Introduction
Sepsis is a very serious inflammatory response,
often occurring due to the host's inability to fight off viral or
bacteria infection, which leads to the loss of function and
eventual failures of various organs (1). Among the numerous organs that are
affected, lung tissue is often primarily affected, causing acute
lung injury (ALI). ALI presents at the beginning of sepsis with an
extremely high incidence rate (1).
ALI involves multiple inflammatory signaling pathways that are
gradually amplified. The cascade reaction of cytokines and
inflammatory mediators ultimately leads to the destruction of
pulmonary capillary endothelial cells and, in the most severe
cases, results in the dysfunction of these cells and increases
pulmonary capillary permeability, increasing the likelihood of
pulmonary edema, causing inflammatory exudation, and eventually
leading to acute respiratory distress syndrome (2). Toll-like receptors (TLRs) participate
in the innate immune and inflammatory responses of the body.
Previous studies have indicated that the knockout or targeted
inhibition of the TLR4 gene significantly reduces the severity of
ALI (3-5).
TLR4 transmits signals following recognition of pathogen-associated
molecular patterns, such as those produced by gram-negative
bacteria, including endotoxin E and Lipid A from lipopolysaccharide
(LPS) (6). LPS binds to TLR4 on the
surface of monocytes, macrophages, neutrophils and other immune
cells after entering the body. This signal is then transduced to
the intracellular domain [Toll/interleukin (IL)-1 receptor region],
which binds to IL-1, thus triggering the expression of nuclear
factor (NF)-κB and various inflammatory cytokines and aggravating
lung injury. The TLR4/NF-κB signaling axis influences the
transmission of inflammatory signals from the extracellular
environment to the cell membrane and cytoplasm and subsequently
enters the nucleus, thus modulating the expression of target genes
(7-9).
MicroRNAs (miRNAs/miRs) are extensively distributed
and belong to RNA fragments that do not encode proteins (10,11).
They participate in multiple physiological processes of the body
and bind to the 3'-untranslated region of target mRNAs (12,13).
Furthermore, it has been reported that miRNAs exert a regulatory
effect in inflammatory responses (14). As regulatory elements of the immune
system and immune responses, miRNAs have attracted an increased
amount of attention (14-16).
miR-223 in particular has a crucial role in innate immunity,
myeloid cell differentiation and cell homeostasis. Various targets
of miR-223 are involved in pathways implicated in the pathogenesis
of inflammation-related diseases (17). Previous studies have demonstrated
that expression levels of miR-223 were increased in blood samples
and inflamed lung samples (18,19).
It has also been reported that miRNA expression can be induced by
tumor necrosis factor-α, IL-1 and TLRs during the activation of
lymphocytes and monocytes (16).
The functions of miRNAs in endothelial cell activation,
pro-inflammatory cytokine expression and heat shock protein remain
elusive. However, to the best of our knowledge, there is currently
little research on miR-223 in rats with sepsis-induced lung injury.
Therefore, the aim of the present study was to reveal the
anti-inflammatory mechanism of miR-223 in septic rats, thereby
providing a novel method for the treatment of inflammatory
responses in lung tissues.
Materials and methods
Materials
A total of 40 specific pathogen free male
Sprague-Dawley rats (n=40; Hebei Province Animal Research Center;
age, 8 weeks; weight, 240-260 g) were used in the cuuremt study.
Rats were housed in a temperature-controlled room (21±2˚C) with a
relative humidity of 30-40% and a 12 h light/dark cycle. All rats
had free access to water and food. Mouse monocyte macrophage
RAW264.7 cells (American Type Culture Collection), ELISA kit for
cytokines (R&D Systems, Inc. cat. no. ML-Elisa-1458), phosphate
buffered saline (EMD Millipore), bicinchoninic acid (Beyotime
Institute of Biotechnology), antibodies (all 1:1,000; all from
Abcam), RNA reverse transcription kit (Roche Diagnostics GmbH),
hematoxylin and eosin (H&E) staining reagent (Beyotime
Institute of Biotechnology) and TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.).
Research objects and grouping
In the present study, SD rats were divided into a
sham group and a sepsis-induced lung injury (sepsis) group
according to whether cecal ligation and puncture was performed. The
RAW264.7 cells were stimulated with LPS and divided into the
following groups, blank, LPS, LPS + control and LPS + mimic. The
present study was approved by the Ethics Committee of the Fourth
Central Hospital of Baoding City (Hebei, China).
Establishment of the rat model of
sepsis-induced lung injury
Following anesthesia via intraperitoneal injection
of pentobarbital sodium (dose, 40 mg/kg), rats underwent laparotomy
via a linear abdominal incision. The cecum was punctured twice at
different sites with an 18-gauge needle and gently pressed until
the feces was squeezed out. The intestines were placed back in the
abdomen and the abdominal incision was subjected to layered
closure. Upon completion of surgery, saline (5 ml/100 g body
weight) was injected subcutaneously into the rats in the sepsis and
sham groups to replace the extracellular fluid that was isolated
during peritonitis. The rats in the sham group underwent a sham
surgery without ligation or puncture of the cecum.
Reverse transcription-quantitative PCR
(RT-qPCR)
Lung tissues and monocytes were homogenized in
TRIzol® reagent and total RNAs were extracted according
to the manufacturer's protocol. Subsequently, 3 µg RNAs were
reverse transcribed into complementary deoxyribose nucleic acids
(cDNAs) at 37˚C for 1 h using the RNA reverse transcription kit.
