Introduction
Globally, colorectal cancer was reported as the
third most commonly diagnosed cancer type for men and the second
most commonly diagnosed cancer type for women in 2018(1). An estimated 551,000 patients died from
colorectal cancer in 2018, which accounts for >5% of
cancer-associated mortality worldwide (1). Despite advancements in treatment
strategies, the prognosis of patients with colorectal cancer
remains poor, with a 5-year overall survival rate of ~40% (2). Several novel therapeutic approaches,
such as aspirin-based chemoprevention and targeting
cyclin-dependent kinase 4/tyrosine-protein kinase Fyn have improved
the treatment efficacy (3-5).
However, the molecular mechanism contributing to colorectal cancer
progression remains elusive, making it difficult to develop
therapeutic approaches for patients. Thus, it remains critical to
determine the molecular mechanism to provide novel targets for the
treatment of colorectal cancer.
MicroRNAs (miRNAs/miRs) are non-coding,
single-stranded, short molecules ubiquitously expressed in human
cells (6). miRNAs degrade mRNAs and
inhibit the translation process by directly binding to the
complementary sites in the 3'-untranslated region (UTR) of target
mRNAs (7). miRNAs regulate several
physiological processes and dysregulation of miRNAs results in the
development of different types of human diseases (8-11).
Aberrant expression or mutation of miRNAs are implicated in the
initiation and development of different types of cancer, including
colorectal cancer (12,13). For example, miR-145 is downregulated
in colorectal cancer, and can directly target transcriptional
regulator ERG to suppress migration and invasion of cancer cells
(12). Several miRNAs, including
miR-934 have been identified as differentially expressed in
colorectal cancer based on human miRNA microarray (14). miR-934 is involved in cancer cell
proliferation, invasion, drug resistance and apoptosis of ovarian
cancer and head and neck squamous cell carcinoma (HNSCC) (15,16).
However, to the best of our knowledge, the role of miR-934 in
colorectal cancer remains known.
The present study aimed to investigate the
biological role of miR-934 in colorectal cancer cells.
Materials and methods
Patients and materials
A total of 40 colorectal tumor tissues and matched
adjacent normal tissues (≥5 cm from tumors) were collected from
patients (24 males and 16 females; including 12 T1, 13 T2, 10 T3
and 5 T4; age range, 49-72 years; median age, 56 years) who
underwent surgery at the Shaanxi Provincial Cancer Hospital (Xi'an,
China) between June 2015 and July 2018. None of these patients
received chemotherapy or radiotherapy before surgery. Tissue
samples were stored at -80˚C until subsequent experimentation. The
present study was approved by the Ethics Committee of Shaanxi
Provincial Cancer Hospital (approval no. 2015-23; Xi'an, China) and
written informed consent was provided by all patients prior to the
study commencement.
Cell culture and transfection
The immortalized human colon cell line FHC and human
colorectal cancer cell lines HCT116 and HT29 were purchased from
the American Type Culture Collection. All cell lines were
authenticated via STR profiling and maintained in DMEM supplemented
with 10% fetal bovine serum (both purchased from Gibco; Thermo
Fisher Scientific, Inc.) at 37˚C in an incubator with 5%
CO2.
miR-negative control (NC) mimic, miR-NC inhibitor,
miR-934 inhibitor and miR-934 mimic were synthesized and purchased
from Suzhou GenePharma Co., Ltd. A total of 50 nM miR-NC mimic
(5'-CUAUCCACCAGGUUGCUUUGACC-3'), miR-NC inhibitor
(5'-UUGUACUACACAAAAGUACUG-3'), miR-934 inhibitor
(5'-CCAGUGUCUCCAGUAGUAGACA-3') or miR-934 mimic
(5'-UGUCUACUACUGGAGACACUGG-3') was transfected into HCT116 cells
using Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Control small interfering (si)RNA (5'-UAAGGCUAUGAAGAGAUAC-3') and
Dickkopf-related protein 2 (DDK2) siRNA (5'-CAGCAGGACGAAUCCAAG-3')
were purchased from Suzhou GenePharma Co., Ltd. A total of 30 nM
control siRNA or DDK2 siRNA was transfected into HCT116 cells using
Lipofectamine 3000 according to the manufacturer's instructions.
The reagent was mixed with siRNA or miRNA mimic or miRNA inhibitor
at room temperature for 10 min. Cells were subjected to reverse
transcription-quantitative (RT-q)PCR and western blot analysis 48 h
post-transfection.
RT-qPCR
Total RNA was extracted from cells and tissues using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific)
and reverse-transcribed into cDNA using the miScript Reverse
Transcription kit (cat. no. 218061; Qiagen GmbH), with the
following temperature protocol: 37˚C for 1 h and 95˚C for 5 min.
qPCR was subsequently performed using the SYBR Premix EX Taq kit
(cat. no. DRR041A; Takara Bio, Inc.), with the following
thermocycling conditions: 95˚C for 2 min; 35 cycles of 95˚C for 10
sec and 55˚C for 30 sec. The miRNA was amplified with the stem loop
primer and universal primers. Relative expression levels were
quantified using the 2-ΔΔCq method (17) and normalized to the internal
reference genes U6 (miRNA) and GAPDH (mRNA). The primer sequences
used for qPCR are listed in Table
I.
 | Table ISequences of primers used for
quantitative PCR. |
Table I
Sequences of primers used for
quantitative PCR.
