Introduction
Cardiovascular disease is the primary complication
and leading cause of death in patients with chronic kidney disease
(CKD) (1). A potentially
life-threatening cardiovascular factor in patients with CKD is the
presence of vascular calcification, which exists in up to 47-83% of
patients with CKD (2-4).
Vascular calcification in patients with CKD can be broadly divided
into two types: Intimal calcification and medial calcification,
with the latter observed more commonly (5). A previous study has suggested that the
mechanism of vascular calcification is a cell-mediated, highly
regulated process similar to osteogenesis (6). During this process, vascular smooth
muscle cells (VSMCs) undergo a series of pathological changes that
eventually result in the generation of osteoblast-like cells
(phenotypic transition) (7).
MicroRNAs (miRNAs/miRs) are a class of small,
single-stranded, non-coding RNAs that modulate gene expression by
binding to the 3' untranslated region (3'UTR) of their target
genes, causing translational suppression and/or mRNA degradation
(8,9). Accumulating evidence indicates that
miRNAs play notable roles in a wide range of biological processes,
including cellular proliferation, apoptosis and differentiation;
therefore, they are involved in a variety of pathologies, most
notable cancer (10). Several
miRNAs have been identified to negatively regulate osteoblast
differentiation by inhibiting mineralization via silencing of the
key transcription factors of osteogenesis, such as runt-related
transcription factor 2 (Runx2), osteopontin (OPN), osterix or
distal-less homeobox 5(11).
The osteogenesis transcription factor Runx2, as an
essential transcription factor in osteoblast differentiation and
chondrocyte maturation, and plays a notable role in the
calcification of VSMCs. Under normal conditions, Runx2 is expressed
at low levels, but is markedly increased in calcified
atherosclerotic plaques, suggesting that Runx2 may be a significant
factor involved in vascular calcification. A previous study
revealed that multiple miRNAs promote osteogenesis through direct
or indirect regulation of Runx2 expression. Zhang et al
(12) reported that miRNA-133a-5p
inhibits the expression of osteoblast differentiation-associated
markers by targeting the 3' UTR of RUNX2. miR-29a and miR-125b
indirectly targeting Runx2 via its co-repressors or co-activators
may lead to increased or decreased regulation of osteoblast
differentiation, respectively (13).
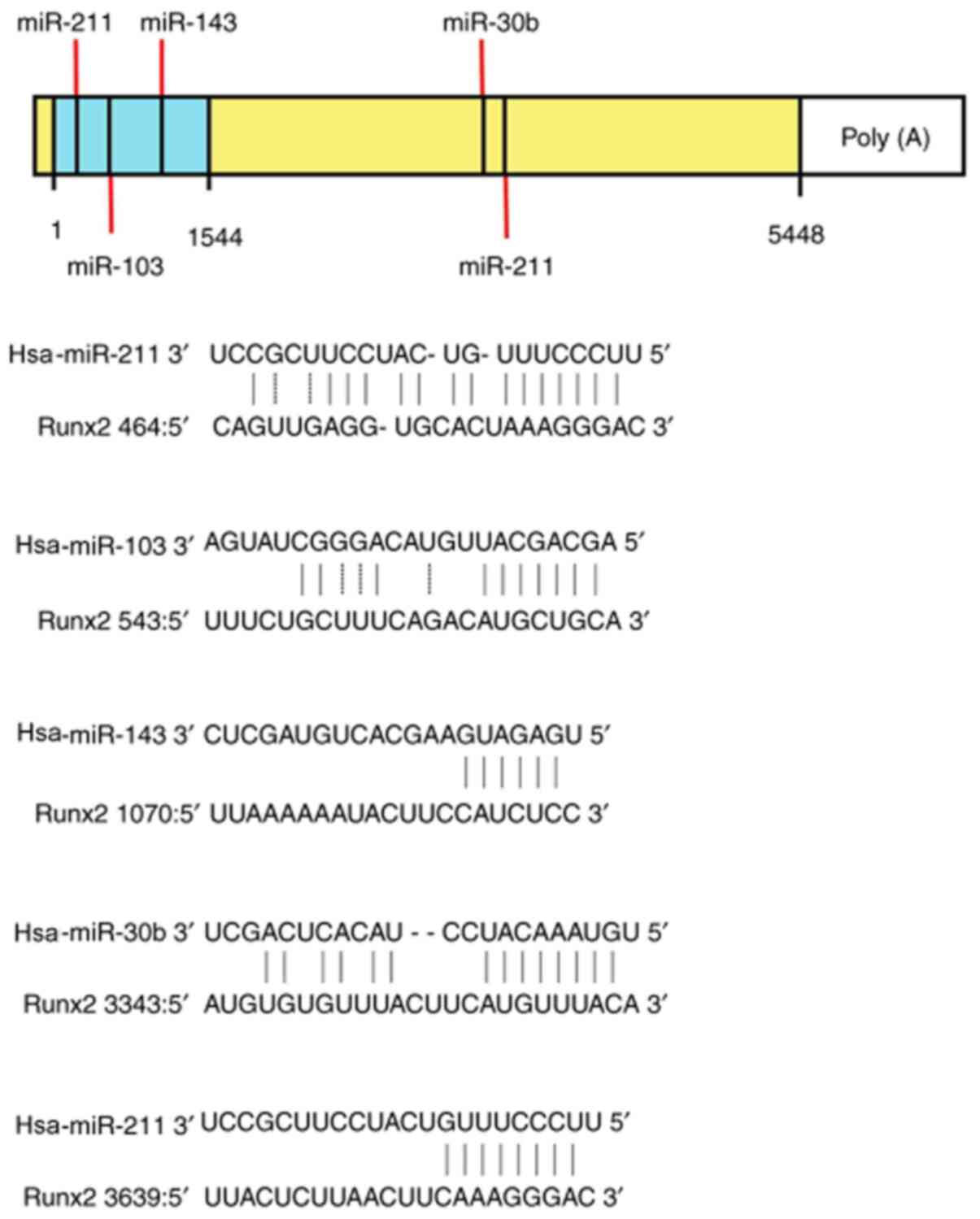
Notably, the miRBase database platform suggested the
sequence-specific base pairing of the ‘seed region’ at the 5' or 3'
ends of miR-30b, -103a, -133a, -143 and -211 with the 3'-UTR
binding sites of Runx2, which regulated its expression. Therefore,
miR-29a, -30b, -103a, -125b, -133a, -143 and -211 were selected to
confirm whether miRNAs were involved in the regulation of vascular
calcification under high phosphorus conditions, and if so, the
underlying mechanisms by which it functions in this context.
Materials and methods
Ethics statement
All experimental programs were reviewed and approved
by The Animal Care and Use Institutional Committee of Hebei Medical
University (approval no. 2020ky189; Shijiazhuang, China).
