Introduction
In Luigi Pirandello's novel ‘One, No One and One
Hundred Thousand’, the protagonist comes to the realization that
everyone he knows and everyone he has ever met has constructed his
persona in their own imagination and that none of these personas
corresponds to the image that he believes himself to be. In a
similar manner, patients, urologists, pathologists, family
physicians and microbiologists have different perceptions on
chronic prostatic inflammation. This condition features a variety
of symptoms that may differ from patient to patient. The most
common symptoms include pain or discomfort in the genital or the
pelvic area, which may or may not be associated with urinary
disorders and/or sexual dysfunction. Premature ejaculation,
hemospermia and increased serum prostate-specific antigen levels
may be also present (1). Patients
with a previous episode of chronic prostatitis (CP) are more likely
to experience future episodes (2).
There are 3 types of chronic prostatic inflammation that share
similar symptoms, namely National Institutes of Health (NIH)
category II chronic bacterial prostatitis (CBP) and cat. III
CP/chronic pelvic pain syndrome (CP/CPPS), in its inflammatory
(IIIB) and non-inflammatory (IIIB) variants (1). These syndromes are characterized by a
rather long-term remitting and relapsing clinical course (that can
last from several months to decades) and are distinguished on the
basis of microbiological findings.
During urology referral service, it was noticed that
patients referred with the tentative diagnosis of prostatitis are
misdiagnosed and actually have a different condition oftentimes.
Several patients that were ultimately found to have a positive
Meares and Stamey test had a prior negative test. A notable number
of patients with a prior diagnosis of CBP and clinical recurrence
were ultimately found to have a negative Meares and Stamey test
while some patients are presenting with different type of
prostatitis overtime. To the best of our knowledge, there is a lot
of outdated information about prostatitis and most studies on
chronic prostatitis are focusing on treatment options, while the
majority of them have a relatively short follow-up period.
In the present study, consecutive cases of chronic
prostatic inflammation and infection were retrospectively reviewed
in order to explore the clinical course (how the disease behaves
over time) and long-term outcome. It should be noticed that no
similar study has been published in the literature up to date.
Materials and methods
Patients
In the present retrospective study, the records of
individual patient visits were analysed. Microbiological data
[Meares and Stamey (MS) or two-glass tests and urethral smear
cultures] and history (including general patient demographics,
medical and surgical history and medication use) obtained from
individuals with CP symptoms visiting the Urology Department of the
Tzaneion Hospital (Piraeus, Greece) between March 2009 and March
2019 were retrospectively evaluated. Patients suffering from
conditions that influence bacterial virulence or host response
(e.g. immunodeficiency, abnormalities of the urogenital system) and
patients who received antibiotics or immunosuppressive treatments
within 4 weeks of the visit were excluded from the study
(clinicopathological and demographic characteristics of included
patients are presented in Table
I).
 | Table IData regarding demography, history,
diagnoses and outcomes. |
Table I
Data regarding demography, history,
diagnoses and outcomes.
| Demography | Value |
|---|
| Mean age (years),
SD | 45.5 (±11.9) |
|
Difference
in mean age among chronic prostatitis subgroups | P=0.449 |
|
Difference
in mean age among cured and uncured patients | P=0.624 |
| History | |
|
Previous
diagnosis of prostatitis (%) | 348/656(53) |
|
Previous
diagnosis of epididymitis (%) | 132/656 (20.1) |
| Initial diagnosis
(Group A and B), (%) | |
|
NIH-IIIB | 96/656(16) |
|
NIH-IIIA | 26/656 (4.3) |
|
Cystitis/Prostatitis | 35/656 (5.8) |
|
NIH-II
(CBP) | 370/656 (61.6) |
|
Cystitis | 6/656 (1.0) |
|
Likelihood
of NIH-II | 68/656 (11.3) |
| Outcome (Group A and
B) (%) | |
|
No cure | 76/656 (12.6) |
|
Cure | 422/656 (70.2) |
|
Unknown | 103/656 (17.1) |
Patient assessment
The patients included were clinically evaluated
(interview, physical examination, National Institutes of Health
Chronic Prostatitis Symptom Index (NIH-CPSI) and International
Prostate Symptom Score (IPSS) questionnaires, ultrasound) and
underwent the MS ‘4-glass’ test, based on cultures of first-void
(VB1), midstream/pre-prostatic massage (VB2), expressed prostatic
secretions (EPS) and post-prostatic massage urine (VB3) specimens.
Several patients underwent the simplified two-glass test, assessing
the sole VB2 and VB3 specimens (1).
Microbiological evaluation
Identification and semi-quantitative assay for
Mycoplasma hominis and Ureaplasma urealyticum were
performed using the Mycoplasma IST-2 kit, according to the
manufacturer's instructions (BioMerieux SA). Chlamydia
trachomatis was detected by direct immunofluorescence
(monoclonal antibodies against lipopolysaccharide membrane
-ab54377-Kallestad Laboratories). Urine samples were cultured
undiluted on blood and MacConkey agar plates (Kallestad
Laboratories) and subjected to centrifugation for microscopic
examination of the sediment (2).
Evaluation of microbiological culture results was performed by two
specialists. Microbiologists performed the evaluation independently
and then compared the results. Any discrepancies were resolved
through a consensus discussion.
Identification of traditional pathogens was
performed by conventional methods and the Vitek-2 Compact
(BioMerieux SA) system and susceptibility testing was performed by
disc diffusion and/or the Vitek-2 system (BioMerieux SA).
Interpretation of susceptibility results was based on Clinical and
Laboratory Standards Institute guidelines and European Committee on
Antimicrobial Susceptibility Testing guidelines (3).