| Primer | Sequence (5'-3') |
|---|
| miR-934
stem-loop |
GTCGTATCCAGTGCGTGTCGTGG
AGTCGGCAATTGCACTGGATACG ACCCAGTGTC |
| miR-934 forward |
GGGTGTCTACTACTGGAGA |
| miR-934 reverse |
CAGTGCGTGTCGTGGAGT |
| U6 forward |
GCTTCGGCAGCACATATACTAA AAT |
| U6 reverse |
CGCTTCACGAATTTGCGTGTCAT |
| DKK2 forward |
CCCCACCAAGGATCATCGG |
| DKK2 reverse |
CCGGGATGTGAGGGGTTAAGA |
| β-catenin
forward |
CACAAGCAGAGTGCTGAAGGTG |
| β-catenin
reverse |
GATTCCTGAGAGTCCAAAGACAG |
| c-Myc forward |
TACCCTCTCAACGACAGCAG |
| c-Myc reverse |
TCTTGACATTCTCCTCGGTG |
| cyclinD1 forward |
CTTCCTCTCCAAAATGCCAG |
| cyclinD1 reverse |
AGAGATGGAAGGGGGAAAGA |
| GAPDH forward |
GTCTCCTCTGACTTCAACAGCG |
| GAPDH reverse |
ACCACCCTGTTGCTGTAGCCAA |
RNA immunoprecipitation (RIP)
RIP was performed using the Magna RIP RNA-Binding
Protein Immunoprecipitation kit (cat. no. 17-700; EMD Millipore),
according to the manufacturer's protocol. After washing to remove
unbound material, RNA was extracted using TRIzol®
reagent. Protein argonaute-2 (AGO2; cat. no. 2897; 1:100) and IgG
(cat. no. 3900; 1:100) antibodies were purchased from Cell
Signaling Technology, Inc. Harvested RNAs were assessed via RT-qPCR
analysis using the aforementioned protocol and primers.
Western blotting
β-catenin (cat. no. 8480; 1:2,000) and
phosphorylated (p)-β-catenin (Ser675; cat. no. 4176; 1:2,000)
antibodies were purchased from Cell Signaling Technology, Inc.
GAPDH (cat. no. ab8245; 1:5,000) antibody was purchased from Abcam.
c-Myc (cat. no. sc40; 1:2,000) and cyclin D1 (cat. no. sc450;
1:2,000) antibodies were purchased from Santa Cruz Biotechnology,
Inc. HRP-conjugated secondary antibodies against mouse (cat. no.
ab205719; 1:10,000) and rabbit (cat. no. ab6721; 1:10,000) were
purchased from Abcam.
Protein lysates from colorectal cancer cells were
prepared using RIPA lysis buffer (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. BCA Protein Assay kit
(cat. no. 23225; Thermo Fisher Scientific, Inc.) was used to
determine the protein concentration. A total of 20 µg proteins per
lane were separated using SDS-PAGE (8% gel) and transferred onto
PVDF membranes. The membranes were blocked with 5% non-fat milk at
25˚C for 1 h, and incubated with the aforementioned primary and
secondary antibodies at 25˚C for 1 h, sequentially. Protein blots
were developed using ECL western blotting substrate (Pierce; Thermo
Fisher Scientific, Inc.) and the intensity of bands was quantified
using ImageJ software (1.52v; National Institutes of Health).
TOP/FOP-flash reporter assay
A total of 50 ng TOP/FOP-Flash vectors (Promega
Corporation) and 5 pmol miR-NC inhibitor or miR-934 inhibitor were
co-transfected into 5,000 HCT116 cells using Lipofectamine 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37˚C for 48 h. The
TOP/FOP ratio was measured to assess the activity of Wnt/β-catenin
signaling via GloMax 20 (Promega Corporation) 48 h
post-transfection.
Cell proliferation and apoptosis
assays
The proliferative ability of HCT116 cells was
assessed using a Cell Counting Kit-8 (CCK-8; cat. no. CK04; Dojindo
Molecular Technologies, Inc.). Cells were seeded into 96-well
plates. CCK-8 solution (10 µl) was added into each well and
incubated for 2, 0, 24, 48, 72 and 96 h after transfection. Cell
proliferation was subsequently analyzed at a wavelength of 450
nm.
Cell apoptosis was assessed using the Annexin
V-FITC/propidium iodide (PI) apoptosis assay kit (cat. no. V13241;
Invitrogen; Thermo Fisher Scientific, Inc.). Briefly, cells were
harvested and suspended in Annexin V binding buffer. PI and Annexin
V-FITC were added to the cells and incubated for 30 min at room
temperature in the dark. Apoptotic cells were subsequently analyzed
using a flow cytometer MACSQuant® X (Miltenyi Biotec
GmbH) and examined on FlowJo (version 10.7; FlowJo LLC).
PI+/Annexin V+ and PI-/Annexin
V+ were classified as apoptotic cells.
Bioinformatics analysis
The miR-934 expression data in 411 colon carcinoma
samples and 380 normal mucosa samples were downloaded from the
GSE115513 dataset using GEO2R (14)
within the Gene Expression Omnibus database (www.ncbi.nlm.nih.gov/gds). GEO2R was also used to
retrieve the top 250 differentially expressed miRNAs between normal
and tumor tissues from GSE115513 with P<0.05. The potential
target genes of miR-934 were predicted using TargetScan software
(version 7.2; www.targetscan.org/vert_72).