Cell culture
A total of 20 adult male Sprague Dawley rats (age, 8
weeks; weight, 200-250 g) were purchased from the Hebei Medical
University Lab Animal Center (Shijiazhuang, China) and allowed free
access to standard rat chow and tap water, in 22±1˚C temperature-
and 40-60% humidity-controlled conditions, with a 12 h dark/light
cycle. The 20 rats were anesthetized with 400 mg/kg chloral hydrate
and intraperitoneally euthanized with 300 mg/kg sodium
pentobarbital (three times the anesthetic dose). Rats were
considered dead after breathing had stopped, cardiac arrest was
confirmed, righting reflex disappeared and the pupils were dilated.
The cause of death for all rats was cardiac arrest. The primary
VSMCs were extracted from the thoracic aorta using a previously
described method (14). The
experiment was usually completed within 30 min and no peritonitis
was observed.
The cells were cultured with DMEM (Gibco; Thermo
Fisher Scientific Inc.) containing 4.5 g/l glucose supplemented
with 15% FBS, at 37˚C (5% CO2) in a humidified
atmosphere. To induce calcification, the VSMCs were seeded at a
density of 5,000 cells/cm2 in six-well dishes and
cultured in 10 mmol/l β-glycerophosphate (β-GP), 10 mol/l insulin
and 50 mg/ml ascorbic acid in the presence of 15% FBS (HyClone;
Cytiva), and the control groups were cultured in identical
conditions but without the β-GP. The culture medium was replaced
every day.
Transfection
The miRNAs were obtained from Guangzhou RiboBio Co.,
Ltd., and the target sequences were as follows: Mimic miR-103,
AGCAGCAUUGUACAGGGCUAUGA; inhibitor miR-103,
UCAUAGCCCUGUACAAUGCUGCU; mimic negative control (NC),
UUUGUACUACACAAAAGUACUG; and inhibitor NC, CAGUACUUUUGUGUAGUACAAA.
The mimic NC and inhibitor NC were both non-targeting. The miRNA
(mimic-103a, inhibitor-103a and control miRNAs) transfection was
performed using Lipofectamine® 2000 transfection reagent
according to the manufacturer's instructions (Invitrogen; Thermo
Fisher Scientific, Inc.). Briefly, 6 µg miRNA and 10 µl
Lipofectamine® 2000 were mixed for 20 min at room
temperature, and subsequently added to the VSMCs cultured in
six-well plates. At 72 h post-transfection, the intracellular RNA
and protein were extracted to detect miR-103 and Runx2 expression
by reverse transcription-quantified PCR (RT-qPCR) and western
blotting, respectively.
Target miRNA forecast of RUNX2
miRBase (http://www.mirbase.org/) online analysis tool was used
to predict the miRNAs targeting Runx2(15). miRBase was developed by
Griffiths-Jones et al (15)
to provide users with a more comprehensive relationship between
miRNA and target genes (15).
RNA sequencing
VSMC cells were treated with normal or 10 mmol/l
β-GP medium, followed by the extraction of total RNA by using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific
Inc.). The quality of RNA was typically measured by using a
NanoDrop spectrophotometer (ND-1000; NanoDrop Technologies; Thermo
Fisher Scientific Inc.) and the integrity was assessed by using gel
electrophoresis to analysis the ratios of 28S to 18S ribosomal
bands. Then, the RNA was used to prepare the miRNA sequencing
library, which included the following steps: 3'-adaptor ligation,
5'-adaptor ligation, cDNA synthesis, PCR amplification and size
selection of ~135 to 155 bp PCR amplified fragments (corresponding
to ~15 to 35 nt small RNAs). The Invitrogen™ Collibri™ kit
(Invitrogen; Thermo Fisher Scientific Inc.). was used to quantify
the sequencing library and 20 pM products were subjected to deep
sequencing using an Illumina-Solexa 1G Genetic Analyzer (Illumina,
Inc.). The pre-miRNA databases (known premiRNA from miRBase v.21
plus the newly predicted pre-miRNAs) and Novoalign software
(http://www.novocraft.com/products/novoalign/.v.2.07.11;
Novocraft Technologies) was used to analysis the data.
Calcification assays
After being cultured with β-GP at 37˚C for 14 days,
the transfected VSMC cells were washed three times with PBS. The
calcium content was determined by spectrophotometry using a calcium
assay kit for 24 h at 4˚C (BioSino Biotechnology & Science,
Inc.), and then by histology using a 1% Alizarin red stain at 4˚C
for 24 h. The specific procedures used were described previously
(14). The results were then
normalized by protein content, which was quantified using a BCA
protein assay kit (Beijing Solarbio Science & Technology Co.,
Ltd.).
Alkaline phosphatase (ALP)
activity
To determine the ALP activity in β-GP stimulated or
miRNA transfected VSMC cells, a phosphorylated nitrophenyl
substrate was performed using an alkaline phosphatase assay kit
(Nanjing Jiancheng Bioengineering Institute), according to the
manufacturer's instructions. The results were calculated according
to the curves generated from the standard samples, and normalized
by protein concentration.
RT-qPCR
Total RNA was extracted from VSMCs using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol and cDNA was
synthesized using superscript reverse transcriptase (Clontech
Laboratories, Inc.) at 37˚C for 15 min and 85˚C for 5 sec. To
investigate the expression of Runx2 mRNA, qPCR was performed using
DyNAmo ColorFlash SYBR Green qPCR kit (Thermo Fisher Scientific,
Inc.) according to the following protocol: 40 Cycles of 95˚C for 5
min, 95˚C for 10 sec, 55˚C for 30 sec and 72˚C for 10 sec. The
mature miR expression levels were also analyzed from total RNA
using the miScript SYBR Green PCR kit (Thermo Fisher Scientific,
Inc.) for miR-103a, miR-29a, miR-133a, miR-30b, miR-143, miR-125b
and miR-211, according to the manufacturer's instructions. The
primer sequences for rat Runx2, GAPDH and the candidate miRNAs are
presented in Table I. The
expression of the selected miRNAs relative to 5s, and of Runx2
relative to GAPDH. The data were analyzed using the Quantstudio™ Dx
v1.0 software (Thermo Fisher Scientific, Inc.).
 | Table IPrimer pairs used for reverse
transcription-quantitative PCR. |
Table I
Primer pairs used for reverse
transcription-quantitative PCR.
