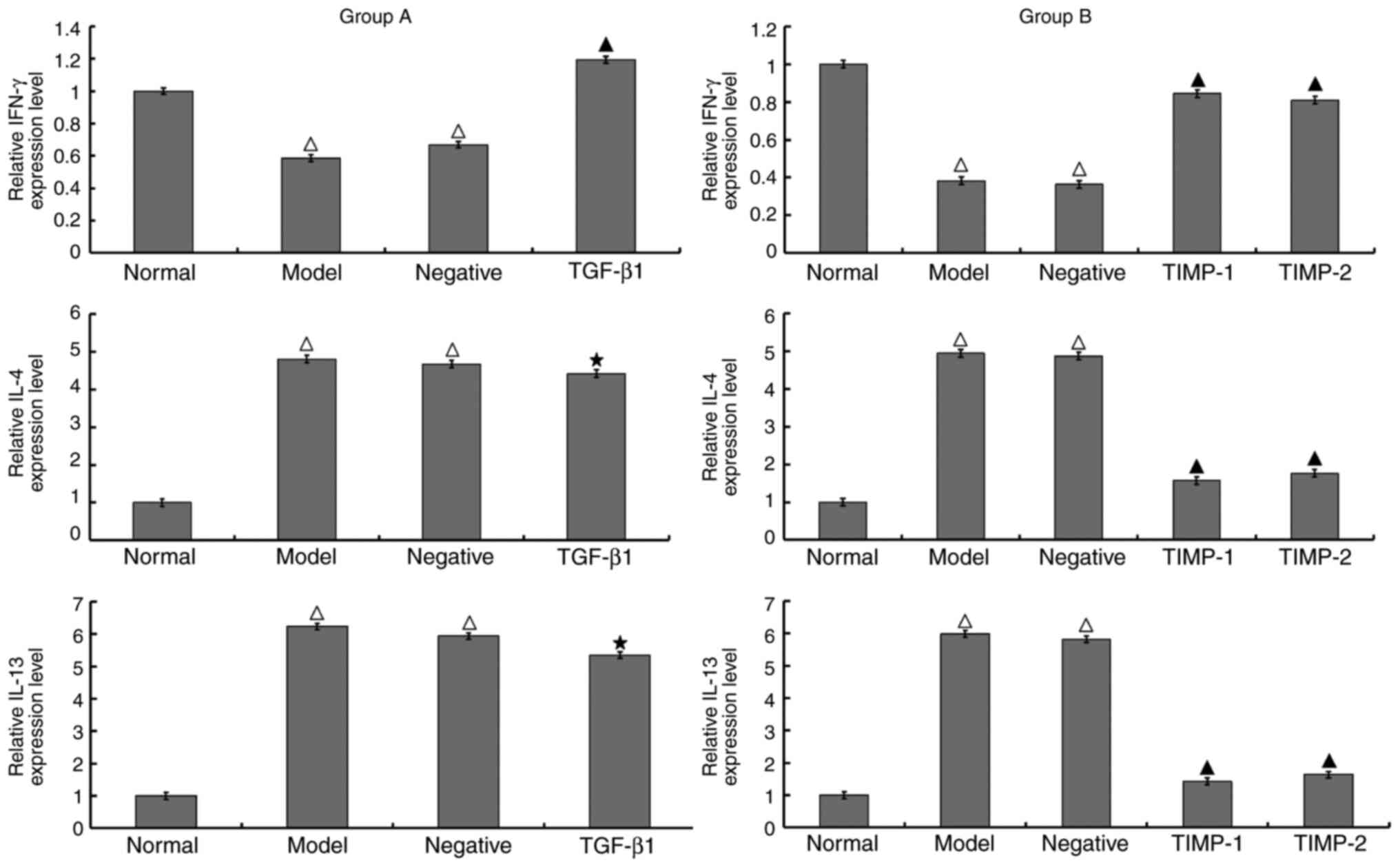
Expression levels of IFN-γ, IL-4 and
IL-13 detected using RT-qPCR
Compared with those of the normal group, the
expression levels of IL-4 and IL-13 were higher and the level of
IFN-γ was lower in the model group. Group A and group B exhibited
the same results. It was observed that in the TGF-β1 siRNA
treatment group (group A), the expression levels of IL-4 and IL-13
were similar to that in the model group; by contrast, the
expression of IFN-γ was significantly increased. In the TIMP-1 and
TIMP-2 siRNA treatment groups (group B), the expression levels of
IL-4 and IL-13 were significantly decreased compared with those in
the model group, and the expression of IFN-γ was significantly
increased. There was no significant difference in the expression
levels of IFN-γ, IL-4 and IL-13 between the negative control group
and the model group of either group A or B (Fig. 4).
