Introduction
The World Health Organisation (WHO) defined obesity
as one of the most dangerous and yet neglected public health issues
which threaten to overwhelm even the most developed countries
(1). The association between
obesity and a higher cancer risk is mainly due to anthropometric
parameters and lifestyle factors which activate different
biological mechanisms. Anthropometric parameters are BMI, weight
increase, and the amount of body fat, particularly visceral fat.
Lifestyle factors include sedentary habits and diet parameters,
such as a hypercaloric and/or low-quality diet (2). Obesity during pregnancy is an
independent risk factor for long-term female malignancies such as
ovarian and breast cancer (3). As
with obesity, cancer has been acknowledged worldwide as a leading
disease with respect to the total number of fatalities, which are
mostly caused by late diagnosis (4). Regarding breast cancer prevention, one
important aspect lies in the use of screening methods such as
mammography. Concerning this, the major issue is related to the
filter used in mammographies in that the filters must have homogen
mass densities, to ensure the uniform exposure of the patient's
breast (5). Cancer is characterized
by uncontrolled cell division. Numerous solutions to this include
the use of cytostatic agents and the development of theoretical
models that describe the use of electrostatic fields in in this
regard (6,7). Therefore, it is imperative to put
effort into both cancer treatment as well as cancer prevention. Of
importance is that there are no approved clinical guidelines for
managing obesity during pregnancy (8,9).
The main objective of this study was to emphasize
the impact of obesity on hepatic function in pregnant women by
analysing the functional tests used in current practice. A further
aim was to identify possible predictors of liver damage by
analyzing specific anthropometric data (10). There was also a focus on visceral
fat which is associated with a number of conditions, including
cardiovascular disease, and insulin resistance during the process
of visceral adipose tissue collection in the abdominal cavity and
surrounding of the internal organs (11).
Materials and methods
General
This study is descriptive, observational and
retrospective and is based on the observation sheets found in the
database of the Institute for the Health of the Mother and Child,
the Obstetrics Gynecology Department of Polizu Hospital. Patients
who presented for consult each trimester of pregnancy were
included. The general demographic data considered included age,
body mass index (BMI), area of origin, and anthropometric data:
Abdominal circumference and a complete set of paraclinical data
from which we extracted these specific liver tests: Aspartate
aminotransferase (AST), alanine transferase (ALT), direct bilirubin
(BD), serum albumin and gamma-glutamyl transferase (GGT) (6-9).
In order to reduce the possibility of bias and to increase the
reproducibility of the study the normal values of these parameters
were produced: Direct bilirubin <1.2 ml/dl, AST <35 µ/l, ALT
<35 µ/l, albumin 3.5-4.5 µ/l, GGT <36 µ/l (12-18).
It should be considered that AST and ALT levels were monitored
dynamically, over several days, describing the variation pattern of
the AST value and respecting the cubic model with a significance
value of P=0.01. This study was approved by the ethics commission
of the National Institute for Maternal and Child
Health-Alessandrescu Rusescu on December 5, 2019 with reference
number 17852/07.11.2019.
Methods
Given the main end-point of this study, which is the
comparison of liver function tests during pregnancy in patients
with normal weight and overweight/obese, the size of the patient
groups should be sufficient to meet this aim (19,20).
For this estimation the program MedCalc 14.1 (sampling-comparison
of two means test) was used. A significance level of 0.05 was
employed to avoid a type 1 (α level-two sided) error and 0.1 to
avoid a type 2 (β) error. Thus, 157 patients (66 in group A and 91
in group B) were included in order to guarantee the detection of a
30% difference between the averages of the two groups with a
computing power equal to 90%. Written informed consent was obtained
from all the patients.
Statistical analysis
For the statistical analysis of the data, the
program MedCalc 14.1 (Stata Corp.) was used as follows: The t-test
for independent variables to compare the differences between two
means, one-way-repeated measures ANOVA for comparing the
differences between three or more means using the Bonferroni
correction as post hoc test, and the Pearson or Spearman
correlation coefficient depending on observing the Gaussian
distribution of data. For all analysis, the confidence interval was
95%; thus, P<0.05 was considered to indicate statistical
significance.
Results
General
The present study included 157 patients divided into
two groups, distributed as follows: Group A: 66 obese pregnant
women (BMI >25 kg/m2) and group B: 91 patients with
normal weight (BMI <25 kg/m2).