| Primer | Sequence,
5'-3' |
|---|
| miR-29a-3p
forward |
AGGAGGTAGCACCATCTGAAATC |
| miR-29a-3p
reverse |
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTAACCG |
| miR-30b-5p
forward |
TCCTCCTGTAAACATCCTACACT |
| miR-30b-5p
reverse |
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAGCTGA |
| miR-103a-3p
forward |
AGAGGTTTGGTCCCCTTCAAC |
| miR-103a-3p
reverse |
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCAGCTG |
| miR-125b-5p
forward |
GCTCCTCCCTGAGACCCTAAC |
| miR-125b-5p
reverse |
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTCACAA |
| miR-133a-3p
forward |
AGAGGTTTGGTCCCCTTCAAC |
| miR-133a-3p
reverse |
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCAGCTG |
| miR-143-3p
forward |
AGGAGGTGAGATGAAGCACTGT |
| miR-143-3p
reverse |
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACGAGCTA |
| miR-211-3p
forward |
GGTGTTCCCTTTGTCATCCTT |
| miR-211-3p
reverse |
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAGGCGA |
| 5S forward |
CTGGTTAGTACTTGGACGGGAGAC |
| 5S reverse |
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCAGGCG |
| Runx2 forward |
CCAGCAGCACTCCATATCTC |
| Runx2 reverse |
CAGCGTCAACACCATCATTC |
| GAPDH forward |
GGAGCGAGATCCCTCCAAAAT |
| GAPDH reverse |
GGCTGTTGTCATACTTCTCATGG |
Western blotting
Protein was extracted from VSMCs using a lysis
buffer containing 150 mmol/l NaCl, 50 mmol/l Tris (pH 7.4), 1%
Triton X-100, 0.1% SDS, 1 mmol/l EDTA, 0.5% sodium deoxycholate, 2
mmol/l sodium pyrophosphate, 1 mmol/k Na3VO4,
0.5 µg/ml leupeptin and 0.1 mmol/l PMSF. The protein concentration
was determined using the BCA method (Sigma-Aldrich; Merck KGaA),
and 40 µg protein per lane was loaded onto 10% SDS-PAGE gels for
electrophoresis. The specific procedures for electrophoresis and
electrotransfer to polyvinylidene difluoride membranes were
performed as previously described (14). The blots were incubated with 5% BSA
(Beijing Solarbio Science & Technology Co., Ltd.)at 37˚C for 1
h, followed by incubation with specific primary antibodies against
Runx2 (1:100 dilution; cat. no. ab23981; Abcam) and GAPDH (1:10,000
dilution; cat. no. AP0063; Bioworld Technology, Inc.) overnight at
4˚C. After rinsing with 1% Tween-Tris buffered saline, the blots
were incubated with peroxidase-conjugated secondary antibody
(1:4,000 dilution; cat. no. TA130016; Origene Technologies Inc.) at
room temperature for 2 h. Immunodetection was performed using an
Enhanced Chemiluminescence kit (Amersham; Cytiva). The expression
level of stained proteins quantified by digital image analysis,
according to the integrated optical density (IOD) of the positive
region. The integrated optical density ratio of the indicated Runx2
and GAPDH bands was measured by using the Image Pro-Plus v.5.0
software (Media Cybernetics, Inc.) to calculate the relative
expression level.
Luciferase assay
pMIR vector of wild-type (WT) RUNX2 3'-UTR
and mutant of the RUNX2 3'-UTR were purchased from Guangzhou
RiboBio Co., Ltd. The sequences of mimic-103a and mimic negative
control (NC) obtained from Guangzhou RiboBio Co., Ltd. were as
follows: 5'-AGCAGCAUUGUACAGGGCUAUGA-3' and
5'-UUCUCCGAACGUGUCACGUTT-3', respectively. VSMC cells
(3x104 cells/well) were co-transfected with 200 ng of
the mimic-103a and 50 ng of the Wild-type or mutant luciferase
plasmid by using Lipofectamine® 2000 transfection
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The
luciferase assays were performed 48 h later with the
dual-luciferase reporter assay system (Promega Corporation) and
FLUOstar photometer (BMG Labtech GmbH). Relative luciferase
activity of each group was expressed as a percentage of Renilla
luciferase activity normalized to the corresponding firefly
luciferase activity. The relative luciferase activity (%)
represented the expression level of RUNX2.
Statistical analysis
SPSS v17.0 software (SPSS, Inc.) was used for
analysis. All experiments were repeated 3 times. The data are
presented as the mean ± standard deviation (unless otherwise
stated). For experiments with 2 groups, statistical analyses were
performed using Student's unpaired t-test. For experiments with
>2 groups, the statistical analyses were performed using one-way
ANOVA. Furthermore, the Student-Newman-Keuls post hoc test was used
to determine the statistical significance of the differences among
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
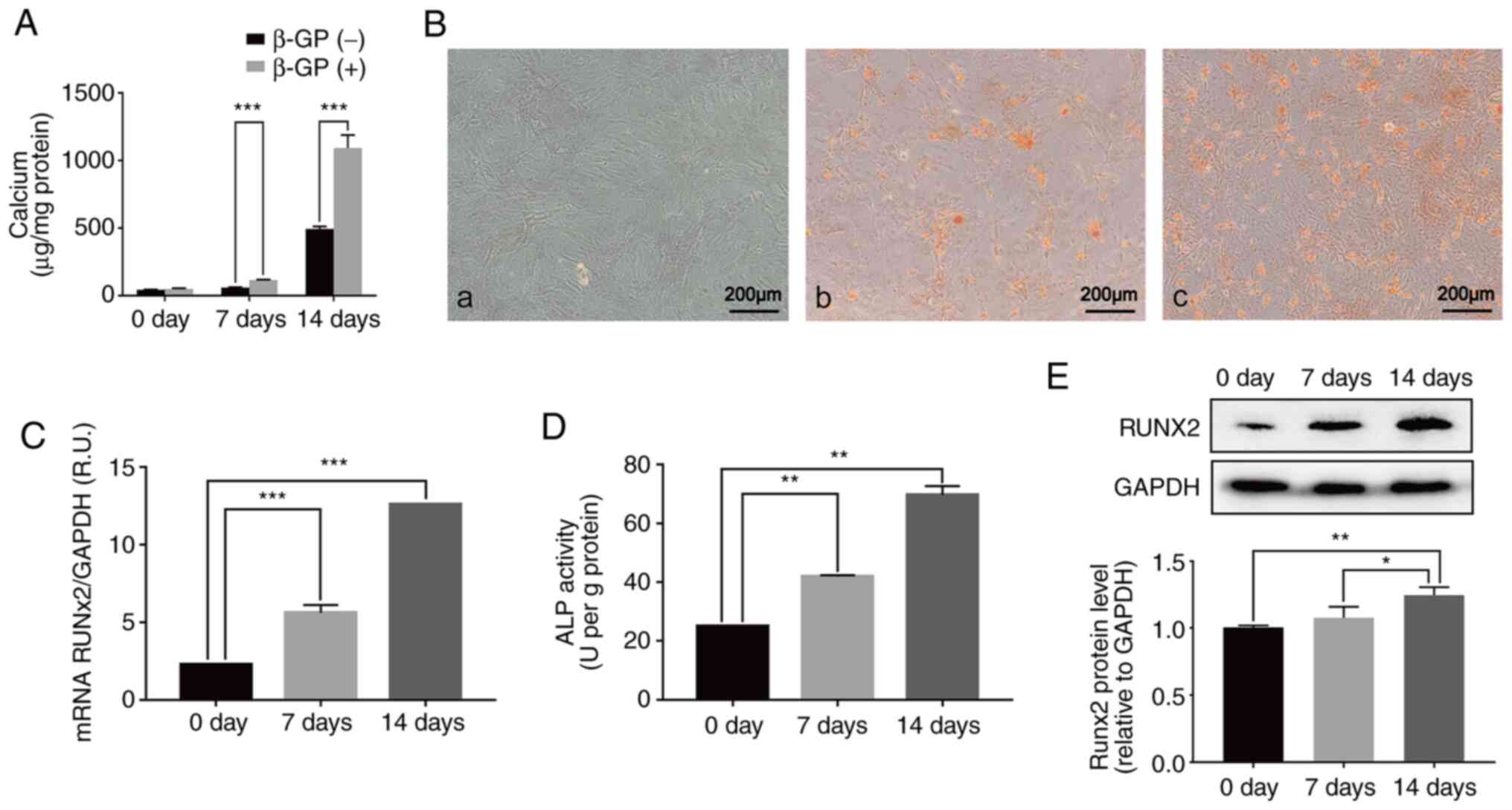
High phosphorus promotes calcification
by inducing the transdifferentiation of VSMCs into osteoblast-like
cells
The calcification of VSMCs cultured in medium with
10 mM β-GP for 0, 7 and 14 days was examined. It was found that
treatment with high phosphorus levels significantly increased the
calcium content of VSMCs in a time-dependent manner, as
demonstrated by the calcium content assay (Fig. 1A). Consistent with the changing
trend of the calcium content results, VSMCs showed a significant
increase in mineralization after 7 days of culture, which was
measured by Alizarin red staining, reaching the highest
calcification level at day 14 (Fig.