Diagnosis
Differential diagnosis included any situation that
causes symptoms similar to those of CP. Diagnosis of CP was based
on the MS test or the two-glass test. For NIH-II, the test was
considered positive if one of the following criteria was fulfilled:
i) Bacteria grew in the culture of EPS and VB3 urine sample and did
not in VB1 and VB2 sample; ii) bacterial colonies in VB3 were
higher in number compared to VB1 and VB2 samples. Given that no
standard cut-off level of the number of bacteria in both urine and
prostate secretion samples is defined by consensus for the
diagnosis of CBP, no lower acceptable level was defined for either
one. Differentiation between NIH-IIIA and IIIB (inflammatory and
non-inflammatory chronic nonbacterial prostatitis) was based on the
presence of leukocytes in the EPS and/or the post-massage urine
sample (2).
EPS/VB3 cultures that were considered negative
(bacteria unable to grow) despite the presence of bacteria in the
EPS/VB3 specimens were rated as cases with a ‘likelihood of
NIH-II’. Cases with a higher number of bacterial colonies in VB1
and VB2 compared to VB3 samples in the presence of positive EPS
were considered as mixed chronic prostatitis/chronic cystitis cases
(inflammation of the prostate and bladder that continues for 2
months or longer).
Appropriate antimicrobials were administered to
confirmed cases of NIH-II according to the antibiogram for a period
of 4 weeks. Patients who were diagnosed with non-bacterial
prostatitis were offered multimodal therapy based on the main
symptom (UPOINT phenotype: e.g. urinary: α-blocker or
antimuscarinic agent; organ-specific: Serenoa repens
preparations; tenderness: Physical therapy) (4).
Evaluation of therapy outcomes
Upon clinical relapse or after four weeks of
therapy, the NIH-CPSI and IPSS questionnaires were re-administered.
Evaluation also included interview, physical examination,
ultrasound and the ‘4-glass’ or ‘2-glass’ tests.
Statistical analysis
In order to analyze the persistence and recurrence
of CP, cases were stratified into two groups. Group 1 consisted of
patients with a single set of CP-like symptoms and were recorded in
up to three visits (including follow-up). Group 2 was made up of
cases with recurring episodes of prostatitis-like symptoms
registered in >3 visits (owing to initial evaluation, symptom
