Introduction
Periodontitis is a destructive disease with
periodontal tissue inflammation, which seriously affects oral
health. In addition to plaque and calculus, certain systemic
diseases are also important contributors to periodontitis (1). Among them, the association between
diabetes and periodontitis has attracted the attention of
researchers (2). Clinical studies
have indicated that the prevalence of periodontitis in diabetic
patients was significantly higher than that in non-diabetic
patients. The immune dysfunction and the decline of the host's
defense ability are important causes of periodontitis in diabetes
(3). Furthermore, diabetes may also
increase apoptosis of osteoblasts and periodontal fibroblasts,
further aggravating the development of periodontitis. Periodontitis
is also a risk factor for diabetes (4). If periodontitis is not effectively
controlled, the bacteria in periodontal pockets and the toxins they
secrete trigger a long-term chronic inflammatory response, damaging
the function of islet β-cells and increasing the risk of diabetes
(5).
Mesenchymal stem cells (MSCs) may be isolated from
bone marrow, fat and umbilical cord. There are numerous
immunosuppressive molecules expressed on the MSCs, which have
certain immune regulation functions (6). MSCs also secrete numerous cytokines to
optimize the local microenvironment and promote tissue repair via
paracrine signaling. At present, MSCs are widely used in the
treatment of diabetes and its complications with good therapeutic
effects (7,8). MSC therapy for diabetic foot has
entered clinical practice, with results indicating that MSCs may
promote healing of diabetic ulcers and repair of nerves (9,10). For
periodontitis, MSCs are able to repair bone defects and reduce
inflammatory response (11).
However, in diabetes, MSC function is seriously damaged and their
apoptosis rate is significantly increased, which affects the
therapeutic effect of MSCs in periodontitis (12). Numerous factors have an important
role in high glucose (HG)-induced damage, including reactive oxygen
species and mitochondrial dysfunction. In addition, hyperosmosis
may cause apoptosis and impaired function of cells. Therefore,
protecting MSCs against HG-induced damage is crucial in the
treatment of diabetic periodontitis.
Nerve growth factor (NGF) is an important nerve
factor that maintains nerve growth, differentiation and axon
production. NGF may reduce cerebral ischemia-reperfusion injury and
promote neuronal repair (13).
Numerous studies have demonstrated that NGF also has tissue and
cell protective functions, reducing apoptosis caused by ischemia
and inflammation (14). The effect
of NGF on MSCs has been widely reported. NGF may induce MSC
differentiation, inhibit MSC apoptosis and enhance MSC paracrine
signaling (15-17).
However, the effect of NGF on immune regulation of MSCs has
remained elusive. In the present study, the effect of NGF on the
therapeutic efficacy of MSCs in diabetic periodontitis was assessed
and the underlying mechanisms were investigated, so as to provide a
novel agent for clinical treatment.
Materials and methods
Cell culture and grouping
MSCs used in the present study were human bone
marrow MSCs (hMSCs) purchased from Guangzhou Saiye Biotechnology
Co. hMSCs were cultured in DMEM/F12 medium (Hyclone; Cytiva)
containing 10% fetal bovine serum (Hyclone; Cytiva). MSCs of the
3rd-5th generation were used for cell and animal experiments. For
the in vitro experiments, cells were divided into four
groups and accordingly cultured in medium containing the following:
Low-glucose (LG) group (5.6 mM glucose), LG+NGF group (5.6 mM
glucose and 10 ng/ml NGF), HG group (30 mM glucose), HG+NGF group
(30 mM glucose and 10 ng/ml NGF) and neurotrophic receptor tyrosine
kinase 1 (TrkA) inhibition group (30 mM glucose, 10 ng/ml NGF and
TrkA inhibitor GW441756). After 48 h of stimulation, the cell
supernatants from each group were collected and the concentrations
of TGF-β (cat. no. EHJ-10099), IL-10 (cat. no. EHJ-10479), TNF-α
(cat. no. EHJ-10039) and IL-6 (cat. no. EHJ-10292) in the
supernatants were detected by ELISA (all purchased from Xiamen
Huijia Biotechnology Co., Ltd.).
Apoptosis experiment
MSCs were digested with 0.25% trypsin (Hyclone;
Cytiva) and seeded in 6-well plates (Corning, Inc.) at a density of
2x104 cells/well. The treatment of MSCs was as
aforementioned. After 48 h of 10 ng/ml NGF stimulation, MSCs were
digested with 0.25% trypsin and suspended in 100 µl PBS. Annexin
V-FITC and PI (Roche Diagnostics) were added, followed by
incubation at room temperature in the dark for 15 min. Binding
buffer (400 µl) was added and the ratio of apoptotic cells was
detected by flow cytometry (BD LSRFortessa X-20; BD Biosciences).
The proportion of apoptotic cells was quantified using FlowJo
software (version 7.6; BD Biosciences).
MTT assay
MSCs were digested with 0.25% trypsin and seeded in
96-well plates at a density of 6x103 cells/well. MSCs
were treated as aforementioned. After 48 h of 10 ng/ml NGF
stimulation, 20 µl MTT solution (5 mg/ml) was added to each well
and the culture was continued for 4 h. After discarding the
supernatant, 150 µl DMSO (Sigma-Aldrich; Merck KGaA) was added to
each well. After 10 min of low-speed oscillation, the absorbance
value of each well at 490 nm was measured with an ELISA plate
reader.
Flow cytometry
After 48 h of NGF stimulation, MSCs were digested
with 0.25% trypsin and suspended in 100 µl PBS. Toll-like receptor
(TLR)4-PE (cat. no. 564215), TLR3-APC (cat. no. 565984), human
leukocyte antigen G (HLA-G)-FITC (cat. no. 751657) (all purchased
from BD Biosciences) were added and used as provided by the
manufacturer, followed by incubation at room temperature in the
dark for 15 min. After washing with PBS three times, the mean
fluorescence intensity of the fluorophores for TLR4, TLR3 and HLA-G
was detected by flow cytometry (BD LSRFortessa X-20; BD
Biosciences). Proportion of apoptotic cells was quantified using
FlowJo software (version 7.6; BD Biosciences).
Co-culture experiment
After 48 h of NGF stimulation, the supernatant was
discarded and fresh medium was added after washing the cells with
PBS for three times. Male, eight-week-old C57BL/6 mice (Charles
River Laboratories, Inc.) were raised in a suitable environment
(temperature, 22˚C; relative humidity, 50%; access to food and
water ad libitum). A total of six mice (20 g; kept under a
12:12 h light and dark cycle) were anesthetized (400 mg/kg chloral
hydrate, intraperitoneal injection). A 1-ml needle was used to
pierce the heart and collect blood from the heart in a terminal
procedure. According to the manufacturer's protocol, lymphocytes
were isolated with lymphocyte separation fluid (Tianjin TBD
Biotechnology Co., Ltd.) from the mouse blood collected, and then
added to MSC medium. According to the treatment of MSCs, the
experiment was divided into five groups: LG-MSC group
(1x105 lymphocytes and 1x104 MSCs were
co-cultured in DMEM/F12 medium containing 5.6 mM glucose),
LG+NGF-MSC group (1x105 lymphocytes and 1x104
MSCs were co-cultured in DMEM/F12 medium containing 5.6 mM glucose
and 10 ng/ml NGF), HG-MSC group (1x105 lymphocytes and
1x104 MSCs were co-cultured in DMEM/F12 medium
containing 30 mM glucose), HG+NGF-MSC group (1x105
lymphocytes and 1x104 MSCs were co-cultured in DMEM/F12
medium containing 30 mM glucose and 10 ng/ml NGF) and
TrkA-inhibited MSC group (1x105 lymphocytes and
1x104 MSCs were co-cultured in DMEM/F12 medium
containing 30 mM glucose, 10 ng/ml NGF and 10 ng/ml GW441756).
