Introduction
Nasopharyngeal carcinoma (NPC) is the most common
type of cancer in southern Asia (1,2). In
Europe, its incidence is rare, with an annual rate varying between
2.1 and 0.4 per 100,000 individuals (3-5).
NPC represents 2% of all cancers developed in the head and neck
(6). The incidence is higher in men
than in women with an M:F ratio of 2.75 (3,4).
Epstein-Barr virus infection is also a factor in most neck and head
cancer cases, along with smoking and alcohol consumption (7-9).
Relative survival for NPC in Europe is 49% at 5 years, with no
differences in survival between sexes (3). HNSCC has been proven to distantly
spread below the clavicles (10).
The chance of dissemination is related to the cervical lymph node
involvement. For N0-N2 tumors, the dissemination occurs in less
than 10% of cases compared with 30% in N2-N3 cases (10).
The lymphatic drainage from the head and neck occurs
through a superficial and deep system. The superficial group of
cervical lymph nodes includes occipital, postauricular, parotid,
facial, submandibular, sub-mental and superficial cervical nodes,
which are situated alongside the external jugular vein. The
profound group of cervical lymph nodes lies along the internal
jugular vein. All of the lymphatic vessels of the head and neck
empty into the deep group, either directly or via the superficial
group of nodes (11). The lymphatic
system, within the axilla region, normally flows from the distal
portion of the upper limb and the chest wall along the axillary
vein toward the subclavian venous system (12).
Like most other locations of HNSCC, the lymphatic
drainage of the nasopharynx (NP) is predominantly directed to the
cervical lymph nodes (13,14). Lymph node metastases are present in
80% of NPC cases, and in 50% of the cases, these are bilateral. The
worst aspect of cancer is its ability to spread or metastasize
(15). Patients with lymph node
metastases have a more favorable prognosis than those with visceral
metastases (16). It is known that
NPC tumors are associated with a high incidence of distant
metastases (17,18). Pulmonary metastases represent the
most common localization, accounting for 66% of total distant
metastases. Other metastatic sites include bone (22%) and liver
(10%) (18-20).
Axillary lymph node metastasis is an uncommon event in HNSCC. There
are very few references in the literature regarding axillary
metastases from HNSCC (21). Only
five reports have been published in the literature, including 10
patients (22). None of these cases
included a co-occurrence of mastitis. Axillary metastases are
mentioned in only 2 of 8 published autopsy studies (10). Upon autopsy reports, between 2 to 9%
of patients with HNSCC tumors were found to have axillary node
metastases, but it is assumed that the incidence is higher, as
impalpable lymph nodes are not routinely examined during autopsy,
except in breast cancer cases and in some malignant melanomas
(23,24).
Case report
We present the case of a 40-year-old woman, a
stay-at-home mother of two children (aged 5 and 8), who lives in
the rural area, with no family history of neoplasm or any other
comorbidities. The onset of the disease was in April 2015, when our
patient started noticing a nasopharyngeal discomfort, nasal
obstruction, rhinorrhea and epistaxis. The ear-nose-throat (ENT)
exam revealed a lesion of 2.3/2 cm in the NP, for which a biopsy
was completed. Clinically, the patient presented bilateral cervical
lymphadenopathy with diameters of up to 3.5 cm. In May 2015, the
diagnosis was confirmed: Stage III, T1N2M0 poorly differentiated
NSCC of the NP.
By November 2015, the patient had undergone 3 cycles
of docetaxel, cisplatin, and 5-fluorouracil (TPF) induction
chemotherapy associated with prophylactic supportive granulocyte
colony-stimulating factor (GCSF) treatment, with PR after induction
chemotherapy, followed by concomitant weekly cisplatin (40
mg/m2) radio-chemotherapy 66 Gray (Gy) on the NP, 58 Gy
on the bilateral cervical lymph nodes and 50 Gy on the bilateral
supraclavicular areas. Both treatment adherence and tolerance were
satisfactory. The post therapeutic brain CT scan, performed in
March 2016, showed PR at the primary NPC, and CR at the
latero-cervical lymph nodes.
On February 2017, the patient reported pain, edema,
erythema and swelling of the left breast. The physical examination
noted left axillary lymphadenopathy and left-sided mastitis. In
order to explore the probability of a coexistent breast cancer
(carcinomatous mastitis), an excisional biopsy was performed on the
left axillary lymph nodes, which excluded a neoplastic process. The
patient received local and oral treatment with anti-inflammatory
drugs (300 mg of ketoprofen per day) and antibiotics
(amoxycillin/clavulanic acid 1,000 mg/62.5 mg, twice a day) for 10
days, after which the mastitis went into slight remission for the
next 4 months.
In June 2017, the inflammation of the left breast
reoccurred along with the worsening of the previous symptoms. Chest
CT scan confirmed left axillary (of 4.5 cm) and supraclavicular
lymph node enlargement (of 3.5 cm), and left mastitis. Left
axillary lymph node excisional biopsy was performed after 10 days
of systemic antibiotic and anti-inflammatory treatment. The
pathology report revealed another inflammatory reaction of the
lymph node, without tumor infiltration.