The PCR mixture contained 0.5 µl Taq polymerases, 1 µl of each
primer and 2 µl of each cDNA sample, with a final volume of 20 µl.
In all amplifications, three repeated wells were set and
quantitative changes in mRNA expression were evaluated by qPCR.
qPCR was subsequently performed using the SYBR-Green Master kit
(Roche Diagnostics). Quantitative analysis was performed using the
ABI 7500 fluorescence PCR amplification instrument (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The reaction system
volume was 25 µl. The thermocycling conditions for qPCR were as
follows: Pre-denaturation at 95˚C for 5 min, denaturation at 95˚
for 30 sec, annealing at 60˚ for 45 sec, extension at 72˚ for 3 min
for 35 cycles, and then a final extension at 72˚C for 5 min. PCR
products were stored at 4˚C. The following primer pairs were used
for qPCR: GAPDH: Forward, 5'-ACAGCAACAGGGTGGTGGAC-3' and reverse,
5'-TTTGAGGGTGCAGCGAACTT-3'; TLR4: Forward,
5'-AAGGCATGGCATGGCTTACAC-3' and reverse,
5'-GGCCAATTTTGTCTCCACAGC-3'; NF-κB: Forward,
5'-CCCAAACCTTGGCATCCTG-3' and reverse, 5'-CCGAACAACACTCAAATCC-3';
miR-223: Forward, 5'-UGUCAGUUUGUCAAAUACCCCAAAA-3' and reverse,
5'-UGUCAGUUUGUCAAAUACCCCAUUU-3'. Finally, Cq values were processed
using the 2-∆∆Cq method (20), with GAPDH serving as the
control.
Observation of pathological changes
following sepsis-induced lung injury via H&E staining
Pneumonectomy was performed 24 h after the rat model
was established in the two groups. The trachea was fixed at 25˚C
with 100% ethanol for 48 h at a distance of 20 mm from the lung.
Following fixation, the paraffin-embedded tissue sections were cut
into slices (thickness, ~5 µm) and histologically stained with
methylene blue at 25˚C for 5 min. The slices were stored at -65˚C
overnight prior to the experiment. At the beginning of the
experiment, the sections were deparaffinized in 65, 70 and 90%
xylene tanks at 25˚C, dehydrated in ethanol with five gradually
increasing concentrations (60, 75, 90, 95 and 98%), gently washed
with deionized water four times and dried in the air. The tissues
were subsequently spread on a sterile glass slide and placed above
an alcohol lamp for 15-30 sec to dry. Eosin Y dye solution was
added dropwise for 3 min for cell plasma staining. Following
cytoplasm staining, the dye solution was diluted with distilled
water to terminate the staining. The stained tissue samples were
cleaned with ethanol and added to the methylene blue dye solution
dropwise for cell nucleus staining. After 60 sec, staining was
ceased by diluting the dye solution with deionized water. A light
microscope (IX70; Olympus) was used to observe cells under five
randomly selected fields of view. Following staining, the lung was
independently evaluated and scored by two experts. For each rat,
the following characteristics of three different lobes were
examined: Interstitial edema, hemorrhage and neutrophil
infiltration.
Tissue protein extraction and western
blot analysis
Cervical dislocation (following anesthesia with
intraperitoneal injection of pentobarbital sodium at a dose of 40
mg/kg) was used as the method of euthanasia. The tissues
surrounding the lungs were dissected and stored in equal parts at
-80˚C and were thawed prior to use. The RIPA
(radioimmunoprecipitation assay) protein lysate (Beyotime Institute
of Biotechnology) was mixed with the tissues evenly, swirled three
times for 15 sec each time and centrifuged at 13,500 x g/min at 4˚C
for 40 min. Total protein concentration was calculated by
bicinchoninic acid (BCA) Protein Assay Kit (Beyotime Institute of
Biotechnology). The protein concentration was obtained according to
450 mm absorbance and the samples were boiled for denaturation.
Subsequently, the proteins were separated via SDS-PAGE and
transferred to a polyvinylidene fluoride membrane (EMD Millipore).
Then non-specific antigen sites were blocked with 5% bovine serum
albumin (Sigma-Aldrich; Merck KGaA) at 25˚C for 1 h and incubated
with TLR4 and NF-κB antibodies (1:1,000; cat. no. ab32536) and
GAPDH (1:1,000; cat. no. ab8245) at 4˚C for 14 h. Finally,
secondary antibodies (HRP-conjugated goat anti-rabbit IgG; 1:5,000;
cat. no. ab6721) were added at 25˚C for 1 h. Immunoreactive bands
were visualized using an enhanced chemiluminescence detection kit
(Amersham; Cytiva). The gray value was analyzed using ImageJ
software (version 1.38; National Institutes of Health).
Transfection of RAW264.7 cells
stimulated by LPS with miR-223 control and mimic
RAW264.7 cells were evenly spread in a six-well
plate with a total volume of 1 ml. LPS was added after 12-16 h of
culture at 37˚C and, after 24 h of stimulation, the stimulated
inflammatory cells were selected for seeding into 24-well plates at
1x105 cells/well. Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) was used to perform
the transfection. The cells were transfected with miR-223 mimic (20
µm) and miR-223 control (20 µm) when the confluency reached 70-80%.