After 48 h of incubation, CD45-PE (cat. no. 560975), CD3-APC (cat.
no. 555335) and CD14-FITC (cat. no. 557153) (all purchased from BD
Biosciences) were added and used as provided by the manufacturer,
followed by incubation at room temperature in the dark for 15 min.
After washing with PBS three times, the ratio of
CD45+CD3+T cells and CD14+
monocytes/macrophages was detected by flow cytometry (BD
LSRFortessa X-20; BD Biosciences). Proportion of apoptotic cells
was quantified using FlowJo software (version 7.6; BD Biosciences).
The use of mice in this experiment was approved by the Ethics
Committee of The Air Force Characteristic Medical Center (Beijing,
China).
Animal experiment
All experiments were approved by the Ethics
Committee of The Air Force Characteristic Medical Center (Beijing,
China) and were performed in accordance with the institutional
regulations. Male, eight-week-old C57BL/6 mice (Charles River) were
raised in a suitable environment (temperature, 22˚C; relative
humidity, 50%; access to food and water ad libitum).
C57BL/6J mice were intraperitoneally injected with 40 mg/kg
streptozotocin once a day for 5 days. Fasting blood glucose levels
of >16.7 mmol/l determined in three consecutive measurements
(interval of 1 day) were considered to indicate that the diabetic
mouse model was successfully constructed and the success rate of
establishing the model was 72.73%. After 1 week of feeding on
normal food, the periodontitis model started to be established, the
brief process is as follows. Mice were first anesthetized with
chloral hydrate (400 mg/kg, intraperitoneal injection).
Subsequently, silk thread (5-mm diameter) was slid into the
proximal and distal adjacent spaces of the maxillary second molars
on both sides of the mouse, and the knots were tied on the buccal
side and pressed against the gingival groove. After 4 weeks of
ligation, the ligated threads were carefully removed. The mice were
divided into four experimental groups: Model group (PBS was
injected through the tail vein, n=10), MSC group (1x106
hMSCs were injected through the tail vein, n=10), NGF group
(1x106 NGF pre-treated hMSCs were injected through the
tail vein, n=10) and TrkA inhibition group (1x106 NGF
and GW441756 pre-treated hMSCs were injected through the tail vein,
n=10). All injections were performed once in each animal. The fur
color, diet and behavior of the mice were monitored daily. After
two weeks, mice were euthanized by anesthesia (400 mg/kg chloral
hydrate, intraperitoneal injection) followed by cervical
dislocation, and the alveolar bones and periodontal tissues were
collected. After fixing with 4% paraformaldehyde, paraffin sections
(4-µm thick) were prepared. Certain sections were stained with
H&E to detect basic pathological changes. Immunohistochemistry
was performed in other sections to detect the number of anti-human
nuclear antibody (ANA)-positive transplanted hMSCs (18), CD3+T cells and
CD68+ macrophages (ANA cat. no. ab215396; CD3 cat. no.
ab16669; CD68 cat. no. ab125212; all purchased from Abcam) and the
antibodies were used as provided by the manufacturer. Transplanted
hMSCs, CD3+T cells and CD68+ macrophages were
directly counted under a light microscope by eye (Eclipse E200;
Nikon Corporation).
Statistical analysis
Statistical analyses were performed using SPSS 19.0
(IBM Corp.). Values are expressed as the mean ± standard deviation.
Data comparisons were performed by ANOVA and Bonferroni's post-hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
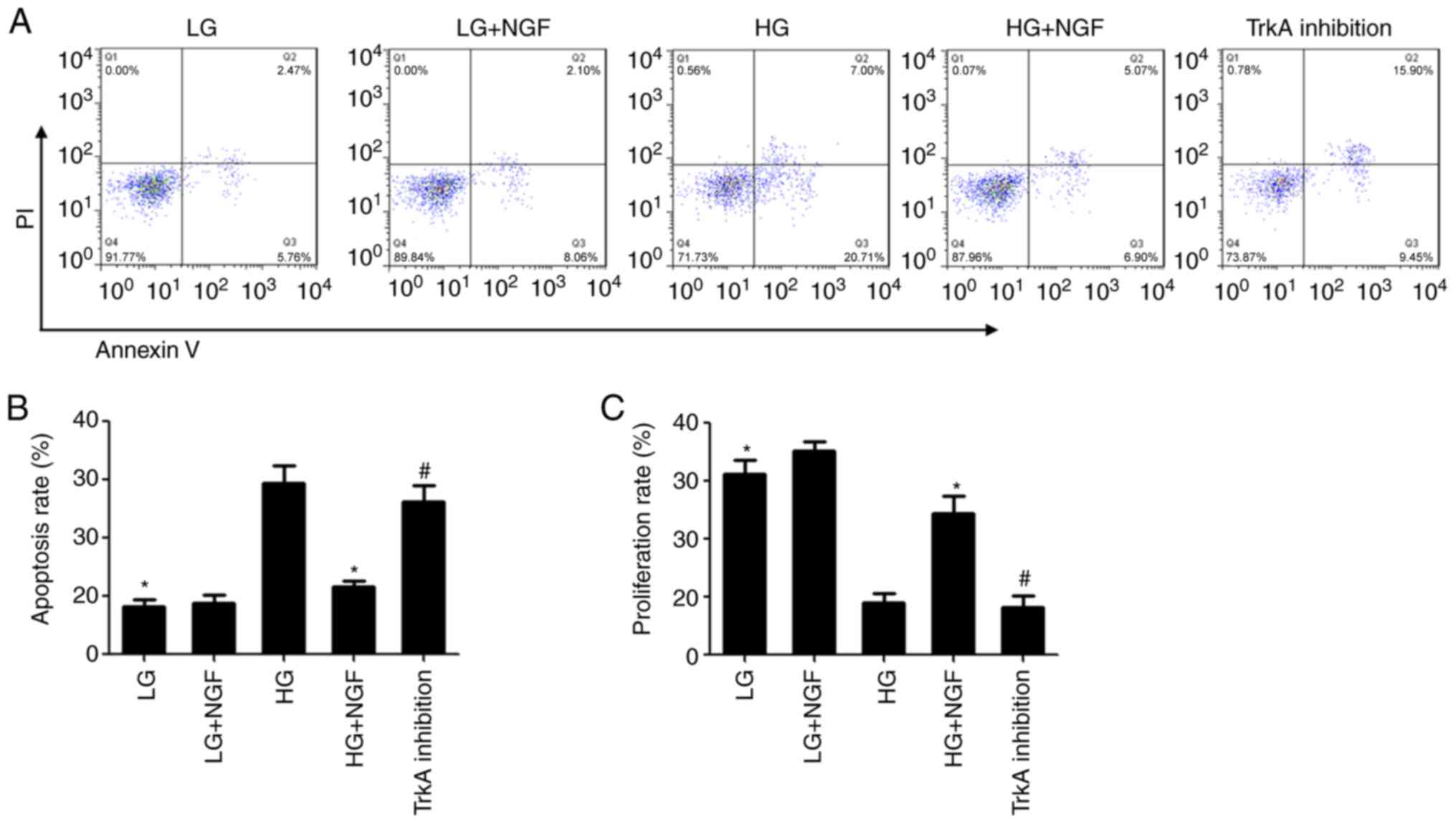
NGF inhibits MSC apoptosis induced by
HG
Flow cytometry showed that there was no significant
difference in the apoptosis rate between the LG and LG+NGF groups.
