Introduction
Chemotherapy, the standard treatment for different
types of cancer, plays an important role in improving the survival
time of patients with cancer (1,2);
however, a large number of patients who receive chemotherapy will
develop chemotherapy resistance, leading to tumor recurrence and
poor prognosis (3). The
pathogenesis of chemotherapeutic resistance in cancer is highly
complex and involves numerous biological processes and molecular
pathways (4,5).
Chemotherapeutic resistance in tumors has been
extensively investigated (6-8).
Previous studies have reported that the process of chemotherapeutic
resistance may be triggered by adaptive mutations in the tumor
(9) or attributed to copy number
variation in certain genes (10).
For example, the high expression of P-glycoprotein protein caused
increased efflux of chemotherapeutic drugs and decreased the
sensitivity of tumor cells to these drugs (11). However, few alternative drugs or
therapeutic strategies aimed at overcoming drug resistance are
available; therefore, an understanding of the specific mechanisms
underlying cancer drug resistance would be beneficial to the
development of drugs for mitigating this chemotherapeutic
resistance.
Colon cancer is the 4th leading cause of
cancer-associated death worldwide (12). The most commonly used clinical
chemotherapeutic agents for the treatment of colon cancer contain
platinum compounds and 5-fluorouracil (5-FU) (13). Patients with colon cancer typically
develop chemotherapy resistance (14), which may be an important cause of
recurrence and poor prognosis. A satisfactory strategy to overcome
colon cancer-related chemotherapeutic resistance remains
unavailable to date. In recent years, biomedical text mining
research, a type of bioinformatics analysis, has been intensively
used to identify information in a more accurate and efficient
manner; thus, serving as an effective tool to identify
differentially expressed genes and to analyze molecular functions
(15). A previous study, using
biomedical text mining, revealed that several drugs have the
potential to be repurposed for colorectal cancer treatment
(16). Furthermore, novel microRNA
biomarkers for the early diagnosis of colorectal cancer and 5-FU
chemotherapeutic resistance were identified (17).
In the present study, data mining was used to
identify genes, then Gene Ontology (GO) analysis was used to
identify the potential association between gene expression and
chemotherapeutic resistance in colon cancer. A deeper understanding
of the mechanisms by which these genes perform their biological
functions would provide further insights into drug resistance in
colon cancer.
Materials and methods
Data mining
From the web-based service platform Human Gene
Function and Network Analysis GenCLiP3 (http://ci.smu.edu.cn/genclip3/analysis.php), two gene
sets were generated using the search terms, ‘cancer recurrence’ and
‘chemotherapy resistance’, respectively. The intersection of the
two gene sets was selected and the data was visualized using a Venn
diagram online (http://bioinformatics.psb.ugent.be/webtools/Venn/).
The intersection of the two gene sets contained 602 genes, which
are associated with cancer recurrence and chemotherapy
resistance.
Analysis of biological processes and
pathways
GO and KEGG pathway enrichment analysis of the genes
generated from the intersection of the two gene sets were performed
using Database for Annotation, Visualization and Integrated
Discovery v6.8 (DAVID 6.8) (https://david.ncifcrf.gov). Among the biological
processes, whose values were above the cut-off, those most
associated with cancer recurrence and chemotherapy resistance were
selected based on available published literature. The pathways
associated with other specific diseases were excluded.
Gene expression
The gene expression data (N=602 genes) was obtained
from The Cancer Genome Atlas dataset (https://tcga-data.nci.nih.gov/tcga/). EdgR and limma
packages were used to calculate the differentially expressed genes
between colon cancers and normal group, with the same parameters
(|logFC|>1, false discovery rate (FDR) <0.05).
Protein-protein interaction (PPI)
networks
PPI networks from the intersection of the associated
genes were generated using the Search Tool for the Retrieval of
Interacting Genes/Proteins (STRING) website (http://string-db.org/), which provides an interactive
platform for assessing the interactions between proteins. The
following parameters were used: i) minimum required interaction
score; ii) medium confidence (0.400); and iii) PPI enrichment
P-value, 1.75e-05.
Survival analysis using Gene
Expression Profiling Interactive Analysis (GEPIA) database
For predicting genes which play a role in drug
resistance in colon cancer, the genes previously identified by
STRING analysis were further analyzed using the GEPIA database
(http://gepia.cancer-pku.cn) to identify
the association between gene expression and survival time among
patients with colon cancer. Specifically, the candidate genes
(N=310) were searched in GEPIA one by one (single gene analysis).
Gene expression and its association with survival were
recorded.
Clinical samples
A total of 33 patients with colon cancer, who
received surgery at Xiang'an Hospital of Xiamen University (Fujian,
China) from January 2015 to December 2019 were included in the
present study. Tumor and adjacent normal tissues (5 cm distance
from the tumor tissue) were collected from patients with colon
cancer. All the patients had complete clinicopathological data,
were diagnosed with colon cancer using histopathology and had not
received any anti-tumor therapy prior to surgery. Patients with
other malignancies or comorbidities and those who were pregnant
were not included in the study. Among the patients included in the
study, there were 19 males and 14 females, aged from 39 to 64
years, with an average age of 63±8.1 years. The postoperative tumor
stage was classified according to the American Joint Committee on
Cancer/International Union Against Cancer TNM staging system, 7th
edition (18), in which there were
5 patients with stage I, 17 patients with stage II, and 11 patients
with stage III cancer. The present study was approved by the Ethics
Committee of Xiang'an Hospital of Xiamen University (Fujian, China)
and all patients provided written informed consent.
Immunohistochemistry (IHC)
Colon cancer tissues and adjacent normal samples
were fixed in 10% neutral formalin for 48 h at room temperature.
Dehydration was done in an ascending alcohol series at 30, 50, 75,
90 and 100% for 10 min at each stage. Subsequently, the samples
were embedded in paraffin wax according to standard laboratory
procedures. Sections (5 µm) were prepared, mounted on glass slides
and dried overnight. The sections were then deparaffinized with
xylene three times for 5 min each time, rehydrated through a
descending alcohol series at 100, 95, 80, 70 and 50% for 2 min at
each stage, and washed with PBS (pH, 7.2-7.4). Antigen retrieval
was performed using citrate-EDTA antigen retrieval solution (cat.
no. P0086; Beyotime Institute of Biotechnology) in a microwave oven
at 100˚C for 15 min. After washing three times with PBS, the
sections were permeabilized for 25 min in 0.2% Triton X-100 at room
temperature. Sections were washed again with PBS, and blocked with
5% BSA (cat. no. A8010; Beijing Solarbio Science & Technology
Co., Ltd.) in PBS for 1 h at room temperature. Primary antibody
incubation was subsequently carried out overnight at 4˚C, followed
by secondary antibody incubation for 1 h at room temperature.
Immunohistochemical reactions were developed using DAB Horseradish
Peroxidase Color Development Kit (cat. no. P0203; Beyotime
Institute of Biotechnology), and nuclei were counterstained with
hematoxylin for 15 sec. The slides were dehydrated using an
ascending alcohol series at 80, 95 and 100% for 2 min at each
stage, followed by xylene permeabilization twice for 5 min each.
Tissue sections were sealed with neutral resins and images were
captured using an optical microscope (Nikon Corporation) from at
least 10 fields of view at x200 magnification. The
immunohistochemical score was calculated based on the distribution
and intensity of staining in the positive cells. The following
classification was used: Negative expression (-, score of 0),
weakly positive expression (+, score of 1), moderately positive
expression (++, score of 2), and strongly positive expression (+++,
score of 3). The results were blindly determined by two experienced
pathologists. In addition, the percentage of stained cells was
scored semi-quantitatively as 1 (0-25%), 2 (26-50%), 3 (51-75%), or
4 (76-100%). Multiplication of the intensity score and percentage
score resulted in a score ranging from 0 to 12 for each tissue.
The following antibodies were used: Anti-CDH2 (cat.
no. 13116; 1:200 dilution; Cell Signaling Technology, Inc.),
anti-LEP (cat. no. ab3583; 1:200 dilution; Abcam), anti-POSTN (cat.
no. ab219056; 1:500 dilution; Abcam), anti-TIMP1 (cat. no.
ab211926; 1:500 dilution; Abcam), anti-VEGFC (cat. no. ab83905;
1:300 dilution; Abcam), anti-rabbit IgG HRP-conjugated antibody
(cat. no. 7074; 1:500 dilution; Cell Signaling Technology, Inc.),
and anti-mouse IgG HRP-conjugated antibody (cat. no. 7076; 1:500
dilution; Cell Signaling Technology, Inc.).
Cell culture
The human HCT116 and LoVo colon cancer cell lines
were purchased from the Cell Bank of the Chinese Academy of
Sciences. Both the cell lines were cultured in DMEM, supplemented
with 10% FBS, and 1% penicillin-streptomycin (all from Gibco;
Thermo Fisher Scientific, Inc.), at 37˚C in a humidified incubator
with 5% CO2.