The sequences were as follows (Dojindo Molecular Technologies,
Inc.): miR-223 mimic: Forward, 5'-CCUACGGAGUUACCAACCUGGC-3' and
reverse, 5'-AAGACUGGCCAGCAUUAUAGAC-3'; miR-223 control: Forward 5'-
GCCAGGACGUUCGAGACGUCAG-3' and reverse,
5'-GCAGCUGCGACGUUACCUUAGA-3'. The transfection reagent was added
into the cells for 12 h of culturing at 37˚C and then replaced with
a normal culture medium to observe the cell state after 48 h.
Detection of IL-6 and IL-1β levels
using an ELISA kit
The supernatant from the blank, LPS, LPS + control
and LPS + mimic groups was collected for analysis. Bronchoalveolar
lavage (BAL) fluid was collected from the blank, LPS, LPS + control
and LPS + mimic groups. The diluted standard substance was
defrosted and diluted according to the 50% ratio gradient. Then 90
µl of standard substance and horseradish peroxidase (HRP)-labeled
working solution were added to the standard well. ELISA plates
(cat. no. 201303; Lianyungang Jinma Biotech Co., Ltd.) were coated
with 100 µl/well of the diluted antigen for 1 h at 37˚C, after
which plates were washed three times with PBS containing 0.05%
Tween 20 and blocked with 200 µl 1% bovine serum albumin and 0.05%
Tween 20 diluted in PBS for 2 h at 37˚C. The plate was cleaned five
times and 90 µl HRP reaction substrate was added to each well in
the dark. After 20 min, the enzymatic reaction was terminated, the
optical density value was detected and the experimental data were
recorded.
Statistical analysis
Experimental results were obtained and analyzed
using Statistical Product and Service Solutions 17.0 software
(SPSS, Inc.). Differences between two groups were analyzed by using
a Student's t-test. Comparisons between three groups was performed
using one-way ANOVA followed by the Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
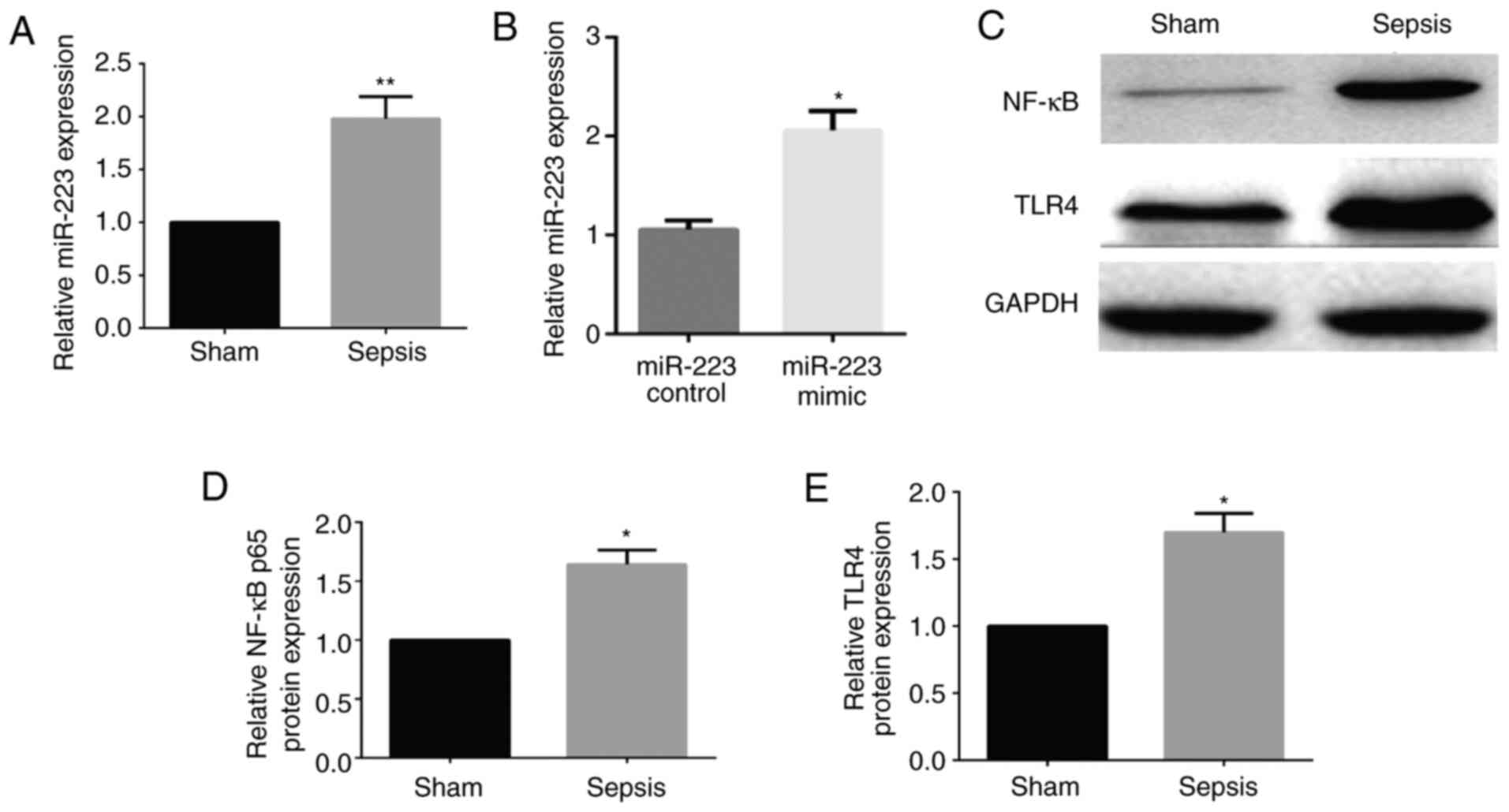
Expression levels of miR-223 and TLR4
and NF-κB proteins in rats with sepsis-induced lung injury
Compared with that in the sham group, the expression
levels of miR-223 and NF-κB and TLR4 proteins were significantly
higher than in the sepsis group (P<0.05), indicating that
miR-223 is associated with the TLR/NF-κB signaling pathway
(Fig. 1).