Compared with that in the LG group, the apoptosis rate of MSCs in
the HG group was significantly increased. NGF was able to
significantly inhibit the apoptosis of MSC caused by HG; compared
with that in the HG group, the apoptosis rate of MSCs in the HG+NGF
group was decreased by 60.98%. TrkA inhibition was able to
completely block this effect of NGF (Fig. 1A and B).
The MTT assay indicated that there was no
significant difference in the viability rate between the LG and
LG+NGF groups. Compared with that in the LG group, the
proliferation rate of MSCs in the HG group was significantly
decreased. Compared with that in the HG group, the viability rate
in the HG+NGF group was increased to 24.5% and the difference was
significant. TrkA inhibition was able to completely block this
effect of NGF (Fig. 1C).
NGF inhibits the transformation of
MSCs into proinflammatory type under HG conditions
As established by numerous studies, MSCs may be
divided into a proinflammatory type and an immunosuppressive type
(19,20). Proinflammatory MSCs have high
expression of TLR4 and low expression of TLR3 and HLA-G.
Immunosuppressive MSCs have high expression of TLR3 and HLA-G and
low expression of TLR4. The present results indicated no
significant difference in TLR4, TLR3 and HLA-G expression between
the LG and LG+NGF groups. HG enhanced TLR4 expression and decreased
TLR3 and HLA-G expression. NGF inhibited the transformation of MSCs
into the proinflammatory type under HG conditions; compared with
that in the HG group, the expression of TLR3 and HLA-G in the
HG+NGF group increased by 68.27% and 1.41-fold, respectively, and
the expression of TLR4 decreased by 40.39%. TrkA inhibition was
able to completely block these effects of NGF (Fig. 2).
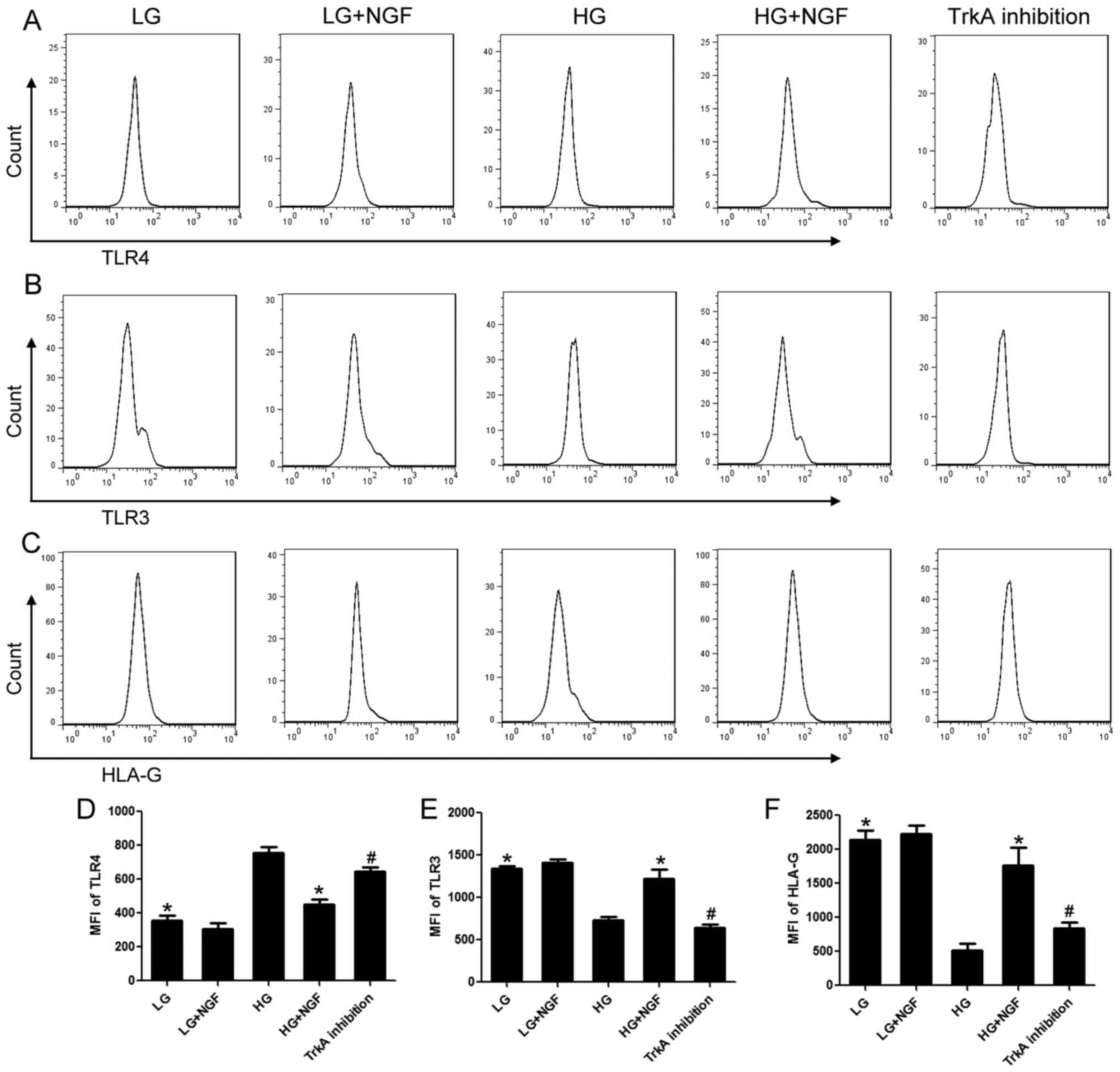 | Figure 2NGF inhibits the transformation of
MSCs into the proinflammatory type under HG conditions. (A) TLR4,
(B) TLR3 and (C) HLA-G expression was detected by flow cytometry.
Quantified levels of (D) TLR4, (E) TLR3 and (F) HLA-G expression.