PI staining
To investigate the sensitivity of the colon cancer
cells to 5-FU, apoptosis was analyzed by staining the cells with
PI. The colon cancer cells were treated with 1 µg/ml 5-FU for 24,
48 h following transfection with siRNA. Subsequently, PI dye (1
µg/ml) was added and the cells were incubated for 10 min at 37˚C in
the dark. The PI fluorescence of the nuclei was observed under a
fluorescent microscope at x100 magnification (Leica Microsystems
GmbH). PI-positive cells in five randomly selected fields of view
were counted for each group.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA was extracted from the colon cancer cells
and human colon cancer tissues using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. The concentration and purity of the
RNA was measured according to the optical density (OD) at 260 nm
and the 260/280 nm ratio, respectively. Only RNA with a 260/280
ratio between 1.8 and 2.0 was used for the experiments. cDNA was
synthesized using a Reverse Transcription kit (Sangon Biotech Co.
Ltd.) under the following conditions: 37˚C for 15 min, 85˚C for 5
sec, and 4˚C for 30 min. Subsequently, the following thermocycling
conditions were used: Initial denaturation at 95˚C for 3 min;
denaturation at 95˚C for 30 sec, annealing at 60˚C for 20 sec for
40 cycles. RT-qPCR was performed using a GoTaq® qPCR
Master Mix (Promega Corporation) on an ABI 7500 qPCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The relative
mRNA expression levels of the target genes were calculated using
the 2-∆∆Cq method and normalized to that of GAPDH
(19). All the primer sequences are
listed in Table I.
 | Table ISequences of the primers and
siRNAs. |
Table I
Sequences of the primers and
siRNAs.
| A, primer sequences
for RT-qPCR |
|---|
| Name | Sequence |
|---|
| GAPDH | F:
5'-GCAAAGTGGAGATTGTTGCCAT-3' |
| GAPDH | R:
5'-CCTTGACTGTGCCGTTGAATTT-3' |
| CDH2 | F:
5'-TGCGGTACAGTGTAACTGGG-3' |
| CDH2 | R:
5'-GAAACCGGGCTATCTGCTCG-3' |
| LEP | F:
5'-TGCCTTCCAGAAACGTGATCC-3' |
| LEP | R:
5'-CTCTGTGGAGTAGCCTGAAGC-3' |
| POSTN | F:
5'-GCTATTCTGACGCCTCAAAACT-3' |
| POSTN | R:
5'-AGCCTCATTACTCGGTGCAAA-3' |
| TIMP1 | F:
5'-AGAGTGTCTGCGGATACTTCC-3' |
| TIMP1 | R:
5'-CCAACAGTGTAGGTCTTGGTG-3 |
| VEGFC | F:
5'-GGCTGGCAACATAACAGAGAA-3' |
| VEGFC | R:
5'-CCCCACATCTATACACACCTCC-3' |
| B, siRNA sequences
used for silencing |
| Name | Sequence |
| CDH2 |
5'-TAAACTTCACATTGAGAAGAG-3' |
| LEP |
5'-TGTGAAATGTCATTGATCCTG-3' |
| POSTN |
5'-ATAATGGTTAATGAAAAGCCC-3' |
| TIMP1 |
5'-TCATCTTGATCTCATAACGCT-3' |
| VEGFC |
5'-TAAAGAAGGTGTTTGTCGCGA-3' |
| Control |
5'-TTCTCCGAACGTGTCACGTTT-3' |
Western blot analysis
The colon cancer cells were lysed on ice using RIPA
lysis buffer with 1% phenylmethylsulfonyl fluoride (Nanjing KeyGen
Biotech Co., Ltd.), then centrifuged at 12,000 x g for 15 min at
4˚C. Total protein was collected, then the concentration of the
samples was quantified using a BCA assay (Nanjing KeyGen Biotech
Co., Ltd.). Next, the proteins (30 µg) were separated using a 10%
SDS-PAGE, then transferred to PVDF membranes using wet transfer.
After incubation with 5% skimmed milk for 2 h at room temperature,
the membranes were incubated with the corresponding primary
antibodies overnight at 4˚C. Subsequently, the membranes were
washed with TBS-Tween-20, containing 0.1% Tween-20, then incubated
with the corresponding secondary horseradish peroxidase-conjugated
antibodies at room temperature for 2 h. The western blots were
visualized using an enhanced chemiluminescence kit (EMD Millipore).
β-actin was used as the loading control. The blots were quantified
using ImageJ software (Version 1.8.0; National Institutes of
Health).
The following antibodies were used: Anti-CDH2 (cat.
no. 14215; 1:1,000 dilution; Cell Signaling Technology, Inc.),
anti-LEP (cat. no. ab3583; 1:1,000 dilution; Abcam), anti-POSTN
(cat. no. ab219056; 1:1,000 dilution; Abcam), anti-TIMP1 (cat. no.
8946; 1:1,000 dilution; Cell Signaling Technology, Inc.),
anti-VEGFC (cat. no. ab83905; 1:1,000 dilution; Abcam),
anti-β-actin (cat. no. sc-47778; 1:3,000 dilution; Santa Cruz
Biotechnology, Inc.), anti-rabbit IgG HRP-linked antibody (cat. no.
7074; 1:5,000 dilution; Cell Signaling Technology, Inc.) and
anti-mouse IgG HRP-linked antibody (cat. no. 7076; 1:5,000
dilution; Cell Signaling Technology, Inc.).
Small interfering (si)RNA
transfection
All the siRNA sequences and the disordered sequence
were synthesized by Sangon Biotech Co., Ltd. and are listed in
Table I. Scrambled siRNA sequences
were used as negative control. The colon cancer cells were cultured
in complete DMEM until the cell density reached 60%, and
transfected with the different siRNAs at a final concentration of
10 nM, and control siRNA at a final concentration of 10 nM using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. After
transfection for 48 h at 37˚C, transfection efficiency was
evaluated by RT-qPCR.
CCK-8 assay
The colon cancer cells in the logarithmic growth
phase were digested with 0.25% trypsin (Gibco; Thermo Fisher
Scientific, Inc.), resuspended, then seeded into 96-well plates, at
a density of 1x103 cells per well. Next, 100 µl complete
medium was added. The cells were cultured for 12 h at 37˚C in a
humidified incubator with 5% CO2. Then, the cells were
treated with different concentrations of 5-FU (0, 0.5, 1, 2 and 4
µg/ml) for 48 h. Subsequently, 10% CCK-8 reagent (Beijing Solarbio
Science and Technology Co., Ltd.) was added to each well according
to the manufacturer's instructions, followed by incubation for 2 h
at 37˚C. The OD at 450 nm was measured using a microplate reader
(BioTek China). Cell-free wells with CCK-8 reagent served as a
negative control.
Statistical analysis
Statistical analysis was performed using SPSS v19.0
(IBM Corporation). The differences between the two independent
groups were analyzed using an unpaired Student's t-test, while the
differences between paired samples was analyzed using a Wilcoxon
signed-rank test. Parametric data was presented as the mean ± SD,
while non-parametric data was presented as the median ±
interquartile range. In addition, the mRNA expression levels of the
target genes in 33 tumor and adjacent normal tissues were analyzed
using a paired Student's t-test and presented as the mean ± SD.
Kaplan-Meier survival curves and statistics (Log-rank) were used to
analyze survival time in the 33 paired samples. P<0.05 was
considered to indicate statistically significant difference. Each
experiment was repeated independently, at least three times.
Results
Data mining strategies
To increase the number of candidate genes associated
with cancer recurrence that are currently known from previous
studies, search terms were used in the GenCLiP3 database, to
preliminary screen genes. The workflow used is shown in Fig. 1A. A total of 1,286 genes were
associated with cancer recurrence and 1,570 genes were associated
with chemoresistance, while a total of 602 genes were shared by
both lists (Fig. 1B). The list of
these genes and their expression levels are shown in Tables SI and SII.