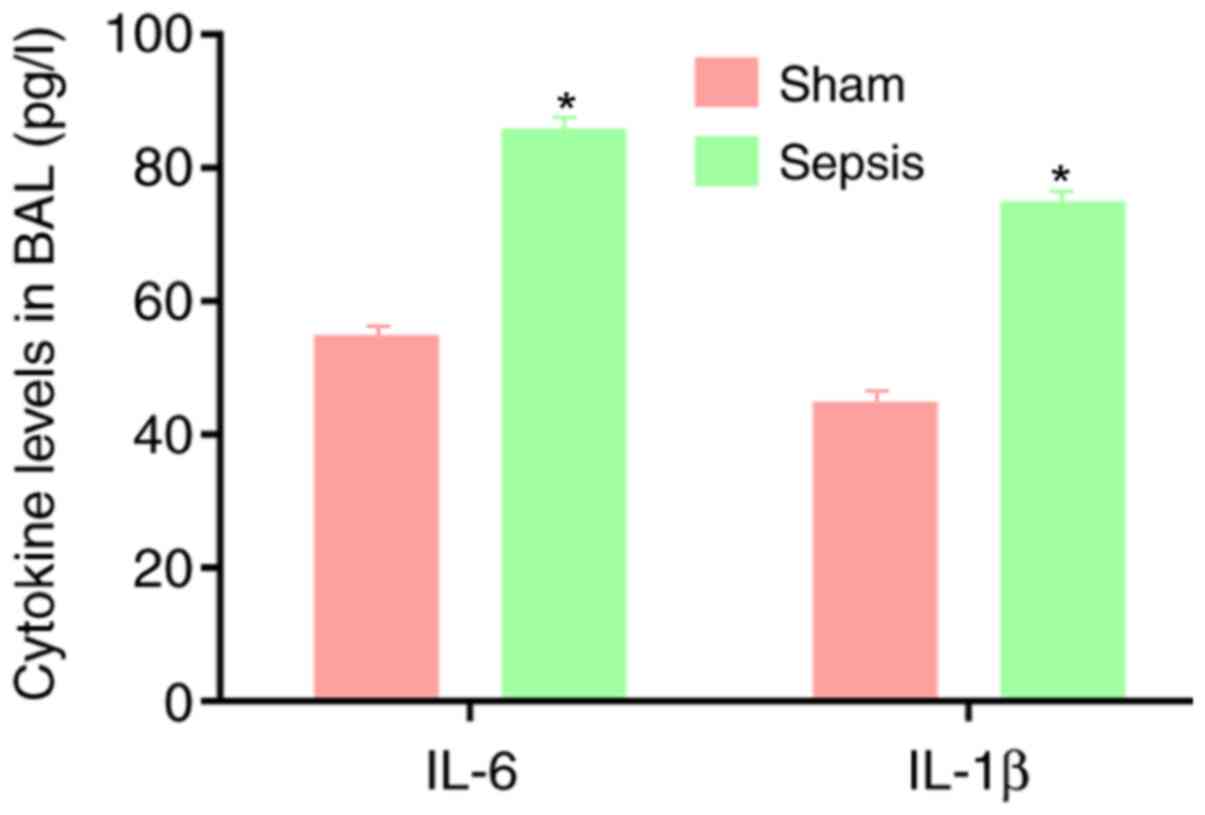
Levels of IL-6 and IL-1β in BAL
fluid
Compared with the sham group, the sepsis group
demonstrated significantly higher IL-6 and IL-1β content in the BAL
fluid, indicating a strong inflammatory response in the sepsis
group (P<0.05; Fig. 2).