In September 2017, due to the persistence of the
clinical signs of mastitis, despite oral antibiotic and
anti-inflammatory treatment, the patient attended our clinic
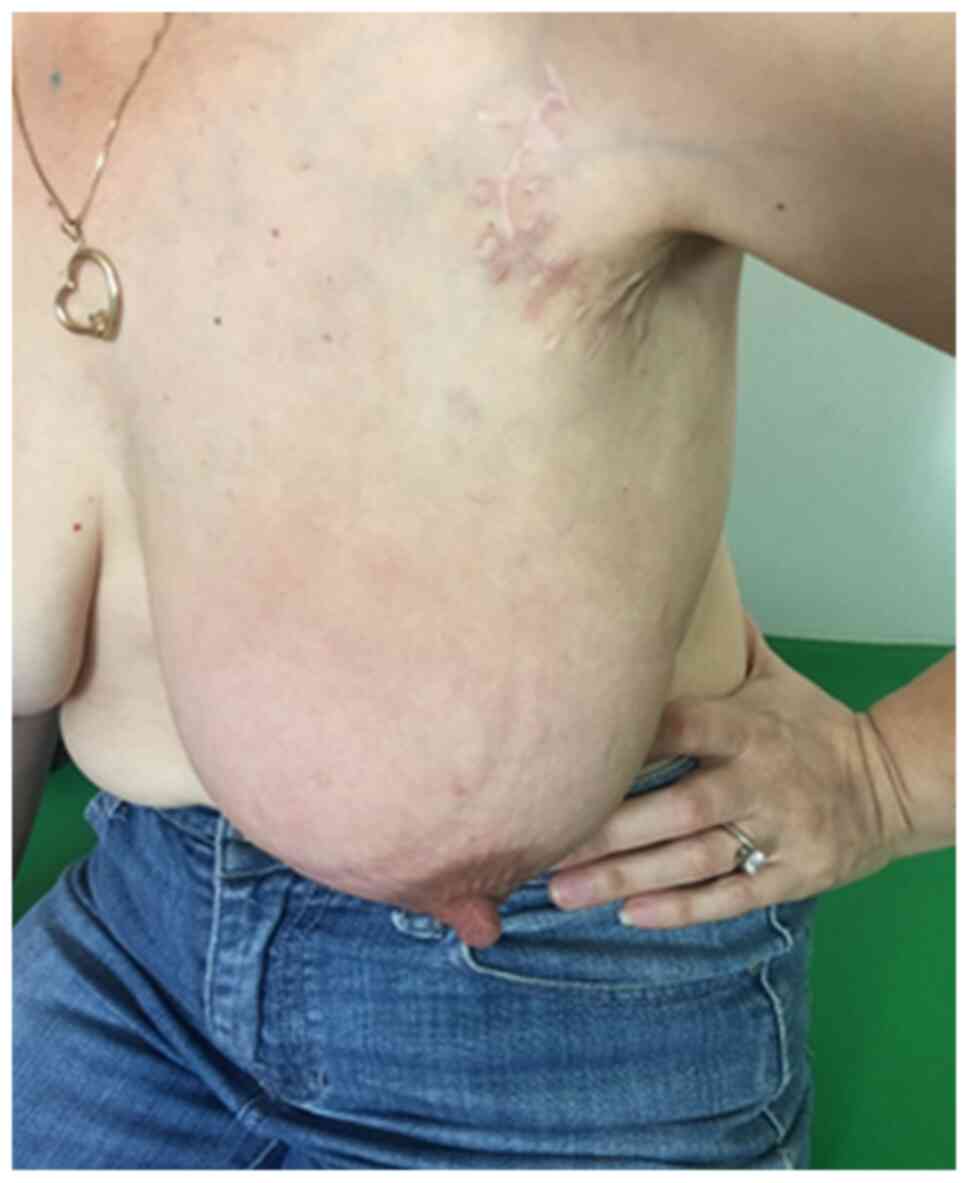
looking for a second medical opinion. Clinical examination revealed
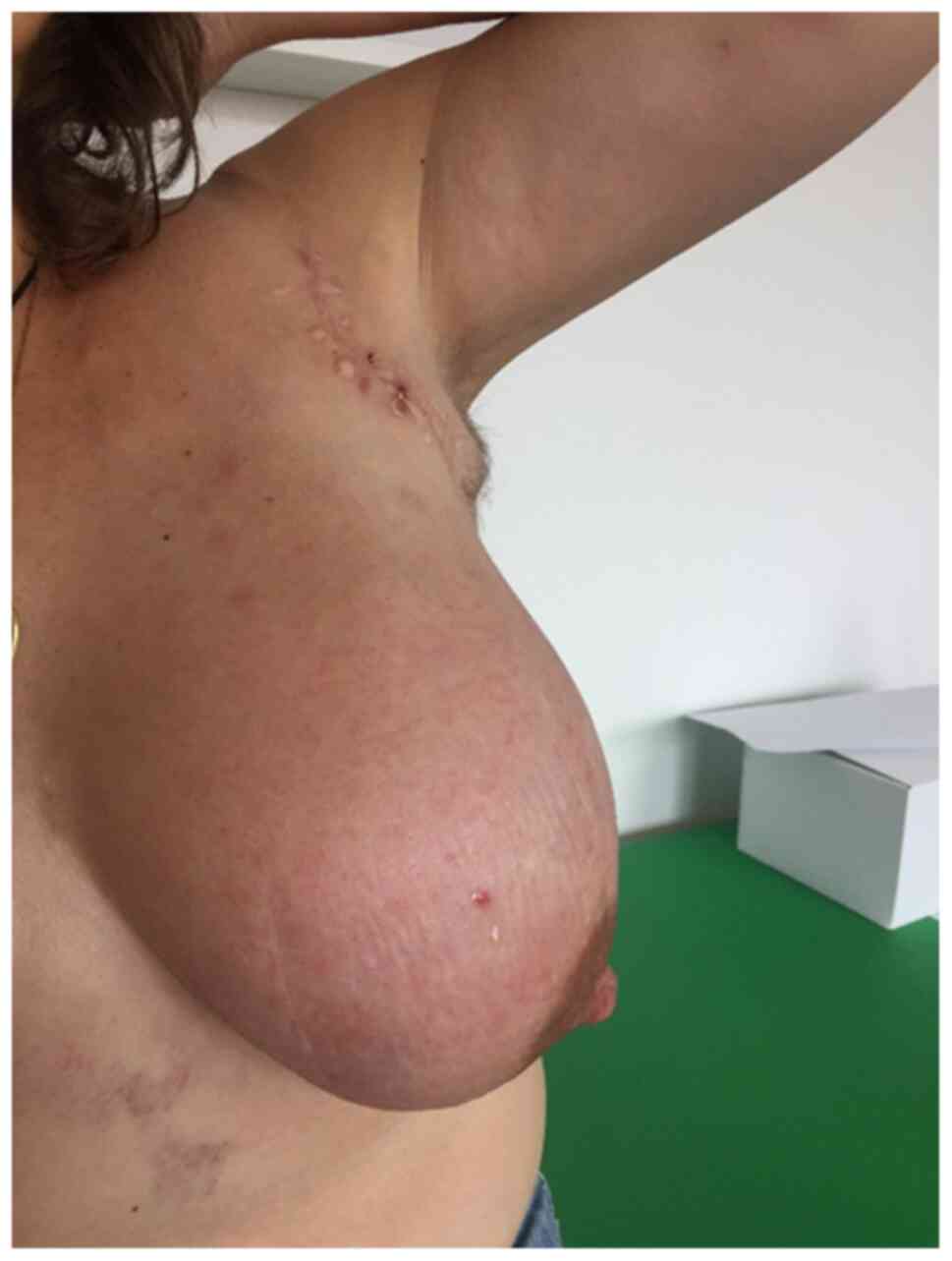
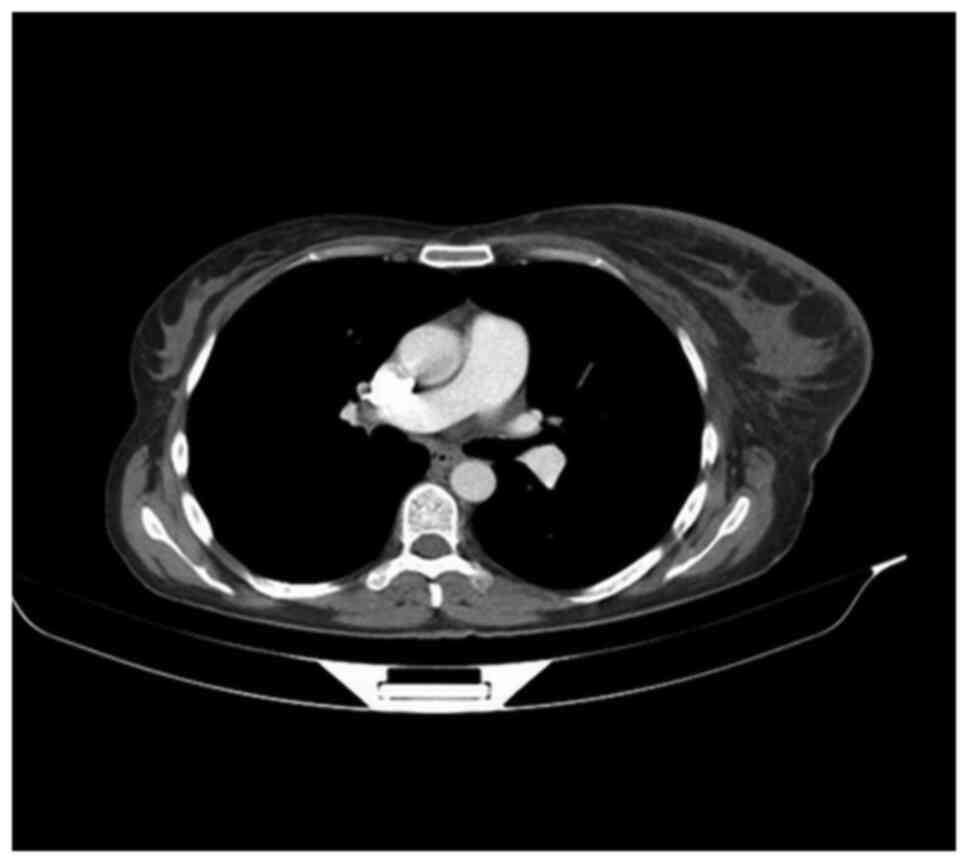
severe mastitis, lymphedema of the left arm and lymphadenopathy in
the left axilla and supraclavicular region (Fig. 1). A new subcutaneous lesion on the
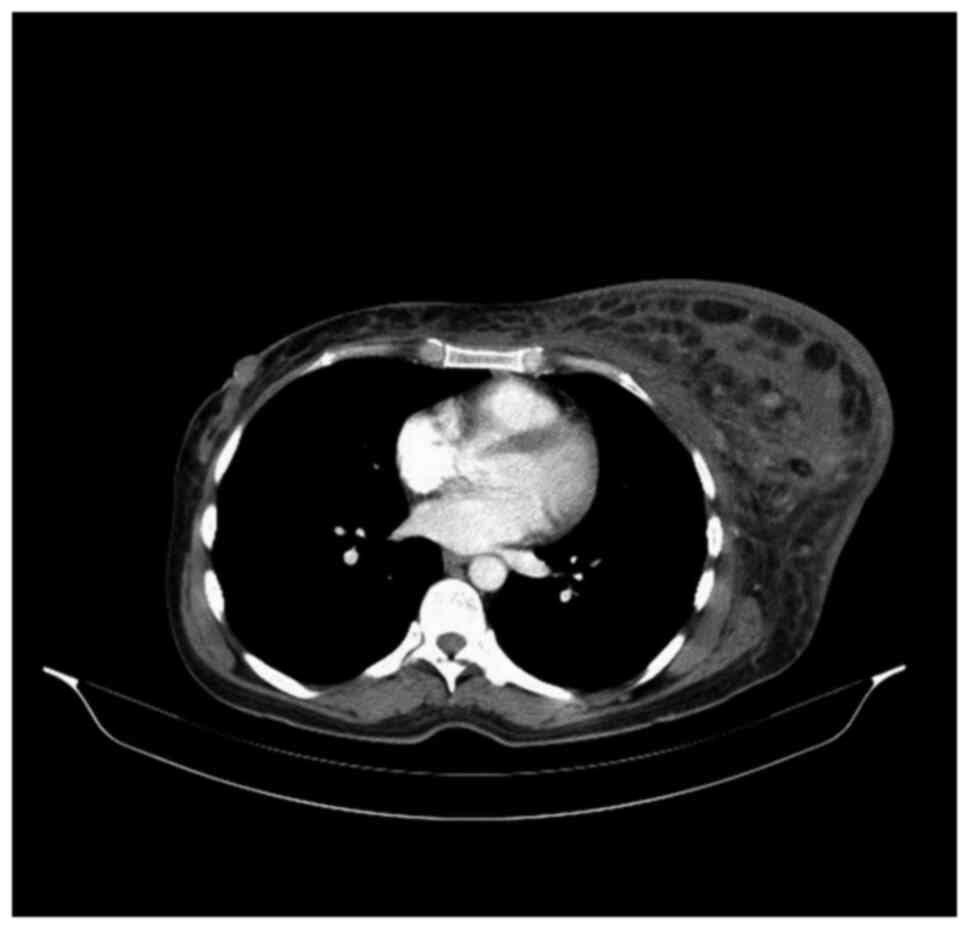
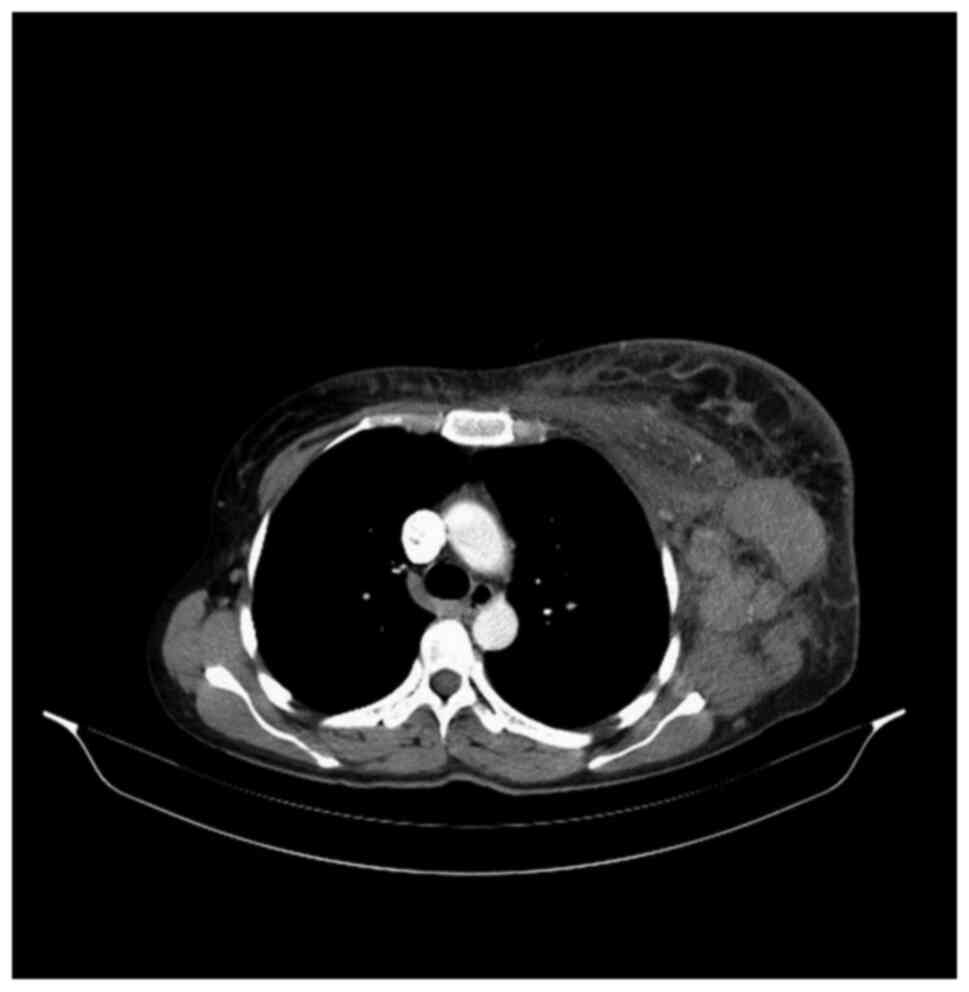
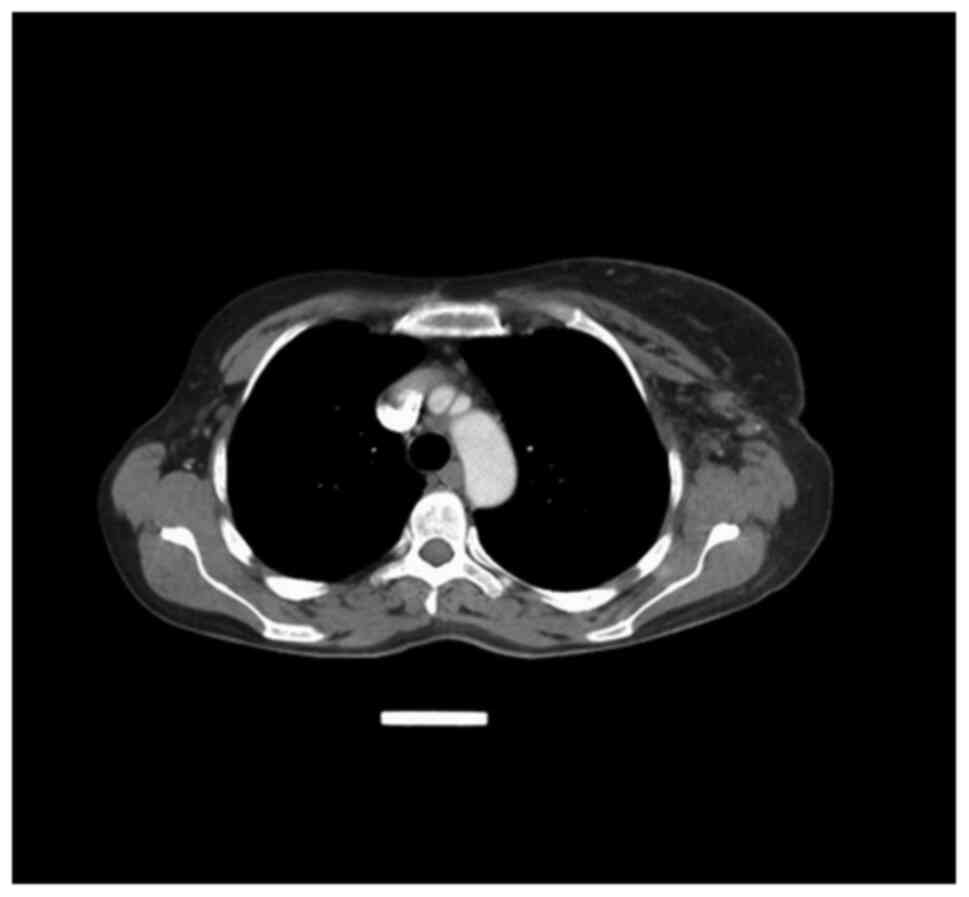
left axilla and thorax was discovered. The total body CT scan
showed a progressive aspect of mastitis and an increase in the
axillary and supraclavicular lymph nodes (Figs. 2 and 3).
