Introduction
Kawasaki disease (KD), also known as mucocutaneous
lymph node syndrome, was first reported by Kawasaki (1) in 1967. KD is an acute febrile disease
that may result in vasculitis of the small- and medium-sized
arteries, particularly the coronary arteries (CA) (2). KD mainly affects infants and children
aged <5 years. Due to the unclear pathogenesis and etiology of
the disease, diagnosis relies on the major clinical features of KD
and other clinically similar diseases should be excluded with known
causes, such as exudative conjunctivitis, exudative pharyngitis,
oral ulcerations, splenomegaly and vesiculobullous or petechial
rashes (3). However, this
frequently results in delayed treatment of pediatric patients with
incomplete KD (3). Principal
clinical findings of classic KD include prolonged fever (≥5 days),
lips and oral cavities (including erythema and cracking of lips,
erythema of oral and pharyngeal mucosa), bilateral conjunctival
congestion, acute non-purulent cervical lymphadenopathy,
polymorphous exanthema and changes in the extremities, such as
erythema and edema of the extremities in the acute phase (4). However, the detailed pathogenesis of
KD has remained elusive (5).
Despite the spontaneous cessation of febrile and other signs of
inflammation, up to 25% of untreated patients develop permanent
damage to the CA, even resulting in CA aneurysm formation (6). The most common cause of mortality from
KD is myocardial infarction due to thrombosis or luminal
myofibroblastic proliferation leading to stenosis (7).
Long non-coding RNAs (lncRNAs), which are >200
nucleotides in length and lack any protein-coding capacity, have
been discovered to be extensively transcribed from the genome
(8). Multiple lines of evidence
indicated that mutations and dysregulations of lncRNAs are related
to a variety of human diseases. LncRNAs are readily detectable in
numerous human body fluids, including plasma, serum, urine and
saliva, making them promising and attractive noninvasive and rapid
diagnostic tools for disease diagnosis and prognosis (9).
The past decade has witnessed a rapid increase in
the number of studies addressing the roles of lncRNAs in diverse
cardiovascular conditions and associated risk factors. For
instance, lncRNAs are now associated with different cardiovascular
diseases, including vascular disease, atherosclerosis, pathological
hypertrophy and development, dyslipidemia and metabolic syndrome
(10).
To the best of our knowledge, the expression of
cardiac disease-related lncRNAs has not been previously
investigated in KD. The present study aimed to explore the levels
of cardiac disease-related lncRNAs in blood specimens of pediatric
patients with and without KD and between acute and convalescent KD.
In addition, their correlation with laboratory parameters was
analyzed and the power of candidate lncRNAs in predicting KD was
determined in order to identify potential biomarkers for the
prediction of the disease.
Materials and methods
Subjects
All specimens were collected at Ningbo Women and
Children's Hospital (Ningbo, China) between March 2017 and December
2017. The study cohort comprised 40 pediatric patients with KD
(cases) and 40 age-matched healthy subjects (controls; children
undergoing physical examination) without any clinical symptoms and
signs of inflammation or infection. All patients with KD had fever
for at least five days and met at least four of the five clinical
criteria for KD (rash, oral mucosal erythema, conjunctival
injection and cervical lymphadenopathy and swelling of the hands
and feet) or three of the five criteria plus CA abnormalities
documented by echocardiogram (3).
Table I presents the demographic
and clinical data of the study subjects. Serum samples were
obtained from healthy children and pediatric patients with KD at
both the acute phase [prior to intravenous immunoglobulin (IVIG)
administration] and convalescent phase (after IVIG therapy)
(11). All patients with KD
received 2 g/kg IVIG and aspirin therapy (Table II).
 | Table IDemographic and clinical
characteristics of the subjects. |
Table I
Demographic and clinical
characteristics of the subjects.
| Item | Healthy controls
(n=40) | Kawasaki disease
(n=40) |
|---|
| Age (years) | 3.16±3.63 | 2.84±1.77 |
| Male sex | 21 (52.5) | 24(60) |
| Average duration of
fever (days) | 0 | 7.97 |
| Lips and oral cavity
changes | - | 37 (92.5) |
| Conjunctival
congestion | - | 33 (82.5) |
| Cervical
lymphadenopathy | - | 30(75) |
| Polymorphous
exanthema | - | 29 (72.5) |
| Changes in the
extremities | - | 24(60) |
| Coronary artery
abnormalities | - | 9 (22.5) |
| IVIG therapy | - | 40(100) |
 | Table IILaboratory data of controls and
patients with acute KD and convalescent KD. |
Table II
Laboratory data of controls and
patients with acute KD and convalescent KD.
| Parameter | Acute KD (n=40) | Convalescent KD
(n=40) | P-value (acute vs.
convalescent KD) | Control (n=40) | P-value (acute KD vs.