Discussion
Hepatic fibrosis is a pathological process, through
which numerous types of chronic liver diseases, including chronic
viral hepatitis, alcoholic hepatitis and drug induced hepatitis
eventually develop into cirrhosis. Hepatic fibrosis causes lobular
alterations and the formation of false lobules and nodules
(1). Hepatic fibrosis can develop
into cirrhosis via various mechanisms, causing serious adverse
consequences, such as portal hypertension and liver failure
(9). Research on the mechanism of
hepatic fibrosis is being currently conducted; increasing
experimental and clinical evidence suggests that cytokines serve an
important role in the regeneration of liver cells, the activation
of hepatic stellate cells and the synthesis and degradation of ECM
(2,3,6). Among
them, the role of Th1/Th2 immune imbalance in hepatic fibrosis is
also becoming increasingly investigated (10).
IFN-γ is an important Th1 factor, as it promotes the
differentiation of Th1 cells and inhibits the differentiation of
Th2 cells (11). IFN-γ can inhibit
the activation and proliferation of myofibroblasts and promote
their apoptosis, thereby suppressing the production of ECM
(12). In addition, IFN-γ can
promote Th1-type and inhibit Th2-type cytokine responses, thereby
inhibiting the proliferation of fibroblasts and the deposition of
ECM (13). The results of a
previous study have demonstrated that IFN-γ also promotes the
apoptosis and directly inhibits the activation of HSCs, thereby
reducing the synthesis and secretion of collagen and other ECM
factors (14). Li et al
(15) used the Th1 cytokine IFN-γ
to treat rats with hepatic fibrosis, which was induced via
thioacetamide injection, and indicated that collagen deposition in
the liver was significantly reduced after IFN-γ treatment.
IL-4 is an important Th2 factor, and it can promote
the activation of B lymphocytes and the differentiation of
CD4+ T cells into Th2 cells (16). Atsukawa et al (17) demonstrated that IL-4 could induce
liver Kupffer cells into multinucleated giant cells and stimulate
the proliferation of HSCs. In vitro experiments by Bergeron
et al (18) indicated that
IL-4 could promote the expression of type I collagen and reduce the
expression of matrix metalloproteinase 2, leading to hepatic
fibrosis. IL-13 is mainly expressed by Th2 cells and is a potent
profibrotic factor. Weng et al (19) confirmed that the cytokines IL-4 and
IL-13 are important drivers of hepatic fibrosis progression, and
IL-13 and IL-4 share a common IL-4 receptor subunit α-STAT6 pathway
and exhibit similar biological functions. Coutinho et al
(20) revealed that IL4, IL-5 and
IL-13 serve important independent roles in liver cirrhosis caused
by schistosomiasis, and suggested that the reason that these
patients were predominantly male may also be associated with Th2
cytokines. IL-4 and IL-13 stimulate collagen synthesis via the
TGF-β1-Smad3 pathway, as it has been indicated that IL-4 and IL-13
can increase the expression of matrix metalloproteinases, separate
the latency-associated peptide-TGF-β1 complex (inactive TGF-β1) and
activate TGF-β1 indirectly (21).
IL4 and IL13 can also independently stimulate the synthesis of
collagen, and IL-4 receptor was found in numerous subtypes of mice
and human fibroblasts (22). In
vitro studies have indicated that IL-4 and IL-13 can stimulate
ECM synthesis from fibroblasts, such as collagen type I, collagen
type III and fibrinogen (23,24).