Compared with that in the HG group, the expression of TLR3 and
HLA-G in the HG+NGF group increased by 68.27% and 1.41-fold, and
the expression of TLR4 decreased by 40.39%. TrkA inhibition
completely blocked these effects of NGF. Values are expressed as
the mean ± standard deviation (n=6). *P<0.05 vs. HG;
#P<0.05 vs. HG+NGF. TrkA, neurotrophic receptor
tyrosine kinase 1; MSC, mesenchymal stem cell; NGF, nerve growth
factor; HG, high glucose; LG, low glucose; TLR, Toll-like receptor;
HLA-G, human leukocyte antigen G; MFI, mean fluorescence
intensity. |
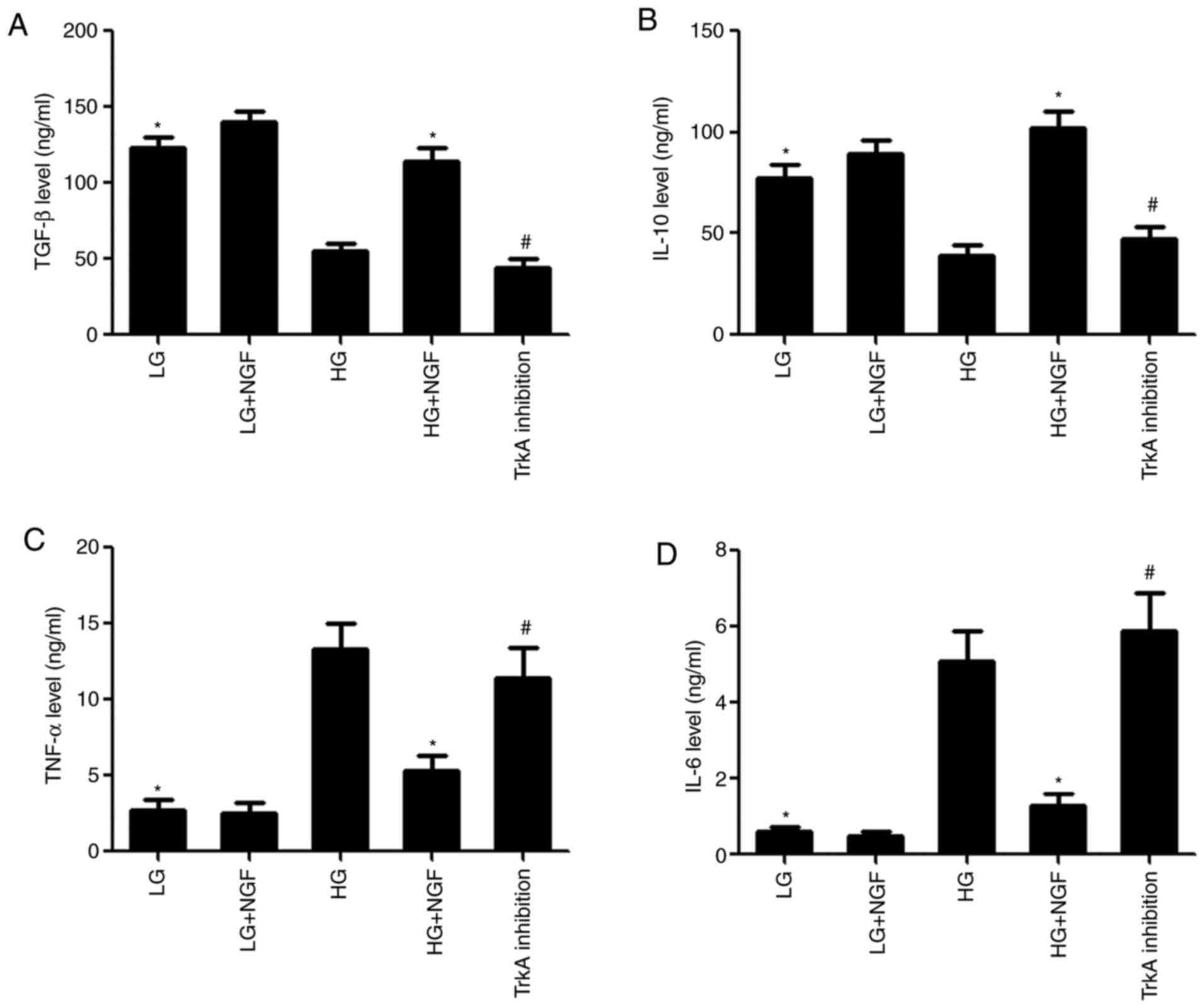
NGF promotes the release of paracrine
immunosuppressive molecules from MSCs under HG conditions
Compared with that in the LG group, the
concentration of TGF-β and IL-10 in the MSC supernatant was
significantly decreased in the HG group, while the concentration of
TNF-α and IL-6 was significantly increased. NGF promoted the
secretion of immunosuppressive molecules from MSCs under HG
conditions; compared with that in the HG group, the concentration
of TGF-β and IL-10 in the HG+NGF group was significantly increased,
while the concentration of TNF-α and IL-6 was significantly
decreased. TrkA inhibition was able to completely block these
effects of NGF (Fig. 3).
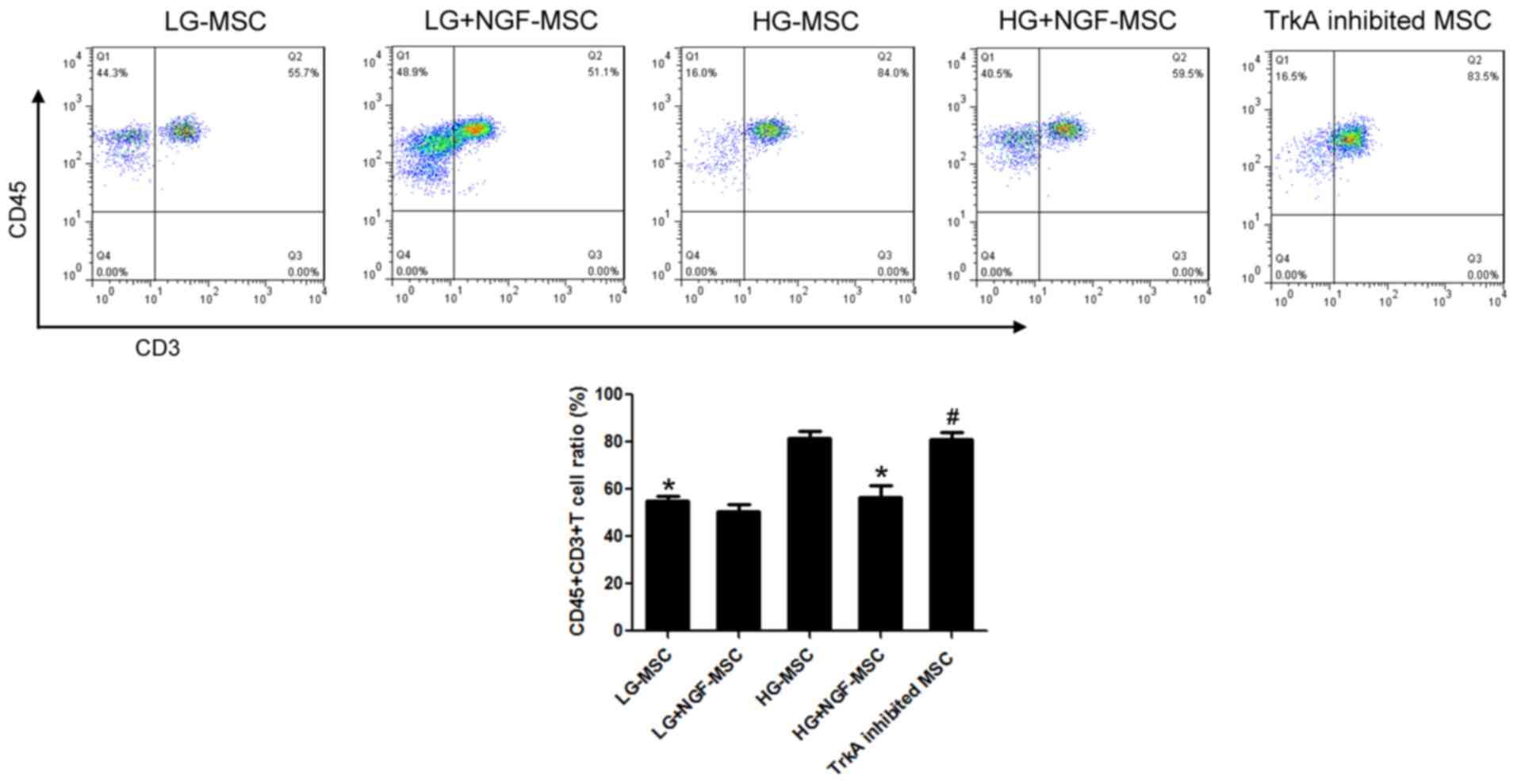
NGF enhances the function of MSCs in
inhibiting T cells under HG conditions
The proportion of CD45+CD3+T
cells among lymphocytes of the HG-MSC group was significantly
increased compared with that in the LG-MSC group. The proportion of
CD45+CD3+T cells in the HG+NGF-MSC group was
56.42%, with a statistically significant difference compared with
that in the HG-MSC group. TrkA inhibition was able to completely
block these effects of NGF (Fig.