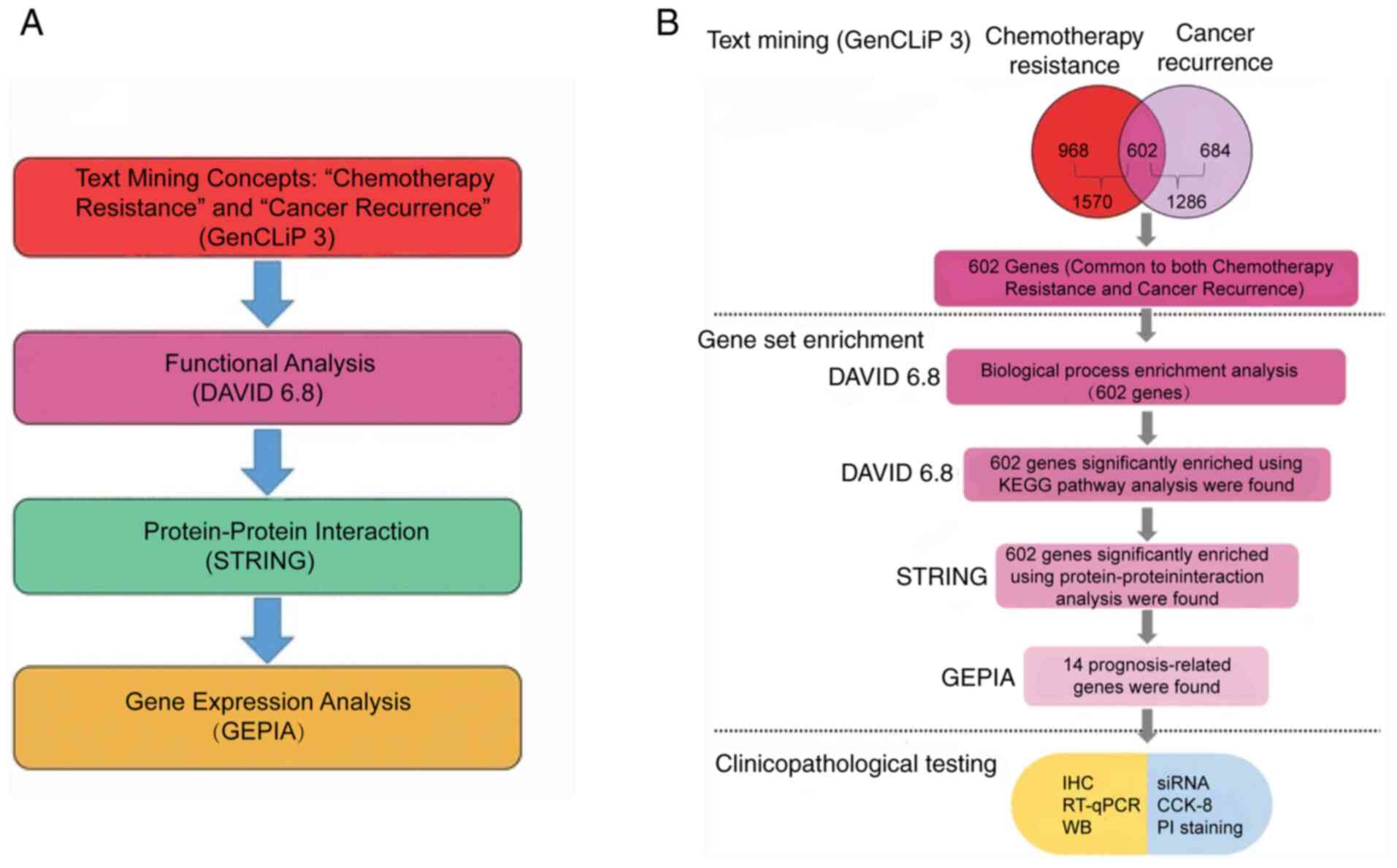 | Figure 1Data mining strategy used in the
present study. (A) Online bioinformatics analysis software was used
to select the genes of interest, in which GenCLiP3 was used to
identify the associated genes using the search terms ‘chemotherapy
resistance’ and ‘cancer recurrence’. Gene Ontology analysis was
performed using DAVID online tool. Protein-protein interaction was
analyzed using the STRING database. The association between the
expression level of the candidate genes and the overall survival of
patients with colon cancer was analyzed using GEPIA. (B) The
workflow used in the present study, where 602 genes were generated
and enriched using KEGG pathway and protein-protein interaction
analysis. Of these 602 genes, 14 prognosis-related genes were
chosen for further investigation. GEPIA, Gene Expression Profiling
Interactive Analysis; DAVID, Database for Annotation, Visualization
and Integrated Discovery; STRING, Search Tool for the Retrieval of
Interacting Genes/Proteins; CCK-8, Cell Counting Kit-8; RT-qPCR,
reverse transcription-quantitative PCR; IHC, immunohistochemistry;
si, small interfering; WB, western blot. |
GO enrichment analysis of gene
sets
The 602 genes were validated by performing GO
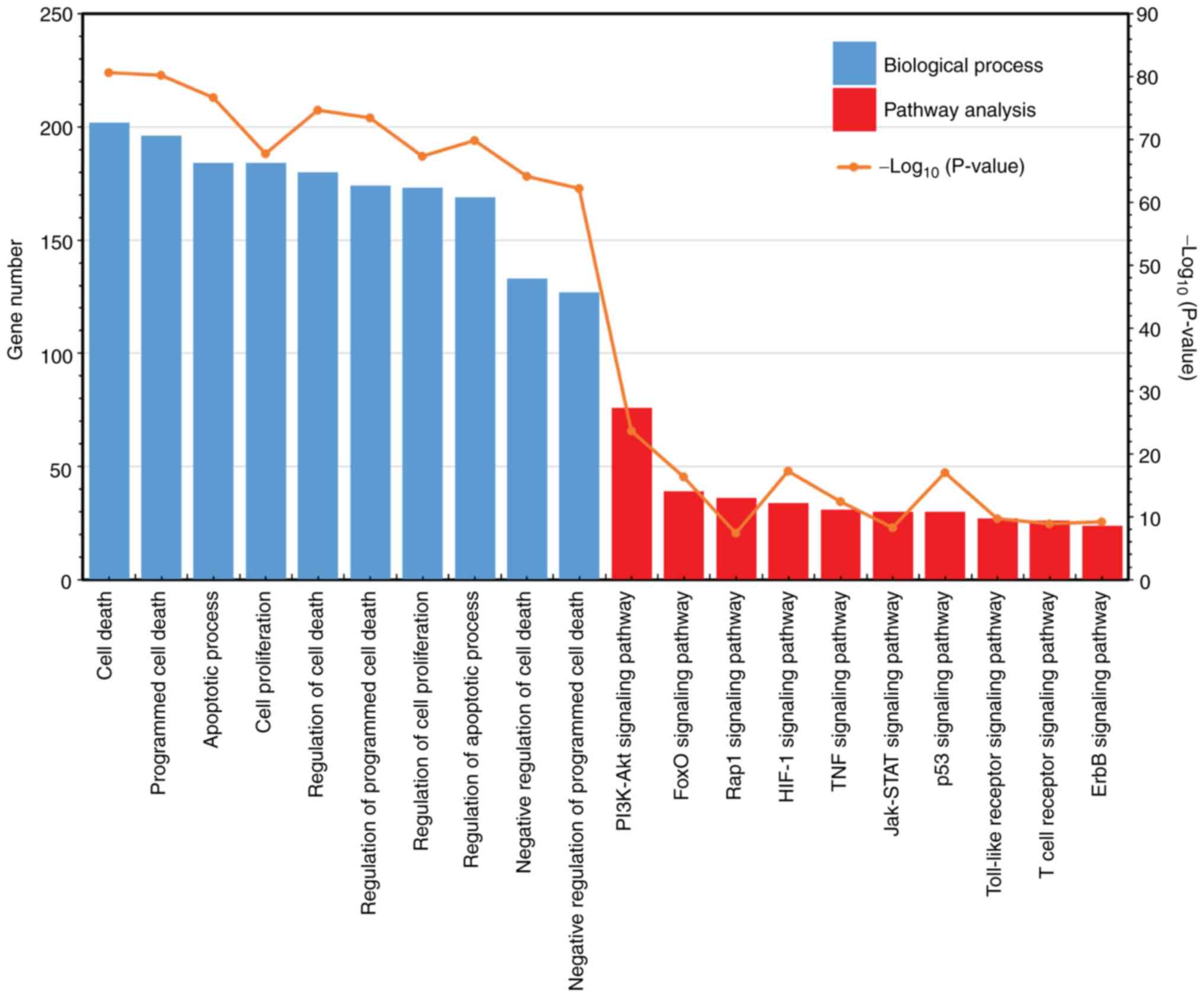
analysis, including biological processes alone. Results
demonstrated that the top ten GO terms were selected and are shown
in Fig. 2. The top 3 enriched
biological processes were ‘cell death’ (P=2.60x10-81),
‘programmed cell death’ (P=6.78x10-81) and ‘apoptotic
process’ (P=2.39x10-77), containing 202, 196 and 184
genes, respectively. Additional highly enriched biological
processes included ‘regulation of programmed cell death’,
‘regulation of apoptotic process’ and ‘regulation of cell
proliferation’.
Furthermore, these 602 genes were significantly
enriched using pathway analysis. KEGG pathway enrichment analyses
were performed using DAVID 6.8. Ten pathways, which were
significantly enriched were also selected. Among these, the top two
pathways were ‘PI3K-Akt signaling pathway’
(P=2.50x10-24) and ‘FoxO signaling pathway’
(P=5.02x10-17), containing 76 and 39 genes,
respectively. Other pathways were the ‘Rap1 signaling pathway’
(N=36, P=4.64x10-8), ‘HIF-1 signaling pathway’ (N=34,
P=6.22x10-18), ‘TNF signaling pathway’ (N=31,
P=4.30x10-13), ‘Jak-STAT signaling pathway’ (N=30,
P=5.95x10-9), ‘p53 signaling pathway’ (N=30,
P=1.07x10-17), ‘Toll-like receptor signaling pathway’
(N=27, P=2.17x10-10), ‘T cell receptor signaling
pathway’ (N= 26, P=1.48x10-9), and ‘ErbB signaling
pathway’ (N=24, P=7.10x10-10).