Dual-luciferase reporter assay
DDK2 3'UTR was amplified from cDNA of HCT116 and
ligated into a pmirGLO plasmid (Promega Corporation). pmirGLO-DDK2
3'UTR-mutant (MUT) was constructed by introducing mutations into
DDK2 3'UTR-wild-type (WT). Cells were transfected with DDK2
3'UTR-WT or DDK2 3'UTR-MUT in combination with miR-NC mimic or
miR-934 mimic, using Lipofectamine 3000. After 48 h, relative
luciferase activity was detected using the Dual Luciferase Reporter
System kit (cat. no. E1910; Promega Corporation). The firefly
luciferase was normalized to Renilla luciferase
activity.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 6.0 software (GraphPad Software, Inc.). All experiments were
performed in triplicate and data are presented as the mean ±
standard deviation. Pearson's correlation analysis was performed
between miR-934 and DDK2 or c-Myc and cyclinD1 expression levels.
Paired Student's t-test was used to compare differences between two
groups of tissues, while unpaired Student's t-test was used to
compare differences between two unpaired groups. One-way analysis
of variance and Fisher's Least Significant Difference post hoc test
were performed to compare differences between multiple groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
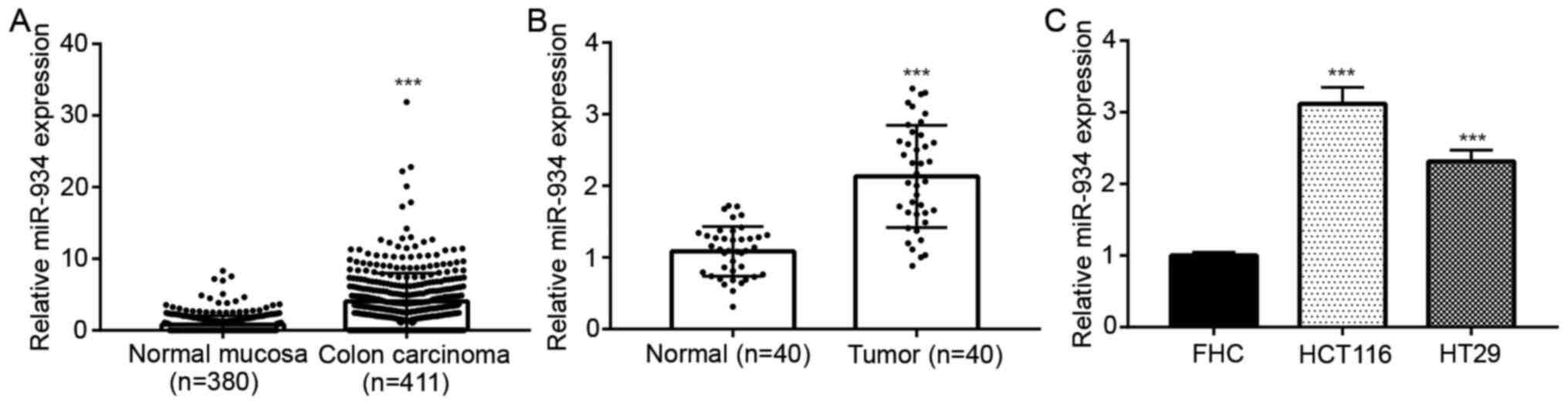
miR-934 expression is upregulated in
colorectal cancer
To investigate the tumor-associated miRNAs in
colorectal cancer, previously published miRNA microarray data were
analyzed. The top 250 differentially expressed miRNAs between colon
carcinoma and normal mucosa were obtained; miR-934 was one of the
most significantly upregulated miRNAs in colon carcinoma (n=411)
compared with normal mucosa (n=380) (Fig. 1A). RT-qPCR analysis was performed to