AST and ALT levels
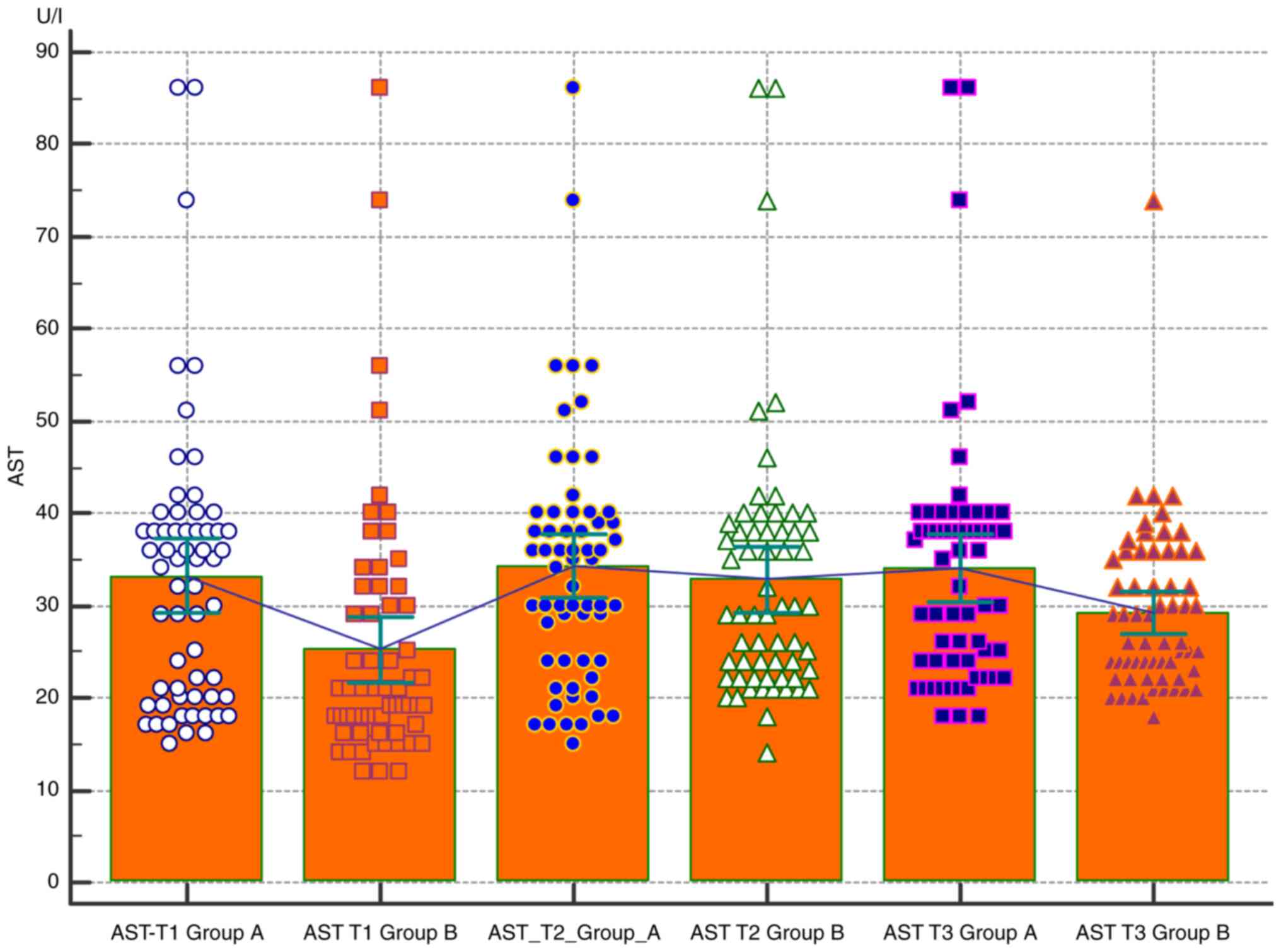
Comparative analysis of the AST level in the two
groups according to the trimester of pregnancy. When comparing the
AST level, it can be observed that obese patients (group A) tend to
have higher values in all trimesters, but no difference compared
with group B was statistically significant (only in the first
trimester is a slight difference detectable between the two groups
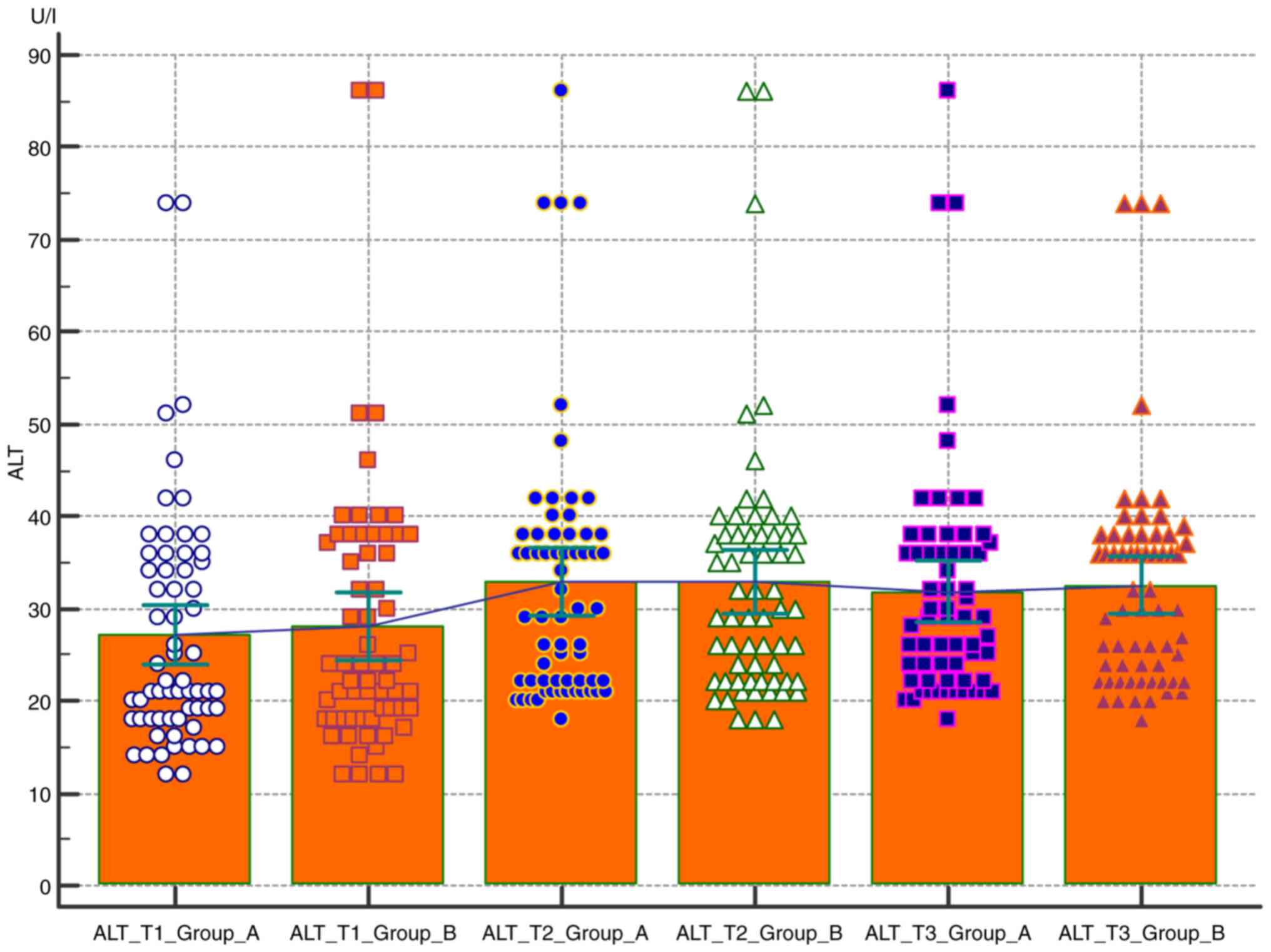
P=0.1) (Table I and Fig. 1). Comparative analysis of the ALT
level in the two groups according to the trimester of pregnancy
revealed no statistically significant differences in the
comparative analysis between the two groups of ALT (Fig. 2).