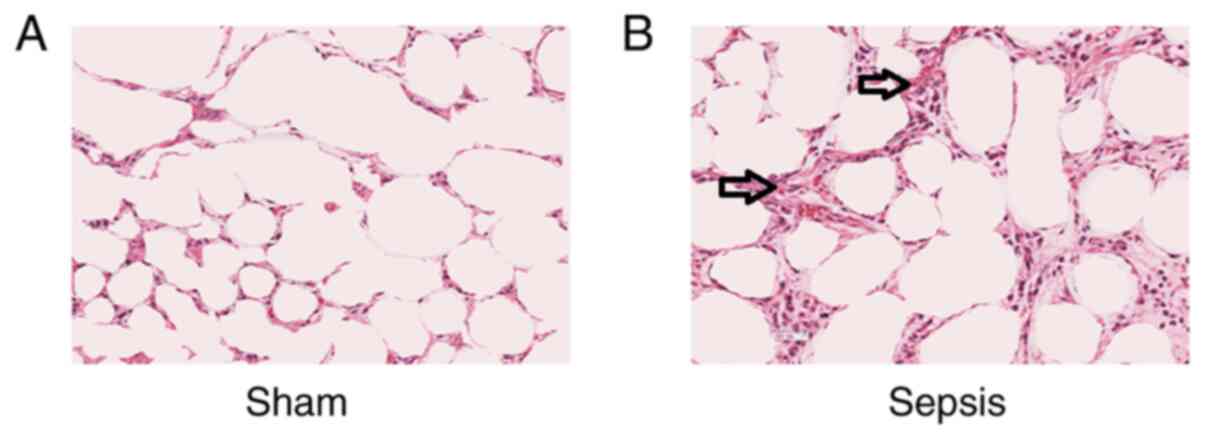
Pathological changes in the alveoli of
rats with sepsis-induced lung injury
H&E staining and microscopic observation of the
lung tissue from the sham group indicated alveoli with a normal
structure and few pathological changes. In the sepsis group, the
alveoli were observed to be surrounded by a large number of
neutrophils, the mesenchyme was swollen, part of the alveolar wall
exhibited fibrosis and the alveolar wall was thickened (Fig. 3), suggesting that the inflammatory
response of cells is enhanced following sepsis.
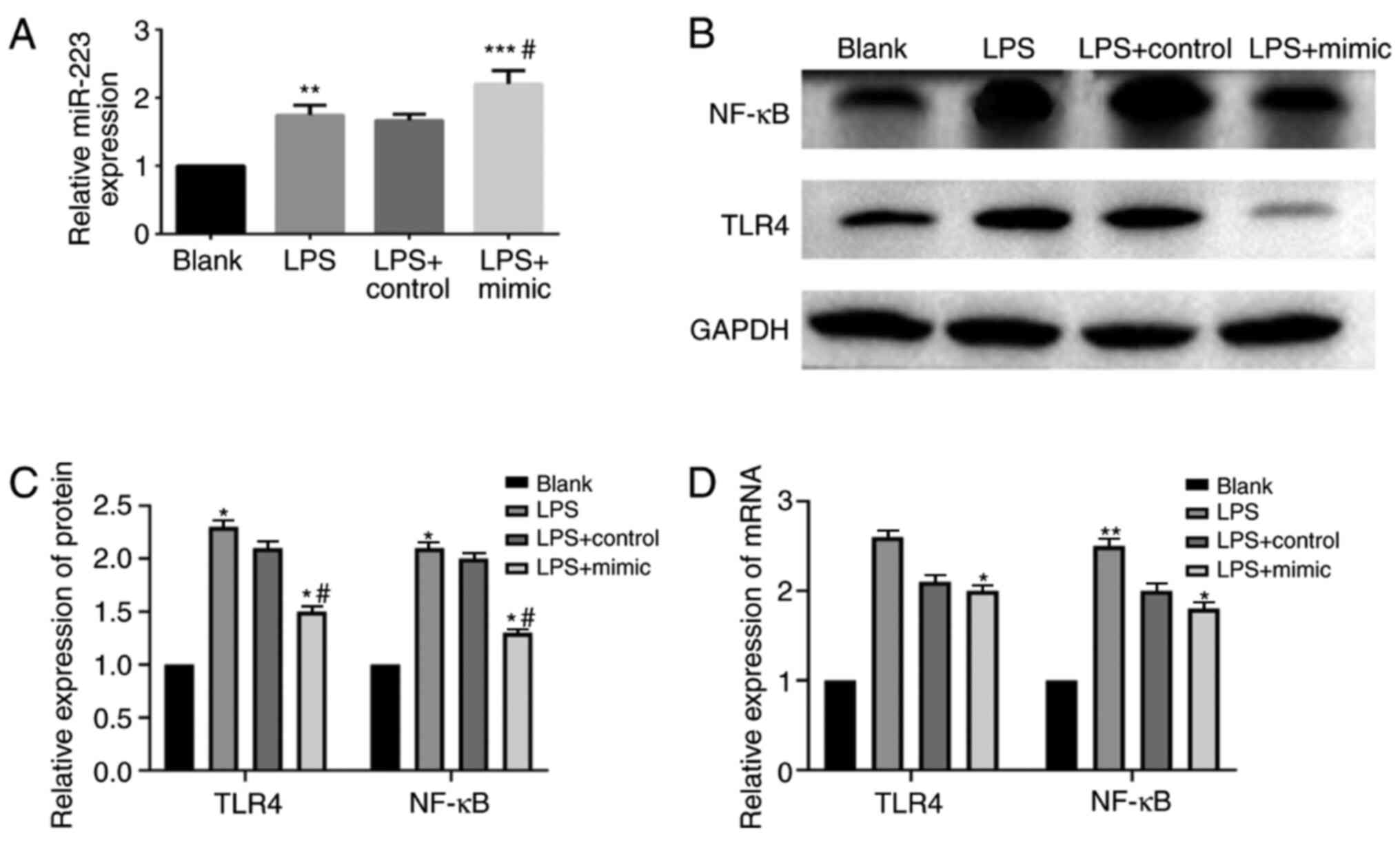
Changes in TLR4 and NF-κB expression
following RAW264.7 cell transfection with the miR-223 mimic and
control
Transfection was verified to be successful (Fig. 1B). Following LPS stimulation, the
expression levels of miR-223 and TLR4 and NF-κB proteins in the
cells of the LPS group were significantly increased when compared
with the blank group (P<0.05). When compared with the other
three groups, miR-223 expression in the LPS + mimic group was the
highest (Fig. 4A). The protein
expression levels of TLR4 and NF-κB in the LPS + mimic group were
significantly reduced when compared with the LPS group and the LPS
+ control group (P<0.05) (Fig.
