Introduction
It was estimated that there were almost 1.3 million
new worldwide cases of prostate cancer and 359,000 associated
deaths in 2018, ranking prostate cancer as the second most common
cancer and the fifth leading cause of cancer-associated mortality
in males (1). Although androgen
deprivation therapy (ADT) has been significant for the treatment of
prostate cancer, numerous patients eventually become insensitive to
the therapy and progress to incurable castration-resistant prostate
cancer (CRPC) (2,3). Therefore, identifying targets
associated with prostate cancer occurrence and development is vital
for the development of novel therapeutic targets.
ChaC glutathione specific γ-glutamylcyclotransferase
1 (CHAC1) was first identified in mammalian cells in 2009 as a new
component of the unfolded protein response (UPR) pathway (4). It is induced in response to
endoplasmic reticulum (ER) stress (5). CHAC1 is a proapoptotic ER stress
protein downstream of the pancreatic EIF2α kinase-ATF4 pathway and
appears to be important for human physiology and disease (6). CHAC1 was observed to have γ-glutamyl
cyclotransferase activity (7) and
overexpression leads to a robust depletion of glutathione (GSH)
(8). A previous study reported that
GSH depletion may stimulate ferroptosis (9). Ferroptosis is a novel programmed cell
death mechanism that is characterized by the accumulation of
reactive oxygen species (ROS) resulting from iron accumulation and
lipid peroxidation (10,11). Considering that GSH is a major
intracellular antioxidant, CHAC1 may have an important role in
cellular oxidative homeostasis (6).
However, the role of CHAC1 in prostate cancer,
particularly in CRPC, remains unclear. The present study found that
overexpression of CHAC1 in two CRPC cell lines, DU145 and 22RV1,
inhibited cell viability and increased the sensitivity to docetaxel
(DTX). The underlying mechanisms were likely associated with the
inductive effects of CHAC1 on ER stress and ferroptosis.
Materials and methods
Cell culture
The human prostate epithelial RWPE-1 cell line was
cultured in complete keratinocyte serum-free medium containing
basal K-SFM (Invitrogen; Thermo Fisher Scientific, Inc.),
supplemented with 50 µg/ml bovine pituitary extract (BPE;
Invitrogen; Thermo Fisher Scientific, Inc.), 5 ng/ml epidermal
growth factor (EGF; R&D Systems, Inc.) and 1%
antibiotic/antimycotic mixture (PSF). Human prostate cancer DU145
cells were cultured in Dulbecco's modified Eagle's medium
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.). The human prostate cancer 22RV1 cell line was
cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc.), supplemented with 10% FBS. All cell lines were obtained from
the Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences and incubated in a humidified atmosphere with 5%
CO2 at 37˚C.
GSH ethyl ester treatment
A total of 5 mM GSH ethyl ester (Sigma-Aldrich;
Merck KGaA) was added to CHAC1-transfected DU145 and 22RV1 cells at
24 h after transfection and incubated at 37˚C for an additional 24
h.
Cell treatment
A total of 50 µM Erastin (MedChemExpress) was added
to DU145 and 22RV1 cells and incubated at 37˚C for 24 h. DTX was
purchased from Sanofi-Aventis Pharmaceuticals. Ferrostatin was
purchased from MedChemExpress. DU145 and 22RV1 cells were treated
with various concentrations (0, 0.1, 1, 5, 10, 20, 50 and 100 nM)
of DTX alone or combined with 1 µM ferrostatin at 37˚C for 48 h.
DTX and ferrostatin were added to CHAC1-transfected DU145 and 22RV1
cells at 24 h after transfection and incubated at 37˚C for an
additional 48 h.
Construction of the plasmid
overexpressing CHAC1
The pcDNA3-Flag-CHAC1 plasmid was constructed by
inserting the cDNA fragment encoding human CHAC1 protein
(NM_024111.6) into the pcDNA3 vector with a Flag tag at its
N-terminal in frame to generate the Flag-tagged CHAC1 fusion
protein. The DNA construct was confirmed by sanger sequencing
(Shanghai Personalbio Technology Co., Ltd.).
Plasmid transfection
Plasmid transfection was performed at 75% cell
density of DU145 or 22RV1. The Flag-tagged CHAC1-overexpressing
plasmid and its control vector was transfected using the
Lipofectamine 3000 transfection reagent (Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol, and incubated in a
humidified atmosphere with 5% CO2 at 37˚C for 48 h.
Following transfection for 48 h, the cells were harvested for
subsequent assays.
Small interfering RNA (siRNA)
transfection
siRNA transfection was performed at 50% cell density
of DU145 or 22RV1. The siRNA specific to CHAC1
(5'-AUCUUCAAGGAGCGUCACCAC-3'; cat. no. SR312343; OriGene
Technologies, Inc.) and its negative control
(5'-GUUAAAUAGCGAUAGGAAUUC-3'; cat. no. SR30002; OriGene
Technologies, Inc.) were commercially purchased. Transfection of
siRNA-CHAC1 and its control (final conc. 10 nM) was performed using
the lipofectamine RNAiMAX reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocols and cells were incubated
in a humidified atmosphere with 5% CO2 at 37˚C for 72 h.
Following transfection, the cells were harvested for subsequent
assays at once.
RNA extraction and reverse
transcription
Total cellular RNA was harvested using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocols. RNA was then reverse transcribed to
cDNA at 37˚C for 15 min and 98˚C for 5 min using the SuperScript
First-Stand Synthesis system (Invitrogen; Thermo Fisher Scientific,
Inc.).