2B). These data suggested that high phosphorus conditions
induce calcification in the VSMCs.
To investigate the underlying mechanisms of VSMC
calcification induced by high phosphorus, the ALP activity and the
mRNA and protein expression of Runx2 were determined. The results
showed that high phosphorus significantly upregulated the ALP
activity and mRNA and protein expression levels of Runx2. The level
of Runx2 mRNA was significantly increased by 56.9 and 81.48% after
7 and 14 days of high phosphorus treatment, respectively, compared
with the normal control group (Fig.
1C). Furthermore, compared with the normal control group, the
protein levels of Runx2 in VSMCs treated with high phosphorus for 7
and 14 days increased significantly, by 19.1 and 25.9%,
respectively (Fig. 1E). The ALP
activity was significantly increased in a time-dependent manner,
similar to the expression of Runx2, in VSMCs treated with high
phosphorus (Fig. 1D). These data
suggested that the transdifferentiation of VSMCs to osteoblast-like
cells may play a prominent role in the high phosphorus-induced
calcification of VSMCs.
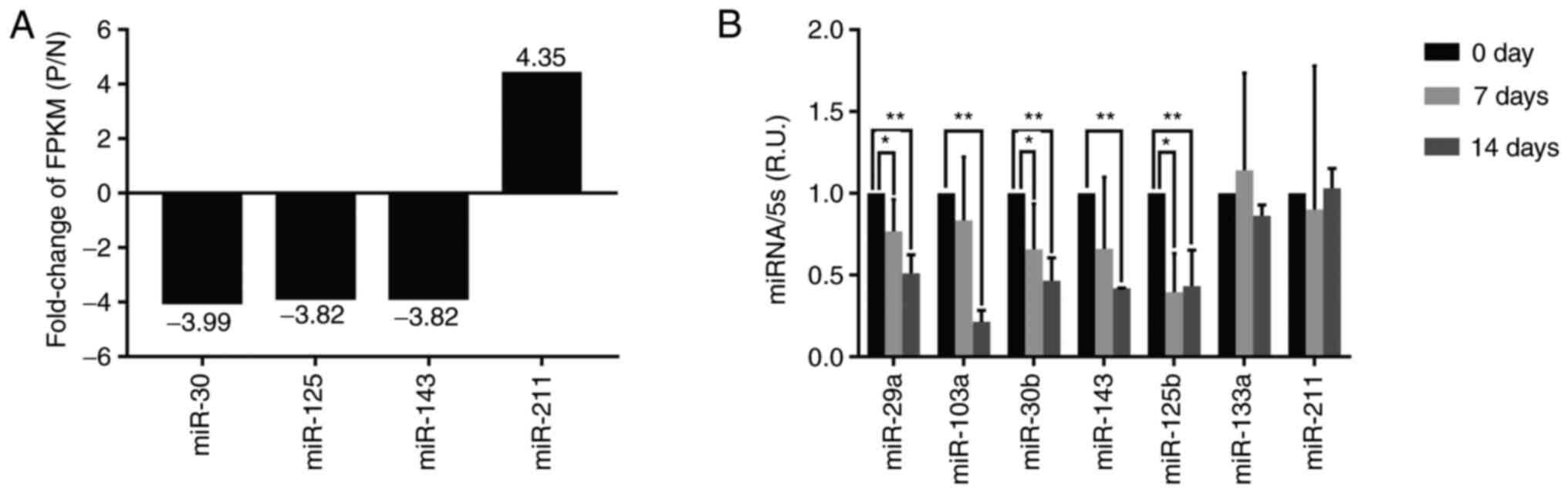
Identification of differentially
expressed miRNAs during the calcification of the VSMCs in high
phosphorus conditions
To further investigate the modulation mechanism of
high phosphorus in the osteoblast-like cell transdifferentiation of
the VSMCs, the expression levels of miRNAs were explored using the
RNA sequencing method. The results, presented in Fig. 2A, show that miR-30, miR-125 and
miR-143 expression levels decreased, whereas miR-211 expression
increased in high phosphorus-treated VSMCs, compared with normal
cells.
Furthermore, VSMCs were treated with 10 mmol/l β-GP
for 0, 7 and 14 days, and the miRNA was extracted for RT-qPCR
analysis. The results of gene expression analysis showed that the
expression levels of miR-29a, miR-30b, miR-103a, miR-125b and
miR-143 significantly decreased in VSMCs cultured with high
phosphorus for 7 days, compared with those in the normal control
group; however, the expression of miR-133a and miR-211 did not
decrease significantly (Fig. 2B).
Moreover, the expression of miR-29a, miR-30b, miR-103a, miR-125b,
miR-143, miR-133a and miR-211 was downregulated by 48.85, 60.56,
53.44, 58.11, 56.78, 36.13 and 31.24% in VSMCs cultured with high
phosphorus for 14 days, compared with the normal control group,
respectively (Fig. 2B). It can be
observed that the expression of miR-103a decreased the most in high
phosphorus-induced VSMCs, and it was therefore hypothesized that
miR-103a was likely to be involved in regulating Runx2 expression
in the high phosphorus-induced VSMCs.