4B and C). qPCR revealed that
the mRNA expression levels of TLR4 and NF-κB in the LPS + mimic
group were significantly higher when compared with those in the
blank group, but they were significantly decreased in the LPS +
mimic group when compared with the LPS group and the LPS + control
group (P<0.01; Fig. 4D). The
results indicate that the inflammatory responses weaken after
miR-223 is elevated and the anti-inflammatory effect of miR-223 is
related to the inhibition of the TLR4/NF-κB pathway.
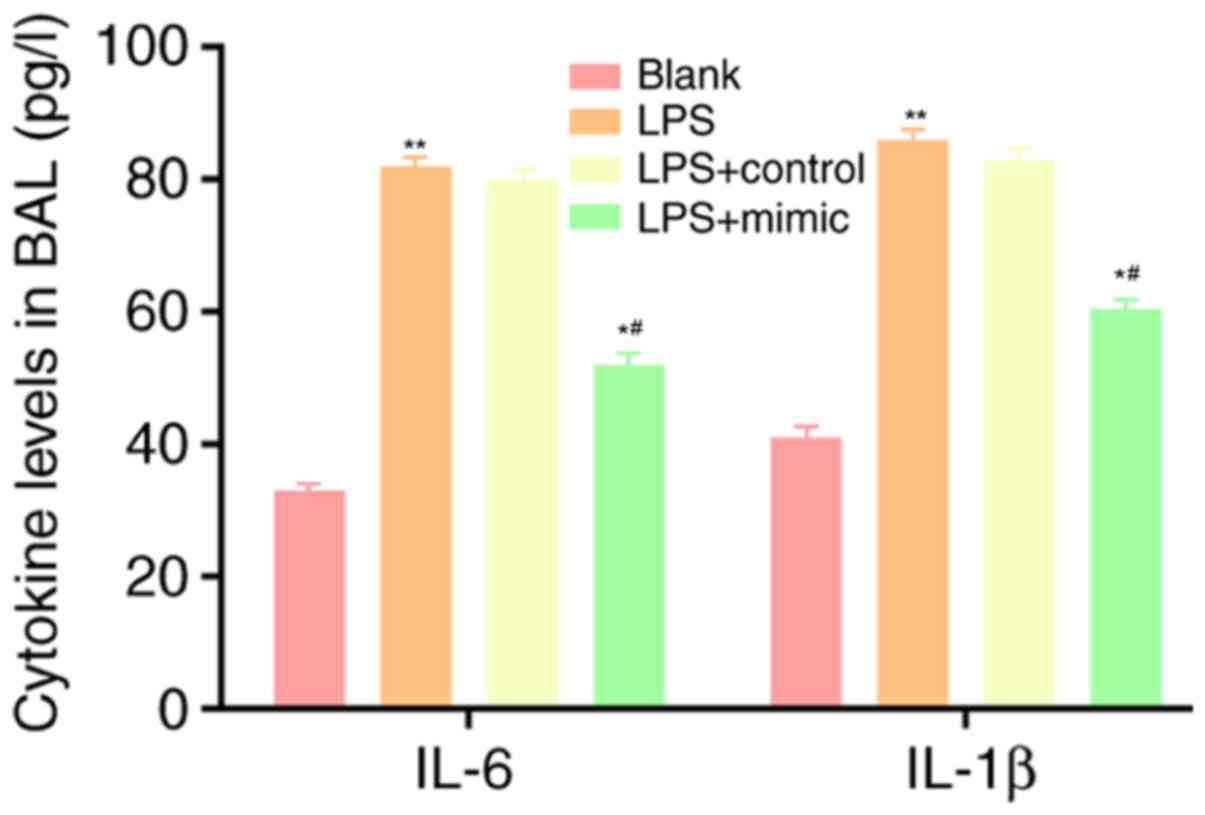
Changes in the content of IL-6 and
IL-1β in the blank, LPS, LPS + control and LPS + mimic groups
Following LPS stimulation, the content of IL-6 and
IL-1β in the cell supernatant increased significantly. Compared
with that in the LPS group and the LPS + control group, the content
of IL-6 and IL-1β in the supernatant of the LPS + mimic group was
significantly decreased (P<0.05; Fig. 5), indicating that miR-223 inhibits
the expression of IL-6 and plays an anti-inflammatory role.
Discussion
Inflammatory responses are a type of immune response
and refer to a stress protection reaction in response to
microorganism infection. However, a persistent inflammatory
response can destroy healthy cells. Inflammatory responses exert
vital effects in host defense and immune responses against
bacterial infection (21). The
purpose and regulation of inflammatory responses are inaccurate and
they often become uncontrolled cascade reactions, which may cause
collateral damage to tissues (22).
Pulmonary infection imposes a heavy burden on public health
worldwide and is the main cause of mortality in the USA (23). Infections caused by gram-negative
bacteria are particularly concerning due to their increasing levels
of antibiotic resistance (24).
High mortality and morbidity rates following bacterial infection
are usually caused by the imbalance of host defense capability
between removing the infection and excessive inflammatory responses
(leading to tissue damage) (25).