The results of the present study suggested that the
expression levels of IL-4 and IL-13 in rats with hepatic fibrosis
induced by CCl4 were significantly increased compared
with those in normal rats, and the expression of IFN-γ was
decreased. This finding is consistent with the results of previous
studies, such as in hepatic fibrosis induced by schistosomiasis
(11,20). Treatment with TGF-β1 siRNA reduced
the degree of hepatic fibrosis, but the expression levels of IL-4
and IL-13 in the liver were not significantly reduced compared with
those of the model group. To hypothesize, the first possible reason
may be that IL-4, IL-13 and TGF-β1 are effective anti-inflammatory
factors. When rats with hepatic fibrosis were injected with TGF-β1
siRNA to block or reduce the expression of TGF-β1, rat liver was
still subjected to damage by CCl4, suggesting that
hepatic inflammation was persistent. To counteract persistent
inflammation, the body may secrete increased levels of compensatory
IL-4 and IL-13. To further hypothesize, the second possible
explanation may be that as the TGF-β1-Smad3 pathway is one of the
mechanisms via which IL-4 and IL-13 stimulate collagen synthesis,
when the expression of TGF-β1 is interfered with, the body may
express more IL-4 and IL-13 due to a feedback mechanism. Treatment
with TIMP-1 siRNA and TIMP-2 siRNA reduced the degree of hepatic
fibrosis and markedly reduced the expression levels of IL-4 and
IL-13 in the liver compared with those of the model group. Although
TGF-β1, TIMP-1 and TIMP-2 are important targets for antifibrotic
treatment, it is hypothesized that TGF-β1 was not an ideal
antifibrotic target, as indicated by the expression of Th2
cytokines. By contrast, TIMP-1 and TIMP-2 were more suitable
targets for treating hepatic fibrosis. Notably, the alterations in
the aforementioned inflammatory factors indicated that Kupffer
cells are a key component of liver fibrosis, which is worthy of
further study. In addition, to the best of our knowledge, there are
no drugs targeting TGF-β1, TIMP-1 or TIMP-2 in the treatment of
liver fibrosis, and the therapeutic effect of antifibrotic drugs
may not be ideal. The treatment of liver fibrosis is still a key
problem to be solved in the clinical practice. Therefore, the
current study can provide novel insights for the development of
antifibrotic drugs.
Acknowledgements
Not applicable.
Funding
Funding: The present study was sponsored by a grant from The
National Natural Science Foundation of China (grant no.
81270503).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YX and KQ were responsible for the conception of
data, data collection and drafting the manuscript. YX, KQ, YS, LX
and XS all participated in the analysis and interpretation of data.
In addition, XS also supervised and funded this study, made
substantial contributions to conception and design and reviewed and
modified the manuscript. YX and KQ confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All animals received humane care that was approved
by the Institutional Animal Care and Ethic Committee the Second
Affiliated Hospital of Chongqing Medical University (Chongqing,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Campana L and Iredale JP: Regression of
liver fibrosis. Semin Liver Dis. 37:1–10. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tsuchida T and Friedman SL: Mechanisms of
hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol.
14:397–411. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Xing ZZ, Huang LY, Wu CR, You H, Ma H and
Jia JD: Activated rat hepatic stellate cells influence Th1/Th2
profile in vitro. World J Gastroenterol. 21:7165–7171.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Schumacher JD and Guo GL: Regulation of
hepatic stellate cells and fibrogenesis by fibroblast growth
factors. Biomed Res Int. 2016(8323747)2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lang Q, Liu Q, Xu N, Qian KL, Qi JH, Sun
YC, Xiao L and Shi XF: The antifibrotic effects of TGF-β1 siRNA on
hepatic fibrosis in rats. Biochem Biophys Res Commun. 409:448–453.
2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cheng X, Zhang M, Xue Y, Sun H, Liu Q and
Shi XF: Effect of tissue inhibitor of metalloproteinase-1 and 2
siRNA on the expression of smad2/3/4 protein in hepatic stellate
cells. Zhonghua Gan Zang Bing Za Zhi. 28:753–759. 2020.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
7
|
Qian KL, Xu N, Lang Q, Qi JH, Sun YC, Xiao
L, Liu Q and Shi XF: Construction and identification of siRNA
eukaryotic expression vectors targeting on TGFβ1, TIMP-1 and TIMP-2
genes in vitro. Zhonghua Gan Zang Bing Za Zhi. 19:291–296.
2011.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
8
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rockey DC, Bell PD and Hill JA: Fibrosis-a
common pathway to organ injury and failure. N Engl J Med.