Quantitative polymerase chain reaction
(qPCR)
SYBR Green (Toyobo Life Science) qPCR was performed
to determine the mRNA expression levels of CHAC1 and β-actin (used
as an internal control). The following primers were used: CHAC1
forward, 5'-TGTGGATTTTCGGGTACGGC-3' and reverse,
5'-CTTGCTTACCTGCTCCCCTT-3'; and β-actin forward,
5'-GTTGCTATCCAGGCTGTGCTA-3' and reverse,
5'-TGTCACGCACGATTTCCCGCT-3'. qPCR was performed on ABI 7500 System
(Applied Biosystems; Thermo Fisher Scientific Inc.) using the
following thermocycling conditions: 95˚C for 30 sec; followed by 40
cycles at 95˚C for 5 sec; 60˚C for 30 sec and 72˚C for 15 sec. mRNA
expression levels of CHAC1 were normalized to β-actin mRNA
expression levels. Relative CHAC1 mRNA levels were calculated using
the comparative 2-∆∆Cq method (12).
Western blot analysis
Cells were harvested and centrifuged at 4˚C, 2,000 x
g for 5 min. Cells were then lysed in 1X sodium dodecyl sulfate
(SDS) loading buffer (Beyotime Institute of Biotechnology). Protein
concentrations of the lysates were measured using the BCA protein
assay kit (Pierce; Thermo Fisher Scientific, Inc.). The lysates
were boiled for 10 min, cooled and then centrifuged at 4˚C, 12,000
x g for 10 min. A total of 30 µg/lane protein extracts were loaded
onto a 10% SDS-polyacrylamide gel and then electrophoresed and
transferred onto a polyvinylidene fluoride membrane (EMD
Millipore). The blots were blocked at room temperature for 1 h in
skimmed milk in tris-buffered saline and incubated with primary
antibodies overnight at 4˚C. Following washing, the blots were
incubated with IR-dye based secondary antibodies (LI-COR) for 1 h
at room temperature. Protein bands were visualized using an Odyssey
scanner (LI-COR Biosciences). The densitometry of the protein bands
was quantified using the Odyssey analyzer software (LI-COR
Biosciences). Rabbit anti-CHAC1 (cat. no. HPA043505; dilution,
1:500; Atlas Antibodies AB), rabbit anti-BIP (cat. no. 3177;
dilution, 1:500; Cell Signaling Technology, Inc.), mouse anti-CHOP
(cat. no. 2895; dilution, 1:500; Cell Signaling Technology, Inc.),
rabbit anti-LC3B (cat. no. 3868; dilution, 1:500; Cell Signaling
Technology, Inc.), rabbit anti-GPX4 (cat. no. HPA058546; dilution,
1:500; Atlas Antibodies AB), rabbit anti-Flag (cat. no. 14793;
dilution, 1:500; Cell Signaling Technology, Inc.), mouse
anti-β-actin (cat. no. A5441; dilution, 1:500; Sigma-Aldrich; Merck
KGaA) were used as primary antibodies and IR-dye based goat
anti-mouse or goat anti-rabbit IgG (cat. nos. 925-68070/926-32211;
dilution, 1:10,000; LI-COR Biosciences) were used as secondary
antibodies.
Cell Counting kit-8 (CCK-8) assay
Cell viability was measured using the CCK-8 regent
(Dojindo Molecular Technologies, Inc.), according to the
manufacturer's protocols.
Glutathione (GSH) measurement
Cell GSH levels were measured using the glutathione
assay kit (cat. no. CS0260; Sigma-Aldrich; Merck KGaA), according
to the manufacturer's protocols.
Detection of intracellular lipid
peroxides
DU145 or 22RV1 cells were seeded onto 96-well plates
at a density of 1x104 cells/well the day before
transfection to perform image-based analysis for intracellular
peroxides. After 16 h, cells were transfected with Flag-tagged
CHAC1-overexpressing plasmid or its control vector using the
Lipofectamine 3000® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocols. A
total of 48 h after transfection at 37˚C, intracellular lipid
peroxides were detected using Hoechst 33342 (Beyotime Institute of
Biotechnology) and Liperflo Reagent (Dojindo Molecular
Technologies, Inc.), according to the manufacturer's protocols.
Images were obtained using the Operetta high-content imaging system
(PerkinElmer, Inc.) and analyzed using the Harmony v.4.8 software
(PerkinElmer, Inc.) to determine intracellular lipid peroxide
levels.
Statistical analysis
All experiments were performed at least in
triplicate. Data are expressed as the mean ± standard error of the
mean. Statistical analysis was performed using a two-tailed
unpaired t-test for two groups and one-way analysis of variance,
followed by Turkey's test, for multiple groups using Prism5
software (GraphPad Software, Inc.).
Results
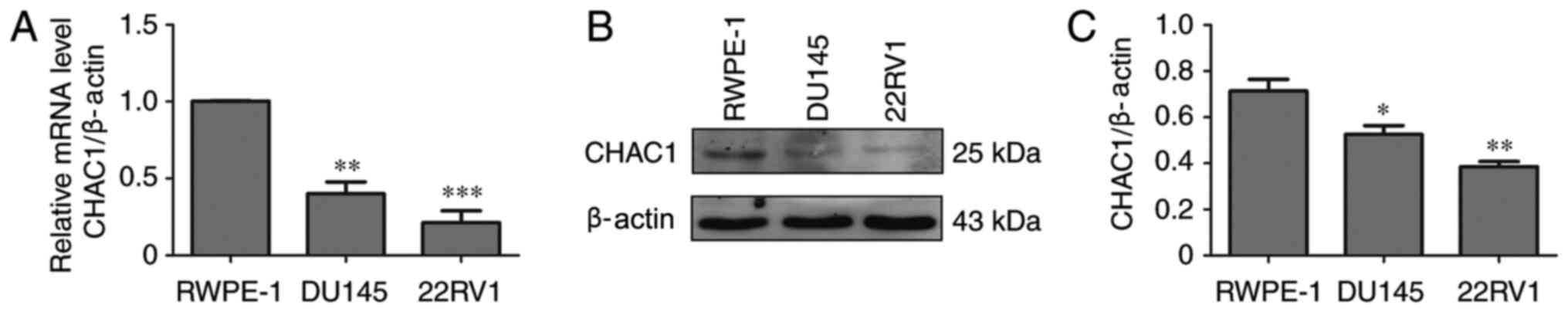
CHAC1 expression levels are decreased
in prostate cancer cells
To determine the function of CHAC1 in prostate
cancer, CHAC1 mRNA and protein levels were measured in non-tumor
human prostate epithelial RWPE-1 cells and two prostate cancer cell
lines, DU145 and 22RV1, by qPCR and western blotting. The results
demonstrated that, compared with RWPE-1 cells, CHAC1 mRNA and
protein levels were significantly decreased in DU145 and 22RV1
cells (P<0.05; Fig. 1).