 | Table IComparative analysis of the AST and
ALT levels in the two groups according to the pregnancy
trimester. |
Table I
Comparative analysis of the AST and
ALT levels in the two groups according to the pregnancy
trimester.
| Paraclinical
data | Mean group A | Mean group B | 95% CI group A | 95% CI group B | Standard error of
difference | P-value |
|---|
| AST T1 | 33.2459 | 25.2623 | 29.2985 to
37.1933 | 21.6698 to
28.8548 | 1.97 | 0.1 |
| AST T2 | 34.3115 | 32.8197 | 30.8527 to
37.7703 | 29.1720 to
36.4674 | 2.757 | 1.0 |
| AST T3 | 34.0656 | 29.2459 | 30.4825 to
37.6487 | 26.9635 to
31.5283 | 2.135 | 0.4 |
| ALT T1 | 27.1270 | 28.0476 | 23.8323 to
30.4217 | 24.3658 to
31.7294 | 2.569 | 1.0 |
| ALT T2 | 32.8889 | 32.8730 | 29.2861 to
36.4917 | 29.3522 to
36.3938 | 2.775 | 1.0 |
| ALT T3 | 31.8413 | 32.5238 | 28.5619 to
35.1206 | 29.4757 to
35.5719 | 2.359 | 1.0 |
GGT, albumin and BD levels
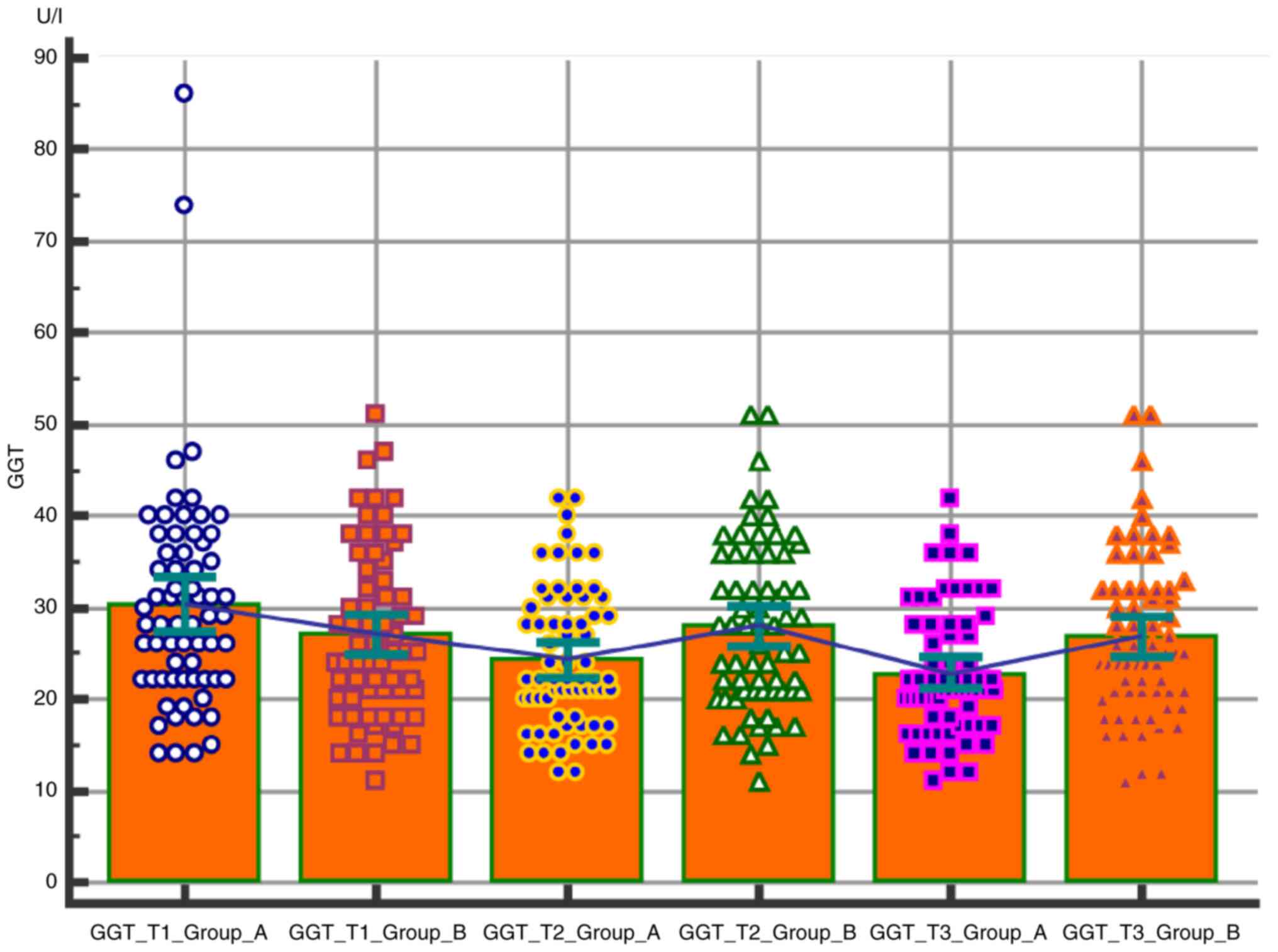
Comparative analysis of the GGT level in the two
groups was conducted according to the pregnancy trimester. The
values of the GGT are higher in the group of obese patients
compared to the normoponderous patients, this difference being best
underlined in the third trimester (Table II and Fig. 3).
 | Table IIPelvic Floor Distress Inventory-20 and
Pelvic Floor Impact Questionnaire-scores in obese and non-obese
patients. |
Table II
Pelvic Floor Distress Inventory-20 and