Previous studies have shown that miR-223 is a small RNA specific to
hematopoietic tissues, and that miR-223 modulates the inflammation
of tissues and organs (26,27).
IL-6, a cytokine secreted by inflammatory cells, has
various roles, and therefore its functions are more complex
depending on the pathophysiology (28,29).
Furthermore, data from animal models indicate that IL-6 plays a
critical role in a variety of pathophysiological events (such as
fever, acute liver reaction and the transition from acute
inflammation to chronic inflammation) (30). However, the innate immune mechanism
of IL-6 requires further investigation. TLRs are transmembrane
glycoprotein families with two domains, among which IL-1R
homologous cytoplasmic signal domain (Toll/IL-1R domain) can bind
to IL to function as inflammatory cytokines, as well as to a
variety of other bacterial and viral peptides (31). TLR predominantly exerts its effects
via the TLR/NF-κB signaling pathway. As TLR ligands, bacteria and
virus peptides usually activate MAPK, NF-κB and the interferon
regulatory factor (IRF)-3/IRF-7 pathway after binding to TLRs. The
final result is to promote the secretion of type I interferon and
mediate the function of inflammatory cytokines, thus controlling
the response to pathogens (31).
Therefore, the role of IL-6 in inflammation may be associated with
the TLR/NF-κB signaling pathway.
In the present study, a lung injury model was
established by cecal ligation and puncture. The expression levels
of miR-223 and TLR4 and NF-κB proteins in the lung tissue cells
from sham and sepsis groups were detected. The content of IL-6 and
IL-1β secreted in BAL fluid was then examined via ELISA. The
results indicated that the expression levels of miR-223 and TLR4
and NF-κB proteins were significantly increased. In addition, the
content of cytokines, IL-6 and IL-1β, was significantly increased
following lung injury. It can therefore be concluded that the
presence of miR-223 may be associated with TLR4 and IL-6 following
lung injury. Microscopic visualization of H&E-stained lung
tissue showed alveoli with normal structures in the sham group. The
pathological results demonstrated that following lung injury, the
lung tissues were destroyed and the inflammatory responses of the
body were enhanced.
The expression levels of miR-223 and TLR4 and NF-κB
proteins in the lung tissues indicate that the content of miR-223
may be associated with TLR4 and IL-6 following lung injury.
Subsequently, the regulatory mechanism among the three at the
cellular level was investigated. RAW264.7 cells were stimulated
with LPS to become inflammatory cells and were then transfected
with miR-223 controls and mimics. Changes in the expression levels
of miR-223 and TLR4 and NF-κB proteins were detected. Following LPS
stimulation of the cells, the expression levels of miR-223 and TLR4
and NF-κB proteins were significantly increased. When compared with
the LPS + control group and the LPS group, the LPS + mimic group
presented significantly increased miR-223, but significantly
decreased expression levels of IL-6 and TLR4 and NF-κB proteins and
mRNAs.
Thus, it can be concluded that miR-223 negatively
regulates the expression of IL-6 in cells and mediates the
TLR4/NF-κB signaling pathway to play an anti-inflammatory role once
the level of IL-6 is decreased. Whether IL-6 is one of the target
genes of miR-223 was not biologically predicted and verified in the
present study. Furthermore, the anti-inflammatory effect of miR-223
was not verified in vivo. However, the present study may
provide a novel therapeutic method for relieving inflammatory
responses in sepsis-induced lung injury.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XM and DT designed the study and performed the
experiments. XM and WL established the animal models, DT and BG
collected the data, ZM and XZ analyzed the data, XM prepared the
manuscript. All authors read and approved the final manuscript. XM
and DT confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
Committee of the Fourth Central Hospital of Baoding City Animal
Center (Hebei, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li G, Zhou CL, Zhou QS and Zou HD:
Galantamine protects against lipopolysaccharide-induced acute lung
injury in rats. Braz J Med Biol Res. 49(e5008)2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Do-Umehara HC, Chen C, Urich D, Zhou L,
Qiu J, Jang S, Zander A, Baker MA, Eilers M, Sporn PH, et al:
Suppression of inflammation and acute lung injury by Miz1 via
repression of C/EBP-δ. Nat Immunol. 14:461–469. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Tauseef M, Knezevic N, Chava KR, Smith M,
Sukriti S, Gianaris N, Obukhov AG, Vogel SM, Schraufnagel DE,
Dietrich A, et al: TLR4 activation of TRPC6-dependent calcium
signaling mediates endotoxin-induced lung vascular permeability and
inflammation. J Exp Med. 209:1953–1968. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
He Z, Chen X, Wang S and Zou Z: Toll-like
receptor 4 monoclonal antibody attenuates
lipopolysaccharide-induced acute lung injury in mice. Exp Ther Med.
8:871–876. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Deng Y, Yang Z, Gao Y, Xu H, Zheng B,
Jiang M, Xu J, He Z and Wang X: Toll-like receptor 4 mediates acute
lung injury induced by high mobility group box-1. PloS One.