CHAC1 inhibits cell viability and
decreases intracellular GSH levels
To determine the function of CHAC1 in prostate
cancer, a CHAC1-overexpression plasmid and CHAC1 siRNA were
constructed. Subsequently CHAC1 protein levels were determined in
DU145 cells. CHAC1 was significantly upregulated in cells
transfected with Flag-tagged CHAC1-overexpressing plasmid, and
downregulated in cells transfected with siRNA specific for CHAC1
(Fig. 2A).
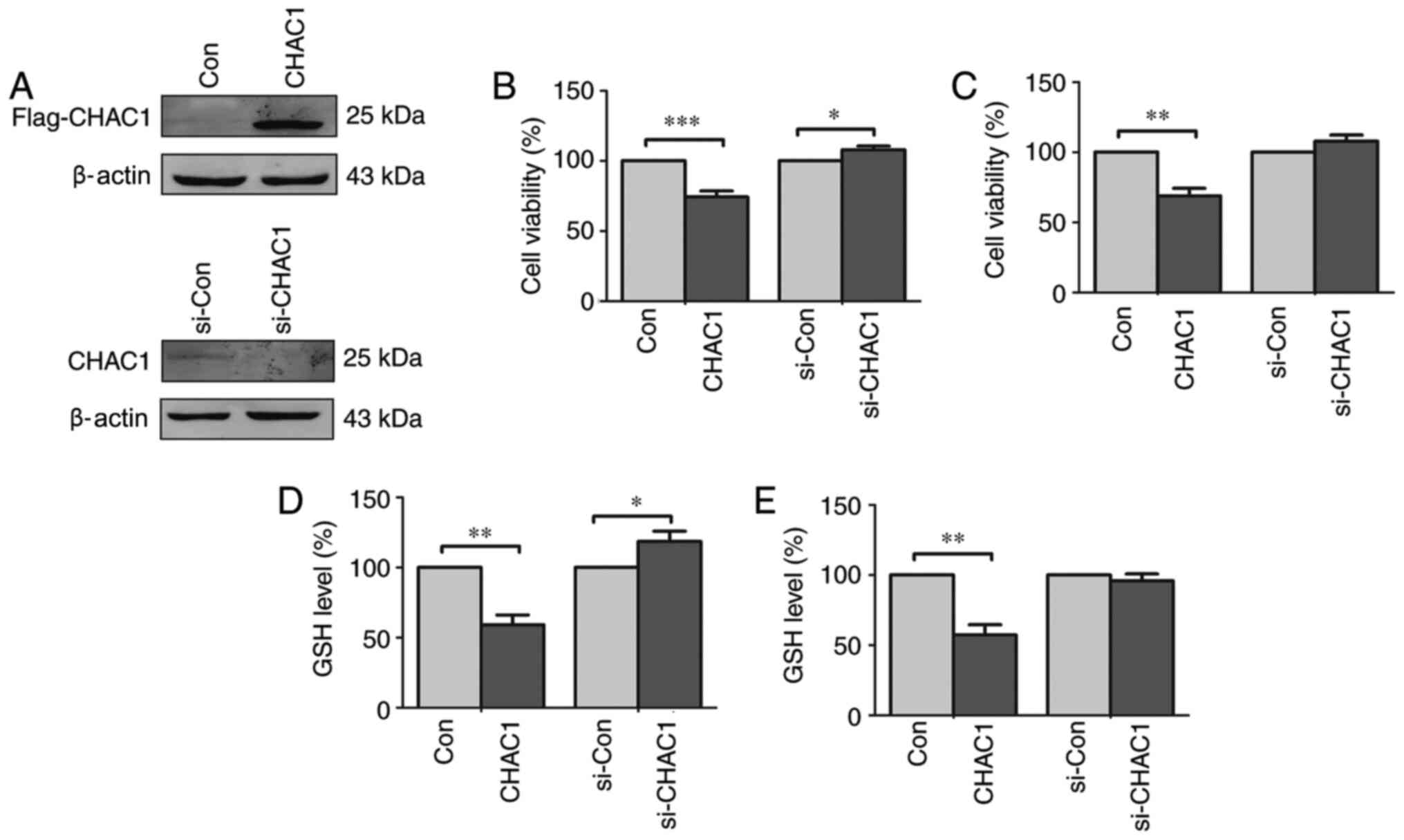 | Figure 2CHAC1 inhibits cell viability and
reduces intracellular GSH levels in DU145 cells and 22RV1 cells.