Pelvic Floor Impact Questionnaire-scores in obese and non-obese
patients.
| Variable | Non-obese (n=44) | Obese (n=30) | P-value |
|---|
| PFIQ-7 | Mean=152.72
SD=69.16 | Mean=191.73
SD=60.00 | P=0.0034 t=3.0248
df=72 |
| PFDI-20 | Mean=156.84
SD=64.33 | Mean=192.5
SD=43.12 | P=0.0097 t=2.6535
df=72 |
| Prolapse degree | I-II=25
III-IV=19 | I-II=8 III-IV=22 | |
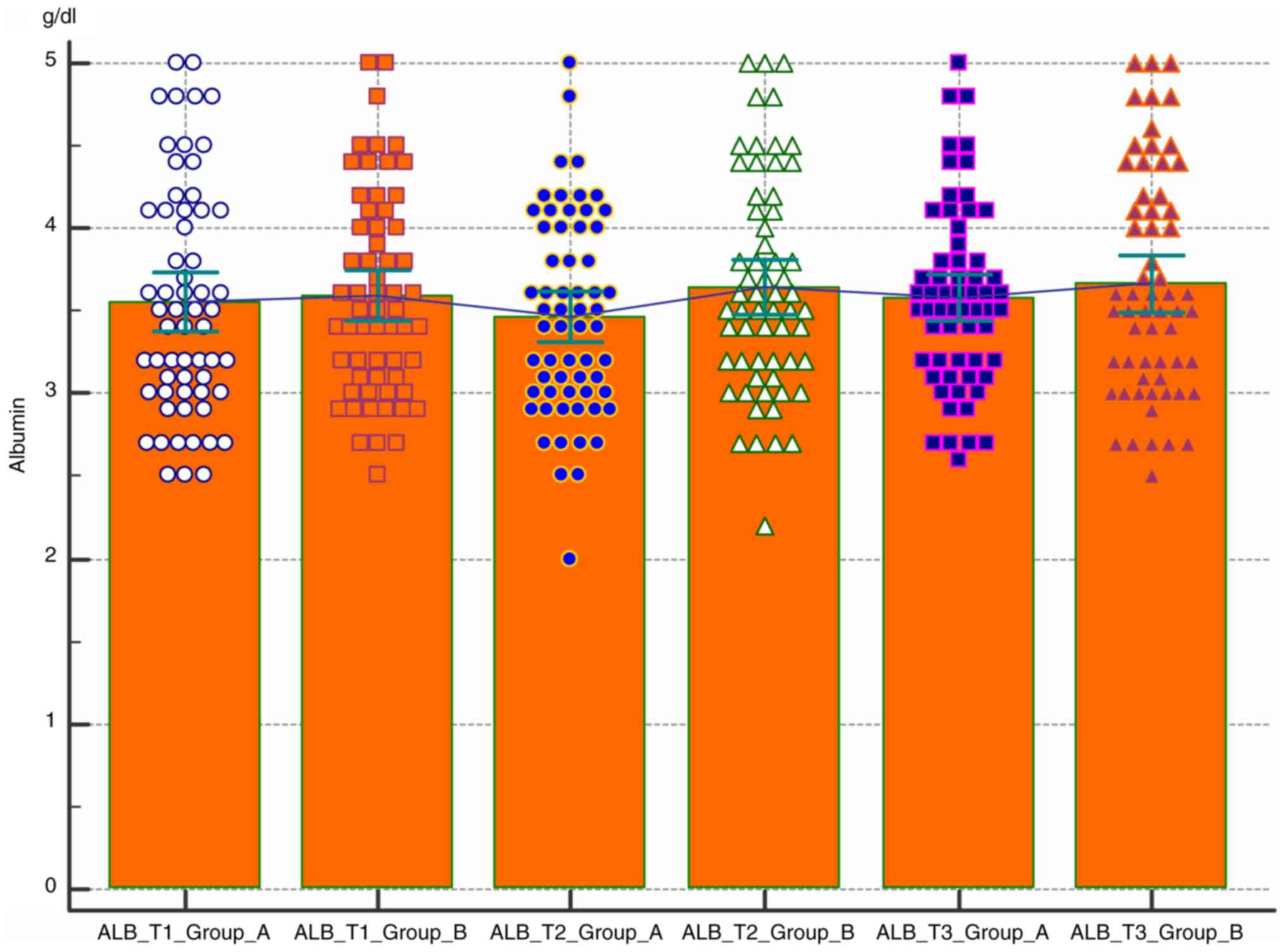
Comparative analysis of the albumin level in the two
groups according to the pregnancy trimester was also performed
(Fig. 4). The level of albumin
remains constant at the lower limit of the normal value of 3.5 µ/l
regardless of the nutritional status. There is no statistically
significant difference between the two groups.