4).
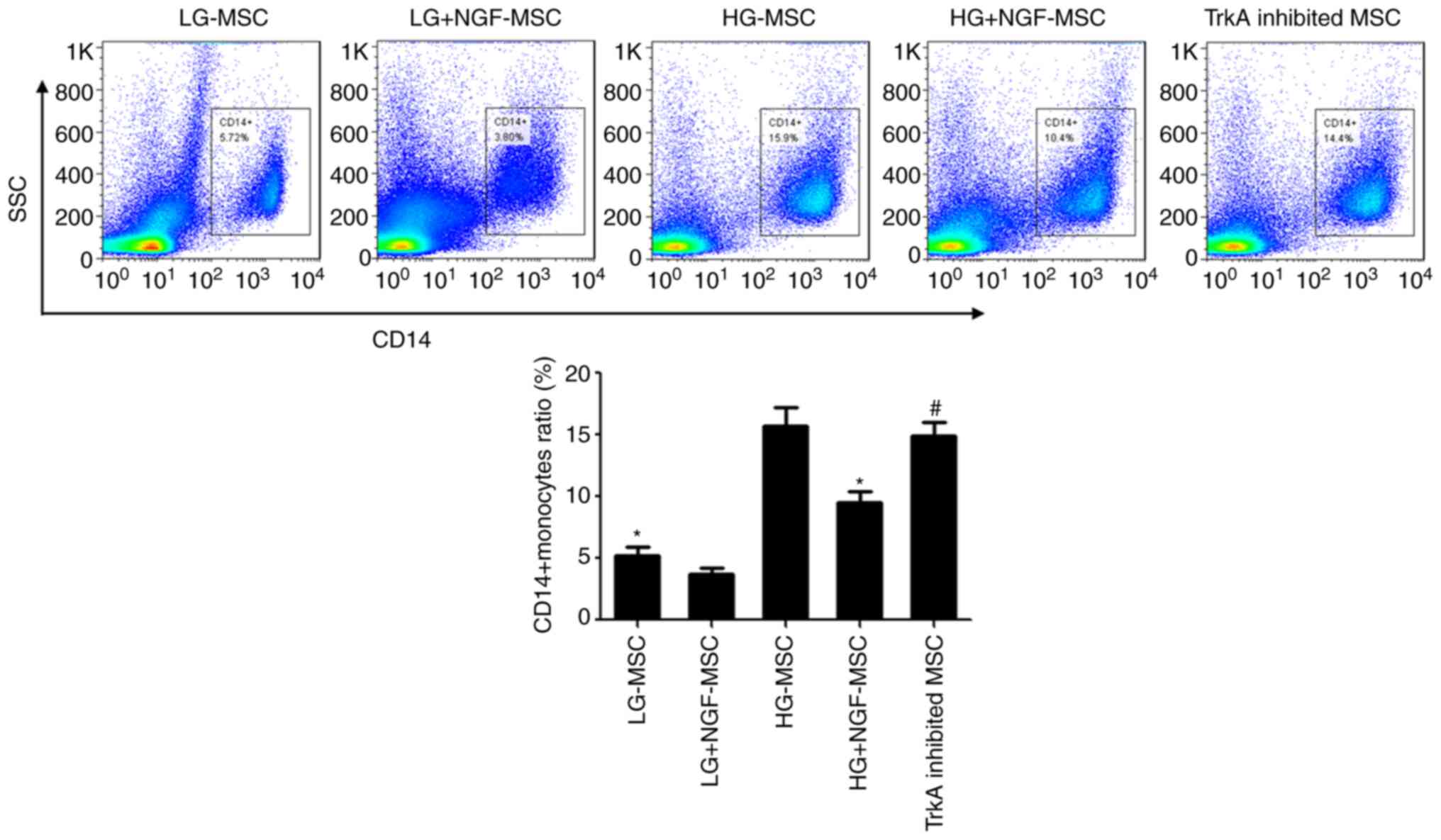
NGF enhances the function of MSCs to
inhibit monocytes/macrophages under HG conditions
In the co-culture experiment, the proportion of
CD14+ monocytes/macrophages in the HG-MSC group was
significantly increased compared with that in the LG-MSC group. The
proportion of CD14+ monocytes/macrophages in the
HG+NGF-MSC group was 9.53%, which was significantly lower than that
in the HG-MSC group. TrkA inhibition was able to completely block
these effects of NGF (Fig. 5).
NGF maintains MSC survival in
periodontal tissue of diabetic mice
The results of the animal experiment are provided in
Fig. 6. There was no significant
difference in body weight between the groups (Fig. 6B). Compared with that in the model
group, alveolar bone loss in the MSC group was significantly
decreased. NGF significantly enhanced the repairing effect of MSCs
and alveolar bone loss in the NGF group was decreased by 43.62%
compared with that in the MSC group (Fig. 6C). Furthermore, immunohistochemistry
with ANA was used to label hMSCs in periodontal tissues. The number
of transplanted MSC cells in the NGF group was 3.11 times that of
the MSC group. TrkA inhibition was able to completely block these
effects of NGF (Fig. 6A and
D). These results indicated that
high glucose could damage MSCs, and NGF had a protective effect and
reduced MSC death in vivo.
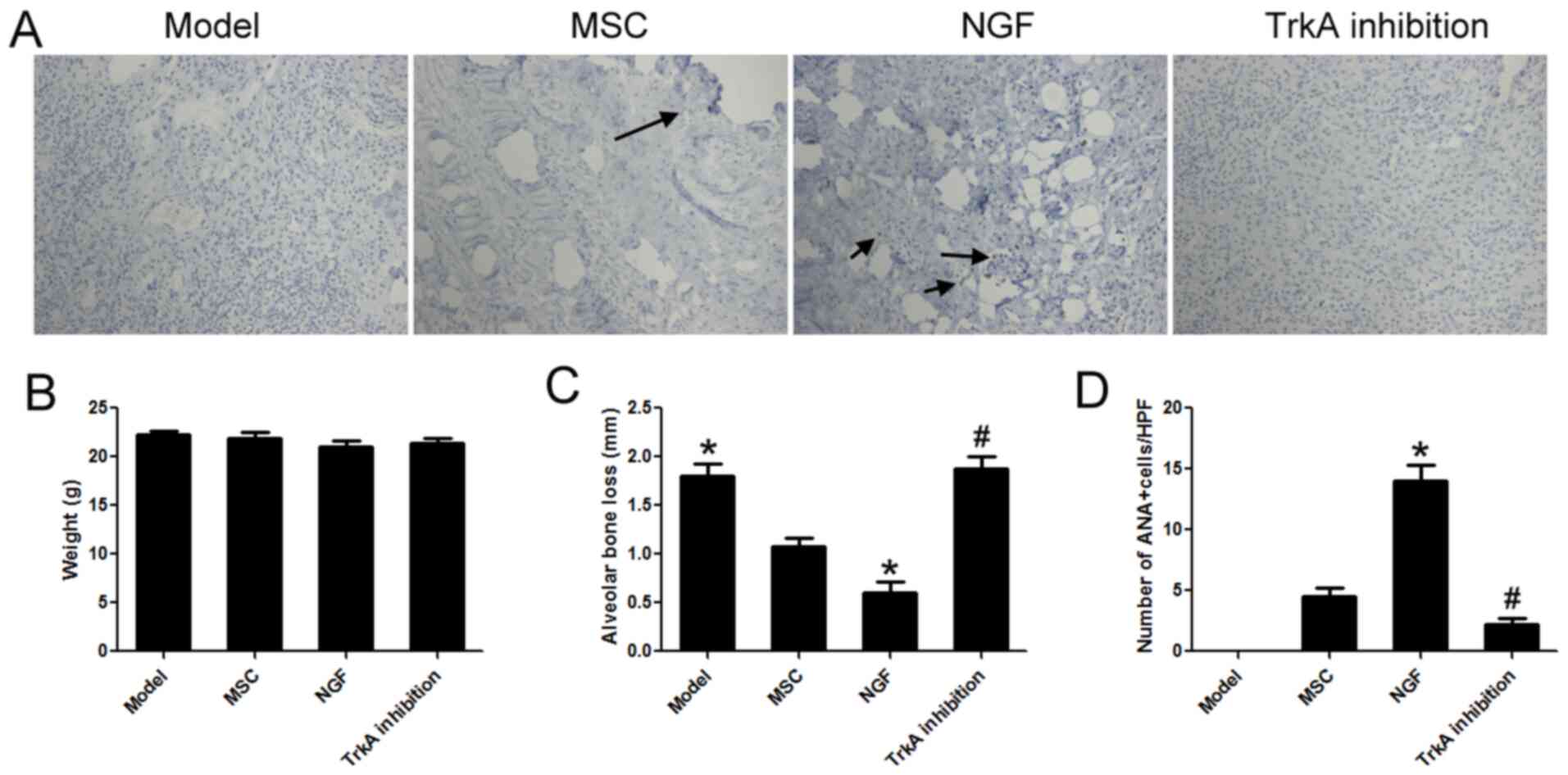 | Figure 6NGF maintains MSC survival in
periodontal tissue of diabetic mice. (A) ANA was used to detect
transplanted human bone marrow MSCs in periodontal tissue. Arrows
indicate ANA+ cells (magnification, x100). (B) There was
no significant difference in body weight between the groups. (C)
Compared with the MSC group, alveolar bone loss in the NGF group
decreased by 43.62%. (D) Compared with the MSC group, the number of
transplanted MSCs in the NGF group increased by 2.11-fold. TrkA
inhibition completely blocked these effects of NGF. Values are
expressed as the mean ± standard deviation (n=6).