The primary NPC tumor and latero-cervical lymph
nodes were in CR. One subcutaneous nodule from the axillary left
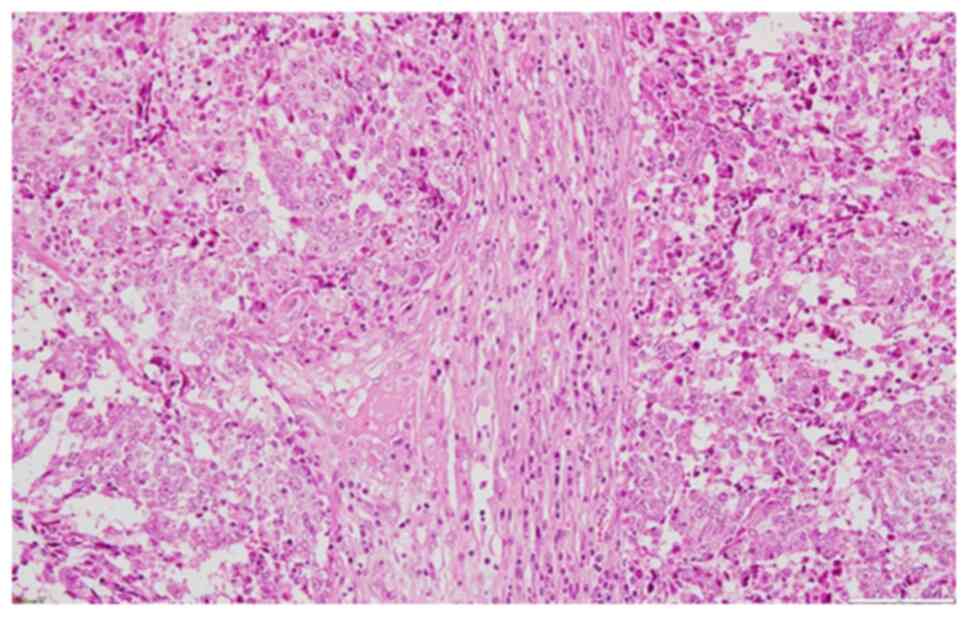
region was excised and sent for pathological examination. The
pathology report described large islands of tumor cells with
prominent nucleoli and admixed inflammatory cells (Fig. 4). IHC staining was done on
paraffin-embedded tissue, with the following antibodies:
Trans-acting T cell-specific transcription factor (GATA-3),
mammaglobin, epithelial membrane antigen (EMA), cytokeratin (CK)
AE1/AE3, CK5, high-molecular-weight cytokeratin (HMWCK) (34BE12),
CK7, p63, neutral peptidase 24.11 (NEP) (CD10), S100, smooth muscle
actin (SMA), p16, chromogranin A (CgA), synaptophysin (Syn). Serial
sections, 3-µm thick, were constructed from one selected