control) | Normal range |
|---|
| D-dimer (µg/l) |
1,768.25±1,670.13 | 721.25±536.72 | <0.001 | Not assessed | - | <500.00 |
| PCT (ng/ml) | 3.70±8.60 | 0.12±0.26 | 0.012 | Not assessed | - | <0.05 |
| BNP (pg/ml) |
1,420.95±1,992.41 | 280.25±430.55 | <0.001 | Not assessed | - | <1,000.00 |
| TP (g/l) | 62.99±5.07 | 78.57±6.67 | <0.001 | 67.89±4.03 | <0.001 | 65.00-85.00 |
| Albumin (g/l) | 36.86±3.57 | 35.62±4.13 | 0.074 | 44.66±6.97 | <0.001 | 40.00-55.00 |
| Sodium (mmol/l) | 134.85±2.02 | 135.68±2.20 | 0.070 | 139.82±1.79 | <0.001 | 137.00-147.00 |
| TG (mmol/l) | 1.39±0.73 | 3.72±11.11 | 0.192 | 1.21±0.61 | 0.24 | +<1.70 |
| LDH (U/l) | 365.75±99.56 | 317.17±103.73 | 0.034 | 298.61±61.49 | <0.001 | 109.00-245.00 |
| CK (U/l) | 59.97±41.82 | 59.42±42.49 | 0.952 | 151.07±70.99 | <0.001 | 38.00-174.00 |
| CK-MB (U/l) | 32.93±15.77 | 35.18±13.29 | 0.529 | 32.69±19.41 | 0.95 | <20.00 |
| ADA (U/l) | 19.20±4.69 | 23.70±5.02 | <0.001 | 17.79±5.22 | 0.20 | <25.00 |
| ALT (U/l) | 67.80±110.50 | 28.80±31.01 | 0.035 | 15.52±7.24 | 0.01 | 9.00-50.00 |
| AST (U/l) | 57.98±122.77 | 49.55±43.55 | 0.688 | 36.50±10.06 | 0.25 | 15.00-40.00 |
| TC (mmol/l) | 3.58±0.62 | 4.15±0.97 | 0.001 | 4.22±0.76 | <0.001 | <5.20 |
| LDL (mmol/l) | 2.06±0.52 | 2.50±0.58 | <0.001 | 2.17±0.52 | 0.36 | <3.12 |
| HDL (mmol/l) | 0.68±0.23 | 0.83±0.95 | 0.328 | 1.17±0.24 | <0.001 | >1.04 |
| Tbil (µmol/l) | 9.28±11.17 | 5.40±2.47 | 0.022 | 5.18±2.59 | 0.03 | 5.00-19.00 |
| TBA (µmol/l) | 26.61±48.46 | 6.03±3.96 | 0.009 | 6.78±4.27 | 0.01 | <6.70 |
| CRP (mg/l) | 77.58±49.86 | 11.11±2.55 | <0.001 | 4.59±2.96 | <0.001 | <6.00 |
Collection of human blood samples and
RNA extraction
Venous blood samples (3 ml/patient) were drawn via
direct venous puncture into tubes containing inert separation gel,
which was collected from each of the patients with KD (both acute
and convalescent phases) and each of the healthy controls.
Subsequently, whole blood was centrifuged to obtain serum within 48
h, which was frozen at -80˚C until further use. In the serum of
patients, the following laboratory parameters were measured:
D-dimer, procalcitonin (PCT), B-type natriuretic peptide (BNP),
total protein (TP), albumin, sodium, triglycerides (TG), lactate
dehydrogenase (LDH), creatine kinase (CK), CK-myocardial band
(CK-MB), adenosine deaminase (ADA), alanine aminotransferase (ALT),
aspartate aminotransferase (AST), total cholesterol (TC),
low-density lipoprotein (LDL), high-density lipoprotein (HDL),
total bilirubin (Tbil), total bile acids (TBA) and C-reactive
protein (CRP). RNA was isolated using TRIzol-LS (Qiagen GmbH),
phenol and chloroform.
Reverse transcription-quantitative PCR
(RT-qPCR)
Complementary (c)DNA was reverse transcribed from
RNA using the HiFi-MMLV cDNA first strand synthesis kit (CoWin
Biosciences). RT-qPCR was performed on a 96-well format Roche
LightCycler 480 real-time PCR machine for detecting lncRNAs in the
case and control groups. The dissociation curve of each sample was
then assessed. The relative fold changes were calculated using the
comparative threshold cycle (Ct) method (12). Larger ΔCt values indicated lower
expression. U6 was used as an internal control. The sequences of
the lncRNA primers are listed in Table III.
 | Table IIIPCR primer pairs. |
Table III
PCR primer pairs.