(A) The representative western blotting image of the expression of
CHAC1 protein in DU145 cells. The top panel was the immunoblot
confirmed by anti-Flag antibody in DU145 cells transfected with
Flag-tagged CHAC1-overexpression plasmid (CHAC1) or with Con. The
bottom panel was the immunoblot confirmed by anti-CHAC1 antibody in
DU145 cells transfected with si-CHAC1 or with si-Con. (B) Cell
viability of DU145 cells transfected with Flag-tagged
CHAC1-overexpression plasmid (CHAC1) or with Con, or transfected
with si-CHAC1 or with si-Con determined by CCK-8 assay. (C) Cell
viability of 22RV1 cells transfected with Flag-tagged
CHAC1-overexpression plasmid (CHAC1) or with negative plasmid
(Con), or transfected with siRNA specific for CHAC1 (si-CHAC1) or
with negative siRNA (si-Con) determined by CCK-8 assay. (D)
Intracellular level of GSH in DU145 cells transfected with
Flag-tagged CHAC1-overexpression plasmid (CHAC1) or with Con, or
transfected with si-CHAC1 or with si-Con. (E) Intracellular level
of GSH in 22RV1 cells transfected with Flag-tagged
CHAC1-overexpression plasmid (CHAC1) or with Con, or transfected
with si-CHAC1 or with si-Con. Data are presented as the mean ±
standard error of the mean from three independent experiments.
*P<0.05, **P<0.01,
***P<0.001. CHAC1, ChaC glutathione specific
gamma-glutamylcyclotransferase 1; Con, negative plasmid; si-CHAC1,
CHAC1 small interfering RNA; si-Con, negative siRNA; CCK-8, Cell
Counting kit-8; GSH, glutathione. |
The CCK-8 assay demonstrated that cell viability was
significantly decreased in DU145 and 22RV1 cells transfected with
CHAC1-overexpressing plasmid (P<0.01), while cell viability was
significantly higher in DU145 cells transfected with CHAC1 siRNA
(P<0.05), but not in 22RV1 cells (P>0.05; Fig. 2B and C). It was also observed that GSH levels
were significantly decreased in DU145 and 22RV1 cells transfected
with CHAC1-overexpressing plasmid (P<0.01), while GSH levels
were significantly increased in DU145 cells transfected with CHAC1
siRNA (P<0.05), but not in 22RV1 cells (Fig. 2D and E).
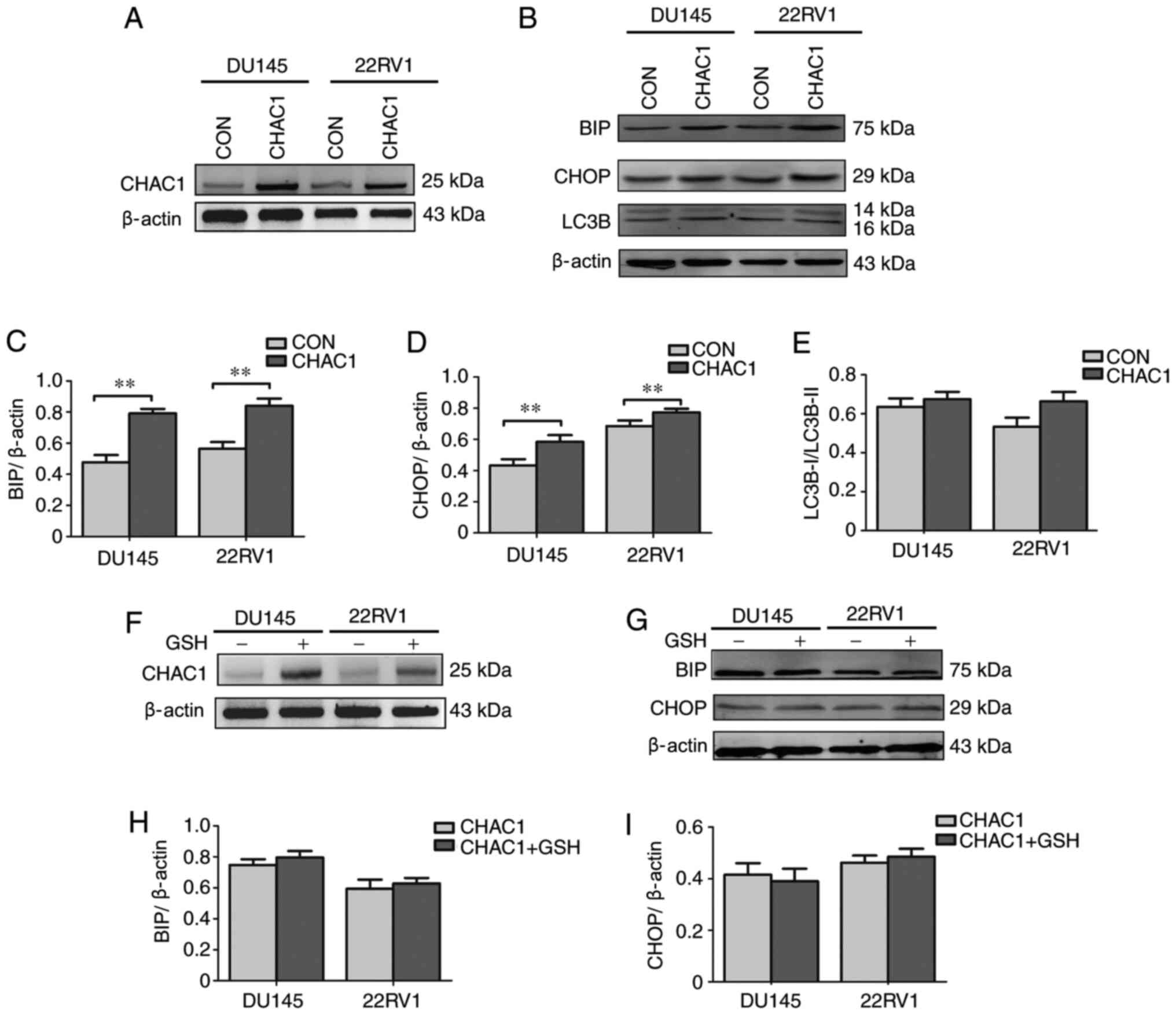
CHAC1 enhances ER stress
The present study subsequently focused on the
associations between CHAC1 and ER stress in prostate cancer by
measuring the expression levels of ER stress-related factors, BIP,
CHOP and LC3B. As shown in Fig.