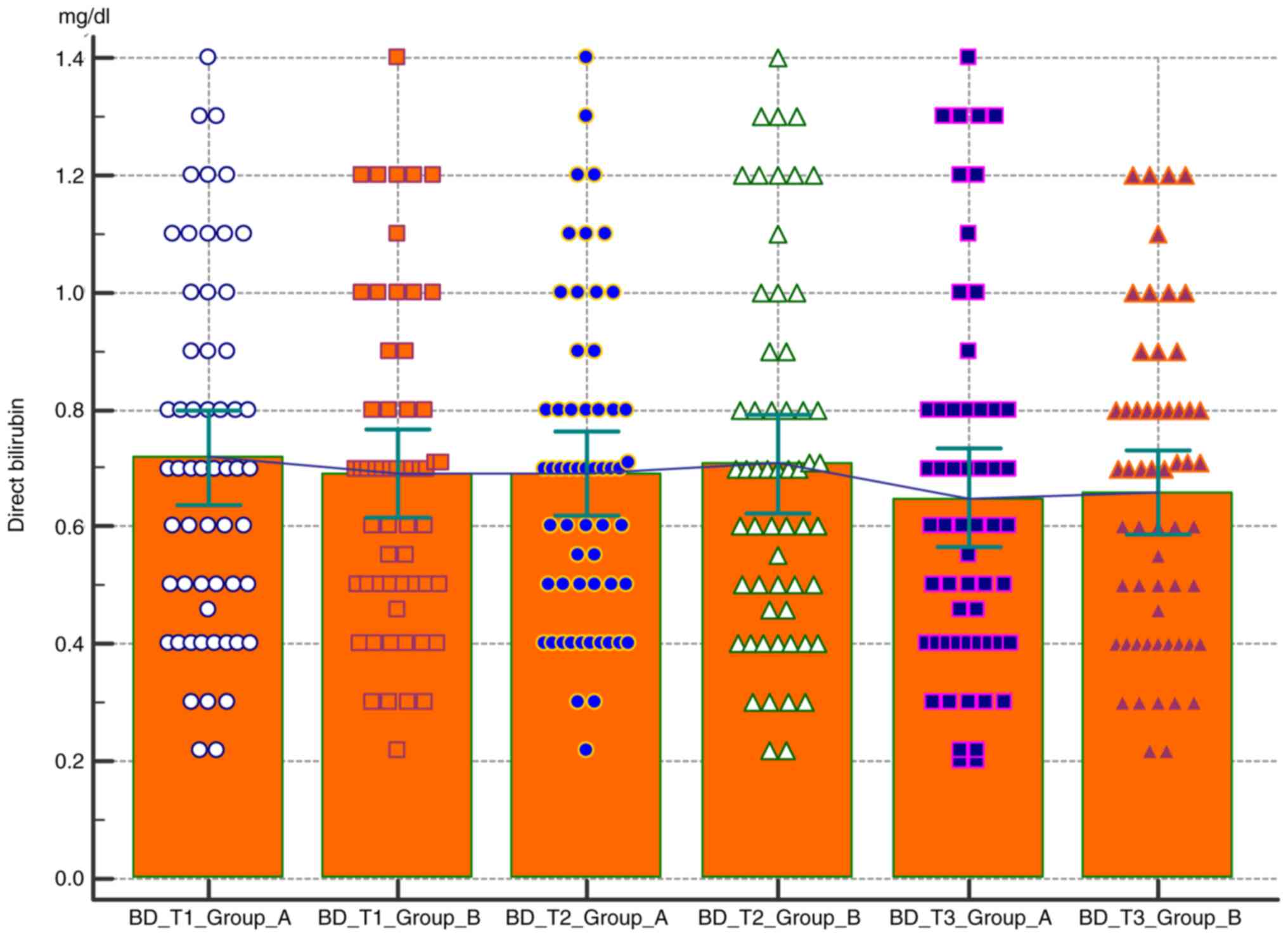
Comparative analysis of the BD level in the two
groups according to the pregnancy trimester was carried out. As in
the case of albumin, direct bilirubin tends to be constant
throughout pregnancy, with no statistically significant difference
between the group of obese patients and the group of norm-weight
patients (Fig. 5 and Table II).
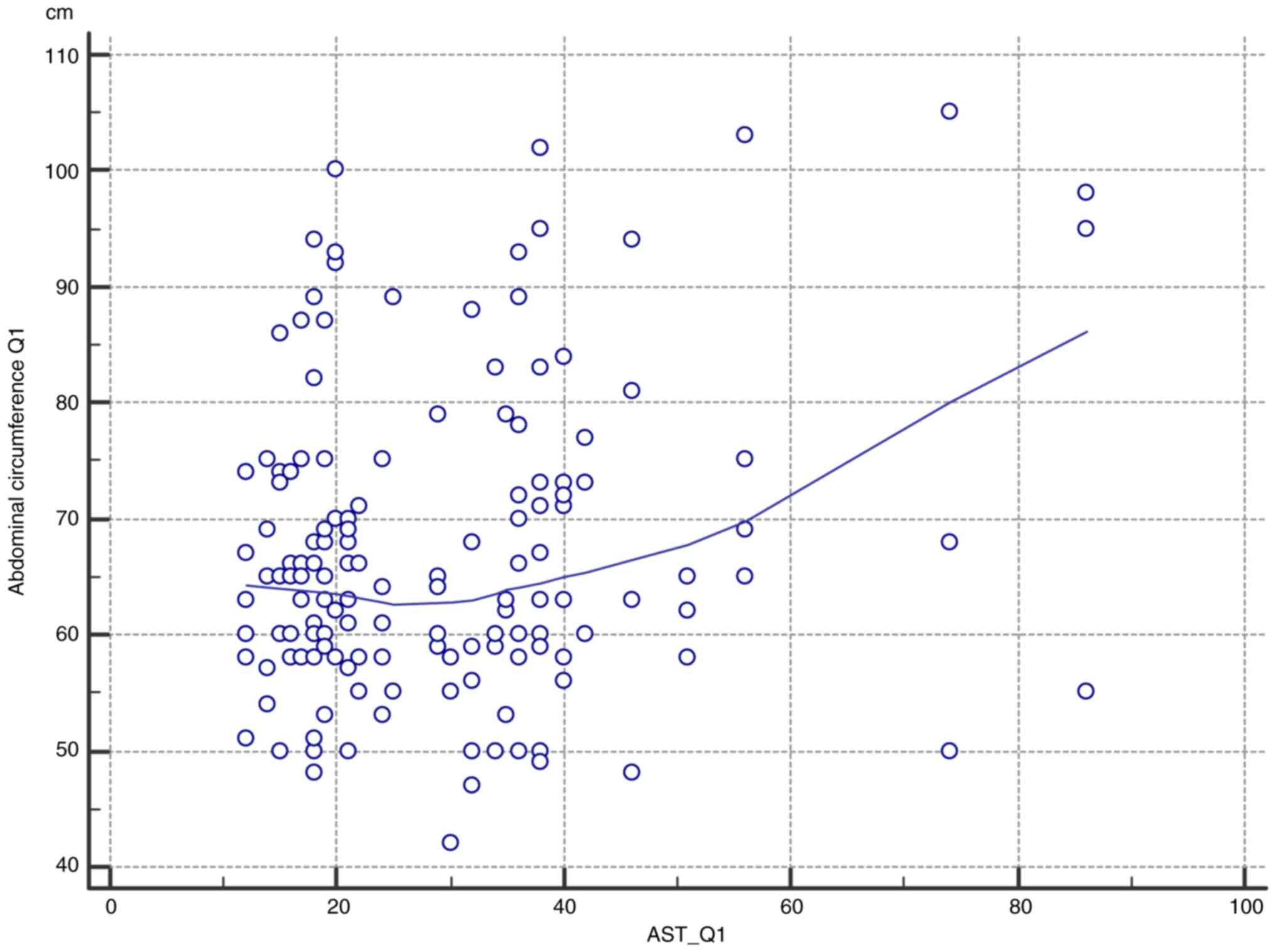
Correlation of circumference and
AST
The correlation between the abdominal circumference
in the first trimester and the value of aspartate transferase was
assessed. In practice, in a patient with a larger abdominal
circumference in the first trimester, higher levels of AST were
evident (Table III).