*P<0.05 vs. MSC; #P<0.05 vs. NGF. TrkA,
neurotrophic receptor tyrosine kinase 1; Q, quadrant; MSC,
mesenchymal stem cell; NGF, nerve growth factor; HG, high glucose;
ANA, anti-nuclear antibody; HPF, high-power field. |
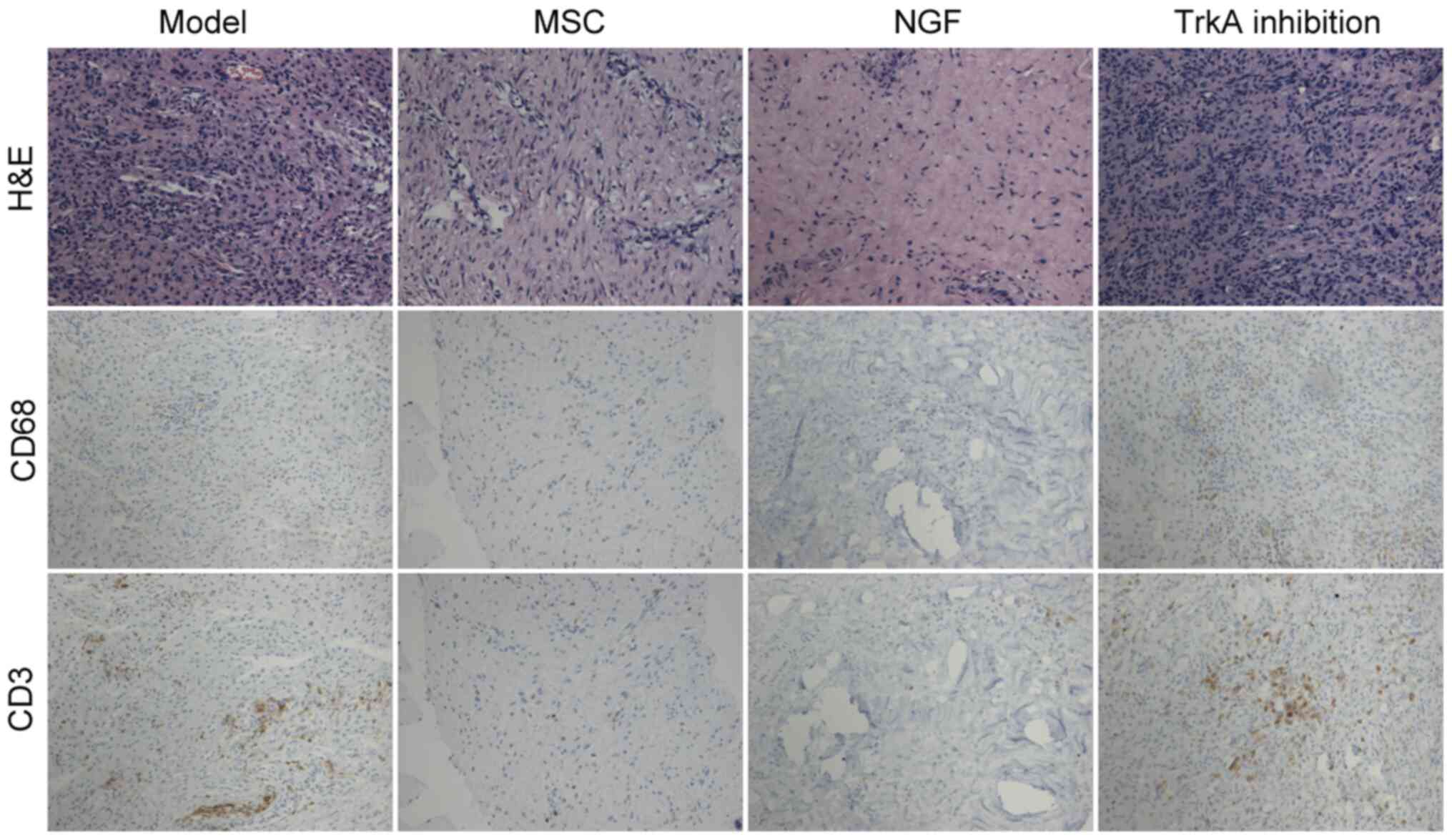
NGF enhances the anti-inflammatory
function of MSCs in diabetic mice
Immunohistochemical analysis of periodontal tissue
indicated that compared with that in the model group, the number of
T cells and macrophages was markedly decreased in the MSC group.
NGF further inhibited the infiltration of T cells and macrophages.
TrkA inhibition blocked these effects of NGF (Fig. 7).
Discussion
Periodontitis is a chronic inflammatory disease of
periodontal tissue, with high incidence and recurrent episodes,
bringing great pain to patients (21). HG and low immunity make diabetes
patients more likely to develop periodontitis. This periodontitis
progresses rapidly and is frequently difficult to cure (22). In severe cases, it is even secondary
to systemic inflammation, aggravating the condition of diabetic
patients (23). In order to study
the effect of diabetes on periodontal tissue, a diabetic mouse
model was constructed in the present study. The fasting blood
glucose in the animals was >16.7 mmol/l for three consecutive
times, suggesting that the diabetic mouse model was successfully
constructed. In these diabetic mice, the periodontitis model was
then constructed. After two weeks, the alveolar bones and
periodontal tissues were collected. H&E and immunohistochemical
results indicated that a large number of T cells and macrophages
were present in periodontal tissues. MSCs have important functions,
such as tissue repair and anti-inflammatory effects. The present
results indicated that MSC transplantation reduced the infiltration
of inflammatory cells and the expression of inflammatory molecules
in periodontal tissues. There are two main mechanisms by which MSCs
exert their anti-inflammatory role. Immunosuppressive molecules
that are highly expressed on the MSCs are able to inhibit T cells
and macrophage-mediated immune responses through cellular contact.