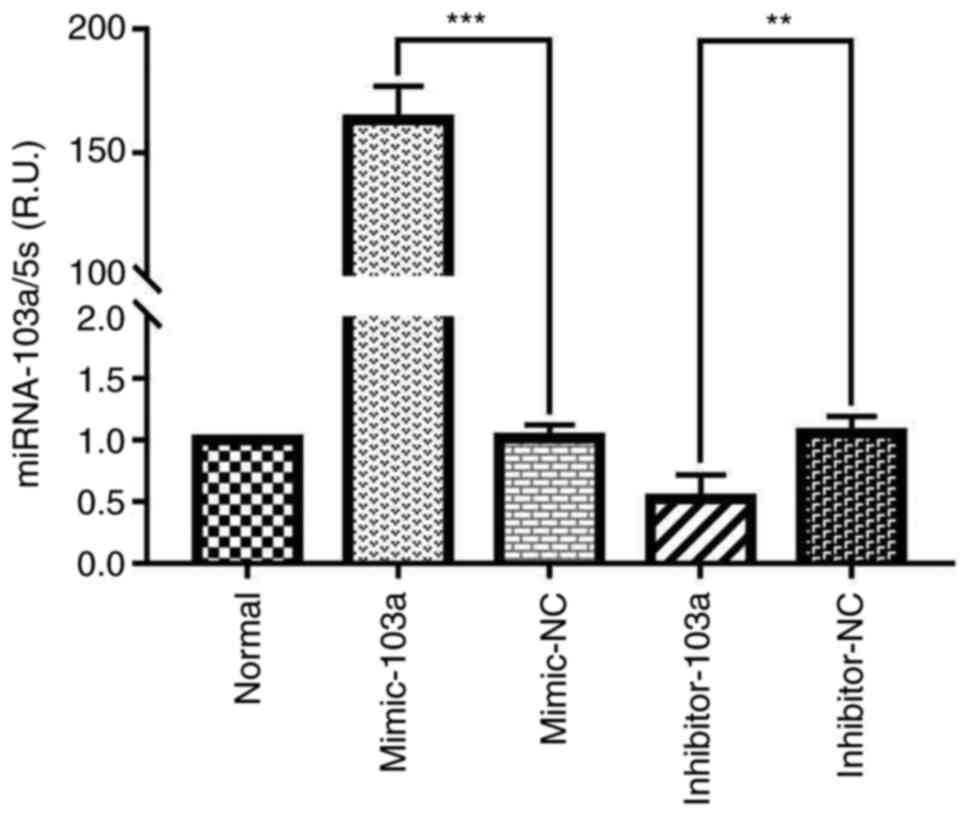
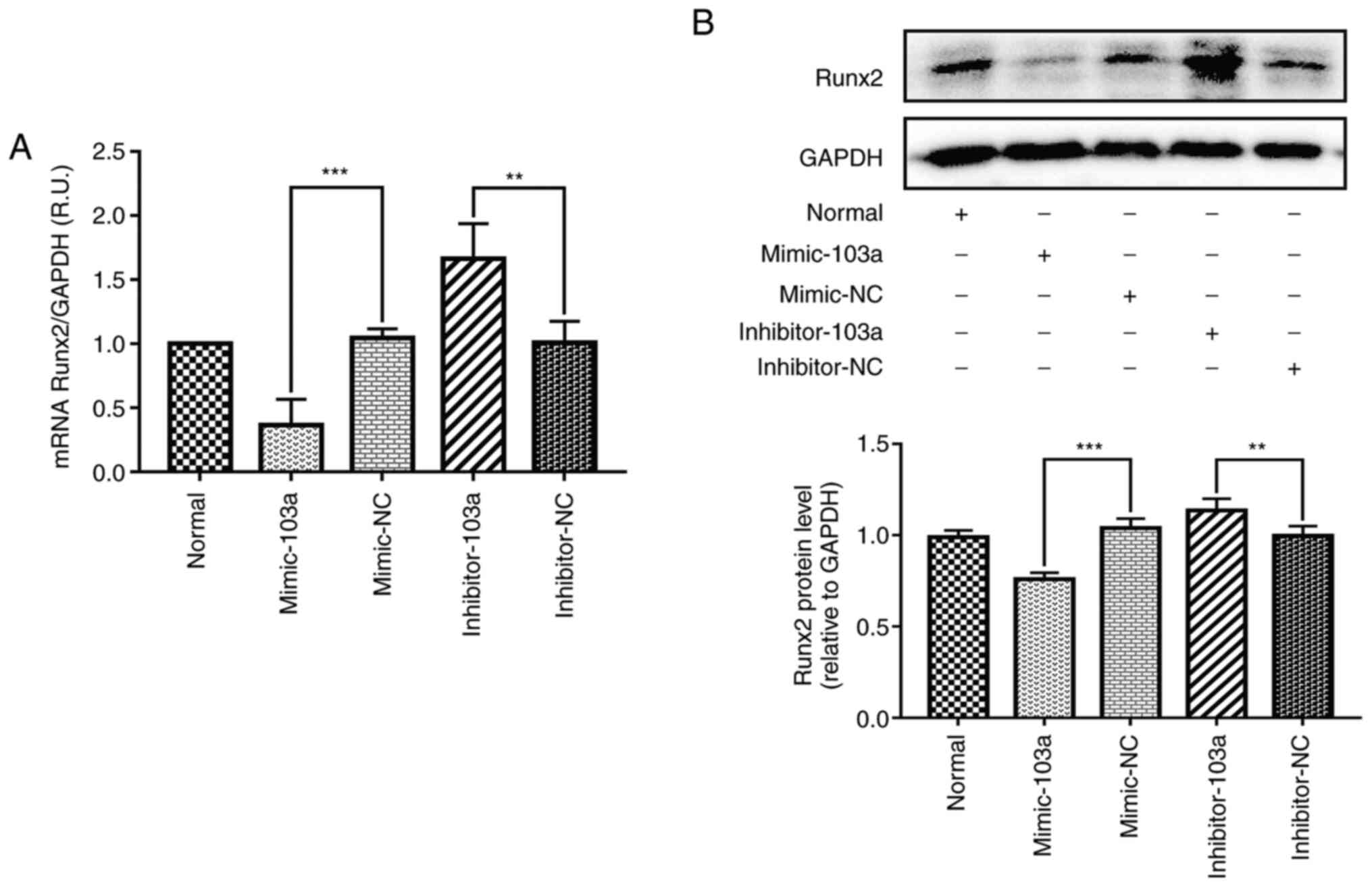
miR-103a inhibits Runx2 expression in
the high phosphorus-induced calcification of VSMCs
Considering that the expression of miR-103a showed
the most significant reduction out of the selected miRNAs in the
VSMC calcification model (at 14 days), miR-103a was selected as the
focus of further study. To determine whether miR-103a is involved
in the regulation of Runx2 in VSMCs, the mRNA and protein levels of
Runx2 were assessed by overexpressing miR-103a (mimic-103a), or
reducing miR-103a expression (inhibitor-103a) in normal cultured
VSMCs. The results showed a 155-fold increase in the mimic-103a,
compared with the mimic-NC group (Fig.
3); and the mRNA and protein levels of Runx2 decreased by 52.7
and 28.8% compared with the mimic-NC, respectively, at 14 days
post-transfection with mimic-103a (Fig.
4A and B).
Furthermore, inhibitor-103a transfection resulted in
a significant decrease in VSMC miR-103a expression, and the
expression levels of Runx2 mRNA and protein were increased by 68.3
and 16.9%, respectively (Fig. 4A
and B).
Taken together, these results demonstrated that
miR-103a plays a notable role in modulating the expression of Runx2
during high phosphorus-induced phenotype transition of VSMCs into
osteoblast-like cells.