| Gene name | Primer
sequence | Annealing
temperature (˚C) |
|---|
| SRA | Sense:
5'-GGAAGCAGGTATGTGATGAC-3' | 58 |
| | Anti-sense:
5'-TACCATCCACTGACTGACCT-3' | |
| SAF | Sense:
5'-ACATCTCAGCCTCTTGGTG-3' | 58 |
| | Anti-sense:
5'-ACAGATGGCGAAATGAGG-3' | |
| DIO3OS | Sense:
5'-CTTCCTGCTCTTCGTTGTCC-3' | 58 |
| | Anti-sense:
5'-TGAGGAGGATTGAGTTGGG-3' | |
| FENDRR | Sense:
5'-AATTGCTGGGCTGCTTTCTA-3' | 58 |
| | Anti-sense:
5'-TTCACAATGGCTCAGTGCTC-3' | |
| HCG22 | Sense:
5'-CGCAGGCACAAATGGATGAG-3' | 58 |
| | Anti-sense:
5'-CTGGTCTCTTTCCGTGGGAC-3' | |
| MHRT | Sense:
5'-CCGACTGCGACTCCTCATAC-3' | 58 |
| | Anti-sense:
5'-GGCTGAAGAGTGAGCCTTGT-3' | |
| U6 | Sense:
5'-GCTTCGGCAGCACATATACTAAAAT-3' | 58 |
| |
Anti-sense:5'-CGCTTCACGAATTTGCGTGTCAT-3' | |
Statistical analysis
All statistical analyses were performed with SPSS
version 24.0 software (IBM Corp.). The mean and standard deviation
(SD) were calculated for lncRNA expression levels. Data are
presented as the mean ± SD of three independent experiments.
Independent-samples Student's t-test was used to compare two
different groups, paired Student's t-test was used to compare the
same group at two different time-points. Pearson correlation
analysis was performed to determine the correlation between levels
of different lncRNAs and between lncRNA levels and clinical
laboratory parameters assessed using the same serum samples. A
receiver operating characteristic (ROC) curve was plotted to
evaluate the diagnostic value of lncRNAs. P<0.05 was considered
to indicate statistical significance.
Results
Demographic data
A total of 40 serum samples from patients with KD
(age, 2.84±1.77 years; 24 males) were collected for the present
study. Furthermore, 40 healthy controls (age, 3.16±3.63 years; 21
males) were included. The mean duration of fever in patients with
KD was 7.97 days (all patients had fever for ≥5 days). Other
symptoms included changes in the lips and oral cavity (37 cases;
92.5%), bilateral conjunctival congestion (33 cases; 82.5%), acute
non-purulent cervical lymphadenopathy (30 cases, 75%), polymorphous
exanthema (29 cases, 72.5%) and changes in the extremities (24
cases, 60.0%). A total of 9 patients (22.5%) were indicated to have
CA abnormalities (Table I).
All patients with KD received 2 g/kg IVIG and
aspirin therapy. As presented in Table
II, between acute KD and convalescent KD, D-dimer (P<0.001),
PCT (P=0.012), BNP (P<0.001), TP (P<0.001), LDH (P=0.034),
ADA (P<0.001), ALT (P=0.035), TC (P=0.001), LDL (P<0.001),
Tbil (P<0.022), TBA (P=0.009) and CRP (P<0.001) differed
significantly. However, the laboratory data of the acute group were
not significantly different with regards to albumin levels, sodium,
TG, CK-MB, CK, AST and HDL. However, when comparing the acute KD
and control groups, TP (P<0.001), albumin (P<0.001), sodium
(P<0.001), CK (P<0.001), LDH (P<0.001), ALT (P=0.005), TC
(P<0.001), HDL (P<0.001), Tbil (P=0.03), TBA (P=0.01) and CRP
(P<0.001) were significantly different. By contrast, TG, CK-MB,
ADA, AST and LDL did not exhibit any significant difference between
acute KD and controls.