paraffin-block with the representative tumor area, and then mounted
on silanized slides, so as to prevent detachment during antigen
unmasking procedures. Tissue sections were deparaffined in xylene
and rehydrated. For unmasking the antigens, we used heat induced
epitope retrieval (HIER) by boiling the slides for 20 min at 98˚C
in target retrieval solution pH 6.0 or 9.0. Endogenous peroxidase
activity was blocked by treatment with 0.5%
H2O2 for 15 min. Subsequently, we applied
ready-to-use (RTU) or concentrated antibodies. For the concentrated
antibodies, the slides were incubated overnight in a refrigerator
at 4˚C with appropriate dilutions of the primary antibodies. The
reaction was visualized by Novolink kit-HRP (horseradish
peroxidase) with diaminobenzidine (DAB) as chromogen. Finally, the
sections were counterstained with hematoxylin, dehydrated,
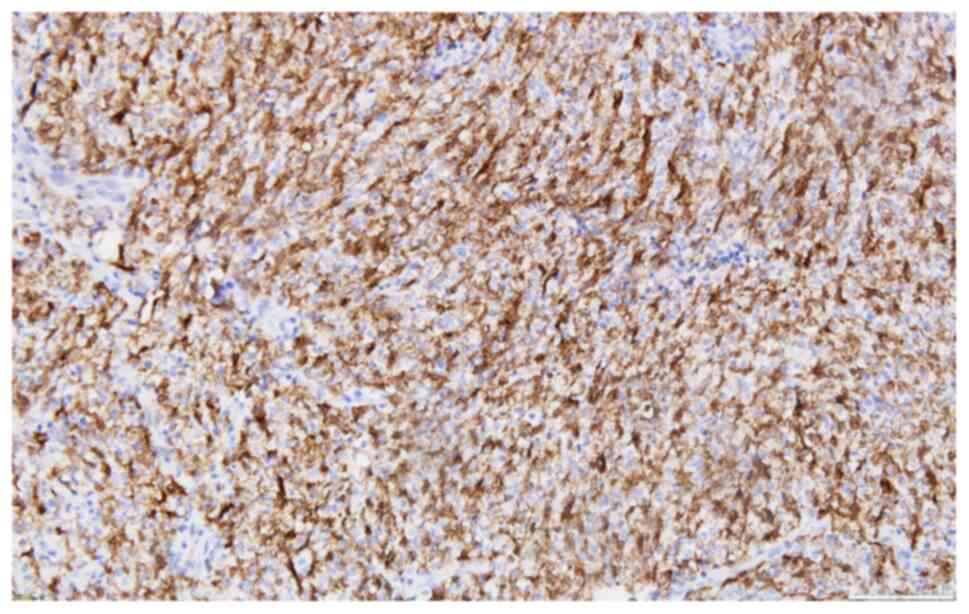
clarified and mounted with Entellan. The tumor cells presented the
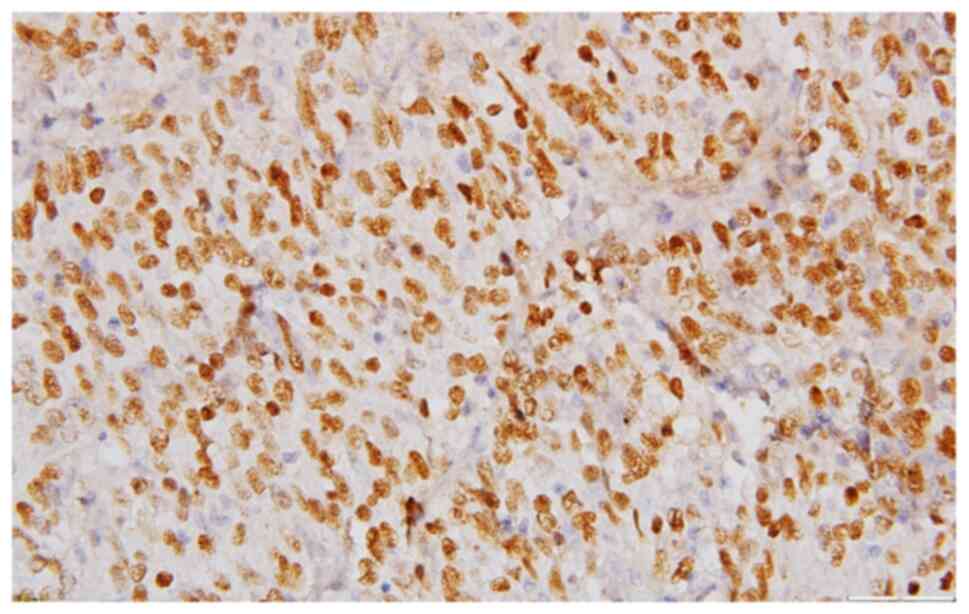
following IHC profile: Extensive expression for p63(+++) (Fig. 5), less extensive reactivity for
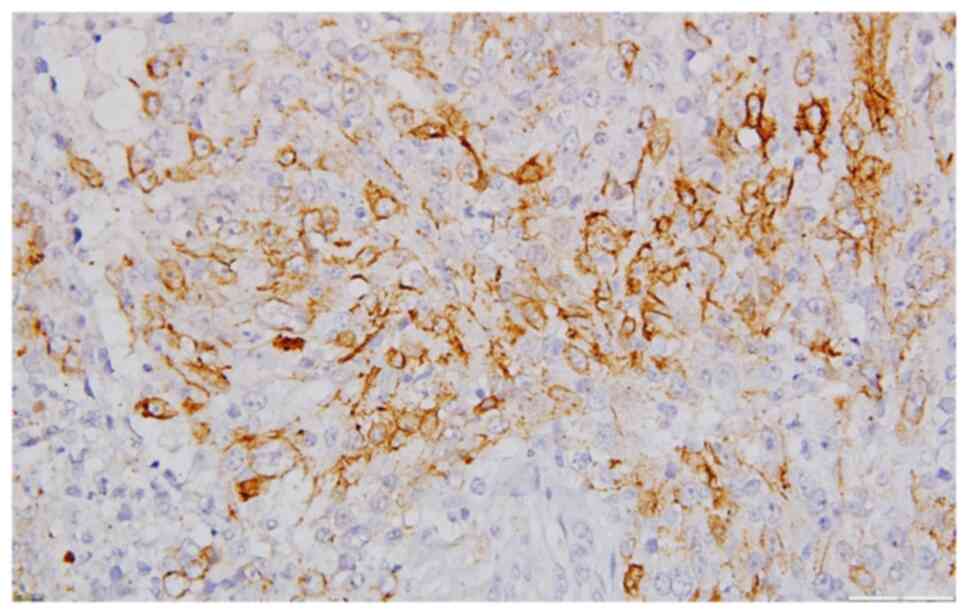
HMWCK(++) (Fig. 6), limited
expression for CK8/18(+), negative reaction for CK7, GATA-3,
thyroid transcription factor 1 (TTF1) and S100. Serum antibodies
against Epstein Barr virus (EBV) were investigated. Early antigen
(EA) immunoglobulin (Ig) G was positive (>150 U/ml); antibodies
against Epstein-Barr nuclear antigen (EBNA) IgG were highly
positive (>600 U/ml), virus capsid antigen (VCA) IgM antibodies
were negative (<10.0 U/ml). Blood deoxyribonucleic acid (DNA)
EBV quantitative polymerase chain reaction (PCR) was undetectable.