In addition, MSCs paracrine numerous anti-inflammatory molecules to
inhibit local inflammatory responses (24).
HG and high oxidative stress may damage the function
of transplanted stem cells in patients with diabetes, which makes
the effect of MSC therapy unsatisfactory (25). The present results indicated that HG
increased MSC apoptosis and inhibited MSC paracrine function.
Therefore, it is of great significance to identify novel targets to
protect MSCs in diabetes. Peripheral neuropathy is an important
complication of diabetes, which may cause itching, abnormal
sensation and diabetic ulcers. Promoting nerve injury repair may
effectively treat diabetic peripheral neuropathy (26). NGF has the function of promoting
nerve differentiation and axon growth and has been used in the
clinic. In recent years, numerous studies have confirmed that NGF
is able to protect cells and repair tissues (27,28).
The present study indicated that NGF inhibited apoptosis of MSCs
caused by HG and restored their damaged paracrine function. The
animal experiment indicated that pretreatment with NGF
significantly improved the survival rate of MSCs in diabetic
periodontal tissues and enhanced their therapeutic effect on
periodontitis. HG-induced damage to MSCs may also be mediated by
other mechanisms, such as hyperosmosis, high oxidative stress and
DNA damage. In the present study, these aspects were not explored,
which is a limitation of the present study.
Numerous studies have indicated that MSCs may have
two different phenotypes. Waterman et al (19) divided MSCs into proinflammatory MSCs
and immunosuppressive MSCs based on their expression of TLR3 and
TLR4. Svobodova et al (20)
also indicated that MSCs with different phenotypes affect the
differentiation of naive T cells into regulatory or proinflammatory
T cells. To better simulate the transplantation of hMSCs into mice
in animal experiments, mouse lymphocytes and hMSCs were co-cultured
in vitro in the present study. Under physiological
conditions, MSCs have a strong anti-inflammatory effect
(immunosuppressive MSCs). However, in the present study, when MSCs
were in an HG environment, immunosuppressive MSCs were converted to
proinflammatory MSCs and the expression of immunosuppressive
protein and secretion of anti-inflammatory molecules were
significantly decreased, while the secretion of pro-inflammatory
molecules was significantly increased. The in vivo
experiments of the present study demonstrated that NGF was able to
inhibit the conversion of MSCs to the proinflammatory type in
diabetes and further decreased the infiltration of T cells and
macrophages in periodontal tissues.
TrkA is the major high-affinity receptor of NGF and
has an irreplaceable role in the growth and development of the
nervous system and the maintenance of neuronal characteristics
(29). The combination of NGF and
TrkA may further activate the MAPK signaling pathway and promote
stem cell proliferation and differentiation (30,31).
Numerous studies have indicated that the TrkA receptor may be
detected on MSCs, and TrkA receptor expression is significantly
increased under HG and ischemic conditions (32-34).
The present study indicated that mainly through the TrkA receptor,
NGF protected MSCs against HG damage and enhanced their
anti-inflammatory effect in diabetes. GW441756 is a highly specific
inhibitor of TrkA. Numerous studies have proved that GW441756 has a
good effect on TrkA inhibition (35,36),
so it was applied in the present study. The present results
demonstrated that TrkA receptor inhibition completely blocked these
functions of NGF.
In conclusion, the present study indicated that NGF
is able to enhance the therapeutic effect of MSCs in diabetic
periodontitis by protecting the cells and promoting the
transformation of MSCs into the immunosuppressive type. The present
study provided a novel agent for stem cell therapy for diabetic
periodontitis.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by Capital's Funds for Health
Improvement and Research (grant no. 2018-2-5122).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JP, YL and SW drafted and revised the manuscript. JP
and YL designed and performed most of the experiments. SW performed
the cell experiments. YX and BL performed the animal experiments.
JP and YL confirm the authenticity of all the raw data. All authors
discussed the results and approved the final submitted version. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animal protocol was approved by the Ethics
Committee of The Air Force Characteristic Medical Center (Beijing,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Amaranath BJ, Das N, Gupta I, Gupta R,
John B and Devi MP: Types of bone destruction and its severity in
chronic periodontitis patients with tobacco smoking habit using
periapical radiographs and transgingival probing: A cross-sectional
study. J Indian Soc Periodontol. 24:20–25. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Datey A, Thaha CSA, Patil SR, Gopalan J
and Chakravortty D: Shockwave therapy efficiently cures
multispecies chronic periodontitis in a humanized rat model. Front
Bioeng Biotechnol. 7(382)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Techatanawat S, Surarit R, Chairatvit K,
Khovidhunkit W, Roytrakul S, Thanakun S, Kobayashi H, Khovidhunkit
SP and Izumi Y: Salivary and serum interleukin-17A and
interleukin-18 levels in patients with type 2 diabetes mellitus
with and without periodontitis. PLoS One.
15(e0228921)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bissett SM, Preshaw PM, Presseau J and
Rapley T: A qualitative study exploring strategies to improve the
inter-professional management of diabetes and periodontitis. Prim
Care Diabetes. 14:126–132. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Balmasova IP, Lomakin YA, Babaev EA,
Tsarev VN, Gabibov AG, Smirnov IV, Knorre VD, Ovchinnikova LA,
Gnuchev NV, Khurs EN, et al: ‘Shielding’ of cytokine induction by
the periodontal microbiome in patients with periodontitis
associated with type 2 diabetes mellitus. Acta Naturae. 11:79–87.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhao W, Zhang H, Yan J and Ma X: An
experimental study on the treatment of diabetes-induced cognitive
disorder mice model with exosomes deriving from mesenchymal stem
cells (MSCs). Pak J Pharm Sci. 32:1965–1970. 2019.PubMed/NCBI
|
|
7
|
Ulyanova O, Askarov M, Kozina L, Karibekov
T, Shaimardanova G, Zhakupova A, Danilova D and Serebrennikova D:
Autologous mesenchymal stem cell transplant in patients with type 1
diabetes mellitus. Exp Clin Transplant. 17 (Suppl 1):S236–S238.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cho J, D'Antuono M, Glicksman M, Wang J
and Jonklaas J: A review of clinical trials: Mesenchymal stem cell
transplant therapy in type 1 and type 2 diabetes mellitus. Am J
Stem Cells. 7:82–93. 2018.PubMed/NCBI
|
|
9
|
Lin Y, Zhang F, Lian XF, Peng WQ and Yin
CY: Mesenchymal stem cell-derived exosomes improve diabetes
mellitus-induced myocardial injury and fibrosis via inhibition of
TGF-β1/Smad2 signaling pathway. Cell Mol Biol (Noisy-le-grand).