detect miR-934 expression in the tissue samples collected in the
present study. Consistently, it was demonstrated that miR-934
expression was upregulated in colorectal tumor tissues compared
with matched adjacent normal tissues from the 40 patients with
colorectal cancer (Fig. 1B). In
addition, miR-934 expression was higher in colorectal cancer cell
lines (HCT116 and HT29) compared with that in the immortalized
colon cells (FHC) (Fig. 1C).
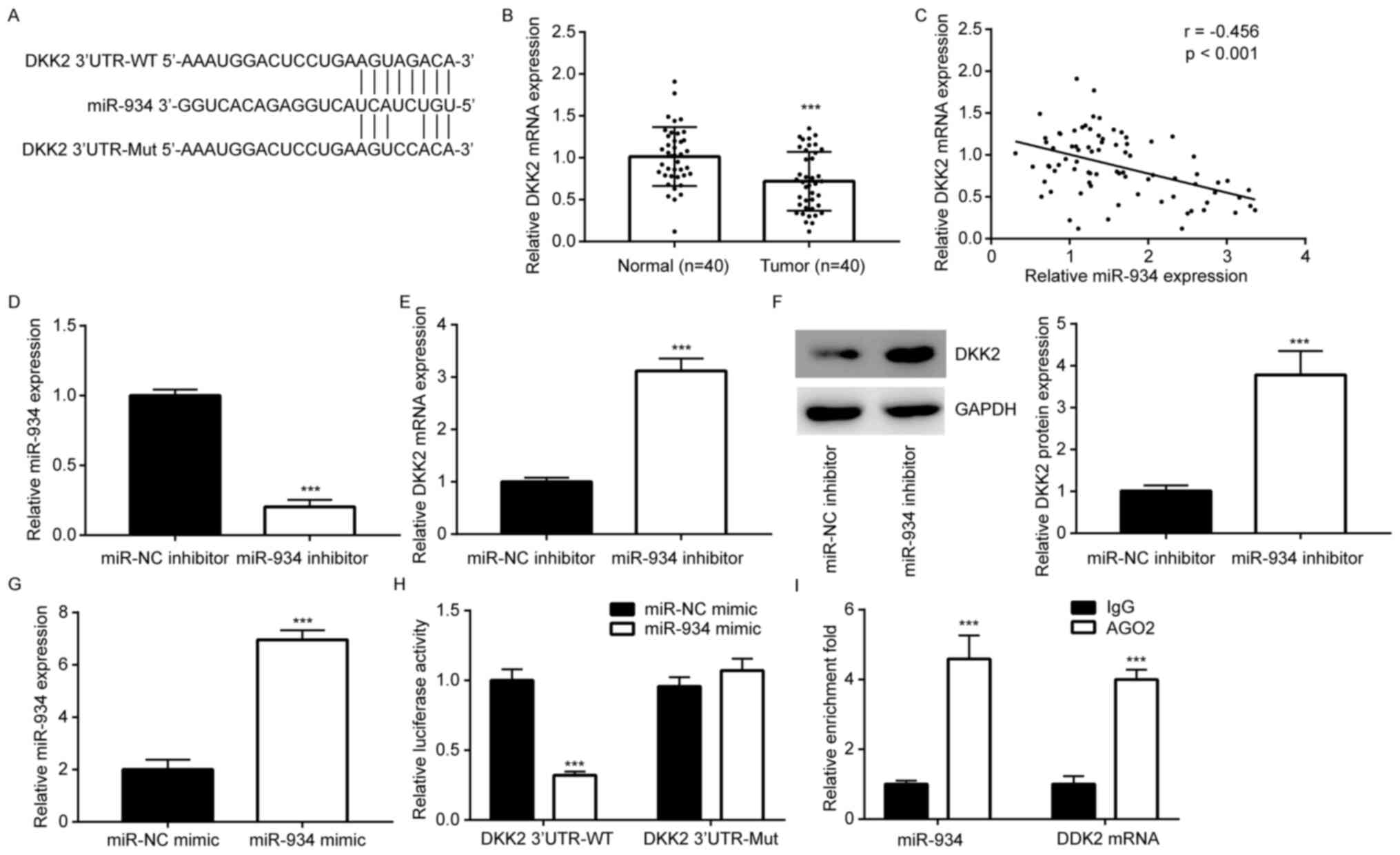
DDK2 is a target gene of miR-934 in
colorectal cancer cells
TargetScan software was used to predict the
potential targets of miR-934. The results demonstrated that there
was a putative binding site for miR-934 in the 3'UTR of DDK2 mRNA
(Fig. 2A). RT-qPCR analysis
demonstrated that DDK2 was downregulated in colorectal tumors
(Fig. 2B). In addition, Pearson's
correlation analysis revealed a negative correlation (r=-0.456)
between miR-934 and DDK2 expression levels in the collected tissue
samples (Fig. 2C). Subsequently,
HCT116 cells were transfected with miR-934 inhibitor to
downregulate miR-934 expression (Fig.
2D). Downregulation of miR-934 increased DDK2 mRNA and protein
levels in HCT116 cells (Fig. 2E and
F). In addition, transfection with
miR-934 mimic increased miR-934 expression in HCT116 cells compared
with that in the miR-NC group (Fig.
2G). Overexpression of miR-934 decreased the relative
luciferase activity of DDK2 3'UTR-WT, while the relative luciferase
activity of DDK2 3'UTR-MUT was not affected by overexpression of
miR-934 (Fig. 2H), suggesting that
miR-934 directly interacted with DDK2 3'UTR in HCT116 cells. To
validate this direct interaction, a RIP assay was performed. The
results demonstrated that AGO2 enriched both DKK2 mRNA and miR-934