persistence and symptom recurrence investigation, as well as
regular follow-up). Statistical analysis was performed with the
SPSS version 11.0 statistical software package (SPSS, Inc.). The
paired t-test was used to analyse differences between means. An
alpha error inferior to 5% (P<0.05) was set as significance
level for each comparison.
Results
Overview
Out of the 2,002 total visits between March 2009 and
March 2019, 218 visits were incompletely recorded and were excluded
from the study. Finally, 1,783 visits for investigation of
prostatitis-like symptoms and routine follow-up were reviewed.
A wide variety of major symptoms, subsidiary
symptoms and symptom combinations was reported by the patients.
Major symptoms were reported to begin slowly and have alternating
periods of absence with moments of worsening. In most cases,
symptoms lasted >3 months prior to diagnosis. However, during
relapse phases, the patients were able to promptly recognise the
symptoms. The most frequent symptom was scrotal/testicular pain,
accounting for almost 40% of the cases.
In total, 656 eligible patients were selected
according to the inclusion/exclusion criteria. More than half of
them (53%) had a previous history of prostatitis. The mean age of
these patients was 45.5±11.9 years. Overall diagnoses and outcomes
are presented in Table I. No
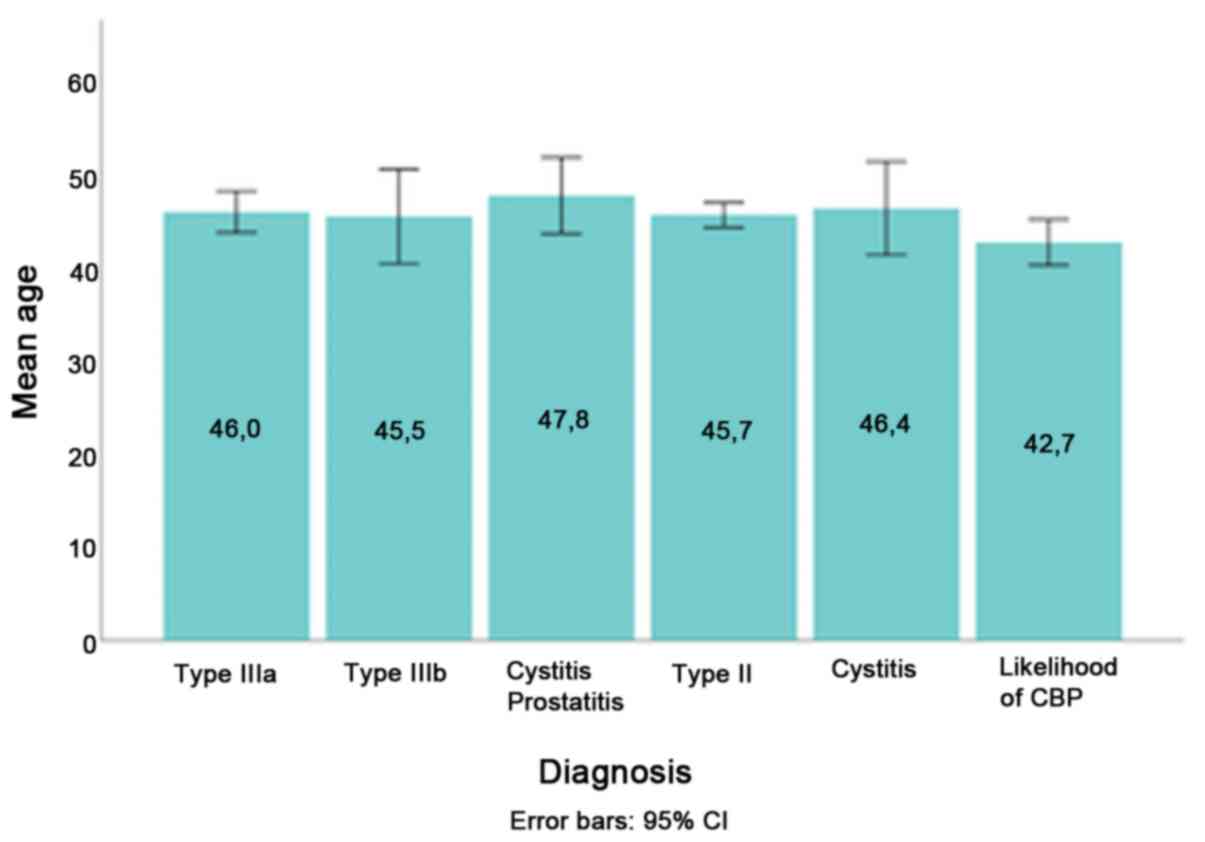
statistically significant difference in mean age was identified
among patients with different chronic prostatitis subtypes
diagnosis [F(5)=0.948; P=0.449]
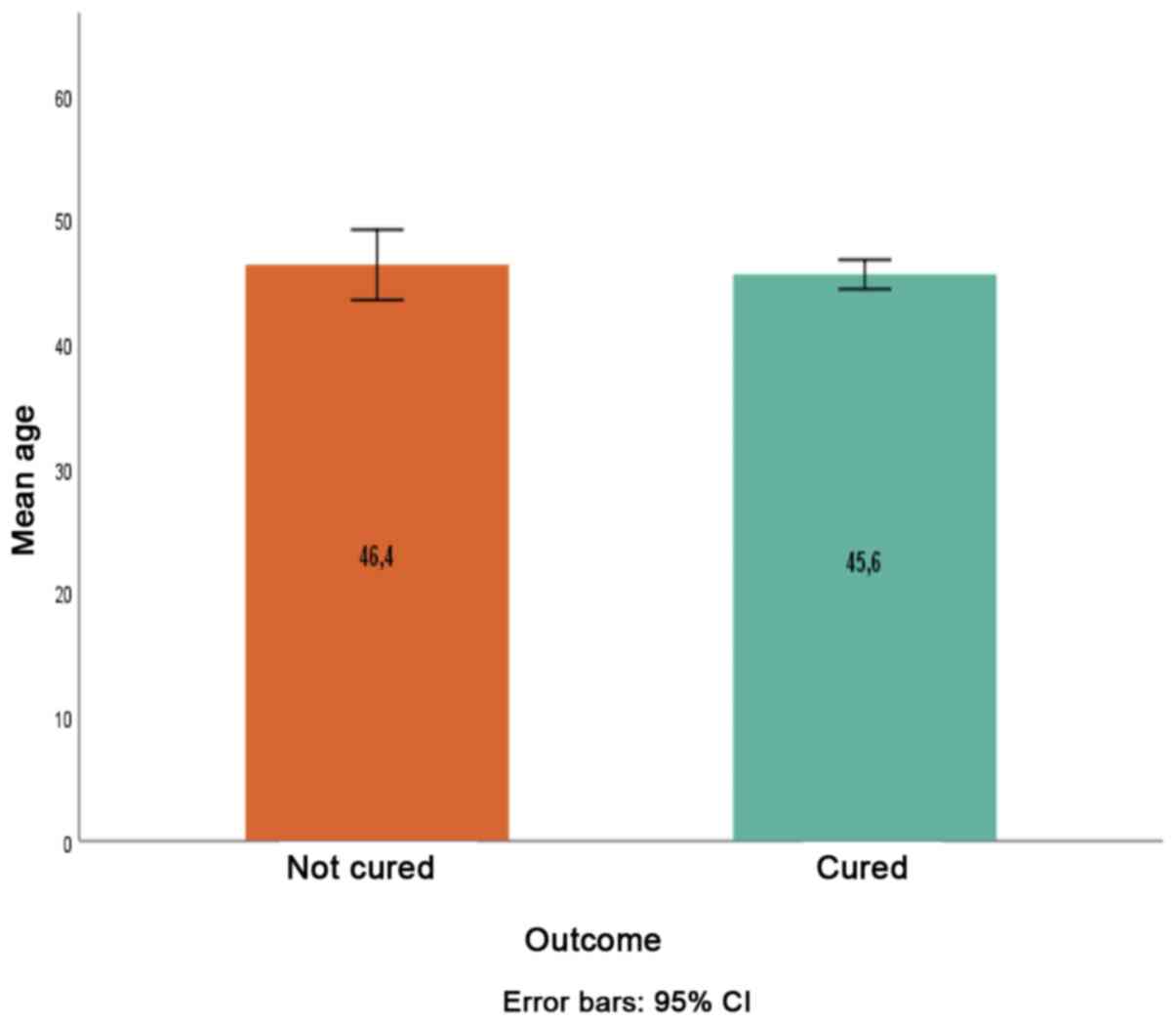
(Fig. 1). The mean age between
cured (45.6±12.1 years) and uncured patients (46.4±11.9 years) was
also not significantly different (P=0.624; Fig.2).
Group 1
Group 1 consisted of 549 cases. A considerable
fraction of patients (44.9%; 247/549) were diagnosed with NIH-II. A
vast variety of bacteria were present in positive cultures, with
Escherichia coli being the most common pathogen. However,
coagulase-negative staphylococci (CoNS) (mainly S. hominis
and S. haemolyticus) and Enterococcus faecalis were
also common isolates. The second most frequent chronic prostatitis
subtype was that of NIH-IIIB (12.39%; 68/149). In 102 cases, some
of (additional) symptoms did not fit the diagnosis and were
attributed to diseases other than prostatitis.