3A-E, BIP and CHOP levels were significantly upregulated in
DU145 and 22RV1 cells following transfection with
CHAC1-overexpressing plasmid (P<0.01), but LC3B levels were not
(P<0.05).
To further determine the association between CHAC1
and ER stress, DU145 and 22RV1 cells were transfected with
CHAC1-overexpressing plasmid and exposed to GSH simultaneously. The
results demonstrated that the increase in BIP and CHOP levels was
decreased by exposure to GSH (Fig.
3F-I). This suggested that CHAC1 may induce BIP and CHOP
expression by decreasing the levels of GSH to further enhance ER
stress.
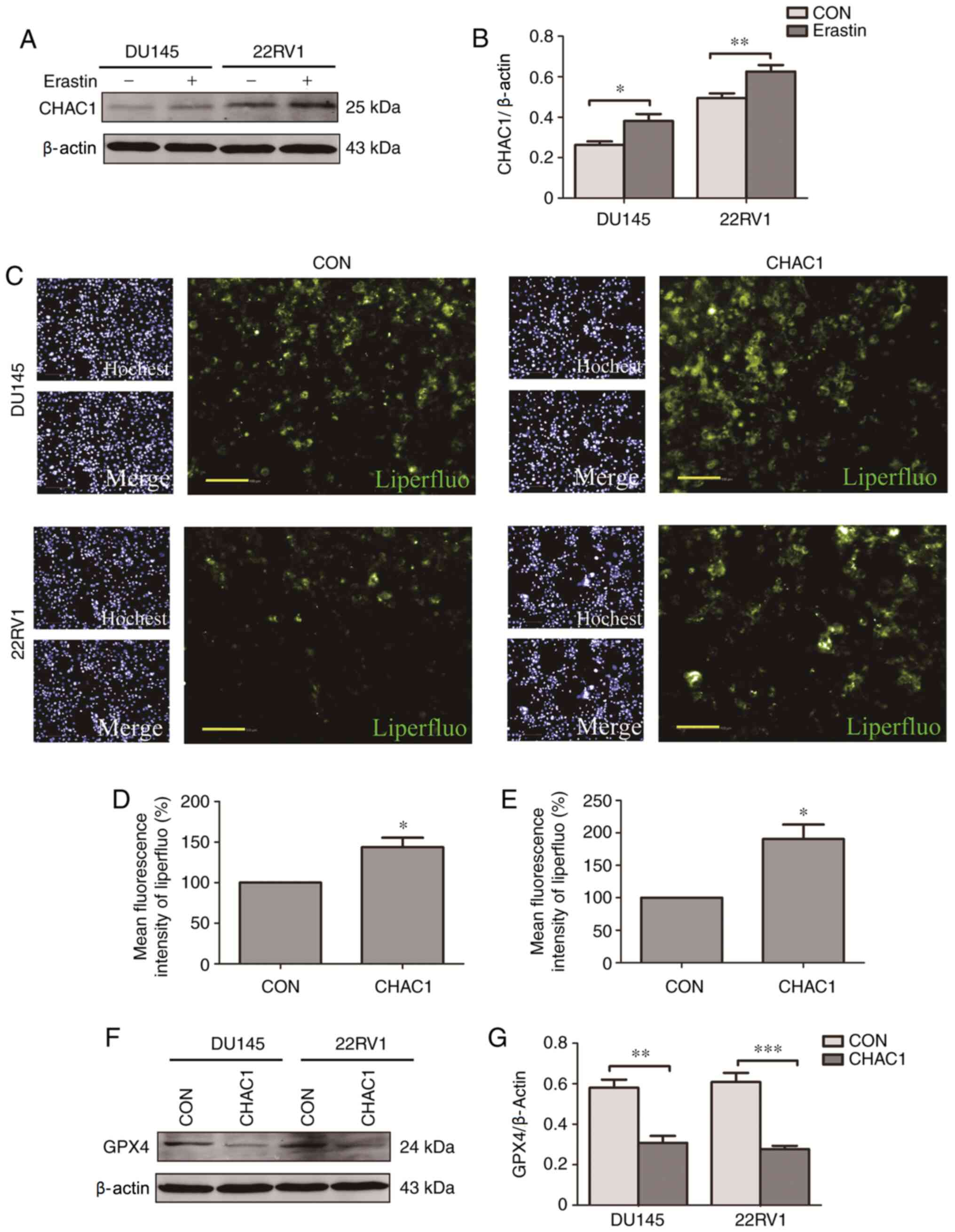
CHAC1 promotes ferroptosis
The role of CHAC1 in ferroptosis was subsequently
investigated. It was observed that CHAC1 protein levels were
significantly upregulated in DU145 and 22RV1 cells treated with
Erastin, a type of ferroptosis activator (P<0.05; Fig. 4A and B). Additionally, intracellular lipid
peroxide levels were significantly increased following transfection
with CHAC1-overexpressing plasmid (P<0.05; Fig. 4C-E), while GPX4 protein levels were
significantly decreased following CHAC1-overexpression (P<0.01;
Fig. 4F and G). These results indicated that CHAC1 may
induce ferroptosis.