Unfortunately, the in situ hybridization for Epstein-Barr
encoding region (EBER) was not able to be performed. Based on
morphological and IHC data, the tumor was diagnosed as metastasis
of an NSCC, most probably from an NPC, based on the positive
serology for the Epstein-Barr virus.
In association to all of the above, our patient
presented anxiety and sleeping difficulties, especially when it
came to the initiation of sleep. Therefore, a psychiatric
evaluation was necessary, which revealed a reactive anxiety
disorder. She was prescribed medazepam 10 mg per day, for four
weeks, and six psychiatric counselling sessions. Gradually, the
symptoms started to alleviate, and after a total of six weeks, they
completely disappeared.
The patient started chemotherapy with cisplatin (75
mg/m2) and docetaxel (75 mg/m2) every 3
weeks, with GCSF support. After 4 cycles, in January 2018, the
edema of the left breast and arm was in remission (Fig. 7). Clinically, the left axillary
lymph nodes decreased and the supraclavicular ones disappeared. The
patient had a mild cough, no fever, but an unpleasant fetid breath.
Chest CT scan confirmed two pulmonary abscesses, PR of the left
axillary lymph nodes and CR of the left supraclavicular nodes.
Chemotherapy was postponed for two months, due to this severe lung
infection. After remission, the same chemotherapy schedule was
restarted and continued until cycle 11.
In September 2018, full-body CT-scan showed
persistence of CR at primary NPC and loco-regional lymph nodes, a
mild diffuse edema of the left breast and a decrease in the left
axillary lymph nodes (at 1.8 cm) (Figs.
8 and 9). Afterwards, our
patient underwent a surgical excision of the left axillary lymph
nodes. The pathological report revealed the presence of 4 lymph
nodes between 0.5 and 2 cm, all of them metastatic NSCC, most
probably originating in the NP. Our patient received adjuvant
radiotherapy of 50 Gy/25 fractions on the axilla, until January
2019. The treatment was well-tolerated. After 9 months of
disease-free interval (DFI), left breast mastitis and a left
axillary lymph node metastasis recurred. The clinical exam showed
left breast mild mastitis and a mobile 3.3 cm axillary
lymphadenopathy, which was programmed for excision.
The retractile scar-tissue was removed surgically,
with a thorough dissection of the fibrous axillary tissue that
resulted from scarring and radiotherapy, while isolating the
lymphadenopathy block of the vascular pedicle and making a
Z-plasty, in order to eliminate the adhesions. The pathology
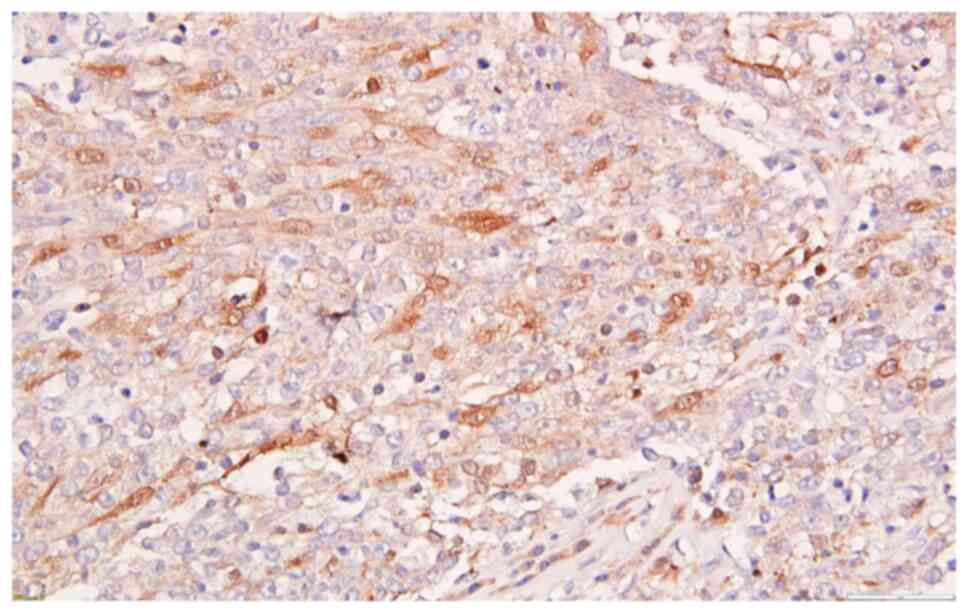
examination, including an extended IHC panel which found positive
reaction for p63, CK5 (Fig. 10),
CK AE1/AE3, focal reactivity for p16 (Fig. 11), cytoplasmic and nuclear and lack
of reactivity for SMA, GATA-3, Syn, chromogranin A (CgA), CK7, EMA,
S100, CD10, confirmed metastasis of a poorly differentiated
NSCC.
The paraffin block from this last biopsy sample was
used to test genomic alteration. The result showed NOTCH1
mutation-V1578 deletion. The patient was invited to be included in
a phase I/II clinical trial abroad, but she refused. The
pre-therapeutic total body CT scan evaluation did not highlight any
other metastasis. Primary NPC tumor and loco-regional lymph nodes
remained in CR. The patient restarted chemotherapy with cisplatin
and docetaxel in January 2020, with the same dosages and following
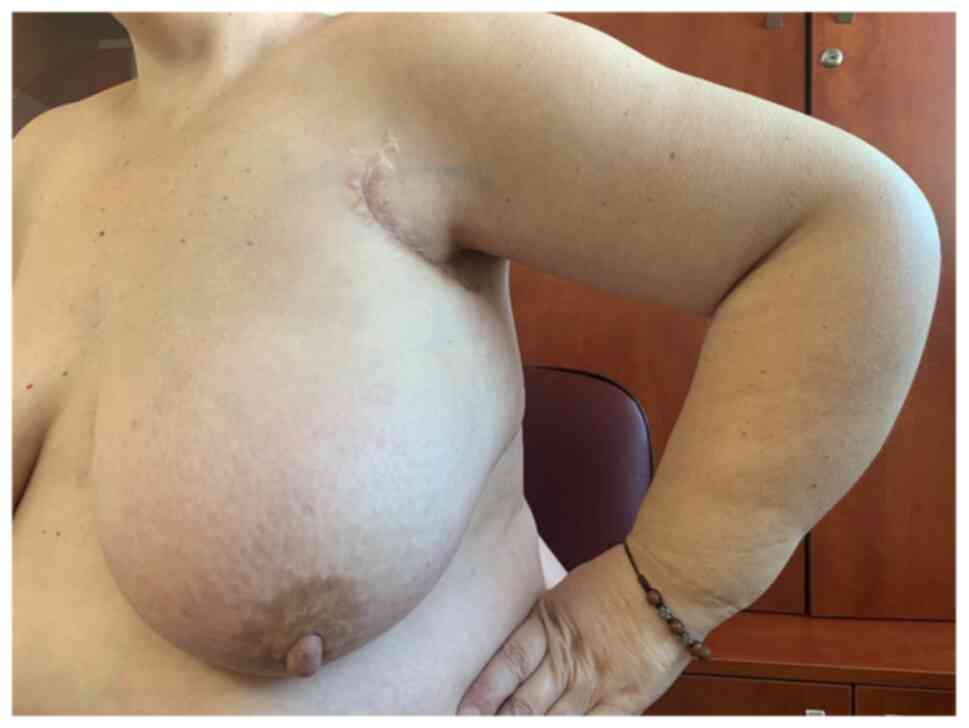
the same schedule, as an ‘adjuvant’ treatment. On April 2020, the
patient was undergoing chemotherapy treatment, and the clinical
symptoms were in complete remission, but the upper left limb edema
persisted (Fig. 12).