 | Table IIICorrelation between abdominal
circumference in the first trimester and the value of aspartate
transferase. |
Table III
Correlation between abdominal
circumference in the first trimester and the value of aspartate
transferase.
| Sample size | 157 |
| Correlation
coefficient r | 0.1997 |
| Significance
level | P=0.0122 |
| 95% confidence
interval for r | 0.04444 to
0.3455 |
Discussion
ALT and AST
Measurement of serum ALT and AST were the most
useful tests for routine diagnosis of liver disease. The effects of
pregnancy on serum levels of ALT and AST are considered somewhat
controversial. In some studies, there was a slight increase in ALT
and/or AST during the second and third trimesters. However, in most
published studies, serum ALT and AST levels did not change during
pregnancy (21,22).
Results of the present study showed that obese
pregnant women tend to have higher values of hepatic transaminases
during all 3 trimesters of pregnancy compared to normal weight
pregnant women, even though it was not statistically significant.
However, it should be considered that AST and ALT levels were
monitored dynamically, over several days; thus, it is necessary to
describe the variation pattern of the AST value (23-29).
It respects the cubic model with a significance value of P=0.01,
which shows that during our follow-up, the AST values tended to
increase during Q2, followed by a definite decrease. However, the
AST values in Q3 are higher than those in Q1 (Table I and Fig. 1). In addition, there were no
significant statistical differences in the comparative analysis
between the two groups regarding AST values. The evolution trend
was linear with a significance level of P=0.05 (Fig. 2).
The GGT values of were higher in the group of obese
patients (group A) compared to the normoponderous patients, this
difference being particularly highlighted in the third trimester
(Table II and Fig. 3).
Both albumin and direct bilirubin levels have
relative constant values throughout pregnancy, with albumin levels
remaining close to the lower normal limits (3.5 µ/l). However, none
of these values indicated statistical significance.
Given the fact that the only liver function test
that varies between the two groups is AST, we performed a Pearson
correlation analysis; the data studied concerns the Gaussian
distribution. Thus, a directly proportional result and a
significant Pearson correlation (r=0.19; P=0.012) were obtained.
Furthermore, a larger abdominal circumference is associated with
higher levels of AST during T1 pregnancy (Table III and Fig. 6).
In conclusion, obesity during pregnancy does not
markedly influence the patient's liver function. However, patients
with greater abdominal circumference are prone to developing a
slight hepatic cytolysis syndrome during pregnancy. The functional
tests of the liver that we used in the present study agree with the
results provided by the specialized studies.
Acknowledgements
This study, together with the efforts in article
publishing were self-founded.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CODT analyzed and interpreted the patients' data
regarding the hepatic function by comparing the functional tests
used in current practice and critically revised the manuscript for
its content. FS analyzed and interpreted the patients' data
regarding the hepatic function by comparing the functional tests
used in current practice and is the corresponding author. AC
identified the possible predictors of liver damage and wrote the
manuscript. AT revised the literature data and analyzed specific
anthropometric patient data. ARP researched the papers that were
included as references. AIM designed the experiments and critically
revised the manuscript and approved the current form of the article
in order to be submitted to the journal. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the ethics commission of
the National Institute for Maternal and Child Health ‘Alessandrescu
Rusescu’ on December 5th, 2019 with ref. no. 17852/07.11.2019.
Written informed consent was provided by the patients.
Patient consent for publication
The patients have given written informed consent for
the publication of the data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
WHO. Geneva, Switzerland: World Health
Organization, 2006. https://www.who.int/whosis/whostat2006.pdf.
|
|
2
|
De Pergola G and Silvestris F: Obesity as
a major risk factor for cancer. J Obes. 2013(291546)2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kessous R, Davidson E, Meirovitz M,
Sergienko R and Sheiner E: Prepregnancy obesity: A risk factor for
future development of ovarian and breast cancer. Eur J Cancer Prev.
26:151–155. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Verga N, Mirea DA, Busca I, Poroschianu
MN, Zarma SF, Grinisteanu L, Anica A, Gheorghe CA, Stan CA, Verga M
and Vasilache R: Optical coherence tomography in oncological
imaging. Rom Rep Phys. 66:75–86. 2014.PubMed/NCBI
|
|
5
|
Scarlat F, Scarisoreanu A and Verga N:
Absorbed dose distributions using the isodensitometric method for
exposures with filter employed for mammographies. Rom Rep Phys.