CHAC1 increases the sensitivity of
prostate cancer cells to DTX
Next, the effect of CHAC1 and ferroptosis on the
sensitivity of prostate cancer cells to DTX was investigated. The
cell viability of DU145 and 22RV1 cells treated with different
concentrations of DTX was measured. As shown in Fig. 5A and B, cell viability was only significantly
inhibited when the concentration of DTX was >5 nM (P<0.01).
However, when prostate cancer cells were treated with 1 nM DTX
following transfection of the CHAC1-overexpressing plasmid, cell
viability was significantly decreased (P<0.001; Fig. 5C and D). Additionally, when prostate cancer
cells were transfected with CHAC1-overexpressing plasmid followed
by co-treatment with 1 nM DTX and 1 µM ferrostatin, a type of
ferroptosis inhibitor, the effect of CHAC1 on decreasing cell
viability was lessened. Cell viability was not significantly
decreased by DTX (P>0.05; Fig.
5E and F). These results
suggested that CHAC1 increases the sensitivity of prostate cancer
cells to DTX by inducing ferroptosis.
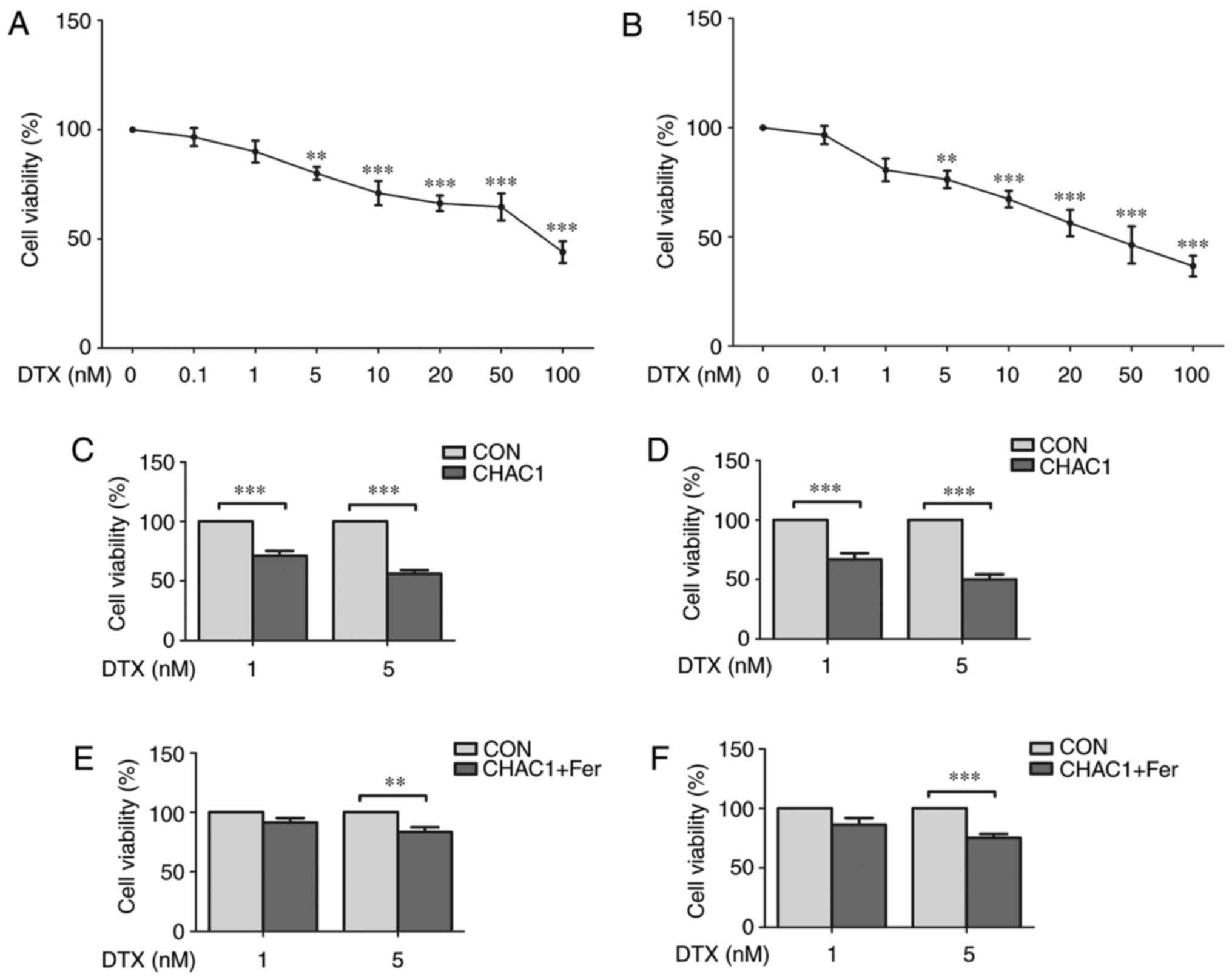 | Figure 5CHAC1 increases the sensitivity of
prostate cancer cells to docetaxel. (A) Cell viability of DU145
cells treated with 0, 0.1, 1, 5, 10, 20, 50 or 100 nM DTX
determined by CCK-8 assay. (B) Cell viability of 22RV1 cells
treated with 0, 0.1, 1, 5, 10, 20, 50 or 100 nM DTX determined by
CCK-8 assay. (C) Cell viability of DU145 cells treated with 1 or 5
nM DTX following transfection with CHAC1-overexpression plasmid
(CHAC1) or with Con determined by CCK-8 assay. (D) Cell viability
of 22RV1 cells treated with 1 or 5 nM DTX following transfection
with CHAC1-overexpression plasmid (CHAC1) or with Con determined by
CCK-8 assay. (E) Cell viability of DU145 cells transfected with
CHAC1-overexpression plasmid was determined by CCK-8 assay
following co-treatment with 1 µM Fer or DMSO (Con) and 1 or 5 nM
DTX. (F) Cell viability of 22RV1 cells transfected with
CHAC1-overexpression plasmid was determined by CCK-8 assay
following co-treatment with 1 µM Fer or DMSO (Con) and 1 or 5 nM
DTX. Data are presented as the mean ± standard error of the mean
from three independent experiments. **P<0.01,
***P<0.001. DTX, docetaxel; CHAC1, ChaC glutathione
specific gamma-glutamylcyclotransferase 1; CCK-8, Cell Counting
kit-8; Con, negative plasmid; Fer, ferrostatin. |
Discussion
The present study demonstrated that CHAC1 expression
levels in prostate cancer cells were significantly decreased,
compared with normal prostate epithelial cells. CHAC1 expression
levels were associated with cell viability and GSH levels. A
previous study demonstrated that CHAC1 acts on cytoplasmic pools
and its primary function was to alter the redox potential that
serves as activation signals (13).