As far as the outcomes determined at the follow-up
visits are concerned, clinical improvement accompanied by bacterial
eradication was reported in 223 patients. A total of 41 patients
reported clinical improvement despite bacterial persistence. In 14
cases, EPS/VB3 cultures were negative (bacteria unable to grow in
culture) despite the presence of bacteria in the sample. Due to the
absence of symptoms, these patients were not further evaluated. The
clinical outcomes of 94 patients remained unknown, whereas 12
patients were diagnosed with diseases other than prostatitis. A
total of six, 36, 29 and 54 patients who were initially diagnosed
with mixed cystitis/prostatitis, NIH-II, NIH-IIIA and NIH-IIIB
received the appropriate treatments and were fully re-evaluated at
the 3rd visit. Initial diagnosis and outcomes determined at the
follow-ups are presented in Table
II.
 | Table IIPresentation of patients with chronic
prostatitis syndromes at the time of initial diagnosis and number
of subsequent conditions assessed at the follow-up in the same
patients. Group 1, first referral patients presenting with CP-like
symptoms assessed in up to three visits. Conditions assessed at
follow-up may exceed the number of initial cases, as patients may
be diagnosed with the same condition multiple times during
follow-up. |
Table II
Presentation of patients with chronic
prostatitis syndromes at the time of initial diagnosis and number
of subsequent conditions assessed at the follow-up in the same
patients. Group 1, first referral patients presenting with CP-like
symptoms assessed in up to three visits. Conditions assessed at
follow-up may exceed the number of initial cases, as patients may
be diagnosed with the same condition multiple times during
follow-up.
| | Newly diagnosed
conditions, cured cases or temporary disease-free cases assessed
during follow-up |
|---|
| Initial
diagnosis | Total patients
initially diagnosed | CBP reinfection/
recurrence | Likelihood of
CBP | Cystitis and CBP | CP/ CPPS IIIA | CP/ CPPS IIIB | Other diagnosis | Unknown outcome | Not cured | Cured |
|---|
| CBP | 247 | See ‘not cured’ | 7 | 5 | 14 | 46 | 3 | 20 | 14 | 139 |
| Likelihood of
CBP | 63 | 22 | See ‘not cured’ | 1 | 10 | 2 | 2 | 7 | 4 | 14 |
| Cystitis and CBP | 31 | 0 | 2 | See ‘not cured’ | 2 | 2 | 0 | 6 | 0 | 19 |
| CP/CPPS IIIA | 38 | 5 | 0 | 0 | See ‘not cured’ | 4 | 0 | 7 | 7 | 15 |
| CP/CPPS IIIB | 68 | 9 | 4 | 0 | 2 | See ‘not cured’ | 7 | 10 | 16 | 20 |
| Other diagnosis | 102 | 0 | 1 | 0 | 1 | 0 | See ‘not cured’ | 44 | 0 | 16 |
| Total | 549 | 36 | 14 | 6 | 29 | 54 | 12 | 94 | 41 | 223 |
Group 2
Group 2 consisted of 107 cases. Most patients
(54.2%) were initially diagnosed with NIH-II, followed by
recurrence or by disease-free periods and transition to NIH-IIIB.
The average time interval between episodes of confirmed NIH-II was
13.9 months (range, 2-56 months). The pathogens most commonly
associated with clinical relapses were Enterococcus
faecalis, CoNS and E. coli. The second most frequent
diagnosis was that of ‘likelihood of NIH-II’, characterized by
negative EPS/VB3 cultures (i.e., bacteria present but unable to
grow in culture) despite the presence of bacteria in the sample.
Most of these cases were re-diagnosed as NIH-II
reinfection/recurrence upon follow-up. Similarly, most NIH-IIIB and
NIH-IIIA cases were re-diagnosed as NIH-II reinfection/recurrence.
The time interval between initial diagnosis and recurrence ranged
between 1 and 23 months (Table
III). The association between initial diagnosis and final
outcome was not statistically significant (P=0.214). However, the
likelihood of cure was significant only for a CBP diagnosis
(P=0.001; Fig.3).
 | Table IIIPresentation of patients with chronic
prostatitis syndromes at the time of initial diagnosis and number
of subsequent conditions occurring thereafter in the same group of
patients. Group 2, patients with recurring episodes of CP-like
symptoms assessed in >3 (and up to18) consecutive visits.
Conditions assessed at follow-up may exceed the number of initial
cases, as patients may be diagnosed with the same condition
multiple times during follow-up. |
Table III
Presentation of patients with chronic
prostatitis syndromes at the time of initial diagnosis and number
of subsequent conditions occurring thereafter in the same group of
patients. Group 2, patients with recurring episodes of CP-like
symptoms assessed in >3 (and up to18) consecutive visits.
Conditions assessed at follow-up may exceed the number of initial
cases, as patients may be diagnosed with the same condition
multiple times during follow-up.