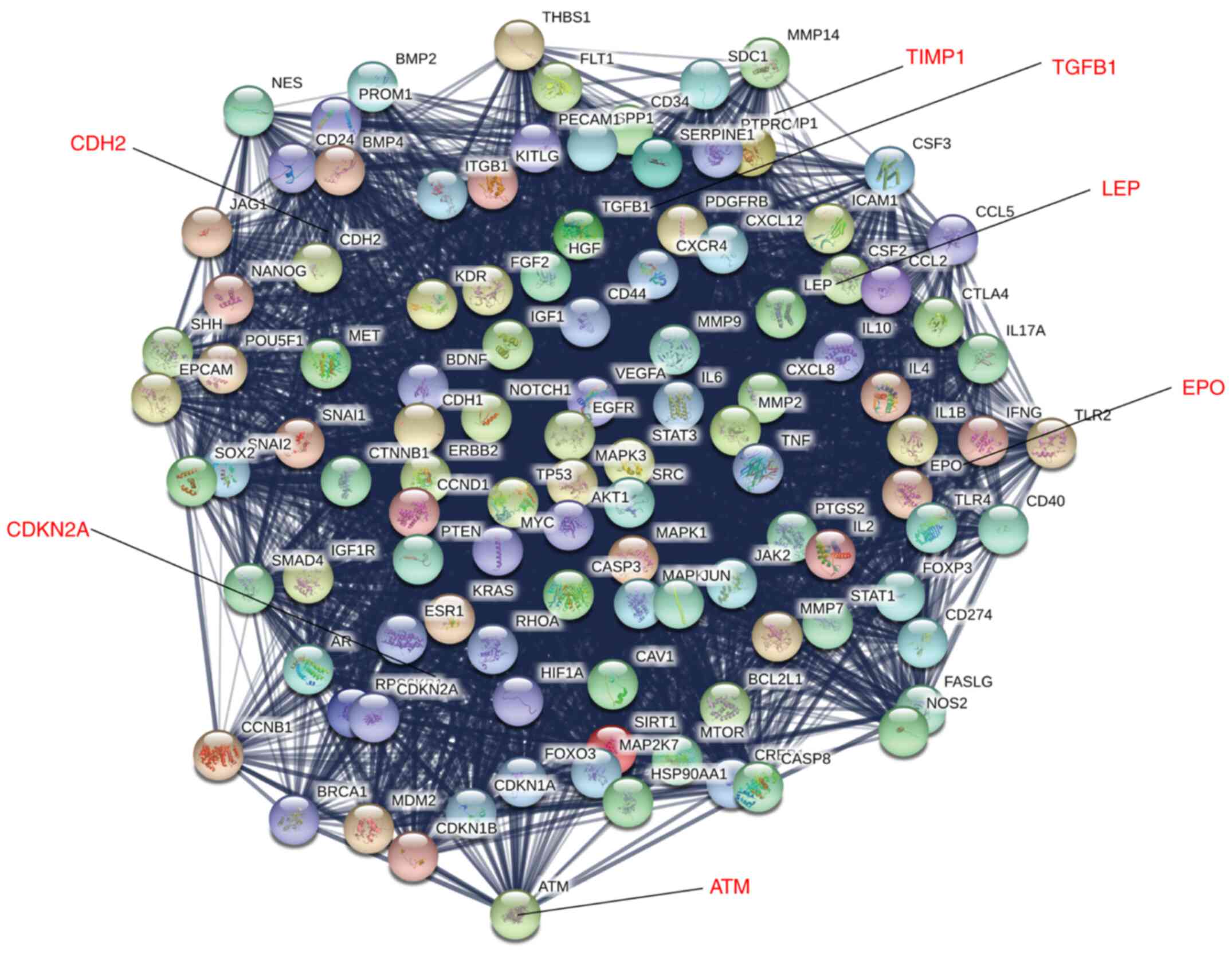
STRING-based analysis of PPI
PPI analysis of the 602 genes was performed using
STRING software. The 602 genes were found to be significantly
enriched using PPI analysis, whereby 4 patterns with strong
interactions were generated, with pattern 1 containing 109 genes
(PPI enrichment P<1.0x10-12; Fig. 3), pattern 2 producing 69 genes (PPI
enrichment P<1.0x10-8; Fig. S1), pattern 3 producing 73 genes
(PPI enrichment P<1.0x10-6; Fig. S2) and pattern 4 producing 59 genes
(PPI enrichment P<1.0x10-5; Fig. S3). These data suggested that the
genes in these patterns formed a tight interaction network.
Candidate genes are associated with
prognosis in patients with colon cancer
After STRING PPI analysis, all the aforementioned
candidate genes (N=310) were searched using the GEPIA database to
identify the genes that were significantly associated with survival
time in patients with colon cancer. The results demonstrated that
14 of 310 prognosis-related genes were highly expressed, and a high
expression level of ATM, CDH2, CDKN2A,
EPO, LEP, TGFB1, TIMP1, PGR,
VEGFC, POSTN, BCL6, CYP19A1,
NOTCH3 and XPA was significantly associated with poor
prognosis in patients with colon cancer (Fig. 4).
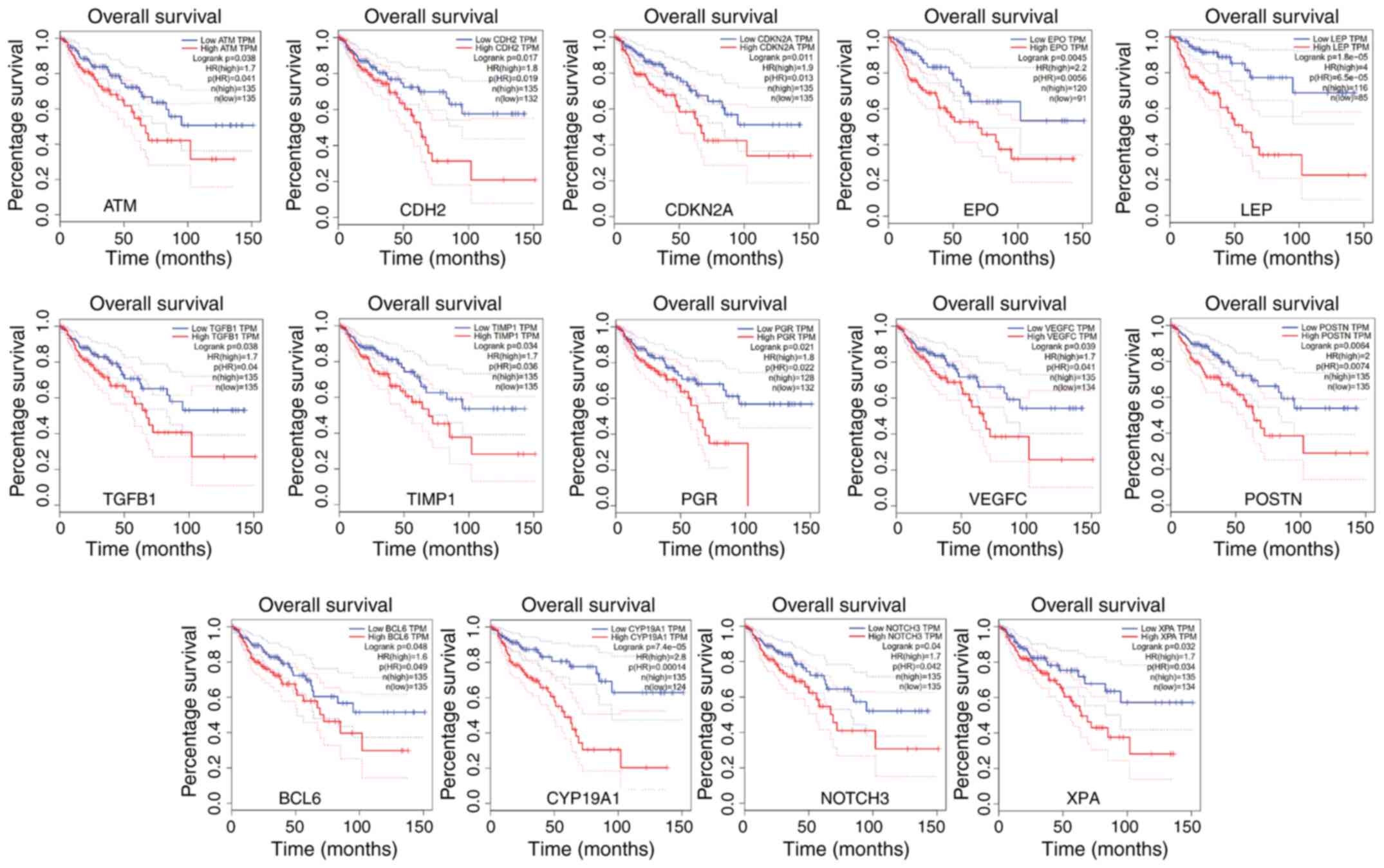 | Figure 4Overall survival analysis was
performed using the GEPIA database. The survival time of patients
with colon cancer and high expression level of ATM,
CDH2, CDKN2A, EPO, LEP, TGFB1,
TIMP1, PGR, VEGFC, POSTN, BCL6,
CYP19A1, NOTCH3 and XPA was significantly
reduced using data from the GEPIA database. GEPIA, Gene Expression
Profiling Interactive Analysis |
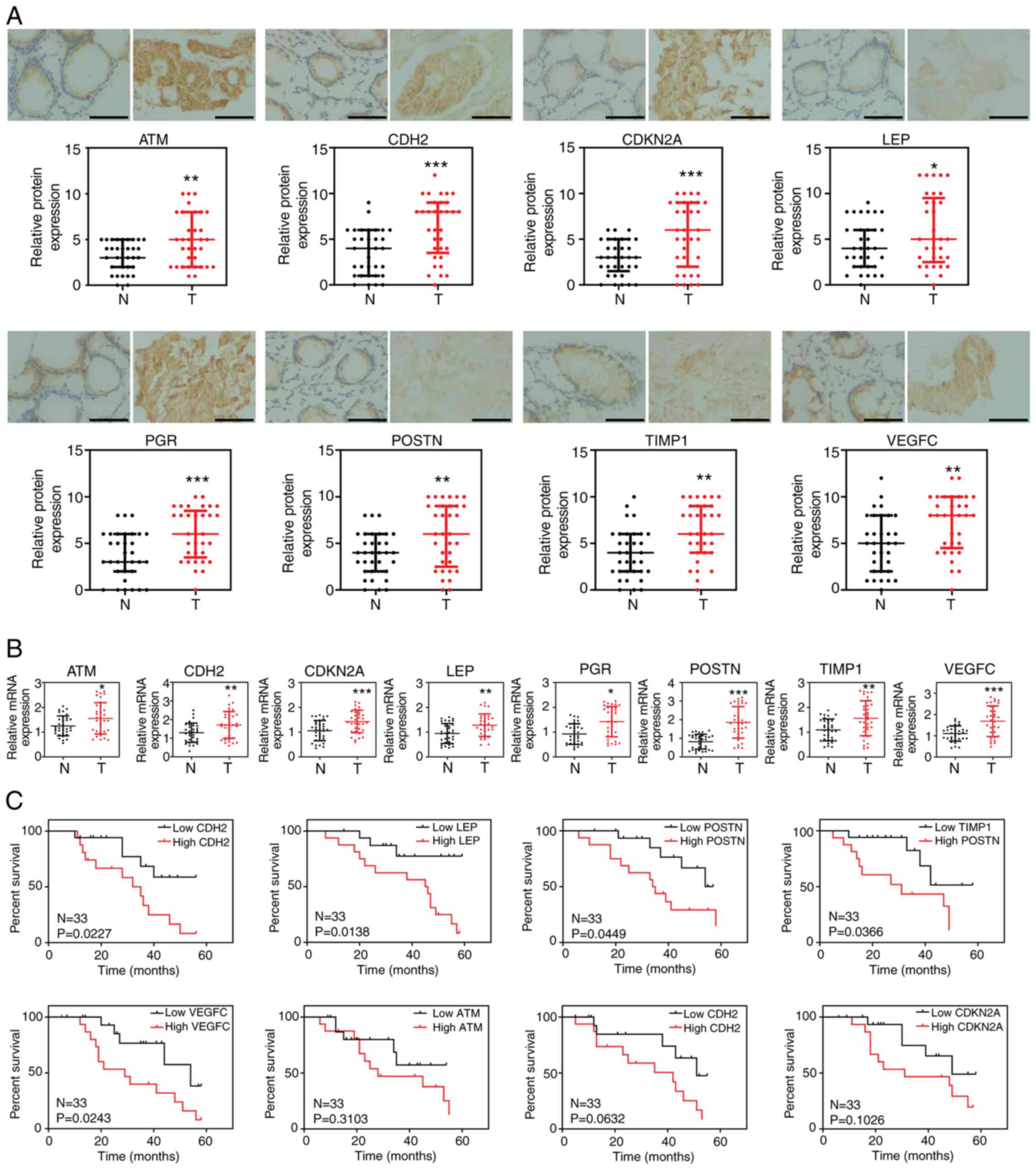
Gene expression in clinical