Discussion
Axillary lymph node involvement is rare in
nasopharyngeal carcinoma (NPC) (21). Yet, they are a frequent site of
regional metastatic disease from breast carcinoma (25). The recognition of axillary
metastasis in patients with HNCC is crucial, especially in patients
with massive metastases or delayed metastases at a low level of the
head and neck (25). This article
presents the case of a young patient, initially diagnosed and
treated for NPC, who presented, in the evolution of the disease,
axillary lymph node metastasis and carcinomatous-like mastitis. At
the time of the axillary recurrence, the primary NPC had CR. Due to
the clinical impression of the inflammatory breast carcinoma, we
began by trying to diagnose a breast cancer, which was disproved
following the results of the IHC and pathological examinations.
Previous literature reports suggest that the involvement of
axillary sites may be related to previous neck surgery, following a
change in lymphatic drainage (11).
Our patient had not undergone prior head and neck surgery.
The pathological aspects corresponded to a
metastasis of a poorly differentiated/undifferentiated tumor, which
is difficult to diagnose based on routine stained slides. The
positive reaction for EMA and CK established the epithelial nature
of the proliferation. Given the clinical aspect of inflammatory
carcinoma combined with axillary lymphadenopathy, the IHC
investigation, performed over two stages (2017 and 2019), was
focused on removing the possibility of a metastasis from a second
primary metachronous breast carcinoma.
The absence of CK7, mammaglobin and GATA3
reactivity, excluded the majority of most common breast carcinoma
subtypes. The diffuse positivity for p63, CK5 and a heterogeneous
one for HMWCK suggested a metastasis from a squamous or
myoepithelial carcinoma, both of which are considered to be
extremely rare subtypes of breast carcinoma. The absence of S100
and SMA reactivity excluded a breast lesion with myoepithelial
differentiation. Knowing that p63 and HMWCK are positive in
metaplastic breast carcinomas with spindle cells and/or squamous
differentiation (26), the
possibility that the axillary lesion represented a metastasis from
these entities could not be completely ruled out. But considering
what Wargotz and Norris (1990) affirmed, namely that before
diagnosing a primary squamous cell carcinoma of the breast it is
necessary to exclude a metastatic squamous cell carcinoma to the
breast, the scenario of a metastasis from the previous diagnosed
NPC was favored (27).
The main arguments for NPC metastasis against a
metastatic metaplastic breast carcinoma with spindle cell and/or
squamous differentiation included: An initial diagnosis of NPC; the
important bilateral latero-cervical lymphadenopathy, staged cN2;
the absence of a primary breast malignancy documented by imaging
and excisional biopsies; the IHC profile of the axillary metastasis
which overlaps with the nasopharyngeal lesion (conducted in another
pathology department); the identification, by next generation
sequencing (NGS), of NOTCH1 V1578 del and missense mutation
of p53, when the NOTCH1 mutation is the second most common mutated
gene (after TP53) in HNSCC (28);
the positive serology for EBV, being well-known that
nonkeratinizing NPC is associated with EBV in almost all cases
(29).
Unfortunately, the in situ hybridization for
EBER, a helpful investigation for establishing the NP as the
primary site for a meta-virus capsid antigen static
undifferentiated or poorly differentiated squamous cell carcinoma,
could not be performed.
Emerging from the main genomic alteration
identified, the NOTCH pathway, targeted therapy appeared to be a
logical therapeutic option for this case. A theory regarding the
favorable evolution of our patient may be due to, partly, the
NOTCH1 mutation, since, more often than not, this mutation
acts as a tumor-suppressor gene (30). The timely recovery surgery of the
axillary metastasis after exclusion of other distant metastasis may
improve the survival of these patients (25). HNSCC is one of the most aggressive
malignancies (31). However, our
patient had a survival of almost 5 years from the diagnosis of the
primary tumor, and of about 3 years from the diagnosis of
recurrence. For this patient, the surgical removal of the axillary
lymph node was completed twice, for two local recurrences. We aimed
to improve local control by adding radiotherapy of the axilla,
after the first complete surgical excision. Chemotherapy was
administered twice, in a ‘neo-adjuvant’ setting, first due to the
initially inoperable recurrence, and second, in an ‘adjuvant’
setting, after the last surgery for the recurrence. In the absence
of clear evidence on the efficacy of adjuvant therapy in this
clinical situation, adjuvant chemotherapy was used for the second
recurrence, which, although surgically removed, did not dismiss the
possibility of systemic micro-metastasis, that cannot be identified
by standard diagnostic methods. The correct duration of this
present ‘adjuvant’ chemotherapy remains an open question. The role
of local radiotherapy and systemic chemotherapy remains to be
established in the future.
This particular case required a multidisciplinary
management, where the oncology, plastic surgery, pathology and
psychiatric medical specialists acted together in order to
establish the correct diagnosis and adopt the appropriate
therapeutic pathway. Currently, neoplastic patients benefit from
personalized therapy, with the ultimate target of offering the
patient a good quality of life.
Acknowledgements
The authors would like to thank the patient for
allowing them to publish her case and to all the healthcare
professionals who helped care for this patient.
Funding
Funding: No funding was received.
Availability of data and materials
Further information regarding this case report is
available from the corresponding author on reasonable request.
Authors' contributions
CMO, NAS and AS were in charge of patient
management, establishing the chemotherapy treatment and follow-up
of the treatment response, and clinical evolution of the patient.
ALCD and CSS were in charge of the laboratory diagnosis: Pathology,
IHC and genomic testing. DG performed the two diagnostic
interventions (biopsy) and the surgical axillary excision. ACB and
IAR performed the psychiatric evaluation, established a treatment
plan and offered psychological support. All authors read and
approved the final manuscript for publication.