| | Newly diagnosed
conditions, cured cases or temporary disease-free cases assessed
during follow-up |
|---|
| Initial
diagnosis | Total patients
initially diagnosed | CBP reinfection/
recurrence | Likelihood of
CBP | Cystitis and CBP | CP/ CPPS IIIA | CP/ CPPS IIIB | Disease- free
period | Unknown
outcome | Cured |
|---|
| CBP | 58 | 76 | 5 | 7 | 17 | 35 | 47 | 21 | 33 |
| Likelihood of
CBP | 27 | 27 | 0 | 1 | 4 | 3 | 6 | 2 | 22 |
| Cystitis and
CBP | 3 | 4 | 1 | 1 | 0 | 1 | 2 | 0 | 0 |
| CP/CPPS IIIA | 9 | 15 | 1 | 0 | 5 | 5 | 6 | 1 | 8 |
| CP/CPPS IIIB | 10 | 12 | 2 | 0 | 2 | 7 | 2 | 3 | 6 |
| Total | 107 | 134 | 9 | 9 | 28 | 51 | 63 | 27 | 69 |
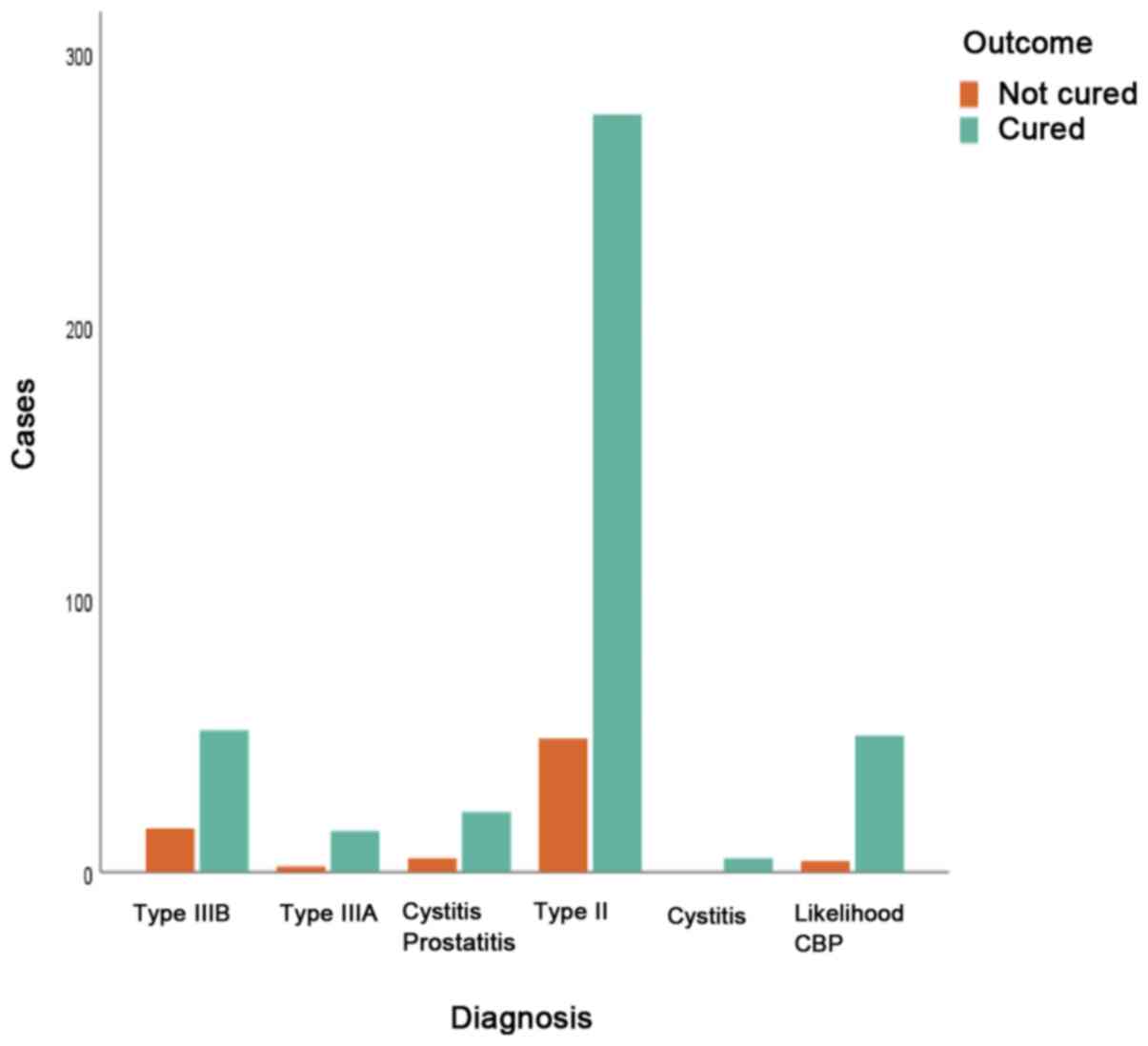
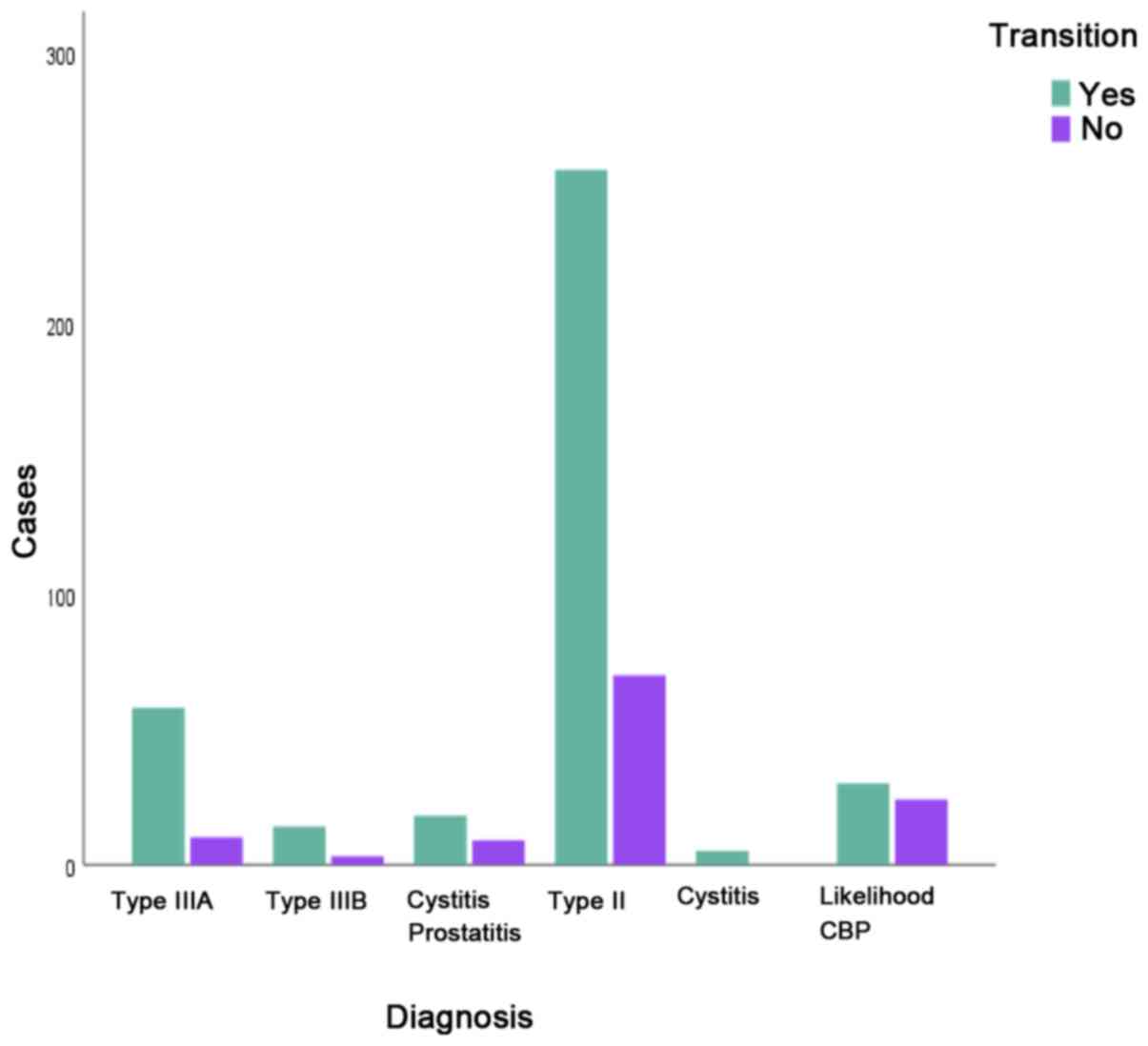
The association between initial diagnosis and
transition was statistically significant [χ2(5)=20.324, P=0.001; Fig.4, Table
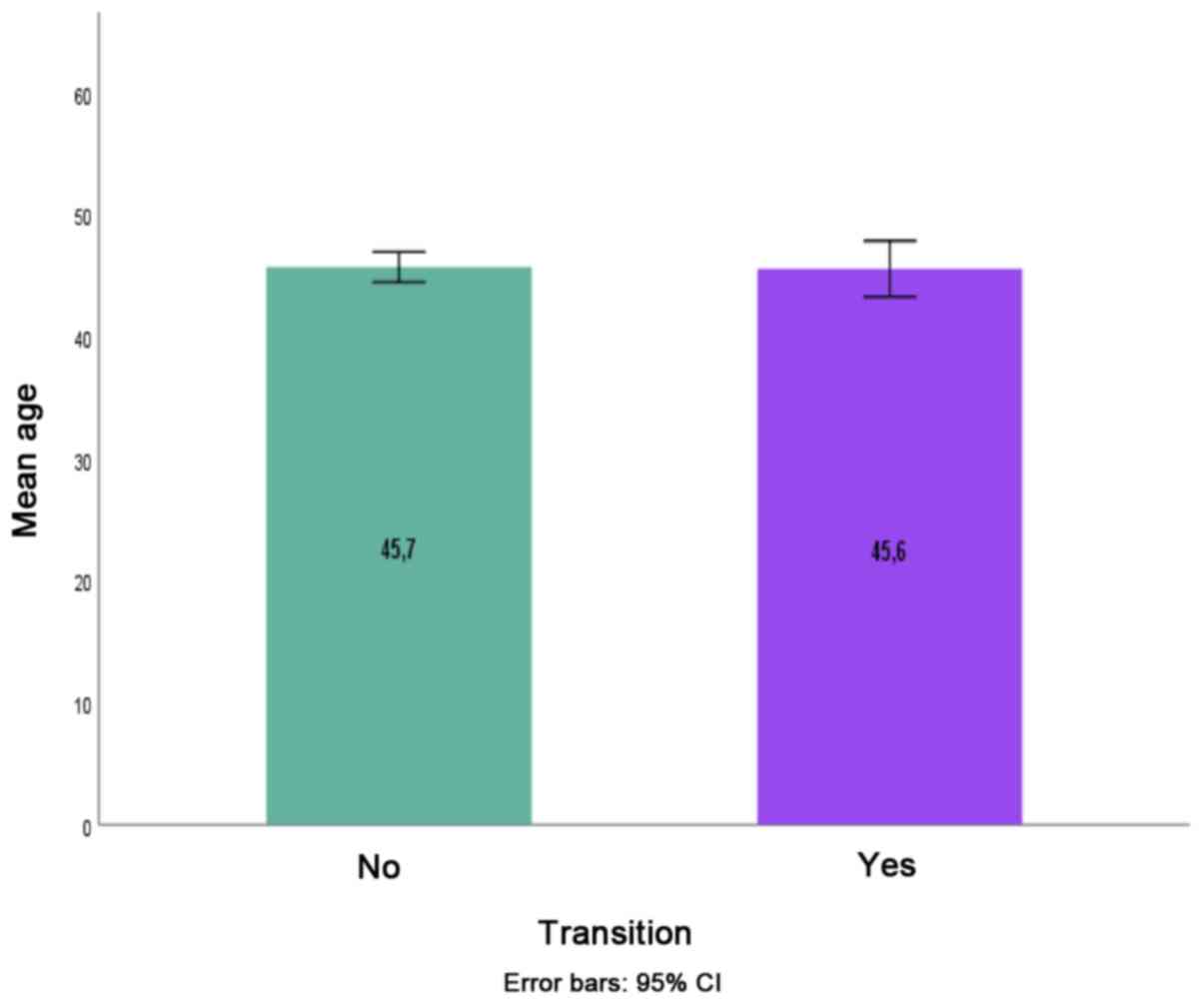
IV]. Age was not associated with transition from a CP subtype
to another CP subtype (P=0.916) (Fig.5).