372:1138–1149. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Weiskirchen R, Weiskirchen S and Tacke F:
Organ and tissue fibrosis: Molecular signals, cellular mechanisms
and translational implications. Mol Aspects Med. 65:2–15.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liu C, Zhang YS, Chen F, Wu XY, Zhang BB,
Wu ZD and Lei JX: Immunopathology in schistosomiasis is regulated
by TLR2, 4- and IFN-γ-activated MSC through modulating Th1/Th2
responses. Stem Cell Res Ther. 11(217)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Euler T, Valesky EM, Meissner M, Hrgovic
I, Kaufmann R, Kippenberger S and Zöller NN: Normal and keloid
fibroblasts are differentially influenced by IFN-γ and
triamcinolone as well as by their combination. Wound Repair Regen.
27:450–461. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kannan AK, Sahu N, Mohanan S, Mohinta S
and August A: IL-2-inducible T-cell kinase modulates TH2-mediated
allergic airway inflammation by suppressing IFN-γ in naive CD4+ T
cells. J Allergy Clin Immunol. 132:811–820.e1-e5. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Oh JE, Shim KY, Lee JI, Choi SI, Baik SK
and Eom YW: 1-Methyl-L-tryptophan promotes the apoptosis of hepatic
stellate cells arrested by interferon-γ by increasing the
expression of IFN-γRβ, IRF-1 and FAS. Int J Mol Med. 40:576–582.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li F, Li QH, Wang JY, Zhan CY, Xie C and
Lu WY: Effects of interferon-gamma liposomes targeted to
platelet-derived growth factor receptor-beta on hepatic fibrosis in
rats. J Control Release. 159:261–270. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cortes-Selva D, Ready A, Gibbs L, Rajwa B
and Fairfax KC: IL-4 promotes stromal cell expansion and is
critical for development of a type-2, but not a type 1 immune
response. Eur J Immunol. 49:428–442. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Atsukawa K, Saito H, Tsukada N, Akiba Y,
Toda K, Kumagai N, Ohishi T, Kamegaya Y and Ishii H: Th1 and Th2
cytokines differentially regulate the transformation of Kupffer
cells into multinucleated giant cells but similarly enhance the
Kupffer cell-induced hepatic stellate cell proliferation. Hepatol
Res. 20:193–206. 2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bergeron C, Pagé N, Barbeau B and Chakir
J: Interleukin-4 promotes airway remodeling in asthma: Regulation
of procollagen I (alpha1) gene by interleukin-4. Chest. 123 (Suppl
3)(424S)2003.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Weng SY, Wang X, Vijayan S, Tang Y, Kim
YO, Padberg K, Regen T, Molokanova O, Chen T, Bopp T, et al: IL-4
Receptor alpha signaling through macrophages differentially
regulates liver fibrosis progression and reversal. EBioMedicine.
29:92–103. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Coutinho HM, Acosta LP, Wu HW, McGarvey
ST, Su L, Langdon GC, Jiz MA, Jarilla B, Olveda RM, Friedman JF and
Kurtis JD: Th2 cytokines are associated with persistent hepatic
fibrosis in human Schistosoma japonicum infection. J Infect Dis.
195:288–295. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Wynn TA: Fibrotic disease and the
T(H)1/T(H)2 paradigm. Nat Rev Immunol. 4:583–594. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Bhogal RK and Bona CA: Regulatory effect
of extracellular signal-regulated kinases (ERK) on type I collagen
synthesis in human dermal fibroblasts stimulated by IL-4 and IL-13.
Int Rev Immunol. 27:472–496. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yan J, Zhang Z, Yang J, Mitch WE and Wang
Y: JAK3/STAT6 Stimulates bone marrow-derived fibroblast activation
in renal fibrosis. J Am Soc Nephrol. 26:3060–3071. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sugimoto R, Enjoji M, Nakamuta M, Ohta S,
Kohjima M, Fukushima M, Kuniyoshi M, Arimura E, Morizono S, Kotoh K
and Nawata H: Effect of IL-4 and IL-13 on collagen production in
cultured LI90 human hepatic stellate cells. Liver Int. 25:420–428.
2005.PubMed/NCBI View Article : Google Scholar
|