LncRNA serum levels in patients with
KD
Initial RT-qPCR analysis included 6 then-known
cardiac-related lncRNAs: Steroid receptor RNA activator (SRA), SAF,
human leukocyte antigen complex group 22 (HCG22), myosin heavy
chain-associated RNA transcript (MHRT), DIO3 opposite strand
upstream RNA (DIO3OS) and forkhead box F1 adjacent non-coding
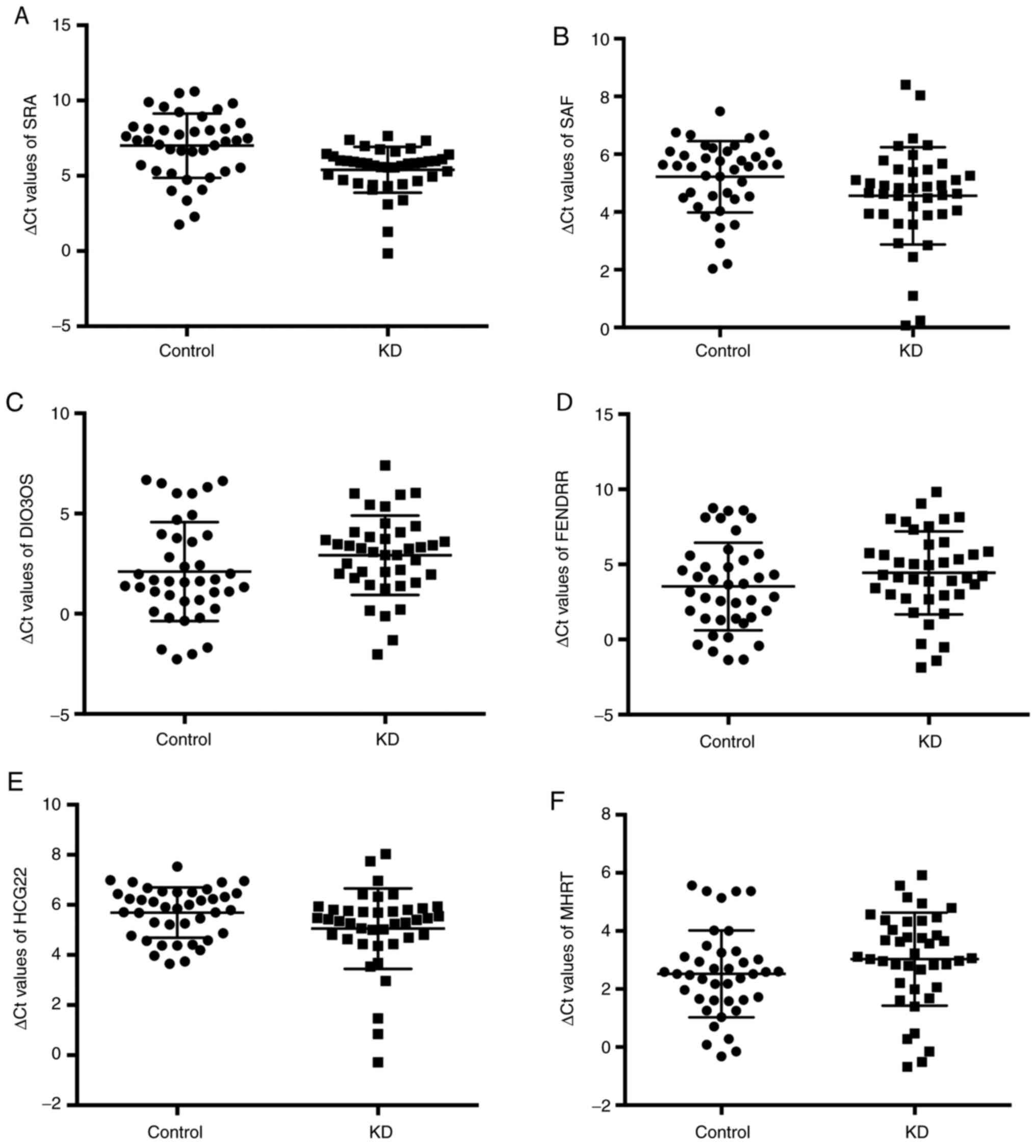
developmental regulatory RNA (FENDRR) (9). Fig. 1
presents the levels of these six lncRNAs. The expression levels of
SRA were higher in patients with KD (∆Ct=5.40±1.53) compared with
those in healthy children (∆Ct=7.00±2.13; P<0.0001). Similarly,
serum HCG22 levels were also higher in patients with KD
(∆Ct=5.05±1.61) compared with those in the controls (∆Ct=5.69±1.00;
P=0.036). Serum SAF, DIO3OS, FENDRR and MHRT levels did not
significantly change in acute KD and controls.
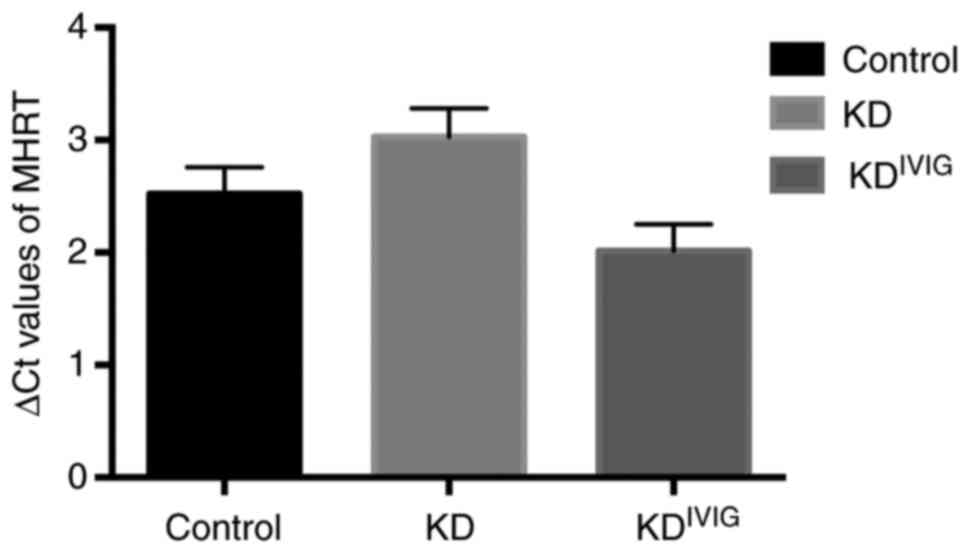
As presented in Fig.