samples
To further confirm the biological functions of these
genes (ATM, CDH2, CDKN2A, EPO,
LEP, TGFB1, TIMP1, PGR, VEGFC,
POSTN, BCL6, CYP19A1, NOTCH3 and
XPA), experiments were performed to verify their expression
level in colon cancer. A total of 33 tumor and adjacent normal
tissues were collected from patients with colon cancer and the
expression levels were analyzed using IHC and RT-qPCR. The results
confirmed that the protein and mRNA expression of ATM,
CDH2, CDKN2A, LEP, PGR, TIMP1,
POSTN and VEGFC were significantly increased in colon
cancer tissues compared with that in the normal adjacent tissues
(Fig. 5A and B), while there were no significant
differences in the expression level of BCL6, EPO,
CYP19A1, TGFB1, NOTCH3 and XPA
(Fig. S4). Notably, the high
expression level of CDH2, LEP, POSTN,
TIMP1 and VEGFC in colon cancer tissues was
significantly associated with poor prognosis in patients with colon
cancer. However, no association was found between ATM,
PGR or CDKN2A and patient survival (Fig. 5C).
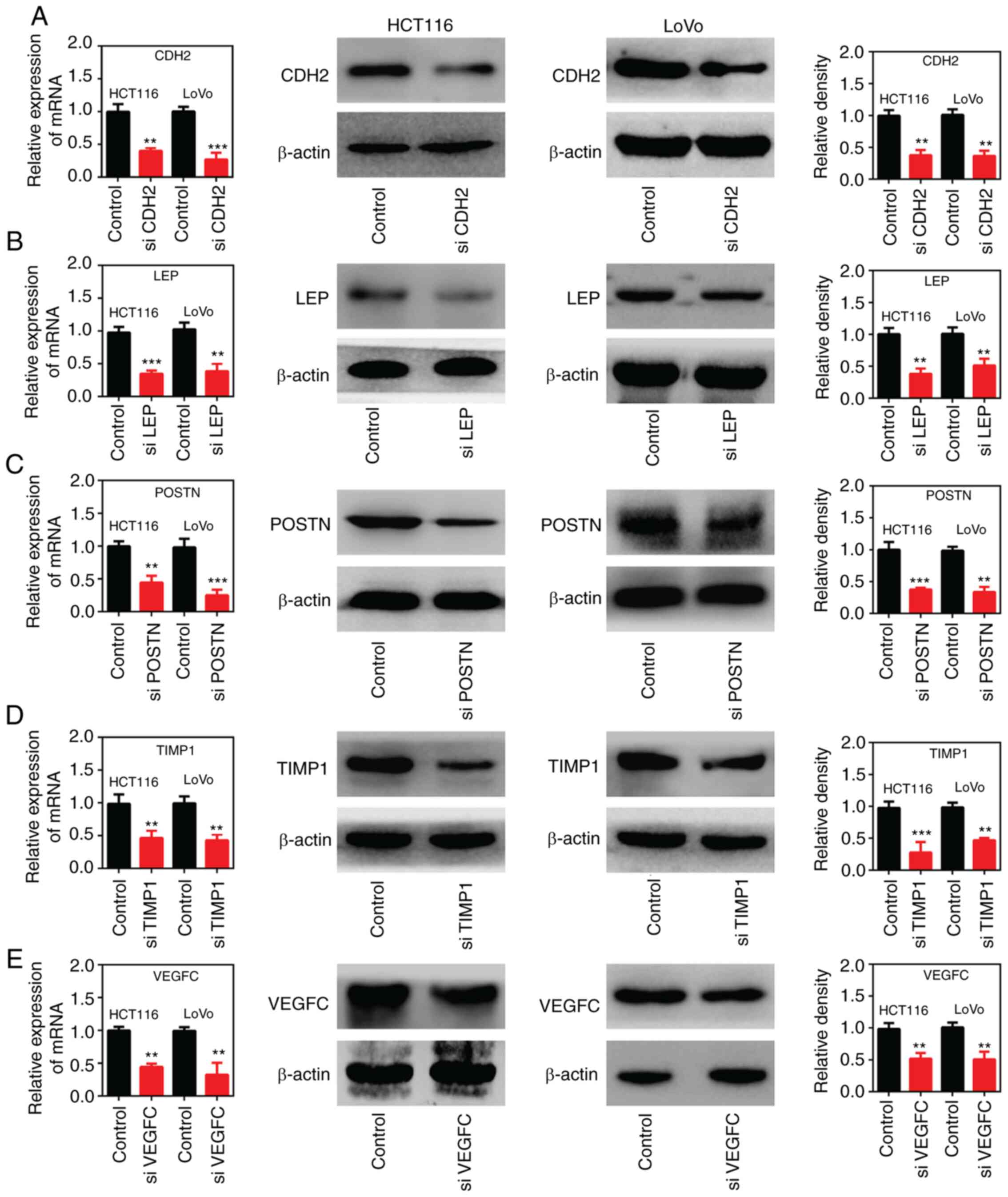
Expression levels of CDH2, LEP, POSTN,
TIMP1 and VEGFC genes were significantly decreased in colon cancer
cells following transfection with siRNA
In the present study, siRNA sequences were designed
to target CDH2, LEP, POSTN, TIMP1 and
VEGFC mRNAs. Subsequently, the cell lines, HCT116 and LoVo
were transfected with the different siRNAs and the expression
levels were evaluated using RT-qPCR and western blot analysis. The
results revealed that the expression levels of these genes were
significantly decreased following transfection with the different
siRNAs compared with that in the cells transfected with siNC
(Fig. 6).