65:123–126. 2019.PubMed/NCBI
|
|
10
|
Fijany A, Sayadi LR, Khoshab N, Banyard
DA, Shaterian A, Alexander M, Lakey JRT, Paydar KZ, Evans GRD and
Widgerow AD: Mesenchymal stem cell dysfunction in diabetes. Mol
Biol Rep. 46:1459–1475. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ouchi T and Nakagawa T: Mesenchymal stem
cell-based tissue regeneration therapies for periodontitis. Regen
Ther. 14:72–78. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Liu X, Wang Z, Song W, Sun W, Hong R,
Pothukuchi A and Xu Q: Systematically transplanted human
gingiva-derived mesenchymal stem cells regulate lipid metabolism
and inflammation in hyperlipidemic mice with periodontitis. Exp
Ther Med. 19:672–682. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lu Z, Lei D, Jiang T, Yang L, Zheng L and
Zhao J: Nerve growth factor from Chinese cobra venom stimulates
chondrogenic differentiation of mesenchymal stem cells. Cell Death
Dis. 8(e2801)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pan Y, Jiao G, Yang J, Guo R, Li J and
Wang C: Insights into the therapeutic potential of heparinized
collagen scaffolds loading human umbilical cord mesenchymal stem
cells and nerve growth factor for the repair of recurrent laryngeal
nerve injury. Tissue Eng Regen Med. 14:317–326. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cho YI, Choi JS, Jeong SY and Yoo HS:
Nerve growth factor (NGF)-conjugated electrospun nanostructures
with topographical cues for neuronal differentiation of mesenchymal
stem cells. Acta Biomater. 6:4725–4733. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
WenBo W, Fei Z, YiHeng D, Wei W, TingMang
Y, WenHao Z, QianRu L and HaiTao L: Human umbilical cord
mesenchymal stem cells overexpressing nerve growth factor
ameliorate diabetic cystopathy in rats. Neurochem Res.
42:3537–3547. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rooney GE, Moran C, McMahon SS, Ritter T,
Maenz M, Flügel A, Dockery P, O'Brien T, Howard L, Windebank AJ and
Barry FP: Gene-modified mesenchymal stem cells express functionally
active nerve growth factor on an engineered poly lactic glycolic
acid (PLGA) substrate. Tissue Eng Part A. 14:681–690.
2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen W, Wu Y, Li L, Yang M, Shen L, Liu G,
Tan J, Zeng W and Zhu C: Adenosine accelerates the healing of
diabetic ischemic ulcers by improving autophagy of endothelial
progenitor cells grown on a biomaterial. Sci Rep.
5(11594)2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Waterman RS, Tomchuck SL, Henkle SL and
Betancourt AM: A new mesenchymal stem cell (MSC) paradigm:
Polarization into a pro-inflammatory MSC1 or an immunosuppressive
MSC2 phenotype. PLoS One. 5(e10088)2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Svobodova E, Krulova M, Zajicova A,
Pokorna K, Prochazkova J, Trosan P and Holan V: The role of mouse
mesenchymal stem cells in differentiation of naive T-cells into
anti-inflammatory regulatory T-cell or proinflammatory helper
T-cell 17 population. Stem Cells Dev. 21:901–910. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Né YGS, Martins BV, Castro MML, Alvarenga
MOP, Fagundes NCF, Magno MB, Maia LC and Lima RR: Is nutritional
intervention an improvement factor in the management of
periodontitis? A systematic review. Clin Nutr. 39:2639–2646.
2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zare Javid A, Bazyar H, Gholinezhad H,
Rahimlou M, Rashidi H, Salehi P and Haghighi-Zadeh MH: The effects
of ginger supplementation on inflammatory, antioxidant, and
periodontal parameters in type 2 diabetes mellitus patients with
chronic periodontitis under non-surgical periodontal therapy. A
double-blind, placebo-controlled trial. Diabetes Metab Syndr Obes.
12:1751–1761. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhu C, Zhao Y, Wu X, Qiang C, Liu J, Shi
J, Gou J, Pei D and Li A: The therapeutic role of baicalein in
combating experimental periodontitis with diabetes via Nrf2
antioxidant signaling pathway. J Periodontal Res. 55:381–391.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hernández-Monjaraz B, Santiago-Osorio E,
Monroy-Garcia A, Ledesma-Martinez E and Mendoza-Núñez VM:
Mesenchymal stem cells of dental origin for inducing tissue
regeneration in periodontitis: A mini-review. Int J Mol Sci.
19(944)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hoveizi E and Tavakol S: Therapeutic
potential of human mesenchymal stem cells derived beta cell
precursors on a nanofibrous scaffold: An approach to treat diabetes
mellitus. J Cell Physiol. 234:10196–10204. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shen Y, Feng Z, Lin C, Hou X, Wang X, Wang
J, Yu Y, Wang L and Sun X: An oligodeoxynucleotide that induces
differentiation of bone marrow mesenchymal stem cells to
osteoblasts in vitro and reduces alveolar bone loss in rats with
periodontitis. Int J Mol Sci. 13:2877–2892. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhu SX, Huang SY, Su YM and Cai P: Growth
and expression of rat bone marrow mesenchymal stem cells modified
by nerve growth factor in diabetic rat bladders. Mol Med Rep.
7:1791–1799. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tomellini E, Touil Y, Lagadec C, Julien S,
Ostyn P, Ziental-Gelus N, Meignan S, Lengrand J, Adriaenssens E,
Polakowska R and Le Bourhis X: Nerve growth factor and proNGF
simultaneously promote symmetric self-renewal, quiescence, and
epithelial to mesenchymal transition to enlarge the breast cancer
stem cell compartment. Stem Cells. 33:342–353. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Valdovinos-Flores C, Limón-Pacheco JH,
León-Rodríguez R, Petrosyan P, Garza-Lombó C and Gonsebatt ME:
Systemic L-buthionine-S-R-sulfoximine treatment increases plasma
NGF and upregulates L-cys/L-cys2 transporter and γ-glutamylcysteine
ligase mRNAs through the NGF/TrkA/Akt/Nrf2 pathway in the striatum.
Front Cell Neurosci. 13(325)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jiang Y and Tuan RS: Role of NGF-TrkA
signaling in calcification of articular chondrocytes. FASEB J.
33:10231–10239. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang J, Yi QT, Gong M, Zhang YQ, Liu D
and Zhu RJ: Upregulation of TRPV1 in spinal dorsal root ganglion by
activating NGF-TrkA pathway contributes to pelvic organ
cross-sensitisation in rats with experimental autoimmune
prostatitis. Andrologia. 51(e13302)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lin C, Ren Z, Yang X, Yang R, Chen Y, Liu
Z, Dai Z, Zhang Y, He Y, Zhang C, et al: Nerve growth factor
(NGF)-TrkA axis in head and neck squamous cell carcinoma triggers
EMT and confers resistance to the EGFR inhibitor erlotinib. Cancer
Lett. 472:81–96. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sanchez-Rodriguez A, Arias-Alvarez M,
Timón P, Bautista JM, Rebollar PG, Lorenzo PL and Garcia-Garcia RM:
Characterization of β-Nerve Growth Factor-TrkA system in male
reproductive tract of rabbit and the relationship between β-NGF and
testosterone levels with seminal quality during sexual maturation.
Theriogenology. 126:206–213. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bogetti ME, Pozo Devoto VM, Rapacioli M,
Flores V and Fiszer de Plazas S: NGF, TrkA-P and neuroprotection
after a hypoxic event in the developing central nervous system. Int
J Dev Neurosci. 71:111–121. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Triaca V, Fico E, Sposato V, Caioli S,
Ciotti MT, Zona C, Mercanti D, La Mendola D, Satriano C, Rizzarelli
E, et al: hNGF peptides elicit the NGF-TrkA signalling pathway in
cholinergic neurons and retain full neurotrophic activity in the
DRG assay. Biomolecules. 10(216)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang L, Peng X, Ai Y, Li L, Zhao S, Liu
Z, Peng Q, Deng S, Huang Y, Mo Y and Huang L: Amitriptyline reduces
sepsis-induced brain damage through TrkA signaling pathway. J Mol
Neurosci. 70:2049–2057. 2020.PubMed/NCBI View Article : Google Scholar
|