2, the serum levels of lncRNA MHRT were upregulated in patients
with convalescent KD (∆Ct=2.01±1.50) compared with those with acute
KD (∆Ct=3.03±1.60; P=0.001) and remained higher compared with those
in healthy controls (∆Ct=2.52±1.50; P=0.027). Serum SRA, SAF,
DIO3OS, FENDRR and HCG22 levels did not significantly change in
patients with KD following IVIG therapy (data not shown).
Evaluation of HCG22 and SRA as novel
biomarkers for KD
On single-factor correlation analysis, BNP (P=0.003)
and CRP (P<0.010) were positively correlated with HCG22 in
patients with acute KD, while TC (P=0.034) and LDL (P=0.016) were
negatively correlated with HCG22 in patients with KD (Table IV). However, there was no
significant correlation between SRA and any biochemical parameter
in patients with KD (data not shown).
 | Table IVSingle-factor correlation analysis
for the association of HCG22 with biochemical parameters between
patients with acute KD and controls. |
Table IV
Single-factor correlation analysis
for the association of HCG22 with biochemical parameters between
patients with acute KD and controls.
| Parameter | Pearson correlation
coefficient | P-value |
|---|
| BNP (pg/ml) | -0.453 | 0.003 |
| TC (mmol/l) | 0.337 | 0.034 |
| LDL (mmol/l) | 0.378 | 0.016 |
| CRP (mg/l) | -0.405 | 0.010 |
Having discovered that the serum levels of HCG22 and
SRA were abnormal in patients with KD, the potential use of HCG22
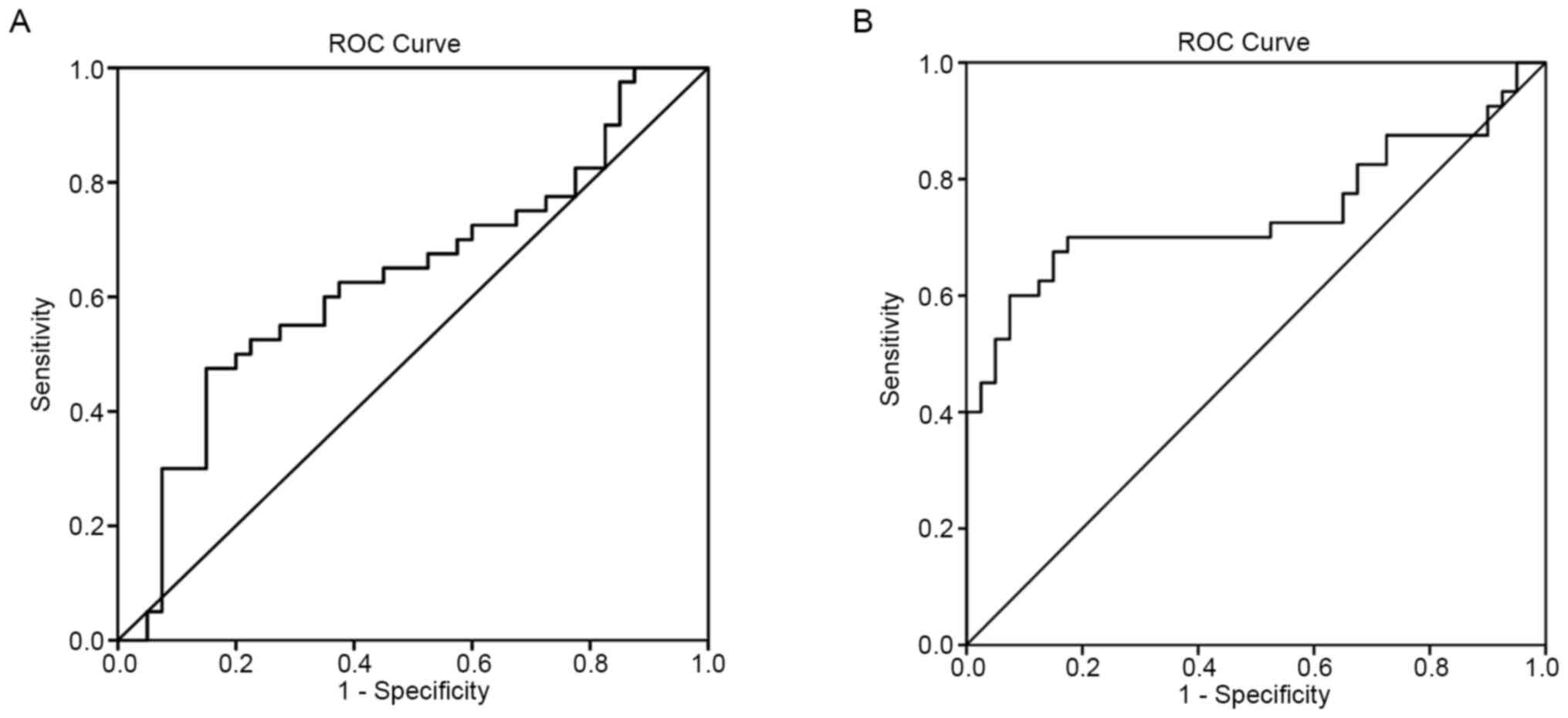
and SRA as diagnostic biomarkers for KD was then tested. ROC
analysis was performed to evaluate the predictive power of HCG22
and SRA for KD. To distinguish between acute KD and controls, the
area under ROC curve (AUC) was 0.633 (95%CI: 0.509-0.757) for HCG22
(Fig. 3A). The sensitivity and
specificity were 55.0 and 72.5%, respectively. The cut-off value
was 5.973. For SRA, the AUC was 0.743 (95% CI: 0.627-0.859;
Fig. 3B) and the sensitivity and
specificity were 70.0 and 82.5%, respectively. The cut-off value
was 7.05.