Results from this study and the previous study (13) suggested a possible role of CHAC1 in
prostate cancer.
Cells may respond to an abrupt accumulation of
secretory proteins within the ER through pathways, including UPR
(14,15). The state of cell health during ER
stress ultimately decides cell fate (16). Previous studies have demonstrated
that an increase in CHAC1 levels was associated with the activation
of ER stress (17,18). In the present study, overexpression
of CHAC1 significantly upregulated the expression of ER
stress-related factors, BIP and CHOP, while co-treatment with GSH
inhibited ER stress. This indicated that CHAC1 may induce BIP and
CHOP expression levels by decreasing GSH levels to further enhance
ER stress. However, in another previous study, CHAC1 siRNA
treatment did not affect BIP and CHOP expression levels, while CHOP
siRNA treatment inhibited CHAC1 expression (4). Therefore, the associations between
CHAC1 and CHOP may be more complex and require further
investigation.
A previous study associated CHAC1 expression levels
with ER stress induction (19),
while the ER stress signaling pathway has been observed to
contribute toward ferroptosis induction (20,21).
The increase in CHAC1 expression levels has been widely regarded as
an indicator for early ferroptosis and have been associated with
GSH degradation and the initiation of ferroptosis (22). This is consistent with the
observations from the present study. Using prostate cancer cells,
it was observed that overexpression of CHAC1 increased
intracellular lipid peroxide levels and decreased GPX4 protein
levels, resulting in the induction of ferroptosis. To the best of
our knowledge, the present study was the first to demonstrate the
effect of CHAC1 on ER stress and ferroptosis in prostate cancer
cells. In addition, the ferroptosis process is defined by the
iron-dependent accumulation of lipid reactive oxygen species and
depletion of plasma membrane polyunsaturated fatty acids. Cancer
cells with high level activities of the RAS-RAF-MEK or
GCN2-eIF2α-ATF4 pathways may be sensitized to this process
(23,24). In future studies, it is worthy
investigating the activation of those pathways.
CRPC therapy is not effective in decreasing
mortality; therefore, the present study investigated whether CHAC1
may increase the sensitivity of prostate cancer cells to DTX by
inducing ferroptosis. CHAC1 significantly increased the sensitivity
of prostate cancer cells to DTX, and the effect was reversed
following co-treatment with a ferroptosis inhibitor. This suggested
an important role of CHAC1 in CRPC therapy. Combined therapy is
gaining increased attention, and several studies have demonstrated
synergistic effects (25-27).
The results of the present study suggested that CHAC1 may be a
potential therapeutic target in combination with other therapeutics
(including DTX) for the treatment of CRPC. Although the effects of
CHAC1 have been confirmed in in vitro models, additional
studies are required in the future to demonstrate its efficacy in
other cell lines and in vivo. This should also be
accompanied by clinical studies assessing the expression levels and
activity levels of CHAC1.
In conclusion, it was found that CHAC1 could inhibit
cell viability and increase the sensitivity of prostate cancer
cells to DTX. The mechanism may involve the induction of ER stress
and ferroptosis. The results of the present study may provide a
potential novel therapeutic target for the treatment of prostate
cancer, including CRPC. Additional studies are required to
investigate the association between CHAC1 levels and the clinical
outcome of patients with prostate cancer.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by grants from National
Natural Science Foundation of China (grant nos. 81904044 and
81904036), Shanghai Municipal Health Commission (grant no.
20174Y0044), Science and Technology Development Fund of Shanghai
Pudong New Area (grant no. PKJ2017-Y14), Talents Training Program
of Pudong Health Commission of Shanghai (grant no. PWRq2017-02) and
Talents Training Program of Seventh People's Hospital of Shanghai
University of Traditional Chinese Medicine (grant nos. XX2017-06
and XX2019-01).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WX and YS conceived the experiments. SH, MZ and XM
conducted the experiments. YY and JZ analyzed the data. YS wrote
the paper. WX and YS revised the paper. WX and YS confirmed the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Xiao L, Tien JC, Vo J, Tan M, Parolia A,
Zhang Y, Wang L, Qiao Y, Shukla S, Wang X, et al: Epigenetic
reprogramming with antisense oligonucleotides enhances the
effectiveness of androgen receptor inhibition in
castration-resistant prostate cancer. Cancer Res. 78:5731–5740.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Liao Y, Guo Z, Xia X, Liu Y, Huang C,
Jiang L, Wang X, Liu J and Huang H: Inhibition of EGFR signaling
with Spautin-1 represents a novel therapeutics for prostate cancer.