Expression levels of CDH2, LEP, POSTN,
TIMP1 and VEGFC are associated with the sensitivity of colon cancer
cells to 5-FU resistance
Following silencing of CDH2, LEP,
POSTN, TIMP1 and VEGFC, the sensitivity of the
colon cancer cells to 5-FU was determined using a CCK-8 assay. The
results revealed that CDH2, LEP, POSTN,
TIMP1 and VEGFC knockdown in colon cancer cells
significantly enhanced their sensitivity to 5-FU. Furthermore,
following treatment with different concentrations of 5-FU, cell
viability and the IC50 values were significantly
decreased in gene-silenced colon cancer cells compared with that in
the cells transfected with siNC (Fig.
7).
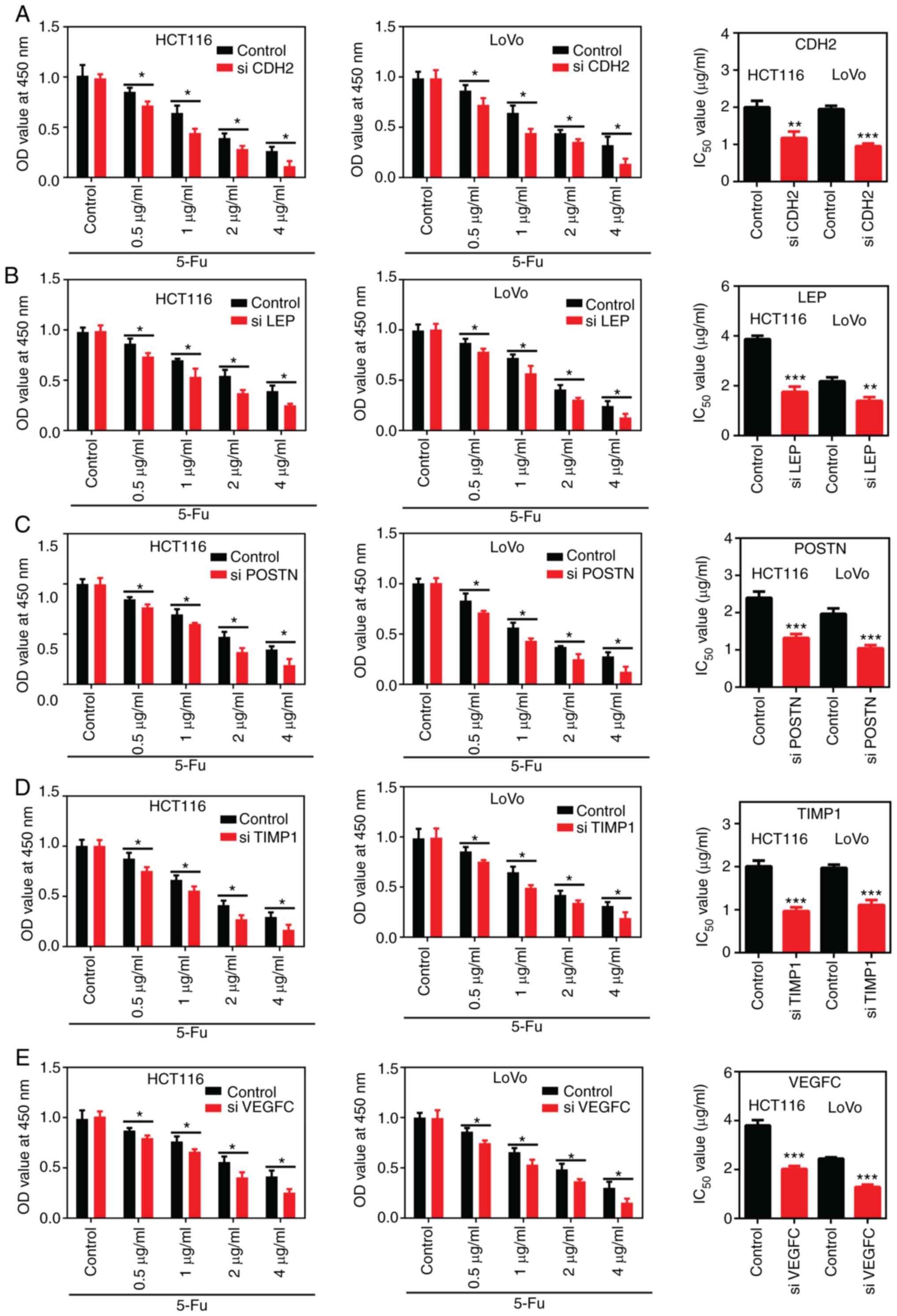 | Figure 7Silencing of CDH2, LEP,
POSTN, TIMP1 and VEGFC enhanced the
chemosensitivity of the colon cancer cells. After different
concentrations (0.5, 1, 2 and 4 µg/ml) of 5-FU treatment, cell
viability and the IC50 values of 5-FU were monitored
using a Cell Counting Kit-8 assay in the HCT116 and LoVo cells
following silencing of (A) CDH2, (B) LEP, (C)
POSTN, (D) TIMP1 and (E) VEGFC. The data was
analyzed using an unpaired Student's t-test and expressed as the
mean ± SD. Each assay was performed independently 3 times.
*P<0.05; **P<0.01;
***P<0.001. OD, optical density; si, small
interfering; 5-FU, 5-fluorouracil. |
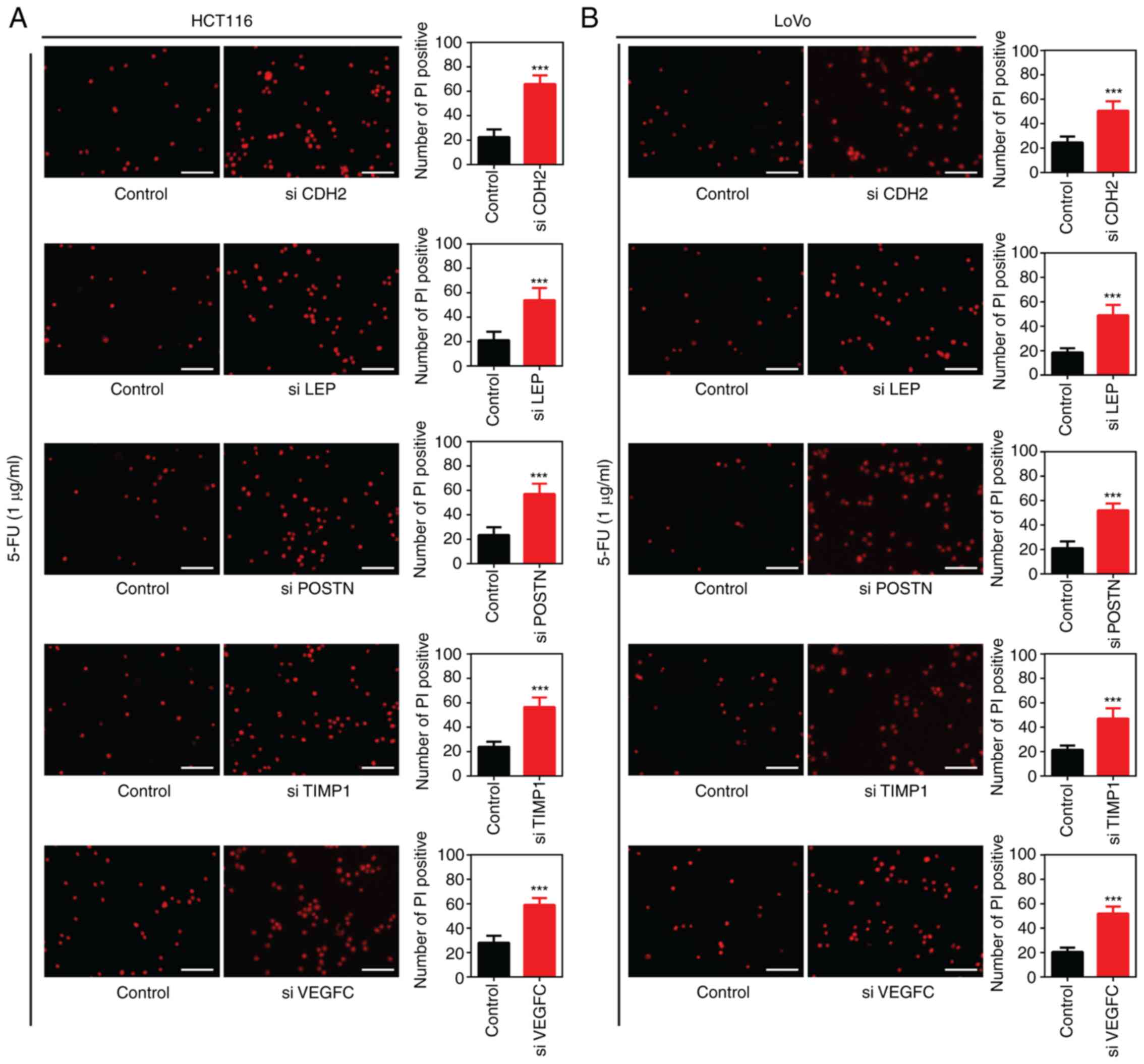
In addition, following silencing of CDH2,
LEP, POSTN, TIMP1 and VEGFC using siRNA
in the HCT116 or LoVo cells and treatment with 5-FU (1 µg/ml) for
24 h, there was a significant increase in the number of PI-positive
cells compared with that in cells transfected with siNC (Fig. 8). Taken together, these results
indicated that siRNA-mediated silencing of CDH2, LEP,
POSTN, TIMP1 and VEGFC enhanced the
sensitivity of the colon cancer cell lines to 5-FU.