in HCT116 cells (Fig. 2I).
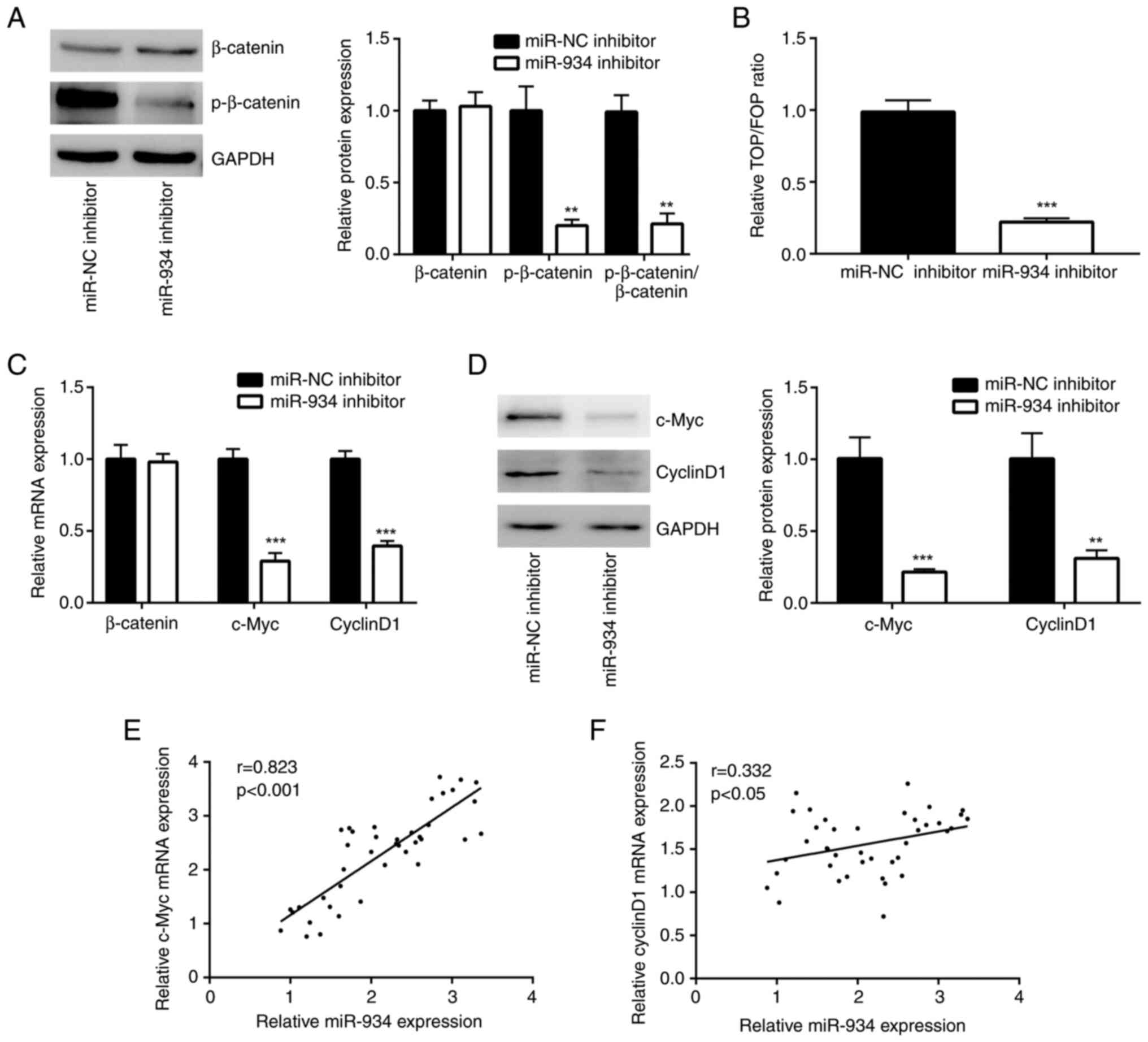
miR-934 activates the Wnt signaling
pathway in colorectal cancer
DDK2 is a well-known negative regulator of Wnt
signaling (18). Thus, β-catenin,
the core effector of the Wnt signaling pathway (18), and p-β-catenin, the activated form
of β-catenin, protein levels were assessed in HCT116 cells. Western
blot analysis demonstrated that downregulation of miR-934 decreased
p-β-catenin levels; however, it did not alter β-catenin expression
in HCT116 cells (Fig. 3A). The
results of the TOP/FOP assay demonstrated that the transcriptional
activity of TOP/FOP was notably repressed in HCT116 cells following
the downregulation of miR-934, suggesting that the Wnt signaling
pathway was inactivated by the miR-934 inhibitor (Fig. 3B). Furthermore, downregulation of
miR-934 decreased the mRNA expression levels of c-Myc and cyclin
D1, which are well-known target genes of Wnt signaling (Fig. 3C) (18). Western blot analysis confirmed that
downregulation of miR-934 also decreased c-Myc and cyclin D1
protein expression levels in HCT116 cells (Fig. 3D). RT-qPCR analysis was performed to
detect c-Myc mRNA expression levels in the 40 tumor samples. The
results demonstrated a notably positive correlation (r=0.823)
between miR-934 and c-Myc mRNA expression in these tumors (Fig. 3E). A relatively weak positive
correlation (r=0.332) was also observed between miR-934 and cyclin
D1 mRNA expression level in tumors (Fig. 3F).
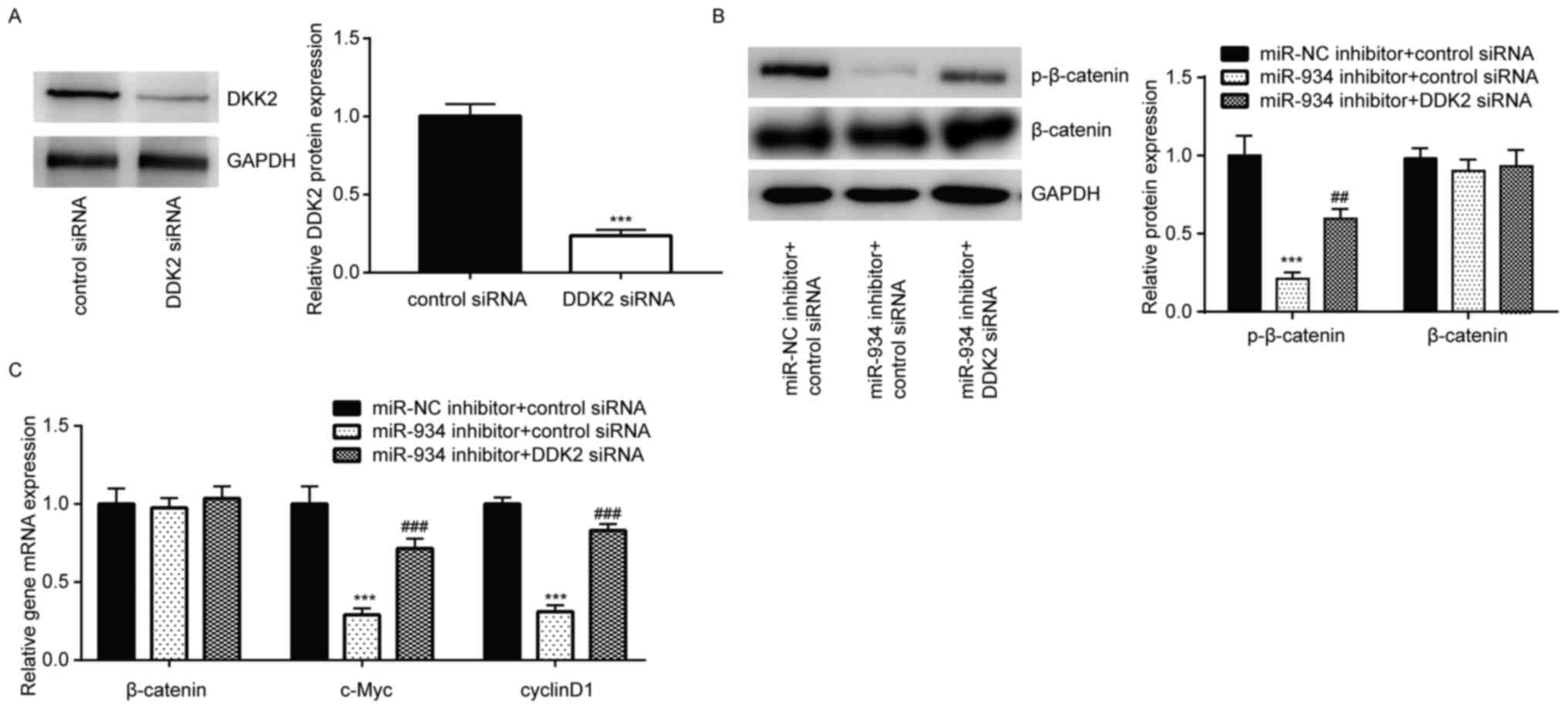
DDK2 silencing rescues inactivation of
Wnt signaling upon downregulation of miR-934 in colorectal cancer
cells
DDK2 siRNA was transfected into HCT116 cells to
downregulate DDK2 expression (Fig.