Overexpression of miR-103a ameliorates
calcification caused by high phosphorus-induced transformation of
VSMCs into osteoblasts
To investigate the effects of miR-103a on cell
phenotype transformation, the expression of miR-103 was inhibited
in normal cultured VSMCs and examined using Alizarin red staining,
as well as calcium content and ALP activity assays. The results of
Alizarin red staining revealed that when miR-103a was inhibited,
the calcium nodules were increased (Fig. 5A). The results of the calcium
content assay demonstrated that following miR-103a-knockdown,
calcium content was significantly increased by x2.4 compared with
the control group (Fig. 5B).
Consistent with the calcium content results, ALP activity assay,
indicated a significant increase in ALP activity following
miR-103a-knockdown (Fig. 5C).
Collectively, these data suggested that the inhibition of miR-103a
promoted the transformation of smooth muscle cells into
osteoblasts.
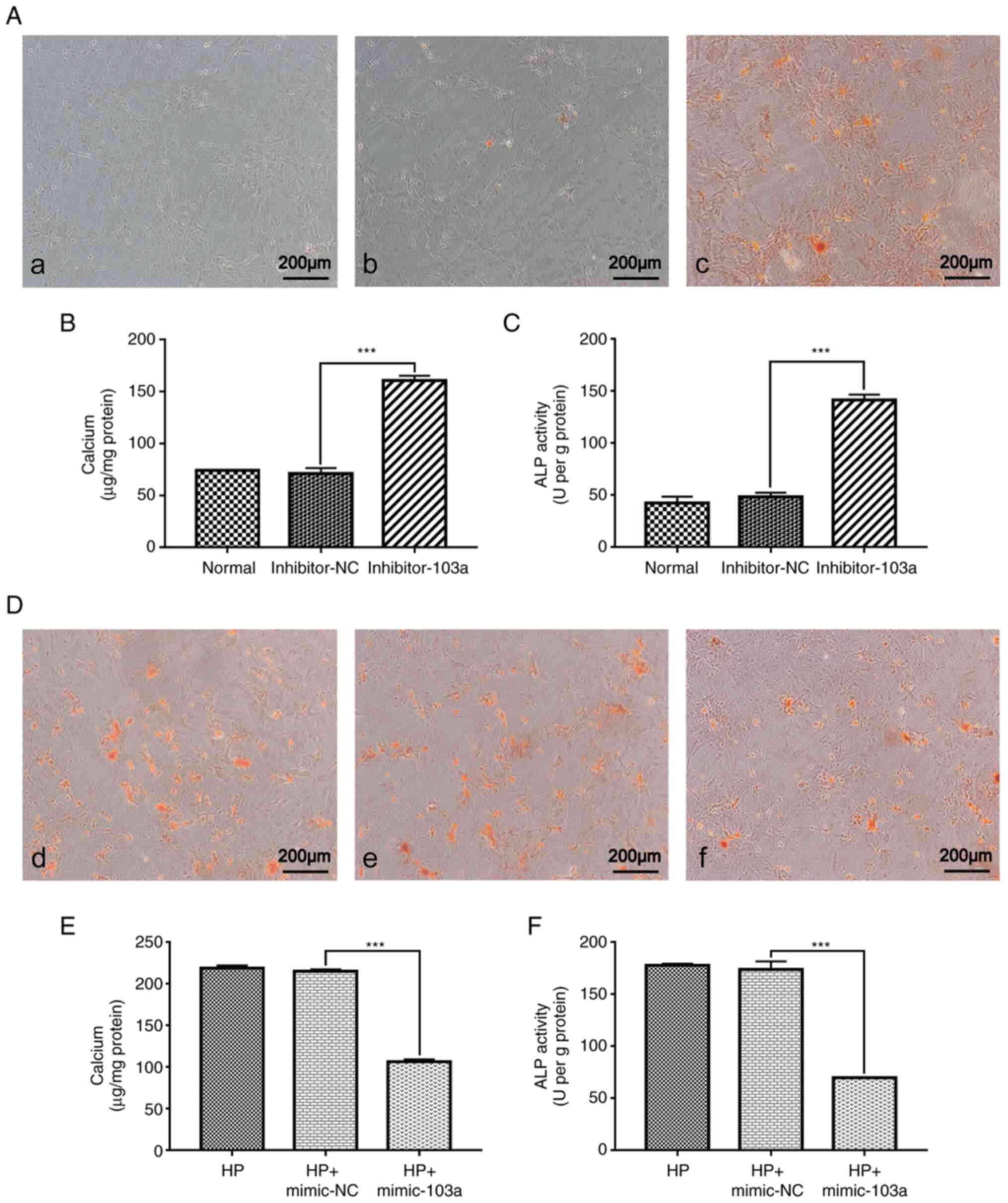 | Figure 5Effect of mimic-103a, inhibitor-103a
or their respective NCs on VSMC phenotype. (A) Rat thoracic VSMCs
treated with (a) normal medium, (b) normal medium with inhibitor NC
or (c) normal medium with inhibitor miR-103a, for 3 days, followed
by Alizarin Red staining; representative microscopic views are
shown. Detection of (B) calcium content and (C) ALP activity after
transfection with inhibitor miR-103a was quantified by
spectrophotometry; both n=5. (D) Rat thoracic VSMCs treated with
(d) 10 mM β-GP, (e) 10 mM β-GP with mimic NC, or (f) normal medium
with mimic miR-103a, for 3 days, followed by Alizarin Red S
staining; representative microscopic views are shown. Detection of
(E) calcium content and (F) ALP activity after transfection with
mimic miR-103a was quantified by spectrophotometry; both n=5.
***P<0.001. VSMCs, vascular smooth muscle cells;
miR-, microRNA; ALP, alkaline phosphatase; NC, negative control;
HP, high phosphorus; β-GP, β-glycerophosphate. |
To further verify the role of miR-103a, mimic-103a
was transfected in VSMCs cultured with high phosphorus and assessed
by using Alizarin red staining, calcium content and ALP activity
assays. The results of Alizarin red staining showed that
overexpression of miR-103a decreased the level of dye incorporation
(Fig. 5D) and that the calcium
content (Fig. 5E) and ALP activity
(Fig. 5F) decreased compared with
the control group.
To investigate whether miR-103 directly targeted the
3'-UTR of Runx2, RUNX2 Wild-type or mutant luciferase plasmid and
miR-103a mimics were co-transfected into the cells and the relative
luciferase activity of each group was then detected. The relative
luciferase activity was significantly reduced in cells transfected
with the miR-103a mimics and the pLuc-RUNX2 3'-UTR Wild-type
plasmid compared with cells transfected with miR-103a natural
control and the pLuc-RUNX2 3'-UTR Wild-type plasmid group. However,
the relative luciferase activity was not significantly increased
between the mutant-RUNX2-transfection and the
Wild-type-RUNX2-transfection in the cells with overexpression of
the miR-103a (Fig. S1).
Taken together, the aforementioned results indicated
that increased expression levels of miR-103a can ameliorate
calcification caused by high phosphorus-induced transformation of
the VSMCs into osteoblasts.