Discussion
In present study, an online public database was
searched, using the terms ‘cancer recurrence’ and ‘chemoresistance’
and two gene sets were obtained, which included 602 shared genes.
Following GO analysis, the 602 genes were mainly associated with
cell death, cell proliferation and apoptosis, which are believed to
play important roles in the development of drug resistance
(14). Besides, KEGG pathway
enrichment analyses showed the top two pathways, of which ‘PI3K-Akt
signaling pathway’, ‘FoxO signaling pathway’, ‘HIF-1 signaling
pathway’, ‘p53 signaling pathway’ and ‘TNF signaling pathway’ have
been reported in previous studies to be significantly associated
with drug resistance in tumors (20-25).
STRING PPI analysis was subsequently performed and a total of 4
patterns with strong interactions were generated. Furthermore, the
candidate genes associated with prognosis in patients with colon
cancer were analyzed using the GEPIA database and the highly
expressed genes, ATM, CDH2, CDKN2A,
EPO, LEP, TGFB1, TIMP1, PGR,
VEGFC, POSTN, BCL6, CYP19A1,
NOTCH3 and XPA were associated with poor prognosis in
patients with colon cancer. To confirm that the genes identified
were associated with chemotherapeutic resistance in colon cancer
cell lines, siRNAs were transfected into the cells, and cell
viability and apoptosis was analyzed following treatment with 5-FU.
The results showed that CDH2, LEP, POSTN,
TIMP1 and VEGFC were significantly increased in the
human colon cancer cells and that their post-transcriptional
silencing enhanced the sensitivity of colon cancer cells to
5-FU.
Cancer drug resistance severely limits the
effectiveness of chemotherapy in patients with cancer and has been
shown to be an important cause of treatment failure and tumor
recurrence in a large cohort of patients (8). Colon cancer is also one of the most
likely tumors to develop chemotherapeutic resistance in clinical
practice (26). There have been
numerous studies on chemotherapeutic resistance in colon cancer. It
is currently hypothesized that the expression of genes, such as
ABCB1 (27,28), ATR (29) and ATM (30) promoted the development of cancer
drug resistance in tumors by enhancing the efflux of
chemotherapeutic drugs or increasing the level of DNA damage
repair. No effective drug candidates, which overcome drug
resistance in colon cancer, have been identified so far; however,
understanding the mechanisms by which drug resistance occurs will
benefit the development of potential therapeutic agents. In the
present study, text mining strategies based on public databases
provided a useful tool to further understand the mechanisms
underlying drug resistance in colon cancer.
CDH2, also known as N-cadherin (31), was increased in epithelial cells
during carcinogenesis and is a marker of epidermal-mesenchymal
transformation (32). Investigation
in colon cancer cells has suggested that CDH2 mRNA
expression was associated with drug resistance in colon cancer
(33).
VEGFC belongs to the vascular endothelial
growth factor family, which is highly expressed in
oxaliplatin-resistant colorectal cancer cells compared with that in
the parental cells (34). In
addition, LEP, TIMP1 and POSTN have been newly
discovered to be associated with drug resistance in the present
study. LEP plays an important role in regulating metabolism
(35). A previous study in
triple-negative breast cancer (TNBC) cells has shown that leptin
signaling increased the mRNA expression of chemoresistance-related
genes, including ABCB1, which contributed to chemotherapy
failure, whereas inhibition of the leptin receptor re-sensitized
the TNBC cells to chemotherapeutics (36).
TIMP1, also known as tissue inhibitor of
metalloproteinases-1, is a multifunctional protein that promotes
cell proliferation and exhibits anti-apoptotic functions (37,38). A
recent study reported that TIMP1 knockdown using short hairpin RNA
in gemcitabine (GEM)-resistant pancreatic ductal adenocarcinoma
cells enhanced GEM sensitivity and reversed chemoresistance by
inducing cell apoptosis (39).
POSTN expresses extracellular matrix periostin, which is
involved in the activation of the PI3K/Akt signaling pathway
(40,41) and was found to be an independent
negative prognostic factor in non-small cell lung carcinoma
(42). In addition, POSTN
protein expression has been confirmed to be positively associated
with cancer drug resistance (43).
The present study has identified the genes associated with drug
resistance in colon cancer; however, the specific mechanisms by
which these candidate genes exert their biological effects on drug
resistance require further investigation. Furthermore, a limitation
of the current study is that the association between the candidate
genes, chemotherapy drugs and prognosis of patients is not
definite.
In summary, identification of genes associated with
cancer drug resistance was achieved using bioinformatics tools,
which were validated using functional experiments. The identified
genes included CDH2, LEP, POSTN, TIMP1
and VEGFC, and therapeutic targeting of these genes may have
considerable clinical benefits in overcoming chemotherapeutic
resistance.
Supplementary Material
The 2nd significant module from the
PPI module. PPIs with high confidence scores are presented as nodes
(90% confidence intervals) and the genes selected for subsequent
experiments were marked in red. PPI, protein-protein
interaction.
The 3nd significant module from the
PPI module. PPIs with high confidence scores are represented as
nodes (90% confidence intervals) and the genes selected for
subsequent experiments were marked in red. PPI, protein-protein
interaction.
The 4nd significant module from the
PPI module. PPIs with high confidence scores are represented as
nodes (90% confidence intervals) and the genes selected for
subsequent experiments were marked in red. PPI, protein-protein
interaction.
Expression levels of BCL6, EPO,
CYP19A1, TGFB1, NOTCH3 and XPA in 33 paired colon cancer tissues
and adjacent normal tissues. (A) Representative images and
statistical analysis of the expression of the target genes using
IHC in 33 paired colon cancer samples. Scale bar, 200 μm. The data
was analyzed using a Wilcoxon signed-rank test and expressed as the
median ± interquartile range. (B) The mRNA expression levels of
target genes were detected using reverse transcription-quantitative
PCR in 33 paired colon cancer tissues. The data was analyzed using
a paired Student’s t-test and expressed as mean ± SD. (C) Survival
analysis between the expression level of target genes and patient
survival time in 33 paired colon cancer tissues. Each assay was
performed independently from 3 repeats. N, normal adjacent tissue;
T, tumor tissue; IHC, immunohistochemistry.
A total of 563 differentially
expressed genes were identified between normal and colon cancer
samples (N=44, T=568) in The Cancer Genome Atlas database.
A total of 39 differentially expressed
microRNAs were identified between normal and colon cancer samples
(N=4, T=179) in The Cancer Genome Atlas database.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by Major Special Project of
Ministry of Science and Technology (grant no.
2017ZX10203206-005-002).
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
WL and RX conceived the study. TL, RX, CL, XCh and
XCa performed the experiments, data analysis and prepared the first
draft of the manuscript. WL supervised the study and revised the
manuscript. WL and RX confirm the authenticity of all the raw data.
All authors approved the final version and agreed to publish the
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Xiang'an Hospital of Xiamen University. All patients provided
written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there have no competing
interests.