4A). As expected, DDK2 silencing reversed the downregulation of
p-β-catenin protein levels induced by miR-934 inhibitor in HCT116
cells (Fig. 4B). In addition,
RT-qPCR analysis demonstrated that DDK2 silencing also reversed the
decreased mRNA expression levels of c-Myc and cyclin D1, induced by
miR-934 inhibitor in HCT116 cells (Fig.
4C). Taken together, these results suggest that miR-934
positively regulated Wnt signaling by targeting DKK2 in colorectal
cancer cells.
miR-934/DKK2 axis controls cell
proliferation and apoptosis in colorectal cancer cells
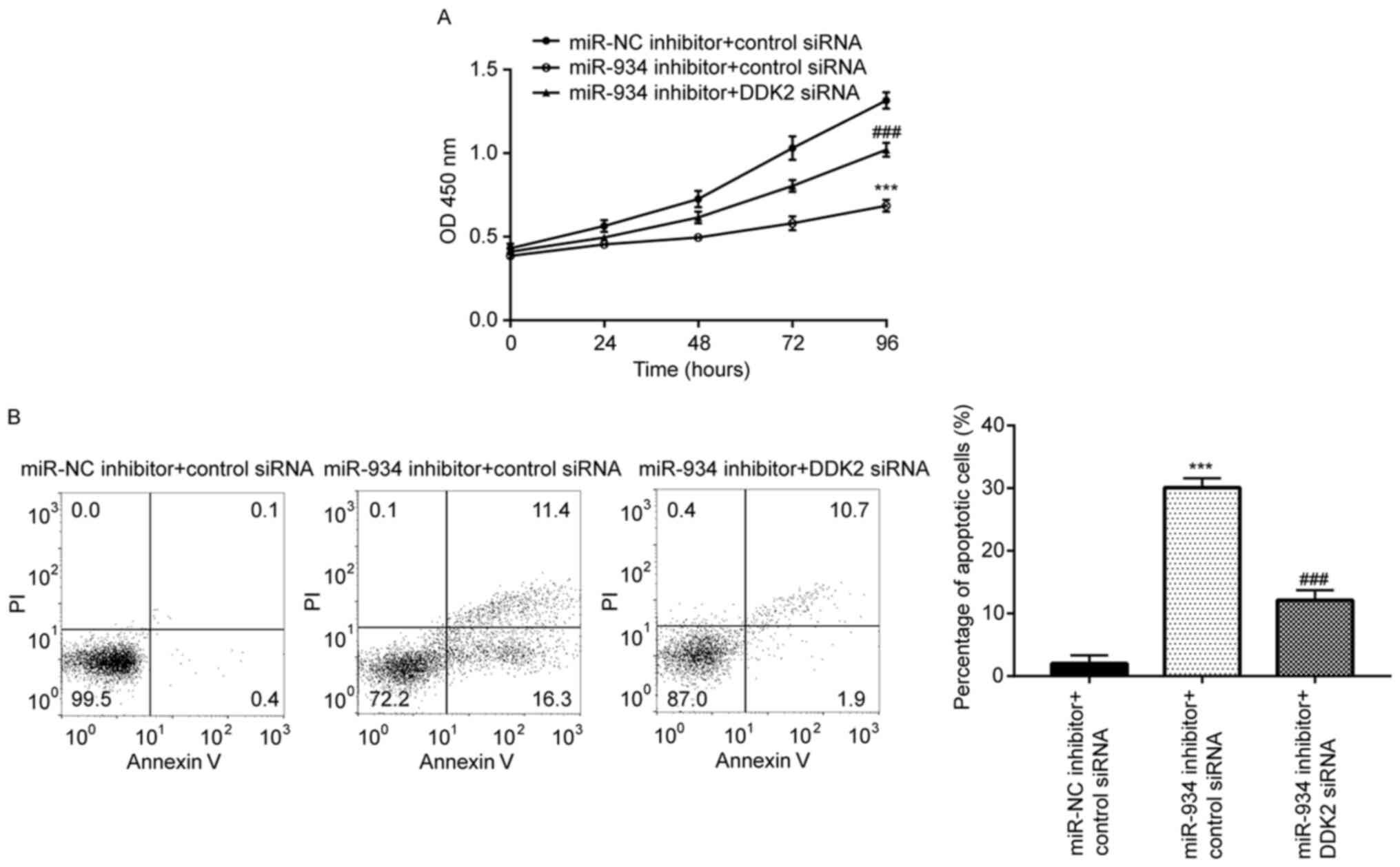
To determine the biological role of miR-934 in
colorectal cancer, the CCK-8 assay was performed to assess the
proliferative ability of HCT116 cells. The results demonstrated
that miR-934 inhibition repressed the proliferation of HCT116
cells, which was reversed following DDK2 knockdown (Fig. 5A). Flow cytometric analysis
demonstrated that miR-934 inhibition induced significant cell
apoptosis in HCT116 cells, which was also reversed following DDK2
knockdown (Fig. 5B). Collectively,
these results suggest that the miR-934/DKK2 axis plays a role in
regulating cell proliferation and apoptosis of colorectal cancer
cells.