65:168–177. 2013.
|
|
6
|
Sardari D and Verga N: Calculation of
externally applied electric field intensity for disruption of
cancer cell proliferation. Electromagn Biol Med. 29:26–30.
2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sardari D and Verga N: A physical model
for study of electromagnetic field interaction with cancer cell.
Prog Electromagn Res Symp. 2:891–893. 2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Adams EJ, Grummer-Strawn L and Chavez G:
Food insecurity is associated with increased risk of obesity in
California women. J Nutr. 133:1070–1074. 2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rooney BL and Schauberger CW: Excess
pregnancy weight gain and long-term obesity: One decade later.
Obstet Gynecol. 100:245–252. 2002.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lynch CM, Sexton DJ, Hession M and
Morrison JJ: Obesity and mode of delivery in primigravid and
multigravid women. Am J Perinatol. 25:163–167. 2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dumitrascu MC, Stanescu AMA, Bejan C,
Sandru F, Toader DO, Radavoi DG, Cotirlet A, Judea-Pusta CT and
Diaconu CC: Obesity and its Implications on Stress Urinary
Incontinence. Rev Chim. 70:3660–3662. 2019.
|
|
12
|
O'Brien TE, Ray JG and Chan WS: Maternal
body mass index and the risk of preeclampsia: A systematic
overview. Epidemiology. 14:368–374. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Soens MA, Birnbach DJ, Ranasinghe JS and
van Zundert A: Obstetric anesthesia for the obese and morbidly
obese patient: An ounce of prevention is worth more than a pound of
treatment. Acta Anaesthesiol Scand. 52:6–19. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ehrenberg HM, Mercer BM and Catalano PM:
The influence of obesity and diabetes on the prevalence of
macrosomia. Am J Obstet Gynecol. 191:964–968. 2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hedderson MM, Williams MA, Holt VL, Weiss
NS and Ferrara A: Body mass index and weight gain prior to
pregnancy and risk of gestational diabetes mellitus. Am J Obstet
Gynecol. 198:409.e1–e7. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chu SY, Callaghan WM, Kim SY, Schmid CH,
Lau J, England LJ and Dietz PM: Maternal obesity and risk of
gestational diabetes mellitus. Diabetes Care. 30:2070–2076.
2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Radaelli T, Varastehpour A, Catalano P and
Hauguel-de Mouzon S: Gestational diabetes induces placental genes
for chronic stress and inflammatory pathways. Diabetes.
52:2951–2958. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yogev Y and Langer O: Pregnancy outcome in
obese and morbidly obese gestational diabetic women. Eur J Obstet
Gynecol Reprod Biol. 137:21–26. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Usha Kiran TS, Hemmadi S, Bethel J and
Evans J: Outcome of pregnancy in a woman with an increased body
mass index. BJOG. 112:768–772. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Brown CD, Higgins M, Donato KA, Rohde FC,
Garrison R, Obarzanek E, Ernst ND and Horan M: Body mass index and
the prevalence of hypertension and dyslipidemia. Obes Res.
8:605–619. 2000.PubMed/NCBI View Article : Google Scholar
|
|
21
|
de Ferranti S and Mozaffarian D: The
perfect storm: Obesity, adipocyte dysfunction, and metabolic
consequences. Clin Chem. 54:945–955. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Boden G: Fatty acid-induced inflammation
and insulin resistance in skeletal muscle and liver. Curr Diab Rep.
6:177–181. 2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Boden G: Role of fatty acids in the
pathogenesis of insulin resistance and NIDDM. Diabetes. 46:3–10.
1997.PubMed/NCBI
|
|
24
|
Hotamisligil GS and Erbay E: Nutrient
sensing and inflammation in metabolic diseases. Nat Rev Immunol.
8:923–934. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Lederman SA, Paxton A, Heymsfield SB, Wang
J, Thornton J and Pierson RN Jr: Body fat and water changes during
pregnancy in women with different body weight and weight gain.
Obstet Gynecol. 90:483–488. 1997.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Okereke NC, Huston-Presley L, Amini SB,
Kalhan S and Catalano PM: Longitudinal changes in energy
expenditure and body composition in obese women with normal and
impaired glucose tolerance. Am J Physiol Endocrinol Metab.
287:E472–E479. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kopp-Hoolihan LE, van Loan MD, Wong WW and
King JC: Fat mass deposition during pregnancy using a
four-component model. J Appl Physiol (1985). 87:196–202.
1999.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Dumitrascu MC, Iliescu M, Petca RC, Sandru
F, Mehedintu P, Farcasanu PD, Maru N, Chibelean C and Petca A: The
chemical pregnancy. Rev Chim. 70:3818–3823. 2019.
|
|
29
|
Alese MO, Moodley J and Naicker T:
Preeclampsia and HELLP syndrome, the role of the liver. J Matern
Fetal Neonatal Med. 34:117–123. 2021.PubMed/NCBI View Article : Google Scholar
|