Discussion
In the present study, miR-103a was identified as a
negative regulator of vascular medial calcification in
vitro, potentially by inhibiting the phenotypic transformation
of VSMCs into osteoblast-like cells, and downregulating Runx2
expression levels and ALP activity.
Osteoblast-like transformation of VSMCs plays a key
role in the pathogenesis of vascular medial calcification (16). It has been reported that VSMCs can
acquire osteoblast-like phenotypes and expression of osteogenic
markers in the process of vascular calcification (17). The results of the present study
showed that the expression of the osteochondrogenic marker Runx2
and the ALP activity were notably increased in calcified VSMCs.
Moreover, high phosphorus promoted Runx2 expression and ALP
activity in a time-dependent manner. Similarly, a previous study
indicated that exposure to elevated phosphorus levels results in
the upregulation of Runx2 expression and ALP activity (18). Runx2 is a notable transcription
factor in the process of VSMC osteo-/chondrogenic
transdifferentiation, which induces the expression of major bone
matrix components, including type I collagen (5), osteocalcin (19) and OPN (20). Runx2 can be transcribed from the
distal P1 promoter or the P2 promoter, resulting in the expression
of two different subtypes (Runx2 I and II), which can be expressed
in ossified and cartilage cells; both subtypes exhibit similar
features in chondrocytes and osteoblasts. However, the mechanisms
underlying the osteogenic differentiation of high
phosphorus-induced VSMCs are not completely understood and
therefore require further research.
As a key regulator of vascular calcification, Runx2
is likely to be involved in multiple levels of VSMC regulation
(13,21). Runx2 has a long 3'-UTR (~4 kb),
presumably containing multiple regulatory elements (12). Therefore, the current study sought
to identify potential miRNAs that bound the 3'-UTR of Runx2, and
that suppressed its translation, as miRNAs have emerged as a novel
regulatory mechanism for gene expression. Previous studies have
shown that several miRNAs are involved in the modulation process of
vascular calcification, such as miRNA-32(21), miRNA-34(13) and miRNA-204(22). Based on the miRNA databases
(http://www.mirbase.org/) and literature reports,
seven miRNAs were selected (miR-29a, 30b, 103a, 125b, 133a, 143 and
211) to analyze the VSMC calcification model at 0, 7 and 14 days.
The miRNA databases predicted the sequence-specific base pairing of
the ‘seed region’ at the 5'- or 3'- of miR-30b, -103a, -133a, -143
and -211 with the 3'-UTR binding sites of mRNA targets of Runx2
(Fig. 6); miR-29a and 125b were
predicted from reports in the literature (23-25).
Previous studies have shown that miR-29a, -30b, -103a, -125b,
-133a, -143 and -211 act as negative regulators of osteoblast
differentiation and subsequent mineralization, functionally
inhibiting the differentiation of osteoprogenitors by attenuating
the essential transcription factor Runx2 (26-28).
The results of the present study revealed lower
levels of miR-29a, 30b, 103a 125b and 143 in VSMCs following
hyperphosphatemia, with an associated increase in Runx2 levels
during the calcification process. However, miR-133a and miR-211 did
not decrease significantly, although they did decrease in a similar
manner to that reported previously (29). These results suggested that miR-133a
and miR-211 may exert their effects on calcification through
additional pathways (30).
In the present study, the expression of miR-103a was
the most significantly reduced of the selected miRNAs in the VSMC
calcification model. This finding indicated that miR-103a may be a
powerful suppressor of VSMC calcification, and may have treatment
potential for the process of vascular calcification. Therefore,
miR-103a was the focus of further investigation herein. To
determine how miR-103a regulated the osteo-/chondrogenic
transdifferentiation of VSMCs, the effect of miR-103a on the
expression of Runx2 protein was determined. The results showed that
miR-103a decreased the expression of Runx2 during the high
phosphorus-induced phenotypic transition of VSMCs into
osteoblast-like cells. Collectively, these findings indicate that
miR-103a plays a notable role in the process of vascular
calcification and ameliorates calcification by downregulating
Runx2, which is consistent with the study by Zuo et al
(31).
To further validate that miR-103 targeted the 3'-UTR
of Runx2, Runx2 wild-type and mutant derivative devoid of
miR-103-binding site were subcloned downstream of the coding region
of the luciferase gene, and then used to transfect VSMCs. However,
no significant downregulation in luciferase activity was observed
following miR-103 overexpression in the present study.
A limitation of the current study was the lack of a
positive finding of the direct link between miR-103 and Runx2.
Moreover, the lack of investigation into the effects of high
phosphorus and miR-103 on different Runx2 isoforms is another
limitation of this article. Therefore, in the future, further
studies are required to investigate the targets of Runx2, to
confirm the direct link between miR-103 and Runx2, and the effects
of high phosphorus and miRNA-103 on different Runx2 isoforms, for
predicting or treating the high level of disease associated with
vascular calcification.
In conclusion, the results of the current study
showed that miR-103 alleviated calcification by inhibiting Runx2
during high phosphorus-induced vascular calcification, which is a
potential novel target for the treatment for this pathological
phenomenon.
Supplementary Material
Relative luciferase activity in VSMCs
transfected with miR-103a mimics by the reporter plasmid with the
3'.UTR of Runx2 or with the mutant 3'.UTR of Runx2. WT, wild-type;
Mut, Mutant; NC, negative control; miR, microRNA; VSMCs, vascular
smooth muscle cells; Runx2, runt-related transcription factor
2.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by The Project of Hebei
Clinical Medicine Outstanding Personnel Training (grant no.
2019139), The Hebei Province Medical Technology Tracking Project
(grant no. G2018050) and Hebei province Key Research and
Development Project (grant no. 20377704D).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JX and YB designed all experiments. LH, HZ and WZ
performed the experiments. MC, DZ, LZ and SZ analyzed the data. LH
and SZ wrote the manuscript. JX and YB confirmed the authenticity
of all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All experimental programs were reviewed and approved
by The Animal Care and Use Institutional Committee of Hebei Medical
University (Shijiazhuang, China) (approval no. 2020ky189).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kottgen A, Russell SD, Loehr LR,
Crainiceanu CM, Rosamond WD, Chang PP, Chambless LE and Coresh J:
Reduced kidney function as a risk factor for incident heart
failure: The atherosclerosis risk in communities (ARIC) study. J Am
Soc Nephrol. 18:1307–1315. 2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tentori F, Blayney MJ, Albert JM,
Gillespie BW, Kerr PG, Bommer J, Young EW, Akizawa T, Akiba T,
Pisoni RL, et al: Mortality risk for dialysis patients with
different levels of serum calcium, phosphorus, and PTH: The
Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney
Dis. 52:519–530. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bolasco P: Effects of the use of
non-calcium phosphate binders in the control and outcome of
vascular calcifications: A review of clinical trials on CKD
patients. Int J Nephrol. 2011(758450)2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Adragao T: Evaluation of vascular
calcifications in CKD patients. Int J Artif Organs. 32:81–86.