 | Table IVLikelihood of transition secondary to
initial diagnosis. |
Table IV
Likelihood of transition secondary to
initial diagnosis.
| Initial
diagnosis | Type IIIB | Type IIIA | Cystitis
prostatitis | Type II (CBP) |
|---|
| Type IIIB | 0 (0,0) | 4 (40,0) | 0 (0,0) | 6 (60,0) |
| Type IIIA | 2 (66,7) | 0 (0,0) | 0 (0,0) | 1 (33,3) |
|
Cystitis-prostatitis | 4 (44,4) | 5 (55,6) | 0 (0,0) | 0 (0,0) |
| Type II (CBP) | 53 (75,7) | 16 (22,9) | 1 (1,4) | 0 (0,0) |
| Likelihood of
CBP | 2 (8,3) | 8 (33,3) | 0 (0,0) | 14 (58,3) |
Discussion
At present, the MS test is considered the gold
standard for the diagnosis of CP syndromes. However, strict
definitions used to classify CP syndromes, together with certain
drawbacks of the MS test (e.g. prostatic secretion cannot be
obtained from all patients, clinical relapses are not always
associated with positive EPS culture) frequently make it difficult
to establish the diagnosis of CP or differentiate between the
different CP syndromes. In the present study, only 29.1% of the MS
tests provided sufficient amounts of EPS; thus, in the remaining
cases (70.9%), the diagnosis was only based on VB3 cultures.
Traditionally, NIH-II is diagnosed by a 10-fold increase in
bacteria in the EPS or VB3 specimens compared with VB1 and
VB2(2). However, in a significant
number of CBP cases in the present study (34%), the increase in
bacterial loads in VB3 specimens was between 2- and 3-fold compared
to VB1 and VB2. In a similar number of cases (32%), leucocyte
counts in VB3 specimens were slightly higher than those in VB1
and/or VB2. Actually, white blood cell (leucocyte) counts have not
been indicated to correlate with symptoms or with the presence or
absence of infection (4). On the
one hand, high counts of leucocytes and positive bacterial cultures
may be present in asymptomatic patients (5); on the other hand, leucocytes may be
absent in symptomatic patients with Gram-positive bacterial
cultures (6,7). Furthermore, in the present study, a
significant number of false-negative cases (classified as
‘likelihood of NIH-II’) was recorded in both groups (63/549 and
27/107 in groups 1 and 2, respectively). Of note, certain patients
may have bacterial infection despite the fact that such pathogens
are unable to grow in cultures of urine specimens. Certain experts
debate the role of Gram-positive organisms other than Enterococci
(8,9) and it has been suggested that urologic
diseases involving Gram-positive bacteria may be easily overlooked
due to the limitations of culture-based assays typically utilized
for urine in hospital microbiology laboratories (10). Negative culture results may also
occur for various other reasons, including for example the presence
of fastidious organisms, the initiation of empirical antibiotic
therapy prior to obtaining an EPS sample, high bacterial count
cut-offs established by laboratories (e.g. a threshold of 50,000
colony-forming units to report a test culture as ‘positive’) or
insufficient sample volumes. Technical difficulties in performing
prostatic massage (e.g. under the circumstances of obesity, rectal
discomfort or recent ejaculation) actually increase the risk of
under-sampling prostatic secretions (11).
According to the Stamey Meyers protocol patients
should avoid ejaculation for 4 days prior to the test. However,
this was not possible for numerous patients. Therefore, recent
ejaculation is the likely reason explaining the low number of
assessable EPS samples in the present study. On the other hand, the
presence of fastidious organisms, anaerobic pathogens or bacteria
not detectable with the usual tests may explain cases characterized
by negative EPS/VB3 cultures despite the actual presence of
bacteria and no recent exposure to antibiotic intake reported. In
the present study, the bacteriologically proven incidence of NIH-II
among males with prostatitis symptoms was high. A possible
explanation is possibly the fact that no lower acceptable level for
bacterial colonies in both urine and prostate secretion samples for
the diagnosis of CBP was defined in the present study. In addition,
the fact that certain Gram-positive bacteria were recognised as
pathogenic may have also contributed to this difference. Similar to
what was reported in the present study, other studies indicated a
high NIH-II incidence and prevalence of Gram-positive bacterial
strains (12-14).
The incidence of NIH-II was even greater, given that obligate
intracellular parasites and intracellular bacterial communities in
the human urinary tract are not detectable by simple urinalysis
(15). A large prospective study of
males with CP indicated that 74% had an infectious etiology.
However, in that study, the most common isolate was Chlamydia
trachomatis (37% of cases) (16).
All of these considerations imply a new
understanding of CP and raise questions about the clinical
usefulness of the standard MS test as a diagnostic tool in males
with suspected CP. Yet, regardless of its drawbacks, the four-glass
test is useful for identifying infections with certain pathogens.
However, this test should be improved in order to increase its
relative sensitivity and specificity for both traditional and
unusual pathogens. Furthermore, as the distinction between NIH type
IIIA and NIH type IIIB may be biased by a non-optimal preparation
of patients (incorrect cleaning of genital area, recent
ejaculation, hyperhydration and the consequent low specific gravity
of the urine), the need for guidelines on the preparation of
patients prior to the SM test is imperative.