J Exp Clin Cancer Res. 38(157)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mungrue IN, Pagnon J, Kohannim O,
Gargalovic PS and Lusis AJ: CHAC1/MGC4504 is a novel proapoptotic
component of the unfolded protein response, downstream of the
ATF4-ATF3-CHOP cascade. J Immunol. 82:466–476. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Perra L, Balloy V, Foussigniere T,
Moissenet D, Petat H, Mungrue IN, Touqui L, Corvol H, Chignard M
and Guillot L: CHAC1 is differentially expressed in normal and
cystic fibrosis bronchial epithelial cells and regulates the
inflammatory response induced by pseudomonas aeruginosa. Front
Immunol. 9(2823)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Crawford RR, Prescott ET, Sylvester CF,
Higdon AN, Shan J, Kilberg MS and Mungrue IN: Human CHAC1 protein
degrades glutathione, and mRNA induction is regulated by the
transcription factors ATF4 and ATF3 and a bipartite ATF/CRE
regulatory element. J Biol Chem. 290:15878–15891. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kumar A, Tikoo S, Maity S, Sengupta S,
Sengupta S, Kaur A and Bachhawat AK: Mammalian proapoptotic factor
ChaC1 and its homologues function as γ-glutamyl cyclotransferases
acting specifically on glutathione. EMBO Rep. 13:1095–1101.
2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ogawa T, Wada Y, Takemura K, Board PG,
Uchida K, Kitagaki K, Tamura T, Suzuki T, Tokairin Y, Nakajima Y
and Eishi Y: CHAC1 overexpression in human gastric parietal cells
with Helicobacter pylori infection in the secretory canaliculi.
Helicobacter. 24(e12598)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yang WS, SriRamaratnam R, Welsch ME,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF, Clish CB, et al: Regulation of ferroptotic cancer cell death by
GPX4. Cell. 156:317–331. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shi ZZ, Fan ZW, Chen YX, Xie XF, Jiang W,
Wang WJ, Qiu YT and Bai J: Ferroptosis in carcinoma: Regulatory
mechanisms and new method for cancer therapy. Onco Targets Ther.
12:11291–11304. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bebber CM, Muller F, Prieto Clemente L,
Weber J and von Karstedt S: Ferroptosis in cancer cell biology.
Cancers (Basel). 12(164)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yadav S, Chawla B, Khursheed MA,
Ramachandran R and Bachhawat AK: The glutathione degrading enzyme,
Chac1, is required for calcium signaling in developing zebrafish:
Redox as an upstream activator of calcium. Biochem J.
476:1857–1873. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chadwick SR and Lajoie P: Endoplasmic
reticulum stress coping mechanisms and lifespan regulation in
health and diseases. Front Cell Dev Biol. 7(84)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zeeshan HM, Lee GH, Kim HR and Chae HJ:
Endoplasmic reticulum stress and associated ROS. Int J Mol Sci.
17(327)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Siwecka N, Rozpedek W, Pytel D,
Wawrzynkiewicz A, Dziki A, Dziki Ł, Diehl JA and Majsterek I: Dual
role of endoplasmic reticulum stress-mediated unfolded protein
response signaling pathway in carcinogenesis. Int J Mol Sci.
20(4354)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Scheffer D, Kulcsár G, Nagyéri G,
Kiss-Merki M, Rékási Z, Maloy M and Czömpöly T: Active mixture of
serum-circulating small molecules selectively inhibits
proliferation and triggers apoptosis in cancer cells via induction
of ER stress. Cell Signal. 65(109426)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cao SS and Kaufman RJ: Endoplasmic
reticulum stress and oxidative stress in cell fate decision and
human disease. Antioxid Redox Signal. 21:396–413. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gagliardi M, Cotella D, Santoro C, Corà D,
Barlev NA, Piacentini M and Corazzari M: Aldo-keto reductases
protect metastatic melanoma from ER stress-independent ferroptosis.
Cell Death Dis. 10(902)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Dixon SJ, Patel DN, Welsch M, Skouta R,
Lee ED, Hayano M, Thomas AG, Gleason CE, Tatonetti NP, Slusher BS
and Stockwell BR: Pharmacological inhibition of cystine-glutamate
exchange induces endoplasmic reticulum stress and ferroptosis.
Elife. 3(e02523)2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lee YS, Lee DH, Choudry HA, Bartlett DL
and Lee YJ: Ferroptosis-induced endoplasmic reticulum stress:
Cross-talk between ferroptosis and apoptosis. Mol Cancer Res.
16:1073–1076. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cao JY and Dixon SJ: Mechanisms of
ferroptosis. Cell Mol Life Sci. 73:2195–2209. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chen MS, Wang SF, Hsu CY, Yin PH, Yeh TS,
Lee HC and Tseng LM: CHAC1 degradation of glutathione enhances
cystine-starvation-induced necroptosis and ferroptosis in human
triple negative breast cancer cells via the GCN2-eIF2α-ATF4
pathway. Oncotarget. 8:114588–114602. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Miao L, Guo S, Lin CM, Liu Q and Huang L:
Nanoformulations for combination or cascade anticancer therapy. Adv
Drug Deliv Rev. 115:3–22. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Xiao B, Ma L and Merlin D:
Nanoparticle-mediated co-delivery of chemotherapeutic agent and
siRNA for combination cancer therapy. Expert Opin Drug Deliv.
14:65–73. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen CK, Law WC, Aalinkeel R, Yu Y, Nair
B, Wu J, Mahajan S, Reynolds JL, Li Y, Lai CK, et al: Biodegradable
cationic polymeric nanocapsules for overcoming multidrug resistance
and enabling drug-gene co-delivery to cancer cells. Nanoscale.
6:1567–1572. 2014.PubMed/NCBI View Article : Google Scholar
|