Ethics approval and consent to
participate
For this case-report we obtained the patient's
informed consent and her permission to use the images that were
taken during her periodical check-ups. An ethical approval from the
hospital was not required.
Patient consent for publication
Written informed consent was obtained from the
patient regarding the publication of this case report and any
accompanying images. A copy of the written consent is available for
review.
Competing interests
The authors declare no competing interests.
References
|
1
|
Kuo DY, Chang MH, Wang SY, Hsieh PY and
Shueng PW: Unusual axillary metastasis of recurrent nasopharyngeal
cancer: A case report. Medicine (Baltimore).
96(e6854)2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mahdavifar N, Ghoncheh M,
Mohammadian-Hafshejani A, Khosravi B and Salehiniya H: Epidemiology
and inequality in the incidence and mortality of nasopharynx cancer
in Asia. Osong Public Health Res Perspect. 7:360–372.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bossi P, Chan AT, Licitra L, Trama A,
Orlandi E, Hui EP, Halámková J, Mattheis S, Baujat B, Hardillo J,
et al: Nasopharyngeal carcinoma: ESMO-EURACAN clinical practice
guidelines for diagnosis, treatment and follow-up †. Ann Oncol.
32:452–465. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108.
2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chang ET and Adami HO: The enigmatic
epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol
Biomarkers Prev. 15:1765–1777. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhou X, Cui J, Macias V, Kajdacsy-Balla
AA, Ye H, Wang J and Rao PN: The progress on genetic analysis of
nasopharyngeal carcinoma. Comp Funct Genomics.
2007(57513)2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fernandes Q, Merhi M, Raza A, Inchakalody
VP, Abdelouahab N, Zar Gul AR, Uddin S and Dermime S: Role of
Epstein-Barr virus in the pathogenesis of head and neck cancers and
its potential as an immunotherapeutic target. Front Oncol.
8(257)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pezzuto F, Buonaguro L, Caponigro F, Ionna
F, Starita N, Annunziata C, Buonaguro FM and Tornesello ML: Update
on head and neck cancer: Current knowledge on epidemiology, risk
factors, molecular features and novel therapies. Oncology.
89:125–136. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sturgis EM, Wei Q and Spitz MR:
Descriptive epidemiology and risk factors for head and neck cancer.
Semin Oncol. 31:726–733. 2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nelson WR and Sisk M: Axillary metastases
from carcinoma of the larynx: A 25-year survival. Head Neck.
16:83–87. 1994.PubMed/NCBI View Article : Google Scholar
|
|
11
|
McKenzie BJ and Loock JW: Axillary nodal
metastasis at primary presentation of an oropharyngeal primary
carcinoma: A case report and review of the literature. J Med Case
Rep. 3(7230)2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rayatt SS, Dancey AL, Fagan J and
Srivastava S: Axillary metastases from recurrent oral carcinoma. Br
J Oral Maxillofac Surg. 42:264–266. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ho FC, Tham IW, Earnest A, Lee KM and Lu
JJ: Patterns of regional lymph node metastasis of nasopharyngeal
carcinoma: A meta-analysis of clinical evidence. BMC Cancer.
12(98)2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Brennan B: Nasopharyngeal carcinoma.
Orphanet J Rare Dis. 1(23)2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dangore-Khasbage S: Local metastasis in
head and neck cancer-an overview. Contemporary Issues in Head and
Neck Cancer Management. IntechOpen, pp152-167, 2015. https://doi.org/10.5772/60072. Accessed 8th July,
2015.
|
|
16
|
Alavi S, Namazie A, Sercarz JA, Wang MB
and Blackwell KE: Distant lymphatic metastasis from head and neck
cancer. Ann Otol Rhinol Laryngol. 108:860–863. 1999.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen MY, Jiang R, Guo L, Zou X, Liu Q, Sun
R, Qiu F, Xia ZJ, Huang HQ, Zhang L, et al: Locoregional
radiotherapy in patients with distant metastases of nasopharyngeal
carcinoma at diagnosis. Chin J Cancer. 32:604–613. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tomao F, Miele E, Spinelli GP, Caprio G,
Ranieri E, Mingazzini P, Zullo A and Tomao S: Axillary and
subcutaneous breast metastases from rhinopharyngeal carcinoma: A
case report and literature review. Anticancer Res. 28:419–424.
2008.PubMed/NCBI
|
|
19
|
Chan AT, Teo PM and Johnson PJ:
Nasopharyngeal carcinoma. Ann Oncol. 13:1007–1015. 2002.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ahmad A and Stefani S: Distant metastases
of nasopharyngeal carcinoma: A study of 256 male patients. J Surg
Oncol. 33:194–197. 1986.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Koch WM: Axillary nodal metastases in head
and neck cancer. Head Neck. 21:269–272. 1999.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wormald R, Sheahan P and Timon C: A case
of head and neck cancer metastasizing to the axillary lymph nodes.
Ear Nose Throat J. 89:E24–E26. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Islam S, Cole CV, Hoffman GR and Brennan
PA: Bilateral axillary metastasis from a primary ethmoidal squamous
cell carcinoma. J Laryngol Otol. 120:353–355. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kowalski LP: Noncervical lymph node
metastasis from head and neck cancer. ORL J Otorhinolaryngol Relat
Spec. 63:252–255. 2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Oo AL, Yamaguchi S, Iwaki H and Amagasa T:
Axillary nodal metastasis from oral and maxillofacial cancers: A
report of 3 cases. J Oral Maxillofac Surg. 62:1019–1024.
2004.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Koker MM and Kleer CG: p63 expression in
breast cancer: A highly sensitive and specific marker of
metaplastic carcinoma. Am J Surg Pathol. 28:1506–1512.
2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wargotz ES and Norris HJ: Metaplastic
carcinomas of the breast. IV. Squamous cell carcinoma of ductal
origin. Cancer. 65:272–276. 1990.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Fukusumi T and Califano JA: The notch
pathway in head and neck squamous cell carcinoma. J Dent Res.
97:645–653. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
El-Naggar AK, Chan JKC, Grandis JR, Takata
T and Slootweg PJ (eds): WHO Classification of Head and Neck
Tumours. Vol. 9. 4th edition. International Agency for Research on
Cancer. IARC, Lyon, 2017.
|
|
30
|
Agrawal N, Frederick MJ, Pickering CR,
Bettegowda C, Chang K, Li RJ, Fakhry C, Xie TX, Zhang J, Wang J, et
al: Exome sequencing of head and neck squamous cell carcinoma
reveals inactivating mutations in NOTCH1. Science. 333:1154–1157.
2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Solomon I, Voiculescu V, Caruntu C, Lupu
M, Popa A, Ilie M, Albulescu R, Caruntu A, Tanase C, Constantin C,
et al: Neuroendocrine factors and head and neck squamous cell
carcinoma: An affair to remember. Dis Markers.
2018(9787831)2018.PubMed/NCBI View Article : Google Scholar
|