References
|
1
|
Holohan C, Van Schaeybroeck S, Longley DB
and Johnston PG: Cancer drug resistance: An evolving paradigm. Nat
Rev Cancer. 13:714–726. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Vasan N, Baselga J and Hyman DM: A view on
drug resistance in cancer. Nature. 575:299–309. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhu H, Luo H, Zhang W, Shen Z, Hu X and
Zhu X: Molecular mechanisms of cisplatin resistance in cervical
cancer. Drug Des Devel Ther. 10:1885–1895. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nikolaou M, Pavlopoulou A, Georgakilas AG
and Kyrodimos E: The challenge of drug resistance in cancer
treatment: A current overview. Clin Exp Metastasis. 35:309–318.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Panda M and Biswal BK: Cell signaling and
cancer: A mechanistic insight into drug resistance. Mol Biol Rep.
46:5645–5659. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Salgia R and Kulkarni P: The
genetic/non-genetic duality of drug ‘resistance’ in cancer. Trends
Cancer. 4:110–118. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kartal-Yandim M, Adan-Gokbulut A and Baran
Y: Molecular mechanisms of drug resistance and its reversal in
cancer. Crit Rev Biotechnol. 36:716–726. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chatterjee N and Bivona TG: Polytherapy
and Targeted Cancer Drug Resistance. Trends Cancer. 5:170–182.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hirose M, Hosoi E, Hamano S and Jalili A:
Multidrug resistance in hematological malignancy. J Med Invest.
50:126–135. 2003.PubMed/NCBI
|
|
10
|
Hackl H, Astanina K and Wieser R:
Molecular and genetic alterations associated with therapy
resistance and relapse of acute myeloid leukemia. J Hematol Oncol.
10(51)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Waghray D and Zhang Q: Inhibit or evade
multidrug resistance P-glycoprotein in cancer treatment. J Med
Chem. 61:5108–5121. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wu C: Systemic therapy for colon cancer.
Surg Oncol Clin N Am. 27:235–242. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hu T, Li Z, Gao CY and Cho CH: Mechanisms
of drug resistance in colon cancer and its therapeutic strategies.
World J Gastroenterol. 22:6876–6889. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Han B, Feng D, Yu X, Zhang Y, Liu Y and
Zhou L: Identification and interaction analysis of molecular
markers in colorectal cancer by integrated bioinformatics analysis.
Med Sci Monit. 24:6059–6069. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Irham LM, Wong HS, Chou WH, Adikusuma W,
Mugiyanto E, Huang WC and Chang WC: Integration of genetic variants
and gene network for drug repurposing in colorectal cancer.
Pharmacol Res. 161(105203)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang X, Zhang H, Shen B and Sun XF: Novel
MicroRNA biomarkers for colorectal cancer early diagnosis and
5-fluorouracil chemotherapy resistance but not prognosis: A atudy
from databases to AI-assisted verifications. Cancers (Basel).
12(E341)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A (eds): AJCC Cancer Staging Manual. 7th
edition. Springer, New York, NY, 2010.
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li Q, Lai Z, Yan Z, Peng J, Jin Y, Wei L
and Lin J: Hedyotis diffusa Willd inhibits proliferation and
induces apoptosis of 5 FU resistant colorectal cancer cells by
regulating the PI3K/AKT signaling pathway. Mol Med Rep. 17:358–365.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li Q, Wei L, Lin S, Chen Y, Lin J and Peng
J: Synergistic effect of kaempferol and 5 fluorouracil on the
growth of colorectal cancer cells by regulating the PI3K/Akt
signaling pathway. Mol Med Rep. 20:728–734. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fallah J and Rini BI: HIF Inhibitors:
Status of current clinical Development. Curr Oncol Rep.
21(6)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Feng X, Liu H, Zhang Z, Gu Y, Qiu H and He
Z: Annexin A2 contributes to cisplatin resistance by activation of
JNK-p53 pathway in non-small cell lung cancer cells. J Exp Clin
Cancer Res. 36(123)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhao F and Lam EW: Role of the forkhead
transcription factor FOXO-FOXM1 axis in cancer and drug resistance.
Front Med. 6:376–380. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lai M, Liu G, Li R, Bai H, Zhao J, Xiao P
and Mei J: Hsa_circ_0079662 induces the resistance mechanism of the
chemotherapy drug oxaliplatin through the TNF-α pathway in human
colon cancer. J Cell Mol Med. 24:5021–5027. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kozovska Z, Gabrisova V and Kucerova L:
Colon cancer: cancer stem cells markers, drug resistance and
treatment. Biomed Pharmacother. 68:911–916. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yuan Z, Shi X, Qiu Y, Jia T, Yuan X, Zou
Y, Liu C, Yu H, Yuan Y, He X, et al: Reversal of P-gp-mediated
multidrug resistance in colon cancer by cinobufagin. Oncol Rep.
37:1815–1825. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hu T, To KK, Wang L, Zhang L, Lu L, Shen
J, Chan RL, Li M, Yeung JH and Cho CH: Reversal of P-glycoprotein
(P-gp) mediated multidrug resistance in colon cancer cells by
cryptotanshinone and dihydrotanshinone of Salvia
miltiorrhiza. Phytomedicine. 21:1264–1272. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Abu-Sanad A, Wang Y, Hasheminasab F,
Panasci J, Noë A, Rosca L, Davidson D, Amrein L, Sharif-Askari B,
Aloyz R, et al: Simultaneous inhibition of ATR and PARP sensitizes
colon cancer cell lines to irinotecan. Front Pharmacol.
6(147)2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhou Y, Wan G, Spizzo R, Ivan C, Mathur R,
Hu X, Ye X, Lu J, Fan F, Xia L, et al: miR-203 induces oxaliplatin
resistance in colorectal cancer cells by negatively regulating ATM
kinase. Mol Oncol. 8:83–92. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Alimperti S and Andreadis ST: CDH2 and
CDH11 act as regulators of stem cell fate decisions. Stem Cell Res
(Amst). 14:270–282. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Loh CY, Chai JY, Tang TF, Wong WF, Sethi
G, Shanmugam MK, Chong PP and Looi CY: The E-cadherin and
N-cadherin switch in epithelial-to-mesenchymal transition:
Signaling, therapeutic implications, and challenges. Cells.
8(E1118)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Serova M, Astorgues-Xerri L, Bieche I,
Albert S, Vidaud M, Benhadji KA, Emami S, Vidaud D, Hammel P,
Theou-Anton N, et al: Epithelial-to-mesenchymal transition and
oncogenic Ras expression in resistance to the protein kinase Cbeta
inhibitor enzastaurin in colon cancer cells. Mol Cancer Ther.
9:1308–1317. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tomida C, Yamagishi N, Nagano H, Uchida T,
Ohno A, Hirasaka K, Nikawa T and Teshima-Kondo S: VEGF
pathway-targeting drugs induce evasive adaptation by activation of
neuropilin-1/cMet in colon cancer cells. Int J Oncol. 52:1350–1362.
2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang Y and Chua S Jr: Leptin function and
regulation. Compr Physiol. 8:351–369. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lipsey CC, Harbuzariu A, Robey RW, Huff
LM, Gottesman MM and Gonzalez-Perez RR: Leptin signaling affects
survival and chemoresistance of estrogen receptor negative breast
cancer. Int J Mol Sci. 21(E3794)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lao G, Ren M, Wang X, Zhang J, Huang Y,
Liu D, Luo H, Yang C and Yan L: Human tissue inhibitor of
metalloproteinases-1 improved wound healing in diabetes through its
anti-apoptotic effect. Exp Dermatol. 28:528–535. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lee JH, Choi JW and Kim YS: Serum TIMP-1
predicts survival outcomes of invasive breast carcinoma patients: A
meta-analysis. Arch Med Res. 42:463–468. 2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Tan Y, Li X, Tian Z, Chen S, Zou J, Lian
G, Chen S, Huang K and Chen Y: TIMP1 down-regulation enhances
gemcitabine sensitivity and reverses chemoresistance in pancreatic
cancer. Biochem Pharmacol. 189(114085)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Liu C, Feng X, Wang B, Wang X, Wang C, Yu
M, Cao G and Wang H: Bone marrow mesenchymal stem cells promote
head and neck cancer progression through Periostin-mediated
phosphoinositide 3-kinase/Akt/mammalian target of rapamycin. Cancer
Sci. 109:688–698. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Xiao ZM, Wang XY and Wang AM: Periostin
induces chemoresistance in colon cancer cells through activation of
the PI3K/Akt/survivin pathway. Biotechnol Appl Biochem. 62:401–406.
2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ratajczak-Wielgomas K, Kmiecik A,
Grzegrzołka J, Piotrowska A, Gomulkiewicz A, Partynska A, Pawelczyk
K, Nowinska K, Podhorska-Okolow M and Dziegiel P: Prognostic
significance of stromal periostin expression in non-small cell lung
cancer. Int J Mol Sci. 21(E7025)2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Park SY, Piao Y, Jeong KJ, Dong J and de
Groot JF: Periostin (POSTN) regulates tumor resistance to
antiangiogenic therapy in glioma models. Mol Cancer Ther.
15:2187–2197. 2016.PubMed/NCBI View Article : Google Scholar
|