Correlation of MHRT with biochemical
parameters
According to the single-factor correlation analysis,
CK (P=0.035) was positively correlated with MHRT in patients with
convalescent KD, while BNP (P=0.003) and ADA (P=0.033) were
negatively correlated with MHRT in patients with convalescent KD
(Table V). No significant
correlation of MHRT with acute KD was observed (data not
shown).
 | Table VSingle-factor correlation analysis
for the association of MHRT with biochemical parameters between
patients with convalescent Kawasaki disease and controls. |
Table V
Single-factor correlation analysis
for the association of MHRT with biochemical parameters between
patients with convalescent Kawasaki disease and controls.
| Parameter | Pearson correlation
coefficient | P-value |
|---|
| BNP (pg/ml) | 0.334 | 0.003 |
| CK (U/l) | -0.334 | 0.035 |
| ADA (U/l) | 0.337 | 0.033 |
Discussion
Evidence suggests that lncRNAs may be involved in
numerous biological processes and lncRNA abnormalities may be
associated with human diseases (13). In addition, lncRNAs were proven to
be highly stable and readily detectable in a number of body fluids,
including saliva, plasma, serum and urine. These characteristics
make lncRNAs diagnostic tools for non-invasive and rapid disease
diagnosis and prognostication; identification of novel biomarkers
in body fluid samples has broad prospects and appeal (14). This strongly suggests the important
role of lncRNAs in pathophysiology and their potential in clinical
application.
LncRNAs SRA and HCG22 are both associated with
cardiovascular disease. SRA is highly expressed in the liver, heart
and skeletal muscle and is associated with human dilated
cardiomyopathy (DCM) (14). HCG22
is a novel mucin-like gene and is located in a mucin-like gene
cluster with DPCR1, mucin 21, cell surface-associated and mucin
22(15). HCG22 expression is high
in lung tissues (16). The
pathogenesis of idiopathic DCM is also related to HCG22 expression
(17). HCG22 has also been detected
in the brain, spleen, thymus, prostate and oral cavity (18). Based on the Ct values of the present
RT-qPCR analysis, both SRA and HCG22 were abundantly expressed in
serum samples of pediatric patients. KD is characterized by
systemic inflammation in all of the medium-sized arteries and in
multiple organs and tissues during the acute fever, but
inflammation of the CA and heart (valvulitis, pericarditis or
myocarditis) results in the most devastating clinical outcomes
(19).
Cardiovascular manifestations may be prominent
during the acute KD episodes and are the major cause of long-term
mortality and morbidity. The results of the present study suggested
that SRA and HCG22 levels in the KD group were higher than those in
the control group. Abnormal lipid metabolism frequently occurs in
pediatric patients with KD and abnormal blood lipid levels are
among the most important risk factors for CA injury (20). TG and LDL-C levels are positively
correlated with atherosclerosis and coronary heart disease, while
HDL-C is a protective factor for coronary heart disease and
atherosclerosis and is negatively correlated with its risk.
Elevated TG and LDL-C and decreased HDL-C levels are reported in
children with KD. They all increase the risk of these children
developing cardiovascular disease at the adolescent stage (21). In the present study, HDL-C was
significantly lower in patients with KD than in healthy controls;
however, TG and LDL-C levels did not significantly differ between
the two groups. Based on single-factor correlation analysis, BNP
and CRP were positively correlated with HCG22 in patients with
acute KD, while TC and LDL were negatively correlated with HCG22 in
patients with acute KD.