Paradoxically, CP may also be considered as a single
‘disease’. As indicated in the present study, type IIIA and type
IIIB may represent the evolution of this disease following an
initial diagnosis of NIH-II, thus representing a condition
characterized by the persistence of CP symptoms despite bacterial
eradication. They may also precede or follow NIH-II relapses and/or
disease-free periods. In such a case, a transition of CBP to NIH
type IIIB and NIH type IIIA and vice versa is not to be
excluded.
CP remains a poorly understood medical condition
characterized by a variety of clinical manifestations and by
transitions between different CP classes during its course The
diagnosis is hard due to the absence of typical clinical symptoms
and the distinction between CP syndromes (bacterial/non-bacterial
and inflammatory/non-inflammatory types) is based on the presence
or absence of bacteria and/or inflammatory cells in the EPS and VB3
specimens. However, a variety of situations may shift the diagnosis
to one or another direction. While the topic of CP remains somewhat
obscure, strict criteria for differentiating types of prostatitis
frequently render the interpretation of the culture results
difficult. As new evidence is added to this understudied field of
research, the current perception of CP may be challenged.
Acknowledgements
The authors wish to thank Dr Kurt G Naber, Assoc.
Professor of Urology at the Technical University of Munich School
of Medicine for his critical suggestions.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
sudy are available from the corresponding author on reasonable
request.
Authors' contributions
SK, ES and RNL conceived and designed the study. SK,
HM and VM performed a literature search. SK, ES and HM acquired the
data and confirm the authenticity of the raw data. SK, ES, RNL and
GP analyzed and interpreted the data. RNL, VM and GP performed
critical analysis and review of the literature. SK, ES and GP
drafted the manuscript. RNL, HM and VM critically revised the
article for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interest.
References
|
1
|
Vaidyanathan R and Mishra VC: Chronic
prostatitis: Current concepts. Indian J Urol. 24:22–27.
2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Stamatiou K, Magri V, Perletti G,
Papadouli V, Recleiti N, Mamali V and Zarkotou O: Chronic prostatic
infection: Microbiological findings in two Mediterranean
populations. Arch Ital Urol Androl. 91:177–181. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kassim A, Omuse G, Premji Z and Revathi G:
Comparison of Clinical Laboratory Standards Institute and European
Committee on Antimicrobial Susceptibility Testing guidelines for
the interpretation of antibiotic susceptibility at a University
teaching hospital in Nairobi, Kenya: A cross-sectional study. Ann
Clin Microbiol Antimicrob. 15(21)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Magri V, Marras E, Restelli A, Wagenlehner
FM and Perletti G: Multimodal therapy for category III chronic
prostatitis/chronic pelvic pain syndrome in UPOINTS phenotyped
patients. Exp Ther Med. 9:658–666. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Nickel JC, Alexander RB, Schaeffer AJ,
Landis JR, Knauss JS and Propert KJ: Chronic Prostatitis
Collaborative Research Network Study Group. Leukocytes and bacteria
in men with chronic prostatitis/chronic pelvic pain syndrome
compared to asymptomatic controls. J Urol. 170:818–822.
2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kohnen PW and Drach GW: Patterns of
inflammation in prostatic hyperplasia: A histologic and
bacteriologic study. J Urol. 121:755–760. 1979.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Potts J and Payne RE: Prostatitis:
Infection, neuromuscular disorder, or pain syndrome? Proper patient
classification is key. Cleve Clin J Med. 74 (Suppl 3):S63–S71.
2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Krieger JN, Ross SO, Limaye AP and Riley
DE: Inconsistent localization of gram-positive bacteria to
prostate-specific specimens from patients with chronic prostatitis.
Urology. 66:721–725. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Naber KG: Management of bacterial
prostatitis: What's new? BJU Int. 101 (Suppl 3):7–10.
2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kline KA and Lewis AL: Gram-Positive
Uropathogens, Polymicrobial Urinary Tract Infection, and the
Emerging Microbiota of the Urinary Tract. Microbiol Spectr.
4(10)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wagenlehner FM, Naber KG, Bschleipfer T,
Brähler E and Weidner W: Prostatitis and male pelvic pain syndrome:
Diagnosis and treatment. Dtsch Arztebl Int. 106:175–183.
2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cai T, Mazzoli S, Meacci F, Boddi V,
Mondaini N, Malossini G and Bartoletti R: Epidemiological features
and resistance pattern in uropathogens isolated from chronic
bacterial prostatitis. J Microbiol. 49:448–454. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Colodner R, Ken-Dror S, Kavenshtock B,
Chazan B and Raz R: Epidemiology and clinical characteristics of
patients with Staphylococcus saprophyticus bacteriuria in Israel.
Infection. 34:278–281. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Novo-Veleiro I, Hernández-Cabrera M,
Cañas-Hernández F, Pisos-Álamo E, Francés-Urmeneta A, Delgado-Yagüe
M, Alvela-Suárez L and Pérez-Arellano JL: Paucisymptomatic
infectious prostatitis as a cause of fever without an apparent
origin. A series of 19 patients. Eur J Clin Microbiol Infect Dis.
32:263–268. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rosen DA, Hooton TM, Stamm WE, Humphrey PA
and Hultgren SJ: Detection of intracellular bacterial communities
in human urinary tract infection. PLoS Med. 4(e329)2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Skerk V, Krhen I, Schonwald S, Cajic V,
Markovinovic L, Roglic S, Zekan S, Andracevic AT and Kruzic V: The
role of unusual pathogens in prostatitis syndrome. Int J Antimicrob
Agents. 24 (Suppl 1):S53–S56. 2004.PubMed/NCBI View Article : Google Scholar
|