Discussion
Aberrant expression of miRNAs is responsible for the
development of colorectal cancer (19-21).
Previously, miR-934 was discovered as a cancer-associated miRNA via
sequencing and experimental validation (14). RNA sequencing data suggested that
miR-934 was one of eight miRNAs associated with alcohol-associated
HNSCC, and the data from in vitro assays demonstrated that
miR-934 facilitated HNSCC cell proliferation and resistance to
apoptosis (16). In bladder cancer,
miR-934 promoted cell proliferation and cell cycle progression by
targeting UBE2N and downregulating CDK6(22). The results of the present study
demonstrated that miR-934 was upregulated in colorectal cancer.
Downregulation of miR-934 inhibited cell proliferation and induced
the apoptosis of HCT116 cells. Collectively, these results suggest
the oncogenic potential of miR-934 in colorectal cancer. However,
the present study did not investigate the biological functions of
miR-934 in colorectal cancer in vivo. Thus, future studies
will aim to establish animal models to determine whether miR-934
promotes colorectal tumor growth and metastasis in vivo.
Wnt/β-catenin signaling is crucial for cell
proliferation, stemness, migration, invasion and resistance to
apoptosis of colorectal cancer (23,24).
Upon activation, the transcription factor β-catenin is
phosphorylated and translocated into the nucleus to activate
transcription of its downstream target genes, such as c-Myc and
cyclin D1(25). DKK2 is an
antagonist of canonical Wnt/β-catenin signaling (26). Downregulation of DKK2 has been
reported in several types of cancer, including colorectal cancer
(27,28). The present study identified DKK2 as
a target gene of miR-934. In addition, the results demonstrated
that miR-934 activated Wnt/β-catenin signaling by targeting DKK2 in
HCT116 cells. DKK2 has previously been reported as a target gene of
miR-154 and miR-27a in in cardiac fibroblasts and bone marrow
stromal cells (29,30). Collectively, the results of the
present study suggest that miR-934 may act as a novel regulator of
DKK2. In addition, miR-934 expression was negatively associated
with DKK2 mRNA expression in the collected patient tissue samples.
DDK2 silencing reversed the biological function of miR-934
inhibition in HCT116 cells. Taken together, these results suggest
that the miR-934/DKK2 axis is involved in proliferation and
apoptosis of colorectal cancer cells.
In conclusion, the results of the present study
demonstrated a role of miR-934 in mediating colorectal cancer cell
proliferation by targeting DKK2, suggesting that miR-934 may be a
potential biomarker for patients with colorectal cancer.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WL acquired the data. LM collected clinical samples.
WL and JZ analyzed the data and wrote the manuscript. JZ supervised
the study. All authors read and approved the final manuscript. WL
and JZ confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
All procedures performed in the present study
involving human participants were supervised and approved by the
Ethics Committee of Shaanxi Provincial Cancer Hospital (approval
no. 2015-23; Xi'an, China) and written informed consent was
provided by all patients prior to the study commencement.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Joachim C, Macni J, Drame M, Pomier A,
Escarmant P, Veronique-Baudin J and Vinh-Hung V: Overall survival
of colorectal cancer by stage at diagnosis: Data from the
Martinique Cancer Registry. Medicine (Baltimore).
98(e16941)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Diao F and Cai S: Aspirin-based
chemoprevention of colorectal cancer: The role for gut microbiota.
Cancer Commun (Lond). 40:633–635. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang Y, Lin R, Ling H, Ke Y, Zeng Y, Xiong
Y, Zhou Q, Zhou F and Zhou Y: Dual inhibition of CDK4 and FYN leads
to selective cell death in KRAS-mutant colorectal cancer. Signal
Transduct Target Ther. 4(52)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Liu S, Lin H, Wang D, Li Q, Luo H, Li G,
Chen X, Li Y, Chen P, Zhai B, et al: PCDH17 increases the
sensitivity of colorectal cancer to 5-fluorouracil treatment by
inducing apoptosis and autophagic cell death. Signal Transduct
Target Ther. 4(53)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297.
2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Alvarez-Garcia I and Miska EA: MicroRNA
functions in animal development and human disease. Development.