The ROC is a comprehensive index used to reflect the
sensitivity and specificity of continuous variables. In the present
study, ROC curves for KD were constructed. The AUC for serum HCG22
to distinguish between KD and normal subjects was 0.633, while the
sensitivity and specificity were 55.0 and 72.5%, respectively. For
SRA, the AUC was 0.743 and the sensitivity and specificity were
70.0 and 82.5%, respectively. The present results indicated that
serum HCG22 and SRA had higher sensitivity and specificity in the
screening for KD, which may be more advantageous than the common
blood biomarkers of KD. Alterations of lncRNAs in the blood may
reflect the underlying mechanisms for a certain disease examined.
In the present study, the number of cases of KD was limited and
lncRNAs expression levels were analyzed in the serum samples of
only 40 patients with KD and 40 healthy controls. More samples
should be analyzed in the future.
KD is characterized by endothelial cell damage,
which may be due to abnormal production of cytokines and production
of cytotoxic antibodies against endothelial cells. Furthermore,
IVIG is an effective method to prevent coronary artery
abnormalities in patients with KD, possibly by inhibiting the
activation of the immune system and reducing endothelial cell
damage (22-25).
The lncRNA MHRT encodes a spliced lncRNA that may act as a
cardioprotective agent of the heart. Based on a study of a similar
gene in mice, the encoded transcript may regulate chromatin
remodeling by acting as a bait for the SMARCA4 chromatin repressor
complex, preventing it from binding to its genomic targets.
Blocking the effects of BRG1 may be crucial to protect the heart
from pathological hypertrophy (26). In the present study, lncRNA MHRT
levels were lower in acute KD, suggesting that lncRNA MHRT is
inhibited during acute KD. LncRNA MHRT levels were upregulated in
convalescent KD following IVIG treatment and were still higher in
the controls. These results suggested that lncRNA MHRT may have a
role during KD following IVIG therapy. In addition, BNP, which is
usually secreted by the myocardium, particularly in the case of
increased intracardiac pressure and myocardial stress, is one of
the important indicators for the diagnosis of human heart failure
and left ventricular dysfunction (27). In the present study, BNP was
significantly decreased in convalescent KD, which suggested
improved cardiac function in pediatric patients with KD during
recovery. BNP negatively correlated with lncRNA MHRT in patients
with convalescent KD, further suggesting that lncRNA MHRT is a
protective factor for KD, indicating that lncRNA MHRT may be a
potential therapeutic target.
In conclusion, the present study suggested that the
serum levels of lncRNAs SRA and HCG22 were upregulated in pediatric
patients with KD compared with those in healthy children. LncRNA
MHRT was upregulated in convalescent KD. The present results
suggested that lncRNAs SRA and HCG22 may act as novel biomarkers
for KD diagnosis and lncRNA MHRT is a novel biomarker for
predicting the clinical prognosis of KD.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by Ningbo Science and
Technology Innovation Team Program (grant nos. 2014B82003 and
2014B82002), the National Natural Science Foundation of China
(grant nos. 81370165 and 81501421), the Natural Science Foundation
of Zhejiang (grant no. Q16H100001), the Natural Science Foundation
of Ningbo (grant no. 2015A610176), the Fang Runhua Fund of Hong
Kong and the K.C. Wong Magna Fund in Ningbo University.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QZ contributed to the conception and design of the
study. JC collected patient serum samples. QZ and DW performed
experimental work and were involved in conceiving the study. HY,
FL, YX, FW, JW, HQ and SB were involved in conceiving the study and
drafting the manuscript. HQ and SB performed the experimental
evaluation. QZ and JC confirmed the authenticity of the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The experimental protocols for the present study
were approved by the Ethics Committee of Ningbo Women and
Children's Hospital (Ningbo, China). Written informed consent was
obtained from the patients' parents prior to study
participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kawasaki T: Acute febrile mucocutaneous
syndrome with lymphoid involvement with specific desquamation of
the fingers and toes in children. Arerugi. 16:178–222.
1967.PubMed/NCBI(In Japanese).
|
|
2
|
Galeotti C, Kaveri SV, Cimaz R, Koné-Paut
I and Bayry J: Predisposing factors, pathogenesis and therapeutic
intervention of Kawasaki disease. Drug Discov Today. 21:1850–1857.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mccrindle BW, Rowley AH, Newburger JW,
Burns JC, Bolger AF, Gewitz M, Baker AL, Jackson MA, Takahashi M,
Shah P, et al: Diagnosis, treatment, and long-term management of
kawasaki disease: A scientific statement for health professionals
from the American Heart Association. Circulation. 135:e927–e999.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bayers S, Shulman ST and Paller AS:
Kawasaki disease: Part I. Diagnosis, clinical features, and
pathogenesis. J Am Acad Dermatol. 69:501.e1–e11. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Takahashi K, Oharaseki T and Yokouchi Y:
Pathogenesis of Kawasaki disease. Clin Exp Immunol. 164 (Suppl
1):S20–S22. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Takahashi K, Oharaseki T and Yokouchi Y:
Update on etio and immunopathogenesis of Kawasaki disease. Curr
Opin Rheumatol. 26:31–36. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Orenstein JM, Shulman ST, Fox LM, Baker
SC, Takahashi M, Bhatti TR, Russo PA, Mierau GW, de Chadarévian JP,
Perlman EJ, et al: Three linked vasculopathic processes
characterize Kawasaki disease: A light and transmission electron
microscopic study. PLoS One. 7(e38998)2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wapinski O and Chang HY: Long noncoding
RNAs and human disease. Trends Cell Biol. 21:354–361.