2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bai Y, Zhang J, Xu J, Cui L, Zhang H and
Zhang S: Alteration of type I collagen in the radial artery of
patients with end-stage renal disease. Am J Med Sci. 349:292–297.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Towler DA and Demer LL: Thematic series on
the pathobiology of vascular calcification: An introduction. Circ
Res. 108:1378–1380. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nitta K and Ogawa T: Vascular
calcification in end-stage renal disease patients. Contrib Nephrol.
185:156–167. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Klimczak D, Paczek L, Jazdzewski K and
Kuch M: MicroRNAs: Powerful regulators and potential diagnostic
tools in cardiovascular disease. Kardiol Pol. 73:1–6.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chiang VS: Withdrawn: MicroRNAs as
potential regulators of docosahexaenoic acid benefits in
Alzheimer's disease. Nutr Neurosci: Mar 14, 2015 (Epub ahead of
print). doi: 10.1179/1476830515Y.0000000014.
|
|
10
|
Mennigen JA, Plagnes-Juan E,
Figueredo-Silva CA, Seiliez I, Panserat S and Skiba-Cassy S: Acute
endocrine and nutritional co-regulation of the hepatic
omy-miRNA-122b and the lipogenic gene fas in rainbow trout,
Oncorhynchus mykiss. Comp Biochem Physiol B Biochem Mol Biol.
169:16–24. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pinto MT, Nicolete LD, Rodrigues ES, Palma
PV, Orellana MD, Kashima S and Covas DT: Overexpression of
hsa-miR-125b during osteoblastic differentiation does not influence
levels of Runx2, osteopontin, and ALPL gene expression. Braz J Med
Biol Res. 46:676–680. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang W, Wu Y, Shiozaki Y, Sugimoto Y,
Takigawa T, Tanaka M, Matsukawa A and Ozaki T: MiRNA-133a-5p
inhibits the expression of osteoblast differentiation-associated
markers by targeting the 3 UTR of RUNX2. DNA Cell Biol. 37:199–209.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Narayanan A, Srinaath N, Rohini M and
Selvamurugan N: Regulation of Runx2 by MicroRNAs in osteoblast
differentiation. Life Sci. 232(116676)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Xu J, Bai Y, Jin J, Zhang J, Zhang S, Cui
L and Zhang H: Magnesium modulates the expression levels of
calcification-associated factors to inhibit calcification in a
time-dependent manner. Exp Ther Med. 9:1028–1034. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Griffiths-Jones S, Grocock RJ, van Dongen
S, Bateman A and Enright AJ: MiRBase: MicroRNA sequences, targets
and gene nomenclature. Nucleic Acids Res. 34:D140–D144.
2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang Z, Jiang Y, Liu N, Ren L, Zhu Y, An Y
and Chen D: Advanced glycation end-product Nε-carboxymethyl-Lysine
accelerates progression of atherosclerotic calcification in
diabetes. Atherosclerosis. 221:387–396. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Du Y, Wang Y, Wang L, Liu B, Tian Q, Liu
CJ, Zhang T, Xu Q, Zhu Y, Ake O, et al: Cartilage oligomeric matrix
protein inhibits vascular smooth muscle calcification by
interacting with bone morphogenetic protein-2. Circ Res.
108:917–928. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Shanahan CM, Crouthamel MH, Kapustin A and
Giachelli CM: Arterial calcification in chronic kidney disease: Key
roles for calcium and phosphate. Circ Res. 109:697–711.
2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tang Q, Tong M, Zheng G, Shen L, Shang P
and Liu H: Masquelet's induced membrane promotes the osteogenic
differentiation of bone marrow mesenchymal stem cells by activating
the Smad and MAPK pathways. Am J Transl Res. 10:1211–1219.
2018.PubMed/NCBI
|
|
20
|
Luo Y, Cao X, Chen J, Gu J, Zhao J and Sun
J: MicroRNA-224 suppresses osteoblast differentiation by inhibiting
SMAD4. J Cell Physiol. 233:6929–6937. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liu J, Xiao X, Shen Y, Chen L, Xu C, Zhao
H, Wu Y, Zhang Q, Zhong J, Tang Z, et al: MicroRNA-32 promotes
calcification in vascular smooth muscle cells: Implications as a
novel marker for coronary artery calcification. PLoS One.
12(e0174138)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang Y, Chen S, Deng C, Li F, Wang Y, Hu
X, Shi F and Dong N: MicroRNA-204 Targets Runx2 to Attenuate
BMP-2-induced osteoblast differentiation of human aortic valve
interstitial cells. J Cardiovasc Pharmacol. 66:63–71.
2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Roberto VP, Tiago DM, Silva IA and Cancela
ML: MiR-29a is an enhancer of mineral deposition in bone-derived
systems. Arch Biochem Biophys. 564:173–183. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wen P, Cao H, Fang L, Ye H, Zhou Y, Jiang
L, Su W, Xu H, He W, Dai C and Yang J: MiR-125b/Ets1 axis regulates
transdifferentiation and calcification of vascular smooth muscle
cells in a high-phosphate environment. Exp Cell Res. 322:302–312.
2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Goettsch C, Rauner M, Pacyna N, Hempel U,
Bornstein SR and Hofbauer LC: MiR-125b regulates calcification of
vascular smooth muscle cells. Am J Pathol. 179:1594–1600.
2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Balderman JA, Lee HY, Mahoney CE, Handy
DE, White K, Annis S, Lebeche D, Hajjar RJ, Loscalzo J and Leopold
JA: Bone morphogenetic protein-2 decreases microRNA-30b and
microRNA-30c to promote vascular smooth muscle cell calcification.
J Am Heart Assoc. 1(e003905)2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sun Z, Cao X, Hu Z, Zhang L, Wang H, Zhou
H, Li D, Zhang S and Xie M: MiR-103 inhibits osteoblast
proliferation mainly through suppressing Cav1.2 expression in
simulated microgravity. Bone. 76:121–128. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li E, Zhang J, Yuan T and Ma B: MiR-143
suppresses osteogenic differentiation by targeting Osterix. Mol
Cell Biochem. 390:69–74. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Panizo S, Naves-Diaz M, Carrillo-Lopez N,
Martinez-Arias L, Fernandez-Martin JL, Ruiz-Torres MP,
Cannata-Andia JB and Rodriguez I: MicroRNAs 29b, 133b, and 211
regulate vascular smooth muscle calcification mediated by high
phosphorus. J Am Soc Nephrol. 27:824–834. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Atlasi Y, Noori R, Gaspar C, Franken P,
Sacchetti A, Rafati H, Mahmoudi T, Decraene C, Calin GA, Merrill BJ
and Fodde R: Wnt signaling regulates the lineage differentiation
potential of mouse embryonic stem cells through Tcf3
down-regulation. PLoS Genet. 9(e1003424)2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zuo B, Zhu J, Li J, Wang C, Zhao X, Cai G,
Li Z, Peng J, Wang P, Shen C, et al: MicroRNA-103a functions as a
mechanosensitive microRNA to inhibit bone formation through
targeting Runx2. J Bone Miner Res. 30:330–345. 2015.PubMed/NCBI View Article : Google Scholar
|