8(e64375)2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang M, Zou L, Feng Y, Chen YJ, Zhou Q,
Ichinose F and Chao W: Toll-like receptor 4 is essential to
preserving cardiac function and survival in low-grade polymicrobial
sepsis. Anesthesiology. 121:1270–1280. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ju M, Liu B, He H, Gu Z, Liu Y, Su Y, Zhu
D, Cang J and Luo Z: MicroRNA-27a alleviates LPS-induced acute lung
injury in mice via inhibiting inflammation and apoptosis through
modulating TLR4/MyD88/NF-κB pathway. Cell Cycle. 17:2001–2018.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hu N, Wang C, Dai X, Zhou M, Gong L, Yu L,
Peng C and Li Y: Phillygenin inhibits LPS-induced activation and
inflammation of LX2 cells by TLR4/MyD88/NF-κB signaling pathway. J
Ethnopharmacol. 248(112361)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cao C, Yin C, Shou S, Wang J, Yu L, Li X
and Chai Y: Ulinastatin protects against LPS-induced acute lung
injury by attenuating TLR4/NF-κB pathway activation and reducing
inflammatory mediators. Shock. 50:595–605. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lee RC, Feinbaum RL and Ambros V: The
C. elegans heterochronic gene lin-4 encodes small RNAs with
antisense complementarity to lin-14. Cell. 75:843–854.
1993.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wightman B, Ha I and Ruvkun G:
Posttranscriptional regulation of the heterochronic gene lin-14 by
lin-4 mediates temporal pattern formation in C. elegans. Cell.
75:855–862. 1993.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lytle JR, Yario TA and Steitz JA: Target
mRNAs are repressed as efficiently by microRNA-binding sites in the
5' UTR as in the 3' UTR. Proc Natl Acad Sci USA. 104:9667–9672.
2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fujiwara T and Sakamoto H: RNA binding
proteins play the leading part of posttranscriptional regulation of
gene expression in nerve. Tanpakushitsu Kakusan Koso. 51:2609–2616.
2006.PubMed/NCBI(In Japanese).
|
|
14
|
Sonkoly E, Stahle M and Pivarcsi A:
MicroRNAs and immunity: Novel players in the regulation of normal
immune function and inflammation. Semin Cancer Biol. 18:131–140.
2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ha M and Kim VN: Regulation of microRNA
biogenesis. Nat Rev Mol Cell Biol. 15:509–524. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Sheedy FJ and O'Neill LA: Adding fuel to
fire: MicroRNAs as a new class of mediators of inflammation. Ann
Rheum Dis. 67 (Suppl 3):i50–i55. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mirjam P, Ken R, Irene H and Tania M:
miR-223: A key regulator in the innate immune response in asthma
and COPD. Front Med (Lausanne). 19(196)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dorhoi A, Iannaccone M, Farinacci M, Faé
KC, Schreiber J, Moura-Alves P, Nouailles G, Mollenkopf HJ,
Oberbeck-Müller D, Jörg S, et al: MicroRNA-223 controls
susceptibility to tuberculosis by regulating lung neutrophil
recruitment. J Clin Invest. 123:4836–4848. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Feng Z, Qi S, Zhang Y, Qi Z, Yan L, Zhou
J, He F, Li Q, Yang Y, Chen Q, et al: Ly6G+ neutrophil-derived
miR-223 inhibits the NLRP3 inflammasome in mitochondrial
DAMP-induced acute lung injury. Cell Death Dis.
8(e3170)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mogensen TH: Pathogen recognition and
inflammatory signaling in innate immune defenses. Clin Microbiol
Rev. 22:240–273. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ulloa L, Brunner M, Ramos L and Deitch EA:
Scientific and clinical challenges in sepsis. Curr Pharm Des.
15:1918–1935. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mathers CD and Loncar D: Projections of
global mortality and burden of disease from 2002 to 2030. PloS Med.
3(e442)2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mamishi S, Mahmoudi S, Naserzadeh N,
Hosseinpour SR, Haghi AM, Bahador A, Abdosalehi MR, Rahmani M and
Pourakbari B: Antibiotic resistance and genotyping of gram-negative
bacteria causing hospital-acquired infection in patients referred
to children's medical center. Infect Drug Resist. 12:3377–3384.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Si-Tahar M, Touqui L and Chignard M:
Innate immunity and inflammation-two facets of the same
anti-infectious reaction. Clin Exp Immunol. 156:194–198.
2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ramkissoon SH, Mainwaring LA, Ogasawara Y,
Keyvanfar K, McCoy JP Jr, Sloand EM, Kajigaya S and Young NS:
Hematopoietic-specific microRNA expression in human cells. Leuk
Res. 30:643–647. 2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Neudecker V, Haneklaus M, Jensen O,
Khailova L, Masterson JC, Tye H, Biette K, Jedlicka P, Brodsky KS,
Gerich ME, et al: Myeloid-derived miR-223 regulates intestinal
inflammation via repression of the NLRP3 inflammasome. J Exp Med.
214:1737–1752. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nishimoto N and Kishimoto T: Interleukin
6: From bench to bedside. Nat Clin Pract Rheumatol. 2:619–626.
2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Heinrich PC, Behrmann I, Haan S, Hermanns
HM, Müller-Newen G and Schaper F: Principles of interleukin
(IL)-6-type cytokine signalling and its regulation. Biochem J.
374:1–20. 2003.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Naugler WE and Karin M: The wolf in
sheep's clothing: The role of interleukin-6 in immunity,
inflammation and cancer. Trends Mol Med. 14:109–119.
2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Takeuchi O and Akira S: Pattern
recognition receptors and inflammation. Cell. 140:805–820.
2010.PubMed/NCBI View Article : Google Scholar
|