2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Schmitz SU, Grote P and Herrmann BG:
Mechanisms of long noncoding RNA function in development and
disease. Cell Mol Life Sci. 73:2491–2509. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sallam T, Sandhu J and Tontonoz P: Long
noncoding RNA discovery in cardiovascular disease: Decoding form to
function. Circ Res. 122(155)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Muta H, Ishii M, Yashiro M, Uehara R and
Nakamura Y: Late intravenous immunoglobulin treatment in patients
with Kawasaki disease. Pediatrics. 129:e291–e297. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yang KC, Yamada KA, Patel AY, Topkara VK,
George I, Cheema FH, Ewald GA, Mann DL and Nerbonne JM: Deep RNA
sequencing reveals dynamic regulation of myocardial noncoding RNAs
in failing human heart and remodeling with mechanical circulatory
support. Circulation. 129:1009–1021. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cooper C, Vincett D, Yan Y, Hamedani MK,
Myal Y and Leygue E: Steroid receptor RNA activator bi-faceted
genetic system: Heads or Tails? Biochimie. 93:1973–1980.
2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yatagai Y, Hirota T, Sakamoto T, Yamada H,
Masuko H, Kaneko Y, Iijima H, Naito T, Noguchi E, Tamari M, et al:
Variants near the HLA complex group 22 gene (HCG22) confer
increased susceptibility to late-onset asthma in Japanese
populations. J Allergy Clin Immunol. 138:281–283.e13.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Taniguchi N, Konno S, Hattori T, Isada A,
Shimizu K, Shimizu K, Shijubo N, Huang SK, Hizawa N and Nishimura
M: The CC16 A38G polymorphism is associated with asymptomatic
airway hyper-responsiveness and development of late-onset asthma.
Ann Allergy Asthma Immunol. 111:376–381.e1. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Meder B, Rühle F, Weis T, Homuth G, Keller
A, Franke J, Peil B, Lorenzo Bermejo J, Frese K, Huge A, et al: A
genome-wide association study identifies 6p21 as novel risk locus
for dilated cardiomyopathy. Eur Heart J. 35:1069–1077.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lu F, Houck JR, Lohavanichbutr P and Chen
C: Transcriptome analysis reveals differentially expressed lncRNAs
between oral squamous cell carcinoma and healthy oral mucosa.
Oncotarget. 8:31521–31531. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Amano S, Hazama F, Kubagawa H, Tasaka K,
Haebara H and Hamashima Y: General pathology of Kawasaki disease:
On the morphological alterations corresponding to the clinical
manifestations. Acta Pathol Jpn. 30:681–694. 1980.PubMed/NCBI
|
|
20
|
Cabana VG, Gidding SS, Getz GS, Chapman J
and Shulman ST: Serum amyloid A and high density lipoprotein
participate in the acute phase response of Kawasaki disease.
Pediatr Res. 42:651–655. 1997.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chiang AN, Hwang B, Shaw GC, Lee BC, Lu
JH, Meng CC and Chou P: Changes in plasma levels of lipids and
lipoprotein composition in patients with Kawasaki disease. Clin
Chim Acta. 260:15–26. 1997.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Galeotti C, Bayry J, Kone-Paut I and
Kaveri SV: Kawasaki disease: Aetiopathogenesis and therapeutic
utility of intravenous immunoglobulin. Autoimmun Rev. 9:441–448.
2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Nonoyama S: Immunological abnormalities
and endothelial cell injury in Kawasaki disease. Acta Paediatr Jpn.
33:752–755. 1991.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mostafavi N, Haghjooy-Javanmard S,
Presidend N, Manssori NS and Kelishadi R: Persistence of
endothelial cell damage late after Kawasaki disease in patients
without coronary artery complications. Adv Biomed Res.
4(25)2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Leung DY: Clinical and immunologic aspects
of Kawasaki disease. Immunodefic Rev. 1:261–271. 1989.PubMed/NCBI
|
|
26
|
Han P, Li W, Lin CH, Yang J, Shang C,
Nuernberg ST, Jin KK, Xu W, Lin CY, Lin CJ, et al: A long noncoding
RNA protects the heart from pathological hypertrophy. Nature.
514:102–106. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
De Lemos JA, Mcguire DK and Drazner MH:
B-type natriuretic peptide in cardiovascular disease. Lancet.
362:316–322. 2003.PubMed/NCBI View Article : Google Scholar
|