132:4653–4662. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yu D, Han GH, Zhao X, Liu X, Xue K, Wang D
and Xu CB: MicroRNA-129-5p suppresses nasopharyngeal carcinoma
lymphangiogenesis and lymph node metastasis by targeting ZIC2. Cell
Oncol (Dordr). 43:249–261. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jacob H, Stanisavljevic L, Storli KE,
Hestetun KE, Dahl O and Myklebust MP: Identification of a
sixteen-microRNA signature as prognostic biomarker for stage II and
III colon cancer. Oncotarget. 8:87837–87847. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li Y, Zhuo ZJ, Zhou H, Liu J, Xiao Z, Xiao
Y, He J and Liu Z: miR-34b/c rs4938723 T>C decreases
neuroblastoma risk: A replication study in the hunan children. Dis
Markers. 2019(6514608)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li S, Wu X, Xu Y, Wu S, Li Z, Chen R,
Huang N, Zhu Z and Xu X: miR-145 suppresses colorectal cancer cell
migration and invasion by targeting an ETS-related gene. Oncol Rep.
36:1917–1926. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Huang W, Yan Y, Liu Y, Lin M, Ma J, Zhang
W, Dai J, Li J, Guo Q, Chen H, et al: Exosomes with low miR-34c-3p
expression promote invasion and migration of non-small cell lung
cancer by upregulating integrin α2β1. Signal Transduct Target Ther.
5(39)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Slattery ML, Herrick JS, Pellatt DF,
Stevens JR, Mullany LE, Wolff E, Hoffman MD, Samowitz WS and Wolff
RK: MicroRNA profiles in colorectal carcinomas, adenomas and normal
colonic mucosa: Variations in miRNA expression and disease
progression. Carcinogenesis. 37:245–261. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hu Y, Zhang Q, Cui J, Liao ZJ, Jiao M,
Zhang YB, Guo YH and Gao YM: Oncogene miR-934 promotes ovarian
cancer cell proliferation and inhibits cell apoptosis through
targeting BRMS1L. Eur Rev Med Pharmacol Sci. 23:5595–5602.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Saad MA, Kuo SZ, Rahimy E, Zou AE,
Korrapati A, Rahimy M, Kim E, Zheng H, Yu MA, Wang-Rodriguez J and
Ongkeko WM: Alcohol-dysregulated miR-30a and miR-934 in head and
neck squamous cell carcinoma. Mol Cancer. 14(181)2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hirata H, Hinoda Y, Nakajima K, Kawamoto
K, Kikuno N, Kawakami K, Yamamura S, Ueno K, Majid S, Saini S, et
al: Wnt antagonist gene DKK2 is epigenetically silenced and
inhibits renal cancer progression through apoptotic and cell cycle
pathways. Clin Cancer Res. 15:5678–5687. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zheng YB, Xiao K, Xiao GC, Tong SL, Ding
Y, Wang QS, Li SB and Hao ZN: MicroRNA-103 promotes tumor growth
and metastasis in colorectal cancer by directly targeting LATS2.
Oncol Lett. 12:2194–2200. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhao D, Ma Y, Li X and Lu X: microRNA-211
promotes invasion and migration of colorectal cancer cells by
targeting FABP4 via PPARγ. J Cell Physiol: Feb 26, 2019. doi:
10.1002/jcp.28190. (Epub ahead of print).
|
|
21
|
Zhang Z, Zhong X, Xiao Y and Chen C:
MicroRNA-296 inhibits colorectal cancer cell growth and enhances
apoptosis by targeting ARRB1-mediated AKT activation. Oncol Rep.
41:619–629. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yan H, Ren S, Lin Q, Yu Y, Chen C, Hua X,
Jin H, Lu Y, Zhang H, Xie Q, et al: Inhibition of UBE2N-dependent
CDK6 protein degradation by miR-934 promotes human bladder cancer
cell growth. FASEB J. 33:12112–12123. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chen G, Gao C, Gao X, Zhang DH, Kuan SF,
Burns TF and Hu J: Wnt/β-catenin pathway activation mediates
adaptive resistance to BRAF inhibition in colorectal cancer. Mol
Cancer Ther. 17:806–813. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bahrami A, Amerizadeh F, ShahidSales S,
Khazaei M, Ghayour-Mobarhan M, Sadeghnia HR, Maftouh M, Hassanian
SM and Avan A: Therapeutic potential of targeting Wnt/β-catenin
pathway in treatment of colorectal cancer: Rational and progress. J
Cell Biochem. 118:1979–1983. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kleszcz R, Szymanska A, Krajka-Kuzniak V,
Baer-Dubowska W and Paluszczak J: Inhibition of CBP/β-catenin and
porcupine attenuates Wnt signaling and induces apoptosis in head
and neck carcinoma cells. Cell Oncol (Dordr). 42:505–520.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhu J, Zhang S, Gu L and Di W: Epigenetic
silencing of DKK2 and Wnt signal pathway components in human
ovarian carcinoma. Carcinogenesis. 33:2334–2343. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kawakita A, Yanamoto S, Yamada S, Naruse
T, Takahashi H, Kawasaki G and Umeda M: MicroRNA-21 promotes oral
cancer invasion via the Wnt/β-catenin pathway by targeting DKK2.
Pathol Oncol Res. 20:253–261. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Deng F, Zhou R, Lin C, Yang S, Wang H, Li
W, Zheng K, Lin W, Li X, Yao X, et al: Tumor-secreted dickkopf2
accelerates aerobic glycolysis and promotes angiogenesis in
colorectal cancer. Theranostics. 9:1001–1014. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sun LY, Bie ZD, Zhang CH, Li H, Li LD and
Yang J: MiR-154 directly suppresses DKK2 to activate Wnt signaling
pathway and enhance activation of cardiac fibroblasts. Cell Biol
Int. 40:1271–1279. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wu X, Gu Q, Chen X, Mi W, Wu T and Huang
H: MiR-27a targets DKK2 and SFRP1 to promote reosseointegration in
the regenerative treatment of peri-implantitis. J Bone Miner Res.
34:123–134. 2019.PubMed/NCBI View Article : Google Scholar
|